- 1Division of Cardiology, Department of Internal Medicine, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea
- 2Division of Cardiology, Department of Internal Medicine, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
- 3Division of Cardiology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea
Background: Although ticagrelor monotherapy after 3-month dual antiplatelet therapy (DAPT) results in a significantly greater net clinical benefit over that with ticagrelor-based 12-month DAPT in patients with acute coronary syndrome (ACS), it remains uncertain whether this effect is dependent on body mass index (BMI). We aimed to evaluate the BMI-dependent effect of these treatment strategies on clinical outcomes.
Methods: This was a pre-specified subgroup analysis from the TICO trial (Ticagrelor Monotherapy After 3 Months in Patients Treated With New Generation Sirolimus-eluting Stent for Acute Coronary Syndrome), evaluating the interaction between BMI and treatment strategies for the primary outcome [composite of major bleeding and adverse cardiac and cerebrovascular events (MACCE): death, myocardial infarction, stent thrombosis, stroke, or target-vessel revascularization]. The secondary outcomes were major bleeding and MACCE.
Results: Based on a pre-specified BMI threshold of 25 kg/m2, 3,056 patients were stratified. Patients with BMI <25 kg/m2 had a higher risk of primary and secondary outcomes than those with BMI ≥25 kg/m2. Regardless of the BMI subgroup, the effects of ticagrelor monotherapy after 3-month DAPT on the primary outcome (pint = 0.61), major bleeding (pint = 0.76), and MACCE (pint = 0.80) were consistent without significant interaction compared with ticagrelor-based 12-month DAPT. The treatment effects according to the BMI quartiles and age, sex, and diabetic status were also consistent without significant interaction.
Conclusion: The BMI-dependent impact of ticagrelor monotherapy after 3-month DAPT compared with 12-month DAPT on clinical outcomes was not heterogeneous in patients with ACS.
Clinical Trial Registration: [www.ClinicalTrials.gov], identifier [NCT02494895].
Introduction
Body mass index (BMI) is a commonly used obesity indicator and independently affects the cardiovascular outcomes in patients with established coronary artery disease (1–3). Numerous studies have demonstrated that patients with a lower or normal BMI tend to be at a greater risk of ischemic and bleeding events after undergoing percutaneous coronary intervention (PCI) compared with those with a higher BMI (1–3). In addition, BMI can affect the efficacy of dual antiplatelet therapy (DAPT) (4, 5). Therefore, evaluating the efficacy and safety of different post-PCI antiplatelet strategies stratified according to BMI may be important for developing an optimal antiplatelet therapy, which considers individual ischemic and bleeding risks.
Recently, the Ticagrelor Monotherapy After 3 Months in Patients Treated With New Generation Sirolimus-Eluting Stent for Acute Coronary Syndrome (TICO) trial showed the superiority of ticagrelor monotherapy after 3-month DAPT over the currently recommended ticagrelor-based 12-month DAPT in the occurrence of net clinical adverse events in patients with acute coronary syndrome (ACS) (6). However, whether the benefit of this TICO trial strategy is BMI dependent remains unclear. Thus, this pre-specified subgroup analysis of the TICO trial conducted an evaluation for the BMI effect on the efficacy and safety of ticagrelor monotherapy after 3-month DAPT versus ticagrelor-based 12-month DAPT after drug-eluting stent (DES) implantation.
Materials and methods
Study design and population
This study is a pre-specified subgroup analysis of the TICO trial. The TICO trial was a multicenter randomized trial investigating the net clinical benefit of ticagrelor monotherapy after 3-month DAPT compared with ticagrelor-based 12-month DAPT in patients with ACS who underwent PCI with ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro; Biotronik AG, Bülach, Switzerland). Detailed explanations, including inclusion and exclusion criteria, have already been described (6, 7). The trial was approved by the institutional review board at each center and was performed in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent.
The TICO trial randomly assigned patients in a 1:1 fashion to receive either ticagrelor monotherapy after 3-month DAPT or ticagrelor-based 12-month DAPT after DES implantation. The baseline BMI was calculated as kg/m2 and collected at the time of randomization. For the present investigation on BMI and clinical outcomes, patients were divided into two pre-specified groups: patients with BMI ≥25 and <25 kg/m2, respectively (Figure 1A) (6). The Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRCECISE-DAPT) score of each patient was calculated using the online calculator with 5 variables in the database (age, hemoglobin, white blood cell count, creatinine clearance, and previous history of bleeding). The high bleeding risk was defined as the PRECISE-DAPT score ≥25 according to previous study (8).
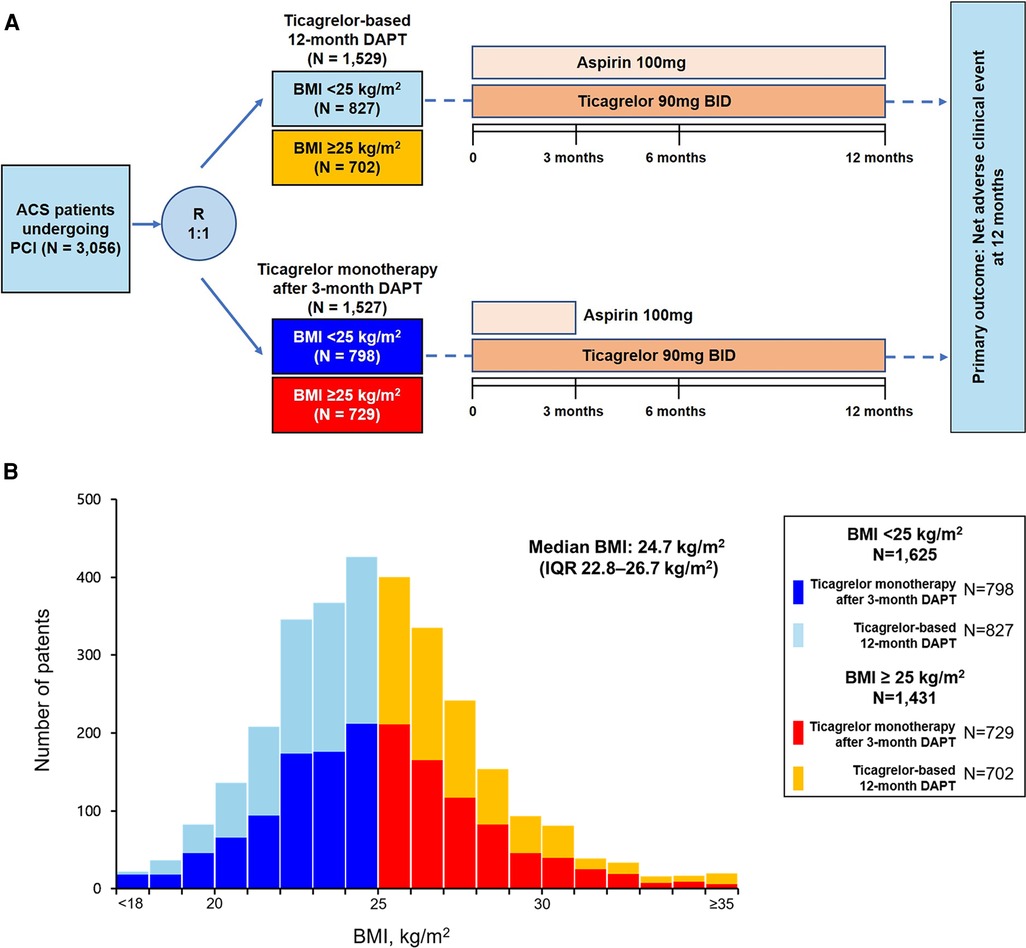
Figure 1. Flowchart of the present study and distribution of BMI. (A) Study flow, (B) distribution of BMI stratified by antiplatelet therapy strategies. ACS, acute coronary syndrome; BMI, body mass index; DAPT, dual antiplatelet therapy; IQR, interquartile range; PCI, percutaneous coronary intervention.
Study outcomes
The primary outcome was the occurrence of a net adverse clinical event, defined as a composite of major adverse cardiac and cerebrovascular events (MACCE) and bleeding, 12 months after PCI (6). MACCE was defined as a composite of all-cause death, myocardial infarction, stent thrombosis, stroke, and target-vessel revascularization (6). Major bleeding was defined according to the Thrombolysis in Myocardial Infarction criteria: intracranial bleeding, hemorrhage with at least 5 g/dl decrease in hemoglobin, or fatal bleeding causing death within 7 days (6). The secondary outcomes were major bleeding and MACCE separately. Other individual primary outcome components, cardiac death, major or minor bleeding, and a composite of cardiac death, myocardial infarction, stent thrombosis, or target-vessel revascularization were also analyzed. Additional bleeding endpoints included the bleeding events according to the Bleeding Academic Research Consortium (BARC) criteria type 3 or 5 (9).
Statistical analysis
BMI-dependent analyses were performed on an intention-to-treat basis. Continuous variables are expressed as mean ± standard deviation and categorical data as frequencies. Baseline and procedural characteristics among the groups were compared using the Student's t-test or Mann–Whitney U test for continuous variables and Chi-square or Fisher's exact test for categorical variables. Log-rank and Kaplan–Meier tests were used to compare the adverse outcome rates according to antiplatelet strategies. Hazard ratios (HRs) for clinical outcomes were assessed using an unadjusted Cox regression model and are shown with 95% confidence interval (CI). The effect heterogeneity among the subgroups was assessed using interaction terms in the Cox proportional hazard model. Proportional hazards models using restricted cubic splines with 3 knots were developed to explore the association between probability of net adverse clinical event, major bleeding, and MACCE, and BMI as continuous variable and depicted graphically. All tests were two-sided. Statistical significance was set at p < 0.05. Statistical analyses were performed using the R Statistical Software (version 3.5.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
The BMI distribution among the patients is presented in Figure 1B. The median BMI was 24.7 (interquartile range, 22.8–26.7) kg/m2. Density plotting showed no significant difference in the BMI distributions between the two antiplatelet strategy groups (Supplementary Figure S1). Of the 3,056 patients randomized in the TICO trial, 1,625 patients (53.2%) had BMIs <25 kg/m2. The baseline characteristics according to the BMI groups and antiplatelet strategies are summarized in Table 1. No significant differences were found in the baseline characteristics according to the antiplatelet strategies in the patients from both the BMI subgroups, except for those who underwent PCI with a transradial approach. However, the baseline characteristics varied according to BMI; patients with BMI <25 kg/m2 were older (62.9 ± 10.1 years vs. 58.7 ± 11.0 years; p < 0.001), more likely to be female (23.0% vs. 17.8%; p = 0.001), had a lower prevalence of hypertension (46.2% vs. 55.2%; p < 0.001) or dyslipidemia (56.1% vs. 65.3%; p < 0.001), and lower hemoglobin levels (14 ± 1.8 vs. 14.6 ± 1.7 g/dl; p < 0.001) and ejection fractions (54.5% vs. 56.7%; p = 0.019), and were more frequently to be at a high bleeding risk (19.6% vs. 15.2%; p = 0.001) compared with those with BMI ≥25 kg/m2 (Table 1 and Supplementary Table S1).
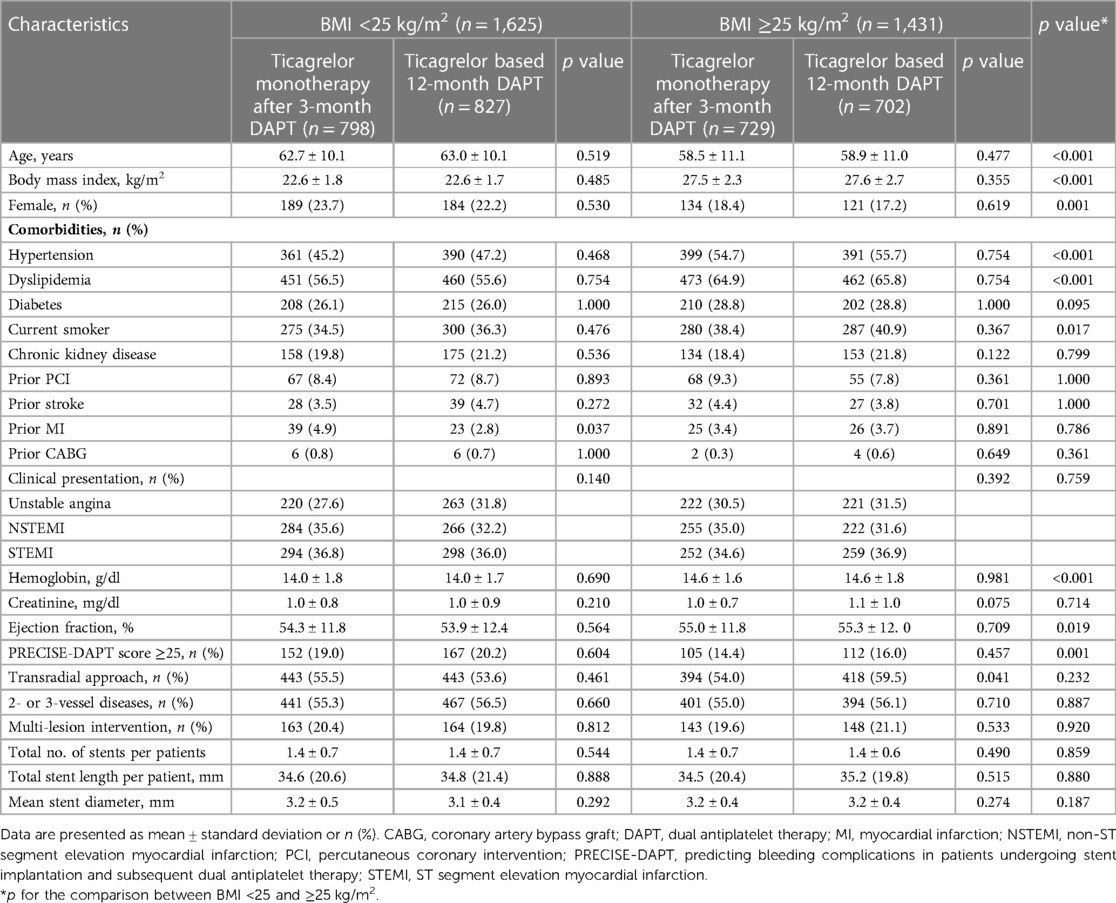
Table 1. Baseline characteristics according to the pre-specified BMI subgroups and antiplatelet strategy.
Effect of BMI on primary and secondary outcomes
The primary outcome of the net adverse clinical event occurred more frequently in patients with BMI <25 kg/m2 than those with BMI ≥25 kg/m2 [100 of 1,625 patients (6.2%) vs. 48 of 1,431 patients (3.4%); p < 0.001] (Figure 2A). Regarding the secondary outcomes, major bleeding [47 of 1,625 patients (2.9%) vs. 23 of 1,431 patients (1.6%); p = 0.018] and MACCE [56 of 1,625 patients (3.4%) vs. 30 of 1,431 patients (2.1%); p = 0.025] occurred more frequently in patients with BMI <25 kg/m2 than those with BMI ≥25 kg/m2 (Figures 2B,C).
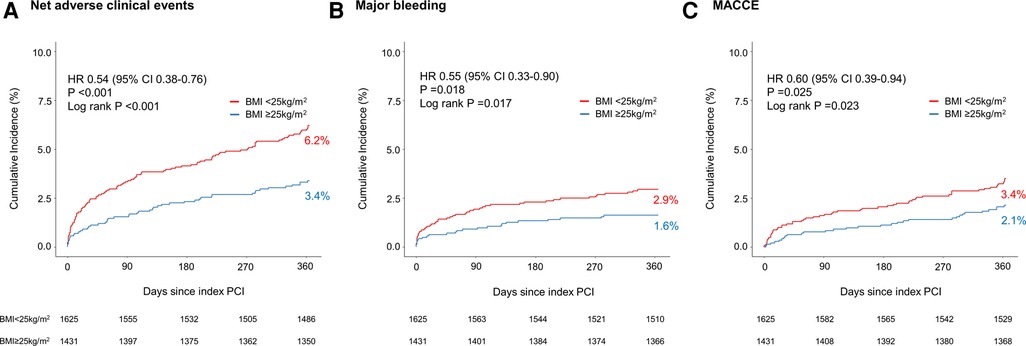
Figure 2. Kaplan–Meier curves for the clinical outcomes between pre-specified BMI subgroups of TICO population. (A) Net adverse clinical events, (B) major bleeding, and (C) major adverse cardiac and cerebrovascular events in patients with either BMI <25 or ≥25 kg/m2. BMI, body mass index; CI, confidence interval; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events; PCI, percutaneous coronary intervention.
Effect of antiplatelet strategy on primary and secondary outcomes according to BMI
In patients with BMI <25 kg/m2, ticagrelor monotherapy after 3-month DAPT resulted in a significant primary outcome reduction than ticagrelor-based 12-month DAPT (4.8% vs. 7.5%; HR 0.63; 95% CI 0.42–0.94; p = 0.024) (Table 2 and Figure 3A). In patients with BMI ≥25 kg/m2, ticagrelor monotherapy after 3-month DAPT compared with ticagrelor-based 12-month DAPT showed a lower primary outcome incidence (2.9% vs. 3.8%; HR 0.76; 95% CI 0.43–1.33; p = 0.337) without significant group interaction (p for interaction = 0.61) (Table 2 and Figure 3A).
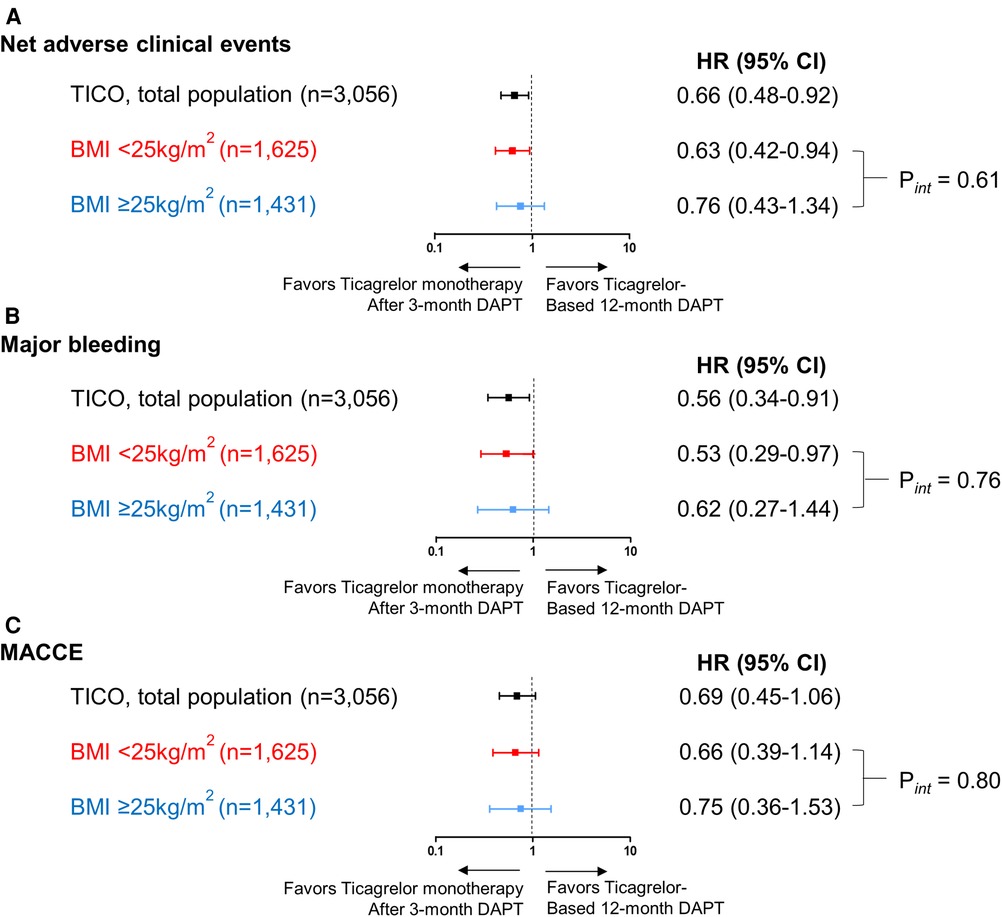
Figure 3. Risk for the primary outcome, major bleeding and MACCE according to the pre-specified BMI subgroups and antiplatelet strategies. Risk for (A) net clinical adverse events, (B) major bleeding and (C) MACCE according to the pre-specified BMI subgroups (BMI <25 and ≥25 kg/m2) and antiplatelet strategies. The squares indicate estimated hazard ratio, and the horizontal lines indicate 95% CI. P for interaction values were derived from Cox regression model. BMI, body mass index; CI, confidence interval; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events.
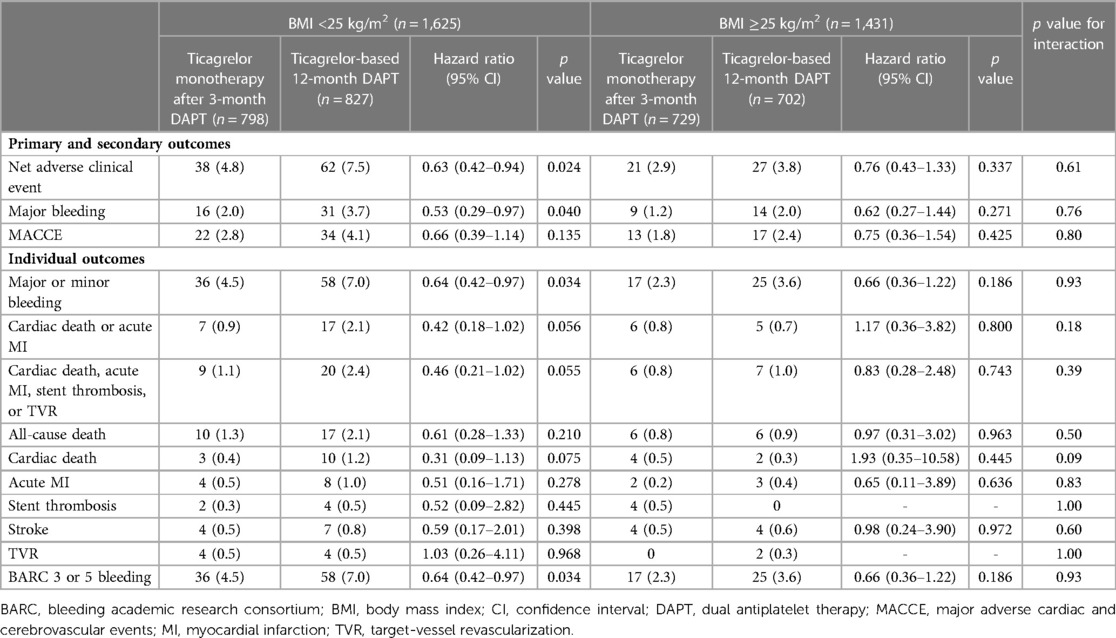
Table 2. Clinical outcomes according to the pre-specified BMI groups and antiplatelet strategy at 12 months follow-up.
Ticagrelor monotherapy after 3-month DAPT showed a lower incidence of major bleeding compared with that of ticagrelor-based 12-month DAPT in patients with BMI <25 kg/m2 (Table 2 and Figure 3B). In patients with BMI ≥25 kg/m2, fewer bleeding events occurred following ticagrelor monotherapy after 3-month DAPT than ticagrelor-based 12-month DAPT without statistical significance. The occurrences of MACCE with the two antiplatelet strategies were not significantly different in both the BMI subsets (Table 2 and Figure 3C). There were no interactions between the BMI subgroup and antiplatelet strategy for major bleeding or MACCE (p for interaction = 0.76 or 0.80, respectively).
The pre-specified 3-month landmark analysis revealed that regardless of BMI <25 or ≥25 kg/m2, the primary outcome incidence was significantly lower in the ticagrelor monotherapy after 3-month DAPT group than in the ticagrelor-based 12-month DAPT group (Figures 4A,B). Similar trends of a lower incidence of major bleeding (Figures 4C,D) and MACCE (Figures 4E,F) in the ticagrelor monotherapy after 3-month DAPT group than in the ticagrelor-based 12-month DAPT group were observed, regardless of the BMI subgroups. Treatment effect analysis according to the BMI quartiles (Q1 to Q4) revealed that the effect of ticagrelor monotherapy after 3-month DAPT was remarkable in patients with BMI quartile 2; however, there was no significant interaction between the BMI quartiles and treatment effects (p for interaction = 0.79) (Supplementary Figure S2). When examined as a continuous variable, there were no significant interactions between the antiplatelet strategies and BMI in terms of net clinical adverse event, major bleeding, and MACCE (Supplementary Figure S3).
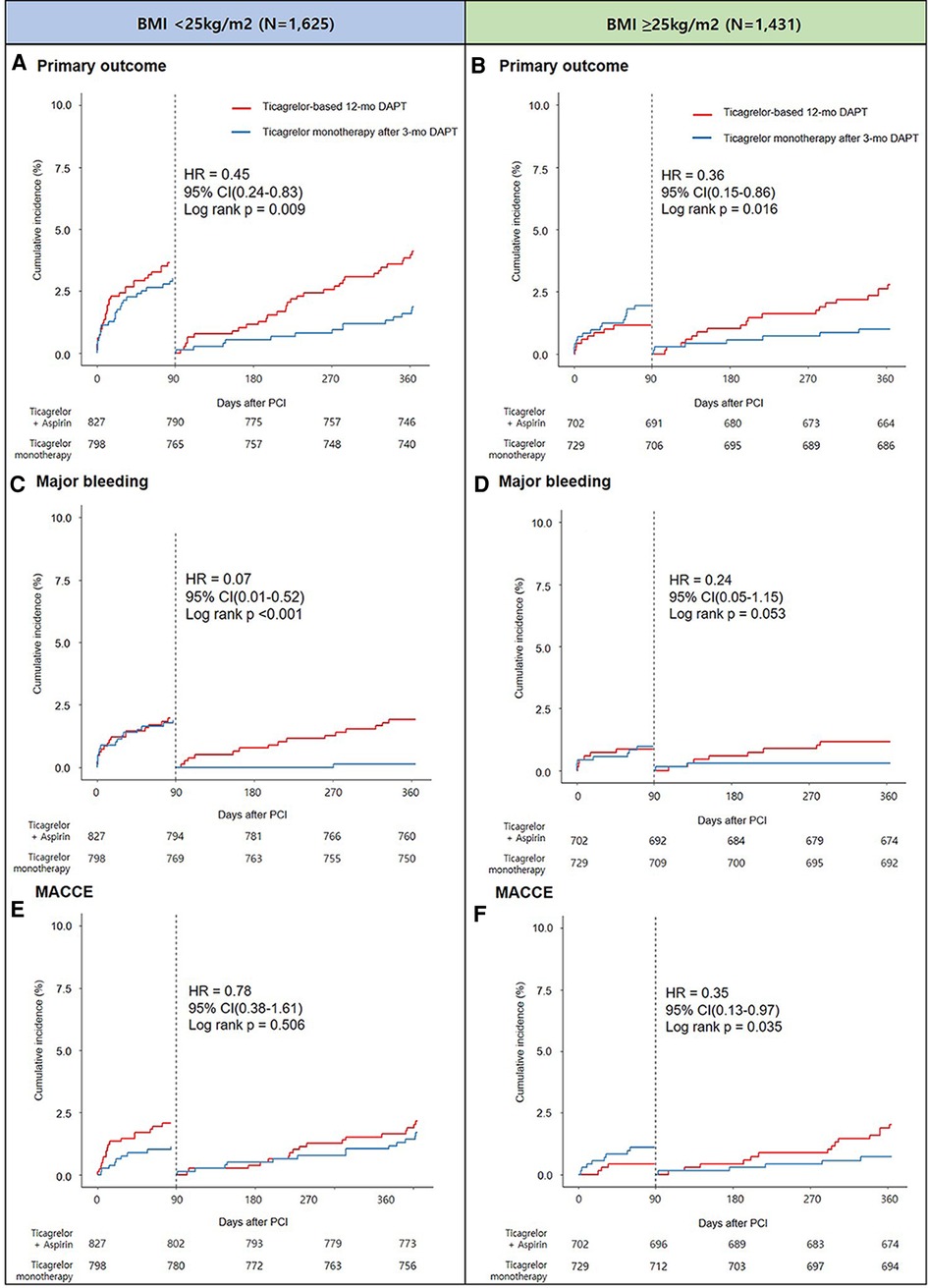
Figure 4. Landmark analysis at 3 months and Kaplan-Meier estimates for primary outcome in patients with BMI <25 kg/m2 (A) and BMI ≥25 kg/m2 (B), major bleeding in patients with BMI <25 kg/m2 (C) and BMI ≥25 kg/m2 (D), and MACCE in patients with BMI <25 kg/m2 (E) and BMI ≥25 kg/m2 (F).
Effect of antiplatelet strategies on primary outcome according to BMI and other covariates, such as age, sex, and diabetes
Interactions between the BMI groups and treatment strategies for the primary outcome according to covariates, such as age, sex, and diabetes, are shown in Supplementary Figure S4. There were no between-group differences in the net adverse clinical events of the two antiplatelet strategies, regardless of the age group (<65 years, p for interaction = 0.16; ≥65 years, p for interaction = 0.28), sex (men, p for interaction = 0.72; women, p for interaction = 0.18), or diabetic status (diabetic, p for interaction = 0.55; non-diabetic, p for interaction = 0.93).
Discussion
In this pre-specified sub-analysis from the TICO trial, we found that: (i) the occurrences of the primary outcome, major bleeding, and MACCE were significantly higher in patients with ACS with low (<25 kg/m2) rather than high (≥25 kg/m2) BMIs; (ii) there were no significant interactions between the antiplatelet strategies and BMI subgroups for the primary outcome, major bleeding, and MACCE, which suggest a consistent BMI-independent effect of ticagrelor monotherapy after 3-month DAPT compared with that of ticagrelor-based 12-month DAPT; and (iii) the effect of ticagrelor monotherapy was consistent across the BMI subgroups, despite other major covariates, such as age (<65 or ≥65 years), sex (men or women), and diabetic status.
BMI may affect the occurrence of ischemic or bleeding. A higher BMI, such as that indicating obesity, is a well-known cardiometabolic risk factor and could be associated with a highly prothrombotic condition due to the increased risk of impaired endothelial function, platelet activation via enhanced oxidative stress, and consequent proinflammatory status, which may accelerate thrombogenicity by increasing the intrinsic platelet reactivity (10, 11). However, several studies have reported a paradoxical association between higher BMI and lower incidence of adverse outcomes, including ischemic events and mortality, called “obesity paradox” (2, 3, 12). The present study was congruent with these previous ones, and demonstrated that patients with low BMI (<25 kg/m2) had a greater risk of ischemic events than those with high BMI (≥25 kg/m2). Patients with a lower BMI who underwent PCI tend to be older, more fragile, and have more frequent renal insufficiency episodes compared with those with a higher BMI (3), which could increase the bleeding risk. Additionally, an association between low BMI and increased post-PCI bleeding risk has been reported in previous observational studies (13, 14), and this consistent association was observed in our study. Therefore, the BMI-dependent effect, especially on a novel antiplatelet therapy after DES implantation needs to be further assessed.
Pharmacokinetic parameters or metabolic regulation of drugs can be altered based on the BMI (15). According to previous observational studies, high BMI was an independent predictor of high residual platelet reactivity with DAPT containing clopidogrel (16, 17). The beneficial effect of ticagrelor compared with clopidogrel was remarkable when body weight was higher than the median value for each sex in the PLATO trial (18). Another study revealed that the inhibition of platelet aggregation after a high loading dose of 600 mg of clopidogrel was less effective in overweight patients compared with normal-weight patients (19), suggesting that the effectiveness of antiplatelet strategies might vary according to the BMI.
In this study, there was no significant interaction between the ticagrelor-based short DAPT strategy and BMI subgroups for the primary and secondary ischemic or bleeding outcomes, which can be attributed to the following reasons. Unlike clopidogrel, ticagrelor is an active metabolite and does not require metabolic activation in the liver (20); therefore, it may be less affected by BMI and prevent the increased residual platelet reactivity observed with clopidogrel use in overweight patients, even after early aspirin discontinuation (16). Although few studies have reported the effects of ticagrelor according to BMI, Nardin et al. reported that there was no evidence that BMI had a significant influence on the effectiveness of ticagrelor maintenance treatment (16). In addition, regarding the DAPT duration with a potent P2Y12 inhibitor regimen, the present subgroup analysis of the TICO trial found that ticagrelor monotherapy after 3-month DAPT showed a consistently beneficial effect in terms of net clinical benefits compared with that of ticagrelor-based 12-month DAPT in patients with both high and low BMIs. Our results suggest that there may be a low attenuation risk of the ticagrelor effect with increasing BMI, and that an aspirin-free strategy can safely decrease the bleeding risk irrespective of BMI. Thus, ticagrelor monotherapy after short-term DAPT might be a safe and effective strategy in patients with ACS after PCI across the BMI groups.
Study limitations
Our study had some limitations. First, although this was a pre-specified subgroup analysis, the BMI subgroups were not specifically powered for the occurrence of the primary or secondary outcomes. Thus, the results obtained in these subgroups should be considered as hypothesis-generating results. Second, because this study was conducted based on the baseline BMI data, the effects of BMI fluctuation or changes during the follow-up period were not evaluated. Third, a BMI cut-off value of 25 kg/m2 was used for this analysis, which is the cut-off for distinguishing normal weight and overweight according to the World Health Organization standards. In addition, our threshold was close to the median value of 24.7 kg/m2, which allows uniform statistical power in the BMI groups. Fourth, our study population comprised a homogeneous Korean ethnicity. Because of anthropometric differences, including body physique and BMI, between eastern and western populations, generalization of our results for other ethnic populations should be performed with caution. Fifth, since the platelet function test was not routinely performed in our cohort, pharmacodynamic data responses to antiplatelet therapy was not available in this study. Given the fact that obesity was found to be an independent predictor of high residual platelet reactivity to DAPT in previous study, further studies on platelet function-guided antiplatelet therapy in cohort involving a larger number of obese patients are needed. Finally, since the TICO trial was conducted exclusively in patients who underwent ultrathin sirolimus-eluting stent implantation, generalization of our results for populations treated with other DESs should be undertaken with caution.
Conclusion
Overall, the benefit of ticagrelor monotherapy after 3-month DAPT over ticagrelor-based 12-month DAPT on net adverse clinical events was uniform across the BMI subgroups in patients with ACS who underwent ultrathin sirolimus-eluting stent implantation. Treatment effects with respect to major bleeding and MACCE were also similar in the BMI subsets. These findings suggest that ticagrelor monotherapy after 3-month DAPT may be an attractive and safe alternative treatment for patients with ACS, regardless of their BMIs.
Data availability statement
The datasets generated for the analyses are not publicly available because of strict government restrictions. Requests to access these datasets should be directed to B-K Kim,a2ltYmtAeXVocy5hYw==.
Ethics statement
The studies involving human participants were reviewed and approved by Yonsei University Health System. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
BK and S-JH did the data analyses and wrote the original draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Cardiovascular Research Center, Seoul, Republic of Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1128834/full#supplementary-material.
References
1. Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. (2010) 3:660–8. doi: 10.1016/j.jcin.2010.03.018
2. Holroyd EW, Sirker A, Kwok CS, Kontopantelis E, Ludman PF, De Belder MA, et al. The relationship of body mass index to percutaneous coronary intervention outcomes: does the obesity paradox exist in contemporary percutaneous coronary intervention cohorts? Insights from the British cardiovascular intervention society registry. JACC Cardiovasc Interv. (2017) 10:1283–92. doi: 10.1016/j.jcin.2017.03.013
3. Kim BG, Hong SJ, Kim BK, Ahn CM, Shin DH, Kim JS, et al. Association between body mass index and clinical outcomes after new-generation drug-eluting stent implantation: Korean multi-center registry data. Atherosclerosis. (2018) 277:155–62. doi: 10.1016/j.atherosclerosis.2018.08.047
4. Rao SV, McCoy LA, Spertus JA, Krone RJ, Singh M, Fitzgerald S, et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the national cardiovascular data registry CathPCI registry. JACC Cardiovasc Interv. (2013) 6:897–904. doi: 10.1016/j.jcin.2013.04.016
5. Cho JY, Lee SY, Yun KH, Kim BK, Hong SJ, Ko JS, et al. Factors related to major bleeding after ticagrelor therapy: results from the TICO trial. J Am Heart Assoc. (2021) 10:e019630. doi: 10.1161/JAHA.120.019630
6. Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. J Am Med Assoc. (2020) 323:2407–16. doi: 10.1001/jama.2020.7580
7. Kim C, Hong SJ, Shin DH, Kim BK, Ahn CM, Kim JS, et al. Randomized evaluation of ticagrelor monotherapy after 3-month dual-antiplatelet therapy in patients with acute coronary syndrome treated with new-generation sirolimus-eluting stents: TICO trial rationale and design. Am Heart J. (2019) 212:45–52. doi: 10.1016/j.ahj.2019.02.015
8. Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. (2017) 389:1025–34. doi: 10.1016/S0140-6736(17)30397-5
9. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
10. Freedman JE. Oxidative stress and platelets. Arterioscler Thromb Vasc Biol. (2008) 28(3):s11–6. doi: 10.1161/ATVBAHA.107.159178
11. Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, et al. Platelet activation in obese women: role of inflammation and oxidant stress. J Am Med Assoc. (2002) 288:2008–14. doi: 10.1001/jama.288.16.2008
12. Neeland IJ, Das SR, Simon DN, Diercks DB, Alexander KP, Wang TY, et al. The obesity paradox, extreme obesity, and long-term outcomes in older adults with ST-segment elevation myocardial infarction: results from the NCDR. Eur Heart J Qual Care Clin Outcomes. (2017) 3:183–91. doi: 10.1093/ehjqcco/qcx010
13. Ndrepepa G, Fusaro M, Cassese S, Guerra E, Schunkert H, Kastrati A. Relation of body mass index to bleeding during percutaneous coronary interventions. Am J Cardiol. (2015) 115:434–40. doi: 10.1016/j.amjcard.2014.11.022
14. Numasawa Y, Kohsaka S, Miyata H, Kawamura A, Noma S, Suzuki M, et al. Impact of body mass index on in-hospital complications in patients undergoing percutaneous coronary intervention in a Japanese real-world multicenter registry. PLoS One. (2015) 10:e0124399. doi: 10.1371/journal.pone.0124399
15. Badimon L, Hernandez Vera R, Padro T, Vilahur G. Antithrombotic therapy in obesity. Thromb Haemost. (2013) 110:681–8. doi: 10.1160/TH12-12-0928
16. Nardin M, Verdoia M, Sartori C, Pergolini P, Rolla R, Barbieri L, et al. Body mass index and platelet reactivity during dual antiplatelet therapy with clopidogrel or ticagrelor. J Cardiovasc Pharmacol. (2015) 66:364–70. doi: 10.1097/FJC.0000000000000288
17. Golukhova EZ, Grigoryan MV, Ryabinina MN, Bulaeva NI, Serebruany VL. Body mass index and plasma P-selectin before coronary stenting predict high residual platelet reactivity at 6 months on dual antiplatelet therapy. Cardiology. (2018) 139:132–6. doi: 10.1159/000485555
18. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
19. Sibbing D, von Beckerath O, Schomig A, Kastrati A, von Beckerath N. Impact of body mass index on platelet aggregation after administration of a high loading dose of 600 mg of clopidogrel before percutaneous coronary intervention. Am J Cardiol. (2007) 100:203–5. doi: 10.1016/j.amjcard.2007.02.081
Keywords: acute coronary sydrome, body mass index, dual antiplatelet therapy, ticagrelor, drug eluting stent
Citation: Kim BG, Hong S-J, Kim B-K, Lee Y-J, Lee S-J, Ahn C-M, Shin D-H, Kim J-S, Ko Y-G, Choi D, Hong M-K and Jang Y (2023) Body mass index affecting ticagrelor monotherapy vs. ticagrelor with aspirin in patients with acute coronary syndrome: A pre-specified sub-analysis of the TICO randomized trial. Front. Cardiovasc. Med. 10:1128834. doi: 10.3389/fcvm.2023.1128834
Received: 21 December 2022; Accepted: 16 March 2023;
Published: 30 March 2023.
Edited by:
Yao-Jun Zhang, Xuzhou Medical University, ChinaReviewed by:
Paul Guedeney, Hôpitaux Universitaires Pitié Salpêtrière, FranceGeorg Gelbenegger, Medical University of Vienna, Austria
© 2023 Kim, Hong, Kim, Lee, Lee, Ahn, Shin, Kim, Ko, Choi, Hong and Jang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Byeong-Keuk Kim a2ltYmtAeXVocy5hYw==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Coronary Artery Disease, a section of the journal Frontiers in Cardiovascular Medicine
 Byung Gyu Kim
Byung Gyu Kim Sung-Jin Hong
Sung-Jin Hong Byeong-Keuk Kim
Byeong-Keuk Kim Yong-Joon Lee2
Yong-Joon Lee2 Seung-Jun Lee
Seung-Jun Lee Jung-Sun Kim
Jung-Sun Kim Young-Guk Ko
Young-Guk Ko Donghoon Choi
Donghoon Choi Myeong-Ki Hong
Myeong-Ki Hong