Abstract
Introduction:
Immune checkpoint inhibitors have advanced the outcomes of many different types of cancer. A rare but extraordinarily severe complication of these agents resembles immune checkpoint inhibitor-related myocarditis, which typically occurs within the first few weeks after treatment initiation with a mortality of 25%–50%.
Case report:
A 57-year-old woman had uneventfully received pembrolizumab for metastatic non-small cell lung cancer for over 2.5 years and was admitted after an out-of-hospital cardiac arrest due to ventricular fibrillation. After successful cardiopulmonary resuscitation, the initial diagnostic work-up showed elevated cardiac enzymes and a limited left-ventricular ejection fraction, while coronary angiography did not show relevant stenosis. Despite cardiac MRI being unsuggestive of myocarditis, myocardial biopsies were obtained and histologically confirmed anti-PD-1 antibody-associated myocarditis. After the initiation of prednisone at 1 mg/kg body weight, the patient gradually recovered and was discharged three weeks later with markedly improved cardiac function.
Conclusion:
This case resembles the first description of a very late onset irMyocarditis, occurring over 2.5 years after the start of treatment. It demonstrates the importance of contemplating that severe immune-related toxicities with a sudden onset clinical presentation may occur even after long uneventful periods of anti-PD-1 immune checkpoint inhibitor treatment. Furthermore, it underlines the critical importance of myocardial biopsies in this setting, especially when cardiac MRI remains inconclusive. Moreover, it demonstrates the necessity and benefits of early immunosuppressive treatment if immune-related myocarditis is considered a differential diagnosis.
Introduction
Since the discovery of immune checkpoint inhibition (ICI) as a novel cancer treatment paradigm, an ever-growing group of substances rapidly became part of standard treatments across major cancer types. For many, ICI revolutionized outcomes and is thus increasingly used in curative intent (1). With a reported incidence between 0.04% and 1.14%, ICI-related myocarditis (irMyocarditis) is a relatively rare, yet potentially lethal immune-related adverse effect (irAE) (2). Importantly, irMyocarditis is considered an early irAE, with a median occurrence of 17 to 34 days after treatment initiation, and 76% of cases develop within the first six weeks of ICI (3–5). However, in the following, we present a rare case of histologically confirmed near fatal irMyocarditis, which occurred after over 2.5 years of pembrolizumab treatment. To our knowledge, this resembles the longest yet reported latency of irMyocarditis.
Case report
A 57-year-old woman experienced sudden cardiac arrest while attending a social event. After a no-flow time of approximately 1 min, a coincidentally present nurse initiated cardiopulmonary resuscitation (CPR). When paramedics arrived, approximately 10 min into CPR, the initial rhythm was ventricular fibrillation. The patient underwent a series of 3 defibrillations and received 200 mg of amiodarone before achieving a return of spontaneous circulation after a total of approximately 17 min.
The patient was then transferred to our center and initially presented with heart failure with a reduced left ventricular ejection fraction (Figure 1A; Supplementary Report S1), which was previously unknown. Laboratory results showed elevated cardiac enzymes (Supplementary Table S1), which further increased initially, likely due to the combination of CPR and myocarditis (Supplementary Table S2). The patient was initially neuro-protectively cooled for 24 h and was successfully weaned from ventilation after 36 h. Subsequently, the patient developed fever and intermittent tachycardic atrial fibrillation, which we treated with piperacillin/tazobactam for suspected aspiration pneumonia, electrolyte supplementation, and metoprolol. Electrocardiograms exhibited no signs of ischemia, even at supraventricular tachycardic frequencies of 180 /min (Figure 1B). Hence, we postponed coronary angiography and reviewed the patient's history.
Figure 1
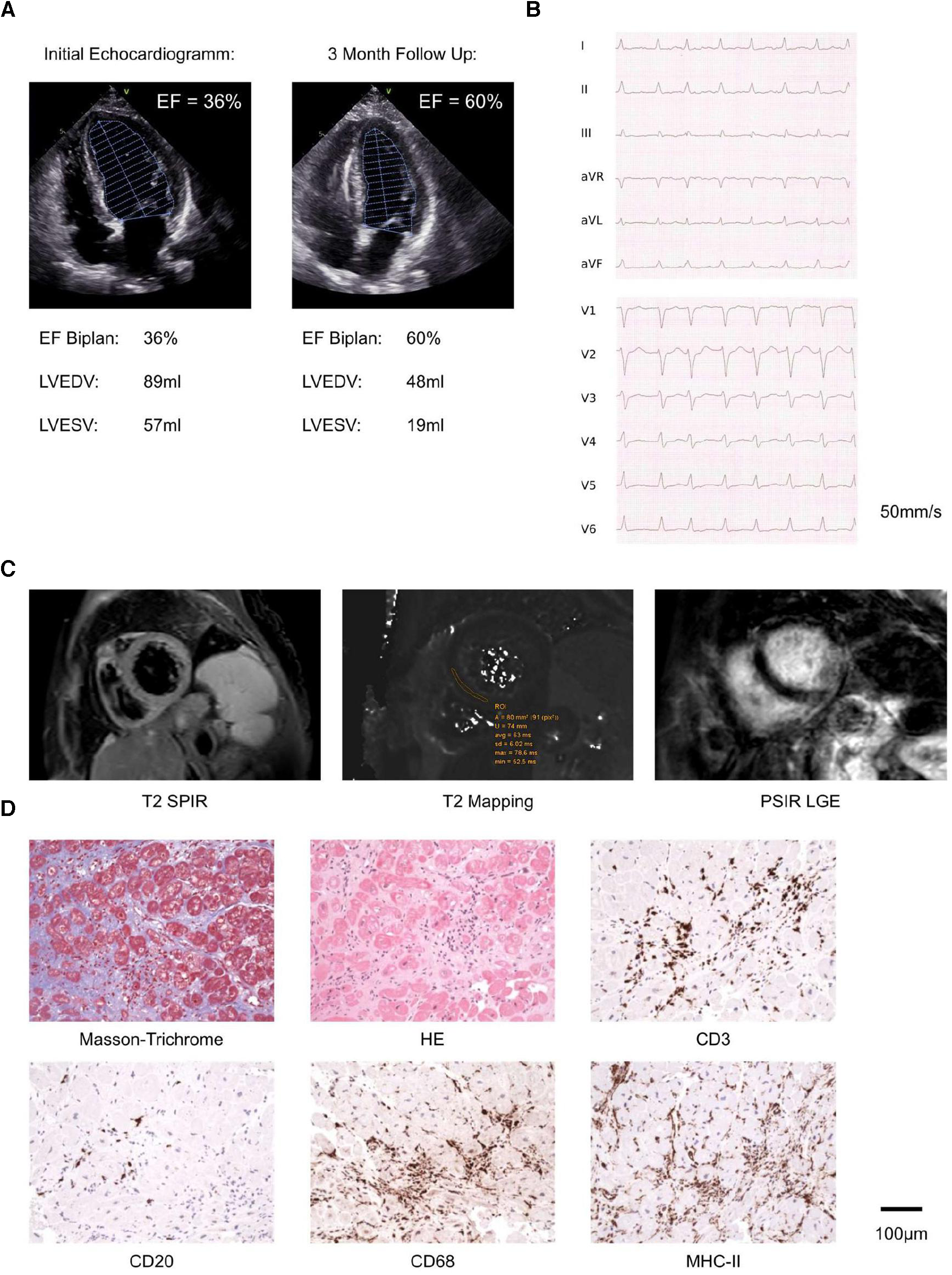
Diagnostics, including echocardiography, radiology and pathology: (A) representative images of initial echocardiography depicting reduced LVEF and 3 months follow up echocardiography depicting improved LVEF. (B) Electrocardiogram recorded during tachycardic (180/min) atrial fibrillation. (C) Representative images from cardiac MRI, recorded one day after initiation of prednisone. (D) Histopathology and Immunohistology of endomyocardial biopsies including Masson Trichrome, HE, CD3 (T lymphocytes), CD20 (B lymphocytes), CD68 (macrophages) and MHCII stains. Scale bar as indicated.
Here we learned the patient had received chemotherapy and a total of 42 infusions of 200 mg pembrolizumab at 3-weekly intervals for over 2.5 years, following the diagnosis of metastatic non-small-cell lung cancer (NSCLC, UICC stage IV). We initiated intravenous prednisone (1 mg/kg body weight) as, despite being improbable, irMyocarditis was considered a differential diagnosis. Cardiac MRI was performed the consecutive day, and MRI-based tissue characterization using Lake Louise Criteria II (LLC II) showed neither myo-/pericardial late gadolinium enhancement nor pericardial thickening or effusion. Furthermore, T2-mapping was not prolonged with an exemplary midventricular septal T2 relaxation time of 60 ms (scanner-specific reference range: 58 ± 5 ms), while native T1 relaxation was prolonged with an exemplary mid-ventricular relaxation time of 974 ms.
In summary, MRI did not indicate peri-/myocarditis (Figure 1C; Supplementary Report S2). Hence, we performed coronary catheterization and etiologically ruled out ischemia (Supplementary Report S3). We then reviewed the case within our center's interdisciplinary CIO iTox Board, dedicated to the management of irAEs, and jointly opted for endomyocardial biopsies even though imaging was inconspicuous. Five samples from the left ventricle were collected and reviewed by an external reference pathologist (KK). The myocardium showed distinct focal but also diffuse interstitial fibrosis, indicative of inflammation, likely subclinically preceding the OHCA by months. In different areas, myocyte necrosis was observed in association with numerous CD3+ T-cells (100 /mm2), a great number of CD68+ macrophages with MHC II expression (200 /mm2), and scattered CD20+ B-lymphocytes, without eosinophilic granulocytes or multinucleated giant cells (Figure 1D). We further carried out nested PCR to rule out cardiotropic pathogens as the underlying cause. EBV-specific DNA sequences were detected at low copy numbers in both endomyocardial biopsies and peripheral leukocyte preparation, most certainly reflecting latent systemic virus persistence. Negative PCR results were obtained for a standardized panel of further cardio-pathogens (Supplementary Table S3). Furthermore, there were no clinical signs of myasthenia or myositis and the patient could not recall pathologies of the thymus, neither did the medical history or CT scans acquired reveal any such alterations.
Taken together, (immuno)histology was consistent with acute lymphocytic myocarditis, as eosinophilic myocarditis, giant cell myocarditis, or amyloidosis were histologically excluded, thus confirming irMyocarditis. Hence, we continued prednisone treatment, on which the patient's clinical condition continuously improved to the state prior to the event. The patient received an implantable cardioverter-defibrillator for secondary prevention, pembrolizumab was permanently discontinued, and with continued heart failure medication, the patient demonstrated normalized LVEF (Figure 1A). The NSCLC remains in remission 4 months after.
Discussion
Summarizing, while irMyocarditis resembles a comparably rare adverse event, it is associated with a high mortality of 25% to 50% (2). While previous case reports and systematic analyses on irMyocarditis exist (4, 6), the case presented herein is extraordinary in multiple aspects and thus provides compelling insights for the management of patients with (very) late-onset: Our patient experienced cardiac arrest after over 2.5 years of treatment without prior irAEs, although irMyocarditis typically manifests early after initiation (3). To our knowledge, this is the longest onset yet reported. With a rapidly growing number of patients treated with ICI, including in curative intent, this case demonstrates the importance of vigilance for irAEs even after long treatment periods. Furthermore, it stresses that early initiation of immunosuppression appears critical for improved outcomes with negligible risks if the specific event may later be attributed to a different etiology and should hence be initiated even when irMyocarditis seems unlikely (7). Moreover, it highlights that irMyocarditis may be present even with inconspicuous MRI. Although cardiac MRI became a pivotal tool in diagnosing myocarditis over the last two decades, it is critical to contemplate that while demonstrating high specificity, sensitivity is reported to be 87% with LLC II (8). Additionally, previous studies showed that sensitivity of cardiac MRI is dependent on underlying cardiac pathology and lowest in cardiomyopathic causes of myocarditis (9). Thus, especially in the context of possible irMyocarditis with inconclusive MRI, myocardial biopsies remain the gold standard of diagnosis. Additionally, our case makes evident the importance of interdisciplinary teams for optimal management of such patients. By reviewing the case in our well-established CIO iTox Board, we swiftly coordinated diagnostics and early immunosuppression, which data suggests is critical for patients’ outcomes (4, 5, 10). With the ever-growing number of ICI-based treatments and expansion beyond targeting PD-1 and CTLA-4, such interdisciplinary teams will become increasingly important in the future to manage these patients’ therapies, especially when irAEs occur. Our case also once more demonstrates, that irAEs can occur after long uneventful treatment periods, which raises the question of optimal length of ICI-based treatments. To this end, initial data indicates the possibility to achieve long-lasting remissions with time-limited treatment periods, which might reduce the risk of irAEs (11). Although mechanisms underlying early-onset and CTLA-4-mediated irMyocarditis are more and more understood and led to the implementation of abatacept as an additional treatment option (12), the biology underlying anti-PD-1 and especially late-onset irMyocarditis is unknown. Hence, further translational studies are crucial to provide optimal care to patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because the manuscript represents a case report. The patient’s data was anonymized and the patient provided written informed consent to the manuscript, which they read and accepted in advance. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RL: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Visualization. KS: Writing – review & editing, Data curation, Visualization. SL: Data curation, Visualization, Writing – review & editing. J-PW: Data curation, Visualization, Writing – review & editing. NK: Data curation, Writing – review & editing. KK: Data curation, Visualization, Writing – review & editing. PB: Conceptualization, Data curation, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
PB is supported by a Mildred Scheel School of Oncology (MSSO) Postdoctoral Fellowship by the German Cancer Aid (No. 70113307).
Acknowledgments
The authors would like to express their sincere gratitude to the patient who generously allowed the utilization of their complete medical record for this case report. Their invaluable contribution has made this case report possible and greatly provides further insight to a to this date research and understanding of this medical case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1328378/full#supplementary-material
References
1.
Tan S Day D Nicholls SJ Segelov E . Immune checkpoint inhibitor therapy in oncology: current uses and future directions: JACC: Cardiooncology state-of-the-art review. JACC CardioOncology. (2022) 4(5):579–97. 10.1016/j.jaccao.2022.09.004
2.
Palaskas N Lopez-Mattei J Durand JB Iliescu C Deswal A . Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9(2):e013757. 10.1161/JAHA.119.013757
3.
Varricchi G Galdiero MR Marone G Criscuolo G Triassi M Bonaduce D et al Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. (2017) 2(4):E000247. 10.1136/esmoopen-2017-000247
4.
Mahmood SS Fradley MG Cohen JV Nohria A Reynolds KL Heinzerling LM et al Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. (2018) 71(16):1755–64. 10.1016/j.jacc.2018.02.037
5.
Osinga TE Oosting SF van der Meer P de Boer RA Kuenen BC Rutgers A et al Immune Checkpoint Inhibitor–Associated Myocarditis. Neth Heart J. (2022) 30(6):295–301. 10.1007/s12471-021-01655-7
6.
Wang DY Salem JE Cohen JV Chandra S Menzer C Ye F et al Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. (2018) 4(12):1721–8. 10.1001/jamaoncol.2018.3923
7.
Maher VE Fernandes LL Weinstock C Tang S Agarwal S Brave M et al Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. (2019) 37(30):2730–7. 10.1200/JCO.19.00318
8.
Luetkens JA Faron A Isaak A Dabir D Kuetting D Feisst A et al Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging. (2019) 1(3):e190010. 10.1148/ryct.2019190010
9.
Francone M Chimenti C Galea N Scopelliti F Verardo R Galea R et al CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging. (2014) 7(3):254–63. 10.1016/j.jcmg.2013.10.011
10.
Escudier M Cautela J Malissen N Ancedy Y Orabona M Pinto J et al Clinical features, management, and outcomes of immune checkpoint inhibitor–related cardiotoxicity. Circulation. (2017) 136(21):2085–7. 10.1161/CIRCULATIONAHA.117.030571
11.
Marron TU Ryan AE Reddy SM Kaczanowska S Younis RH Thakkar D et al Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunother Cancer. (2021) 9(3):E001901. 10.1136/jitc-2020-001901
12.
Salem J-E Bretagne M Abbar B Leonard-Louis S Ederhy S Redheuil A et al Abatacept/ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis. Cancer Discovery. (2023) 13(5):1100–15. 10.1158/2159-8290.Cd-22-1180
Summary
Keywords
cancer immunotherapy, immune checkpoint inhibition, immune related adverse effects (irAEs), immune-related myocarditis, pembrolizumab
Citation
Lewis RI, Seuthe K, Lennartz S, Weber J-P, Kreuzberg N, Klingel K and Bröckelmann PJ (2024) Case Report: Sudden very late-onset near fatal PD1 inhibitor-associated myocarditis with out-of-hospital cardiac arrest after >2.5 years of pembrolizumab treatment. Front. Cardiovasc. Med. 11:1328378. doi: 10.3389/fcvm.2024.1328378
Received
05 December 2023
Accepted
25 January 2024
Published
19 February 2024
Volume
11 - 2024
Edited by
Alessandro Inno, Ospedale Sacro Cuore Don Calabria, Italy
Reviewed by
Luigi Tarantini, IRCCS Local Health Authority of Reggio Emilia, Italy
Joshua Eric Levenson, University of Pittsburgh Medical Center, United States
Updates
Copyright
© 2024 Lewis, Seuthe, Lennartz, Weber, Kreuzberg, Klingel and Bröckelmann.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Paul J. Bröckelmann paul.broeckelmann@uk-koeln.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.