Abstract
Background:
Several cardiovascular (CV) risk factors are reported to be associated with abnormal cardiac structure in children and adults. However, no study has assessed the association between clustering of multiple CV risk factors and left ventricular geometric (LVG) remodeling. We examined the association between clustering of CV risk factors and LVG remodeling among Chinese children.
Methods:
This cross-sectional study included 1,406 children aged 6–11 years. Clustering of CV risk factors was quantified as the sum of the number of five CV risk factors (abdominal obesity, elevated blood pressure, high fasting blood glucose, high triglycerides and low high-density lipoprotein cholesterol). Based on left ventricular mass index and relative wall thickness (RWT), left ventricular hypertrophy (LVH), high RWT and LVG remodeling [concentric remodeling (CR), eccentric hypertrophy (EH) and concentric hypertrophy (CH)] were defined.
Results:
Compared to participants without CV risk factor, those with 1, 2 and ≥3 risk factors were at increased risk of LVH [ORs (95% CIs): 3.49 (2.19–5.56), 5.53 (3.20–9.55), and 19.19 (9.67–38.08), respectively]; corresponding values for high RWT were 2.47 (1.63–3.74), 3.76 (2.25–6.27), and 5.47 (2.65–11.28). Similar associations between clustering of CV risk factors and LVG remodeling were found [CR: 1.71 (1.06–2.76), 2.83 (1.54–5.18), and 3.82 (1.37–10.62); EH: 2.42 (1.42–4.11), 4.23 (2.24–7.96), and 16.86 (7.70–36.92); CH: 14.92 (4.41–50.47), 23.15 (6.32–84.83), and 71.19 (17.09–296.56)].
Conclusion:
CV risk factors in isolation and combination were associated with an increased risk of LVH, high RWT and LVG remodeling among children, emphasizing the need to consider multiple risk factors when assessing the risk of cardiac outcomes.
1. Introduction
Left ventricular hypertrophy (LVH) is a common target organ damage in youth with hypertension (1). LVH and left ventricular geometric (LVG) remodeling, which are surrogate markers of abnormal cardiac structure, and are independently associated with cardiovascular disease (CVD) morbidity and mortality (2, 3), stroke (4, 5) and cognitive impairment (6, 7).
Previous studies have documented the association between several cardiovascular (CV) risk factors separately and left ventricular structure in both children (8, 9) and adults (10, 11). Indeed, abdominal obesity (12, 13), elevated blood pressure (9, 14), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) (15, 16) are independently associated with cardiac structural remodeling in youth. Importantly, these CV risk factors usually co-exist in the population (17, 18) and the clustering of several CV risk factors could have a larger effect on CVD than the sole presence of individual CV risk factor (19). Although the clustering of at least three CV risk factors is defined as metabolic syndrome (MetS), this definition remains controversial. MetS is a binary definition (yes: number of the CV risk factors ≥3 vs. no: number <3) while those with 1 or 2 CV risk factors cannot be simply recognized as metabolicaly healthy. In 2017, the American Academy of Pediatrics also emphasized the need to consider CV risk factors clustering rather than MetS itself (20). Furthermore, a recent review also recommended a validated and unified continuous CV risk score for children (21). Accordingly, a continuous risk score of the clustering of the same five components from MetS may better identify CVD risk. Indeed, an international study of 2,427 children and adolescents aged 6–17 years showed that clustering of CV risk factors performed better than MetS in identifying high carotid intima-media thickness (cIMT, a surrogate marker of subclinical atherosclerosis) (22).
In this study, we investigated the association of clustering of CV risk factors (including the five risk factors used in the definition of MetS) with LVH, high relative wall thickness (RWT), and LVG remodeling [including concentric remodeling (CR), eccentric hypertrophy (EH), and concentric hypertrophy (CH)] among Chinese children aged 6–11 years.
2. Materials and methods
2.1. Participants
Data came from the baseline survey of “Huantai Childhood Cardiovascular Health Cohort Study” conducted in Huantai County, Zibo City, Shandong Province, China, conducted from November 2017 to January 2018. The survey has been described elsewhere (23). In brief, 1,406 children aged 6–11 years from one primary school were included in this study. All children and their parents/guardians provided written informed consent before examinations. Collected data included demographic information (sex and age), anthropometric examinations [height, weight, waist circumference (WC) and blood pressure (BP)], lifestyle factors (sleep duration, screen time, physical activity and intake of vegetables and fruits), fasting blood assays (glucose and lipid profiles) and an echocardiogram examination. The study protocol was approved by the institutional review board at the School of Public Health, Shandong University (approval number: 20160308).
2.2. Collections of CV risk factors
Anthropometric examinations were performed by trained staff according to a standard protocol in the school setting. Height and weight were measured twice in light clothes without shoes using a calibrated scale with stadiometer (HGM-300; Shengyuan Co. Ltd., China). WC was measured twice at 1 cm distance above the horizontal level of umbilicus using a flexible plastic tape. Mean values of height, weight, and WC, as well as body mass index (BMI, kg/m2), respectively, were calculated for data analysis. The BP in a seated position was measured three times consecutively at the heart level at about 20-second intervals, using the validated and calibrated electronic sphygmomanometer (HEM 7012; Omron, Osaka, Japan) (24). The last two BP readings were averaged for data analysis.
Venous blood samples were drawn after an overnight fast (for at least 10 h). Fasting blood glucose (FBG), TG and HDL-C were assayed using an automatic analyzer (Beckman Coulter AU480; Mishima, Shizuoka, Japan).
2.3. Measurements of left ventricular structure indices
A same experienced sonographer who was blinded with the study protocol measured the left ventricular structure using a portable color Doppler ultrasound machine (CX30; Royal Philips, Amsterdam, the Netherlands) equipped with an S4-2 convex array transducer (frequency of 2–4 MHz), according to recommendations proposed by the American Society of Echocardiography (25). Using the R-wave apex of the electrocardiogram as the standard phase, the left ventricular end-diastolic internal dimension (LVID) was obtained by measuring the echogenicity of the left ventricular septum from the left ventricular surface to the left ventricular posterior wall. In addition, the vertical distance between the two points on the time axis was measured from the upper edge of the front edge echo line to the upper edge of the rear edge echo line of the measured structure to obtain interventricular septal thickness (IVST) and left ventricular posterior wall thickness (LVPWT). Intra-observer reproducibility of IVST values [intra-class correlation coefficient (ICC) = 0.92] and LVPWT values (ICC = 0.95) was evaluated in the same 20 subjects by the same sonographer who measured twice. Left ventricular mass (LVM, g) was calculated using the Devereux's formula as 0.8 × 1.04 × [(IVST + LVID + LVPWT)3−(LVID)3] + 0.6 (26). Additionally, left ventricular mass index (LVMI, g/m2.7) was calculated as LVM divided by height to the power of 2.7 (27). RWT was calculated as (LVPWT + IVST)/LVID (28).
2.4. Definitions of CV risk factors and LVG patterns
CV risk factors including abdominal obesity, elevated BP, high FBG, high TG and low HDL-C were defined separately as recommended by the Society of Pediatrics, Chinese Medical Association (29). Abdominal obesity was defined as WC ≥90th percentile values for sex and age (30, 31). Elevated BP was defined as systolic and/or diastolic BP ≥95th percentile values for sex, age and height (32). High FBG (≥5.60 mmol/L), high TG (≥1.47 mmol/L) and low HDL-C (<1.03 mmol/L) were defined according to recommendations from the Society of Pediatrics, Chinese Medical Association (29). The clustering of CV risk factors was defined as the sum of the number of risk factors (abdominal obesity, elevated BP, high FBG, high TG and low HDL-C) with the range spanning from 0 to 4. As only few children had more than 3 risk factors, we combined those with 3–4 risk factors into a same group as ≥3, and the clustering was further categorized as 0, 1, 2 and ≥3. Additionally, we re-defined these CV risk factors according to the same distribution of the present study population, namely the corresponding 90th, 85th and 80th sex- and age-specific percentile values of WC, systolic/diastolic BP, FBG and TG, and 10th, 15th and 20th percentile values of HDL-C.
LVH was defined as LVMI ≥90th percentile values for sex and age of this population, and high RWT was defined as RWT ≥90th percentile values for sex and age of this population (the sex- and age-specific percentile cutoffs of LVMI and RWT are provided in Supplementary Table S1). LVG patterns were defined as normal geometry (normal LVMI and normal RWT), CR (normal LVMI and high RWT), EH (LVH and normal RWT) and CH (LVH and high RWT).
2.5. Statistical analysis
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA), and a two-sided P < 0.05 was considered statistically significant. Continuous variables were presented as means ± standard deviations and categorical variables as numbers (percentages). Students' t test and chi-square test were used to examine differences in characteristics by the LVH status (presence vs. absence) or the RWT status (high vs. normal). Pearson correlation analysis was performed between single CV risk factors and LVMI and RWT. Potential covariates used for adjustment included sex, age, sleep duration (<9 vs. ≥9 h per day), screen time (≤2 vs. >2 h per day), physical activity (<1 vs. ≥1 h per day), and intake of vegetables and fruits (<5 vs. ≥5 servings per day). Adjusted LVMI and RWT levels between each CV risk factor status (abnormal vs. normal) and across the number of CV risk factors (0, 1, 2 and ≥3) were compared using covariance analysis. We also conducted trend analysis for LVMI and RWT levels according to the number of CV risk factors using multivariate linear regression analysis. Binary logistic regression analysis was used to calculate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of LVH and high RWT, and multi-class logistic regression analysis for LVG remodeling with the normal geometry as the reference category.
3. Results
3.1. Characteristics of study participants
A total of 1,406 children (boys: 52.8%) aged 6–11 years were included in this study. Characteristics of children by the LVH status are shown in Table 1. Children with LVH (n = 133) had higher levels of BMI, WC, systolic and diastolic BP, and TG, and a lower level of HDL-C than those without LVH (n = 1,273). Similar characteristics were found between children with high RWT and those with normal RWT (Supplementary Table S2).
Table 1
| Characteristics | Total (n = 1,406) | LVH status | ||
|---|---|---|---|---|
| Presence (n = 133) | Absence (n = 1,273) | P valuea | ||
| Age, years | 8.93 ± 1.50 | 8.87 ± 1.53 | 8.93 ± 1.50 | 0.613 |
| Height, cm | 136.40 ± 10.67 | 135.73 ± 12.72 | 136.46 ± 10.44 | 0.524 |
| Body mass index, kg/m2 | 18.21 ± 3.46 | 22.54 ± 4.41 | 17.76 ± 3.01 | <0.001 |
| Waist circumference, cm | 63.03 ± 9.78 | 73.05 ± 12.94 | 61.98 ± 8.75 | <0.001 |
| Systolic BP, mmHg | 106.43 ± 9.20 | 108.68 ± 9.75 | 106.19 ± 9.11 | 0.003 |
| Diastolic BP, mmHg | 63.60 ± 6.66 | 65.31 ± 7.47 | 63.42 ± 6.55 | 0.006 |
| FBG, mmol/L | 4.73 ± 0.56 | 4.81 ± 0.59 | 4.72 ± 0.55 | 0.104 |
| TG, mmol/L | 0.76 ± 0.35 | 0.97 ± 0.47 | 0.74 ± 0.32 | <0.001 |
| HDL-C, mmol/L | 1.58 ± 0.38 | 1.44 ± 0.36 | 1.60 ± 0.38 | <0.001 |
| Abdominal obesity, n (%) | 440 (31.29) | 96 (72.18) | 344 (27.02) | <0.001 |
| Elevated BP, n (%) | 215 (15.29) | 28 (21.05) | 187 (14.69) | 0.052 |
| High FBG, n (%) | 88 (6.26) | 15 (11.28) | 73 (5.73) | 0.012 |
| High TG, n (%) | 64 (4.55) | 19 (14.29) | 45 (3.53) | <0.001 |
| Low HDL-C, n (%) | 76 (5.41) | 16 (12.03) | 60 (4.71) | <0.001 |
| Short sleep duration, n (%) | 229 (16.29) | 11 (8.27) | 218 (17.12) | 0.009 |
| Long screen time, n (%) | 62 (4.41) | 9 (6.77) | 53 (4.16) | 0.164 |
| Insufficient physical activity, n (%) | 796 (56.61) | 78 (58.65) | 718 (56.40) | 0.619 |
| Insufficient intake of fruits and/or vegetables, n (%) | 1,142 (81.22) | 115 (86.47) | 1,027 (80.68) | 0.104 |
Characteristics of the children according to the LVH status.
Continuous variables are presented as means ± standard deviations. BP, blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; TG, triglycerides.
Group differences between the LVH status.
3.2. Association of single CV risk factor with LVG remodeling
Abdominal obesity, high TG and low HDL-C were associated with the risk of LVH irrespective of definitions of CV risk factors (Table 2). Elevated BP was associated with LVH when using percentile values of this population (Table 2). High FBG was associated with LVH when using recommended guideline (Table 2). Similarly, regardless of definitions of CV risk factors, abdominal obesity and high TG were associated with increased risk of high RWT, while associations of elevated BP, high FBG and low HDL-C with high RWT varied slightly based on different definitions of CV risk factors (Table 2). Besides, there were positive correlations between specific CV risk factors (including WC, SBP, DBP, FBG, and TG; Supplementary Table S3) and LVMI and RWT, and HDL-C was related inversely with LVMI (r = −0.12, P < 0.001; Supplementary Table S3). Additionally, covariance analysis showed significant associations of each CV risk factor with LVMI and RWT levels (Supplementary Table S4).
Table 2
| CV risk factors | LVH | High RWT | ||||
|---|---|---|---|---|---|---|
| n (%)b | OR (95% CI) | P value | n (%)b | OR (95% CI) | P value | |
| Abdominal obesity | ||||||
| Recommendeda (n = 440) | 96 (21.82) | 6.85 (4.58–10.24) | <0.001 | 79 (17.95) | 3.42 (2.38–4.91) | <0.001 |
| P90 (n = 146) | 53 (36.30) | 8.52 (5.62–12.93) | <0.001 | 37 (25.34) | 4.10 (2.66–6.33) | <0.001 |
| P85 (n = 216) | 68 (31.48) | 8.10 (5.49–11.95) | <0.001 | 53 (24.54) | 4.40 (2.99–6.47) | <0.001 |
| P80 (n = 286) | 84 (29.37) | 9.08 (6.16–13.39) | <0.001 | 65 (22.73) | 4.26 (2.95–6.15) | <0.001 |
| Elevated BP | ||||||
| Recommendeda (n = 215) | 28 (13.02) | 1.48 (0.95–2.32) | 0.087 | 26 (12.09) | 1.31 (0.83–2.07) | 0.240 |
| P90 (n = 234) | 34 (14.53) | 1.77 (1.16–2.69) | 0.008 | 37 (15.81) | 2.00 (1.33–3.00) | 0.001 |
| P85 (n = 341) | 44 (12.90) | 1.57 (1.07–2.31) | 0.022 | 48 (14.08) | 1.75 (1.20–2.55) | 0.003 |
| P80 (n = 446) | 53 (11.88) | 1.46 (1.01–2.11) | 0.044 | 56 (12.56) | 1.50 (1.05–2.16) | 0.026 |
| High FBG | ||||||
| Recommendeda (n = 88) | 15 (17.05) | 2.21 (1.21–4.06) | 0.010 | 15 (17.05) | 1.94 (1.06–3.53) | 0.031 |
| P90 (n = 148) | 19 (12.84) | 1.52 (0.90–2.56) | 0.118 | 20 (13.51) | 1.48 (0.89–2.46) | 0.132 |
| P85 (n = 217) | 25 (11.52) | 1.34 (0.84–2.14) | 0.219 | 30 (13.82) | 1.56 (1.01–2.42) | 0.044 |
| P80 (n = 288) | 34 (11.81) | 1.43 (0.94–2.18) | 0.091 | 41 (14.24) | 1.71 (1.15–2.53) | 0.008 |
| High TG | ||||||
| Recommendeda (n = 64) | 19 (29.69) | 5.15 (2.83–9.38) | <0.001 | 12 (18.75) | 2.18 (1.12–4.26) | 0.022 |
| P90 (n = 150) | 32 (21.33) | 3.10 (1.99–4.84) | <0.001 | 25 (16.67) | 1.98 (1.23–3.17) | 0.005 |
| P85 (n = 220) | 42 (19.09) | 2.88 (1.93–4.31) | <0.001 | 33 (15.00) | 1.80 (1.18–2.74) | 0.007 |
| P80 (n = 291) | 54 (18.56) | 3.02 (2.07–4.40) | <0.001 | 41 (14.09) | 1.71 (1.15–2.52) | 0.008 |
| Low HDL-C | ||||||
| Recommendeda (n = 76) | 16 (21.05) | 2.90 (1.61–5.25) | <0.001 | 16 (21.05) | 2.71 (1.51–4.86) | 0.001 |
| P10 (n = 132) | 27 (20.45) | 2.89 (1.80–4.63) | <0.001 | 19 (14.39) | 1.67 (0.99–2.82) | 0.055 |
| P15 (n = 200) | 31 (15.50) | 2.03 (1.31–3.15) | 0.002 | 25 (12.50) | 1.41 (0.89–2.25) | 0.143 |
| P20 (n = 277) | 40 (14.44) | 1.90 (1.28–2.83) | 0.002 | 37 (13.36) | 1.57 (1.05–2.35) | 0.028 |
Associations of each CV risk factor with LVH and high RWT.
Binary logistic regression models were performed separately for each cardiovascular risk factor adjusting for sex, age, sleep duration, screen time, physical activity, and intake of vegetables and fruits. P90, P85 and P80 represented the corresponding sex- and age-specific percentile values of waist circumference, systolic/diastolic BP, FBG and TG based on the present population, and P10, P15 and P20 for HDL-C. BP, blood pressure; CI, confidence interval; CV, cardiovascular; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; OR, odds ratio; RWT, relative wall thickness; TG, triglycerides.
Recommended guideline from the Society of Pediatrics, Chinese Medical Association.
Percentages are calculated as: (the number of subjects with LVH or high RWT)/(the number of those with specific CV risk factors)×100%.
Abdominal obesity (OR = 2.34, 95% CI: 1.53–3.59) and low HDL-C (OR = 2.36, 95% CI: 1.12–4.97) were associated with CR. Elevated BP (90th or 85th) and high TG (85th) defined using the percentiles were also associated with CR (Supplementary Table S5). Abdominal obesity, elevated BP, high TG, and low HDL-C increased the risk of EH with ORs (95% CIs) of 5.26 (3.34–8.29), 1.72 (1.03–2.89), 5.92 (2.92–11.98), and 2.58 (1.21–5.47), respectively; these associations were mostly similar when defining CV risk factors based on the percentiles (Supplementary Table S5). Abdominal obesity, high FBG, high TG, and low HDL-C also increased the risk of CH, with ORs (95% CIs) of 20.96 (8.13–54.03), 3.33 (1.38–8.06), 4.82 (1.86–12.52), and 4.58 (1.92–10.92), respectively; similar associations were found when CV risk factors were defined using the percentiles for definitions (Supplementary Table S5).
3.3. Association of clustering of CV risk factors with LVG remodeling
There were 787 (55.97%) children without CV risk factor, 413 (29.37%) with one, 156 (11.10%) with two, and 50 (3.56%) with at least three. LVMI and RWT levels were higher along increasing number of CV risk factors (P for linear trend <0.001), after adjustment for sex, age, sleep duration, screen time, physical activity, and intake of vegetables and fruits (Figures 1, 2).
Figure 1
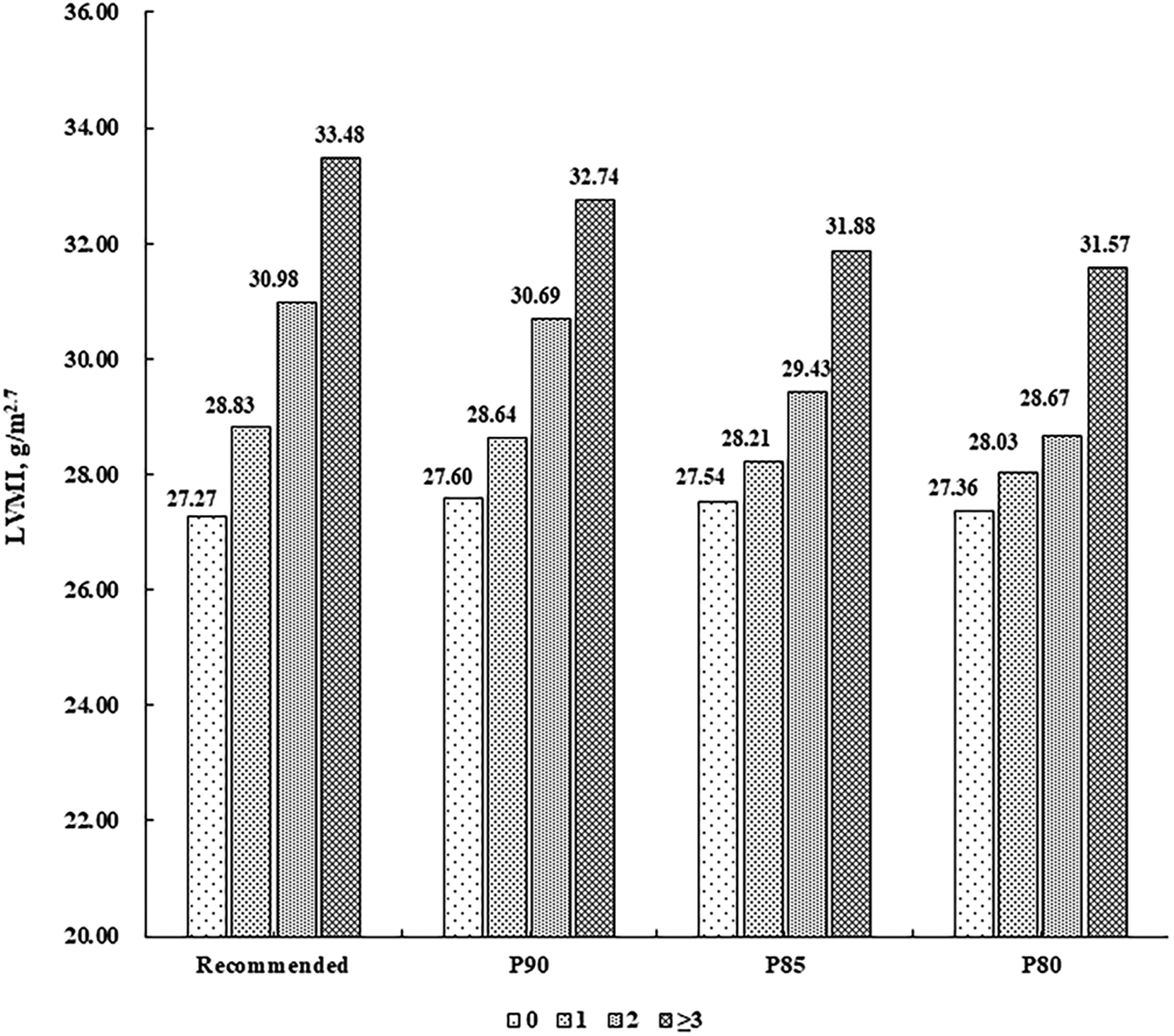
Mean levels of LVMI (g/m2.7) according to the number of cardiovascular risk factors. Cardiovascular risk factors were defined according to the recommended guideline from the Society of Pediatrics, Chinese Medical Association and the corresponding sex- and age-specific percentile values based on the present population, respectively (P90, P85 and P80 for waist circumference, systolic/diastolic blood pressure, fasting blood glucose, and triglycerides, and P10, P15 and P20 for high-density lipoprotein cholesterol). LVMI, left ventricular mass index.
Figure 2
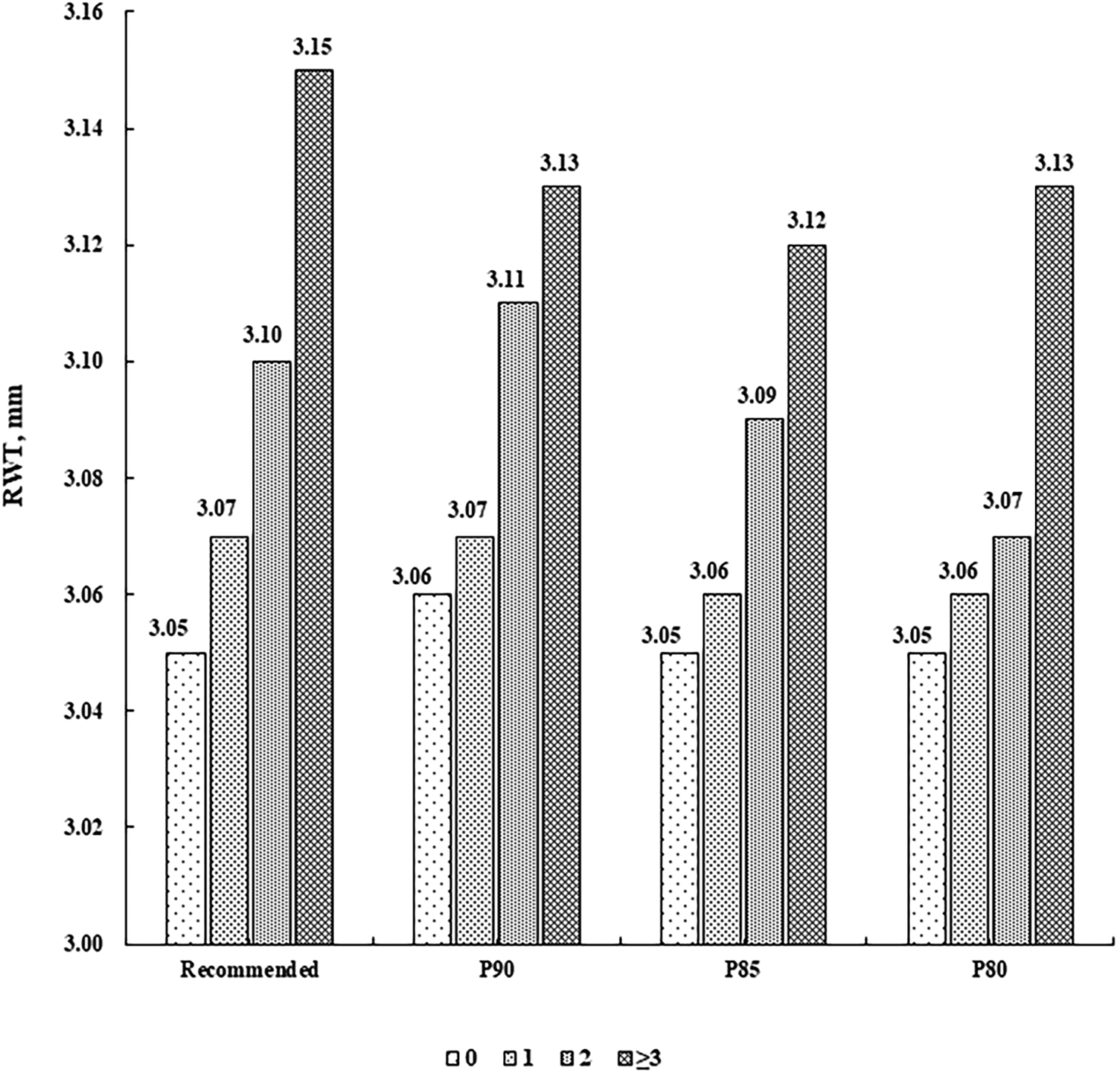
Mean levels of RWT (mm) according to the number of cardiovascular risk factors. Cardiovascular risk factors were defined according to the recommended guideline from the Society of Pediatrics, Chinese Medical Association and the corresponding sex- and age-specific percentile values based on the present population, respectively (P90, P85 and P80 for waist circumference, systolic/diastolic blood pressure, fasting blood glucose, and triglycerides, and P10, P15 and P20 for high-density lipoprotein cholesterol). RWT, relative wall thickness.
The prevalence of LVH and high RWT increased progressively across the clustering of CV risk factors (Table 3). Compared with children without CV risk factor, those with 1, 2 and ≥3 CV risk factors were associated with an increased risk of LVH, with ORs (95% CIs) of 3.49 (2.19–5.56), 5.53 (3.20–9.55), and 19.19 (9.67–38.08), respectively (Table 3). Similarly, the more number of CV risk factors was associated with a higher risk of high RWT [2.47 (1.63–3.74) for 1, 3.76 (2.25–6.27) for 2, and 5.47 (2.65–11.28) for ≥3, Table 3]. Similar associations were found when CV risk factors were defined based on the percentiles (Table 3).
Table 3
| Cut-offs | Number of CV risk factors | LVH | High RWT | ||
|---|---|---|---|---|---|
| n (%) | OR (95% CI) | n (%) | OR (95% CI) | ||
| Recommendeda | 0 (n = 787) | 31 (3.94) | Reference | 45 (5.72) | Reference |
| 1 (n = 413) | 52 (12.59) | 3.49 (2.19–5.56) | 54 (13.08) | 2.47 (1.63–3.74) | |
| 2 (n = 156) | 29 (18.59) | 5.53 (3.20–9.55) | 28 (17.95) | 3.76 (2.25–6.27) | |
| ≥3 (n = 50) | 21 (42.00) | 19.19 (9.67–38.08) | 12 (24.00) | 5.47 (2.65–11.28) | |
| P90 | 0 (n = 853) | 42 (4.92) | Reference | 53 (6.21) | Reference |
| 1 (n = 367) | 43 (11.72) | 2.54 (1.62–3.96) | 49 (13.35) | 2.34 (1.56–3.54) | |
| 2 (n = 129) | 27 (20.93) | 5.11 (3.00–8.69) | 26 (20.16) | 3.89 (2.32–6.52) | |
| ≥3 (n = 57) | 21 (36.84) | 11.19 (5.96–20.98) | 11 (19.30) | 3.73 (1.82–7.65) | |
| P85 | 0 (n = 684) | 32 (4.68) | Reference | 40 (5.85) | Reference |
| 1 (n = 398) | 35 (8.79) | 2.03 (1.23–3.34) | 43 (10.80) | 1.95 (1.25–3.07) | |
| 2 (n = 209) | 31 (14.83) | 3.43 (2.03–5.79) | 30 (14.35) | 2.69 (1.62–4.45) | |
| ≥3 (n = 115) | 35 (30.43) | 9.23 (5.38–15.83) | 26 (22.61) | 4.87 (2.83–8.39) | |
| P80 | 0 (n = 529) | 18 (3.40) | Reference | 31 (5.86) | Reference |
| 1 (n = 413) | 35 (8.47) | 2.78 (1.55–5.01) | 34 (8.23) | 1.42 (0.86–2.36) | |
| 2 (n = 282) | 27 (9.57) | 2.96 (1.59–5.49) | 34 (12.06) | 2.20 (1.32–3.68) | |
| ≥3 (n = 182) | 53 (29.12) | 12.08 (6.81–21.44) | 40 (21.98) | 4.50 (2.71–7.47) | |
Associations of the clustering of CV risk factors with LVH and high RWT.
Binary logistic regression models were performed with adjustment for sex, age, sleep duration, screen time, physical activity, and intake of vegetables and fruits. P90, P85 and P80 represented the corresponding sex- and age-specific percentile values of waist circumference, systolic/diastolic blood pressure, fasting blood glucose and triglycerides based on the present population, and P10, P15 and P20 for high-density lipoprotein cholesterol. CI, confidence interval; CV, cardiovascular; LVH, left ventricular hypertrophy; OR, odds ratio; RWT, relative wall thickness.
Recommended guideline from the Society of Pediatrics, Chinese Medical Association.
The clustering of CV risk factors was associated with LVG remodeling including CR, EH and CH (Table 4). The corresponding ORs (95% CIs) of CR were 1.71 (1.06–2.76), 2.83 (1.54–5.18), and 3.82 (1.37–10.62), respectively, for children with 1, 2 and ≥3 CV risk factors; those of EH were 2.42 (1.42–4.11), 4.23 (2.24–7.96), and 16.86 (7.70–36.92), respectively; those of CH were 14.92 (4.41–50.47), 23.15 (6.32–84.83), and 71.19 (17.09–296.56), respectively. Similar associations of the clustering of CV risk factors with CR, EH and CH were found when using the percentiles for definitions.
Table 4
| Cut-offs | Number of CV risk factors | CR | EH | CH | |||
|---|---|---|---|---|---|---|---|
| n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | ||
| Recommendeda | 0 (n = 787) | 42 (5.34) | Reference | 28 (3.56) | Reference | 3 (0.38) | Reference |
| 1 (n = 413) | 33 (7.99) | 1.71 (1.06–2.76) | 31 (7.51) | 2.42 (1.42–4.11) | 21 (5.08) | 14.92 (4.41–50.47) | |
| 2 (n = 156) | 17 (10.90) | 2.83 (1.54–5.18) | 18 (11.54) | 4.23 (2.24–7.96) | 11 (7.05) | 23.15 (6.32–84.83) | |
| ≥3 (n = 50) | 5 (10.00) | 3.82 (1.37–10.62) | 14 (28.00) | 16.86 (7.70–36.92) | 7 (14.00) | 71.19 (17.09–296.56) | |
| P90 | 0 (n = 853) | 46 (5.39) | Reference | 35 (4.10) | Reference | 7 (0.82) | Reference |
| 1 (n = 367) | 31 (8.45) | 1.79 (1.11–2.89) | 25 (6.81) | 1.85 (1.09–3.16) | 18 (4.90) | 6.63 (2.74–16.05) | |
| 2 (n = 129) | 13 (10.08) | 2.55 (1.32–4.92) | 14 (10.85) | 3.52 (1.81–6.84) | 13 (10.08) | 15.46 (5.97–40.02) | |
| ≥3 (n = 57) | 7 (12.28) | 4.33 (1.79–10.48) | 17 (29.82) | 13.12 (6.52–26.41) | 4 (7.02) | 14.49 (3.99–52.65) | |
| P85 | 0 (n = 684) | 34 (4.97) | Reference | 26 (3.80) | Reference | 6 (0.88) | Reference |
| 1 (n = 398) | 31 (7.79) | 1.70 (1.03–2.83) | 23 (5.78) | 1.72 (0.97–3.07) | 12 (3.02) | 3.77 (1.40–10.15) | |
| 2 (n = 209) | 19 (9.09) | 2.23 (1.23–4.03) | 20 (9.57) | 2.95 (1.60–5.45) | 11 (5.26) | 6.62 (2.40–18.28) | |
| ≥3 (n = 115) | 13 (11.30) | 3.71 (1.86–7.41) | 22 (19.13) | 8.23 (4.39–15.45) | 13 (11.30) | 20.24 (7.41–55.30) | |
| P80 | 0 (n = 529) | 27 (5.10) | Reference | 14 (2.65) | Reference | 4 (0.76) | Reference |
| 1 (n = 413) | 26 (6.30) | 1.30 (0.74–2.27) | 27 (6.54) | 2.85 (1.47–5.53) | 8 (1.94) | 2.81 (0.84–9.44) | |
| 2 (n = 282) | 27 (9.57) | 2.19 (1.25–3.84) | 20 (7.09) | 3.06 (1.51–6.19) | 7 (2.48) | 3.48 (1.00–12.06) | |
| ≥3 (n = 182) | 17 (9.34) | 2.70 (1.42–5.15) | 30 (16.48) | 9.75 (4.97–19.10) | 23 (12.64) | 24.82 (8.39–73.46) | |
Association of the clustering of CV risk factors with LVG.
Multi-class logistic regression models were performed with adjustment for sex, age, sleep duration, screen time, physical activity, and intake of vegetables and fruits. P90, P85 and P80 represented the corresponding sex- and age-specific percentile values of waist circumference, systolic/diastolic blood pressure, fasting blood glucose and triglycerides based on the present population, and P10, P15 and P20 for high-density lipoprotein cholesterol. CH, concentric hypertrophy; CI, confidence interval; CR, concentric remodeling; CV, cardiovascular; EH, eccentric hypertrophy; LVG, left ventricular geometric; OR, odds ratio.
Recommended guideline from the Society of Pediatrics, Chinese Medical Association.
4. Discussion
In this cross-sectional study among 1,406 children aged 6–11 years from China, we found that increased numbers of CV risk factors were associated with the higher levels of LVMI and RWT. The clustering of CV risk factors progressively increased the risk of LVH, high RWT and LVG remodeling (CR, EH and CH). These findings indicate a strong association between the number of CV risk factors and the risk of LVG remodeling among children.
Consistent with previous studies, we found that specific CV risk factors were significantly associated with LVMI and RWT. A retrospective study including 160 overweight and obese children and adolescents aged 6–18 years found that WC above the 90th percentile values was associated with increased LVMI and CH (33). A cross-sectional study of 303 adolescents (mean age 15.6 years) showed an independent relationship between BP and LVMI (9). A cross-sectional study of 70 children with obesity (median age 14 years) found positive correlations of TG and the TG-to-HDL-C ratio with LVMI and RWT (15). Associations of the TG-to-HDL-C ratio with LVMI and RWT were also found in a study including 884 outpatient children and adolescents aged 6–16 years (16). In the present study, WC, SBP, DBP, FBG, and TG were positively related with LVMI and RWT, along with inverse correlation between HDL-C and LVMI. Our findings, combined with prior research, may suggest that monitoring CV risk factors among children holds practical value for the prevention of subclinical target organ damage.
Comparatively, abdominal obesity among all five CV risk factors had the largest effect on the cardiac remodeling. Obesity has been considered as a state of chronic metabolic disorder (34), and obesity-related hemodynamic factors (e.g., increased circulating blood volume and cardiac output) and metabolic factors (e.g., insulin resistance, visceral fat deposition and secretion of adipokines) may contribute to a series of adaptations/alterations in cardiac structure and function (35). Besides, obesity frequently promotes other CV risk factors such as hypertension, dyslipidemia, and glucose intolerance (36), and there is also an additive effect between obesity and BP on cardiac remodeling (37). Evidence shows that elevated BP is a prominent contributor to cardiac remodeling (38), however, a weaker association between elevated BP (vs. abdominal obesity) and cardiac remodeling was found in our study. This may suggest that other underlying mechanisms other than BP-related responses may play a vital role. Further studies are warranted to clarify the pathophysiologic mechanisms.
Furthermore, our study also showed that LVMI and RWT levels increased markedly according to the number of CV risk factors. Similarly, a cross-sectional study of 830 young US adults aged 24–43 years found that LVMI levels increased according to the number of abnormal MetS components (39). In another cross-sectional study of 291 children (mean age 11.7 years), a comprehensive CV risk score based on five MetS components had a positive correlation with cIMT levels (40). Overall, these findings support an additive effect of these risk factors on continuous subclinical CVD markers.
A recent combined analysis of three population-based studies of 2,427 children and adolescents aged 6–17 years showed that a graded risk score based on five MetS components was superior to dichotomous classification of MetS to predict high cIMT (22). Additionally, our findings that having ≥3 CV risk factors was associated with the highest risk of LVG remodeling, followed by having two and one CV risk factors, indicate a dose-response association between the clustering of CV risk factors and LVG remodeling. Of note, children who did not meet the definition of MetS (i.e., number of CV risk factors as 1 or 2) were also at an increased risk of LVG remodeling, re-emphasizing the continuous nature of the association between CV risk factors and cardiac outcomes. Therefore, our findings supported that it is important to focus on clustering of CV risk factors among children and adolescents rather than the arbitrarily defined MetS (20). Moreover, irrespective of what are cutoffs of CV risk factors, it is essential to keep an optimal levels of health factors. As exemplified in a child cohort study from China, the ideal levels of four behavior factors (i.e., diet, physical activity, nicotine exposure, sleep heath) as well as four health indicators (i.e., BMI, blood lipids, blood glucose, and blood pressure) were conducive to reducing the abnormal cardiovascular structures (41).
To our knowledge, this is the first study examining the association between the clustering of CV risk factors and LVG remodeling among Chinese children. Meanwhile, there are several limitations in this study that should be considered. First, this study included children aged 6–11 years from only one primary school, making our results less generalizable. Further studies in children with different ages from other regions are needed to further validate the associations observed in this study. Second, the cross-sectional data used in this study preclude concluding on the causal link of CV risk factors on cardiac outcomes. Third, although different CV risk factors had different effects on the cardiac structure, and we did not allocate different weights to specific risk factors because of unavailable weights, we partially addressed this issue by considering these CV risk factors along percentile values of the present population.
5. Conclusion
CV risk factors in isolation and combination were associated with an increased risk of LVH, high RWT and LVG remodeling among children, emphasizing the need to consider multiple risk factors when assessing the risk of cardiac outcomes.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by School of Public Health, Shandong University (approval number: 20160308). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
QL contributed to writing of the original draft, formal analysis, reviewing and editing. HW contributed to data curation, formal analysis, reviewing and editing. CZ, MZ, and PB contributed to conceptualization, methodology, reviewing and editing. BX contributed to conceptualization, methodology, reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81673195, 81722039). The funders had no role in the design, analysis, or submission of the prepared manuscript.
Acknowledgments
We thank all the participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1236730/full#supplementary-material
References
1.
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. (2004) 114:555–76. 10.1542/peds.114.s2.iv
2.
EskerudIGerdtsELarsenTHLonnebakkenMT. Left ventricular hypertrophy contributes to myocardial ischemia in non-obstructive coronary artery disease (the microcad study). Int J Cardiol. (2019) 286:1–6. 10.1016/j.ijcard.2019.03.059
3.
AkintoyeEMahmoudKShokrMSandioAMallikethi-ReddySSheikhMet alRacial/ethnic differences in the prognostic utility of left ventricular mass Index for incident cardiovascular disease. Clin Cardiol. (2018) 41:502–9. 10.1002/clc.22914
4.
WangSXueHZouYSunKFuCWangHet alLeft ventricular hypertrophy, abnormal ventricular geometry and relative wall thickness are associated with increased risk of stroke in hypertensive patients among the Han Chinese. Hypertens Res. (2014) 37:870–4. 10.1038/hr.2014.88
5.
O'NealWTAlmahmoudMFQureshiWTSolimanEZ. Electrocardiographic and echocardiographic left ventricular hypertrophy in the prediction of stroke in the elderly. J Stroke Cerebrovasc Dis. (2015) 24:1991–7. 10.1016/j.jstrokecerebrovasdis.2015.04.044
6.
NorbyFLChenLYSolimanEZGottesmanRFMosleyTHAlonsoA. Association of left ventricular hypertrophy with cognitive decline and dementia risk over 20 years: the atherosclerosis risk in communities-neurocognitive study (Aric-Ncs). Am Heart J. (2018) 204:58–67. 10.1016/j.ahj.2018.07.007
7.
MoazzamiKOstovanehMRAmbale VenkateshBHabibiMYoneyamaKWuCet alLeft ventricular hypertrophy and remodeling and risk of cognitive impairment and dementia: mesa (multi-ethnic study of atherosclerosis). Hypertension. (2018) 71:429–36. 10.1161/HYPERTENSIONAHA.117.10289
8.
JingLNeviusCDFridayCMSueverJDPulenthiranAMejia-SpiegelerAet alAmbulatory systolic blood pressure and obesity are independently associated with left ventricular hypertrophic remodeling in children. J Cardiovasc Magn Reson. (2017) 19:86. 10.1186/s12968-017-0401-3
9.
UrbinaEMMendizabalBBeckerRCDanielsSRFalknerBEHamdaniGet alAssociation of blood pressure level with left ventricular mass in adolescents. Hypertension. (2019) 74:590–6. 10.1161/HYPERTENSIONAHA.119.13027
10.
KishiSGiddingSSReisJPColangeloLAVenkateshBAArmstrongACet alAssociation of insulin resistance and glycemic metabolic abnormalities with lv structure and function in middle age: the cardia study. JACC Cardiovasc Imaging. (2017) 10:105–14. 10.1016/j.jcmg.2016.02.033
11.
PietriPGeorgiopoulosGTsiachrisDKordalisAVlachopoulosCVyssoulisGet alTriglycerides are related to left ventricular mass in hypertensive patients independently of other cardiometabolic risk factors: the effect of gender. Sci Rep. (2020) 10:13253. 10.1038/s41598-020-70237-1
12.
RodicioMMDomenech de MiguelVGuinda JimenezMCigarran GuldrisSLopez FrancoMMEstany GestalAet alEarly cardiac abnormalities in obese children and their relationship with adiposity. Nutrition. (2018) 46:83–9. 10.1016/j.nut.2017.09.001
13.
MehtaSK. Waist circumference to height ratio and left ventricular mass in children and adolescents. Cardiol Young. (2016) 26:658–62. 10.1017/S1047951115000803
14.
MaugeriAHruskovaJJakubikJBarchittaMLo ReOKunzovaSet alIndependent effects of hypertension and obesity on left ventricular mass and geometry: evidence from the cardiovision 2030 study. J Clin Med. (2019) 8:370. 10.3390/jcm8030370
15.
BjelakovicBStefanuttiCVukovicVKavaricNSaranacLKlisicAet alLipid profile and left ventricular geometry pattern in obese children. Lipids Health Dis. (2020) 19:109. 10.1186/s12944-020-01285-9
16.
Di BonitoPMoioNScillaCCavutoLSibilioGSanguignoEet alUsefulness of the high triglyceride-to-hdl cholesterol ratio to identify cardiometabolic risk factors and preclinical signs of organ damage in outpatient children. Diabetes Care. (2012) 35:158–62. 10.2337/dc11-1456
17.
CamhiSMKatzmarzykPT. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int J Pediatr Obes. (2010) 5:122–9. 10.3109/17477160903111763
18.
SmoakCGBurkeGLWebberLSHarshaDWSrinivasanSRBerensonGS. Relation of obesity to clustering of cardiovascular disease risk factors in children and young adults: the Bogalusa heart study. Am J Epidemiol. (1987) 125:364–72. 10.1093/oxfordjournals.aje.a114543
19.
ChungSTOnuzuruikeAUMaggeSN. Cardiometabolic risk in obese children. Ann N Y Acad Sci. (2018) 1411:166–83. 10.1111/nyas.13602
20.
MaggeSNGoodmanEArmstrongSC, Committee on Nutrition, Section on Endocrinology, Section on Obesity. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. (2017) 140:e20171603. 10.1542/peds.2017-1603
21.
KamelMSmithBTWahiGCarsleySBirkenCSAndersonLN. Continuous cardiometabolic risk score definitions in early childhood: a scoping review. Obes Rev. (2018) 19:1688–99. 10.1111/obr.12748
22.
ZhaoMCasertaCAMedeirosCCMLopez-BermejoAKolliasAZhangQet alMetabolic syndrome, clustering of cardiovascular risk factors and high carotid intima-media thickness in children and adolescents. J Hypertens. (2020) 38:618–24. 10.1097/HJH.0000000000002318
23.
YangLLZhangQZhangYQSunJHZhaoMXiB. Design of huantai childhood cardiovascular health cohort study. Chin J Prev Med. (2020) 54:1461–4. 10.3760/cma.j.cn112150-20200610-00857
24.
MengLHHouDQShanXYMiJ. Accuracy evaluation of omron hem-7012 electronic sphygmomanometers in measuring blood pressure of children and adolescents. Chin J Hypertens. (2013) 21:158–62. 10.16439/j.cnki.1673-7245.2013.02.036
25.
LangRMBierigMDevereuxRBFlachskampfFAFosterEPellikkaPAet alRecommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. (2005) 18:1440–63. 10.1016/j.echo.2005.10.005
26.
DevereuxRBAlonsoDRLutasEMGottliebGJCampoESachsIet alEchocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. J Am Soc Echocardiogr. (1986) 57:450–8. 10.1016/0002-9149(86)90771-X
27.
de SimoneGDanielsSRDevereuxRBMeyerRARomanMJDedivitiisOet alLeft-ventricular mass and body size in normotensive children and adults-assessment of allometric relations and impact of overweight. J Am Coll Cardiol. (1992) 20:1251–60. 10.1016/0735-1097(92)90385-z
28.
ReichekNDevereuxRB. Reliable estimation of peak left ventricular systolic pressure by M-mode echographic-determined End-diastolic relative wall thickness: identification of severe valvular aortic stenosis in adult patients. Am Heart J. (1982) 103:202–3. 10.1016/0002-8703(82)90493-8
29.
The Society of Pediatrics, The Subspecialty Group of Endocrinologic Hereditary and Metabolic Diseases, The Subspecialty Group of Cardiology, The Subspecialty Group of Child Health Care, Chinese Medical Association. The definition and prevention recommends of metabolic syndrome in Chinese children and adolescents. Chin J Pediatr. (2012) 50:420–2. 10.3760/cma.j.issn.0578-1310.2012.06.005
30.
ZongXNLiHZhangYQ. Percentile reference value of waist circumference for Chinese children aged 3-7 years. Chin J Epidemiol. (2020) 41:1286–90. 10.3760/cma.j.cn112338-20190827-00629
31.
MaGSJiCYMaJMiJSungRYXiongFet alWaist circumference reference values for screening cardiovascular risk factors in Chinese children and adolescents aged 7-18 years. Chin J Epidemiol. (2010) 31:609–15. 10.3760/cma.j.issn.0254-6450.2010.06.003
32.
FanHYanYKMiJ. Updating blood pressure references for Chinese children aged 3-17 years. Chin J Hypertens. (2017) 25:428–35. 10.16439/j.cnki.1673-7245.2017.05.009
33.
TrandafirLMRussuGMoscaluMMironILupuVVLeon ConstantinMMet alWaist circumference a clinical criterion for prediction of cardio-vascular complications in children and adolescences with overweight and obesity. Medicine. (2020) 99:e20923. 10.1097/MD.0000000000020923
34.
PoirierPGilesTDBrayGAHongYSternJSPi-SunyerFXet alObesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. (2006) 113:898–918. 10.1161/CIRCULATIONAHA.106.171016
35.
BradyTM. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr Hypertens Rep. (2016) 18:3. 10.1007/s11906-015-0608-3
36.
BastienMPoirierPLemieuxIDespresJP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. (2014) 56:369–81. 10.1016/j.pcad.2013.10.016
37.
WoodiwissAJNortonGR. Obesity and left ventricular hypertrophy: the hypertension connection. Curr Hypertens Rep. (2015) 17:539. 10.1007/s11906-015-0539-z
38.
YildizMOktayAAStewartMHMilaniRVVenturaHOLavieCJ. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis. (2020) 63:10–21. 10.1016/j.pcad.2019.11.009
39.
PatelDASrinivasanSRChenWBerensonGS. Influence of the metabolic syndrome versus the sum of its individual components on left ventricular geometry in young adults (from the Bogalusa heart study). Am J Cardiol. (2009) 104:69–73. 10.1016/j.amjcard.2009.02.063
40.
GootyVDSinaikoARRyderJRDengelDRJacobsDRJrSteinbergerJ. Association between carotid intima media thickness, age, and cardiovascular risk factors in children and adolescents. Metab Syndr Relat Disord. (2018) 16:122–6. 10.1089/met.2017.0149
41.
ShuWLiMXiaoHAmaerjiangNKhattabNMZunongJet alValidation of “life’s essential 8” metrics with cardiovascular structural Status in children: the PROC study in China. J Am Heart Assoc. (2023) 12:e029077. 10.1161/JAHA.122.029077
Summary
Keywords
cardiovascular risk factor, children, geometric remodeling, left ventricle, Chinese
Citation
Liu Q, Wang H, Zhao M, Zhang C, Bovet P and Xi B (2023) Association between clustering of cardiovascular risk factors and left ventricular geometric remodeling in Chinese children. Front. Cardiovasc. Med. 10:1236730. doi: 10.3389/fcvm.2023.1236730
Received
13 June 2023
Accepted
04 August 2023
Published
17 August 2023
Volume
10 - 2023
Edited by
Zhen-Yu Zhang, KU Leuven, Belgium
Reviewed by
Mirjam Močnik, University Clinical Centre Maribor, Slovenia Nataša Marčun Varda, Maribor University Medical Centre, Slovenia
Updates
Copyright
© 2023 Liu, Wang, Zhao, Zhang, Bovet and Xi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Xi xibo2007@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.