- 1Department of General Internal Medicine, Mayo Clinic, Jacksonville, FL, United States
- 2Department of Cardiovascular Medicine, Mayo Clinic, Jacksonville, FL, United States
- 3Division of Cardiovascular Medicine, Department of Medicine, University of Florida, Gainesville, FL, United States
- 4Center for Clinical and Translational Science, Mayo Clinic, Rochester, MN, United States
- 5Mayo Clinic Graduate School of Biomedical Sciences, Mayo Clinic, Rochester, MN, United States
- 6Department of Immunology, Mayo Clinic, Jacksonville, FL, United States
Background: Defective connective tissue structure may cause individuals with hypermobile Ehlers-Danlos syndrome (hEDS) or hypermobility spectrum disorders (HSD) to develop cardiac defects.
Methods: We conducted a retrospective chart review of adult patients treated in the EDS Clinic from November 1, 2019, to June 20, 2022 to identify those with cardiac defects. Echocardiogram data were collected using a data collection service. All EDS Clinic patients were evaluated by a single physician and diagnosed according to the 2017 EDS diagnostic criteria. Patient demographic, family and cardiac history were extracted from self-reported responses from a REDCap clinical intake questionnaire. Patients with at least 1 available echocardiogram (ECHO) were selected for the study (n = 568).
Results: The prevalence of aortic root dilation in patients with hEDS was 2.7% and for HSD was 0.6%, with larger measurements for males than females and with age. Based on self-reported cardiac history that was verified from the medical record, patients with hEDS with bradycardia (p = 0.034) or brain aneurysm (p = 0.015) had a significantly larger average adult aortic root z-score. In contrast, patients with HSD that self-reported dysautonomia (p = 0.019) had a significantly larger average aortic root z-score. The prevalence of diagnosed mitral valve prolapse in patients with hEDS was 3.5% and HSD was 1.8%. Variants of uncertain significance were identified in 16 of 84 patients that received genetic testing based on family history.
Conclusions: These data reveal a low prevalence of cardiac defects in a large cohort of well-characterized hEDS and HSD patients. Differences in cardiovascular issues were not observed between patients with hEDS vs. HSD; and our findings suggest that cardiac defects in patients with hEDS or HSD are similar to the general population.
1 Introduction
Hypermobile Ehlers-Danlos syndrome (hEDS) and hypermobility spectrum disorders (HSD) are common connective tissue disorders that have a strong genetic basis from family history yet no identified genetic variant/s (1, 2). Other forms of EDS such as vascular EDS are associated with joint hypermobility and serious cardiovascular complications including arterial aneurysms, dissections, and organ rupture (3, 4). In contrast, mild, nonprogressive aortic root dilatation (ARD) and mitral valve prolapse (MVP) have been associated with hEDS and HSD, which have been reported to occur in 6%–21% of patients with hEDS/HSD (5–7). In 2017 new diagnostic criteria for hypermobile EDS were created that separated patients into hEDS or HSD categories. Although the presence of ARD and MVP are part of the diagnostic criteria distinguishing hEDS from HSD, their presence is not required for a diagnosis of hEDS (2). Based on previous reports that ARD and MVP were present more often in patients with symptomatic hypermobility than the general population, the 2017 diagnostic criteria indicated an echocardiographic assessment for cardiovascular complications (2). As a result, echocardiography is often a routine part of the evaluation of joint hypermobility syndromes.
However, recent studies have produced contradictory findings. In a study published in 2017, Ritter et al. examined mainly pediatric patients diagnosed with hEDS age 7–21 and reported ARD in 14.2% (Z-score >2.0) or 5.5% (Z-score >3.0) of patients (6). Asher et al. combined their findings from adult patients diagnosed with hEDS, HSD and classical EDS and found ARD in 1.6% and MVP in 6.4% of patients (5). A study by Paige et al. that examined 95 pediatric patients diagnosed with cEDS (n = 12) or hEDS (n = 83) using the 2017 diagnostic criteria found only one patient with mild ARV and another with mild ascending aorta dilation (8). Recently, Rashed et al. examined 258 mainly adult patients (94%) diagnosed with hEDS or HSD using the 2017 diagnostic criteria at their specialty Cardiovascular Genetics Program and reported 15.2% ARD and 7.5% MVP in hEDS/HSD combined (7). They also reported that ARD occurs more often in patients diagnosed with hEDS (20.7%) than with HSD (7.7%) and occurs at a similar prevalence between females and males, although was mild in >90% of females and moderate-to-severe in 50% of males (7). They also observed cervical artery dissection (CeAD, n = 2), spontaneous coronary artery dissection (SCAD, n = 2), and SCAD plus celiac artery pseudoaneurysm (n = 1) in several patients (1.9%). We established a specialty EDS Clinic in the fall of 2019 (9). Based on previous studies, we hypothesized that hEDS and HSD patients diagnosed in our clinic would have a higher rate of abnormal echocardiogram (ECHO) findings than the general population. In this study we retrospectively investigated a large cohort of adult patients diagnosed with hEDS or HSD (n = 568) using the 2017 diagnostic criteria in order to determine the prevalence of patients with ARD, MVP or other cardiac complications and to determine whether cardiac defects or comorbidities differed between patients with hEDS or HSD.
2 Methods
2.1 Ethics statement
This retrospective study was approved by the Mayo Clinic Institutional Review Board (IRB# 19-011260) and informed consent was waived by the IRB for all patients. The research conformed to the principles outlined in the Declaration of Helsinki.
2.2 Study design
All patients seen at the EDS Clinic were evaluated by the same physician who is an EDS specialist and diagnosed according to the 2017 EDS diagnostic criteria (2). Adult patients received a diagnosis of hEDS or HSD, which we reported previously have a high overlap of symptoms and comorbidities (10). Patients that attended the EDS Clinic but did not obtain a diagnosis of hEDS or HSD were also examined. These patients are not healthy, but have many symptoms and comorbidities similar to hEDS and HSD patients. It is possible that some of these patients have symptomatic hypermobility for joints other than those assessed by the Beighton score or were stiffer as occurs with age and so may not have met the diagnostic criteria. A full medical history and physical exam were obtained at the EDS Clinic visit. A retrospective chart review of patients seen at the EDS Clinic at Mayo Clinic in Jacksonville, Florida was conducted from November 1, 2019, to June 20, 2022. From the total cohort of 999 patients, 233 patients were diagnosed with hEDS (met criteria 1, 2 and 3) with 170 having at least one echocardiogram (ECHO) and 148 with an aortic root measurement. Additionally, 551 patients were diagnosed with HSD (met criteria 1 and 3 but NOT 2) with 398 having at least one ECHO and 333 with an aortic root measurement. From all individuals visiting the EDS Clinic during that time window, 8 had another connective tissue disorder (did not meet criteria 3) and were not included in the study, and 158 did not meet criteria 1, 2, or 3 with 101 of these patients having at least one ECHO and 77 with an aortic root measurement. For multiple reasons, data were not reported for 49 patients for a total of 784 patients in the study. Only 568 of the patients had at least one ECHO in their medical record and were used in the study.
2.3 Patient REDCap intake questionnaire
Self-reported demographics, patient history and cardiac history were obtained from all patients who attended the EDS Clinic using a Research Electronic Data Capture (REDCap) Intake Questionnaire, as reported previously (10).
2.4 Echocardiography measurements
Echocardiogram data were collected retrospectively using the cardiovascular DataMart; a data collection service offered by the Mayo Clinic that extracts data from the electronic medical record (EMR). Echocardiogram data available in the EMR for patients seen at the EDS Clinic was included in the study, including echocardiograms that were obtained prior to the patient being seen at the EDS Clinic. This was a retrospective study and so no active followup occurred.
The aortic root diameter was measured at end-diastole at the onset of the QRS from the leading edge of the aortic root from the parasternal long axis image on transthoracic echocardiography. See the Supplementary Excel File for the echocardiography data dictionary that describes how the specific measurements were obtained. To understand the cardiac complications of aortic root dilation (ARD), aortic root z-scores for adults were calculated by the study team (not DataMart) using the Devereux formula (11). This involved considering the aortic root diameter and patient age while accounting for weight and height through body surface area (BSA) with the calculation below, following recent recommendations by (12, 13):
A z-score greater than 2 was considered dilated. A diagnosis of MVP and/or mitral valve regurgitation (MVR) was confirmed by an echocardiologist that was an expert in these analyses which were conducted in the Department of Cardiovascular Medicine at Mayo Clinic.
2.5 Genetic testing
A patient with a diagnosis of hEDS or HSD is not indicated for genetic testing because there is no known gene with a causal relationship for these diagnoses. However, some clinical features or medical/ family history indicate suspicion of a genetic abnormality such as a family history of aneurysm, cleft palate, retinal detachment, autism spectrum disorder or other genetic disorders, for example. Based on the physician's assessment of patient history, 84 patients were selected for genetic testing which was conducted using the Invitae Connective Tissue Disorder Gene Panel (test code # 434340) which includes a panel of 92 connective tissue genes that were analyzed by Invitae. Findings were reported as benign, a variant of uncertain significance (VUS) or pathogenic.
2.6 Statistical analyses
Fisher's exact, Student's t test, and Pearson correlation were performed using Python. All graphs and analysis were completed using Python.
3 Results
3.1 Demographics
A total of 784 patients were included in the study that had been diagnosed at the EDS Clinic with hEDS or HSD from November 1, 2019, to June 20, 2022. Of those, 568 patients (aged 16–65) had at least one ECHO in their medical record at any time (including prior to the study window) that was reviewed for the study. Demographics for patients with hEDS (Table 1) or HSD (Table 2) were very similar for individuals that had at least 1 ECHO compared to their overall disease cohort indicating that the ECHO subset was representative of the larger group of hEDS or HSD patients. Most patients with hEDS (90.0%) or HSD (95.7%) were female (Tables 1, 2). Additionally, the majority of patients with at least 1 ECHO were 25–34.9 years old, White (>90%) and not Hispanic or Latinx (90%) (Tables 1, 2). The majority of patients were diagnosed with HSD (67.9%, n = 398) vs. hEDS (29.1%, n = 170). Patients with hEDS had a higher number of average Features A (∼5) than HSD patients (∼2), which is part of the diagnostic criteria (2), but similar average Beighton scores (hEDS ∼6 vs. HSD ∼5) (Tables 1, 2). For those with Feature A characteristics, most were similar between patients with hEDS and HSD except for mild skin hyperextensibility which was more prominent in patients with hEDS (Tables 1, 2).
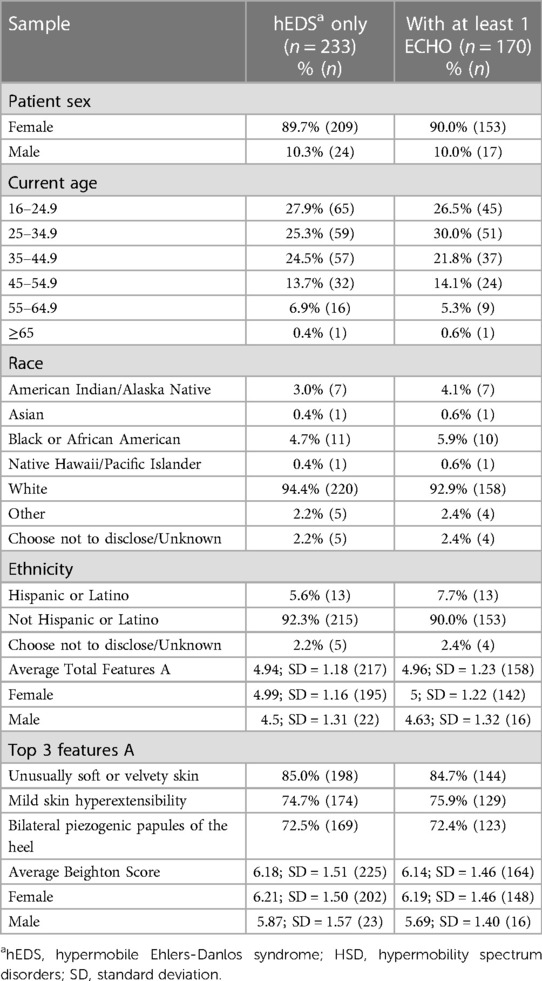
Table 1. Demographics and clinical features of female and male hEDS patients diagnosed at the EDS clinic (n = 233) and the subsample that had at least 1 echocardiogram data available (n = 170).
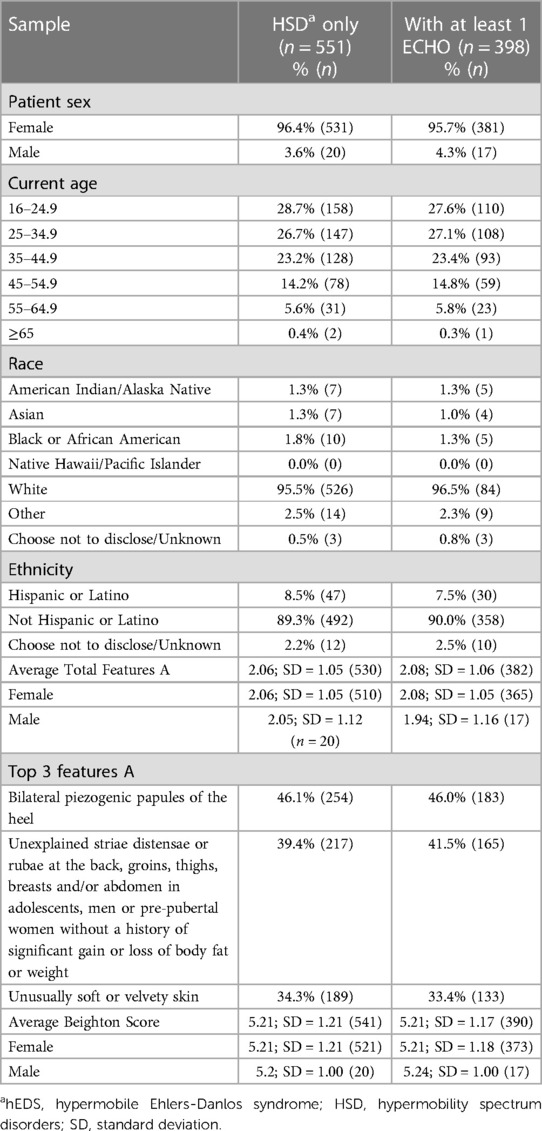
Table 2. Demographics and clinical features of female and male HSD patients diagnosed at the EDS clinic (n = 551) and the subsample that had at least 1 echocardiogram data available (n = 398).
3.2 Aortic root dilatation
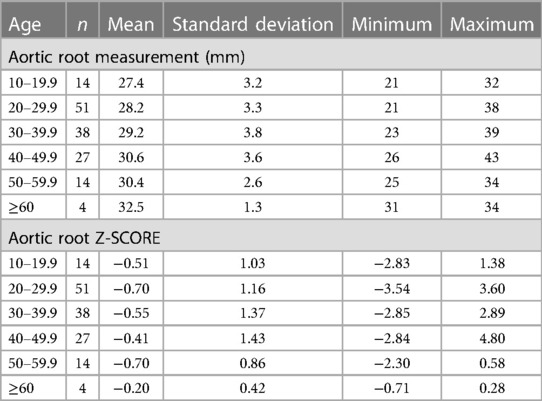
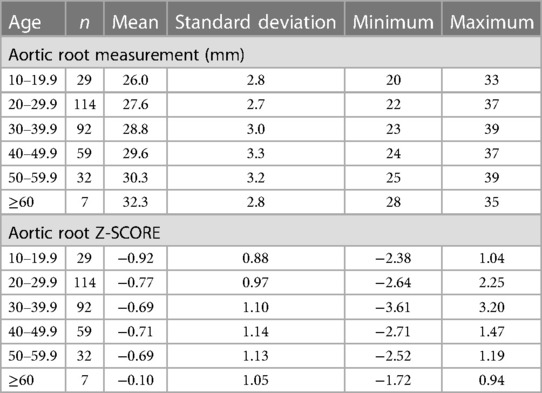
Of the 568 hEDS and HSD patients with at least one ECHO, 481 (84.7%) had an aortic root diameter measurement (Tables 3, 4, Supplementary Figure S1). The average aortic root z-score after Devereux z-score normalization at the first ECHO for patients with hEDS was −0.58 (n = 148), and for HSD was −0.73 (n = 333), indicating that most patients did not have dilated aortic roots. There was no significant difference in average aortic root diameter between patients with HSD (28.5 mm, n = 333) compared to hEDS (29.1 mm, n = 148, p = 0.070) (Figure 1A) or for average aortic root z-score when HSD (−0.73, n = 333) was compared to hEDS (−0.58, n = 148, p = 0.185) (Figure 1B). In a study by Asher et al. the average root diameter after Devereux z-score normalization in EDS patients was −0.97 ± 1.2 at the time of the first ECHO indicating that our findings are similar to previous reports (5). We had 101 patients from the EDS Clinic who were not diagnosed with hEDS or HSD that had an available ECHO and 77 with recorded aortic root measurements. Note that these were not healthy individuals. These patients attended the EDS Clinic because of suspicion for hEDS or HSD but were not diagnosed with either condition. They had many comorbidities based on the intake questionnaire. We found that there was no statistically significant difference in the average aortic root diameter between patients with hEDS (p = 0.076, t test) or HSD (p = 0.425, t test) and this group of patients.
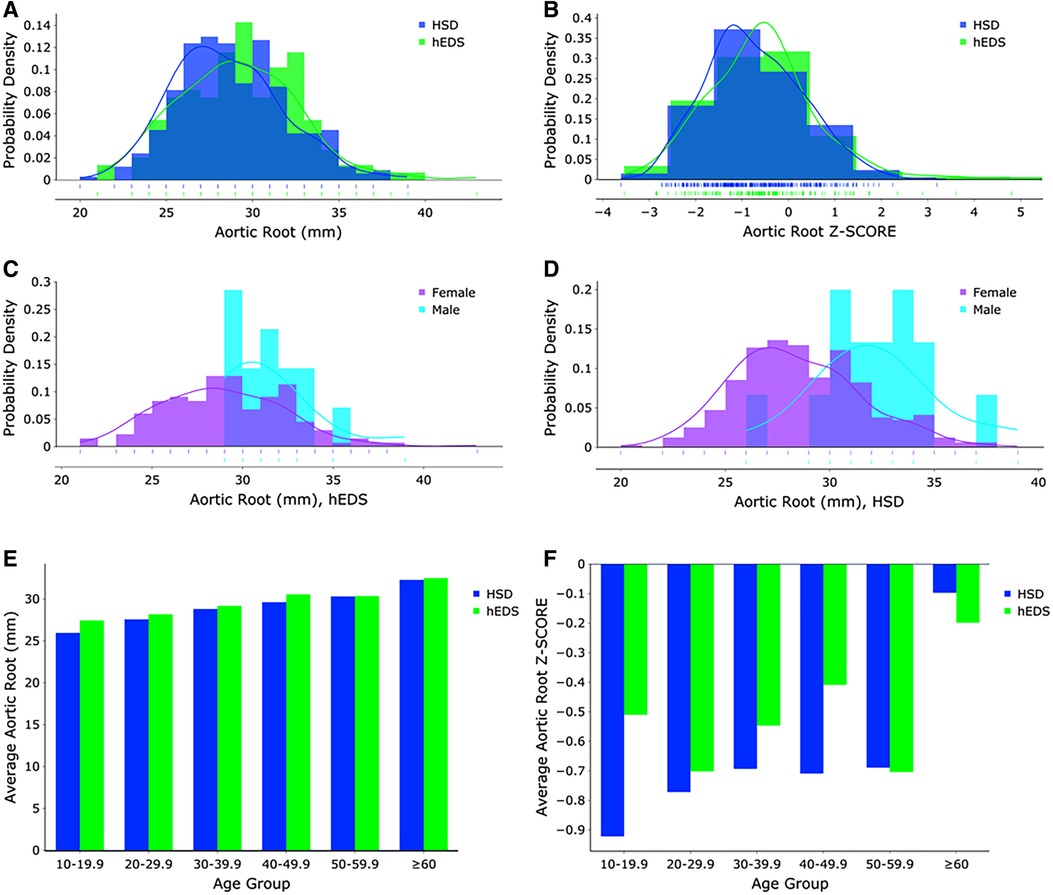
Figure 1. (A) Aortic root measurements (mm) and (B) z-score distributions for hEDS (blue) and HSD (green) patients (n = 481). Aortic root measurements (mm) for (C) hEDS and (D) HSD females (purple) and males (turquoise). (E) Aortic root measurements (mm) and (F) z-score distributions for hEDS (blue) and HSD (green) patients according to age group.
As expected, male patients with hEDS had significantly larger average aortic root diameters compared to females (females n = 133, diameter 28.85 mm, z-score −0.58; males n = 15, diameter 31.53 mm, z-score −0.52, p = 0.005) (Figure 1C). Similarly male patients with HSD also had significantly larger average aortic root diameters compared to females (females n = 317, diameter 28.32 mm, z-score −0.73; males n = 16, diameter 32.13 mm, z-score −0.68, p < 0.0001) (Figure 1D). However, there was no significant difference by sex in average z-scores in patients with hEDS (p = 0.851) or HSD (p = 0.854) which is expected because the z-score controls for larger body size (typically larger in males) and increased size with age (12). Also as expected, aortic root diameter increased with increasing age in patients diagnosed with hEDS or HSD (Tables 3, 4, Figures 1E,F, Supplementary Figure S2) (13). The largest average aortic root diameter and average z-score were found in patients with hEDS or HSD who were 60 years or older (Tables 3, 4, Figures 1E,F, Supplementary Figure S2), similar to results of a previous study of aortic root diameter in patients with hEDS by age (5).
We did not find a significant relationship between aortic root diameter and self-reported cardiac comorbidities from our intake questionnaire in patients diagnosed with hEDS except for a borderline significant relationship for bradycardia (p = 0.085) (Table 5). However, bradycardia was significant when examining the average aortic root z-score (p = 0.034) in patients with hEDS along with brain aneurysm (p = 0.015) (Table 6). We found that patients diagnosed with HSD that self-reported chest pain (p = 0.044) or hypertension (p = 0.008) had significantly larger aortic root diameters while white coat hypertension was borderline significant (p = 0.072) (Table 7). HSD patients with dysautonomia (p = 0.019) had significantly larger average aortic root z-scores (i.e., less negative) and patients who self-reported chest pain were borderline significant for larger z-scores (p = 0.070) (Table 8). We also found an absence of valvular complications in patients with hEDS or HSD with aortic root dilation. Almost all aortic root dilation patients had trivial regurgitation in their mitral, tricuspid, or pulmonary valve.
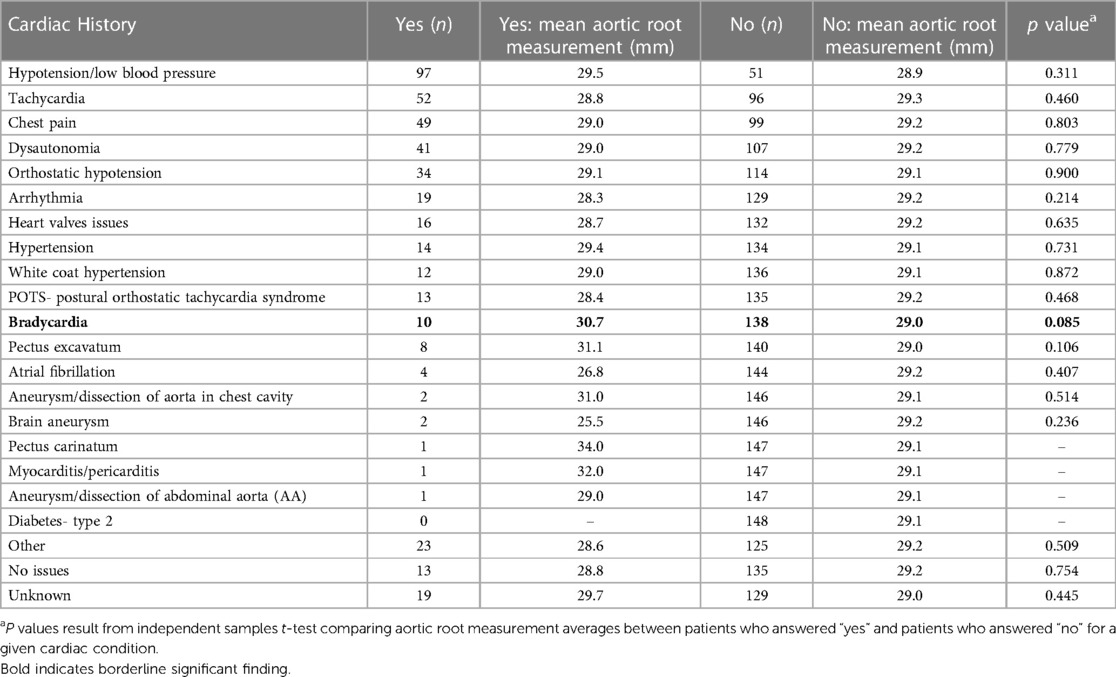
Table 5. Aortic root measurement averages by frequency of self-reported cardiac comorbidities for patients with hEDS (n = 148).
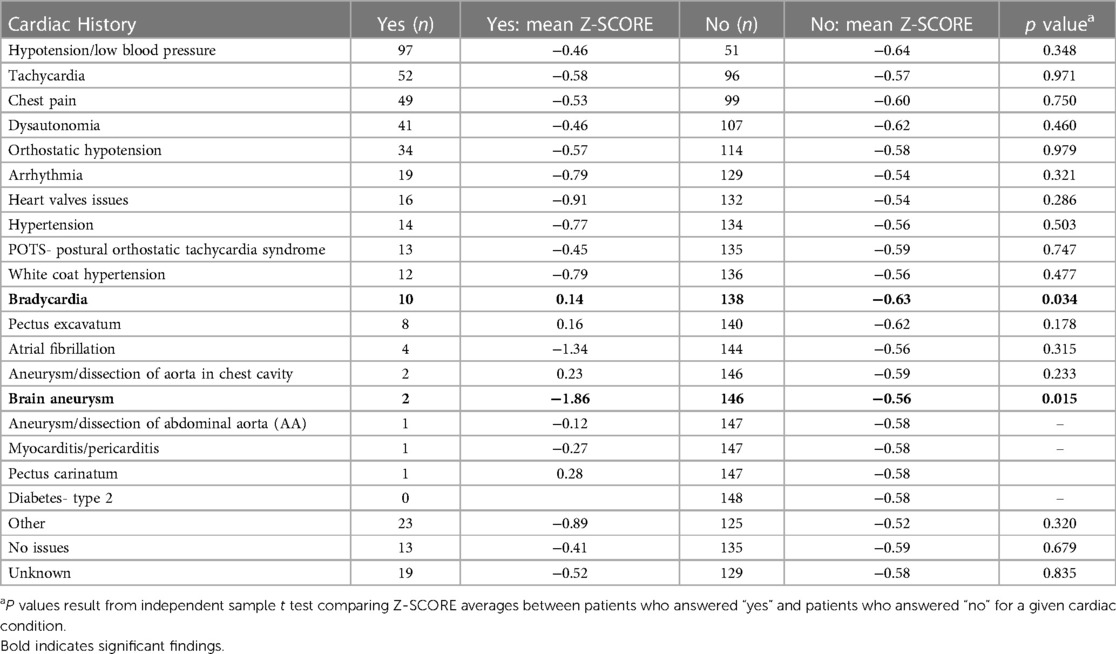
Table 6. Aortic root Z-SCORE averages ordered by frequency of self-reported cardiac comorbidities in hEDS patients with aortic root measurements (n = 148).
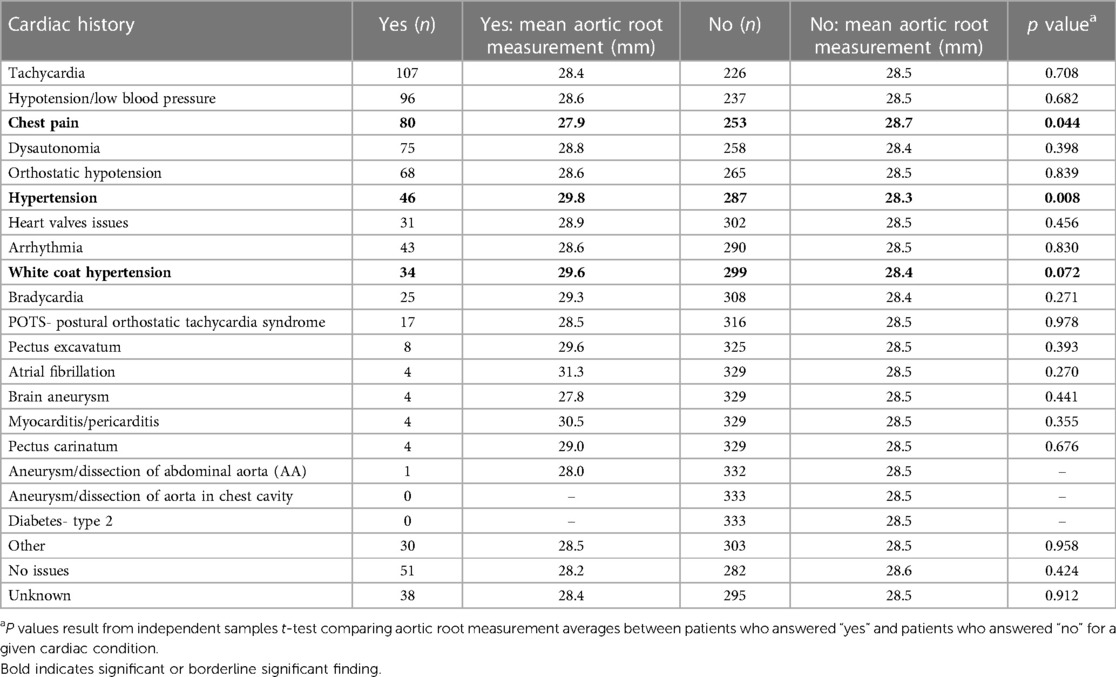
Table 7. Aortic root measurement averages by frequency of self-reported cardiac comorbidities for patients with HSD (n = 333).
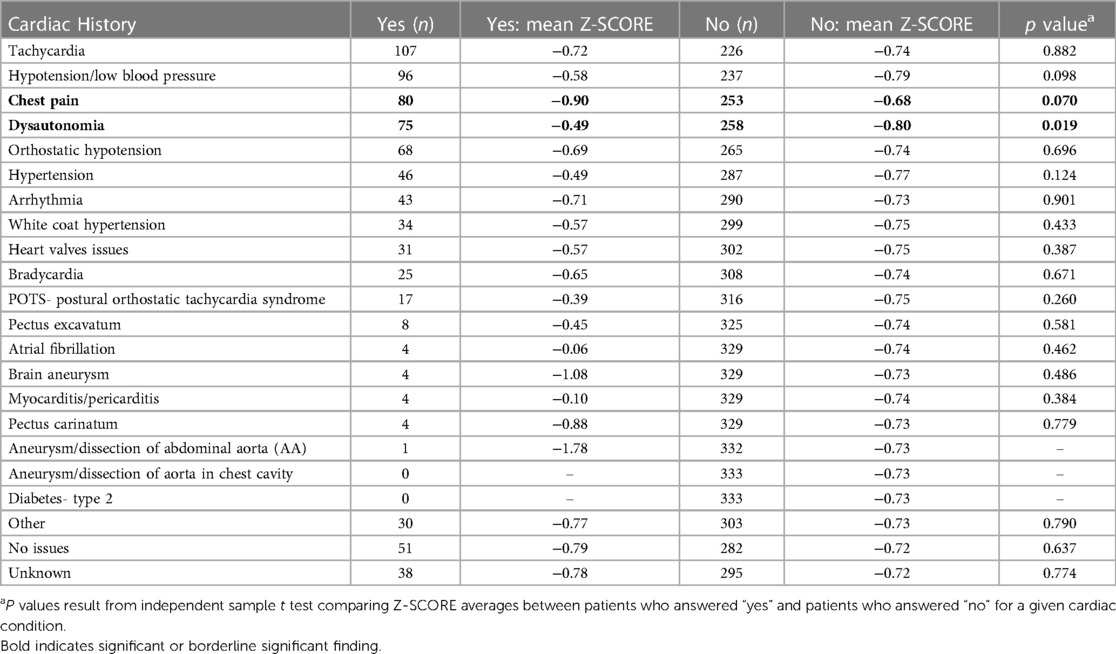
Table 8. Aortic root Z-SCORE averages ordered by frequency of self-reported cardiac comorbidities in HSD patients with aortic root measurements (n = 333).
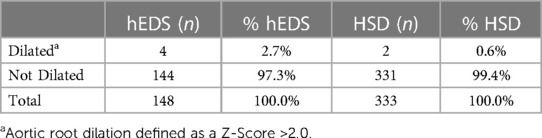
We found that 6 out of 481 patients in this study had aortic root dilation based on a z-score >2.0 (Table 9). All six were female and were under 45 years of age at first ECHO, with four of the six patients diagnosed with hEDS and two with HSD (Table 9). Based on these findings, the prevalence of aortic root dilation was 2.7% in patients with hEDS (n = 148) and 0.6% in patients with HSD (n = 333) (Table 10). Patients with a dilated aortic root who underwent a second ECHO (n = 2) did not display an increase in either aortic root z-score or aortic root diameter measurement (Table 9). Self-reported cardiac comorbidities are listed for the 6 patients with aortic root dilation in Table 11. Two of the six patients with aortic root dilation self-reported having postural orthostatic tachycardia syndrome (POTS) (one with hEDS and one with HSD) (Table 11). This compared to 13 hEDS patients (n = 148, 8.8%), 17 HSD patients (n = 333, 5.1%) and 2 patients that were not diagnosed with hEDS or HSD (n = 101, 2%) in this study (Supplementary Table S1). POTS did not occur more often in hEDS (p = 0.311, Fisher's Exact test) or HSD (p = 0.100, Fisher's Exact test) patients with aortic root dilation. We had only three patients with a z-score greater than 3, indicating clinically important dilation (Table 9, Supplementary Table S2). When we examined whether these patients had a self-reported family history of heritable aortic disease, we found that one patient reported a family history of brain aneurysm and another patient reported a family history of abdominal aortic aneurysm (Supplementary Table S2). Of these patients only the patient with HSD and a z-score >3.0 had the connective tissue gene panel run, but the results were negative (Table 9).
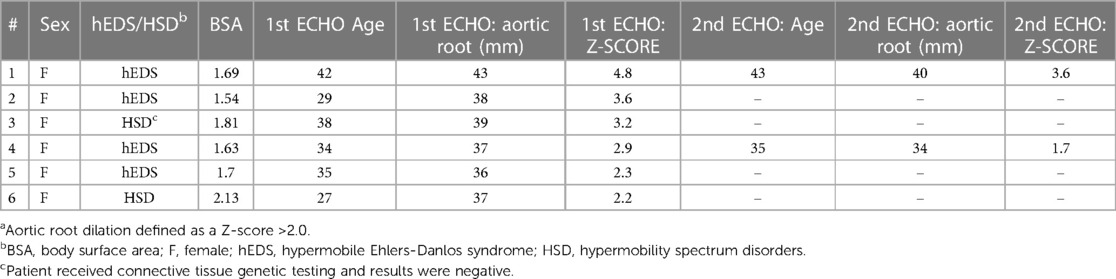
Table 9. Aortic root Z-SCORE and measurements at first and second echocardiogram for all patients with a dilated aortic roota (n = 6).
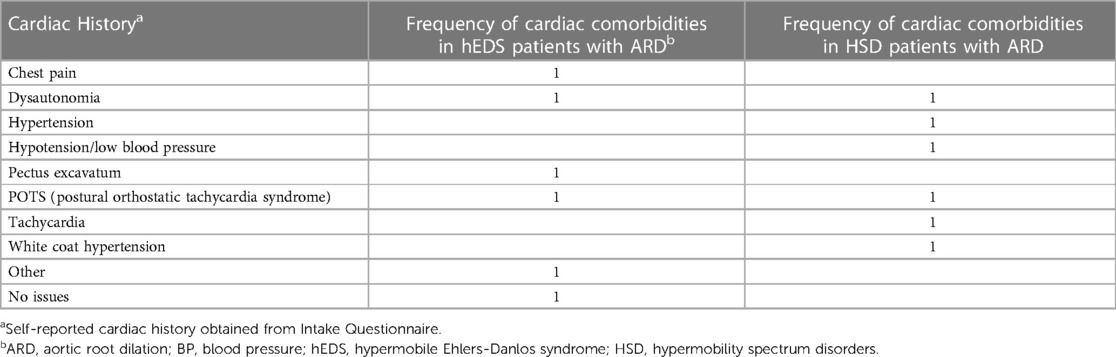
Table 11. Self-reported cardiac history for patients with hEDS (n = 4) or HSD (n = 2) with aortic root dilation (ARD).
3.3 Valvular regurgitation
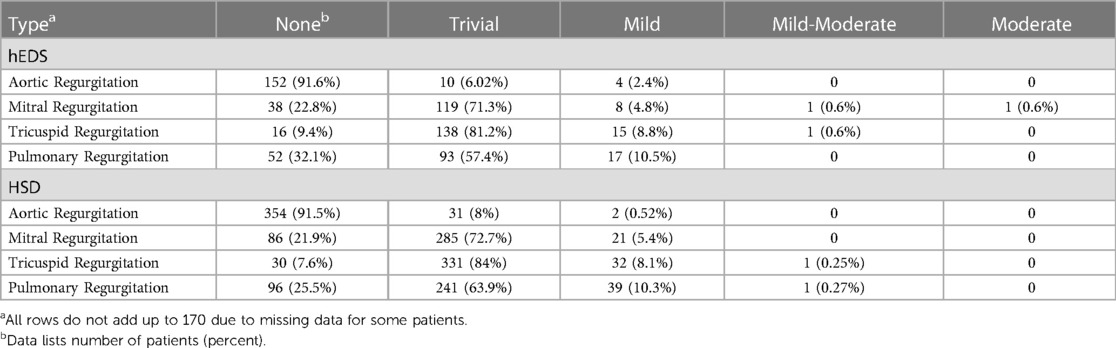
The majority of hEDS patients were found to have trivial mitral (71.3%), tricuspid (81.2%) or pulmonary (57.4%) regurgitation at their first ECHO (Table 12). A similar result was found for patients diagnosed with HSD who had trivial mitral (72.7%), tricuspid (84%) or pulmonary (63.9%) regurgitation at their first ECHO (Table 12). Less than 10% of hEDS patients were found to have mild aortic (2.4%), mitral (4.8%), tricuspid (8.8%) or pulmonary (10.5%) regurgitation at first ECHO (Table 12). Similarly, less than 10% of HSD patients were found to have mild aortic (0.5%), mitral (5.4%), tricuspid (8.1%) or pulmonary (10.3%) regurgitation at first ECHO (Table 12). Mild aortic regurgitation occurred more often in patients diagnosed with hEDS (2.4%) compared to HSD (0.5%). Around 2% or less of patients with hEDS or HSD had thickened or sclerotic valves by ECHO (Supplementary Table S3).
3.4 Mitral valve prolapse
The prevalence of diagnosed mitral valve prolapse (MVP) in patients with hEDS was 6/170 (3.5%) vs. 7/398 (1.8%) in patients with HSD. There was no significant difference in prevalence for MVP between patients diagnosed with hEDS vs. HSD (p = 0.224). All 13 cases of MVP that were identified (plus 2 possible cases making 15) occurred in women with hEDS or HSD who ranged in age from 18 to 70 (Table 13, Supplementary Figure S3). Bi-leaflet MVP was the most common type of MVP observed in hEDS or HSD women (Supplementary Table S4). Cardiac conditions associated with MVP are listed in Supplementary Table S5. The severity of MVP was noted as trivial in 8 patients, mild in 5 patients and moderate in 2 patients (Table 13, Supplementary Table S6). There were two female patients with HSD who had trivial MVP that also developed arrhythmia, and two patients with mild regurgitation that had thickened mitral valves (Supplementary Tables S5–S7). None of the patients with hEDS or HSD with MVP had severe regurgitation.

Table 13. Severity of mitral valve regurgitation (MVR) and impressions for all possible mitral valve prolapse (MVP) patients (n = 15).
3.5 Genetic testing
Genetic testing was conducted on 84 patients from the EDS Clinic (36 with hEDS and 48 with HSD) during the study window. No connective tissue-related genes have been identified as causative/pathogenic in patients with hEDS or HSD and so genetic testing is not indicated or routinely conducted in these patients. Selection for obtaining genetic testing is based on family and medical history. A finding of a genetic variant was identified in 16 (19%) patients, but all were variants of uncertain significance (VUS) (Supplementary Table S8). Twelve of the 16 patients with genetic variants were diagnosed with HSD (75%) vs. hEDS (25%). Six of the 16 patients (37.5%) with a genetic variant had a cardiac abnormality including issues like MVP and mild aortic regurgitation (Supplementary Table S8). Four patients had genetic variants in collagen genes, but only one of these patients had mild pulmonary valve regurgitation (Supplementary Table S8). Three patients had polymorphisms in filamin A (FLNA) with one patient having mild aortic regurgitation and another patient having mitral valve anterior leaflet prolapse, moderate mitral valve regurgitation and mild aortic and pulmonary regurgitation. And two patients were carriers for variants in lysl hydrolase (PLOD1), which is associated with the kyphoscoliotic form of EDS (Supplementary Table S8). Some variants listed in Supplementary Table S8 are not specifically related to connective tissue issues, aortic or valve disease such as HFE and MYH7.
4 Discussion
Hypermobile forms of EDS, which are the most common subtype of EDS, are inherited in an autosomal dominant manner. The genetic variant(s) contributing to hEDS and HSD are unknown. This study aimed to understand the prevalence of cardiovascular complications in adult patients with hEDS vs. HSD using a large well-defined cohort from the Mayo EDS Clinic diagnosed using the 2017 classification. Previous studies had combined data for hEDS and HSD or used a combination of diagnoses including classical EDS. A number of previous studies found that 6%–21% of patients with symptomatic hypermobility are at risk of ARD or MVP (6, 7), which prompted the requirement to examine ARD and MVP in the 2017 diagnostic process. However, other studies have reported a low prevalence similar to our results (5, 8, 14).
We found in this study that the overall prevalence of ARD in patients with hEDS was 2.7% and with HSD was 0.6% (Table 10). The prevalence of MVP in patients in this study with hEDS was 3.5% or HSD was 1.8%, similar to the prevalence of MVP in the general population of 2.7% (15). In contrast, Rashed et al. reported a prevalence of ARD of 15.2% and MVP of 7.5% in patients diagnosed with hEDS and HSD combined (7). Rashed et al. found a significant difference between patients, with ARD and MVP being higher in patients diagnosed with hEDS as expected based on the diagnostic criteria. However, we found no significant difference between hEDS and HSD patients in our study. Although the 2017 diagnostic criteria require an assessment of ARD and MVP, confirming a diagnosis of hEDS does not require the presence of these cardiovascular complications. Thus, our findings from a large cohort of well-defined hEDS and HSD patients indicate that the prevalence of MVP is similar to the general population and similar to the findings of several other studies (5, 8, 14), but those studies did not evaluate patients only using the 2017 diagnostic criteria. In contrast, Rashed et al. used the 2017 diagnostic criteria, mainly in adults (94%) with similar methods to evaluate ARD as our study (7). Reasons that we may have found a different prevalence than Rashed et al. is that we examined a higher number of patients, and patients were referred in the Rashed study to a specialty Cardiovascular Genetics Program which may have biased toward patients with more cardiovascular issues (7).
We found very few patients with hEDS or HSD that had a dilated aortic root in this study. Additionally, we did not find a significant difference in z-score between patients with hEDS or HSD, which is perhaps not surprising considering the high degree of overlap in many clinical features we and others have found between the two diagnoses (5, 10, 16). We were not using a pediatric z-score but one that incorporated body size area (BSA) as recently recommended for adults (12, 13). There was however no significant difference in aortic root measurements in our study between patients who were diagnosed with hEDS or HSD. Finding a difference in the z-score for an individual patient reveals the limitations of z-score normalization using the Boston and Devereux formulas, as highlighted by Ritter et al. (6). Because of this, clinical interpretation of a patient's z-score should be carefully considered. The average aortic root diameter for males with hEDS or HSD in this study was greater than the average reported by Rashed et al. (7). However, Rashed et al. had a lower number of male patients (n = 25) than our study (n = 34) and also reported 4 out of 25 male patients with aortic root dilatation whereas no males had dilatation in our study (7). This disparity can be explained by small sample sizes for males. For females, the average aortic root measurement for those with dilatation in our study was also greater. However, increasing prevalence of aortic dilatation by age was observed in both studies.
Although MVR and MVP are known to increase with age (17), we found that 11 out of 15 (73%) confirmed or suspected cases of MVP occurred in patients with hEDS or HSD that were under 50 years of age (Table 13). Thus, our study does not suggest that MVP occurs more often in older patients with hEDS or HSD. However, this may be related to the fact that hEDS and HSD are typically diagnosed in patients under age 50 and that patients experience joint stiffening with age (reduced hypermobility), so that a patient that was diagnosed when young may not fulfill the diagnostic criteria when they are past age 50.
Genetic testing was conducted on a subset of patients in this study based on family and/or medical history. Because no genetic variant has been identified for hEDS or HSD, conducting genetic testing on all patients is not currently indicated. Only a small percentage of those tested were found to have genetic variants, all of which were VUS. Rashed et al. also examined whether there was a relationship between genetic variants and cardiac issues in patients with hEDS/HSD (7). They found that of 22 patients with ARD and five patients with extra-aortic manifestations who underwent genetic testing, results were either negative or identified as a VUS like our findings (7). In our study, there was a low number of genetic variants identified for patients with hEDS or HSD, consistent with the literature. Research is needed to investigate connective tissue VUS identified in patients with hEDS and HSD to determine whether they are related to disease severity in general or to cardiovascular outcomes. Although genetic testing is not currently indicated for patients with hEDS or HSD, certain indicators in the family or medical history may warrant genetic testing and echocardiology evaluation.
The strengths of this study are a large sample size of well characterized hEDS and HSD patients, availability of a data collection service to collect ECHO data from the medical record, independent measurement of aortic root diameters, and a large amount of information available about patients including cardiac history from an intake questionnaire. Another benefit of the study was a unique setting with a single provider who is an EDS specialist who evaluated the patients at an EDS Clinic, heart disease specialists to interpret ECHO readings, and basic researchers to assess trends in the data. Limitations of the study include a self-reported assessment of cardiovascular history and the small number of males with a diagnosis of hEDS or HSD. Another limitation is the lack of genetic screening for all patients and the lack of healthy controls for comparison to hEDS/HSD. We showed previously that 64% of the individuals that attended our EDS Clinic but were not diagnosed with hEDS or HSD were diagnosed with fibromyalgia (10). A study by Kozanoglu et al. found that females with joint hypermobility and fibromyalgia were 9× more likely to have MVP than patients with fibromyalgia alone (18). Our findings suggest that MVP may not occur at a high rate in this group of patients at our EDS Clinic, but further research is needed. Our previous study showed that patients that are not diagnosed with hEDS or HSD at our EDS Clinic have significantly fewer symptoms and comorbidities compared to hEDS and HSD patients, suggesting that they may not be the same patient groups (18). However, studies comparing rates of ARD and MVP in patients with hEDS and HSD compared to normal healthy controls are needed.
This study has implications for clinicians. Our findings suggest that an ECHO may not be routinely needed in all newly diagnosed patients with hEDS or HSD because only a small number of cardiac defects were identified, and those complications were predominantly mild. Additionally, cardiac testing, which may not have been conducted at the time of diagnostic evaluation, may delay the diagnosis for patients using the 2017 diagnostic criteria, which requires assessment of ARD and/or MVP. However, determining whether a patient has ARD and/or MVP can only be assessed by ECHO. Even though there is a low prevalence of events in patients with hEDS and HSD, if ECHOs are not routinely performed these cardiovascular complications could be missed. Although our study is the largest to date to examine this issue, more research is needed with larger numbers because some recent studies such as Rashed et al. have found significant numbers of patients with hEDS/HSD with ARD and MVP (7). Future iterations of the hEDS/HSD diagnostic criteria should be revised related to cardiovascular findings based on the most recent research. Overall, our findings indicate that cardiac defects in patients with hEDS/HSD are similar to the general population.
5 Conclusions
Patients with hEDS and HSD in our study had few cardiovascular complications suggesting that routine echocardiography as part of the initial workup may not be warranted. However, other current studies using similar criteria have found a higher prevalence of ARD and MVP in patients with hEDS and HSD than our study and the general population indicating that more research is needed. Overall, we did not observe a difference in cardiovascular complications in patients with hEDS vs. HSD, which were mild, infrequent and similar to the general population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Mayo Clinic Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was waived by the IRB for this retrospective study.
Author contributions
DK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. KB: Data curation, Formal Analysis, Funding acquisition, Writing – original draft, Writing – review & editing. AS: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. BM: Data curation, Writing – original draft, Writing – review & editing. SG: Data curation, Writing – review & editing. MS: Data curation, Writing – review & editing. SK: Data curation, Visualization, Writing – review & editing. AD: Data curation, Writing – review & editing. AJ: Data curation, Writing – review & editing. EW: Data curation, Writing – review & editing. AK: Data curation, Writing – review & editing. DG: Data curation, Writing – review & editing. SP: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. DF: Data curation, Formal Analysis, Funding acquisition, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This study was partially supported by funding from the Mayo Clinic RACER Award to DRTK; Mayo Clinic's Division of General Internal Medicine to DRTK, KAB, DF; the Ralph E. Pounds and Kathy Olesker Pounds Fund in Research Related to Chronic Pain to DF; The Ehlers-Danlos Foundation Micro Grant to DRTK, DF; National Institutes of Health R01 HL164520, R21 AI154927, R21 AI152318, R21 AI145356 to DF and R21 AI163302 to KAB and TL1 TR002380 to ERW, DF; and American Heart Association 20TPA35490415 to DF and 23SCEFIA1153413 to KAB.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1332508/full#supplementary-material
References
1. Malfait F, Castori M, Francomano CA, Giunta C, Kosho T, Byers PH. The Ehlers-Danlos syndromes. Nat Rev Dis Primers. (2020) 6(1):64. doi: 10.1038/s41572-020-0194-9
2. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. (2017) 175(1):8–26. doi: 10.1002/ajmg.c.31552
3. Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, et al. Hypermobile Ehlers-Danlos syndrome (A.K.A. Ehlers-Danlos syndrome type III and Ehlers-Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet C Semin Med Genet. (2017) 175(1):48–69. doi: 10.1002/ajmg.c.31538
4. Eagleton MJ. Arterial complications of vascular Ehlers-Danlos syndrome. J Vasc Surg. (2016) 64(6):1869–80. doi: 10.1016/j.jvs.2016.06.120
5. Asher SB, Chen R, Kallish S. Mitral valve prolapse and aortic root dilation in adults with hypermobile Ehlers-Danlos syndrome and related disorders. Am J Med Genet A. (2018) 176(9):1838–44. doi: 10.1002/ajmg.a.40364
6. Ritter A, Atzinger C, Hays B, James J, Shikany A, Neilson D, et al. Natural history of aortic root dilation through young adulthood in a hypermobile Ehlers-Danlos syndrome cohort. Am J Med Genet A. (2017) 173(6):1467–72. doi: 10.1002/ajmg.a.38243
7. Rashed ER, Ruiz Maya T, Black J, Fettig V, Kadian-Dodov D, Olin JW, et al. Cardiovascular manifestations of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Vasc Med. (2022) 27(3):283–9. doi: 10.1177/1358863X211067566
8. Paige SL, Lechich KM, Tierney ESS, Collins RT 2nd. Cardiac involvement in classical or hypermobile Ehlers-Danlos syndrome is uncommon. Genet Med. (2020) 22(10):1583–8. doi: 10.1038/s41436-020-0856-8
9. Knight DRT, Confiado SM, Bruno KA, Fairweather D, Seymour-Sonnier AM, Jain A, et al. Establishing an Ehlers-Danlos syndrome clinic: lessons learned. SN Compr Clin Med. (2022) 4(1):138. doi: 10.1007/s42399-022-01218-w
10. Fairweather D, Bruno KA, Darakjian AA, Bruce BK, Gehin JM, Kotha A, et al. High overlap in patients diagnosed with hypermobile Ehlers-Danlos syndrome or hypermobile spectrum disorders with fibromyalgia and 40 self-reported symptoms and comorbidities. Front Med. (2023) 10:1096180. doi: 10.3389/fmed.2023.1096180
11. Devereux RB, de Simone G, Arnett DK, Best LG, Boerwinkle E, Howard BV, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. (2012) 110(8):1189–94. doi: 10.1016/j.amjcard.2012.05.063
12. Saura D, Dulgheru R, Caballero L, Bernard A, Kou S, Gonjilashvili N, et al. Two-dimensional transthoracic echocardiographic normal reference ranges for proximal aorta dimensions: results from the EAVCI NORRE study. Eur Heart J Cardiovasc Imaging. (2017) 18(2):167–79. doi: 10.1093/ehjci/jew053
13. Patel HN, Miyoshi T, Addetia K, Citro R, Daimon M, Gutierrez Fajardo P, et al. Normal values of aortic root size according to age, sex, and race: results of the World Alliance of Societies of Echocardiography study. J Am Soc Echocardiogr. (2022) 35(3):267–74. doi: 10.1016/j.echo.2021.09.011
14. Rauser-Foltz KK, Starr LJ, Yetman AT. Utilization of echocardiography in Ehlers-Danlos syndrome. Congenit Heart Dis. (2019) 14(5):864–7. doi: 10.1111/chd.12824
15. Padang R, Enriquez-Sarano M, Pislaru SV, Maalouf JF, Nkomo VT, Mankad SV, et al. Coexistent bicuspid aortic valve and mitral valve prolapse: epidemiology, phenotypic spectrum, and clinical implications. Eur Heart J Cardiovasc Imaging. (2019) 20(6):677–86. doi: 10.1093/ehjci/jey166
16. Miller E, Grosel JM. A review of Ehlers-Danlos syndrome. JAAPA. (2020) 33(4):23–8. doi: 10.1097/01.JAA.0000657160.48246.91
17. Douedi S, Douedi H. Mitral regurgitation. Statpearls [Internet]. Treasure Island, FL: StatPearls Publishing (2024). PMID: 31985928
Keywords: echocardiogram, aortic root dilation, hypermobility, valve prolapse, prevalence
Citation: Knight DRT, Bruno KA, Singh A, Munipalli B, Gajarawala S, Solomon M, Kocsis SC, Darakjian AA, Jain A, Whelan ER, Kotha A, Gorelov DJ, Phillips SD and Fairweather D (2024) Cardiac defects of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders: a retrospective cohort study. Front. Cardiovasc. Med. 11:1332508. doi: 10.3389/fcvm.2024.1332508
Received: 3 November 2023; Accepted: 16 February 2024;
Published: 18 March 2024.
Edited by:
Olivier M. Vanakker, Ghent University, BelgiumReviewed by:
Jirko Kühnisch, Charité University Medicine Berlin, GermanySiddharth Prakash, University of Texas Health Science Center at Houston, United States
Amy Kontorovich, Icahn School of Medicine at Mount Sinai, United States
© 2024 Knight, Bruno, Singh, Munipalli, Gajarawala, Solomon, Kocsis, Darakjian, Jain, Whelan, Kotha, Gorelov, Phillips and Fairweather. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dacre R. T. Knight S25pZ2h0LkRhY3JlQG1heW8uZWR1
†These authors share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Dacre R. T. Knight
Dacre R. T. Knight Katelyn A. Bruno
Katelyn A. Bruno Ayush Singh
Ayush Singh Bala Munipalli
Bala Munipalli Shilpa Gajarawala1
Shilpa Gajarawala1 Emily R. Whelan
Emily R. Whelan David J. Gorelov
David J. Gorelov DeLisa Fairweather
DeLisa Fairweather