- 13rd Department of Internal Medicine, First Faculty of Medicine, General University Hospital, Charles University, Prague, Czechia
- 2Dialysis Center Ohradni, B. Braun Avitum, Prague, Czechia
- 3Dialysis Center Taborska, B. Braun Avitum, Prague, Czechia
- 4Department of Nephrology, General University Hospital, First Faculty of Medicine, Charles University, Prague, Czechia
- 5Dialysis Center Cerny Most, B. Braun Avitum, Prague, Czechia
- 6Dialysis Center Motol, Fresenius Medical Care, Prague, Czechia
- 7Dialysis Center Uhersky Brod, B. Braun Avitum, Uhersky Brod, Czechia
Introduction: Heart failure (HF) is a serious complication of end-stage kidney disease (ESKD). However, most data come from retrospective studies that included patients on chronic hemodialysis at the time of its initiation. These patients are frequently overhydrated, which significantly influences the echocardiogram findings. The primary aim of this study was to analyze the prevalence of heart failure and its phenotypes. The secondary aims were (1) to describe the potential of N-terminal pro-brain natriuretic peptide (NTproBNP) for HF diagnosis in ESKD patients on hemodialysis, (2) to analyze the frequency of abnormal left ventricular geometry, and (3) to describe the differences between various HF phenotypes in this population.
Methods: We included all patients on chronic hemodialysis for at least 3 months from five hemodialysis units who were willing to participate, had no living kidney transplant donor, and had a life expectancy longer than 6 months at the time of inclusion. Detailed echocardiography together with hemodynamic calculations, dialysis arteriovenous fistula flow volume calculation, and basic lab analysis were performed in conditions of clinical stability. Excess of severe overhydration was excluded by clinical examination and by employing bioimpedance.
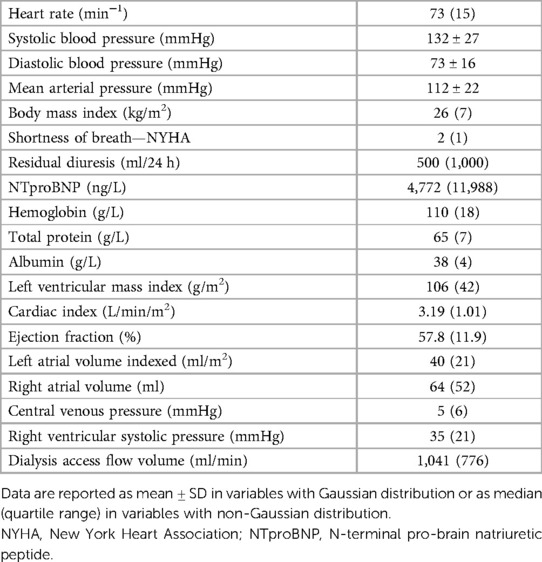
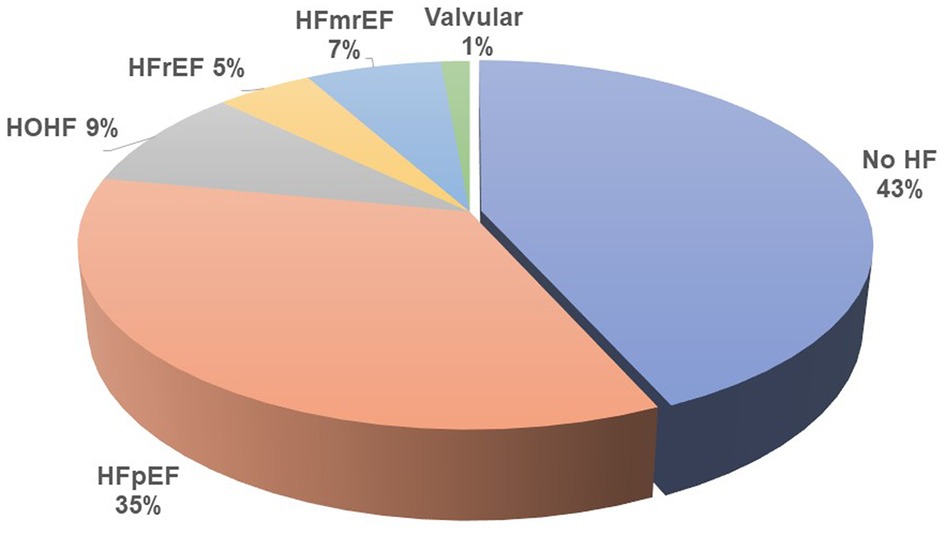
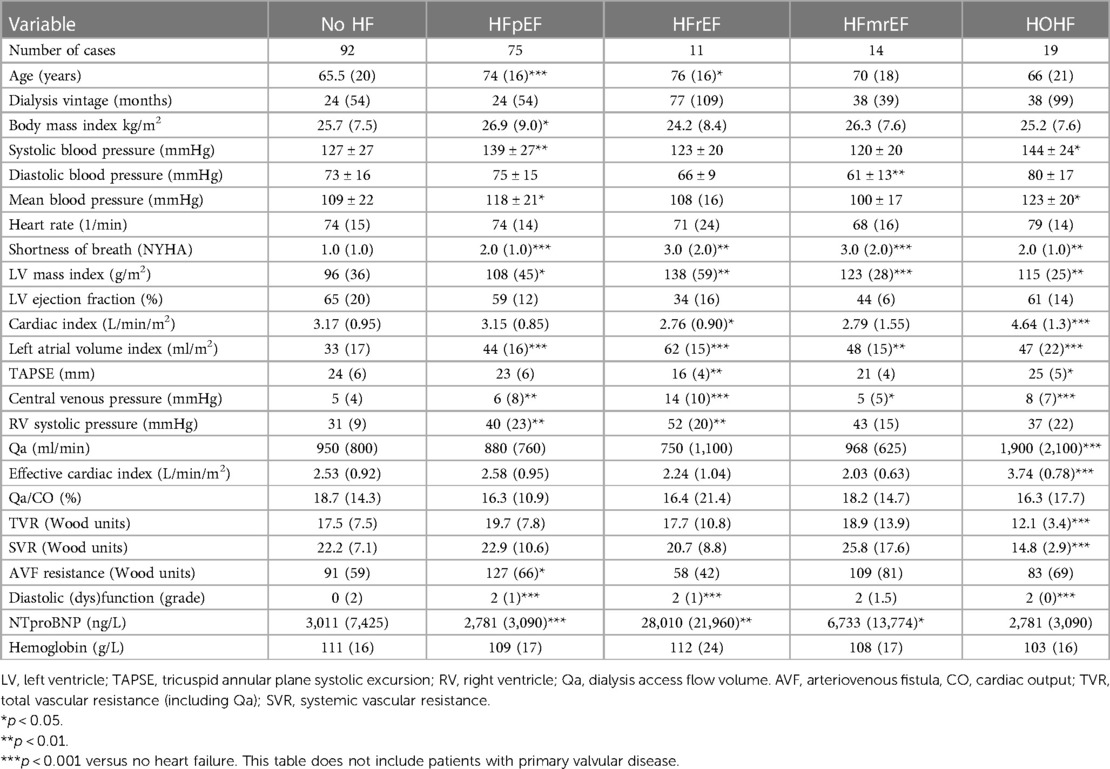
Results: A total of 214 patients aged 66.4 ± 14.6 years were included. HF was diagnosed in 57% of them. Among patients with HF, HF with preserved ejection fraction (HFpEF) was, by far, the most common phenotype and occurred in 35%, while HF with reduced ejection fraction (HFrEF) occurred only in 7%, HF with mildly reduced ejection fraction (HFmrEF) in 7%, and high-output HF in 9%. Patients with HFpEF differed from patients with no HF significantly in the following: they were older (62 ± 14 vs. 70 ± 14, p = 0.002) and had a higher left ventricular mass index [96(36) vs. 108(45), p = 0.015], higher left atrial index [33(12) vs. 44(16), p < 0.0001], and higher estimated central venous pressure [5(4) vs. 6(8), p = 0.004] and pulmonary artery systolic pressure [31(9) vs. 40(23), p = 0.006] but slightly lower tricuspid annular plane systolic excursion (TAPSE): 22 ± 5 vs. 24 ± 5, p = 0.04. NTproBNP had low sensitivity and specificity for diagnosing HF or HFpEF: with the use of the cutoff value of 8,296 ng/L, the sensitivity of HF diagnosis was only 52% while the specificity was 79%. However, NTproBNP levels were significantly related to echocardiographic variables, most significantly to the indexed left atrial volume (R = 0.56, p < 10−5) and to the estimated systolic pulmonary arterial pressure (R = 0.50, p < 10−5).
Conclusions: HFpEF was by far the most common heart failure phenotype in patients on chronic hemodialysis and was followed by high-output HF. Patients suffering from HFpEF were older and had not only typical echocardiographic changes but also higher hydration that mirrored increased filling pressures of both ventricles than in those of patients without HF.
1. Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is a clinical syndrome characterized by HF symptoms, left ventricular ejection fraction (LVEF) ≥ 50%, and evidence of cardiac dysfunction (e.g., abnormal LV filling and elevated filling pressures) (1, 2). In the absence of pericardial or valvular disease, diastolic left ventricular dysfunction results from increased stiffness (of cardiomyocytes and extracellular matrix) and/or slower relaxation (1). The arterial wall also changes due to decreased proportion of elastin and calcium deposition. Thus impaired ventriculoatrial coupling also characterizes HFpEF (3). These changes are accelerated in patients with chronic kidney disease.
The prevalence of chronic kidney disease (CKD) is growing worldwide. In developed countries, this is mainly due to the epidemics of obesity and metabolic syndrome that include two important causes of CKD—hypertension and type 2 diabetes mellitus. Patients, whose CKD progresses into end-stage kidney disease (ESKD), need renal function replacement therapy. Hemodialysis is the most frequent method by far. However, ESKD and hemodialysis itself are associated with vast hemodynamic, metabolic, and endocrine changes that lead to significant functional and morphological changes in many organs, including the cardiovascular system (4). Indeed, cardiovascular complications are responsible for significant morbidity and represent the most frequent cause of death in this population. Moreover, cardiovascular changes develop much faster in ESKD patients than that in the general population, and this acceleration of pathological changes is responsible for the shorter lifespan of ESKD patients (5). Practically, all heart structures could be affected (4), and heart failure (HF) is a frequent consequence.
CKD and especially ESKD are characterized by sodium and water retention and thus with a high risk of overhydration. In patients on chronic hemodialysis, fluids are removed by ultrafiltration, which is directed by the difference between actual and dry weight (weight of the patient at the end of dialysis sessions). Setting an appropriate dry weight is difficult and based on clinical experience, lab results, and bioimpedance. Therefore, water overload is not rare in hemodialysis patients. Its signs and symptoms are very similar to heart failure (6) and chronic overload is associated with higher mortality (7). Distinguishing water overload from real HF is therefore not easy, especially for retrospective studies analyzing hospital discharge letters. Interestingly, most of our knowledge about HF prevalence in ESKD patients on hemodialysis comes from such retrospective studies (8, 9).
Briefly, HF seems to be frequent among patients on chronic hemodialysis, and many mechanisms have been discovered. However, most data come from retrospective studies that did not quantify the role of actual hydration and where the diagnosis of HFpEF was not based on the current recommendations. To overcome these limitations, we started a prospective observational study named CZecking HF in CKD (10) (registered as ISRCTN 18275480) in 2020. Here we present the baseline data with regard to HF prevalence and phenotypes in a central European population. All patients were examined by detailed expert echocardiography, and lack of significant overhydration was confirmed by both ultrasonography and bioimpedance. The primary aim of this study was to analyze the prevalence of heart failure and its phenotypes. The secondary aims were (1) to describe the potential of N-terminal pro-brain natriuretic peptide (NTproBNP) for HF diagnosis in ESKD patients on hemodialysis; (2) to analyze the frequency of abnormal left ventricular geometry; and (3) to describe the differences between various HF phenotypes in this population.
2. Materials and methods
In the CZecking HF in CKD study, all prevalent patients on chronic hemodialysis (>3 months) that fulfill the broad eligibility criteria came for the index examination, after which they were followed every 6–12 months lifelong or till kidney transplantation. The study design has already been published (10), and the inclusion of patients is ongoing. Briefly, all patients of collaborating hemodialysis units were asked to participate in this study, and the exclusion criteria were only planned kidney transplantation from a living donor within 3 months of the index visit or life expectancy <6 months at V1 visit of any reason. Here, we present baseline data (Visit 1) of patients included till 30 June 2022. The study was approved by the Ethical Committee of the General University Hospital in Prague and is registered in the ISRCTN database. We explained the principles of the study to all patients, and they signed informed consent.
The following data were recorded: basic medical history data, full blood count, levels of albumin, total blood protein and NTproBNP, echocardiography, volume status estimation, heart rhythm analysis, and hemodialysis fistula flow volume calculation. Pulmonary arterial systolic pressure was estimated as a sum of the central venous pressure and the tricuspid regurgitation gradient (if present).
Expert echocardiography was performed using a matrix echocardiography probe of Vivid E9 device (General Electric, Vingmed, Norway), as well as detailed analysis of the volumes of heart cavities, quantification of valvular disease, diastolic dysfunction according to the recent guidelines (11), and cardiac output calculation (using the left ventricular outflow tract diameter and velocity time interval). Echocardiography and all other examinations were performed within 1 h on a non-dialysis day. Two examiners experienced in cardio-nephrology provided all examinations (AV, JM).
Hydration status was assessed as the central venous pressure with the use of the diameter and collapsibility of the inferior vena cava (12) and by bioimpedance (Body Composition Monitor (BCM), Fresenius Medical Care (FMC), Germany). Patients with significant overhydration (>5 L) were not included. Hemodialysis fistula flow volume was analyzed by duplex Doppler ultrasonography at the level of the brachial artery as described previously (13). Hemodynamic calculations (effective cardiac output, vascular resistance, access resistance) were based on echocardiographic data and vascular access flow measurement.
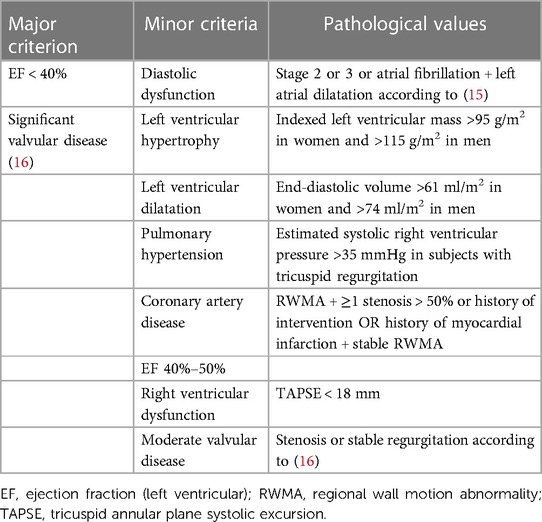
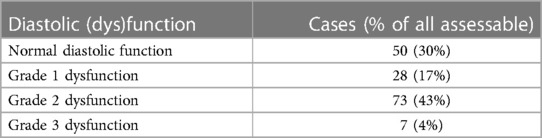
Heart failure diagnosis is established by the guidelines of the European Society of Cardiology (14). HF phenotypes were diagnosed according to the same guidelines (HFpEF), HF with mildly reduced ejection fraction (HFmrEF), HF with reduced ejection fraction (HFrEF)), and two other phenotypes were added: (1) high-output heart failure (HOHF defined by HF signs and symptoms + cardiac index >3.9 L/min/m2) and (2) significant primary valvular disease. The diagnosis of heart failure was set by a cardiologist experienced in cardio-nephrology (JM, AV) and was based on a combination of HF signs and symptoms that improve after each hemodialysis and documentation of structural heart disease by echocardiography. The latter was based on the modified ADQI criteria (10) (see Table 1).
Assessments were performed according to the official guidelines as stated by the references. The ejection fraction was calculated by the biplane methods of disks as recommended (11). For the HF diagnosis according to this definition, the presence of at least one major or two minor criteria was necessary. We also recorded the diagnosis of any HF phenotype according to the nephrologists (based on the clinical picture, patients’ history, and/or echocardiography performed elsewhere in the history).
2.1. Statistical analysis
The normality of data distribution was tested by the Lilliefors test. Data are reported as mean ± SD in variables with Gaussian distribution or as median (quartile range) in variables with non-Gaussian distribution. The difference between (sub) groups was tested by unpaired t-test in the case of Gaussian distribution of data and by Mann–Whitney U test. The relation between variables was calculated by the Spearman correlation analysis. Receiver-operating characteristics were calculated to be considered predictors of HF or, specifically, HFpEF.
3. Results
We report data from 214 patients, aged 66.4 ± 14.6 years, of which 65% were males, the mean dialysis vintage is 47 months (median 26 months), and 98.5% of included patients had Caucasian race. The most frequent causes of CKD were diabetes mellitus (31%), hypertension (23%), and polycystic kidney disease (7%). Hemodialysis vascular access was arteriovenous fistula in 73%, arteriovenous graft in 19%, and catheter in 8%. Other baseline data are presented in Table 2. Eight selected patients were not included in this analysis because of significant overhydration as defined above. A decrease in the dry weight setting was recommended, and the patients were re-examined and then entered the main study.
Heart failure was diagnosed in 122 (57%) of all included patients by the cardiologists, but only in 25% by the referring nephrologists. HFpEF was the leading HF phenotype (see Figure 1). Patients suffering from HFpEF were older and had higher left ventricular mass, left atrial volume, and pulmonary artery blood pressure (see Table 3 for details). The referring nephrologists diagnosed correctly significant valvular disease in 100%, HFrEF in 80%, HFmrEF in 43%, HFpEF in 31%, and HOHF in 18%.
3.1. Other findings
Normal left ventricular geometry was present in only 20% of patients. Out of the patients with abnormal geometry, 44% had concentric hypertrophy, 33% had concentric remodeling, and 24% had eccentric hypertrophy. The left ventricular diastolic function could be formally assessed in 158 (74%) patients (see Table 4).
Left ventricular dilatation was diagnosed in 24% of all patients. Pulmonary artery systolic blood pressure estimation was possible in 122 patients, and, in those, pulmonary hypertension (defined by the estimated peak systolic pulmonary pressure of 35 mmHg or higher) occurred in 56 (46%) patients. The non-sinus rhythm was recorded in 19% of examinations.
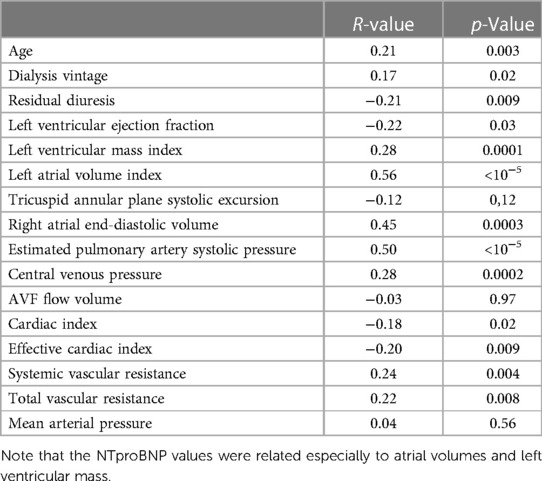
NTproBNP was high in the whole group (see Table 1 for details) but significantly higher in patients with any type of HF [6,935 (17,575) vs. 3,011 (7,425), p = 0.00007]. It was significantly related to many variables tested in this study (Table 5), and by most, it was positively related to the left atrial volume and the estimated peak pulmonary systolic pressure. However, NTproBNP was inversely related to the left ventricular ejection fraction in this study. This biomarker had low sensitivity and specificity for diagnosing HF or HFpEF: with the use of the cutoff value of 8,296 ng/L, the sensitivity of HF diagnosis was only 52% while the specificity was 79% (see Figure 2 for more details). The sensitivity and specificity increased only slightly when only symptomatic HF patients were included (NYHA 2–4) (Figure 2).
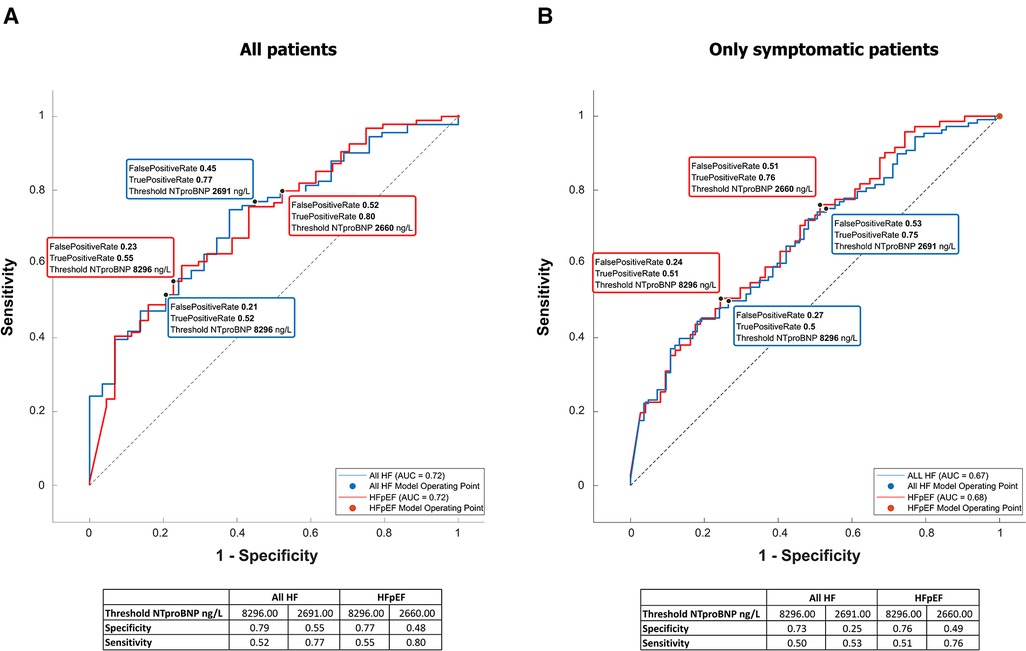
Figure 2. NTproBNP for the diagnosis of heart failure and HFpEF. Receiver-operating curves for NTproBNP differentiating between HF or specifically HFpEF and no HF. The left graph (A) is based on all included patients, while the right one (B) included only patients with shortness of breath (NYHA II–IV).
4. Discussion
The main findings of this study are as follows: (1) HF was highly prevalent in ESKD patients on chronic hemodialysis. HFpEF and HOHF were the leading phenotypes. (2) HF and especially HFpEF were underdiagnosed by nephrologists. (3) Normal left ventricular geometry was found in only 20% of patients, which explained the high prevalence of diastolic dysfunction. (4) NTproBNP was not a precise marker of HF in patients on chronic hemodialysis.
Our study diagnosed HF of any phenotype in 57% of all patients on maintenance hemodialysis. For comparison, Harnett et al. (17) reported a 31% prevalence of HF among hemodialysis patients in a study that started in 1982 (i.e., before the concept of HFpEF) and included significantly younger patients. In 2004, Cheung et al. (18) reported HF in 40% of prevalent hemodialysis patients in the HEMO study, again on younger patients. Stack and Bloembergen (19) found HF in 36% of patients entering chronic hemodialysis, while Antlanger et al. (20) reported recently a 70% prevalence of HF. Although we could explain the differences by including older and more complicated patients in the hemodialysis programs nowadays, the main difference is most probably in the definition of HF itself: Harnett et al. (17) used a clinical HF diagnosis (without the need for echocardiography), and in the Stack’s study (19), patients were recorded as having HF if the diagnosis occurred in the discharge letters. The more recent study by Antlanger et al. (20) used the 2012 ESC guidelines for HF diagnosis (21). Moreover, the older studies did not specifically rule out overload that could mimic HF. Nevertheless, the diagnosis of HF was associated with increased mortality (17, 19, 20).
Metabolic syndrome is a known risk factor of HFpEF in the general population (22). In this study, two main etiologies of CKD are included, i.e., type 2 diabetes mellitus and hypertension, which are parts of the metabolic syndrome. Moreover, the high very prevalence of HFpEF was also not surprising in light of the very frequent left ventricular hypertrophy (LVH) or concentric remodeling and diastolic dysfunction in this study. Similar findings were also reported by Antlanger et al. (20), although they used HFpEF diagnosis differently from the recent one. LVH is linked to increased mortality (23), and many causes of LVH were identified in ESKD patients, such as arterial hypertension and cyclic fluid overload (24), and also higher levels of the fibroblast-growth factor-23 (25). Indeed, the histological structure of LVH in ESKD patients differs from LVH of other etiologies (26). Nowadays, echocardiography could detect myocardial fibrosis of both ventricles and of the left atrium through speckle-tracking (strain analysis) (27). Interestingly, HFpEF (and HOHF) were the most frequent phenotypes underdiagnosed by the referring nephrologists. It highlights lower awareness of these two phenotypes by non-cardiologists that are known also in the non-CKD population (28). HOHF is a specific HF phenotype that was even missed by the guidelines (29). A total of 60% of our HOHF cases had high dialysis fistula flow (>1500 ml/min), which was considered the main reason for HOHF. They were indicated for flow-reducing surgery. Other known contributing factors in developed countries include obesity, anemia, or hepatic disease. Also, HOHF is linked to higher mortality (30). High-flow AVFs have recently been associated with increased myocardial fibrosis (31).
NTproBNP levels increase with the worsening of glomerular filtration in CKD patients (32), and, also in our study, the levels were very high. Although the NTproBNP levels were higher in HF patients and in patients with respective HF phenotypes than those in non-HF controls, NTproBNP is not a good HF marker according to our study due to high false-positive rates. Claus et al. (33) did a similar conclusion, and according to their findings, the sensitivity and specificity could increase by adding other biomarkers, such as growth differentiation factor-15 and circulating neprilysin. Interestingly, NTproBNP values were related especially not only to the volumes of both atria and left ventricular mass index (see Table 5), i.e., to variables that mirror long-term volume overload, but also to higher AVF flow (34). A similar conclusion was published recently (35). Although it is not sure whether NTproBNP level is a marker of HF in ESKD patients or not, a recent meta-analysis documented a gradual increase in cardiovascular and all-cause mortality in ESKD patients with high NTproBNP values (36). Moreover, increased neprilysin levels independently predicted the composite of CV events and cardiac events in HD patients (37).
The main limitation of this study is that the hemodynamic data were obtained non-invasively, but the wide use of the right heart catheterization is no more justifiable nowadays. Although we made every effort to examine only patients with optimal hydration, improper dry weight setting could have played a role in some cases. Moreover, this study was performed in one European country, and 98.5% of the included patients had Caucasian race. The next limitation is the cross-sectional design of this study and inclusion of prevalent patients with various time spent on hemodialysis.
5. Conclusions
Heart failure is present in more than half of ESKD patients on chronic hemodialysis, but it was frequently missed by the nephrologists. HFpEF and HOHF were the leading phenotypes. Impaired left ventricular geometry occurred in the vast majority of patients, and it probably explained the high prevalence of HFpEF. Based on the current knowledge, adequate dry weight setting and avoidance of high-flow AVFs could be causal precautions, especially in patients with HFpEF. NTproBNP is not helpful in HF diagnosis among patients on chronic hemodialysis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the General University Hospital in Prague, Czech Republic. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Echocardiography, heart failure assessment, patient selection: JM and AV. Nephrological part of the project: SP, KM, ZH, VB, KR, MT, and MK. Arteriovenous fistula flow volume and bioimpedance: KB and VL. Data collection and management: LK. Statistical analysis: MS. Protocol design and manuscript preparation: VT, JM, ZH, and SP. All authors contributed to the article and approved the submitted version.
Funding
This trial was supported by the Ministry of Health, Czech Republic-DRO (General University Hospital in Prague-VFN), 00064165, and by grant No. NU22-02-00014 of the Grant Agency for Health Research of the Czech Republic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. (2011) 32(6):670–9. doi: 10.1093/eurheartj/ehq426
2. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40(40):3297–317. doi: 10.1093/eurheartj/ehz641
3. Borlaug BA, Kass DA. Ventricular–vascular interaction in heart failure. Cardiol Clin. (2011) 29(3):447–59. doi: 10.1016/j.ccl.2011.06.004
4. Malik J. Heart disease in chronic kidney disease—review of the mechanisms and the role of dialysis access. J Vasc Access. (2018) 19(1):3–11. doi: 10.5301/jva.5000815
5. Moromizato T, Kohagura K, Tokuyama K, Shiohira Y, Toma S, Uehara H, et al. Predictors of survival in chronic hemodialysis patients: a 10-year longitudinal follow-up analysis. Am J Nephrol. (2021) 52(2):108–18. doi: 10.1159/000513951
6. Han BA-O, Song SH, Yoo JS, Park H, Kim J, Choi E. Association between OH/ECW and echocardiographic parameters in CKD5 patients not undergoing dialysis. PLoS One. (2018) 13(4):e0195202. doi: 10.1371/journal.pone.0195202. (1932–6203).29630661
7. Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol. (2017) 28(8):2491–7. doi: 10.1681/ASN.2016121341
8. Modi ZJ, Lu Y, Ji N, Kapke A, Selewski DT, Dietrich X, et al. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease an analysis of the US renal data system. JAMA Cardiol. (2019) 4(4):353–62. doi: 10.1001/jamacardio.2019.0375
9. Inampudi C, Akintoye E, Jayanna MB, Asleh R, Briasouli A, Alvarez P, et al. Trends in in-hospital mortality, length of stay, nonroutine discharge, and cost among end-stage renal disease patients on dialysis hospitalized with heart failure (2001–2014). J Card Fail. (2019) 25(7):524–33. doi: 10.1016/j.cardfail.2019.02.020
10. Malik J, Valerianova A, Pesickova SS, Hruskova Z, Bednarova V, Michalek P, et al. CZecking heart failure in patients with advanced chronic kidney disease (Czech HF-CKD): study protocol. J Vasc Access. (2022). doi: 10.1177/11297298221099843
11. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2016) 17(4):412. doi: 10.1093/ehjci/jew041
12. Kircher BJ, Himelman Rb, Fau-Schiller NB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol (1990);66(4):493–6 doi: 10.1016/0002-9149(90)90711-9
13. Kudlicka J, Kavan J, Tuka V, Malik J. More precise diagnosis of access stenosis: ultrasonography versus angiography. J Vasc Access. (2012) 13(3):310–4. doi: 10.5301/jva.5000047
14. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24(1):4–131. doi: 10.1002/ejhf.2333
15. Toida T, Toida R, Yamashita R, Komiya N, Uezono S, Komatsu H, et al. Grading of left ventricular diastolic dysfunction with preserved systolic function by the 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations contributes to predicting cardiovascular events in hemodialysis patients. Cardiorenal Med. (2019) 9(3):190–200. doi: 10.1159/000496064
16. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2013) 14(7):611–44. doi: 10.1093/ehjci/jet105
17. Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients—prevalence, incidence, prognosis and risk factors. Kidney Int. (1995) 47(3):884–90. doi: 10.1038/ki.1995.132
18. Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO study. Kidney Int. (2004) 65(6):2380–9. doi: 10.1111/j.1523-1755.2004.00657.x
19. Stack AG, Bloembergen WE. A cross-sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis. (2001) 38(5):992–1000. doi: 10.1053/ajkd.2001.28588
20. Antlanger M, Aschauer S, Kopecky C, Hecking M, Kovarik JJ, Werzowa J, et al. Heart failure with preserved and reduced ejection fraction in hemodialysis patients: prevalence, disease prediction and prognosis. Kidney Blood Pres Res. (2017) 42(1):165–76. doi: 10.1159/000473868
21. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2012) 14(8):803–69. doi: 10.1093/eurjhf/hfs105
22. Savji N., Meijers W. C., Bartz T. M., Bhambhani V., Cushman M., Nayor M., et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail (2018) 6(8):701–9. doi: 10.1016/j.jchf.2018.05.018
23. London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. (2001) 12(12):2759–67. doi: 10.1681/ASN.V12122759
24. Ozkahya M, Toz H, Qzerkan F, Duman S, Ok E, Basci A, et al. Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol. (2002) 15(6):655–60. PMID: 12495279
25. Stevens KK, McQuarrie EP, Sands W, Hillyard DZ, Patel RK, Mark PB, Jardine AG. Fibroblast growth factor 23 predicts left ventricular mass and induces cell adhesion molecule formation. Int J Nephrol (2011) 2011:297070. doi: 10.4061/2011/297070
26. Mall G, Huther W, Schneider J, Lundin P, Ritz E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant. (1990) 5(1):39–44. doi: 10.1093/ndt/5.1.39
27. Lisi M, Cameli M, Mandoli GE, Pastore MC, Righini FM, D'Ascenzi F, et al. Detection of myocardial fibrosis by speckle-tracking echocardiography: from prediction to clinical applications. Heart Fail Rev. (2022) 27(5):1857–67. doi: 10.1007/s10741-022-10214-0
28. Shear FE. Novel paradigms in the therapeutic management of heart failure with preserved ejection fraction: clinical perspectives. Am J Cardiovasc Dis. (2019) 9(5):91–108. PMID: 31763061; PMCID: PMC6872467
29. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail (2016) 18(8):891–975. doi: 10.1002/ejhf.592
30. Reddy YNV, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-output heart failure: a 15-year experience. J Am Coll Cardiol. (2016) 68(5):473–82. doi: 10.1016/j.jacc.2016.05.043
31. Choi YS, Lee IJ, An JN, Song YR, Kim SG, Lee HS, et al. High-flow arteriovenous fistula and myocardial fibrosis in hemodialysis patients with non-contrast cardiac magnetic resonance imaging. Front Cardiovasc Med. (2022) 27(9):922593. doi: 10.3389/fcvm.2022.922593
32. Weng SC, Chen YC, Hsu CY, Lin CS, Tarng DC, Lin SY. Impacts of heart failure and physical performance on long-term mortality in old patients with chronic kidney disease. Front Cardiovasc Med. (2021) 4(8):680098. doi: 10.3389/fcvm.2021.680098
33. Claus RA-O, Berliner D, Bavendiek U, Vodovar N, Lichtinghagen R, David S, et al. Soluble neprilysin, NT-proBNP, and growth differentiation factor−15 as biomarkers for heart failure in dialysis patients (SONGBIRD). Clin Res Cardiol (2020);109(8):1035–47 doi: 10.1007/s00392-020-01597-x
34. Malik J, Valerianova A, Tuka V, Trachta P, Bednarova V, Hruskova Z, et al. The effect of high-flow arteriovenous fistulas on systemic haemodynamics and brain oxygenation. ESC Heart Fail. (2021) 8(3):2165–71. doi: 10.1002/ehf2.13305
35. Wang Y, Cao X, Yu J, Zhang Y, Li X, Chen X, et al. Association of N-terminal pro-brain natriuretic peptide with volume Status and cardiac function in hemodialysis patients. Front Cardiovasc Med. (2021) 22(8):646402. doi: 10.3389/fcvm.2021.646402
36. Harrison TG, Shukalek CB, Hemmelgarn BR, Zarnke KB, Ronksley PE, Iragorri N, et al. Association of NT-proBNP and BNP with future clinical outcomes in patients with ESKD: a systematic review and meta-analysis. Am J Kidney Dis. (2020) 76(2):233–47. doi: 10.1053/j.ajkd.2019.12.017
Keywords: heart failure, end-stage renal disease, HFPEF, high-output heart failure, echocardiography
Citation: Malik J, Valerianova A, Pesickova SS, Michalickova K, Hladinova Z, Hruskova Z, Bednarova V, Rocinova K, Tothova M, Kratochvilova M, Kaiserova L, Buryskova Salajova K, Lejsek V, Sevcik M and Tesar V (2023) Heart failure with preserved ejection fraction is the most frequent but commonly overlooked phenotype in patients on chronic hemodialysis. Front. Cardiovasc. Med. 10:1130618. doi: 10.3389/fcvm.2023.1130618
Received: 23 December 2022; Accepted: 9 May 2023;
Published: 1 June 2023.
Edited by:
Matteo Lisi, Santa Maria delle Croci Hospital, ItalyReviewed by:
Husam Salah, University of Arkansas for Medical Sciences, United StatesGiuditta Benincasa, University of Campania Luigi Vanvitelli, Italy
© 2023 Malik, Valerianova, Pesickova, Michalickova, Hladinova, Hruskova, Bednarova, Rocinova, Tothova, Kratochvilova, Kaiserova, Buryskova Salajova, Lejsek, Sevcik and Tesar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Malik amFuLm1hbGlrQHZmbi5jeg==
 Jan Malik
Jan Malik Anna Valerianova1
Anna Valerianova1 Satu Sinikka Pesickova
Satu Sinikka Pesickova Zdenka Hruskova
Zdenka Hruskova Vladimira Bednarova
Vladimira Bednarova Vladimir Tesar
Vladimir Tesar