- 1Heart Center, Department of Cardiovascular Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, China
- 2The Second School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
- 3Zhejiang Provincial People's Hospital, Qingdao University, Hangzhou, China
Background: Although great progress has been made in caring for patients with acute coronary syndrome (ACS), the incidence of heart failure (HF) after discharge remains high after ACS.
Aims: We aimed to investigate the risk predictors for new-onset HF and build a simple nomogram to optimize the clinical management of female patients.
Methods: The clinical data of 319 female patients with ACS between January 1, 2021 and January 1, 2022, were obtained from the Zhejiang Provincial People’s Hospital. Multivariate logistic regression analysis was carried out to build the prediction model among all participants and then verified by 10-fold cross-validation. The discrimination, calibration, and clinical usefulness of the prediction model were assessed using receiver operating characteristic curve, calibration curve, and decision curve analyses.
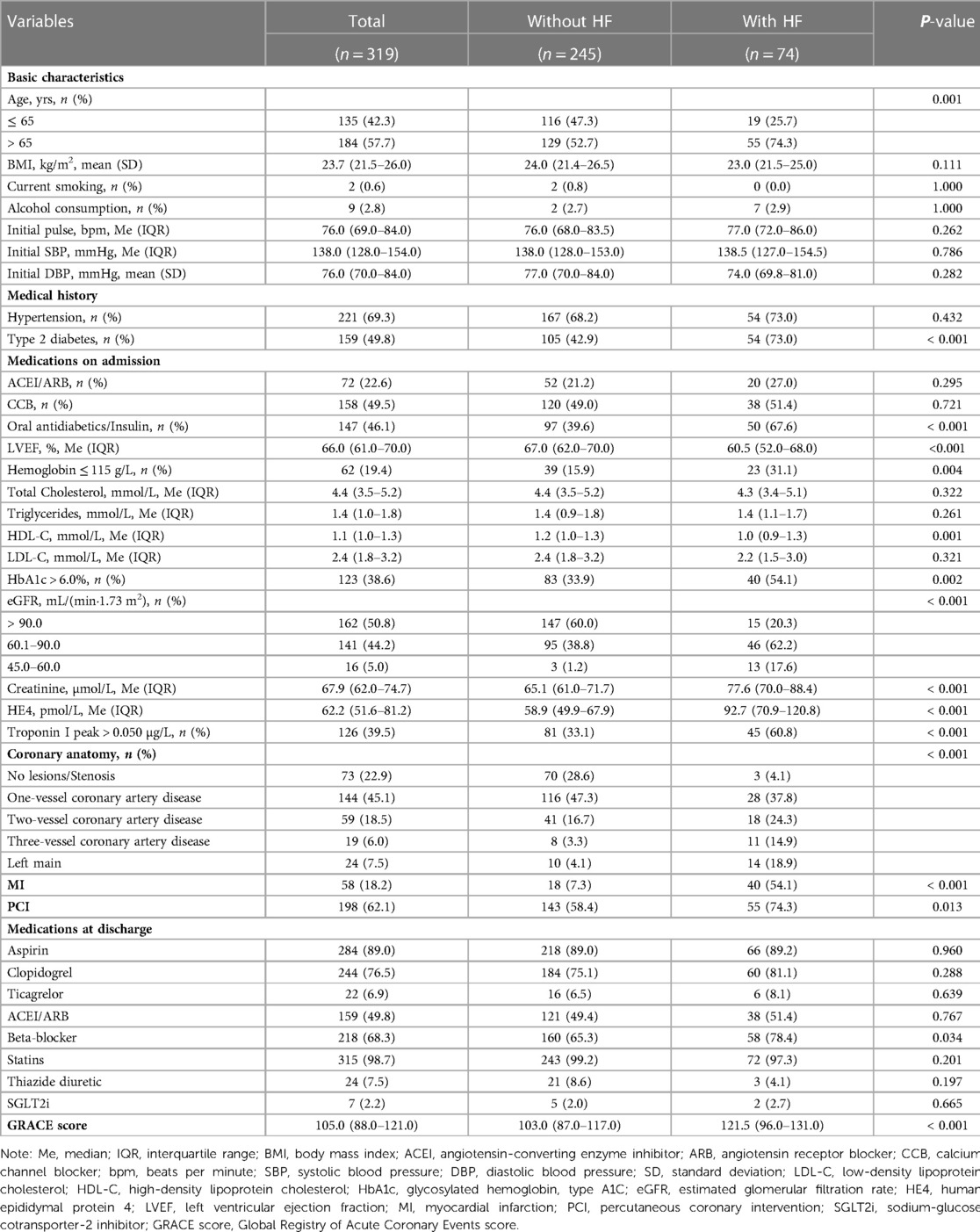
Results: This study analyzed 15 potential independent risk predictors of new-onset HF in 319 female patients with ACS. The incidence of HF onset was 23.2%. The following 5 independent risk predictors were filtered out as most relevant for predicting 12-month HF onset: left ventricular ejection fraction ≤ 60.5%, high-density lipoprotein ≤ 1.055 mmol/L, human epididymal protein 4 > 69.6 pmol/L, creatinine > 71.95 µmol/L, and diagnosis of myocardial infarction (MI).
Conclusion: Our nomogram, which used five easily obtained clinical variables, could be a useful tool to help identify female individuals with ACS who are at high risk of developing HF after discharge and facilitate communication between female patients and physicians.
Introduction
Although great progress has been made in the care of patients with acute coronary syndrome (ACS), the incidence of heart failure (HF) at discharge remains high after ACS (1). In addition, sex differences in new-onset HF after ACS are noteworthy. A recent prospective study reported that whether under unadjusted analysis or multivariate adjustment, women carried a higher risk of HF onset than men (2, 3). Moreover, compared to men, women with coronary artery disease will be older and have higher risk of hypertension, diabetes, and congestive HF (4). However, as we know, the symptoms and signs of HF provide limited diagnostic accuracy (5), while female patients present a more atypical clinical HF status (6, 7). Additionally, it has been reported that nearly two out of three female patients with coronary artery disease show persistent symptoms and ischemic signals, but coronary angiography (CAG) shows no obstructive coronary lesions, which significantly affects their prognosis (8, 9). Therefore, we aimed to detect the risk predictors for new-onset HF and build a simple nomogram to optimize the clinical management of female patients.
Materials and methods
Study patients
We conducted a single-center, retrospective, observational cohort study in Zhejiang Provincial People's Hospital (Hang Zhou, China). A total of 319 female patients who experienced ACS and underwent CAG from January 1, 2021 to January 1, 2022, were included in this study and followed up for one year by telephone, and all subsequent hospitalizations, emergency admissions, and outpatient visits were reviewed. The development of a new-onset HF event (New York Heart Association heart failure classes ranging from II to IV) was our main observation outcome. When screening the included population, we excluded patients with heart failure or those who had previously underwent percutaneous coronary intervention (PCI) or coronary artery bypass grafting. In addition, patients were diagnosed with ACS, including myocardial infarction (MI) and unstable angina (UA), according to the criteria of the 2020 ESC Guidelines (10), while HF was diagnosed according to the criteria of the 2021 ESC Guidelines (11). This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board of Zhejiang Provincial People's Hospital (Hangzhou, China), registration number: QT2022337. Informed consent was waived due to the retrospective nature of the study.
Data collection and variables
Clinical information was obtained from the patients' medical records, including demographics [age, body mass index (BMI), history of smoking, and alcohol consumption]; history of hypertension and type 2 diabetes; vital signs at admission (pulse, systolic blood pressure (SBP), and diastolic blood pressure (DBP)); left ventricular ejection fraction (LVEF); administration of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACEI or ARB), calcium channel blocker (CCB), oral antidiabetics, and insulin 3 months before admission; results of coronary angiography; whether PCI was performed; and medications at discharge [aspirin, clopidogrel, ticagrelor, ACEI or ARB, beta-blocker, statins, thiazide diuretic, and sodium-glucose cotransporter-2 inhibitor (SGLT2i)]. In addition, we collected the results of patients' laboratory findings during hospitalization, including hemoglobin, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glycosylated hemoglobin, type A1C (HbA1c), estimated glomerular filtration rate (eGFR), serum creatinine, human epididymis protein 4 (HE4), and troponin I peak levels. In addition, the Global Registry of Acute Coronary Events (GRACE) score at discharge was calculated (10) for subsequent comparison with our model's prediction ability.
Model establishment and validation
We used univariate and multivariate logistic regression models to identify the relationship between the variables and new-onset HF. We preliminarily detected potential risk factors using univariate analysis. Potential risk factors (P < 0.05) were further analyzed using multicollinearity analysis. If there were variables with a tolerance lower than 0.2 or a variance inflation factor (VIF) higher than 5, we incorporated more meaningful variables based on the empirical screening into the multivariable analysis. Receiver operating characteristic curves were used to detect the relevant cutoff values for selected continuous potential risk predictor variables. To facilitate clinical use, we converted these meaningful continuous variables into binary variables according to the cutoff points. Multivariate logistic regression was performed to build a stepwise nomogram model for new-onset HF within 12 months after ACS. The nomogram was built using forward stepwise method with a threshold of P < 0.1 based on the Akaike information criterion. We evaluated the discriminative ability of the model using receiver operating characteristic (ROC) curve analysis and detected the calibration capability between the model probability curve and the ideal calibration curve using a calibration curve. Later, we verified the accuracy of the model by 10-fold cross-validation and evaluated the clinical usefulness of the prognostic nomogram model using decision curve analysis (DCA).
Statistical analysis
Means (standard deviation) or medians (interquartile range) were used for continuous variables, and numbers and percentages were used for categorical variables. The associations between HF and variables were tested using Student's t-test, Mann–Whitney U test, χ2 test, Fisher's exact test, and logistic regression model. Statistical analyses were two-tailed with 95% confidence intervals (CIs). Statistical significance was set at P < 0.05. Statistical analyses were performed using IBM SPSS Statistics (version 26.0; SPSS Inc., Chicago, IL, USA) and STATA version 15.0 (StataCorp LLC., College Station, TX, USA).
Results
Baseline characteristics
We retrospectively analyzed the clinical data collected from Zhejiang Provincial People's Hospital, summarized in Table 1. Three hundred and nineteen female patients were included in this study, and 245 (76.8%) had not developed new-onset HF after ACS, while 74 (23.2%) had. Overall, fifty-five (74.3%) patients of the with-HF group were over 65 years old, and 54 (73.0%) had a history of type 2 diabetes, with 50 (67.6%) patients taking oral antidiabetics or insulin. Patients in the with-HF group had lower LVEF, hemoglobin, HDL-C, and eGFR at admission, but higher levels of HbA1c, creatinine, HE4, and troponin I peak. The number of patients in the with-HF group with coronary artery stenosis ≥50% with 2- or 3-vessel disease was more than that in the without-HF group. Forty (54.1%) patients who had developed new-onset HF experienced myocardial infarction at that time, while 55 (74.3%) had PCI performed. Patients in the with-HF group had a higher proportion rate of beta blockers at discharge. Besides, the mean GRACE score of patients with-HF group was significantly higher.
Potential risk factors for developing new-onset HF after ACS among women
Table 2 presents the results of the univariate logistic regression analyses performed to test the relationship between variables and new-onset HF. Multicollinearity analyses were performed to assess multicollinearity between variables. The risk of HF among female patients was associated with higher prevalence of type 2 diabetes (OR = 3.600, 95%CI, 2.032–6.379, P < 0.001), use of oral antidiabetics or insulin (OR = 3.179, 95%CI, 1.823–5.509, P < 0.001), morbidity of MI (OR =, 14.837 95%CI, 7.647–28.787, P < 0.001), and proportion of performed PCI (OR = 2.065, 95%CI, 1.156–3.688, P = 0.014). In addition, the risk of HF among patients with HbA1c > 6.0% was higher than that of those with HbA1c ≤ 6.0% (OR = 2.296, 95%CI, 1.354–3.894, P = 0.002). Moreover, the continuous variables of LVEF, HDL-C, creatinine, and HE4 levels were potential predictors of 12-month incidence of HF in the univariate analysis (P < 0.001); the categorical variables of age, eGFR, troponin I peak, hemoglobin, and the number of coronary arteries showed a statistically significant difference between the without-HF and with-HF groups (P < 0.05). Multicollinearity analyses revealed that the tolerances of the history of diabetes and antidiabetic drugs or insulin prescriptions were smaller than 0.2, and the VIFs were larger than 5, confirming that the regression models were affected by the presence of multicollinearity. Considering that diabetes mellitus may be regarded as equivalent to coronary heart disease (12) and one of the most important risk factors for cardiovascular disease (13), we incorporated type 2 diabetes into the multivariate analysis.
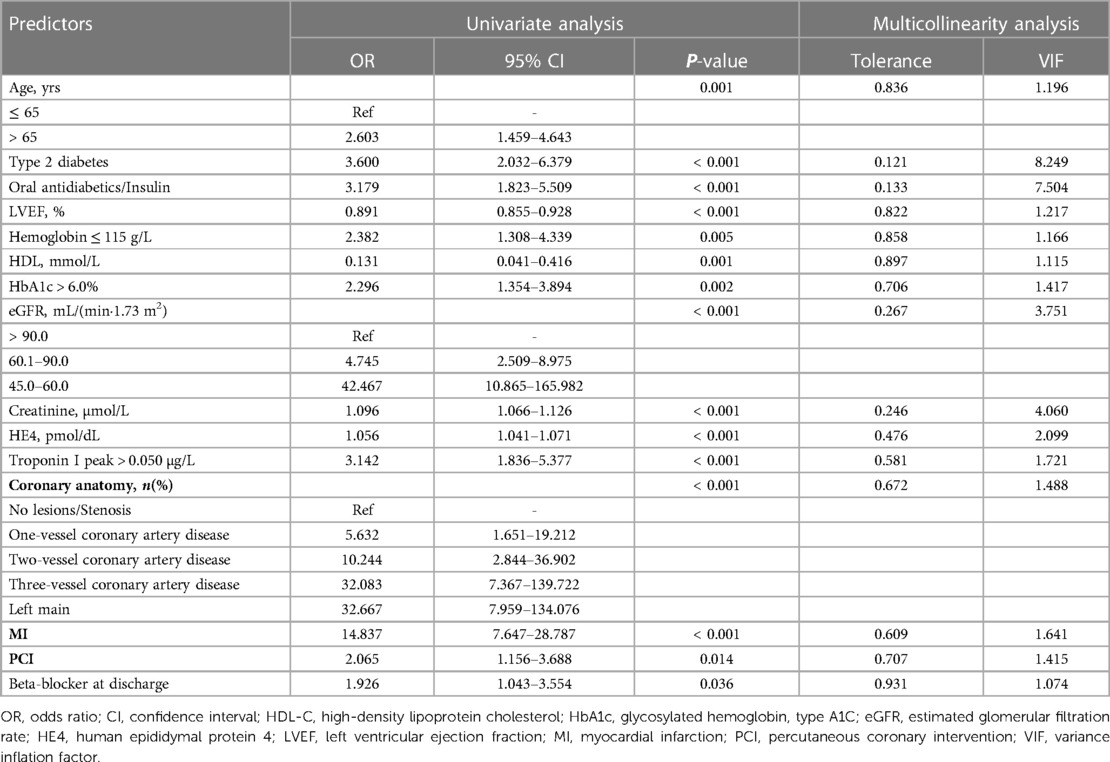
Table 2. Potential independent predictors for new-onset HF in female patients after acute coronary syndrome.
A prognostic nomogram for 12-month incidence of HF
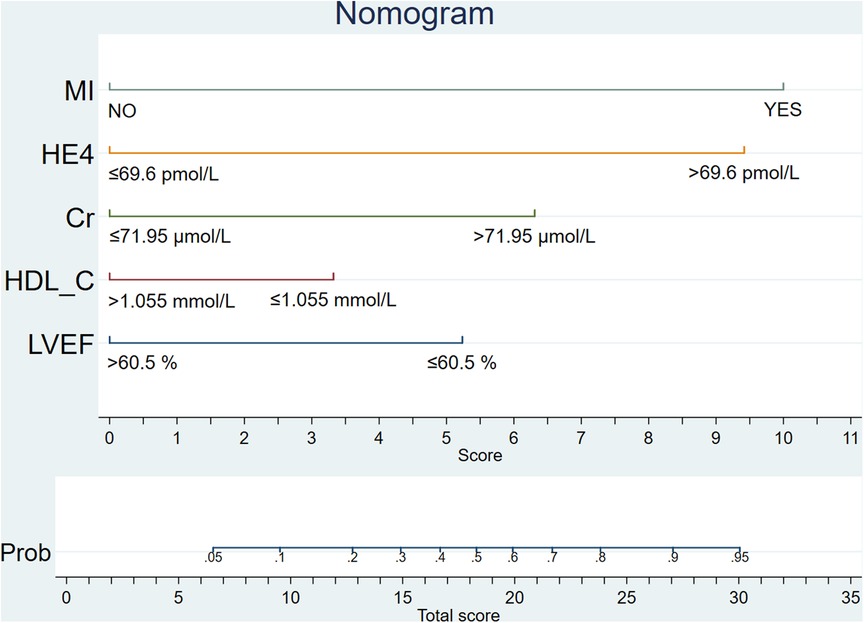
To facilitate the convenience of the model, we dichotomized the standard cutoff points for continuous variables, including LVEF (cutoff value = 60.5%), HDL-C (cutoff value = 1.055 mmol/L), creatinine (cutoff value = 71.95 µmol/L), and HE4 (cutoff value = 69.6 pmol/L). Fifteen clinical factors were analyzed to test their relationships with HF using a multivariate logistic model following forward stepwise process and five variables were filtered out from the initial 15: LVEF, HE4, creatinine, HDL-C, and MI (Table 3). The incidence rate of HF could be evaluated using the stepwise nomogram shown in Figure 1. The performance of the model was estimated using ROC curve analysis, and the C-index was 0.922 (sensitivity, 83.8%; specificity, 88.2%), indicating a great diagnostic performance (Figure 2). The calibration χ2 statistic for the models was 7.32, indicating an excellent goodness-of-fit (P = 0.292). And its performance of prediction ability was better than that of GRACE score (AUC = 0.708, sensitivity = 63.5%, and specificity = 74.7%) (Supplementary Figure S1).
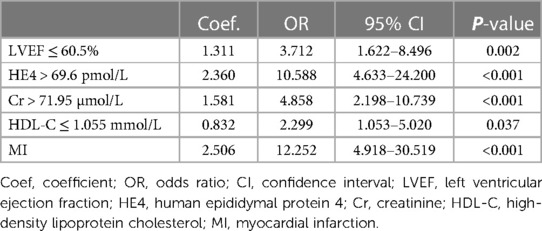
Table 3. Multivariate model for predicting new-onset heart failure in female patients after acute coronary syndrome.
Nomogram model verification
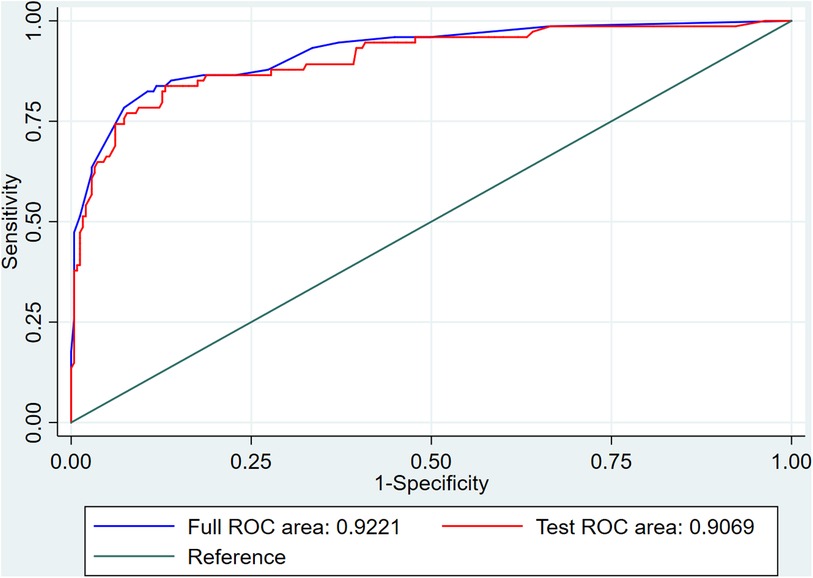
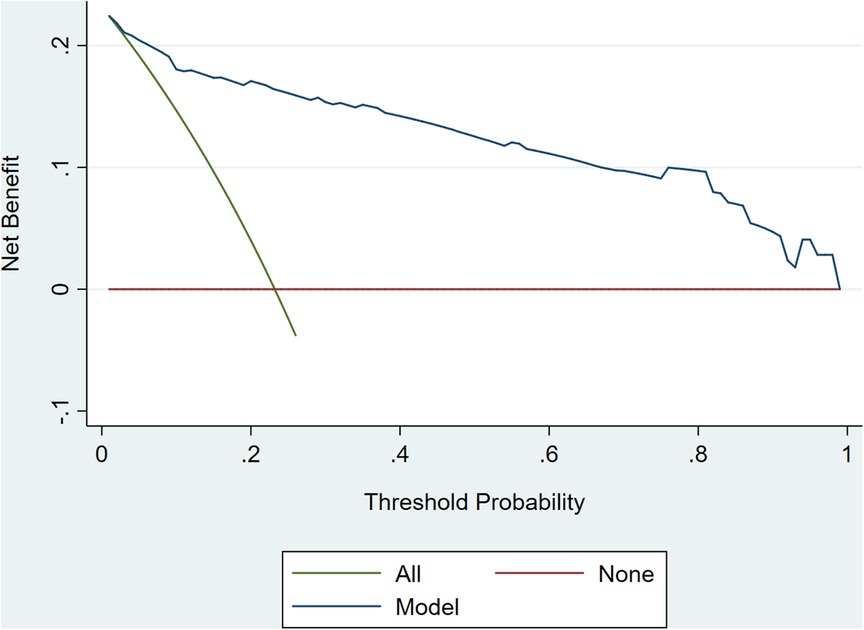
The internal 10-fold cross-validation verification showed that the stepwise nomogram could accurately predict the C-index of the incidence of HF among female patients within 12 months after ACS, which was 0.907. Furthermore, the calibration curve demonstrated high consistency between the predicted survival probability and actual survival proportion (Figure 3), which was assessed by the Hosmer–Lemeshow statistic (P = 0.778). Additionally, decision curve analysis showed great positive net benefits in the model under a threshold probability in the primary cohort (Figure 4), indicating the potential beneficial clinical impact of the model.
Discussion
Our study analyzed 15 potential independent risk predictors of new-onset HF in 319 female patients with ACS who underwent CAG in Zhejiang Provincial People's Hospital. Seventy-four female patients developed new-onset HF in our study, accounting for 23.2% of all participants with ACS. The following 5 independent risk predictors were screened using stepwise regression for the nomogram: incident MI, LVEF ≤ 60.5%, HE4 > 69.6 pmol/L, HDL-C ≤ 1.055 mmol/L, and creatinine > 71.95 µmol/L. Furthermore, an easy-to-use prediction nomogram was developed for the first time in this study. And our findings of the study are hypothesis generating for future studies. Encouragingly, the model presented an excellent performance in predicting the incidence of new-onset HF (AUC = 0.922, sensitivity = 83.8%, and specificity = 88.2%) and was validated internally using 10-fold cross-validation with AUC = 0.907. Moreover, the developed model presented a superior performance in clinical settings, as shown in the results of the calibration curve and decision curve analysis.
Compared with other models, our model showed some specific strengths. Three risk stratification tools are available. The GRACE score (10) was built to assess the risk of patients who experience ACS with or without STEMI, including future all-cause mortality and MI. Several GRACE risk scores have been established for different patient groups and for predicting different outcomes (14–17). The Thrombolysis in Myocardial Infarction (TIMI) score (18, 19), based on seven clinical variables, is one of the most widely used risk evaluation tools. Nevertheless, the TIMI risk score for UA and NSTEMI validated in a trial (18) showed that the usefulness for prognosis was poor in a real-world dataset of 1-year (20). Subsequently, history, ECG, age, risk factors, and troponin (HEART score) (21), a predictor of outcome in patients with chest pain, outperformed both GRACE and TIMI scores in identifying low-risk ACS (22–24).
Although these risk scores were validated, they did not include important factors. For example, as we emphasized, sex differences cannot be ignored in the prognosis of ACS, especially for HF onset; however, women are often under-represented in large clinical trials (25). Some new factors might contribute to the identification of high-risk patients that were not included in these risk scores. In addition, these risk scores mainly enrolled people from Western countries, and their prognostic study in Asian populations was not sufficiently detailed. Moreover, focusing on the occurrence probability of major adverse cardiovascular and cerebrovascular diseases would weaken the ability to predict the risk of HF onset, an important and high-incidence complication after ACS. Some risk scores were apparently inconvenient to use, with too many factors required. Finally, these tools are mainly used for risk assessment of short-term prognosis.
Considering these shortcomings, our model has attempted to address these issues. First, specifically targeting female patients with ACS is one of the most significant strengths of our nomogram model, which can more accurately assess the incidence of HF in women. The main endpoint of the present study was new-onset HF, which might further refine the management and prognosis of female patients after ACS. Furthermore, all analyses were conducted after adjusting for confounding factors, which were selected from the variables considered reasonable confounders. In our study, we considered all the general cardiovascular risk factors mentioned in the Framingham prediction model (26), including age, SBP, antihypertensive drug use (ACEI or ARB and CCB in our study), TC and HDL-C, smoking status, and diabetes mellitus. Other important factors were also considered. For example, a study conducted by Núñez et al. (2) reported that lower baseline LVEF was associated with a higher risk of new-onset HF, whereas women displayed a higher risk in certain ranges of preserved LVEF, which partly explains our experiment. We identified HE4, a protein that reflects ongoing cardiac fibrosis (27), as a meaningful factor in predicting HF onset, which was consistent with other studies (28, 29). Kumar et al. (30) conducted a study showing that the risk of new-onset HF after ACS was closely related to MI, which remains the most common cause of HF (31). Consistent with previous studies, which reported that plasma creatinine was associated with an increased risk of HF (32, 33), creatinine played an important role in our prediction model. A higher creatinine level reflected a greater prevalence of atherosclerosis and risk factors of aging, as described in another study (34). Therefore, knowledge about HF would make an important contribution to developing new guidelines to reduce its incidence among women.
However, our study was subject to inherent limitations associated with retrospective analysis. First, selection or recall bias was possible in our study. Second, although our model adjusted for confounders and mediators, several confounders that were not included might play an important role, such as other undetected cardiac biomarkers. Third, to clarify the good discrimination and calibration of the model through internal verification, the model requires external validation before we can determine its applicability to other patient populations. Finally, the results of our study might be weakened because of the small sample size. Therefore, we will address these limitations in subsequent prospective studies.
In conclusion, HF is a common complication in patients with ACS, particularly in women. LVEF, HE4, HDL-C, creatinine, and MI were independent risk factors for new-onset HF after ACS among female patients. The novel nomogram that we developed can identify genuine high-risk patients and facilitate communication between female patients and physicians. The prediction model may provide an important early warning for high risk of HF in female patients with ACS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board of Zhejiang Provincial People's Hospital (Hangzhou, China), registration number: QT2022337. Informed consent was waived due to the retrospective nature of the study.
Author contributions
QY conceived of the study and wrote the manuscript. LY and JS performed statistical analyses of the clinical information and interpreted the results. QZ provided suggestions on study design. XZ and LW enrolled subjects and obtained the clinical details. LW (corresponding author) directed the project and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China under Grant No. 81670447, the National Natural Science Foundation of Zhejiang Province under Grant No. LY15H020006, Zhejiang Province Key Subject of Medicine (Neurological Rehabilitation) and the Traditional Chinese Medicine Program of Zhejiang Province under Grant No. 2017ZZ001, and the Zhejiang Provincial Health Commission Project under Grant No. 2017KY559. LW was sponsored by the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1131813/full#supplementary-material.
References
1. Cordero A, Rodríguez-Mañero M, Bertomeu-González V, García-Acuña JM, Baluja A, Agra-Bermejo R, et al. New-onset heart failure after acute coronary syndrome in patients without heart failure or left ventricular dysfunction. Rev Esp Cardiol (Engl Ed). (2021) 74(6):494–501. English, Spanish. doi: doi: 10.1016/j.rec.2020.03.011
2. Núñez J, Lorenzo M, Miñana G, Palau P, Monmeneu JV, López-Lereu MP, et al. Sex differences on new-onset heart failure in patients with known or suspected coronary artery disease. Eur J Prev Cardiol. (2021) 28(15):1711–9. doi: 10.1093/eurjpc/zwab078
3. Rossello X, Ferreira JP, Caimari F, Lamiral Z, Sharma A, Mehta C, et al. Influence of sex, age and race on coronary and heart failure events in patients with diabetes and post-acute coronary syndrome. Clin Res Cardiol. (2021) 110(10):1612–24. doi: 10.1007/s00392-021-01859-2
4. Kornacewicz-Jach Z. Coronary artery disease in women—how to prevent heart failure after myocardial infarction? Przegl Lek. (2016) 73(6):399–401.29671301
5. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European society of cardiology and endorsed by the European society of intensive care medicine. Eur J Heart Fail. (2010) 12(5):423–33. doi: 10.1093/eurjhf/hfq045
6. Eisenberg E, Di Palo KE, Piña IL. Sex differences in heart failure. Clin Cardiol. (2018) 41(2):211–6. doi: 10.1002/clc.22917
7. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J. (2019) 40(47):3859–3868c. doi: 10.1093/eurheartj/ehz835
8. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. (2017) 135(11):1075–92. doi: 10.1161/CIRCULATIONAHA.116.024534
9. Wei J, Cheng S, Bairey Merz CN. Coronary microvascular dysfunction causing cardiac ischemia in women. JAMA. (2019) 322(23):2334–5. doi: 10.1001/jama.2019.15736
10. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
12. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. (2012) 126(15):1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402
13. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. (2018) 17(1):83. doi: 10.1186/s12933-018-0728-6
14. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. (2004) 291(22):2727–33. doi: 10.1001/jama.291.22.2727
15. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). Br Med J. (2006) 333(7578):1091. doi: 10.1136/bmj.38985.646481.55
16. Fox KA, Fitzgerald G, Puymirat E, Huang W, Carruthers K, Simon T, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open. (2014) 4(2):e004425. doi: 10.1136/bmjopen-2013-004425
17. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. (2003) 163(19):2345–53. doi: 10.1001/archinte.163.19.2345
18. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. (2000) 284(7):835–42. doi: 10.1001/jama.284.7.835
19. Morrow DA, Antman EM, Parsons L, de Lemos JA, Cannon CP, Giugliano RP, et al. Application of the TIMI risk score for ST-elevation MI in the national registry of myocardial infarction 3. JAMA. (2001) 286(11):1356–9. doi: 10.1001/jama.286.11.1356
20. Singh M, Reeder GS, Jacobsen SJ, Weston S, Killian J, Roger VL. Scores for post-myocardial infarction risk stratification in the community. Circulation. (2002) 106(18):2309–14. doi: 10.1161/01.cir.0000036598.12888.de
21. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. (2008) 16(6):191–6. doi: 10.1007/BF03086144
22. Backus BE, Six AJ, Kelder JC, Bosschaert MA, Mast EG, Mosterd A, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. (2013) 168(3):2153–8. doi: 10.1016/j.ijcard.2013.01.255
23. Six AJ, Cullen L, Backus BE, Greenslade J, Parsonage W, Aldous S, et al. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pathw Cardiol. (2013) 12(3):121–6. doi: 10.1097/HPC.0b013e31828b327e
24. Poldervaart JM, Langedijk M, Backus BE, Dekker IMC, Six AJ, Doevendans PA, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. (2017) 227:656–61. doi: 10.1016/j.ijcard.2016.10.080
25. Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. (2002) 162(15):1682–8. doi: 10.1001/archinte.162.15.1682
26. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the framingham heart study. Circulation. (2008) 117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579
27. Yamamoto M, Hanatani S, Araki S, Izumiya Y, Yamada T, Nakanishi N, et al. HE4 Predicts progressive fibrosis and cardiovascular events in patients with dilated cardiomyopathy. J Am Heart Assoc. (2021) 10(15):e021069. doi: 10.1161/JAHA.120.021069
28. de Boer RA, Cao Q, Postmus D, Damman K, Voors AA, Jaarsma T, et al. The WAP four-disulfide core domain protein HE4: a novel biomarker for heart failure. JACC Heart Fail. (2013) 1(2):164–9. doi: 10.1016/j.jchf.2012.11.005
29. Piek A, Meijers WC, Schroten NF, Gansevoort RT, de Boer RA, Silljé HH. HE4 Serum levels are associated with heart failure severity in patients with chronic heart failure. J Card Fail. (2017 Jan) 23(1):12–9. doi: 10.1016/j.cardfail.2016.05.002
30. Kumar A, Cannon CP. The role of statins in the prevention of heart failure after acute coronary syndrome. Heart Fail Clin. (2008) 4(2):129–39. doi: 10.1016/j.hfc.2008.01.010
31. Roger VL. Epidemiology of heart failure. Circ Res. (2013) 113(6):646–59. doi: 10.1161/CIRCRESAHA.113.300268
32. Schytz PA, Nissen AB, Torp-Pedersen C, Gislason GH, Nelveg-Kristensen KE, Hommel K, et al. Creatinine increase following initiation of antihypertensives is associated with cardiovascular risk: a nationwide cohort study. J Hypertens. (2020) 38(12):2519–26. doi: 10.1097/HJH.0000000000002573
33. Núñez J, Garcia S, Núñez E, Bonanad C, Bodí V, Miñana G, et al. Early serum creatinine changes and outcomes in patients admitted for acute heart failure: the cardio-renal syndrome revisited. Eur Heart J Acute Cardiovasc Care. (2017) 6(5):430–40. doi: 10.1177/2048872614540094
Keywords: acute coronary syndrome, heart failure, nomogram, women, 10-fold cross-validation
Citation: Yan Q, Ye L, Zhang Q, Song J, Zhang X, Wu L and Wang L (2023) Nomogram to predict the incidence of new-onset heart failure after acute coronary syndrome among women. Front. Cardiovasc. Med. 10:1131813. doi: 10.3389/fcvm.2023.1131813
Received: 26 December 2022; Accepted: 8 March 2023;
Published: 24 March 2023.
Edited by:
Hamidreza Goodarzynejad, University Health Network, CanadaReviewed by:
Nicholas Wettersten, VA San Diego Healthcare System, United StatesAlexander E Berezin, Zaporizhia State Medical University, Ukraine
Hong Cheng, Capital Medical University, China
© 2023 Yan, Ye, Zhang, Song, Zhang, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Wang d2FuZ2xobmV3QDEyNi5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Sex and Gender in Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
 Qiqi Yan
Qiqi Yan Lifang Ye1,†
Lifang Ye1,† Lihong Wang
Lihong Wang