Abstract
Cogan's syndrome (CS) is recognized as a form of variable vasculitis. This report presents the case of a middle-aged woman experiencing recurrent coronary artery stenosis, accompanied by a history of non-syphilis keratitis, vestibular auditory symptoms, and venous thrombosis. Positron emission tomography/computed tomography revealed an elevated uptake of (18)F-fluorodeoxyglucose in the subclavian artery, common carotid artery, aortic arch, and thoracic aorta. A diagnosis of Cogan's syndrome was made. The aim of this study was to increase clinicians’ awareness of the vascular manifestations in CS and to emphasize the importance of thorough history taking. CS should be included in the differential diagnosis when patients present with recurrent coronary artery stenosis.
Introduction
Cogan's syndrome (CS) is a rare autoimmune disease, often categorized as a variable vasculitis, and is most commonly diagnosed during the third or fourth decade of life, with a similar prevalence in women and men. The presence of non-syphilitic keratitis and vestibuloauditory symptoms within 2 years is indicative of typical CS (1). Aortitis may affect approximately 10% of CS patients (2). This case report highlights a rare case of coronary artery stenosis in the context of CS, underscoring the need for vigilance in clinical practice.
Case description
A 49-year-old female patient was admitted in 2021 with a complaint of chest tightness and a shortness of breath, symptoms that had been present for a year and were exacerbated by satiety and physical activity. She was diagnosed with non-ST segment elevation myocardial infarction with stenosis of the left main trunk (LM) and right coronary artery (RCA) (Figure 1) and had undergone percutaneous trans-luminal coronary angioplasty and intervention, followed by secondary prevention and treatment for coronary heart disease. Recently, the aforementioned symptoms have re-emerged. The patient reported no fever, rash, oral or genital ulcers, erythema nodosum, or intermittent claudication.
Figure 1
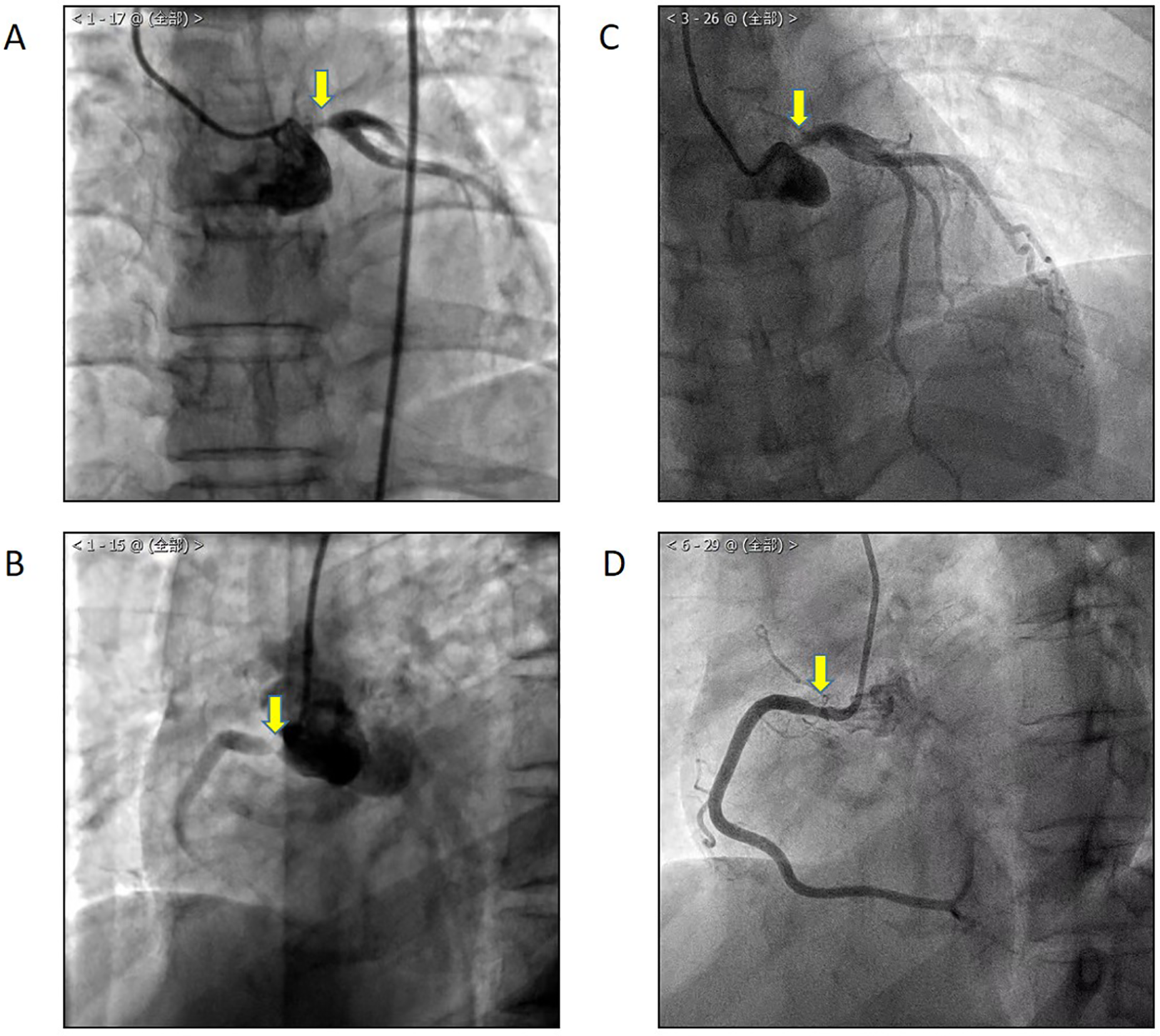
In 2020, CAG showed LM (A) and RCA (B) stenosis. One year after percutaneous coronary intervention, the patient suffered from chest tightness and a shortness of breath again, and CAG showed LM (C) and RCA (D) in-stent restenosis.
A comprehensive medical history (Figure 2) revealed a history of sudden hearing loss, vertigo, and nausea, with recurrent episodes from 9 years ago. She had experienced episodes of redness and blurred vision in both eyes 8 years ago, which improved with antibiotic and corticosteroid treatment. Over the years, she had also suffered from symptoms of vertigo and blurred vision, accompanied by photophobia, which were alleviated with glucocorticoid use, although her hearing progressively declined. In addition, she had a history of limb venous thrombosis, which responded to anticoagulant treatment. There were no special family and psychosocial histories and genetic disorders.
Figure 2
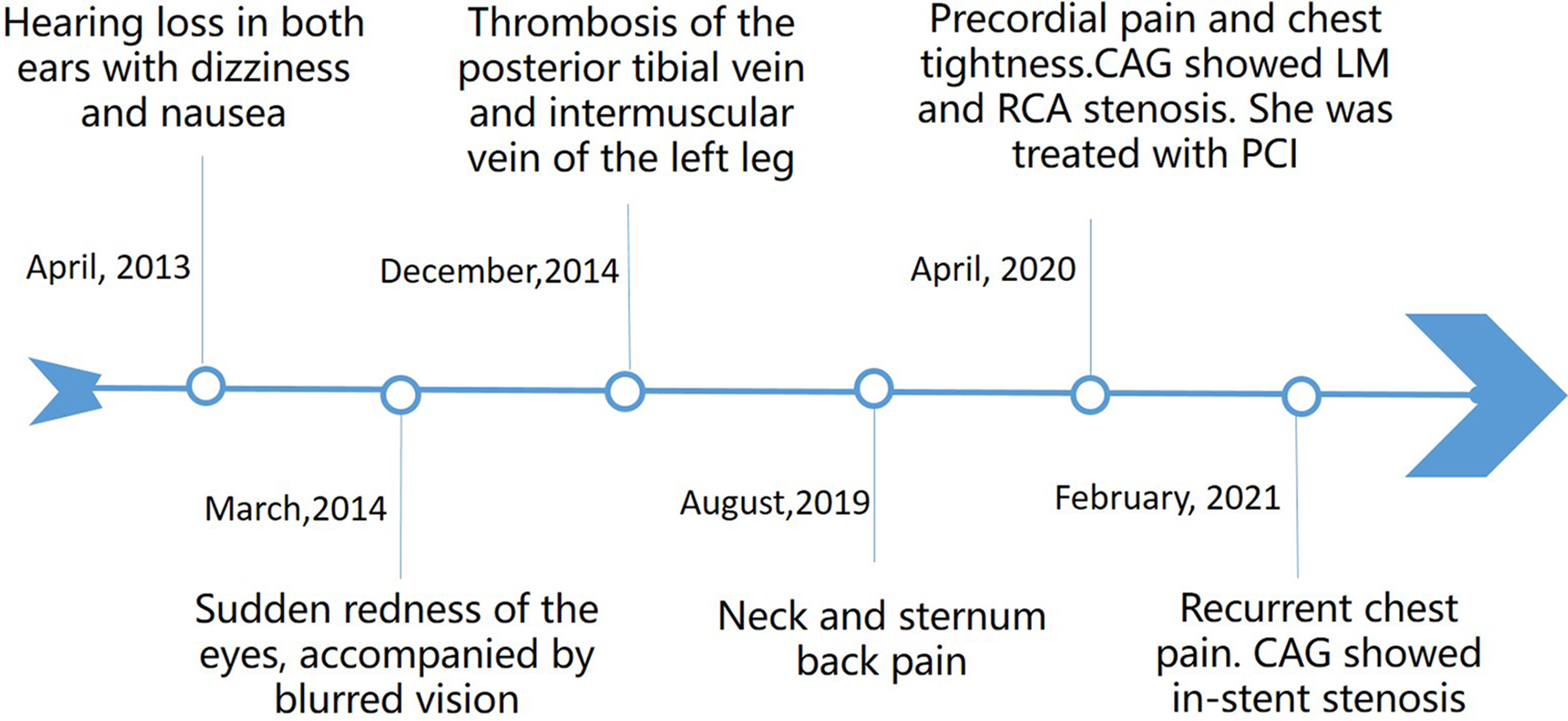
A timeline with the relevant data from the episode of care.
Physical examination findings included a nebula on the lateral edge of the cornea in both eyes, decreased hearing in both ears, and reduced pulsation in the radial, brachial, and dorsal pedal arteries, particularly on the right side. Vascular murmurs were detected in the auscultation areas of the bilateral carotid, subclavian, renal, and iliac arteries and abdominal aorta.
In laboratory tests, the erythrocyte sedimentation rate was 97 mm/1 h, C-reactive protein was above 20 mg/L, complement C3 was 1.72 g/L, complement C4 was normal, immunoglobulin and serum immunoglobulin G4 were normal, and IL-6 was 49.67 pg/ml. Antinuclear antibody profile, neutrophil cytoplasmic antibody, antiphospholipid antibody, interferon-gamma release assay for mycobacterium tuberculosis, syphilis antibody, and tumor marker tests were all negative, and n-terminal prohormone B-type natriuretic peptide was 185.00 pg/ml.
Upon review, coronary angiography (CAG) showed in-stent restenosis (Figure 1). Echocardiography revealed thinning of the left ventricular inferior wall basal segment, mild aortic regurgitation, and decreased left ventricular diastolic function. Positron emission tomography/computed tomography (PET/CT) demonstrated an increased uptake of (18)F-fluorodeoxyglucose (18F-FDG) in the aorta and its primary branches, as well as in the coronary arteries (Figure 3).
Figure 3
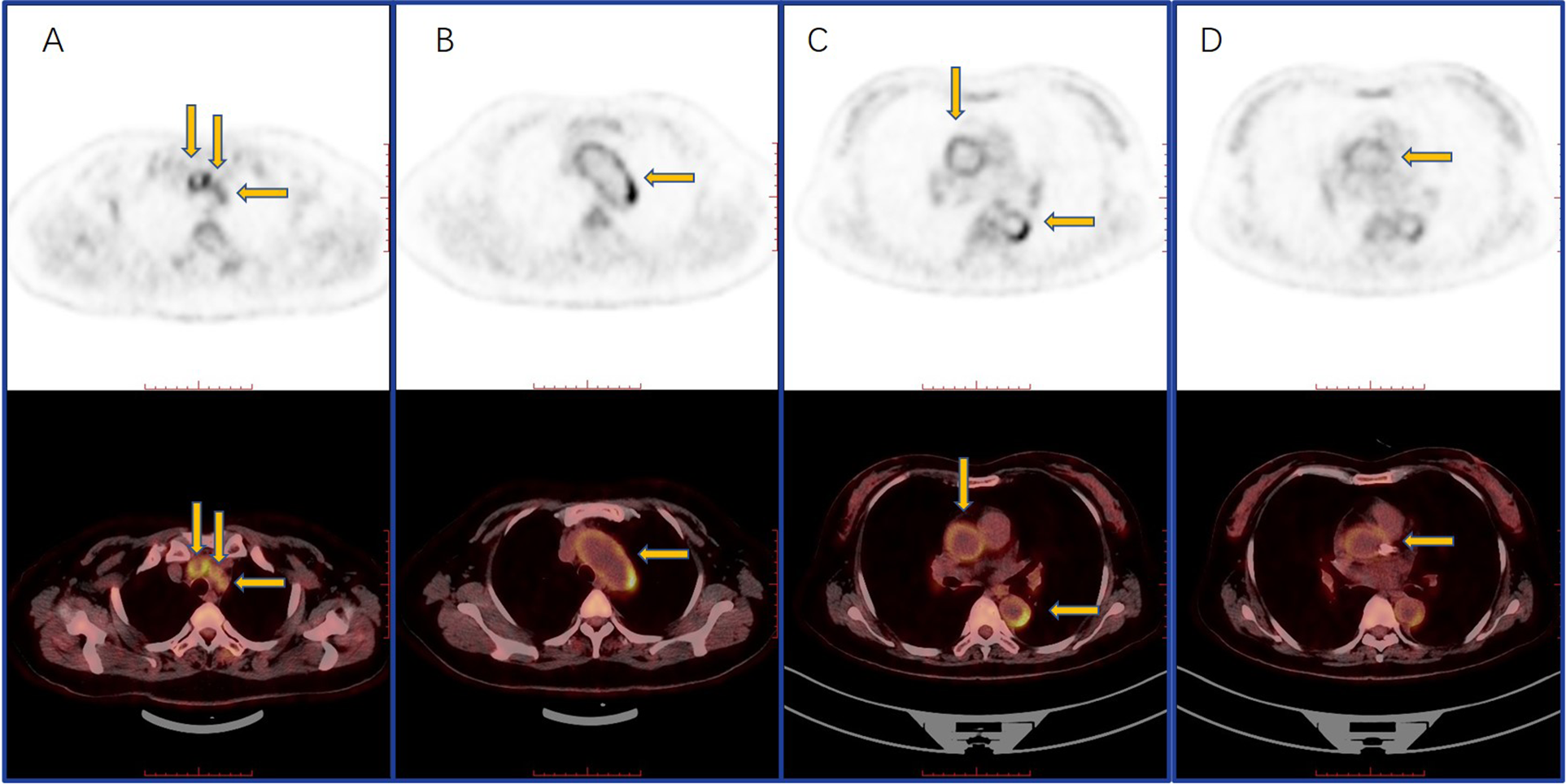
PET-CT showed an increased circular and linear 18F-FDG uptake in the subclavian and common carotid arteries (A), aortic arch (B), thoracic aorta (C), and coronary (D).
Given the patient's ocular, auditory, vestibular, and vascular symptoms, a diagnosis of CS was confirmed. We conducted a differential diagnosis to rule out the following conditions: (1) Takayasu’s arteritis: the patient presented with multiple arterial stenoses, necessitating the consideration of Takayasu’s arteritis. However, the occurrence of stromal keratitis and sensorineural hearing loss did not align with the typical manifestations of this condition. (2) Antiphospholipid syndrome: the patient's history of recurrent thrombosis and multiple arterial stenoses raised the possibility of antiphospholipid syndrome. However, the absence of phospholipid antibodies in all tests negated this diagnosis. (3) Behçet's syndrome: despite the need to consider Behçet's syndrome given the patient's symptoms, the lack of oral and genital ulcers, erythema nodosum, and other characteristic skin changes, as well as the absence of ocular involvement, did not fulfill the criteria for this diagnosis.
The patient was managed conservatively without undergoing repeat percutaneous coronary intervention (rePCI) or bypass surgery involving the internal mammary artery (IMA) to the left anterior descending artery (LAD). The patient was managed conservatively with aspirin, clopidogrel, and isosorbide mononitrate. In tandem with this approach, we initiated a therapeutic regimen that included a combination of corticosteroids and cyclophosphamide to address vasculitis at the same time. The treatment began with prednisone at a dosage of 50 mg once daily, complemented by intravenous cyclophosphamide at 0.4 g administered biweekly. Subsequently, we implemented a gradual reduction in prednisone and transitioned to methotrexate at 15 mg weekly to alleviate the potential long-term adverse effects associated with cyclophosphamide.
The patient maintained remission over the subsequent 3 years, with no recurrence of chest pain, stable vision and hearing, and no further episodes of venous thrombosis. Laboratory tests and coronary angiography remained stable. The patient had good adherence and tolerability to the intervention, and no adverse or unanticipated events occurred.
Discussion
This case highlights the importance of considering Cogan's syndrome in the differential diagnosis for patients presenting with recurrent coronary artery stenosis. The patient's history of ocular abnormalities, hearing loss with vertigo, and subsequent development of multiple artery stenoses and venous thrombosis, along with PET/CT (a valuable tool in the diagnostic and therapeutic management of vasculitis due to its comprehensive and detailed imaging capabilities) findings of a high 18F-FDG uptake in the aorta and its branches, supports this diagnosis. We conducted a differential diagnosis to rule out other conditions. The limitation was that the patient was not assessed using intravascular ultrasound (IVUS) or fractional flow reserve (FFR) during the coronary angiography. A previous study (3) has suggested that the histological presence of inflammatory cells in the vessel walls of CS patients may be indicative of the disease's vasculitic nature, which can be managed with immunosuppressive therapy (4). When faced with such cases, clinicians are encouraged to conduct thorough history taking and consider CS as part of the differential diagnosis.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Anzhen Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GN: Data curation, Funding acquisition, Writing – original draft. ZN: Data curation, Resources, Writing – review & editing. MJ: Data curation, Writing – review & editing. PL: Supervision, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Beijing Municipality (7222043 and 7232038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CS, Cogan's syndrome; CAG, coronary angiography; LM, left main trunk; PET/CT, positron emission tomography/computed tomography; 18F-FDG, (18)F-fluorodeoxyglucose.
References
1.
Espinoza GM Wheeler J Temprano KK Keller AP . Cogan’s syndrome: clinical presentations and update on treatment. Curr Allergy Asthma Rep. (2020) 20(9):46. 10.1007/s11882-020-00945-1
2.
Kim JS Park JB Joo JC Seol MD Yoon JW Park HK et al A case of Cogan’s syndrome with angina. Korean Circ J. (2010) 40(12):680–3. 10.4070/kcj.2010.40.12.680
3.
Stone JR Bruneval P Angelini A Bartoloni G Basso C Batoroeva L et al Consensus statement on surgical pathology of the aorta from the society for cardiovascular pathology and the association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol. (2015) 24(5):267–78. 10.1016/j.carpath.2015.05.001
4.
Mora P Calzetti G Ghirardini S Rubino P Gandolfi S Orsoni J. Cogan’s syndrome: state of the art of systemic immunosuppressive treatment in adult and pediatric patients. Autoimmun Rev. (2017) 16:385–90. 10.1016/j.autrev.2017.02.009
Summary
Keywords
Cogan’s syndrome, variable vasculitis, recurrent coronary stenosis, case report, coronary angiography
Citation
Na G, Nan Z, Jingjing M and Lili P (2024) A case report of Cogan's syndrome with recurrent coronary stenosis. Front. Cardiovasc. Med. 11:1451113. doi: 10.3389/fcvm.2024.1451113
Received
18 June 2024
Accepted
26 August 2024
Published
12 September 2024
Volume
11 - 2024
Edited by
Tommaso Gori, Johannes Gutenberg University Mainz, Germany
Reviewed by
Pawel Latacz, The Brothers of Saint John of God Hospital, Poland
Aparna Irodi, Christian Medical College and Hospital, India
Updates
Copyright
© 2024 Na, Nan, Jingjing and Lili.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Pan Lili lilypansxmu@sina.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.