- 1Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Division of Cardiology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 3Centre for Public Health, School of Medicine, Dentistry, and Biomedical Sciences, Queen’s University Belfast, Belfast, United Kingdom
- 4School of Medicine and Public Health, University of Newcastle, Newcastle, NSW, Australia
Objective: Systemic arterial hypertension (HT) is a major modifiable risk factor for cardiovascular disease (CVDs), associated with all-cause death (ACD). Understanding its progression from the early state to late complications should lead to more timely intensification of treatment. This study aimed to construct a real-world cohort profile of HT and to estimate transition probabilities from the uncomplicated state to any of these long-term complications; chronic kidney disease (CKD), coronary artery disease (CAD), stroke, and ACD.
Methods: This real-world cohort study used routine clinical practice data for all adult patients diagnosed with HT in the Ramathibodi Hospital, Thailand from 2010 to 2022. A multi-state model was developed based on the following: state 1-uncomplicated HT, 2-CKD, 3-CAD, 4-stroke, and 5-ACD. Transition probabilities were estimated using Kaplan-Meier method.
Results: A total of 144,149 patients were initially classified as having uncomplicated HT. The transition probabilities (95% CI) from the initial state to CKD, CAD, stroke, and ACD at 10-years were 19.6% (19.3%, 20.0%), 18.2% (17.9%, 18.6%), 7.4% (7.1%, 7.6%), and 1.7% (1.5%, 1.8%), respectively. Once in the intermediate-states of CKD, CAD, and stroke, 10-year transition probabilities to death were 7.5% (6.8%, 8.4%), 9.0% (8.2%, 9.9%), and 10.8% (9.3%, 12.5%).
Conclusions: In this 13-year cohort, CKD was observed as the most common complication, followed by CAD and stroke. Among these, stroke carried the highest risk of ACD, followed by CAD and CKD. These findings provide improved understanding of disease progression to guide appropriate prevention measures. Further investigations of prognostic factors and treatment effectiveness are warranted.
Introduction
Systemic arterial hypertension (HT) is a major modifiable risk factor of cardiovascular diseases (CVDs) and is associated with all-cause death (ACD) (1). This common non-communicable disease is characterized by persistent elevation of arterial blood pressure (BP), diagnosed as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg (2–4). About 1.28 billion adults worldwide are estimated to have HT, with almost half (46%) of them being unaware of the condition, hence the moniker “silent killer” as per the World Health Organization 2021 report (4). In Thailand, 25% of adults were diagnosed with HT according to the National Health Survey of 2014 (5), and HT is estimated to account for two-thirds of stroke and half of atherosclerotic coronary artery disease (CAD) events (6).
The etiology of HT involves the complex interplay of increased dietary salt intake, obesity, activation of neuro-hormonal systems such as the sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS), as well as genetic predisposition (7), causing multi-system effects and long-term complications. Among these complications, chronic kidney disease (CKD) (8, 9), coronary artery disease (10, 11), stroke (12, 13) are frequent risks that cause premature death in HT patients (14–18). Progressive glomerulo-sclerosis is commonly observed in hypertensive CKD patients, with prevalence ranging from 60% to 90%, dependent on CKD stage and cause (19), and a previous study reported 30.9% incidence of CKD observed in hypertensive cohort (20). Furthermore, there is a bi-directional association, where sustained HT results in poorer kidney function, and progressive kidney function decline leads to worsening BP control. HT accelerates cholesterol-dependent atherogenesis and promotes atherosclerotic plaque development in the cerebrovascular circulation increasing the risk of ischemic stroke (21), and atherosclerotic CAD (22). Globally, CAD and stroke rank second and third among the causes of mortality and disability (23), and HT remains the most important risk factor of CAD and stroke (24–26).
HT and its complications have an impact on both individual-level quality of life and population-level economic health burden. A review estimated that the United States spent 193 billion USD in 2017 on HT management, not including economic loss caused by reduced productivity or increased premature death; direct cost (e.g., drugs, diagnostics, hospitalization, consultations) per person were estimated to be 6,250 USD (27). The financial burden is further increased when treating associated complications; for example, post-stroke hospitalization cost 15,415 USD per person in 2004 (28). Therefore, improved understanding of HT progression or transition to other conditions will lead to more appropriate, effective and timely treatment strategies.
A multi-state model of HT progression including CKD, CAD and stroke allows the estimation of time for disease progression between each disease state. Mortality associated with individual HT complications has been reported, e.g., stroke (N = 503) in Ethiopia (29), myocardial infarction (N = 2,336) (12) and CKD (N = 9,361) in the US (30), with several studies conducted in Asian populations using multi-state modelling. One study included 13,933 elderly Chinese subjects aged 60 years or older to assess prognostic factors and transitions from healthy state to cardio-metabolic disease and multi-comorbidity (respectively defined as one and two or more complications among HT, diabetes, CAD, and stroke), and death (31). However, this study also included non-hypertensive patients. A previous study in Italy reported association of non-communicable diseases including HT with adverse cardiovascular events and death (32). An Iranian study evaluated 3,002 hypertensive patients aged 50 years or older (33) to develop an illness-death model, known as a basic multi-state model, to estimate transition probabilities and prognostic factors for changing states from CVD-free HT to any CVD and ACD. However, this study considered both CAD and stroke as composite CVD events when, in reality, they have different prognoses. In addition, although CKD plays an important role in disease progression, this was not considered. Furthermore, HT disease progression in Southeast Asia may differ relative to other countries and ethnicities due to different prognostic factors, treatments, healthcare system, clinical practice guidelines, resource availability and accessibility, and reimbursement schemes.
As such, this large-scale, real-world dataset was constructed from a Thai teaching hospital. The study cohort sought to estimate the transition probabilities from HT without complication to any long-term complications including CKD, CAD, stroke, and ACD as well as transition probabilities between complications.
Materials and methods
Data design
This retrospective cohort study retrieved all routine patient electronic medical records from January 2010 to December 2022 from Ramathibodi Hospital, Bangkok, Thailand. Patients aged 18 years and over, diagnosed with HT, and followed up for more than one visit and longer than 30 days were identified and included. The study was approved by Ramathibodi Human Research Ethics Committee (COA.MURA 2021/512).
Data sources and features
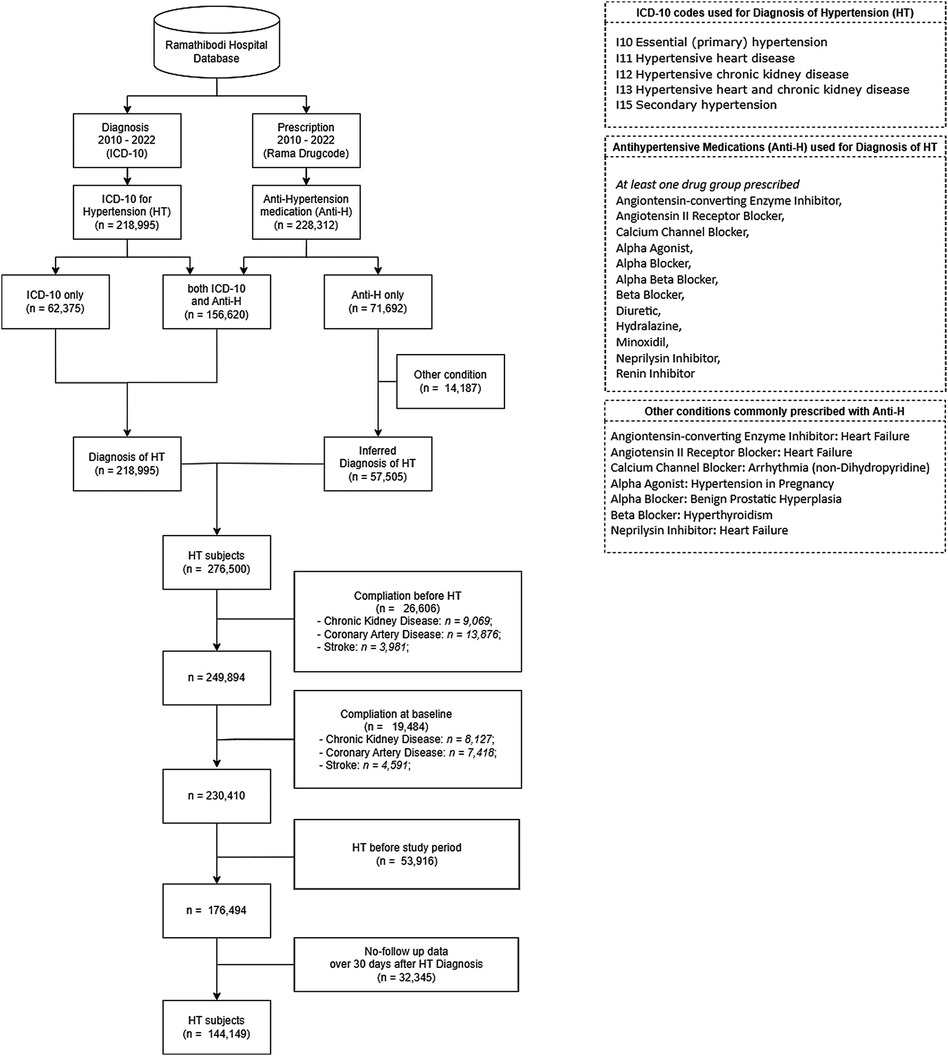
Data from both routine clinical visits and hospital admission records were retrieved to assess the illness progression and identify conditions of interest based on the International classification of Diseases (ICD) Ninth (ICD-9) (34) and Tenth Revisions (ICD-10) (35). To develop the cohort, ICD-10 codes I10, I11, I12, I13 and I15 for HT were retrieved. To correct for potential under-coding (36), medication data were also retrieved to identify those taking alpha/beta blockers (BBs), calcium channel blockers (CCBs), agents acting on the renin-angiotensin system [i.e., angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs)], and other HT related drugs (i.e., diuretics, minoxidil, and reserpine). However, beta blockers are also commonly prescribed to control other conditions such as hyperthyroidism. Therefore, patients who were prescribed a single anti-hypertensive medication along with other indications such as hyperthyroidism were excluded. We also used a 5 year “look back” period (2005–2010) to exclude those participants who were diagnosed or developed complications before study initiation. The data flow for identification of HT cohort is described in Figure 1.
Other covariates were extracted including: demographic data (i.e., age and sex), physical data (i.e., BP, heart and respiratory rates per minute, body temperature, and body mass index); laboratory data [i.e., lipid profiles (i.e., total cholesterol, high-density lipoprotein, low-density lipoprotein), kidney function [i.e., urine creatinine, estimated glomerular filtration rate (eGFR), urinary albumin-to-creatinine ratio], complete blood counts (i.e., hemoglobin, white blood cell count, red blood cell count) and troponin)] and prescribed medications (e.g., anti-hypertensive drugs, diabetic drugs, dyslipidemia drugs, drugs for atrial fibrillation, etc.).
Laboratory data were standardized to conventional units, while medications were converted to generic drugs and drug classes based on prescription and pharmacy dispensing information. Data across different information systems were linked and merged using de-identified hospital numbers and visit dates as shown in Supplement Figure 1. Given that patients were followed up at varying time intervals, data were aggregated to 180 day-intervals where possible.
Complications of interest
Complications of interest associated with HT progression included CKD, CAD, stroke, and ACD. CKD was identified by ICD-10 and ICD-9 codes for renal replacement therapy. Also, eGFR was used to identify those who were not coded but had eGFR less than 60 ml/min/1.73 m2 on two consecutive occasions more than 90 days apart. The estimated GFR (ml/min/1.73 m2) was calculated based on the CKD epidemiology collaboration (CKD-EPI) equations (37). Similarly, CAD was identified using ICD-10 and ICD-9 codes as well as procedure codes for percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG), and troponin results higher than 14 ng/ml. Patients identified from troponin levels were verified using electrocardiogram findings within two weeks prior to or after the troponin test. Stroke was characterized as ischemic, hemorrhagic or transient ischemic attack, identified using ICD-10 codes. ACD and date were identified and retrieved from hospital databases. All ICD codes and criteria used to infer diagnoses are reported in Supplementary Table S1.
Statistical analysis
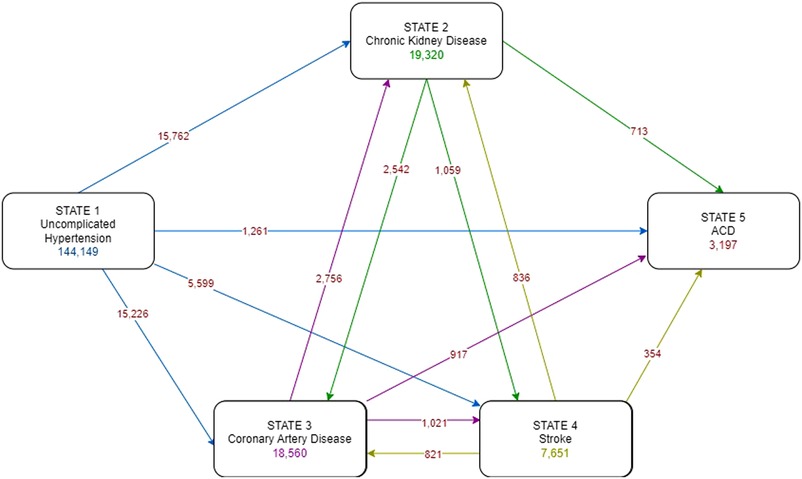
A multi-state model was developed, which consisted of three intermediate states (i.e., CKD, CAD, stroke) and the absorbing state of ACD, see Figure 2. Patients could directly move or transition from state 1 (complication-free) to state 2 (CKD), state 3 (CAD), state 4 (stroke), or even state 5 (ACD), whichever occurred first. Once they entered an intermediate state (2, 3, or 4), they could transition between them (i.e., 2 → 3, 2 → 4, 3 → 2, 3 → 4, 4 → 2, 4 → 3), then to ACD (e.g., 2 → 3 → 5, 2 → 4 → 5, 3 → 4 → 5, etc.), or directly to ACD (i.e., 2 → 5, 3 → 5, 4 → 5). Time from the initial state (date at HT diagnosis) to each intermediate or absorbing state (date at diagnosis of CKD, CAD, stroke, ACD), whichever occurred first, was calculated if such a transition was observed. Time from entry to the intermediate state to another state or absorbing state was also calculated if patients had subsequent transitions. In addition, patients were censored: (a) at 31st December 2022 if they were still complication free, (b) at the date of last encounter if lost to follow up.
The transition probabilities for 13 transition pathways from initial state to each intermediate and absorbing states (i.e., CKD, CAD, Stroke, and ACD), transitions between intermediate states, and intermediate states to ACD were estimated using a Kaplan–Meier test (38). Simultaneous transition to multiple complication states were considered accordingly, such as development of two complications recorded at the same visit was included in two analyses for both transition pathways. Transition probabilities for hazard functions were estimated using the lifelines package (39) (version 0.27.0) and Python (version 3.9.7) (40) within a Spyder integrated development environment (version 5.1.5) (41).
Results
Baseline characteristics
A total of 276,500 HT patients aged 18 years or over were identified using the ICD-10 codes and/or antihypertensive medications. Of these, 132,351 were excluded due to: (a) development of a complication before HT diagnosis (26,606 subjects: 13,876 patients with CAD, 9,069 with CKD and 3,981 with stroke with one patient that may have more than one complication at the same time), (b) complication diagnosed together with HT (19,484 subjects: 8,127 patients with CKD, 7,418 with CAD and 4,591 with stroke), (c) HT diagnosis preceding the study period (53,916 subjects), or (d) no follow up after 30 days from HT diagnosis (32,345 subjects). The remaining 144,149 patients were included in the study cohort, see Figure 1.
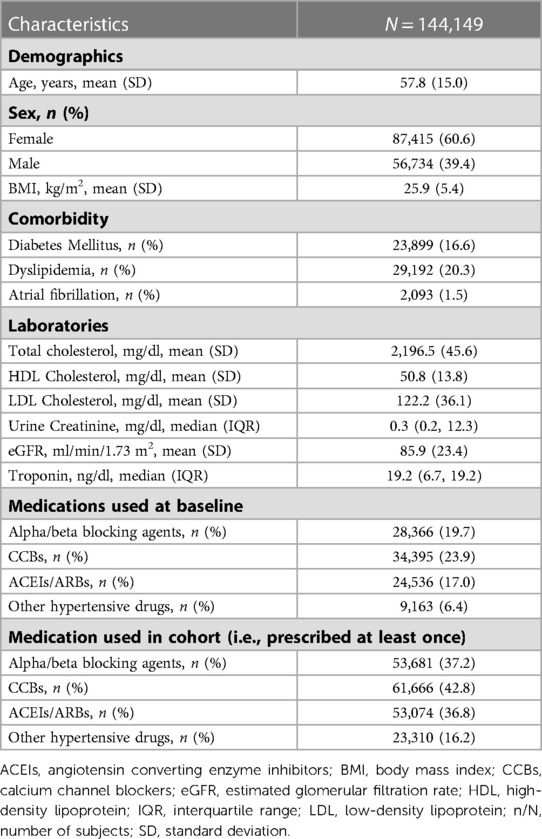
Baseline characteristics for study participants are presented in Table 1. The mean age was 57.8 ± 15.0 years with 60.6% being female. Average BMI was 25.9 ± 5.4 kg/m2 and average eGFR was 85.9 ± 25.4 ml/min/1.73m2. CCBs were the most commonly prescribed antihypertensive medication (42.8%). In addition, the median follow-up time was 3.6 years (range: 0.08–13.00) and the median number of visits was 33 (IQR: 11–76).
Transition probabilities
As per the multi-state diagram in Figure 2, a total of 144,149 patients were included. Of these, 15,762 (10.9%), 15,226 (10.6%), 5,599 (3.9%) and 1,261 (0.9%) patients moved from the initial uncomplicated state directly to state 2-CKD, state 3-CAD, state 4-stroke, and state 5-ACD, respectively; the remaining 107,308 patients remained complication-free by the end of the study period, see Supplementary Table S2. Upon moving to an intermediate state (i.e., state 2-CKD, state 3-CAD, state 4-stroke), some patients further moved between other complication states, or ACD. As a result, 19,320 (13.4%), 18,560 (12.9%), and 7,651 (5.3%) patients were at subsequent risk of state 2-CKD, state 3-CAD, and state 4-stroke, see Figure 2. The transition probabilities for each state are presented in Figure 3. The transition probabilities (95% CI) for moving from an uncomplicated HT state to CKD, CAD, stroke, and ACD at 10 years were 19.6% (19.3%–20.0%), 18.2% (17.9%–18.6%), 7.4% (7.1%–7.6%), and 1.7% (1.5%–1.8%), respectively (Figure 3A).
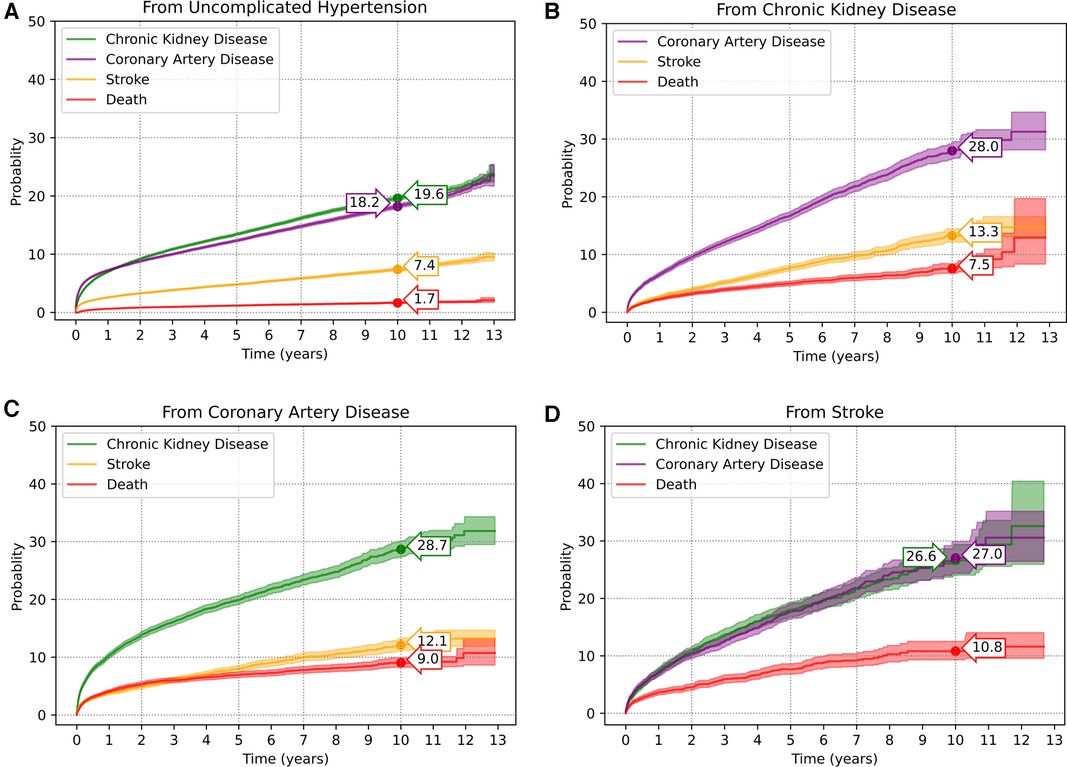
Figure 3. Transition probabilities of complication occurrences in hypertensive patients: (A) from uncomplicated HT to complications; (B) from chronic kidney disease to other complications; (C) from coronary artery disease to other complications; (D) from stroke to other complications.
Upon reaching the CKD state, HT patients had 10-year transition probabilities (95% CI) of 28.0% (26.6%–29.4%), 13.3% (12.1%–14.5%), and 7.5% (6.8%–8.4%) to progress to CAD, stroke, and ACD, respectively (Figure 3B). HT patients with CAD progressed to CKD, stroke, and ACD with 10-year transition probabilities of 28.7% (27.3%–30.1%), 12.1% (11.1%–13.2%), and 9.0% (8.2%–9.9%), respectively (Figure 3C). HT patients with stroke progressed to CKD, CAD, and ACD with 10-year transition probabilities (95% CI) of 26.6% (24.1%–29.4%), 27.0% (24.4%–29.9%), and 10.8% (9.3%–12.5%), respectively (Figure 3D). Further details on the transition probabilities of progressing to each state at 2-, 5-, 7- and 10-years are shown in Supplementary Tables S3–S6.
Discussion
This study followed a retrospective cohort of 144,149 HT patients and their subsequent development of long-term complications from Ramathibodi Hospital, Bangkok, Thailand from 2010 to 2022. This large real-world Asian cohort allowed the estimation of the transition probabilities in individuals who were initially complication-free to progressive complication states and ACD. We found that HT patients were most likely to transition to CKD followed by CAD and then stroke with associated 10-year transition probabilities of 19.6%, 18.2%, and 7.4%, respectively. Among these complications, stroke had the highest risk of mortality followed by CAD in contrast to CKD, which had the lowest risk of death.
In addition to HT and traditional atherosclerotic risk factors, CKD also has been associated with accelerated atherosclerosis (42, 43). In our study, hypertensive patients with CKD had 10-year transition probabilities of 28.0% and 13.3% to CAD and stroke, respectively, which is similar to that reported previously, where HT patients with CKD had greater risk of developing CAD (44, 45) and stroke (46, 47) compared to uncomplicated HT patients. Conversely, patients with CAD and stroke were most likely to transition to CKD, with 10-year transition probabilities of 28.7% and 26.6% respectively, suggesting a bi-directional relationship (48, 49).
Once any complication had developed, HT patients were more likely to move to the absorbing state of ACD, with 10-year probabilities of 10.8%, 9.0% and 7.5% from each of the stroke, CAD, and CKD states, respectively. Although previous studies reported an elevated risk of mortality of 24% to 70% with decreased kidney function (50), our finding showed much more modest risk, which may be due to the Universal Healthcare Coverage in Thailand which has been available since 2002, including peritoneal dialysis since 2008 (51, 52). Subsidizing care improves treatment accessibility, reducing the mortality rate (53–55).
As shown in Figure 3, the transition from CKD to CAD increased at a higher rate over time compared to other competing states, with a sharp increase in the first year. Previous studies have reported that kidney disease and decreased renal function are risk factors for CAD (43, 56). American Heart Association Councils on Kidney recommend the patients with CKD be considered in the highest risk group for subsequent cardiovascular event (57). Conversely, transition from CAD to CKD was also observed to be the highest at 28.7% (27.3%–30.1%) for 10-years transition probability, compared to stroke and uncomplicated HT at 26.6% (24.1%–29.4%) and 19.6% (19.3%–20.0%) respectively.
The two-year transition probability for ACD was the highest for patients with CAD at 5.3% (5.0%–5.7%), with stroke as the leading cause of ACD for the remaining study period followed by CAD, i.e., 7.7% (6.8%–8.6%) vs. 6.9% (6.5%–7.4%) at 5 years, 9.2% (8.1%–10.4%) vs. 7.8% (7.2%–8.4%) at 7 years, and 10.8% (9.3%–12.5%) vs. 9.0% (8.2%–9.9%) at 10 years. While some previous studies reported conflicting findings (58, 59), others were similar to ours (60, 61). This likely reflects differing levels of cardiac care and access to revascularization procedures and should be further investigated.
To prevent late complications, BP control through lifestyle changes, including low-salt intake, and increased physical activity, effectively lowers BP and prevents target organ damage and its CVD sequelae (62, 63). Pharmacological therapy is very effective in lowering BP and in preventing CVD outcomes in most patients (64). Awareness of early complication such as declining of renal function is the early surrogate of CVD prevention, especially stroke and CAD which are the major cause of premature death in hypertensive patients (65, 66). Microalbuminuria has been shown to be an early marker of hypertensive renal disease (67). Pharmacologic therapy to reduce microalbuminuria is associated with delayed progression of renal disease (68). Furthermore, statin therapy should be beneficial for HT patients with CKD due to their demonstrated safety and efficacy for both lowering lipid levels and preventing CVD events in pre-end stage CKD (69, 70).
Our findings offer insight into HT progression in a Southeast Asian population. This large real-world cohort of HT patients allowed the estimation of transition probabilities of multiple complications simultaneously (13 transition pathways overall). Further prognostic factors should be considered to aid clinicians and patients delay disease progression, particularly in high-risk patients. In addition, the effectiveness of different HT treatments, cost-effectiveness or utility analysis should be undertaken for improved resource planning and utilization, especially in limited settings.
Some limitations could not be avoided. Our results are based on routine clinical practice data, thus, follow-up time intervals varied across individual patients, and lab test data and prescriptions also varied, leading to the aggregation of data in 180-day windows. Date of diagnosis for each state may be inaccurate if patients were diagnosed elsewhere prior to attending Ramathibodi Hospital. An inter-hospital data linkage should be sought to minimize this limitation. Prognostic factors for moving to complication-states are not considered and beyond the scope of this current study. Further studies should be conducted to simultaneously consider important prognostic factors such as age, body mass index, anti-hypertensive treatments, comorbidities (e.g., diabetes, dyslipidemia, etc.) in a multi-state model.
Conclusions
A well-characterized cohort of 144,149 HT patients was constructed based on 13-year real-world clinical data. CKD was observed to be the most common complication in hypertensive patients, followed by CAD and then stroke according to 10-year risks. Among complication states, stroke had the highest risk of mortality followed by CAD, while CKD had the lowest. Early detection of declining renal function and tight BP control has been shown to reduce late complications. Tailoring antihypertensive medication to the clinical setting to achieve a lower BP goal is critical. This finding might help physicians better understand disease progression and guide appropriate and timely prevention measures. Further prognostic factors, treatment effectiveness/safety, and economic evaluation are warranted.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data can be shared on request to Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine Ramathibodi Hospital, Mahidol University. Requests to access these datasets should be directed to https://www.rama.mahidol.ac.th/ceb/CEBdatawarehouse/Submittheproposal.
Ethics statement
The studies involving human participants were reviewed and approved by Ramathibodi Human Research Ethics Committee (COA.MURA 2021/512). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
HT: Conceptualization, Data Curation, Data analysis, Interpretation, Writing—Original Draft, Visualization; SB: Conceptualization, Methodology, Interpretation, Writing—Review & Editing; NU: Methodology, Data analysis; KT: Conceptualization, Methodology, Writing—Review & Editing; TL: Conceptualization, Methodology, Data Analysis, Interpretation, Writing—Review & Editing, Supervision; OP: Methodology, Writing—Review & Editing; AP: Data curation, Methodology, Data analysis, Interpretation; GM: Writing—Review & Editing; JA: Writing—Review & Editing; AT: Conceptualization, Methodology, Data analysis, Writing—Review & Editing, Supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Research Council of Thailand (NRCT) N42A640323. The sponsor had no role in the design or conduct of the study.
Acknowledgments
Funding from the National Research Council of Thailand (Grant number N42A640323) is gratefully acknowledged. We thank CEB Data Warehouse Working Group for their expert advice and support throughout this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1170010/full#supplementary-material.
References
1. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (pure): a prospective cohort study. Lancet (London, England). (2020) 395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2
2. Kunanon S, Chattranukulchai P, Chotruangnapa C, Kositanurit W, Methavigul K, Boonyasirinant T, et al. Thai Guidelines on the Treatment of Hypertension: Executive Summary. J Med Assoc Thai. (2019) 104:1729–38. doi: 10.35755/jmedassocthai.2021.10.12199
3. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Esc/esh guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology (esc) and the European society of hypertension (esh). Eur Heart J. (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
4. (WHO) WHO. Hypertension World Health Organization Newsroom: World Health Organization (WHO) (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/hypertension (Updated March 16, 2023; Cited March 29, 2023).
5. Sukonthasarn A, Audhya R, Sitthisook S, Chattranukulchai P, Roubsanthisuk W, Saengwattanaroj S, et al. Thai Guidelines on the Treatment of Hypertension (2019).
6. World Health Organization. Country Office for T. Hypertension care in Thailand: best practices and challenges, 2019. Bangkok: World Health Organization. Country Office for Thailand (2019).
7. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. (2019) 15(6):367–85. doi: 10.1038/s41581-019-0145-4
8. Tsuchida-Nishiwaki M, Uchida HA, Takeuchi H, Nishiwaki N, Maeshima Y, Saito C, et al. Association of blood pressure and renal outcome in patients with chronic kidney disease; a post hoc analysis of from-J study. Sci Rep. (2021) 11(1):14990. doi: 10.1038/s41598-021-94467-z
9. Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage. Hypertension. (2004) 44(5):595–601. doi: 10.1161/01.HYP.0000145180.38707.84
10. Escobar E. Hypertension and coronary heart disease. J Hum Hypertens. (2002) 16(1):S61–S3. doi: 10.1038/sj.jhh.1001345
11. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. (2020) 75(2):285–92. doi: 10.1161/HYPERTENSIONAHA.119.14240
12. Kannel WB, Sorlie P, Castelli WP, McGee D. Blood pressure and survival after myocardial infarction: the framingham study. Am J Cardiol. (1980) 45(2):326–30. doi: 10.1016/0002-9149(80)90654-2
13. Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. (2008) 7(6):476–84. doi: 10.1016/j.cmet.2008.03.010
14. Muller DC, Murphy N, Johansson M, Ferrari P, Tsilidis KK, Boutron-Ruault M-C, et al. Modifiable causes of premature death in middle-age in Western Europe: results from the epic cohort study. BMC Med. (2016) 14(1):87. doi: 10.1186/s12916-016-0630-6
15. Eslami A, Naghibi Irvani SS, Ramezankhani A, Fekri N, Asadi K, Azizi F, et al. Incidence and associated risk factors for premature death in the Tehran lipid and glucose study cohort, Iran. BMC Public Health. (2019) 19(1):719. doi: 10.1186/s12889-019-7056-y
16. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-Specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. (2002) 360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8
17. Wang M, Wu T, Yu C, Gao W, Lv J, Wu Y, et al. Association between blood pressure levels and cardiovascular deaths: a 20-year follow-up study in rural China. BMJ Open. (2020) 10(2):e035190. doi: 10.1136/bmjopen-2019-035190
18. Kuriakose A, Nair Anish TS, Soman B, Varghese RT, Sreelal TP, Mendez AM, et al. Rate and risk of all cause mortality among people with known hypertension in a rural community of southern kerala, India: the results from the prolife cohort. Int J Prev Med. (2014) 5(5):596–603. PMID: 24932391.24932391
19. Ku E, Lee BJ, Wei J, Weir MR. Hypertension in ckd: core curriculum 2019. Am J Kidney Dis. (2019) 74(1):120–31. doi: 10.1053/j.ajkd.2018.12.044
20. Chia YC, Ching SM. Hypertension and the development of new onset chronic kidney disease over a 10 year period: a retrospective cohort study in a primary care setting in Malaysia. BMC Nephrol. (2012) 13:173. doi: 10.1186/1471-2369-13-173
21. Yu JG, Zhou RR, Cai GJ. From hypertension to stroke: mechanisms and potential prevention strategies. CNS Neurosci Ther. (2011) 17(5):577–84. doi: 10.1111/j.1755-5949.2011.00264.x
22. Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. (2000) 342(7):454–60. doi: 10.1056/nejm200002173420702
23. Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. (2017) 16(11):877–97. doi: 10.1016/S1474-4422(17)30299-5
24. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): a case-control study. Lancet. (2010) 376(9735):112–23. doi: 10.1016/s0140-6736(10)60834-3
25. Janwanishstaporn S, Karaketklang K, Krittayaphong R. National trend in heart failure hospitalization and outcome under public health insurance system in Thailand 2008–2013. BMC Cardiovasc Disord. (2022) 22(1):203. doi: 10.1186/s12872-022-02629-2
26. Nilanont Y, Nidhinandana S, Suwanwela NC, Hanchaiphiboolkul S, Pimpak T, Tatsanavivat P, et al. Quality of acute ischemic stroke care in Thailand: a prospective multicenter countrywide cohort study. J Stroke Cerebrovasc Dis. (2014) 23(2):213–9. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.001
27. Wierzejska E, Giernaś B, Lipiak A, Karasiewicz M, Cofta M, Staszewski R. A global perspective on the costs of hypertension: a systematic review. Arch Med Sci. (2020) 16(5):1078–91. doi: 10.5114/aoms.2020.92689
28. Anis AH, Sun H, Singh S, Woolcott J, Nosyk B, Brisson M. A cost-utility analysis of losartan versus atenolol in the treatment of hypertension with left ventricular hypertrophy. Pharmacoeconomics. (2006) 24(4):387–400. doi: 10.2165/00019053-200624040-00008
29. Hagos Gufue Z, Gizaw NF, Ayele W, Yifru YM, Hailu NA, Welesemayat ET, et al. Survival of stroke patients according to hypertension Status in northern Ethiopia: seven years retrospective cohort study. Vasc Health Risk Manag. (2020) 16:389–401. doi: 10.2147/VHRM.S247667
30. Aggarwal R, Petrie B, Bala W, Chiu N. Mortality outcomes with intensive blood pressure targets in chronic kidney disease patients. Hypertension. (2019) 73(6):1275–82. doi: 10.1161/HYPERTENSIONAHA.119.12697
31. Zhang H, Duan X, Rong P, Dang Y, Yan M, Zhao Y, et al. Effects of potential risk factors on the development of cardiometabolic multimorbidity and mortality among the elders in China. Front Cardiovasc Med. (2022) 9:966217. doi: 10.3389/fcvm.2022.966217
32. Pugliese NR, De Biase N, Gargani L, Mazzola M, Conte L, Fabiani I, et al. Predicting the transition to and progression of heart failure with preserved ejection fraction: a weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur J Prev Cardiol. (2021) 28(15):1650–61. doi: 10.1093/eurjpc/zwaa129
33. Ramezankhani A, Blaha MJ, Mirbolouk M, Azizi F, Hadaegh F. Multi-State analysis of hypertension and mortality: application of semi-markov model in a longitudinal cohort study. BMC Cardiovasc Disord. (2020) 20(1):321. doi: 10.1186/s12872-020-01599-7
34. (WHO) WHO. Icd-9 : International Statistical Classification of Diseases and Related Health Problems : Ninth Revision (1978). Available at: https://apps.who.int/iris/handle/10665/39473 (Cited September 11, 2022).
35. (WHO) WHO. Icd-10 : International Statistical Classification of Diseases and Related Health Problems : Tenth Revision (2004). Available at: https://apps.who.int/iris/handle/10665/42980 (Cited September 11, 2022).
36. Morley KI, Wallace J, Denaxas SC, Hunter RJ, Patel RS, Perel P, et al. Defining disease phenotypes using national linked electronic health records: a case study of atrial fibrillation. PLoS One. (2014) 9(11):e110900. doi: 10.1371/journal.pone.0110900
37. Sapp PA, Riley TM, Tindall AM, Sullivan VK, Johnston EA, Petersen KS, et al. Chapter 22—nutrition and atherosclerotic cardiovascular disease. In: Marriott BP, Birt DF, Stallings VA, Yates AA, editors. Present knowledge in nutrition (eleventh edition). London: Academic Press (2020). p. 393–411.
38. Goel MK, Khanna P, Kishore J. Understanding survival analysis: kaplan-meier estimate. Int J Ayurveda Res. (2010) 1(4):274–8. doi: 10.4103/0974-7788.76794
39. Davidson-Pilon C. Lifelines: survival analysis in python. J Open Source Softw. (2019) 4(40):1317. doi: 10.21105/joss.01317
41. Raybaut P. Spyder-Documentation (2009). Available at: pythonhosted.org
42. Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. (2005) 16(2):529–38. doi: 10.1681/asn.2004080656
43. Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and coronary artery disease: jacc state-of-the-art review. J Am Coll Cardiol. (2019) 74(14):1823–38. doi: 10.1016/j.jacc.2019.08.1017
44. Saad M, Karam B, Faddoul G, Douaihy YE, Yacoub H, Baydoun H, et al. Is kidney function affecting the management of myocardial infarction? A retrospective cohort study in patients with normal kidney function, chronic kidney disease stage III-V, and esrd. Int J Nephrol Renovasc Dis. (2016) 9:5–10. doi: 10.2147/ijnrd.S91567
45. Banerjee D, Rosano G, Herzog CA. Management of heart failure patient with ckd. Clin J Am Soc Nephrol. (2021) 16(7):1131–9. doi: 10.2215/cjn.14180920
46. Nayak-Rao S, Shenoy MP. Stroke in patients with chronic kidney disease…: how do we approach and manage it? Indian J Nephrol. (2017) 27(3):167–71. doi: 10.4103/0971-4065.202405
47. Ghoshal S, Freedman BI. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol. (2019) 50(4):229–39. doi: 10.1159/000502446
48. van der Burgh AC, Geurts S, Ikram MA, Hoorn EJ, Kavousi M, Chaker L. Bidirectional association between kidney function and atrial fibrillation: a population-based cohort study. J Am Heart Assoc. (2022) 11(10):e025303. doi: 10.1161/JAHA.122.025303
49. Chavda V, Chaurasia B, Deora H, Umana GE. Chronic kidney disease and stroke: a bi-directional risk cascade and therapeutic update. Brain Disorders. (2021) 3:100017. doi: 10.1016/j.dscb.2021.100017
50. Synhaeve N, van Alebeek M, Arntz R, Maaijwee N, Rutten-Jacobs L, Schoonderwaldt H, et al. Kidney dysfunction increases mortality and incident events after young stroke: the future study. Cerebrovasc Dis. (2016) 42:224–31. doi: 10.1159/000444683
51. Chuengsaman P, Kasemsup V. Pd first policy: Thailand's response to the challenge of meeting the needs of patients with End-stage renal disease. Semin Nephrol. (2017) 37(3):287–95. doi: 10.1016/j.semnephrol.2017.02.008
52. Kanjanabuch T, Takkavatakarn K. Global dialysis perspective: Thailand. Kidney360. (2020) 1(7):671–5. doi: 10.34067/kid.0000762020
53. Chou CY, Wang SM, Liang CC, Chang CT, Liu JH, Wang IK, et al. Peritoneal dialysis is associated with a better survival in cirrhotic patients with chronic kidney disease. Medicine (Baltimore). (2016) 95(4):e2465. doi: 10.1097/md.0000000000002465
54. Tekkarişmaz N, Torun D. Long-Term clinical outcomes of peritoneal dialysis patients: 9-year experience of a single centre in Turkey. Turk J Med Sci. (2020) 50(2):386–97. doi: 10.3906/sag-1909-98
55. Assanatham M, Pattanaprateep O, Chuasuwan A, Vareesangthip K, Supasyndh O, Lumpaopong A, et al. Economic evaluation of peritoneal dialysis and hemodialysis in Thai population with End-stage kidney disease. BMC Health Serv Res. (2022) 22(1):1384. doi: 10.1186/s12913-022-08827-0
56. Cai Q, Mukku VK, Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. (2013) 9(4):331–9. doi: 10.2174/1573403x10666140214122234
57. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease. Circulation. (2003) 108(17):2154–69. doi: 10.1161/01.CIR.0000095676.90936.80
58. Chwojnicki K, Wierucki Ł, Zagożdżon P, Wojtyniak B, Nyka WM, Zdrojewski T. Long-Term mortality after stroke is higher than after myocardial infarction. Neurol Sci. (2016) 37(6):891–8. doi: 10.1007/s10072-016-2502-4
59. Vaartjes I, van Dis I, Grobbee DE, Bots ML. The dynamics of mortality in follow-up time after an acute myocardial infarction, lower extremity arterial disease and ischemic stroke. BMC Cardiovasc Disord. (2010) 10(1):57. doi: 10.1186/1471-2261-10-57
60. Wilhelmsen L, Köster M, Harmsen P, Lappas G. Differences between coronary disease and stroke in incidence, case fatality, and risk factors, but few differences in risk factors for fatal and non-fatal events. Eur Heart J. (2005) 26(18):1916–22. doi: 10.1093/eurheartj/ehi412
61. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Global Health. (2017) 2(2):e000298. doi: 10.1136/bmjgh-2017-000298
62. Antonakoudis G, Poulimenos L, Kifnidis K, Zouras C, Antonakoudis H. Blood pressure control and cardiovascular risk reduction. Hippokratia. (2007) 11(3):114–9. PMID: 19582204.19582204
63. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: jacc health promotion series. J Am Coll Cardiol. (2018) 72(11):1278–93. doi: 10.1016/j.jacc.2018.07.008
64. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Br Med J. (2009) 338:b1665. doi: 10.1136/bmj.b1665
65. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375(9731):2073–81. doi: 10.1016/s0140-6736(10)60674-5
66. Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. (2003) 41(1):47–55. doi: 10.1016/s0735-1097(02)02663-3
67. Poudel B, Yadav BK, Nepal AK, Jha B, Raut KB. Prevalence and association of microalbuminuria in essential hypertensive patients. N Am J Med Sci. (2012) 4(8):331–5. doi: 10.4103/1947-2714.99501
68. Janssen WM, de Jong PE, de Zeeuw D. Hypertension and renal disease: role of microalbuminuria. J Hypertens Suppl. (1996) 14(5):S173–7. PMID: 9120675.9120675
69. Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol. (2018) 14(12):727–49. doi: 10.1038/s41581-018-0072-9
Keywords: cohort profile, hypertension, hypertension progression, multi-state model, real-world data, survival analysis, transition probability
Citation: Teza H, Boonmanunt S, Unwanatham N, Thadanipon K, Limpijankit T, Pattanaprateep O, Pattanateepapon A, McKay GJ, Attia J and Thakkinstian A (2023) Evaluation of transitions from early hypertension to hypertensive chronic kidney disease, coronary artery disease, stroke and mortality: a Thai real-world data cohort. Front. Cardiovasc. Med. 10:1170010. doi: 10.3389/fcvm.2023.1170010
Received: 2 March 2023; Accepted: 11 April 2023;
Published: 2 May 2023.
Edited by:
Nicola Riccardo Pugliese, University of Pisa, Italy© 2023 Teza, Boonmanunt, Unwanatham, Thadanipon, Limpijankit, Pattanaprateep, Pattanateepapon, McKay, Attia and Thakkinstian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suparee Boonmanunt c3VwYXJlZS5ib29AbWFoaWRvbC5lZHU= Anuchate Pattanateepapon YW51Y2hhdGUuZ2FiQG1haGlkb2wuZWR1
Abbreviations ACD, All-Cause Death; ACEI, Angiotensin Converting Enzyme Inhibitor; BMI, Body Mass Index; CAD, Coronary Artery Disease; CCB, Calcium Channel Blocker; CI, Confidence Interval; CKD, Chronic Kidney Disease; CVD, Cardiovascular Disease; DBP, Diastolic Blood Pressure; eGFR, estimated Glomerular Filtration Rate; GFR, Glomerular Filtration Rate; HDL, High-Density Lipoprotein; HT, Hypertension; ICD, International Classification of Diseases; IQR, Interquartile range; LDL, Low-Density Lipoprotein; n, number of subjects; RAAS, Renin-Angiotensin-Aldosterone System; SBP, Systolic Blood Pressure; SD, Standard Deviation; USD, United States Dollar.
†ORCID Htun Teza orcid.org/0000-0002-1076-9513 Suparee Boonmanunt orcid.org/0000-0003-0474-9986 Nattawut Unwanatham orcid.org/0000-0001-9174-3448 Kunlawat Thadanipon orcid.org/0000-0001-6324-2312 Thosaphol Limpijankit orcid.org/0000-0002-1457-350X Oraluck Pattanaprateep orcid.org/0000-0001-9570-2635 Anuchate Pattanateepapon orcid.org/0000-0003-1246-9482 Gareth J. McKay orcid.org/0000-0001-8197-6280 John Attia orcid.org/0000-0001-9800-1308 Ammarin Thakkinstian orcid.org/0000-0001-9991-386X
 Htun Teza
Htun Teza Suparee Boonmanunt
Suparee Boonmanunt Nattawut Unwanatham1,†
Nattawut Unwanatham1,† John Attia
John Attia Ammarin Thakkinstian
Ammarin Thakkinstian