- Department of Cardiology, Lihuili Hospital Affiliated to Ningbo University, Ningbo University, Ningbo Institute of Innovation for Combined Medicine and Engineering, Ningbo, China
Background: Observational studies have suggested that irritability is associated with a higher risk of cardiovascular disease (CVD). However, the potential causal association is not clear. Therefore, we used Mendelian randomization (MR) analysis to assess the causal association of irritability with CVD risk.
Methods: A two-sample MR analysis was performed to confirm the causal association of irritability with the risk of several common CVDs. The exposure data were derived from the UK biobank involving 90,282 cases and 232,386 controls, and outcome data were collected from the published genome-wide association studies (GWAS) and FinnGen database. Inverse-variance weighted (IVW), MR-Egger, and weighted median methods were performed to assess the causal association. Furthermore, the mediating effect of smoking, insomnia, and depressed affect was explored by using a two-step MR.
Results: The MR analysis indicated that genetically predicted irritability increased the risk of CVD, including coronary artery disease (CAD) (Odds ratio, OR: 2.989; 95% confidence interval, CI: 1.521–5.874, p = 0.001), myocardial infarction (MI) (OR: 2.329, 95% CI: 1.145–4.737, p = 0.020), coronary angioplasty (OR: 5.989, 95% CI: 1.696–21.153, p = 0.005), atrial fibrillation (AF) (OR: 4.646, 95% CI: 1.268–17.026, p = 0.02), hypertensive heart disease (HHD) (OR: 8.203; 95% CI: 1.614–41.698, p = 0.011), non-ischemic cardiomyopathy (NIC) (OR: 5.186; 95% CI: 1.994–13.487, p = 0.001), heart failure (HF) (OR: 2.253; 95% CI: 1.327–3.828, p = 0.003), stroke (OR: 2.334; 95% CI: 1.270–4.292, p = 0.006), ischemic stroke (IS) (OR: 2.249; 95% CI: 1.156–4.374, p = 0.017), and ischemic stroke of large-artery atherosclerosis ISla (OR: 14.326; 95% CI: 2.750–74.540, p = 0.002). The analysis also indicated that smoking, insomnia, and depressed affect play an important role in the process of irritability leading to cardiovascular disease.
Conclusion: Our findings support the first genetic evidence of the causality of genetically predicted irritability with the risk of developing into CVDs. Our results deliver a viewpoint that more early active interventions to manage an individual's anger and related unhealthy lifestyle habits are needed to prevent the occurrence of adverse cardiovascular events.
1. Introduction
Cardiovascular disease (CVD) is a general term for cardiac and vascular diseases such as coronary artery disease (CAD), hypertension, atrial fibrillation (AF), heart failure (HF), and stroke (1, 2). CVDs are the main cause of morbidity, disability, and mortality worldwide, causing a substantial burden on the economy, society, and healthcare. CVDs caused approximately 19 million deaths globally in 2020, with an increase of 18.7% in 10 years (3). The identification of risk factors is expected to mitigate the occurrence and development of CVDs through early prevention and intervention (4).
Irritability is best described by impulsiveness, anger, and hostility and is a tendency to overreact to aversive stimuli with negative affect (5). Some observational studies have shown that irritability is significantly related to various CVDs. A recent cohort study showed that irritability is independently associated with severe CAD (6). In addition, some prospective studies suggested that the level of anger had a dose–response relationship with CAD (7, 8). Meanwhile, irritability also increased the risk of AF (9), HF (10), hypertension (11), and stroke (12).
Although the increasing evidence indicates that irritability is a risk factor for CVDs, it is unclear whether this association merely stems from irritability. Since most findings are based on observational studies, the role of confounding factors and reverse causality cannot be ignored (13). Clarifying the causality between irritability and CVDs is helpful in better preventing the potential risk of adverse cardiovascular events.
Mendelian randomization (MR) analysis is an epidemiological tool that confirms a causal association between a trait and an outcome by applying genetic variants as proxies (14). Because genetic variants have the excellent feature that it is randomly allocated when passed from parents to offspring, the potential confounders and reverse causation bias of observational studies are effectively reduced and circumvented to reinforce causal inference (15).
Nevertheless, so far, no MR studies have focused on the causal inference between irritability and CVDs. In this study, the objective is to assess the potential causal association between irritability and various CVDs by implementing a two-sample MR analysis and a two-step MR analysis.
2. Methods
2.1. Study design and data sources
A summary of the study design is presented in Figure 1. Two-sample MR analyses were performed to evaluate the causality between genetic susceptibility to irritability and the risk of several CVDs. Moreover, we used a two-step MR to estimate whether the causal relationship is mediated by CVD risk factors such as smoking, insomnia, and depressed affect.
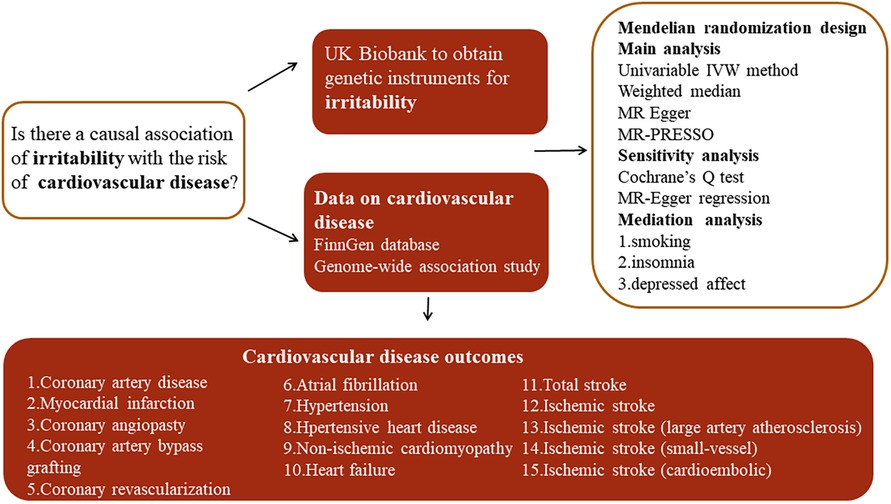
Figure 1. Overview of the study design. MR-PRESSO, MR pleiotropy residual sum and outlier test; IVW, inverse variance weighted.
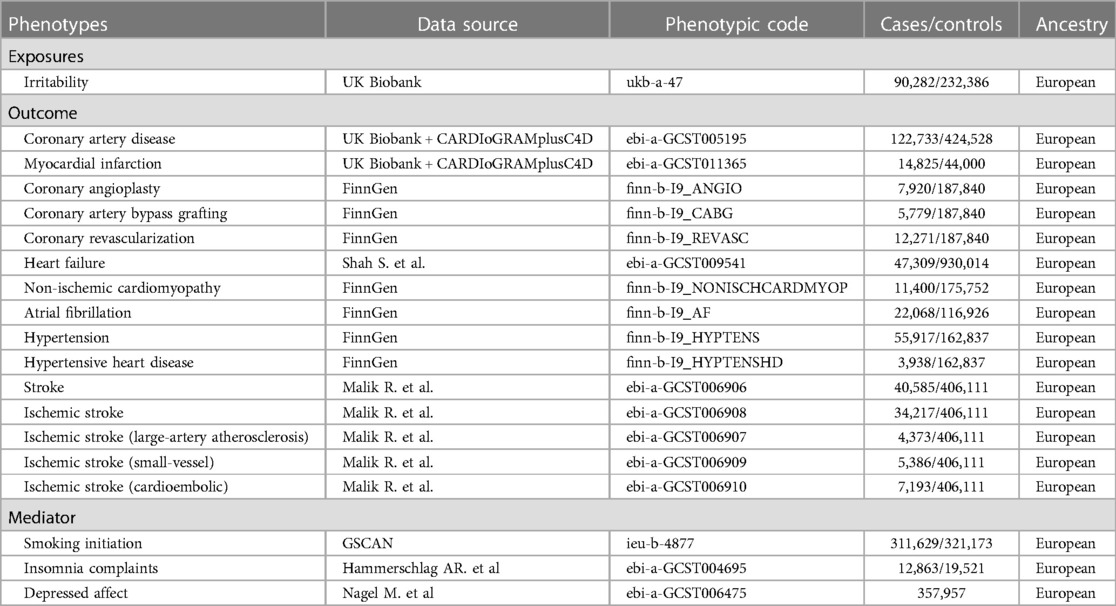
Genetic variants (single-nucleotide polymorphisms (SNPs)) were applied as instrumental variables (IVs) to explore the causal relationship. The whole process followed the three main assumptions in MR analysis: (a) IVs are robustly related to exposure factors; (b) IVs are not associated with confounding factors; and (c) IVs influence the risk of the outcomes only via exposure (10). Summary-level data for irritability were obtained from the UK Biobank (UKB) involving 90,282 cases and 232,386 controls. The question “Are you an irritable person?” was used to reflect irritability in the data sources. The data for various CVDs were derived from recently publicly available genome-wide association studies (GWASs) and the FinnGen GWAS database, including coronary artery disease (CAD), myocardial infarction (MI), coronary angioplasty (CA), coronary artery bypass grafting (CABG), coronary revascularization (CR), atrial fibrillation (AF), hypertension (HTN), hypertensive heart disease (HHD), non-ischemic cardiomyopathy (NIC), stroke, ischemic stroke (IS), ischemic stroke of large-artery atherosclerosis (ISla), ischemic stroke of small-vessel (ISsv), and cardioembolic ischemic stroke (ISce). All summary statistics associated with irritability and CVDs in the present study were retrieved from the IEU OpenGWAS Project (https://gwas.mrcieu.ac.uk/), and a detailed description is listed in Table 1.
2.2. Selection of instrumental variables
SNPs were identified as IVs at the genome-wide significance threshold (p < 5 × 10−8). To ensure independence between the included SNPs as IVs, we set the linkage disequilibrium (LD) threshold for grouping to r2 < 0.001 and a genetic distance of 10,000 kb. PhenoScanner (16) was used to search for each included SNP to estimate the associations with potential confounders (BMI, hypertension, smoking, insomnia, and depressed affect) and exclude those strongly associated with the confounders (p < 5 × 10−8). The details of the used SNPs between exposure (irritability, smoking, insomnia, and depressed affect) and outcomes (CVDs) are given in Supplementary Table S1–S43. F-statistics were used to eliminate the weak instrument bias of the used SNPs. An F-statistic >10 indicated the correlation between the IVs, and exposure was considered sufficiently strong and avoided a weak instrument bias (17).
2.3. Statistical analyses
R packages (TwoSampleMR and MR-PRESSO) and R software (version 4.2.0) were performed for the statistical analysis. Multiple MR methods were employed to assess the causal association between irritability and CVDs. Each method has different assumptions for achieving effective IVs. Inverse-variance weighted (IVW) was used as the main method in MR analyses, as the results of the IVW method are generally most reliable when the total IVs are valid (13). MR-pleiotropy residual sum and outlier (MR-PRESSO) were used to discover outliers in IVW linear regression and correct MR estimates after removing outliers when they exist (18). In addition, weighted median (19) and MR-Egger (20) methods were applied as secondary supplements. Several methods were implemented for sensitivity analysis to confirm the validity and robustness of the results. The heterogeneity of the used SNPs was assessed through Cochrane's Q test; if heterogeneity existed (p < 0.05), the multiplicative random effects IVW was considered as the main method to obtain a robust and conservative result. Moreover, MR-Egger regression analysis was performed to assess whether a directional pleiotropy of the used IVs existed by its intercept.
2.4. Mediation MR analysis
A design of mediation analysis is shown in Figure 2. Some recognized risk factors of CVD are significantly associated with irritability, such as smoking, insomnia, and depressed affect. Therefore, a two-step MR was used to estimate to what extent the causal relationship was mediated by these risk factors. In the first step, the causality of irritability on these potential mediators was confirmed. In the second step, we used the IVs that were significantly associated with the mediators to assess the causal association between the mediators and the risk of CVDs causally associated with irritability.
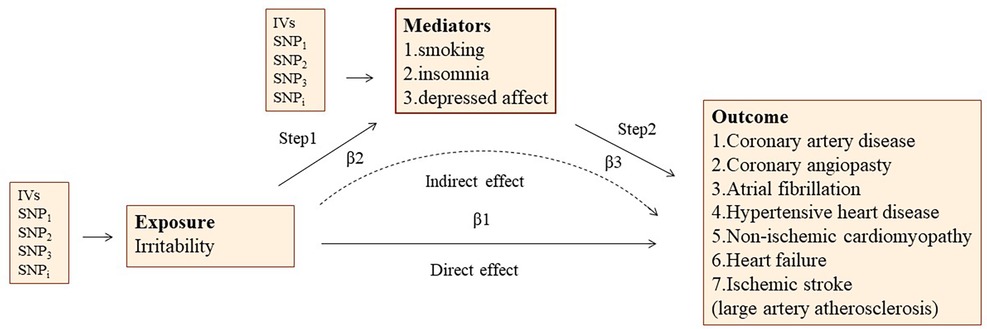
Figure 2. The overview of two-step mediation analysis of irritability on various CVDs via potential mediators. IVs, instrumental variables. In the first step, the causality of irritability on potential mediators was confirmed. In the second step, IVs significantly associated with the mediators are used to assess the causal effect between mediators and the risk of CVDs causally associated with irritability. Direct effect, the effect of irritability on CVD risk after adjusting for the mediators (β1–β2*β3). Indirect effect, the effect of irritability on CVD risk through the mediator (β2*β3).
3. Results
3.1. Selection of instrumental variables
Detailed characteristics of SNPs selected to predict irritability are displayed in Supplementary Table S1. All SNPs satisfied established screening criteria in Methods. There was no weak tool bias in the IV strength test since the F-statistic of all SNPs >10.
3.2. Causal estimates of genetic susceptibility to irritability and CVD risk
The genetically predicted results between irritability and various CVDs are presented in Figure 3. The IVW analyses as the main analysis method indicated that genetically predicted irritability was positively associated with the risk of several CVDs, including CAD (OR: 2.989, 95% CI: 1.521–5.874, p = 0.001), MI (OR: 2.329, 95% CI: 1.145–4.737, p = 0.020), CA (OR: 5.989, 95% CI: 1.696–21.153, p = 0.005), AF (OR: 4.646, 95% CI: 1.268–17.026, p 0.02), HHD (OR: 8.203, 95% CI: 1.614–41.698, p = 0.011), NIC (OR: 5.186, 95% CI: 1.994–13.487, p = 0.001), HF (OR: 2.253, 95% CI: 1.327–3.828, p = 0.003), stroke (OR: 2.334, 95% CI: 1.270–4.292, p = 0.006), IS (OR: 2.249, 95% CI: 1.156–4.374, p = 0.017), and ISla (OR: 14.326, 95% CI: 2.750–74.540, p = 0.002). Nevertheless, insufficient evidence indicated that genetically predicted irritability had a remarkable causal relationship with the prevalence of other CVDs such as CABG, CR, HTN, ISsv, and ISce (Figure 3).
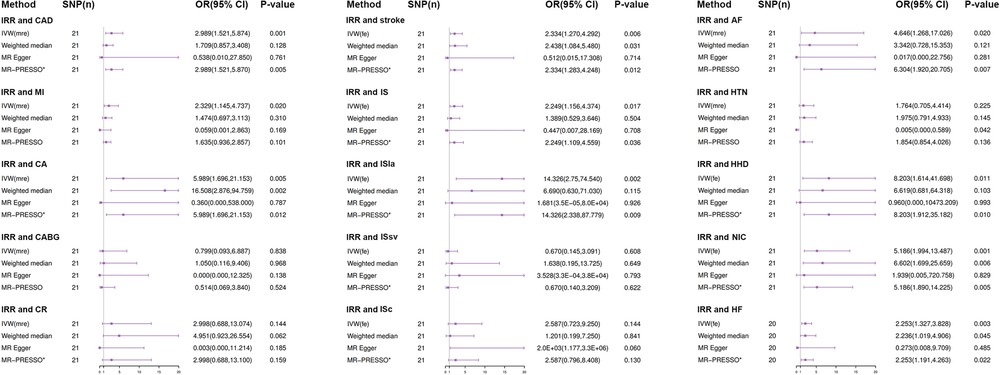
Figure 3. Results from Mendelian randomization analysis of irritability and CVD risk. SNP, Single-nucleotide polymorphism; OR, odd ratio; CI, confidence interval; IVW (fixed effects), fixed effects inverse-variance weighted; IVW (MR effects), multiplicative random inverse-variance weighted; MR-PRESSO, MR-pleiotropy residual sum and outlier; *No outlier was detected; IRR, irritability; CAD, coronary artery disease; MI, myocardial infarction; CA, coronary angioplasty; CABG, coronary artery bypass grafting; CR, coronary revascularization (CA or CABG); AF, atrial fibrillation; HTN, hypertension; HHF, hypertensive heart disease; NIC, non-ischemic cardiomyopathy; HF, heart failure; IS, ischemic stroke; ISla, ischemic stroke (large-artery atherosclerosis); ISsv, ischemic stroke (small-vessel); ISce, ischemic stroke (cardioembolic).
The effect estimator of most statistical analysis models remained generally directionally consistent with IVW analysis, such as weighted median, MR-Egger, and MR-PRESSO. In all the causal analyses, most MR-PRESSO analysis methods did not detect outliers, demonstrating the reliability of the causal relationship estimates in this study (Figure 3). Moreover, scatter plots showed the associations between irritability and various CVD risks (Supplementary Figure S1).
3.3. MR sensitivity analysis
In Cochran's Q test, we found that there was heterogeneity between the used SNPs in part analyses of causal effects (p-values of Cochran's Q statistics <0.05) such as CAD, MI, CA, CABG, CR, AF, and HTN. Consequently, multiplicative random effects IVW was used in these causal effect analyses, and IVW analysis was implemented with a fixed-effects model in other effect analyses (Table 2). In addition, apart from HTN, we did not observe that the intercepts of the MR-Egger analysis deviated from zero in all studies, demonstrating that there was no horizontal pleiotropy (Table 2 and Supplementary Figure S1).

Table 2. Heterogeneity and pleiotropy tests for the associations between irritability with various cVDs.
3.4. Mediation MR analysis results
Considering the higher propensity for smoking, insomnia, and depressed affect in the irritable population, these factors may be mediators causing the adverse effect of irritability on various CVDs. We assessed the mediating pathways of smoking initiation, insomnia, and depressed affect in irritability on the risk of several CVDs by performing a two-step MR analysis (Figure 2).
In the first step, we investigated the causal effect of irritability on smoking initiation (IVW: OR: 1.911, 95% CI: 1.276–2.886, p = 0.002), irritability on insomnia (IVW: OR: 4.324, 95% CI: 2.359–7.929, p < 0.001), and irritability on depressed affect (IVW: OR: 3.327, 95% CI: 2.682–4.127, p < 0.001) (Table 3 and Supplementary Table S44).
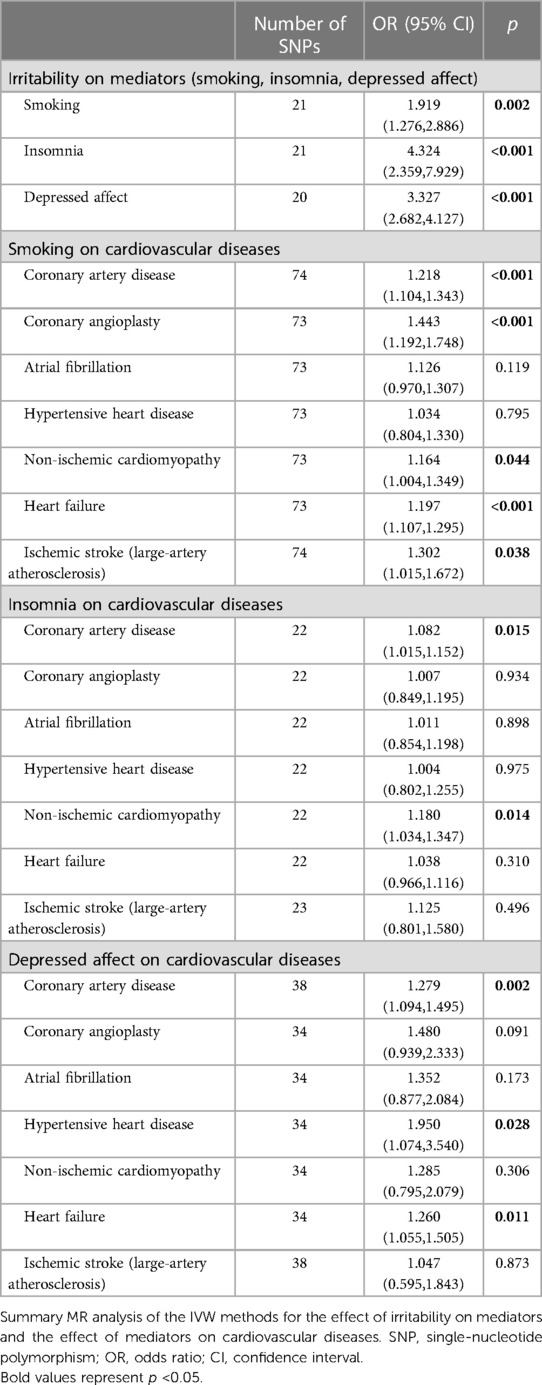
Table 3. The mediation analysis results of irritability on cardiovascular diseases via smoking, insomnia, and depressed affect.
In a second step, we confirmed the causal effect association of mediators on coronary artery disease (CAD), coronary angioplasty (CA), atrial fibrillation (AF), hypertensive heart disease (HHD), non-ischemic cardiomyopathy (NIC), heart failure (HF), and ischemic stroke of large-artery atherosclerosis (ISla). MR-Egger and weighted median estimations were consistent with IVW MR analysis in the direction (Table 3 and Supplementary Table S45–S47).
Finally, we assessed the causal effect of irritability on several CVDs through the mediating effect of smoking (irritability on CAD: mediated proportion = 12%, OR = 1.137; irritability on CA: mediated proportion = 13%, OR = 1.270; irritability on NIC: mediated proportion = 6%, OR = 1.104; irritability on HF: mediated proportion = 14%, OR = 1.125; irritability on ISla: mediated proportion = 6%, OR = 1.188), insomnia (irritability on CAD: mediated proportion = 10%, OR = 1.122; irritability on NIC: mediated proportion = 15%, OR = 1.274), and depressed affect (irritability on CAD: mediated proportion = 27%, OR = 1.344; irritability on CA: mediated proportion = 26%, OR = 1.602; irritability on HHD: mediated proportion = 38%, OR = 2.232; irritability on HF: mediated proportion = 34%, OR = 1.320) (Table 4).
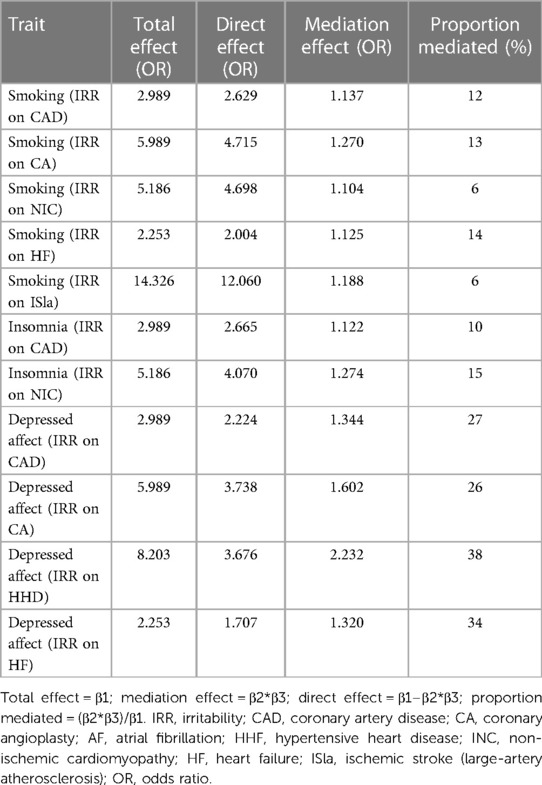
Table 4. The mediation effect of irritability on cardiovascular diseases via smoking, insomnia, and depressed affect.
4. Discussion
To our knowledge, many observational studies have found evidence of a relationship between irritability and CVD risk. With the various MR analyses, we systematically confirmed the potential causal relationships between irritability susceptibility and a broad range of CVD risks for the first time. The MR evidence of our study indicates that genetic predisposition to irritability has adverse causal associations with many CVD risks, including CAD, AF, HHD, NIC, HF, and ISla. In addition, insufficient MR evidence supports a potential causal association between genetic predisposition to irritability and the risk of HTN, ISsv, and ISce. Given the fact that the irritable population has a high propensity for smoking (21), insomnia (22), and depressed affect (23, 24), the results of the mediation MR analysis in our study show that these factors play an important role in the process of irritability leading to cardiovascular disease. The MR results are robust due to the use of different MR analysis methods and strong instruments.
Recently, increasing attention has been focused on the role of psychosocial factors in CVDs. Irritability is generally best described by impulsiveness, anger, and hostility and is a tendency to overreact to aversive stimuli with negative affect. Observational studies have shown that irritability is significantly related to an increased risk of various CVDs, which was supported by the present MR analysis.
In specific CVDs, our estimates were consistent with the findings of previous studies.
A cohort study of 225 patients with suspected CAD found that high irritable scores had a direct relationship with the presence of significant luminal stenosis (6). Another cohort study, including 506 women with suspected CAD, reported that hostile affect and aggressive responses were associated with poorer event-free survival and a higher risk of adverse events (25). In addition, a prospective study indicated that anger increased the risk of CAD during an average of 10.9 years of follow-up (8). We used MR analysis to further prove their relevance. Irritability increased the risk of CAD and MI; also, irritable people are more likely to accept to undergo coronary angioplasty. Moreover, we found that smoking, insomnia, and depressed affect mediated the occurrence of CAD.
In a study involving 6,292 Japanese people, it was found that men with higher “anger-in” scores had a 50% increased risk of hypertension compared with those with lower scores, but no such association was found in women (26). A recent study suggested that there should be focused attention on anger as a potential risk factor for hypertension (27). We did not find any association between irritability and hypertension in our MR study, but we found that irritable people had a tendency to smoke. Men are more inclined to smoke and smoking is a risk factor for hypertension. This may partly explain why the association of anger with hypertension is significant only in men. Moreover, a study confirmed that anger may cause left ventricular hypertrophy in hypertensive patients (28). We confirmed the causal relationship between irritability and hypertensive heart disease. Although irritability is not directly related to hypertension, irritability-related bad behaviors are risk factors for hypertension. They may play a significant mediatory role in the development of hypertension.
A meta-analysis demonstrated that the incidence of AF was increased by 15% for patients with anger (9). A Swedish cohort study also found that anger increased the risk of AF (29). In contrast, another study showed that no significant associations were observed between anger, anxiety, and chronic stress and the development of AF (30). Our results of MR analysis provided evidence that genetically predicted irritability increased the incidence of AF.
A Swedish cohort study found that anger increased the risk of HF (HR = 1.19) (29). A prospective study containing 13,171 black and white participants found that the incidence of HF was greater among participants with high trait anger (HR: 1.44) compared with those with low or moderate trait anger. Adjustment for comorbidities and depressive symptoms attenuated the estimated relative hazard in men to 1.26 (10). An angry temperament may cause a rise in the levels of catecholamine, resulting in transient left ventricular dysfunction. Recently, anger has been found associated with worse resting left ventricular diastolic pressure (31). These findings are consistent with our findings. Our results indicated that irritability increased the risk of NIC and HF. The potential association of irritability with HF was partly mediated by depressed affect (34%).
Anger has been suggested as a triggering factor for stroke (32). A study reported that Finnish male patients with the highest level of expressed anger were at twice the risk of stroke of men who reported the lowest level of anger, after adjustments for risk factors (12). Another study including 13,851 black and white participants obtained similar results that participants with high trait anger had a 2.82 times greater risk for hemorrhagic and ischemic strokes combined and a 2.93 times greater risk for ischemic strokes alone than their counterparts with low trait anger during an average of 6.4 years follow-up (33). Our results demonstrated that irritability increased the risk of total stroke, IS, and ISla. Smoking plays a part of the intermediary role in the process of disease formation occurrence of ISla.
Several hypotheses have explained the association between an angry temperament and CVD. Highly hostile individuals constantly have a high level of catecholamine and cortisol secretion in response to anger-provoking stimuli (5) and they display increased cortisol excretion during daytime hours along with an activation of the hypothalamic–hypophyseal axis (34, 35). This causes endothelial injury, increased vascular rigidity, and the disruption of vulnerable plaques. In addition, an angry temperament may lead to augmented platelet aggregation, decreased fibrinolytic potential, and increased plasma viscosity by enhancing inflammatory and prothrombotic responses (36). Moreover, behavioral factors may also have a significant impact on the relationship between irritability and CVD risk. Our two-step MR analysis revealed that irritability is significantly linked to an increased risk of smoking, insomnia, and depressed affect, which have all been identified as major risk factors for CVD. Controlling these behavioral factors in irritable individuals could be an effective measure to reduce the incidence of CVD in this population. These mechanisms theoretically can help us explain our observed association between irritability and various CVDs, but more comprehensive and reliable studies are needed to confirm these hypotheses.
There are several noteworthy advantages in our study. The current evidence on the relationship between irritability and the risk of CVDs is almost based on observational studies, and the findings may be limited by sample size, residual confusion, and reverse causality. Due to the random allocation of genetic variants, the MR analysis can help obtain a more robust causal effect between exposure and outcome by the avoidance of these limitations. In the present MR study, multiple MR methods were performed to confirm the causality between irritability and the risk of CVDs for the first time. Moreover, several sensitivity analyses were used to estimate heterogeneity and pleiotropy. Lastly, we used a two-step MR to perform a mediation analysis and indicated that the causal relationship of irritability on various CVDs may be mediated by smoking, insomnia, and depression.
Inevitably, certain limitations should be acknowledged. First, all participants involved in our study were Europeans. However, risk factors, prevalence, and mortality are different in different ethnicities. Therefore, in other ethnicities and populations, our findings possibly cannot explain the potential causality of irritability and CVDs. In addition, we tried our best to avoid sample overlap, but the datasets of CAD and MI were partly prepared from UKB data, which may cause slightly biased MR estimates. Therefore, we further confirmed the causality of irritability by highlighting the necessity of angioplasty intervention, because coronary angioplasty is the main diagnosis and treatment method for patients with coronary heart disease. Finally, a part MR-PRESSO analysis method detected outliers (AF, MI, and CABG), but the result of the MR-Egger regression analysis showed that there was no horizontal pleiotropy. Therefore, the results of the MR analysis should be interpreted with caution.
5. Conclusions
This MR study supports the first genetic evidence that genetically predicted irritability may be implicated in the development of CVD. We found the existence of adverse associations between irritability and the risk of CAD, AF, HF, HHD, NIC, and ISla, and these associations may partly be driven by smoking, insomnia, and depressed affect. Our results deliver a viewpoint that the identification of irritability as a risk factor or trigger of CVD is important because more early active interventions to manage an individual's anger may prevent the occurrence of cardiovascular adverse events. Moreover, correcting the unhealthy lifestyle habits of irritable individuals is an effective clinical intervention for reducing the risk of CVD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Author contributions
JL designed and funded the study. DC and YF were primarily involved in data acquisition, MR analysis, and drafting of the manuscript. HL, YS, and YB draft the manuscript and prepared the figures and tables. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (grant no. 81870255), the Natural Science Foundation of the Zhejiang Province (grant no. LY21H020001), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (grant no. 2021KY306), and the Natural Science Foundation of Ningbo Municipality (grant no. 2021J296).
Acknowledgments
We acknowledge the efforts of the participants and investigators of the FinnGen studies and UK biobank, and we owe a note of special mention to the IEU Open GWAS project for publicly providing summary data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1174329/full#supplementary-material
References
1. Roth GA, Mensah GA, Fuster V. The global burden of cardiovascular diseases and risks: a compass for global action. J Am Coll Cardiol. (2020) 76(25):2980–1. doi: 10.1016/j.jacc.2020.11.021
2. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 Acc/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. (2019) 140(11):e596–e646. doi: 10.1161/cir.0000000000000678
3. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics – 2022 update: a report from the American Heart Association. Circulation. (2022) 145(8):e153–e639. doi: 10.1161/cir.0000000000001052
4. Roger VL, Sidney S, Fairchild AL, Howard VJ, Labarthe DR, Shay CM, et al. Recommendations for cardiovascular health and disease surveillance for 2030 and beyond: a policy statement from the American Heart Association. Circulation. (2020) 141(9):e104–19. doi: 10.1161/cir.0000000000000756
5. Suarez EC, Kuhn CM, Schanberg SM, Williams RB Jr, Zimmermann EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosom Med. (1998) 60(1):78–88. doi: 10.1097/00006842-199801000-00017
6. Vecsey-Nagy M, Szilveszter B, Kolossváry M, Boussoussou M, Vattay B, Gonda X, et al. Association between affective temperaments and severe coronary artery disease. J Affect Disord. (2021) 295:914–9. doi: 10.1016/j.jad.2021.08.063
7. Kawachi I, Sparrow D, Spiro A 3rd, Vokonas P, Weiss ST. A prospective study of anger and coronary heart disease. The normative aging study. Circulation. (1996) 94(9):2090–5. doi: 10.1161/01.cir.94.9.2090
8. Kubzansky LD, Cole SR, Kawachi I, Vokonas P, Sparrow D. Shared and unique contributions of anger, anxiety, and depression to coronary heart disease: a prospective study in the normative aging study. Ann Behav Med. (2006) 31(1):21–9. doi: 10.1207/s15324796abm3101_5
9. Wu H, Li C, Li B, Zheng T, Feng K, Wu Y. Psychological factors and risk of atrial fibrillation: a meta-analysis and systematic review. Int J Cardiol. (2022) 362:85–92. doi: 10.1016/j.ijcard.2022.05.048
10. Kucharska-Newton AM, Williams JE, Chang PP, Stearns SC, Sueta CA, Blecker SB, et al. Anger proneness, gender, and the risk of heart failure. J Card Fail. (2014) 20(12):1020–6. doi: 10.1016/j.cardfail.2014.09.010
11. Kőrösi B, Gyöngyösi H, Batta D, László A, Kovács I, Tislér A, et al. Evaluation of affective temperaments and arterial stiffness in different hypertension phenotypes. Hypertens Res. (2021) 44(1):47–54. doi: 10.1038/s41440-020-0513-2
12. Everson SA, Kaplan GA, Goldberg DE, Lakka TA, Sivenius J, Salonen JT. Anger expression and incident stroke: prospective evidence from the Kuopio ischemic heart disease study. Stroke. (1999) 30(3):523–8. doi: 10.1161/01.str.30.3.523
13. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27(8):1133–63. doi: 10.1002/sim.3034
14. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32(1):1–22. doi: 10.1093/ije/dyg070
15. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
16. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. Phenoscanner V2: an expanded tool for searching human genotype–phenotype associations. Bioinformatics. (2019) 35(22):4851–3. doi: 10.1093/bioinformatics/btz469
17. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40(3):755–64. doi: 10.1093/ije/dyr036
18. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
19. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40(4):304–14. doi: 10.1002/gepi.21965
20. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
21. Bisol LW, Soldado F, Albuquerque C, Lorenzi TM, Lara DR. Emotional and affective temperaments and cigarette smoking in a large sample. J Affect Disord. (2010) 127(1–3):89–95. doi: 10.1016/j.jad.2010.04.016
22. Oniszczenko W, Rzeszutek M, Stanisławiak E. Affective temperaments, mood, and insomnia symptoms in a nonclinical sample. Behav Sleep Med. (2019) 17(3):355–63. doi: 10.1080/15402002.2017.1357121
23. Eyre O, Hughes RA, Thapar AK, Leibenluft E, Stringaris A, Davey Smith G, et al. Childhood neurodevelopmental difficulties and risk of adolescent depression: the role of irritability. J Child Psychol Psychiatry. (2019) 60(8):866–74. doi: 10.1111/jcpp.13053
24. Carter Leno V, Wright N, Pickles A, Bedford R, Zaidman-Zait A, Kerns C, et al. Longitudinal associations between early childhood irritability and adolescent depression symptoms in autistic children are mediated by peer relationships but not educational engagement. Dev Psychopathol. (2023):1–11. doi: 10.1017/s0954579422001316
25. Olson MB, Krantz DS, Kelsey SF, Pepine CJ, Sopko G, Handberg E, et al. Hostility scores are associated with increased risk of cardiovascular events in women undergoing coronary angiography: a report from the NHLBI-sponsored WISE study. Psychosom Med. (2005) 67(4):546–52. doi: 10.1097/01.psy.0000170830.99263.4e
26. Ohira T. Psychological distress and cardiovascular disease: the circulatory risk in communities study (CIRCS). J Epidemiol. (2010) 20(3):185–91. doi: 10.2188/jea.je20100011
27. Gavrilova L, Zawadzki MJ. Testing the associations between state and trait anxiety, anger, sadness, and ambulatory blood pressure and whether race impacts these relationships. Ann Behav Med. (2023) 57(1):38–49. doi: 10.1093/abm/kaab098
28. Vecsey-Nagy M, Szilveszter B, Kolossváry M, Boussoussou M, Vattay B, Gonda X, et al. Cyclothymic affective temperament is independently associated with left ventricular hypertrophy in chronic hypertensive patients. J Psychosom Res. (2022) 160:110988. doi: 10.1016/j.jpsychores.2022.110988
29. Titova OE, Baron JA, Michaëlsson K, Larsson SC. Anger frequency and risk of cardiovascular morbidity and mortality. Eur Heart J Open. (2022) 2(4):oeac050. doi: 10.1093/ehjopen/oeac050
30. Garg PK, O'Neal WT, Diez-Roux AV, Alonso A, Soliman EZ, Heckbert S. Negative affect and risk of atrial fibrillation: MESA. J Am Heart Assoc. (2019) 8(1):e010603. doi: 10.1161/jaha.118.010603
31. Harris KM, Gottdiener JS, Gottlieb SS, Burg MM, Li S, Krantz DS. Impact of mental stress and anger on indices of diastolic function in patients with heart failure. J Card Fail. (2020) 26(11):1006–10. doi: 10.1016/j.cardfail.2020.07.008
32. Choi-Kwon S, Kim JS. Anger, a result and cause of stroke: a narrative review. J Stroke. (2022) 24(3):311–22. doi: 10.5853/jos.2022.02516
33. Williams JE, Nieto FJ, Sanford CP, Couper DJ, Tyroler HA. The association between trait anger and incident stroke risk: the atherosclerosis risk in communities (ARIC) study. Stroke. (2002) 33(1):13–9. doi: 10.1161/hs0102.101625
34. Pope MK, Smith TW. Cortisol excretion in high and low cynically hostile men. Psychosom Med. (1991) 53(4):386–92. doi: 10.1097/00006842-199107000-00004
35. Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain Behav Immun. (2010) 24(2):215–9. doi: 10.1016/j.bbi.2009.08.009
Keywords: cardiovascular disease, irritability, coronary artery disease, heart failure, Mendelian randomization
Citation: Cai D, Fu Y, Song Y, Lin H, Ba Y and Lian J (2023) A causal relationship between irritability and cardiovascular diseases: a Mendelian randomization study. Front. Cardiovasc. Med. 10:1174329. doi: 10.3389/fcvm.2023.1174329
Received: 26 February 2023; Accepted: 10 May 2023;
Published: 30 May 2023.
Edited by:
János Nemcsik, Semmelweis University, HungaryReviewed by:
Amirmohammad Khalaji, Tehran University of Medical Sciences, IranXenia Gonda, Semmelweis University, Hungary
© Cai, Fu, Song, Lin, Ba and Lian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangfang Lian aGptcGluQDE2My5jb20=
†These authors have contributed equally to this work
 Dihui Cai
Dihui Cai Yin Fu
Yin Fu Yongfei Song
Yongfei Song Hui Lin
Hui Lin Jiangfang Lian
Jiangfang Lian