Abstract
Background:
Primary cardiac lymphoma is an extremely rare malignant lymphoma, with clinical manifestations related to its location. We reported the diagnosis and treatment of primary cardiac lymphoma in a patient presented with atrioventricular block.
Case presentation:
A 64 year-old man was admitted to our hospital because of symptoms of a tired heart and shortness of breath. The initial electrocardiogram revealed a third-degree atrioventricular block. Computed tomography scan showed an irregularly shaped right heart, irregular clusters, and relatively weakly enhanced areas in the right auricle, atrium, and ventricle. The local boundary between the lesion, pericardium, and left atrium was unclear, and the ventricular septum was irregular and thickened. Multiple irregular gray neoplasms with less smooth surfaces were observed, with a maximum diameter of approximately 7 cm. Pathological findings confirmed a non-germinal center B cell subtype of diffuse large B-cell lymphoma. After surgical resection of the tumor and implantation of a permanent pacemaker, the symptoms of the patient were significantly improved, allowing subsequent chemotherapy.
Conclusion:
Surgical resection and placement of a permanent pacemaker were effective treatments for a patient with primary cardiac lymphoma presented with atrioventricular block.
1 Introduction
Primary cardiac tumors are particularly rare, with a prevalence ranging from 0.001% to 0.03%, with 1.3% of primary cardiac tumors and 0.5% of extranodal lymphomas being primary cardiac lymphomas (1). Primary cardiac lymphoma, which always involves the right atrium and ventricle, is three times more common in women than men, with 64 years being the median age for disease diagnosis (2). Primary cardiac lymphoma is defined as follows: (1) the heart or pericardium is affected by malignant lymphoma as demonstrated by autopsy (3); and (2) tumor tissue consisting of cardiac or pericardial lymphoma or lymphoma infiltration in the myocardium causing cardiac symptoms at initial diagnosis (4). Primary cardiac lymphomas are largely reported as isolated cases owing to their rarity and difficulty in their diagnosis. Thus, owing to late diagnosis, the prognosis of primary cardiac lymphoma is poor, with the median survival after diagnosis being 7 months (5).
The main clinical manifestations of primary cardiac lymphoma are cardiac symptoms induced by lymphoma infiltration of the myocardium. Other signs may be observed, including mediastinal lymph node enlargement, pleural exudation, and pulmonary embolism (6). Primary cardiac lymphoma is easily misdiagnosed in patients presenting with atrioventricular block only (7). We reports a case of third-degree atrioventricular block secondary to primary cardiac lymphoma that provides theoretical and empirical evidence for the diagnosis and treatment of this disease.
2 Case description
A 64 year-old man was admitted to our hospital on September 2, 2022, owing to symptoms of a tachycardia and shortness of breath. Physical examination at admission revealed the following: body temperature: 36.5°C, heart rate: 51 times/min, pulse: 51 beats/min; blood pressure: 102/68 mmHg, poor spirit, edema of the trunk, yellow sclera, slight cyanosis of the lip, no filling of the jugular vein, coarse breath sounds in both lungs, no enlargement of the heart boundary, arrhythmia, soft abdomen, and severe pitting edema of the lower limbs. Moreover, laboratory tests revealed the following: cardiac troponin I (cTNI) < 0.02 ng/ml (normal range: <0.53 ng/ml), creatinine-kinase-MB: 5.49 ng/ml (normal range: <5.0 ng/ml), myohemoglobin: 216.82 ng/ml (normal range: <110 ng/ml), D-dimer: 1214.27 ng/ml (normal range: <200 ng/ml), and B-type natriuretic peptide: 2460.72 pg/ml (normal range: <100 pg/ml). Electrocardiography indicated sinus rhythm, third-degree atrioventricular block, full-lead low voltage, QT interval prolongation, and borderline escape rhythm. Echocardiography revealed a hypoechoic mass extending from the right atrium to the right ventricle and obstructing the forward flow of the tricuspid valve. The size of the mass was approximately 66 × 40 mm (Figure 1). Computed tomography revealed the following: (1) irregular shape of the right heart, with the enhanced right auricle, right atrium, and ventricle exhibiting irregular clusters and relatively weakly enhanced areas. The CT value after enhancement was approximately 70–90 HU, while the larger cross-section was approximately 6.2 × 6.9 cm. The local boundaries between the lesion, pericardium, and left atrium were unclear, while the ventricular septum was irregular and thickened; (2) the filling of the superior vena cava and left and right brachiocephalic veins was not uniform; (3) the pulmonary artery and its branches in the upper, middle, and lower lobes of the right lung were poorly developed, while the local lumen was suspected to be uneven, exhibiting a few patchy and slightly low-density shadows. A suspicious low density shadow was observed at the edge of the upper and lower pulmonary arteries of the left lung; (4) the two lungs were dispersed with flaky shadows of increased density, the volume of the lower lobe of the left lung was reduced, a dense shadow was present in some lung tissues, and the enhancement was visible; (5) the bronchial segment of the left inferior lobe of the lung was slightly narrowed, while part of the bronchus of the left inferior lobe was unclear; (6) pericardial effusion, bilateral pleural effusion, left interlobar fissure, and adjacent lung hypoinflation; (7) the thoracic section of the esophageal wall was slightly thickened, approximately 0.4 cm; (8) swelling of left axilla, chest wall, and back soft tissue; (9) abdominal fluid, the local adipose space in the abdominal area was seen as lamellar and slightly high-density shadows; (10) multiple small veins in inferior vena cava and right lobe of liver were developed in advance; and (11) the adipose space in the abdominal cavity was blurred, with multiple pieces being increasingly flocculent, while the fascia around both kidneys was thickened (Figure 2).
Figure 1
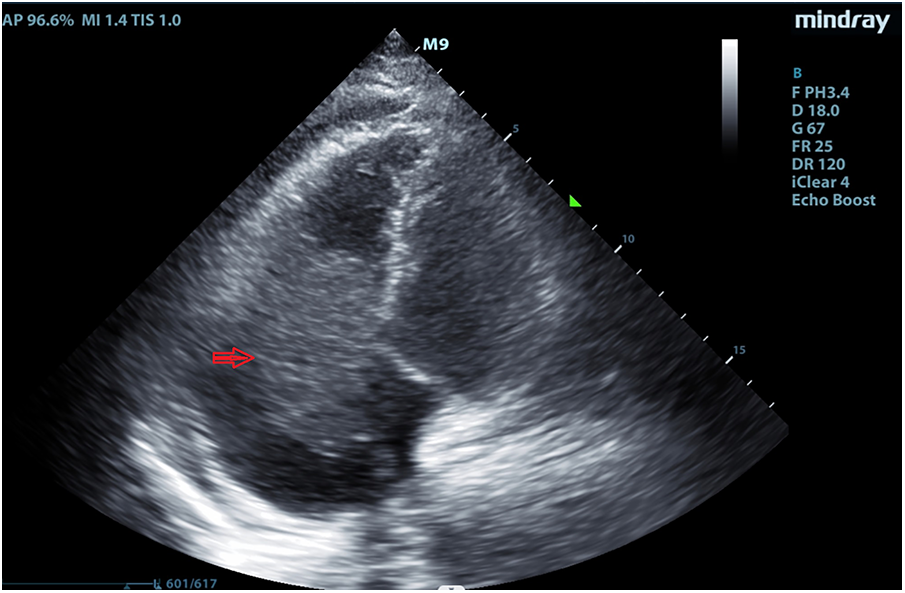
Echocardiography revealed a hypoechoic mass extending from the right atrium to the right ventricle in September 2, 2022.
Figure 2
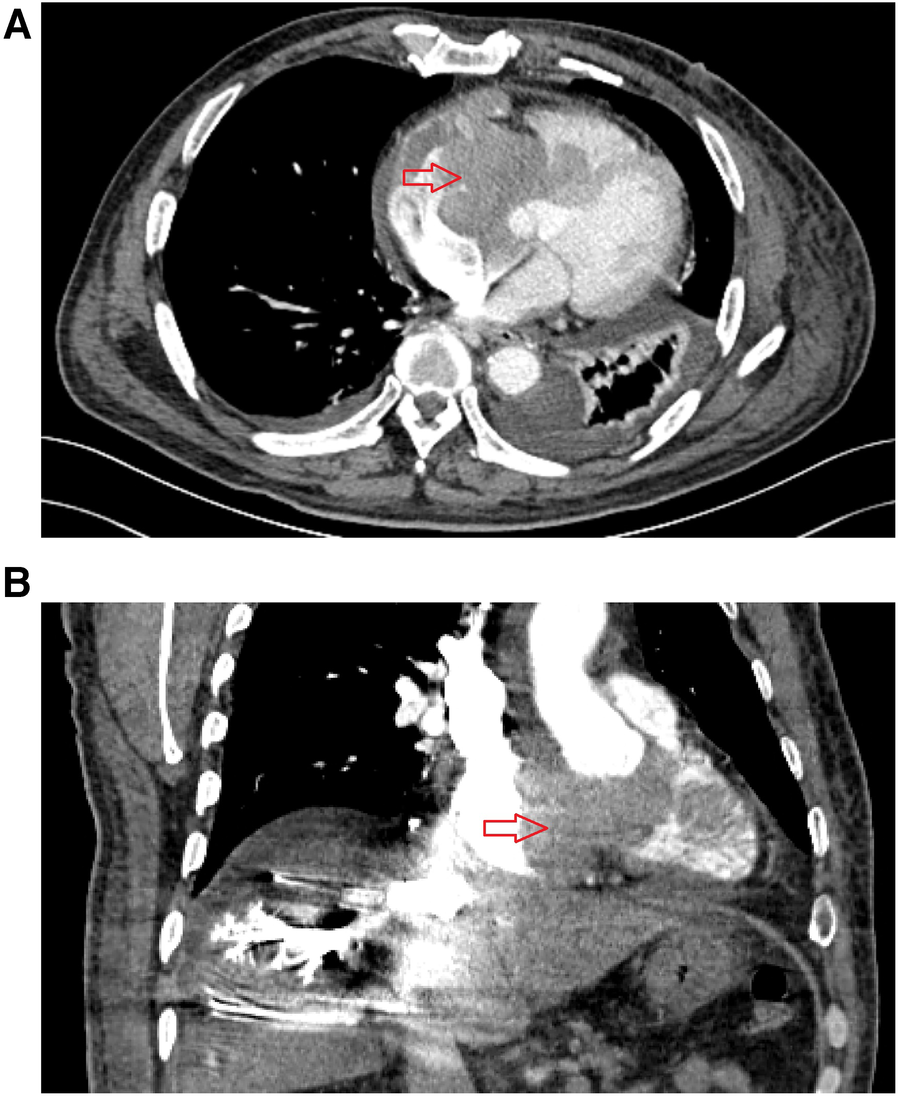
Preoperative enhanced computed tomography results for patient in September 2, 2022. (A) The cross-sectional image shows a mass in the right atrium, with irregular patchy enhancement in the right atrial appendage, right atrium, and right ventricular region, with a CT value of approximately 89 Hounsfield units. The interventricular septum is irregularly thickened. (B) Coronal view shows a mass in the right atrium, uneven opacification in some areas of the superior vena cava, delayed opacification of the inferior vena cava and veins in the right lobe of the liver.
September 3, 2022, hematological tests revealed the following: reduced lymphocyte ratio (8.1%, normal range: 20%–50%), platelet count (88 × 109, normal range: 100–300 × 109), prothrombin time activity (36%, normal range: 70%–130%), and level of plasma fibrinogen (1.7 g/L, normal range: 2–4 g/L), whereas elevated neutrophil ratio (76.3%, normal range: 40%–75%), level of high-sensitivity C-reactive protein (40.25 mg/L, normal range: 0–10 mg/L), prothrombin time (23.1 s, normal range: 9.6–12.8 s), international normalized ratio (2.05, normal range: 0.88–1.15), activated partial thromboplastin time (46 s, normal range: 24.8–33.8 s), and levels of D-dimer (8.52 μg/ml, normal range: <200 ng/ml), fibrinogen degradation product (25.5 μg/ml, normal range: 0–5 μg/ml), cTNI (74.8 pg/ml), myohemoglobin (476.54 ng/ml), N-terminal brain natriuretic peptide (700.7 pg/ml, normal range: <100 pg/ml), alanine transaminase (ALT) (1,202 U/L, normal range: 9–50 U/L), aspartate transaminase (AST) (806 U/L, normal range: 15–40 U/L), total bilirubin (72.1 μmol/L, normal range: <26 μmol/L), direct bilirubin (40.8 μmol/L, normal range: <4 μmol/L), indirect bilirubin (31.3 μmol/L, normal range: <22 μmol/L), urea (27.97 mmol/L, normal range: 3.6–9.5 mmol/L), creatinine (203 μmol/L, normal range: 57–111 μmol/L), and uric acid (1,265 Μmol/L, normal range: 208–428 μmol/L). On September 4, 2022, the patient underwent preoperative preparation, and the right atrial and right ventricular masses were removed using tracheal intubation cardiopulmonary bypass. Pericardial mediastinal drainage was performed and a temporary cardiac pacemaker was implanted during surgery. Intraoperative findings included the following: (1) multiple irregular gray neoplasms in the right atrium, which was characterized by less smooth surfaces and a maximum diameter of approximately 7 cm. The base was located in the atrial septum, which had grown compact with the tricuspid valve without gaps. An increased number of mural thrombi were present around the tumor body of the right atrium, with some thrombi being embedded in the dressing muscle of the right atrium; (2) several new organisms with a diameter of approximately 3 cm were observed in the right ventricular chamber, which straddled the tricuspid valve opening, resulting in severe blockage of the tricuspid valve; (3) the tumor profile was yellow and fish-like; (4) some tumors were not densely adhered to the tricuspid septum, while the tricuspid ring was slightly enlarged, and the water injection test showed mild regurgitation. Postoperative pathological examination revealed a bunch of grayish white tissue with a total volume of 7.5 × 6.5 × 3 cm. Focal hemorrhage and necrosis were observed in this section. Microscopically, the lymphocytic phenotype was diffuse and scattered among histiocytes, small lymphocytes, and nuclear fragments. Immunohistochemical examination revealed the following: medium large heterogeneous lymphocyte CD20 (+), CD19 (+), CD22 (+), CD10 (−), BCL-6 (+), MUM-1 (+), CD30 (−), BCL-2 (+, 90%), C-MYC (+, 50%), Ki-67(+, 90%), CD3 (−), CD5 (−), CyclinD1 (−), TDT (−), p53 (+, 70%), and PCK (−). Fluorescence in situ hybridization analysis showed no separation rearrangement of BCL-6, BCL-2, and MYC. Therefore, the patient was diagnosed as having the non-germinal center B cell (non-GCB) subtype of diffuse large B-cell lymphoma. BCL-2, C-MYC double expression. Bone marrow biopsy and flow cytometry revealed no lymphocytic infiltration. On September 5, 2022, the patient presented elevated levels of ALT (204 U/L), AST (297 U/L), total bilirubin (100 μmol/L), direct bilirubin (35 μmol/L), indirect bilirubin (65 μmol/L), urea (27.88 mmol/L), creatinine (280 μmol/L), uric acid (1,045 μmol/L), as well as increased prothrombin time (17.8 s), international normalized ratio (1.46), and activated partial thromboplastin time (50.9 s), whereas prothrombin time activity (55%), and the level of plasma fibrinogen (1.93 g/L) were significantly reduced. A permanent pacemaker was placed after the patient stabilized.
On October 19, 2022, echocardiography revealed an enlarged left atrium and a hypoechoic mass extending from the right atrium to the right ventricle with an irregular shape and a size of approximately 85 × 54 mm was (Figure 3A). The boundary between the mass and tricuspid septum was unclear, while the atrial septum was infiltrated by the mass, suggesting tumor recurrence and severe tricuspid stenosis. The patient was administered an rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen. On November 1, 2022, echocardiography revealed the irregular shape of the right atrium, a solid mass measuring 25 × 21 mm, adhesion to the root of the tricuspid septum and lower atrial septum, enlargement of the left and right atria, widening of the aortic sinus, severe tricuspid regurgitation, and arrhythmia, with an ejection fraction of 71% (Figure 3B). Chest computed tomography showed exudation of the mediastinum and pericardium, slightly increased levels of blood and fluid, pneumonia, bilateral pleural effusion with atelectasis of the adjacent lung tissue, slightly narrowed left dorsal segment bronchus of the lower lobe of the lung due to compression, and aortic and coronary area calcification. Next-generation sequencing of the mass indicated a class I variation of CD79B (+), C.587A > C, which suggesting The adenine (A) at the 587th base position of the CD79B gene is replaced by cytosine (C). After three months post-surgery, the patient died due to severe lung infection following chemotherapy.
Figure 3
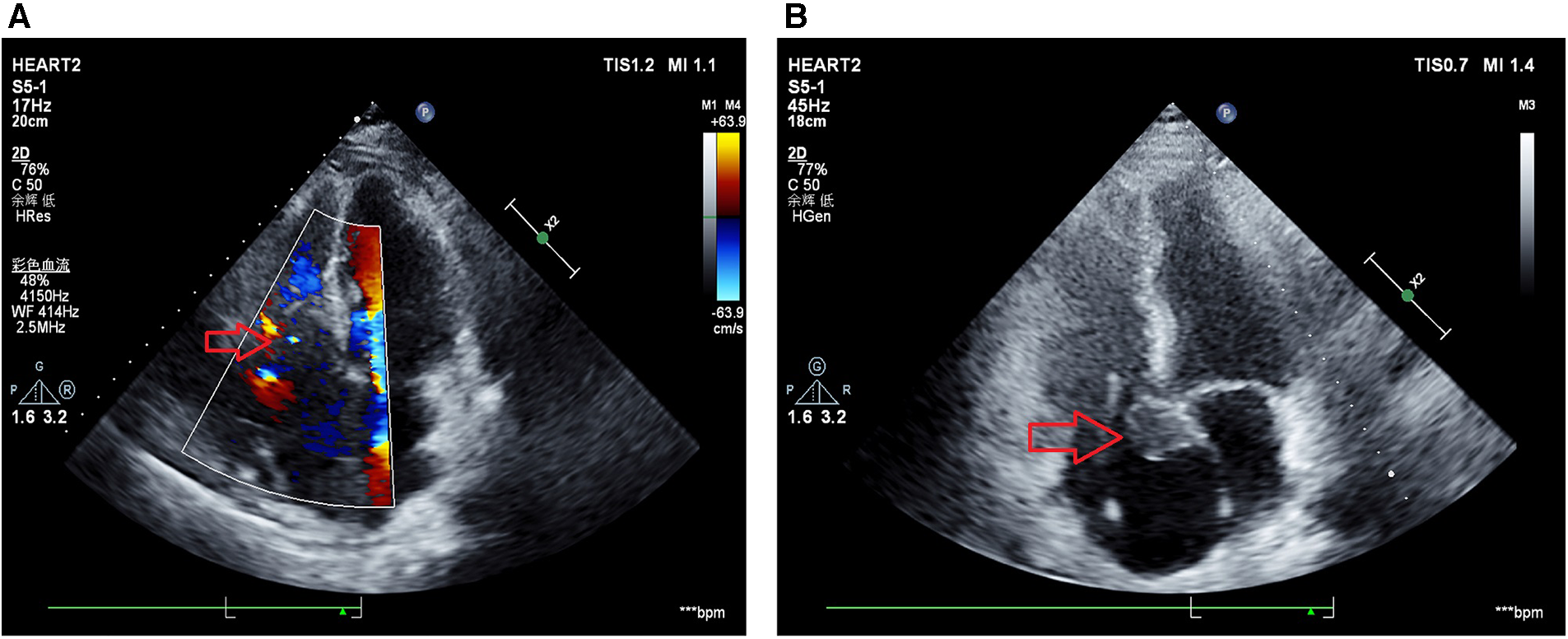
Echocardiography results after surgical resection. (A) Echocardiogram results at October 19, 2022 indicated a hypoechoic mass with irregular shape measuring approximately 85 mm × 54 mm is observed from the right atrium to the right ventricle. The boundary between the mass and the tricuspid valve is unclear, and the mass infiltrates the interatrial septum, suggesting tumor recurrence (with an increased size compared to pre-surgery) and severe tricuspid valve stenosis. (B) Echocardiogram results at November 1, 2022 indicated irregularly shaped solid mass in the right atrium, measuring 25 mm × 21 mm, adhering to the septal leaflet and inferior interatrial septum, with enlargement of the left and right atria, dilation of the aortic sinus, and severe tricuspid regurgitation.
3 Discussion
Primary cardiac lymphoma, of which diffuse B-cell lymphoma is the main pathological type, is extremely rare. The disease often involves the right atrium, right ventricle, atrial septum, and inferior vena cava. We reported a patient with highly aggressive CD20 (+) B-cell lymphoma, which invaded the right atrium, right ventricle, and atrial septum and involved the tricuspid septum, resulting in severe tricuspid stenosis. The clinical manifestations of primary cardiac lymphoma lack specificity and are closely related to tumor size, location, and complications. Right heart tumors can cause venous congestion, and their enlargement aggravates the disease. Tumors can invade the atrioventricular valve and cause stenosis or incomplete closure of the valve. The main clinical symptoms include congestive heart failure (31%–78%), chest pain (19%–46%), and arrhythmia (8%–56%) (8, 9). Other less common symptoms include the superior vena cava syndrome (10–12), odynophagia (6), and embolic phenomena (13). In this paper, we noted that the patient presented with dyspnea, edema, multiple serous effusions, hepatic and renal dysfunction, coagulation dysfunction, sinus rhythm, third-degree atrioventricular block, full-lead low voltage, QT interval prolongation, and a borderline escape rhythm.
Numerous studies have reported cases of primary cardiac lymphoma accompanied by atrioventricular block (Table 1) (14–25). The age of patients included in these studies ranged from 36.0 to 80.0 years, with 58.3% of them being men. Of the 12 included patients, 2 had primary cardiac T-cell lymphoma and most had complete atrioventricular block. All patients underwent surgical resection, pacemaker implantation, and chemotherapy, resulting in the complete remission or reduced mass size of tumors and a better prognosis. The primary treatment for cardiac lymphoma was chemotherapy; however, some patients presented with extensive myocardial involvement, which could cause sudden cardiac arrest (26, 27). Thus, surgical resection was performed first in patients at high risk of sudden cardiac events due to cardiac involvement. An alternative method for treating atrioventricular block was surgical epicardial lead implantation, particularly in patients presenting with broad cardiac involvement. Complete atrioventricular block was always caused by infiltration of the conduction system by primary cardiac lymphoma, and pacemaker lead implantation was always performed owing to bradycardia-induced heart failure (27).
Table 1
| Study | Gender | Age (years) | Disease status | Clinical symptoms | Imaging diagnosis | Treatments | Prognosis |
|---|---|---|---|---|---|---|---|
| Frikha et al. (14) | Male | 64.0 | Non-Hodgkin large B-cell lymphoma | Cardiac tamponade and paroxysmal third-degree atrioventricular block | Echocardiography, computed tomography, coronary angiography | Surgical resection and R-CHOP | The size of the mass was reduced |
| Crisel et al. (15) | Male | 55.0 | Diffuse large B-cell lymphoma | Complete atrioventricular block | Echocardiography, cardiac magnetic resonance imaging | Pacemaker implantation and R-EPOCH | No identifiable tumor |
| Chen et al. (16) | Male | 36.0 | Primary diffuse cardiac large B-cell lymphoma | Atrioventricular block and paroxysmal ventricular tachycardia | Echocardiography, computed tomography | COP, CHOP, and R-CHOP | Adequate global systolic and diastolic functions, with significant reduction of myocardial thickness and resolution of pericardial effusion |
| Wang et al. (17) | Female | 70.0 | Primary cardiac T-cell lymphoma | Complete atrioventricular block and torsades de pointes | Echocardiography, computed tomography | CHOP | Complete remission |
| Sekar et al. (18) | Male | 76.0 | High-grade non-Hodgkin's lymphoma of B-cell type | Acute coronary syndrome and atrioventricular block | Echocardiography, computed tomography | R-GCVP | Tumor recurrence |
| Jang et al. (19) | Male | 56.0 | Diffuse large B-cell lymphoma | Atrioventricular block | Echocardiography, cardiac magnetic resonance imaging, positron emission tomography-computed tomography | Surgical resection, pacemaker implantation, R-CHOP | Complete remission |
| Usry et al. (20) | Female | 80.0 | C-MYC positive, EBV-negative, Burkitt lymphoma | Symptomatic complete heart block | Echocardiography, cardiac magnetic resonance imaging, coronary angiography | Pacemaker implantation and R-CHOP | The size of the mass was reduced |
| Al Mawed et al. (21) | Female | 73.0 | Diffuse large B-cell non-Hodgkin lymphoma of non-germ-cell type | Atrial flutter and atrioventricular block | Echocardiography, cardiac magnetic resonance imaging, positron emission tomography-computed tomography | Pacemaker implantation, R-mini-CHOP and R-CHOP | Complete remission |
| Hu et al. (22) | Male | 70.0 | Non-germinal centre diffuse large B-cell lymphoma | Complete atrioventricular block | Echocardiography, computed tomography, positron emission tomography-computed tomography | R-EPOCH | The size of the mass was reduced |
| Chen et al. (23) | Female | 47.0 | Primary cardiac T-cell lymphoma | Complete atrioventricular block | Echocardiography, cardiac magnetic resonance imaging, computed tomography, positron emission tomography-computed tomography | MTX-CHOP | Complete remission |
| Shigeno et al. (24) | Female | 55.0 | Diffuse large B-cell lymphoma | Complete atrioventricular block | Echocardiography, computed tomography | Surgical resection, epicardial lead implantation, R-CHOP | Complete remission |
| Mao et al. (25) | Male | 64.0 | Primary diffuse cardiac large B-cell lymphoma | Complete atrioventricular block | Echocardiography, computed tomography, positron emission tomography-computed tomography | Pacemaker implantation, R-COP and R-CDOP | The size of the mass was reduced |
The summary results for primary cardiac lymphoma with atrioventricular block.
Accurate diagnosis at an early stage is particularly important for patients with primary cardiac lymphoma (28). Imaging examinations could be considered useful tools for detecting masses in the heart; however, imaging examinations do not provide a definitive diagnosis. However, echocardiography can provide information regarding the location, size, and activity of the tumor, tumor involvement in the atrioventricular valve, and cardiac function. Once echocardiography suggests a malignant tumor in the heart, computed tomography and cardiac magnetic resonance imaging can be performed to observe tumor infiltration and the relationship with surrounding tissues, providing information for surgical planning. Furthermore, positron emission tomography-computed tomography can comprehensively determine the presence of distant metastasis and the presence of other extracardiac tumors. These diagnostic tools can provide further information guiding personalized treatments. Of note, endomyocardial biopsy is considered a valuable tool for diagnosing intracardiac masses and arrhythmogenic cardiomyopathies (23). In our paper, the patient underwent echocardiography and computed tomography to detect the tumor location, while an endomyocardial biopsy was performed to assess the primary cardiac lymphoma.
4 Conclusions
This paper described the clinical course of a patient with a non-GCB subtype of diffuse large B-cell lymphoma diagnosed via endomyocardial biopsy. Echocardiography and computed tomography were used as imaging diagnostic tools, and the patient underwent surgical resection of the right atrial and right ventricular masses through tracheal intubation cardiopulmonary bypass. Furthermore, a permanent pacemaker was placed after the patient stabilized, followed by administration of R-CHOP as the chemotherapy regimen. After chemotherapy, the size of the tumor was reduced, indicating remission.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because standard care is performed in this case report. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. WZ: Data curation, Formal Analysis, Writing – review & editing. JX: Data curation, Formal Analysis, Writing – review & editing. YZ: Conceptualization, Formal Analysis, Project administration, Writing – review & editing. LT: Data curation, Formal Analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
He S Cao Y Qin W Chen W Yin L Chai H et al Prevalence of primary cardiac tumor malignancies in retrospective studies over six decades: a systematic review and meta-analysis. Oncotarget. (2017) 8:43284–94. 10.18632/oncotarget.17378
2.
Sheppard M Angelini A Raad M Savelieva I . Tumours of the heart. In: CammAJFLuscherTSerruysPW, editors. The ESC Textbook of Cardiovascular Medicine. London: Blackwell (2006). p. 535–52.
3.
Curtsinger CR Wilson MJ Yoneda K . Primary cardiac lymphoma. Cancer. (1989) 64:521–5. 10.1002/1097-0142(19890715)64:2%3C521::AID-CNCR2820640227%3E3.0.CO;2-T
4.
Zaharia L Gill PS . Primary cardiac lymphoma. Am J Clin Oncol. (1991) 14:142–5. 10.1097/00000421-199104000-00009
5.
Miguel CE Bestetti RB . Primary cardiac lymphoma. Int J Cardiol. (2011) 149:358–63. 10.1016/j.ijcard.2010.02.016
6.
Antoniades L Eftychiou C Petrou PM Bagatzounis A Minas M . Primary cardiac lymphoma: case report and brief review of the literature. Echocardiography. (2009) 26:214–9. 10.1111/j.1540-8175.2008.00757.x
7.
Montiel V Maziers N Dereme T . Primary cardiac lymphoma and complete atrio-ventricular block: case report and review of the literature. Acta Cardiol. (2007) 62:55–8. 10.2143/AC.62.1.2019372
8.
Gordon MJ Danilova O Spurgeon S Danilov AV . Cardiac non-Hodgkin’s lymphoma: clinical characteristics and trends in survival. Eur J Haematol. (2016) 97:445–52. 10.1111/ejh.12751
9.
Carras S Berger F Chalabreysse L Callet-Bauchut E Cordier J-F Salles G et al Primary cardiac lymphoma: diagnosis, treatment and outcome in a modern series. Hematol Oncol. (2017) 35:510–9. 10.1002/hon.2301
10.
Habertheuer A Ehrlich M Wiedemann D Mora B Rath C Kocher A . A rare case of primary cardiac B cell lymphoma. J Cardiothorac Surg. (2014) 9:14. 10.1186/1749-8090-9-14
11.
Johri A Baetz T Isotalo PA Nolan RL Sanfilippo AJ Ropchan G . Primary cardiac diffuse large B cell lymphoma presenting with superior vena cava syndrome. Can J Cardiol. (2009) 25:e210–2. 10.1016/S0828-282X(09)70110-2
12.
van Rooijen CR Scholtens AM de Jong CN Saraber CE van de Donk NWCJ . Primary cardiac lymphoma with central nervous system relapse. Clin Case Rep. (2017) 5:1454–8. 10.1002/ccr3.1094
13.
Liu X Liu P Li J . EBV+ diffuse large B-cell lymphoma arising within atrial myxoma in Chinese immunocompetent patient. Int J Clin Exp Pathol. (2015) 8:2159–64.
14.
Frikha Z Abid L Abid D Mallek S Frikha I Abdennadher M et al Cardiac tamponade and paroxysmal third-degree atrioventricular block revealing a primary cardiac non-Hodgkin large B-cell lymphoma of the right ventricle: a case report. J Med Case Rep. (2011) 5:433. 10.1186/1752-1947-5-433
15.
Crisel RK Knight BP Kim SS . Reversible, complete atrioventricular block caused by primary cardiac lymphoma in a nonimmunocompromised patient. J Cardiovasc Electrophysiol. (2012) 23:1386–9. 10.1111/j.1540-8167.2012.02343.x
16.
Chen K-W Chang J-H Yeh S-P Lu C-R . Primary cardiac B-cell lymphoma with atrioventricular block and paroxysmal ventricular tachycardia. J Cardiothorac Surg. (2012) 7:70. 10.1186/1749-8090-7-70
17.
Wang T-L Lai C-H Liou J-Y Lo H-M Shyu K-G . Complete AV block and torsades de pointes in a case of primary cardiac T-cell lymphoma. Acta Cardiol Sin. (2015) 31:245–8. 10.6515/acs20141117a
18.
Sekar B Swami G Ibrahim A Hanna A Payne MN Seale JR et al 76-year-old Gentlemen with primary cardiac lymphoma presenting as acute coronary syndrome and atrioventricular block. BJR Case Rep. (2016) 2:20150466. 10.1259/bjrcr.20150466
19.
Jang G-W Chung H Kim W-S Lee JM . Primary cardiac lymphoma manifesting as an atrioventricular block in a renal transplantation recipient. JACC Case Rep. (2020) 2:600–3. 10.1016/j.jaccas.2020.01.028
20.
Usry CR Wilson AS Bush KNV . Primary cardiac lymphoma manifesting as complete heart block. Case Rep Cardiol. (2020) 2020:3825312. 10.1155/2020/3825312
21.
Al Mawed M Brockmeier J Haertel D Ellermeier M Hartmann F Gielen S . From inoperable to back to life: a case report of successfully treated obstructive right ventricular primary cardiac lymphoma. Eur Heart J Case Rep. (2022) 6:ytac051. 10.1093/ehjcr/ytac051
22.
Hu B Zhao J Liang X Ren C Li N Liang C . A case of complete atrioventricular block associated with primary cardiac lymphoma reversed without cardiac pacemaker implantation. J Int Med Res. (2022) 50:3000605221089780. 10.1177/03000605221089780
23.
Chen P Hao Y Qiu X Xiao X Zhu W Xu Y et al Case report: primary cardiac T-cell lymphoma with complete atrio-ventricular block diagnosed by endomyocardial biopsy. Front Immunol. (2022) 13:890059. 10.3389/fimmu.2022.890059
24.
Shigeno R Okada T Koyama T Furukawa Y . Surgical resection and epicardial lead implantation for primary cardiac lymphoma with a complete atrioventricular block: a case report. Eur Heart J Case Rep. (2023) 7:ytad035. 10.1093/ehjcr/ytad035
25.
Mao J Xu Y Zhu M Wang L Hou Y . Case report: complete atrio-ventricular block successfully reversed in newly diagnosed primary cardiac B-cell lymphoma. Front Med. (2023) 10:1119286. 10.3389/fmed.2023.1119286
26.
Sultan I Aranda-Michel E Habertheuer A Kilic A Arnaoutakis G Bianco V et al Long-term outcomes of primary cardiac lymphoma. Circulation. (2020) 142:2194–5. 10.1161/CIRCULATIONAHA.120.047215
27.
Ottaviani G Matturri L Rossi L Jones D . Sudden death due to lymphomatous infiltration of the cardiac conduction system. Cardiovasc Pathol. (2003) 12:77–81. 10.1016/S1054-8807(02)00168-0
28.
Csizmar CM Sachs Z Cayci Z Bu L Linden MA . Primary cardiac lymphoma: three case reports and a review of the literature. Open J Blood Dis. (2021) 11:120–32. 10.4236/ojbd.2021.114012
Summary
Keywords
cardiac lymphoma, atrioventricular block, diffuse large B-cell lymphoma, case report, diagnosis, treatment
Citation
Liu J, Zheng Y, Zhang W, Xia J, Zhang Y and Tang L (2024) Case Report: A case of third-degree atrioventricular block associated with primary cardiac lymphoma. Front. Cardiovasc. Med. 11:1356134. doi: 10.3389/fcvm.2024.1356134
Received
15 December 2023
Accepted
05 February 2024
Published
28 February 2024
Volume
11 - 2024
Edited by
Monica Cattaneo, MultiMedica (IRCCS), Italy
Reviewed by
Giulia Iannaccone, Catholic University of the Sacred Heart, Italy
Wilhelm Mistiaen, University of Antwerp, Belgium
Updates
Copyright
© 2024 Liu, Zheng, Zhang, Xia, Zhang and Tang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Yongheng Zhang mfqq_258383@sohu.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.