Abstract
Background:
Growing evidence suggests that growth differentiation factor-15 (GDF-15) may contribute to adverse clinical outcomes, such as major cardiovascular events and all-cause mortality. However, there is little information about its relationship to hypertension. This meta-analysis aimed to quantitatively evaluate the relationship between circulating GDF-15 and hypertension prevalence.
Methods:
Databases searched included PubMed, Web of Science, and Embase, from inception to August 2024. The inclusion criteria were studies reporting hypertension prevalence in at least three GDF-15 categories.
Result:
A total of 24 studies from 21 articles with 35,904 participants and 23,925 hypertensive cases were included in this meta-analysis. Compared with individuals with a low level of circulating GDF-15, those with high GDF-15 level had a higher prevalence of hypertension [odds ratios (OR) 1.60, 95% confidence interval (CI) 1.37–1.88, P < 0.001). In the dose-response meta-analysis, the prevalence of hypertension increased by 24% with every 1 ng/ml increase in GDF-15 (OR 1.24, 95% CI 1.16–1.33, P < 0.001). However, the dose-response curve was nonlinear (P-nonlinearity < 0.001), plateauing or even decreasing slightly after GDF-15 concentrations reached approximately 5.5 ng/ml. Significant heterogeneity was detected in the pooled analysis and meta-regression analysis suggested that participants’ age and the prevalence of diabetes significantly accounted for the heterogeneity.
Conclusions:
Circulating GDF-15 is positively and non-linearly associated with the prevalence of hypertension, with a plateau or slight decline after reaching a certain GDF-15 dose.
Systematic Review Registration: https://inplasy.com/inplasy-2023-3-0082/, identifier (INPLASY202330082).
Introduction
Hypertension has always been a major public health issue that affects over a billion people worldwide (1). Elevated blood pressure is the leading risk factor for cardiovascular diseases (CVD) and chronic kidney disease (CKD) and their associated mortality (2). Unfortunately, the pathogenesis of hypertension is complex and has not yet been fully elucidated (3). Many risk factors contribute to the development of hypertension, including genetic, pathophysiological, environmental and lifestyle factors (4, 5). Thus, it is necessary to identify suitable biomarkers for predicting the development of hypertension.
Growth differentiation factor-15 (GDF-15) is a member of the transforming growth factor β superfamily that is widely expressed in multiple mammalian tissues, including liver, kidney, prostate, intestinal mucosa (6). Evidence suggests that GDF-15 regulates appetite, energy balance, body weight, lipid metabolism, and immune function, and protects the body from oxidative stress, inflammation, and damage (7, 8). GDF-15 is sensitive to external stimuli with a rapid increase in its circulating levels, and therefore it is well-established as a stress-responsive factor (9). Numerous prospective studies have shown that an elevated level of circulating GDF-15 is a powerful predictor of incident adverse clinical outcomes (e.g., major cardiovascular events and all-cause death) (10–12). However, the ability of GDF-15 to predict the risk of developing hypertension in prospective studies has not been well-studied. In a population-based prospective cohort study, circulating GDF-15 was significantly and positively associated with hypertension at baseline examination, and it was also an independent predictor of hypertensive heart failure during long-term follow-up (13). A recent cross-sectional study showed that circulating GDF-15 levels were significantly higher in patients with grade 2 hypertension than those with grade 1 hypertension and healthy individuals (14). These findings suggest that circulating GDF-15 may be used as a candidate predictor for incident hypertension.
Despite the lack of prospective studies exploring the relationship between circulating GDF-15 and the risk of hypertension, the past dozen years have seen numerous studies providing information on hypertension prevalence across different GDF-15 concentration intervals in different populations. In this study, we performed a dose-response meta-analysis based on all eligible studies to quantitatively evaluate the relationship between circulating GDF-15 and the prevalence of hypertension.
Materials and methods
The current systematic review followed PRISMA reporting guidelines (15), and the protocol was registered on INPLASY (registration number: INPLASY202330082). Due to the fact this was a systematic review, no ethics approval was needed.
Search strategy
Three databases, including PubMed, Embase, and Web of Science, were systematically searched through August 30, 2024. We developed a search strategy by combining the following medical subject headings and free text words: (“hypertension” or “high blood pressure” or “blood pressure”) AND (“GDF-15” or “growth differentiation factor-15” or “MIC-1” or “macrophage inhibitory cytokine-1” or “NAG-1” or “NSAID activated gene-1”). Additionally, a manual search of reference lists of included articles was conducted to identify additional eligible studies.
Study selection
The studies were selected according to the PICOS framework with the following criteria: Population: no restrictions; Exposure: stratification into three or more categories based on GDF-15 levels; Comparators: the highest GDF-15 quantile vs. the remaining quantiles or per 1 ng/ml increment; Outcomes: prevalence of hypertension; Study design: no restrictions. Two researchers independently searched the literature using the standardized screening process. They first screened titles and abstracts against predetermined criteria. Then, in the second phase, they thoroughly evaluated the full texts of potentially relevant studies. Discrepancies between reviewers were arbitrated through consultation with a senior investigator to achieve consensus. Exclusion criteria were as follows: (1) there were no circulating GDF-15 categories or they were only divided into two categories, which made it impossible to perform the dose-response analysis; (2) there were no reports of hypertensive cases in each of the GDF-15 categories, or the number could not be calculated; (3) circulating GDF-15 concentrations cannot be converted to ng/ml, or were not expressed as such; (4) the types of studies were conference abstracts, case reports, reviews, comments, or editorials; (5) the samples were overlapped with those from another study; and (6) non-English language articles. We employed the Cohen's Kappa statistic to assess the consistency between the two reviewers in the selection process. A Kappa value of ≤0.2 signified poor consistency, 0.21–0.40 fair consistency, 0.41–0.60 moderate consistency, 0.61–0.80 good consistency, and 0.81–1.00 very good consistency (16).
Data extraction and quality evaluation
Two investigators extracted the data and assessed the methodological quality independently, and a third investigator resolved disagreements. We extracted data on the first author, publishing year, location, study design, sample type, GDF-15 assay, and the definition of hypertension. Several participant characteristics were also recorded, including the source population, sample size, age of the participants, the percentage of male participants, body mass index (BMI), smoking status, and diabetes prevalence. Additionally, we extracted the number of hypertensive cases and participants, and GDF-15 concentrations by category. In order to evaluate the methodological quality of selected studies, the Newcastle-Ottawa Scale (NOS) was used, which consists of three domains: selection, comparability, and outcome (17). We classified studies scoring 7–9 points as high-quality, 4–6 points as moderate-quality, and 0–3 points as low-quality.
Statistical analysis
As a first step, a random-effects meta-analysis was conducted to compare hypertension prevalence among individuals with high and low GDF-15 levels. In this analysis, the high layer is defined as circulating GDF-15 levels in the highest tertiles, quartiles, or quintiles, and low layers are the remaining quantiles. Pooled effect sizes were presented odds ratio (OR) and 95% confidence interval (95% CI).
Based on previously reported methods (18, 19), a dose-response relationship between circulating GDF-15 and hypertension prevalence was investigated. Study-specific ORs and 95% CIs were first estimated for every 1 ng/ml increase in GDF-15 concentration in each study, then these effect sizes were pooled using a random-effects model. For this purpose, the median or mean level of GDF-15, the number of hypertensive patients, and the number of participants in each category are required. When GDF-15 dosages were given as a range, we took the middle point. If open ended lowest or highest GDF-15 categories are reported, the midpoint is estimated by assuming that the width of the category equals that of the adjacent one. In the absence of information about the number of hypertensive cases or participants in each GDF-15 category, we assumed that these individuals were equally assigned into each group. We explored the possibility of the nonlinear relationship between circulating GDF-15 and hypertension prevalence by modeling exposure levels using restricted cubic splines with three knots at fixed percentiles (10%, 50% and 90%) of GDF-15 distribution. A nonlinear P-value was calculated by determining whether the second spline coefficient of the model was equal to zero.
I 2 statistic was used to assess heterogeneity, where a value of I2 greater than 50% indicated statistical heterogeneity. In the case of I2 > 50%, a random-effects model would be used; otherwise, a fixed-effects model would be adopted. Pre-specified subgroup and meta-regression analyses were carried out to evaluate potential sources of heterogeneity. By omitting 1 study at a time, sensitivity analysis was performed. An Egger's test and funnel plot were used to assess publication bias.
Statistical analyses were performed using Stata software (version 15.0; StataCorp LP, USA). For this meta-analysis, we employed the following Stata commands: “metan” for primary effect size estimation, “metabias” for publication bias assessment, “metaninf” for sensitivity analyses, “metreg” for meta-regression, and “glst” for dose-response analysis. P < 0.05 was considered statistically significant.
Results
Literature search
We initially found 1,156 records in PubMed, Web of Science, and Embase databases, as well as 9 additional records in other sources. After removing 492 duplicates, we screened 673 articles for eligibility. By reviewing titles and abstracts, we excluded 622 records. Of the 51 studies remaining for full-text evaluation, 30 were further excluded: 26 for lack of relevant outcomes, 2 for insufficient GDF-15 categories, 1 for inappropriate GDF-15 units, and 1 for missing categorical data. Finally, a total of 21 articles containing 24 independent studies were included in our meta-analysis (Figure 1) (20–40). The Kappa score for screening titles and abstracts was 0.90, and for full-text screening, it was 0.93, indicating “very good” interrater agreement.
Figure 1
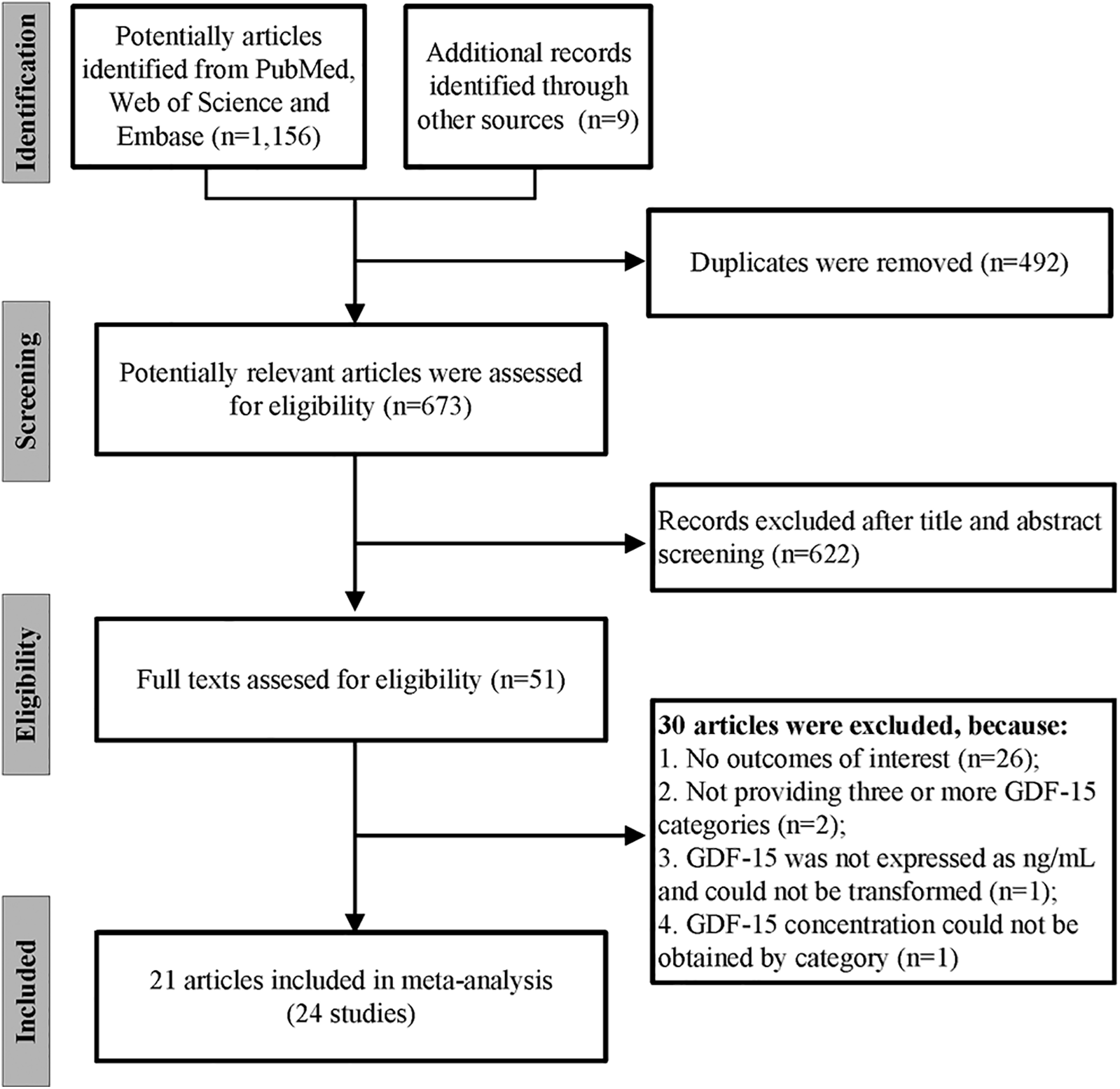
The flow chart of the study selection process.
Characteristics of selected studies
Table 1 summarizes the characteristics of 24 studies from 21 articles. Studies were published from 2009 to 2023, covering 35,904 participants, of whom 21,514 (59.9%) were men. In the 24 studies included, 5 were conducted in Europe (Sweden, Spain, Norway, Germany), 9 in the USA, 9 in Asia (Singapore, Japan, China, Taiwan), and 1 in South Africa. All studies were prospective cohort design, except for one (26) which was a cross-sectional design. The study population involved both clinical and non-clinical populations, including community-dwelling populations, patients with CVD, CKD, and other diseases. The concentration of circulating GDF-15 in the blood was measured by immunoradiometric assay (IRMA) in 2 studies, enzyme-linked immunosorbent assay (ELISA) in 12 studies, electro chemiluminescence immunoassay (ECLIA) in 8 studies, latex turbidimetric immunoassay (LTIA) in 1 study, and Milliplex map kits in 1 study. Half of the studies used serum samples, and the other half used plasma samples. Ten studies gave a definition of hypertension, including systolic blood pressure (SBP) ≥140 mm Hg, diastolic blood pressure (DBP) ≥90 mm Hg, the use of antihypertensives medications, electronic medical records, or self-report. All studies reported the age of participants, while most included studies also reported BMI, smoking prevalence, and diabetes prevalence.
Table 1
| Author/year | Region | Study design | Study population | Sample size (% male) | Sample types | GDF-15 assays | Definition of hypertension | Age, year | BMI, kg/m2 | Smoking, % | Diabetes, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lind (2009) (20) | Sweden | Cohort study | Community residents aged 70 years | 1,004 (50.0) | Serum | IRMA | SBP ≥140 mm Hg, DBP ≥90 mm Hg, or using antihypertensive drugs | 70.0 | 26.6 | 10.8 | 11.8 |
| Bonaca (2011) (21) | USA | Cohort study | Patients after ACS | 3,501 (78.9) | Serum | IRMA | N/A | 58.1 | 29.5 | 36.3 | 17.4 |
| Rohatgi (2012) (22) | USA | Cohort study | Community-living adults | 3,219 (45.0) | Plasma | ELISA | SBP ≥140 mm Hg, DBP ≥90 mm Hg, or using antihypertensive drugs | 43.3 | 29.4 | 29.9 | 11.5 |
| Wallentin (2013) (23) | Sweden | Cohort study | Community-living elderly | 940 (100) | Plasma | ECLIA | N/A | 71.0 | 26.2 | 21.0 | 10.7 |
| Cotter (2015) (24) | USA | Cohort study | Patients admitted for acute heart failure | 1,088 (62.6) | Serum | ECLIA | N/A | 72.1 | 29.2 | 12.8 | 48.3 |
| Chan (2016) (HFrEF) (25) | Singapore | Cohort study | Patients with heart failure | 730 (84.7) | Plasma | ELISA | N/A | 59.8 | 26.0 | N/A | 55.1 |
| Chan (2016) (HFpEF) (25) | Singapore | Cohort study | Patients with heart failure | 186 (43.0) | Plasma | ELISA | N/A | 68.3 | 27.8 | N/A | 56.5 |
| Martinez (2017) (26) | USA | Cross-sectional study | Patients with COPD | 694 (52.9) | Plasma | ELISA | Self-report or using antihypertensive drugs | 63.6 | 27.3 | 29.8 | 8.7 |
| Nair (2017) (C-PROBE cohort) (27) | USA | Cohort study | Non-dialysis CKD patients | 224 (40.0) | Plasma | ELISA | SBP ≥140 mm Hg, DBP ≥90 mm Hg, or using antihypertensive drugs | 58 | N/A | 12.5 | 38.8 |
| Nair (2017) (SKS cohort) (27) | USA | Cohort study | Non-dialysis CKD patients | 297 (82.9) | Plasma | ELISA | SBP ≥140 mm Hg, DBP ≥90 mm Hg, or using antihypertensive drugs | 62 | N/A | 15.7 | 56.3 |
| Tuegel (2018) (28) | USA | Cohort study | Non-dialysis CKD patients | 618 (60.7) | Plasma | ELISA | N/A | 58.5 | 31.6 | 13.6 | 45.1 |
| Sanchis (2019) (29) | Spain | Cohort study | Elderly patients with ACS | 208 (54.8) | Plasma | ECLIA | N/A | 78.3 | N/A | 10.1 | 48.1 |
| Zelniker (2019) (30) | USA | Cohort study | Patients with NSTE-ACS | 4,330 (64.8) | Plasma | ECLIA | N/A | 64.0 | N/A | 25.0 | 32.4 |
| Myhre (2020) (31) | Norway | Cohort study | COVID-19 patients | 123 (58.0) | Serum | ECLIA | Electronic medical records | 59.6 | 28.3 | 4.8 | 17.1 |
| Oba (2020) (32) | Japan | Cohort study | Patients with cardiometabolic disease | 275 (39.0) | Serum | LTIA | N/A | 78 | 23 | 10.0 | 52.0 |
| Arnold (2020) (33) | Germany | Cohort study | Patients with osteoarthritis | 636 (35.7) | Serum | ECLIA | Self-report | 65.0 | 27.8 | 11.6 | 8.8 |
| Wada (2020) (34) | Japan | Cohort study | Patients with suspected or known CAD | 2,418 (67.2) | Serum | ELISA | N/A | 70.6 | 24.3 | 17.7 | 45.0 |
| Vermeulen (2020) (35) | South Africa | Cohort study | Apparently healthy adults | 1,189 (48.2) | Serum | Milliplex MAP kits | N/A | 24.6 | 25.1 | 23.8 | N/A |
| Negishi (2021) (36) | Japan | Cohort study | Outpatients with cardiovascular risk factors | 3,562 (46.0) | Serum | ECLIA | SBP ≥140 mm Hg, DBP ≥90 mm Hg, or using antihypertensive drugs | 65.0 | 24.2 | 12.1 | 24.5 |
| Chang (2021) (37) | Taiwan | Cohort study | Hemodialysis patients | 170 (54.1) | Plasma | ELISA | N/A | 63.6 | N/A | N/A | 44.7 |
| Echouffo (2021) (38) | USA | Cohort study | Community-living elderly | 3,792 (41.0) | Serum | ECLIA | SBP ≥130 mm Hg, DBP ≥80 mm Hg, or using antihypertensive drugs | 80.0 | 28.3 | 6.8 | 33.8 |
| Yang (2022) (Diabetic cohort) (39) | China | Cohort study | Patients with ischemic stroke | 978 (59.5) | Serum | ELISA | N/A | 62.4 | N/A | 30.7 | 100.0 |
| Yang (2022) (Non-diabetic cohort) (39) | China | Cohort study | Patients with ischemic stroke | 2,023 (66.5) | Serum | ELISA | N/A | 62.3 | N/A | 40.1 | 0.0 |
| Wang (2023) (40) | China | Cohort study | Patients with CAD | 3,699 (72.0) | Plasma | ELISA | SBP ≥140 mm Hg, DBP ≥90 mm Hg, or using antihypertensive drugs | 61.3 | 25.5 | 45.7 | 31.9 |
The general characteristics of the included studies.
HfrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; C-PROBE, clinical phenotyping and resource biobank; SKS, Seattle kidney study; ACS, acute coronary syndrome; NSTE-ACS, non-ST-segment elevation acute coronary syndromes; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; CAD, coronary artery disease; IRMA, immunoradiometric assay; ELISA, enzyme-linked immunosorbent assay; ECLIA, electro chemiluminescence immunoassay; LTIA, latex turbidimetric immunoassay; FPG, fasting plasma glucose; BMI, body mass index.
The number of hypertensive cases and participants as well as the concentration of GDF-15 in each category were displayed in Supplementary Table 1, where 13 studies had three GDF-15 categories, and 11 had four categories. The total number of hypertensive cases in all the studies was 23,925.
Based on NOS scale, 2 studies (29, 37) were rated as moderate quality (NOS score = 6), and the remaining 22 were rated as high quality (NOS score ≥7). Details of the appraisal are shown in Supplementary Table 2.
Hypertension prevalence in high vs. low circulating GDF-15 concentration
We first performed a two-class meta-analysis, in which the first highest GDF-15 quantile was classified as the high layer, while the remaining GDF-15 quantiles were categorized as the low layer. Based on the pooled analysis, participants in the high GDF-15 layer were 1.60 times more likely for incident hypertension compared with individuals in low GDF-15 layer (OR 1.54, 95% CI 1.37–1.88, P < 0.001; Figure 2). A random-effects model was used due to the significant heterogeneity (I2 = 82.7%).
Figure 2
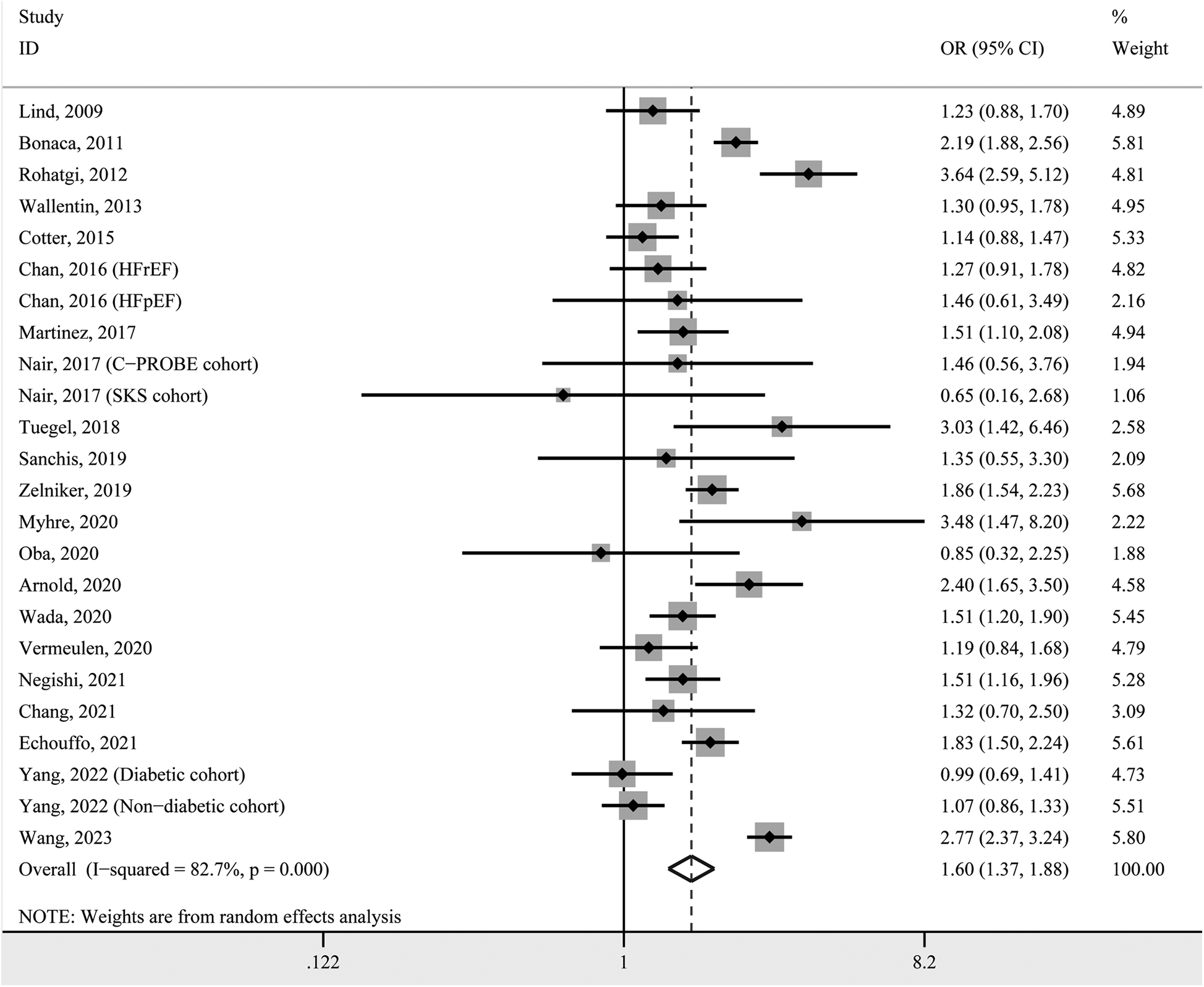
Forest plot of hypertension prevalence in individuals with high GDF-15 concentration vs. those with low GDF-15 concentration. OR, odds ratio; CI, confidence interval.
Dose-response correlation between circulating GDF-15 concentration and hypertension prevalence
To obtain a pooled effect size for every 1 ng/ml increase in circulating GDF-15, we calculated study-specific ORs and 95% CIs in each included study and then pooled them. The pooled result indicated that the prevalence of hypertension increased by 24% with each 1 ng/ml increase in circulating GDF-15 (OR 1.24, 95% CI 1.16–1.33, P < 0.001; Figure 3). Due to significant heterogeneity (I2 = 91.5%), these effect sizes were still pooled using the random-effects model.
Figure 3
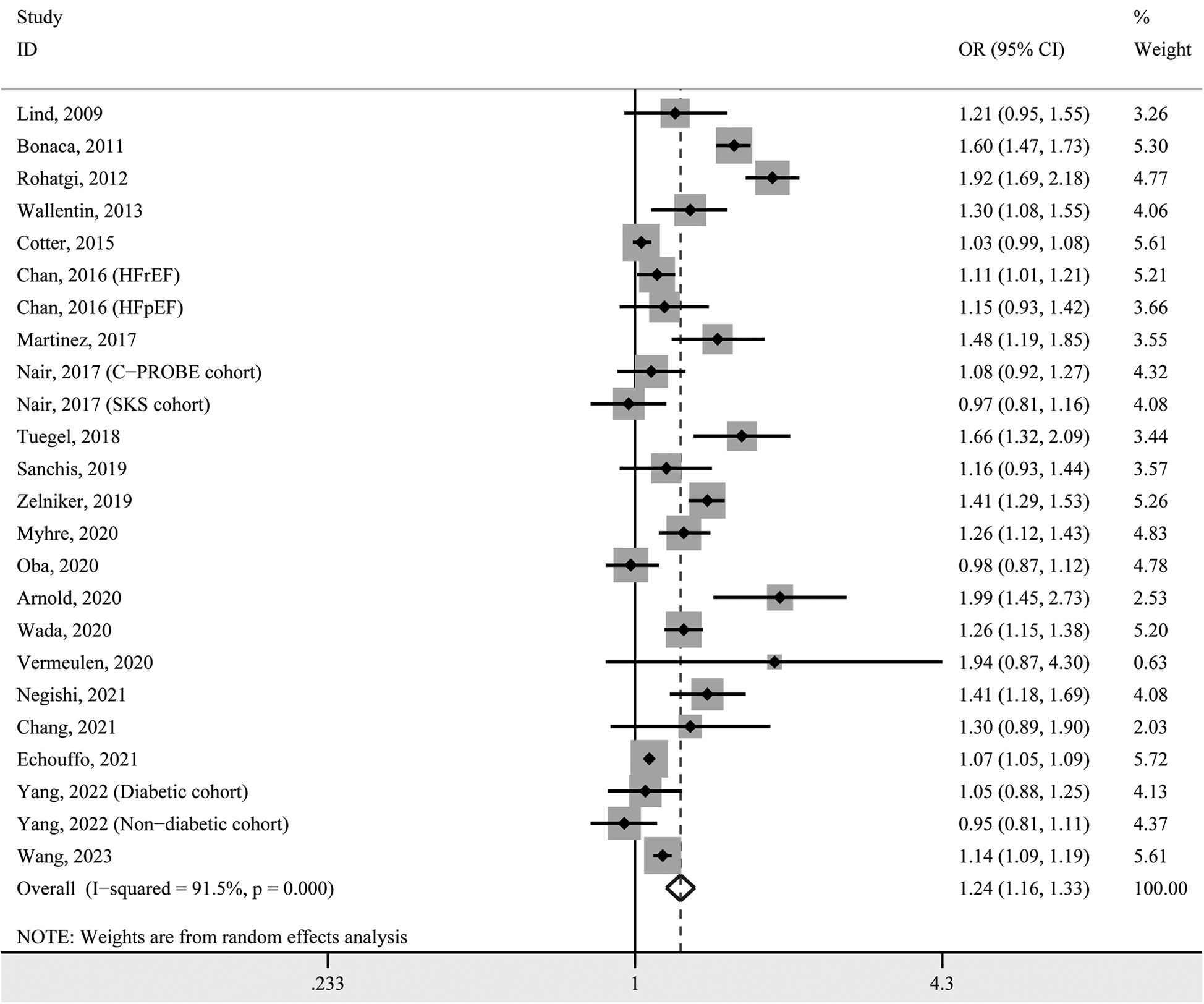
Forest plot for study-specific prevalence of hypertension for per 1 ng/ml increase in GDF-15 concentration. OR, odds ratio; CI, confidence interval.
In Figure 4, the dose-response curve indicates a nonlinear correlation between GDF-15 concentration and the prevalence of hypertension (P-nonlinearity < 0.001). As circulating GDF-15 concentration increased, the OR for hypertension prevalence increased, but after reaching approximately 5.5 ng/ml, the dose-response curve plateaued or even decreased slightly.
Figure 4
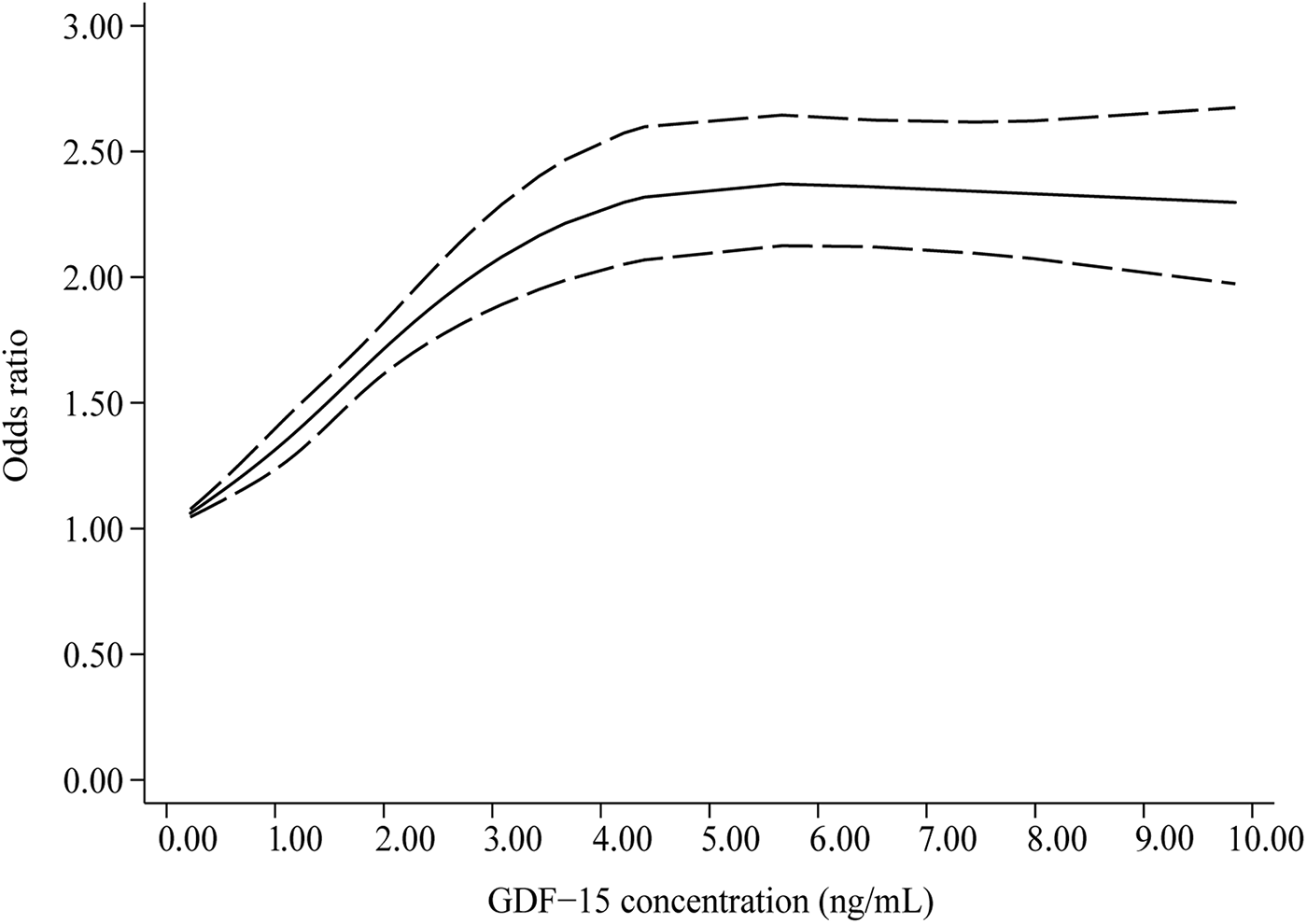
Dose-response correlation between circulating GDF-15 concentration and hypertension prevalence (Pnon−linearity < 0.001).
Subgroup analysis
Subgroup analysis was performed to assess the sources of heterogeneity, as well as to determine whether a variety of clinical factors affected the dose-response relationship. The following factors were taken into account when stratifying studies, including study areas, source populations, study designs, sample types, and GDF-15 detection methods. In terms of the source population, we divided them into four categories: non-clinical populations (20, 22, 23, 35, 38), patients with severe CVD (21, 24, 25, 29, 30, 34, 39, 40), patients with CKD (27, 28, 37), and patients with other diseases (26, 31–33, 36). Supplementary Table 3 illustrates that heterogeneity persisted across all subgroups, with I2 statistics ranging from 53.1% to 96.5%. However, the dose-response relationship was not significant for patients with CKD (P = 0.120). Nonsignificant dose-response relationships also were observed in 3 subgroups with only 1 study, namely, African participants (P = 0.104), GDF-15 detection methods by LTIA (P = 0.754), and Milliplex map kits (P = 0.104).
Meta-regression analysis
A meta-regression analysis was carried out with the prevalence of hypertension (OR, each 1 ng/ml GDF-15 increase) as the dependent variable, and several continuity variables including age, the percentage of male sex, BMI, sample sizes, smoking prevalence, and diabetes prevalence as independent variables. Supplementary Table 4 shows that there were negative correlations between the prevalence of hypertension and age (P = 0.007) as well as diabetes prevalence (P = 0.026). Other independent variables were not significantly correlated with the prevalence of hypertension (all P > 0.05).
Sensitivity analysis and bias of publication
A sensitivity analysis showed that excluding any single study had no effect on the pooled effect size for each 1 ng/ml increase in GDF-15 (Supplementary Figure 1). There was, however, considerable asymmetry in the funnel plot (Supplementary Figure 2) and Egger's test indicated that publication bias may exist (P = 0.012).
Discussion
This study represents the inaugural meta-analysis to quantify the association between circulating GDF-15 levels and the prevalence of hypertension. Our findings initially suggest that elevated GDF-15 levels are associated with a higher prevalence of hypertension. Subsequent dose-response meta-analysis revealed that each 1 ng/ml increment in GDF-15 concentration corresponds to a 24% increase in hypertension prevalence. Nevertheless, the dose-response curve exhibited a plateau or slight decline at higher GDF-15 concentrations, indicating that the prevalence of hypertension does not increase linearly with rising GDF-15 levels. These findings suggest that GDF-15 concentration may elevate as a compensatory mechanism in hypertensive patients. However, this compensatory response appears to have a threshold, beyond which GDF-15 levels cease to increase and may potentially decrease.
Besides being a risk factor for adverse cardiovascular events such as stroke, heart failure and cardiovascular death, hypertension itself is a common cardiovascular disease (41, 42). It is established that obesity, chronic inflammation, oxidative stress and atherosclerosis are relevant to the development of hypertension, beyond its relationship with genetic factors, eating habits, and lifestyle (43, 44). Interestingly, however, although multiple studies have been done to investigate the relationship between circulating GDF-15 and the risk factors associated with hypertension (7–9), little work has been done to explore the relationship between GDF-15 and the risk of hypertension. During the latest years, a number of meta-analyses already summarized the diagnostic and prognostic value of circulating GDF-15 for diverse human diseases. GDF-15, for instance, has been identified as a promising candidate biomarker for gynecological tumors, digestive system tumors, and mitochondrial diseases in several meta-analyses (45–47). It has also been shown that higher concentration of GDF-15 was a significant predictor of adverse cardiovascular events, cardiovascular mortality and all-cause mortality in a variety of acute and chronic diseases (10–12). However, none of the above meta-analyses evaluated the dose-response relationship between GDF-15 and adverse events.
A recent study from our team (48) demonstrated that there is a positive and non-linear association between circulating GDF-15 and CKD progression and poor outcome. Similar to the present study, the linear response was only observed in GDF-15 concentration range of 0–3 ng/ml, but the curve showed a gentle slope over 3 ng/ml. In the prior meta-analysis, we included 7,813 participants, but fewer than half of these were included in the dose-response study. In contrast, we included more studies and samples in our present meta-analysis, which was comprised of 24 studies involving 35,904 participants and 23,925 hypertensive individuals. It is therefore reasonable to believe that the relatively small sample size prevented us from observing a plateau or decline in the prior curve after a specific concentration of GDF-15. The finding also suggests that exogenous GDF-15 supplementation may be advantageous for the prevention or treatment of chronic metabolic diseases, such as hypertension. In fact, this potential therapeutic benefit has been demonstrated in recent murine studies through the administration of GDF-15. In mouse models of non-alcoholic steatohepatitis (NASH), overexpression of GDF-15 was shown to mitigate the progression of NASH, and this mitigation was evidenced by reduced expression of inflammatory and fibrotic genes, as well as decreased levels of liver enzymes, liver weight, and liver triglyceride content (49). Conversely, deletion of the GDF-15 gene resulted in the opposite outcomes (49). The protective effect was also observed in mouse models of diabetic nephropathy, which indicated that renal and systemic inflammation, the AGE/RAGE axis and its downstream inflammatory and adhesion molecules were significantly inhibited when GDF-15 was overexpressed (50). Indeed, GDF-15 has been suggested to be a biologically active protein with therapeutic potential in metabolic disorders, given that it could effectively improve cardiovascular risk factors such as oxidative stress, insulin resistance, and dyslipidemia (51). However, the dose-response curve observed in this study indicates that GDF-15 may have a dual role in hypertension pathophysiology, potentially serving as an adaptive protective mechanism during early disease stages, while chronic exposure to elevated concentrations may induce receptor-mediated pathophysiological alterations. At the molecular level, GDF-15 modulates key biological processes including appetite suppression, energy homeostasis, and vascular remodeling through GFRAL receptor activation (52). Based on established receptor pharmacology principles, prolonged exposure to high ligand concentrations can result in two potential mechanisms: receptor desensitization, which reduces the efficiency of intracellular signal transduction, and receptor occupancy saturation, where further increases in ligand concentration do not elicit additional biological effects (53, 54). The observed plateau phenomenon in circulating GDF-15 levels among hypertensive patients may reflect functional adaptations within the GFRAL receptor system under chronic hyperactivation. These compensatory changes could potentially impair both the endogenous blood pressure-regulating capacity of GDF-15 and the therapeutic efficacy of exogenous interventions targeting this pathway.
Notably, the hypertension cases analyzed in this meta-analysis predominantly represent secondary hypertension arising from comorbid conditions rather than essential hypertension. A substantial proportion of the cohort consisted of CKD patients, where the pathophysiological landscape reveals a complex interplay between GDF-15 kinetics and hypertensive mechanisms. In this population, compromised renal clearance capacity leads to progressive GDF-15 accumulation, which interacts bidirectionally with hypertension drivers such as sodium retention and fluid overload (55, 56). The cytokine demonstrates paradoxical regulatory effects: while its natriuretic properties through renal tubular action theoretically promote blood pressure reduction, concurrent uremic toxin accumulation and persistent volume expansion create counterregulatory hypertensive forces. This pathophysiological interplay may establish a concentration-dependent equilibrium within specific GDF-15 thresholds, clinically manifesting as blood pressure stabilization despite ongoing renal deterioration. Consequently, in CKD-associated hypertension, GDF-15 may exert its influence through multiple pathways, which encompass not only the regulatory mechanisms activated by renal impairment but also potential pharmacological mechanisms involving receptor desensitization and saturation.
In the context of metabolic hypertension, the secretion of GDF-15 exhibits a stress-responsive pattern (9). Obesity, insulin resistance, and inflammatory cascades enhance the transcription of GDF-15 through SMAD-signaling pathways (57), thereby mitigating the elevation of blood pressure induced by metabolic stress. Paradoxically, chronic hypersecretion of GDF-15 can lead to receptor desensitization and may suppress its own synthesis through negative feedback mechanisms. These autoregulatory processes interact with intricate blood pressure regulatory networks, resulting in the nonlinear relationship between GDF-15 levels and hypertension observed in clinical settings. While the dose-response characteristics identified in this study offer new insights into the role of GDF-15 in the pathophysiology of hypertension, the precise mechanisms remain to be elucidated. Future research should focus on the following areas: firstly, the development of GFRAL receptor knockout animal models to test the receptor-dependent hypothesis; secondly, the implementation of prospective cohort studies to elucidate the spatiotemporal relationship between dynamic changes in GDF-15 and blood pressure trajectories; and thirdly, the investigation of the functional heterogeneity of the GDF-15/GFRAL axis in hypertensive patients with diverse clinical phenotypes. These studies are expected to provide a theoretical basis for precise stratified management of hypertension and the development of therapeutic targets.
There is a considerable strength in this meta-analysis because a large number of studies were included, and every study was included in the dose-response analysis, whereas our aforementioned meta-analysis, which included 14 studies, only included a few dose-response studies (48). Another strength is that we explored the sources of heterogeneity using both subgroup analysis and meta-regression, and the latter suggested that age and diabetes prevalence contribute to heterogeneity. Moreover, our sensitivity analysis indicated that the pooled results were robust. There are also several potential limitations to be considered. Firstly, both the clinical and nonclinical populations were included in this meta-analysis, and clinical populations included patients with CKD, severe CVD, and other diseases. In this study, we were unable to determine whether these diseases had an effect on hypertension prevalence. However, in nonclinical populations (20, 22, 23, 35, 38), the prevalence of hypertension increased by 39% with every 1 ng/ml increase in GDF-15, similar to the overall pooled result. Secondly, more than half of the included studies did not give definitions of hypertension. Moreover, some patients self-reported blood pressure data (26, 33), which is likely to be inaccurate and underreported. Therefore, hypertension prevalence might be inflated or deflated in some included studies. Thirdly, according to the asymmetric funnel plot, there may be potential publication biases, which may be explained by the exclusion of non-English articles and grey literature such as conference abstracts. However, it is important to recognize that an asymmetric funnel plot may not solely be attributed to publication bias; significant levels of heterogeneity can also result in such an asymmetry (58). Fourthly, the original studies we selected did not directly investigate the association between GDF-15 and hypertension; instead, they examined GDF-15 in relation to other medical conditions. Thus, the hypertension-related data extracted for this meta-analysis were limited to baseline information—specifically, the number of hypertensive patients across GDF-15 categories. Consequently, we could not adjust for confounding factors in assessing the dose-response relationship between GDF-15 and hypertension. Additionally, circulating GDF-15 levels are influenced by various factors, including physiological (e.g., exercise, diet, and weight changes) and pathological conditions (59, 60). Therefore, the dose-response relationship observed in this meta-analysis may not fully represent the true association. Finally, this study is subject to selection bias, primarily because it included only studies that reported hypertension prevalence with at least three GDF-15 categories. Consequently, the results of the two-class meta-analysis, which compared hypertension prevalence between high and low circulating GDF-15 levels, should be interpreted with caution. Furthermore, selection bias may have been introduced by the inclusion criteria that restricted the analysis to English-language studies and full-text articles.
Conclusions
This dose-response meta-analysis suggested that circulating GDF-15 is positively and non-linearly associated with the prevalence of hypertension. A slight decreasing trend in the dose-response curve implies that the administration of GDF-15 may be beneficial for preventing or treating hypertension. However, to determine the efficacy and impact of GDF-15 supplementation on hypertension or other chronic diseases in humans, it is essential to conduct prospective studies, including clinical trials.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ZY: Data curation, Investigation, Methodology, Writing – original draft. JG: Data curation, Investigation, Methodology, Writing – original draft. ZZ: Conceptualization, Formal analysis, Validation, Writing – review & editing. LL: Data curation, Investigation, Software, Writing – review & editing. SH: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1500882/full#supplementary-material
Supplementary Figure 1Sensitivity analysis of pooled effect estimates for each 1 ng/mL increase in circulating GDF-15 by excluding single study at a time.
Supplementary Figure 2Funnel plot for assessing publication bias for each 1 ng/mL increase in circulating GDF-15.
References
1.
Srikanth S Mondal A Aggarwal S Alle NR Odugbemi O Nayak PR et al Key hypertension breakthroughs and emerging trends from the AHA’s scientific sessions. Curr Probl Cardiol. (2024) 49:102434. 10.1016/j.cpcardiol.2024.102434
2.
Jiao Y Li W Zhang Q Jiang Q . Gut microbiota and hypertension: a bibliometric analysis of recent research (2014–2023). Front Nutr. (2023) 10:1253803. 10.3389/fnut.2023.1253803
3.
Ma J Chen X . Advances in pathogenesis and treatment of essential hypertension. Front Cardiovasc Med. (2022) 9:1003852. 10.3389/fcvm.2022.1003852
4.
Kobari E Tanaka K Nagao M Okazaki K Hayashi F Kazama S et al Impact of lifestyle and psychosocial factors on the onset of hypertension after the great east Japan earthquake: a 7-year follow-up of the Fukushima health management survey. Hypertens Res. (2022) 45:1609–21. 10.1038/s41440-022-00968-3
5.
Laxmi Golmei P Srivastava S Kumar S . Single nucleotide polymorphism-based biomarker in primary hypertension. Eur J Pharmacol. (2024) 972:176584. 10.1016/j.ejphar.2024.176584
6.
Shibasaki I Otani N Ouchi M Fukuda T Matsuoka T Hirota S et al Utility of growth differentiation factor-15 as a predictor of cardiovascular surgery outcomes: current research and future directions. J Cardiol. (2024) 83:211–8. 10.1016/j.jjcc.2023.08.013
7.
Klein AB Kleinert M Richter EA Clemmensen C . GDF15 in appetite and exercise: essential player or coincidental bystander?Endocrinology. (2022) 163:bqab242. 10.1210/endocr/bqab242
8.
Moon JS Goeminne LJE Kim JT Tian JW Kim SH Nga HT et al Growth differentiation factor 15 protects against the aging-mediated systemic inflammatory response in humans and mice. Aging Cell. (2020) 19:e13195. 10.1111/acel.13195
9.
Kim KH Lee MS . GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH. Biochim Biophys Acta Gen Subj. (2021) 1865:129834. 10.1016/j.bbagen.2020.129834
10.
Wang Y Zhen C Wang R Wang G . Growth-differentiation factor-15 predicts adverse cardiac events in patients with acute coronary syndrome: a meta-analysis. Am J Emerg Med. (2019) 37:1346–52. 10.1016/j.ajem.2019.04.035
11.
Xie S Li Q Luk AOY Lan HY Chan PKS Bayés-Genís A et al Major adverse cardiovascular events and mortality prediction by circulating GDF-15 in patients with type 2 diabetes: a systematic review and meta-analysis. Biomolecules. (2022) 12:934. 10.3390/biom12070934
12.
Luo JW Duan WH Song L Yu YQ Shi DZ . A meta-analysis of growth differentiation factor-15 and prognosis in chronic heart failure. Front Cardiovasc Med. (2021) 8:630818. 10.3389/fcvm.2021.630818
13.
Fernandez C Rysä J Ström K Nilsson J Engström G Orho-Melander M et al Circulating protein biomarkers predict incident hypertensive heart failure independently of N-terminal pro-B-type natriuretic peptide levels. ESC Heart Fail. (2020) 7:1891–9. 10.1002/ehf2.12757
14.
Sökmen E Uçar C Sivri S Çelik M Güçlü K . Relationship of growth differentiation factor-15 with aortic stiffness in essential hypertension. Future Sci OA. (2019) 5:Fso406. 10.2144/fsoa-2019-0029
15.
Liberati A Altman DG Tetzlaff J Mulrow C Gøtzsche PC Ioannidis JP et al The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. (2009) 151:W65–94. 10.7326/0003-4819-151-4-200908180-00136
16.
McHugh ML . Interrater reliability: the kappa statistic. Biochem Med (Zagreb). (2012) 22:276–82. 10.11613/BM.2012.031
17.
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z
18.
Berlin JA Longnecker MP Greenland S . Meta-analysis of epidemiologic dose-response data. Epidemiology. (1993) 4:218–28. 10.1097/00001648-199305000-00005
19.
Orsini N Li R Wolk A Khudyakov P Spiegelman D . Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. 10.1093/aje/kwr265
20.
Lind L Wallentin L Kempf T Tapken H Quint A Lindahl B et al Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the prospective investigation of the vasculature in Uppsala seniors (PIVUS) study. Eur Heart J. (2009) 30:2346–53. 10.1093/eurheartj/ehp261
21.
Bonaca MP Morrow DA Braunwald E Cannon CP Jiang S Breher S et al Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. (2011) 31:203–10. 10.1161/ATVBAHA.110.213512
22.
Rohatgi A Patel P Das SR Ayers CR Khera A Martinez-Rumayor A et al Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas heart study. Clin Chem. (2012) 58:172–82. 10.1373/clinchem.2011.171926
23.
Wallentin L Zethelius B Berglund L Eggers KM Lind L Lindahl B et al GDF-15 for prognostication of cardiovascular and cancer morbidity and mortality in men. PLoS One. (2013) 8:e78797. 10.1371/journal.pone.0078797
24.
Cotter G Voors AA Prescott MF Felker GM Filippatos G Greenberg BH et al Growth differentiation factor 15 (GDF-15) in patients admitted for acute heart failure: results from the RELAX-AHF study. Eur J Heart Fail. (2015) 17:1133–43. 10.1002/ejhf.331
25.
Chan MM Santhanakrishnan R Chong JP Chen Z Tai BC Liew OW et al Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. (2016) 18:81–8. 10.1002/ejhf.431
26.
Martinez CH Freeman CM Nelson JD Murray S Wang X Budoff MJ et al GDF-15 plasma levels in chronic obstructive pulmonary disease are associated with subclinical coronary artery disease. Respir Res. (2017) 18:42. 10.1186/s12931-017-0521-1
27.
Nair V Robinson-Cohen C Smith MR Bellovich KA Bhat ZY Bobadilla M et al Growth differentiation factor-15 and risk of CKD progression. J Am Soc Nephrol. (2017) 28:2233–40. 10.1681/asn.2016080919
28.
Tuegel C Katz R Alam M Bhat Z Bellovich K de Boer I et al GDF-15, galectin 3, soluble ST2, and risk of mortality and cardiovascular events in CKD. Am J Kidney Dis. (2018) 72:519–28. 10.1053/j.ajkd.2018.03.025
29.
Sanchis J Ruiz V Bonanad C Sastre C Ruescas A Diaz M et al Growth differentiation factor 15 and geriatric conditions in acute coronary syndrome. Int J Cardiol. (2019) 290:15–20. 10.1016/j.ijcard.2019.05.034
30.
Zelniker TA Jarolim P Silverman MG Bohula EA Park JG Bonaca MP et al Prognostic role of GDF-15 across the spectrum of clinical risk in patients with NSTE-ACS. Clin Chem Lab Med. (2019) 57:1084–92. 10.1515/cclm-2018-1081
31.
Myhre PL Prebensen C Strand H Roysland R Jonassen CM Rangberg A et al Growth differentiation factor 15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation. (2020) 142:2128–37. 10.1161/CIRCULATIONAHA.120.050360
32.
Oba K Ishikawa J Tamura Y Fujita Y Ito M Iizuka A et al Serum growth differentiation factor 15 level is associated with muscle strength and lower extremity function in older patients with cardiometabolic disease. Geriatr Gerontol Int. (2020) 20:980–7. 10.1111/ggi.14021
33.
Arnold N Rehm M Buchele G Peter RS Brenner RE Gunther KP et al Growth differentiation factor-15 as a potent predictor of long-term mortality among subjects with osteoarthritis. J Clin Med. (2020) 9:3107. 10.3390/jcm9103107
34.
Wada H Suzuki M Matsuda M Ajiro Y Shinozaki T Sakagami S et al Impact of smoking status on growth differentiation factor 15 and mortality in patients with suspected or known coronary artery disease: the ANOX study. J Am Heart Assoc. (2020) 9:e018217. 10.1161/JAHA.120.018217
35.
Vermeulen B Schutte AE Gafane-Matemane LF Kruger R . Growth differentiating factor-15 and its association with traditional cardiovascular risk factors: the African-PREDICT study. Nutr Metab Cardiovasc Dis. (2020) 30:925–31. 10.1016/j.numecd.2020.03.001
36.
Negishi K Hoshide S Shimpo M Kanegae H Kario K . Growth differentiation factor-15 predicts death and stroke event in outpatients with cardiovascular risk factors: the J-HOP study. J Am Heart Assoc. (2021) 10:e022601. 10.1161/JAHA.121.022601
37.
Chang JF Chen PC Hsieh CY Liou JC . A growth differentiation factor 15-based risk score model to predict mortality in hemodialysis patients. Diagnostics (Basel). (2021) 11:286. 10.3390/diagnostics11020286
38.
Echouffo-Tcheugui JB Daya N Matsushita K Wang D Ndumele CE Al Rifai M et al Growth differentiation factor (GDF)-15 and cardiometabolic outcomes among older adults: the atherosclerosis risk in communities study. Clin Chem. (2021) 67:653–61. 10.1093/clinchem/hvaa332
39.
Yang P Zhu Z Shi M Yin J Zang Y Zhong C et al Association of serum growth differentiation factor-15 levels with the risks of death and vascular events in patients with ischemic stroke: the role of diabetes. Nutr Metab Cardiovasc Dis. (2022) 32:616–23. 10.1016/j.numecd.2021.12.005
40.
Wang J Han LN Ai DS Wang XY Zhang WJ Xu XR et al Growth differentiation factor 15 predicts cardiovascular events in stable coronary artery disease. J Geriatr Cardiol. (2023) 20:527–37. 10.26599/1671-5411.2023.07.007
41.
Oliveros E Patel H Kyung S Fugar S Goldberg A Madan N et al Hypertension in older adults: assessment, management, and challenges. Clin Cardiol. (2020) 43:99–107. 10.1002/clc.23303
42.
Wang Z Zheng Y Ruan H Li L Zhang M Duan L et al The impact of hypertension on the prognosis of patients with hypertrophic cardiomyopathy: a single-center retrospective study. PeerJ. (2023) 11:e14614. 10.7717/peerj.14614
43.
Wyszyńska J Łuszczki E Sobek G Mazur A Dereń K . Association and risk factors for hypertension and dyslipidemia in young adults from Poland. Int J Environ Res Public Health. (2023) 20:982. 10.3390/ijerph20020982
44.
Ali N Mahmud F Akter SA Islam S Sumon AH Barman DN et al The prevalence of general obesity, abdominal obesity, and hypertension and its related risk factors among young adult students in Bangladesh. J Clin Hypertens (Greenwich). (2022) 24:1339–49. 10.1111/jch.14560
45.
Roy D Modi A Purohit P Khokhar M Goyal M Sharma S et al Growth differentiation factor-15 as a candidate biomarker in gynecologic malignancies: a meta-analysis. Cancer Invest. (2022) 40:901–10. 10.1080/07357907.2022.2133138
46.
Wang Y Jiang T Jiang M Gu S . Appraising growth differentiation factor 15 as a promising biomarker in digestive system tumors: a meta-analysis. BMC Cancer. (2019) 19:177. 10.1186/s12885-019-5385-y
47.
Lin Y Ji K Ma X Liu S Li W Zhao Y et al Accuracy of FGF-21 and GDF-15 for the diagnosis of mitochondrial disorders: a meta-analysis. Ann Clin Transl Neurol. (2020) 7:1204–13. 10.1002/acn3.51104
48.
Zhou Z Liu H Ju H Chen H Jin H Sun M . Circulating GDF-15 in relation to the progression and prognosis of chronic kidney disease: a systematic review and dose-response meta-analysis. Eur J Intern Med. (2023) 110:77–85. 10.1016/j.ejim.2023.01.026
49.
Kim KH Kim SH Han DH Jo YS Lee YH Lee MS . Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci Rep. (2018) 8:6789. 10.1038/s41598-018-25098-0
50.
Chen J Peng H Chen C Wang Y Sang T Cai Z et al NAG-1/GDF15 inhibits diabetic nephropathy via inhibiting AGE/RAGE-mediated inflammation signaling pathways in C57BL/6 mice and HK-2 cells. Life Sci. (2022) 311:121142. 10.1016/j.lfs.2022.121142
51.
Baek SJ Eling T . Growth differentiation factor 15 (GDF15): a survival protein with therapeutic potential in metabolic diseases. Pharmacol Ther. (2019) 198:46–58. 10.1016/j.pharmthera.2019.02.008
52.
Emmerson PJ Wang F Du Y Liu Q Pickard RT Gonciarz MD et al The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. (2017) 23:1215–9. 10.1038/nm.4393
53.
Moore GJ Ridway H Gadanec LK Apostolopoulos V Zulli A Swiderski J et al Structural features influencing the bioactive conformation of angiotensin II and angiotensin A: relationship between receptor desensitization, addiction, and the blood-brain barrier. Int J Mol Sci. (2024) 25:5779. 10.3390/ijms25115779
54.
Mahmood A Ahmed K Zhang Y . β-adrenergic receptor desensitization/down-regulation in heart failure: a friend or foe?Front Cardiovasc Med. (2022) 9:925692. 10.3389/fcvm.2022.925692
55.
Tang Y Liu T Sun S Peng Y Huang X Wang S et al Role and mechanism of growth differentiation factor 15 in chronic kidney disease. J Inflamm Res. (2024) 17:2861–71. 10.2147/jir.S451398
56.
Nagata D Hishida E . Elucidating the complex interplay between chronic kidney disease and hypertension. Hypertens Res. (2024) 47:3409–22. 10.1038/s41440-024-01937-8
57.
Adela R Banerjee SK . GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res. (2015) 2015:490842. 10.1155/2015/490842
58.
Peters JL Sutton AJ Jones DR Abrams KR Rushton L . Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. (2007) 26:4544–62. 10.1002/sim.2889
59.
Yıldırım Ayaz E Mesci B Üner ÖE Kaya FN Dincer B İşman FK et al The effect of exercise on GDF-15 levels in individuals with prediabetes: a randomized controlled trial. J Diabetes Investig. (2025) 16:656–69. 10.1111/jdi.14404
60.
Wang D Townsend LK DesOrmeaux GJ Frangos SM Batchuluun B Dumont L et al GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature. (2023) 619:143–50. 10.1038/s41586-023-06249-4
Summary
Keywords
growth differentiation factor-15, hypertension, prevalence, dose-response relationship, meta-analysis
Citation
Yu Z, Gao J, Zhou Z, Li L and Hu S (2025) Circulating growth differentiation factor-15 concentration and hypertension risk: a dose-response meta-analysis. Front. Cardiovasc. Med. 12:1500882. doi: 10.3389/fcvm.2025.1500882
Received
19 October 2024
Accepted
18 April 2025
Published
30 April 2025
Volume
12 - 2025
Edited by
Naresh Chandra Bal, KIIT University, India
Reviewed by
Bijayashree Sahu, KIIT University, India
Hiba Behayaa, University of Babylon, Iraq
Updates
Copyright
© 2025 Yu, Gao, Zhou, Li and Hu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Sanqiang Hu lygfyhsq@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.