Abstract
Transcatheter Aortic Valve Replacement (TAVR) is currently the preferred treatment option not only for high-surgical-risk patients with severe aortic stenosis (AS) but also increasingly for those with intermediate and low surgical risk. Coronary artery disease (CAD) is one of the most common complications in severe AS patients, making percutaneous coronary intervention (PCI) a frequent clinical requirement in cases of severe AS complicated by CAD. The coexistence of Aortic Tortuosity and aortic stenosis is extremely rare.We report a case of an elderly male with severe aortic stenosis combined with coronary artery disease, who underwent successful transcarotid TAVR and PCI due to a Z-shaped fold tortuosity in the aortic arch, making femoral access challenging. The patient was able to ambulate the following day and was discharged on the fifth postoperative day with stable follow-up. As of this writing, no related reports of combined transcarotid TAVR and PCI have been published.
Introduction
Aortic stenosis (AS) is a common valvular heart disease with a poor prognosis. With increasing evidence supporting the efficacy of TAVR in intermediate- and low-risk AS patients, TAVR is becoming a viable treatment option for more patients with aortic valve disease (1, 2). Coronary artery disease (CAD) is a common comorbidity in patients undergoing TAVR, with a prevalence of about 50% that increases with age and impacts treatment strategy and prognosis (3–5). While one-stage TAVR combined with PCI has shown clinical success, the superiority of simultaneous vs. staged intervention remains controversial and requires further data (3, 6–9). Although TAVR typically uses a transfemoral approach, alternative access routes can be chosen if femoral access is challenging. Herein, we present a case of severe AS with CAD in an elderly male, treated with transcarotid TAVR combined with PCI due to a tortuous aortic arch, the first reported case of this kind as of this writing, no related reports have been published.
Case report
A 77-year-old male, height 159 cm, weight 58 kg, was admitted to Wuhan Asia Heart Hospital on June 6, 2024, with a 2-year history of intermittent chest tightness and palpitations, worsened over the prior two weeks. Previous treatments at another hospital for coronary artery disease included rotational atherectomy and drug-coated balloon angioplasty of the left anterior descending artery. The patient had a 10-year history of diabetes mellitus. Auscultation of the first aortic valve area revealed a grade 3/6 systolic murmur.
N-terminal pro-B-type natriuretic peptide (NT-proBNP) was 7,417.00 pg/ml and Hyper-sensitive cardiac troponin I (hs-cTnI)was 0.0766 ng/ml. Electrocardiography (ECG) showed atrial flutter with a rapid ventricular response, and ST-T segment changes. Transthoracic echocardiography (TTE) showed: Type 0 bicuspid aortic valve with severe stenosis and mild-to-moderate regurgitation, regional wall motion abnormalities in the left ventricle, mild-to-moderate mitral regurgitation, ascending aortic dilation, interventricular septal thickening, reduced left ventricular systolic function, left ventricular end-diastolic diameter of 5.7 cm, left ventricular outflow tract diameter of 2.5 cm, ejection fraction of 45%, peak aortic valve velocity of 4.9 m/s, peak transvalvular pressure gradient of 95 mmHg, mean transvalvular pressure gradient of 57 mmHg, and aortic valve area of 0.6 cm². Chest computed tomography (CT) showed dilation of the ascending aorta, and atherosclerosis in the thoracic aorta, with a local Z-shaped tortuosity in the descending aorta. He was diagnosed with severe AS complicated with CAD.
Preoperative CT indicated that the aortic valve has a Type 0 L-R bicuspid configuration with a horizontal alignment. The annulus has an average diameter of 25.2 mm, and the outflow tract measures 24 mm. The calcification score is 1,063 mm³, with calcification mainly located on the leaflets, commissures, and root. The leaflets are thickened, with calcification in the left anterior descending (LAD) artery. Coronary ostium height is adequate, presenting a low risk of obstruction, and the heart shows a horizontal orientation at a 71° angle.
This patient had severe AS combined with CAD. His descending aortic arch has a localized tortuosity (Figures 1A,B), while the left common carotid artery is free from stenosis or calcification, and the Willis circle is complete. The right vertebral artery is dominant, and the pathway from the left common carotid artery to the annulus is feasible, the left subclavian artery was relatively small in diameter, and the right subclavian artery showed marked angulation (Figure 1C). Chest CT demonstrated a minimal descending aortic diameter of 21 mm at the narrowest segment, with no significant caliber change compared to adjacent normal segments and no evidence of collateral vessel formation (Figure 1D). Following Multi-Disciplinary Team (MDT) discussion and with the consent of the family, a transcarotid, one-stage TAVR procedure combined with PCI was planned.
Figure 1
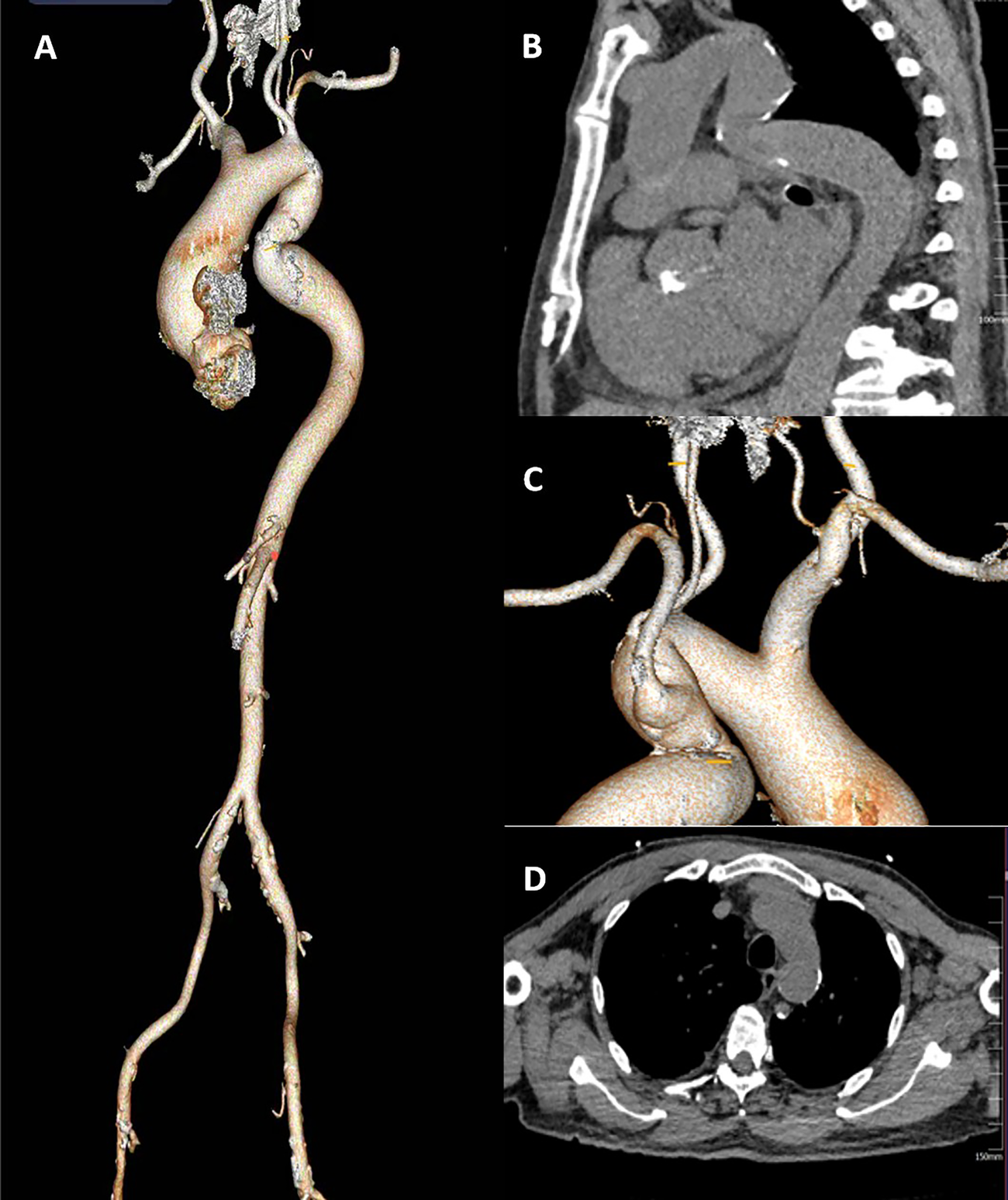
(A) the full CT of the aorta. (B) a Z-shaped fold tortuosity in the descending aortic arch.(C) the left common carotid artery is free from stenosis or calcification, the Willis circle is complete. The right vertebral artery is dominant. (D) The narrowest segment of the descending aorta).
On June 8, 2024, right radial artery access was obtained under local anesthesia with 1% lidocaine. Coronary angiography (CAG) revealed 90% diffuse stenosis in the proximal left anterior descending (LAD) artery and 70% segmental stenosis in the Diagonal Branches(D) (Figure 2A). Intravascular ultrasound (IVUS) revealed severe calcified lesions in the proximal LAD. A shockwave balloon (3.0 × 12 mm) was deployed in the proximal LAD, delivering 80 pulses, followed by the implantation of three drug-eluting stents (2.5 mm × 33 mm, 2.75 mm × 26 mm, and 3.0 mm × 15 mm). IVUS assessment confirmed good stent apposition, with a minimal stent area (MSA) greater than 6.0 mm² (Figure 2B).
Figure 2
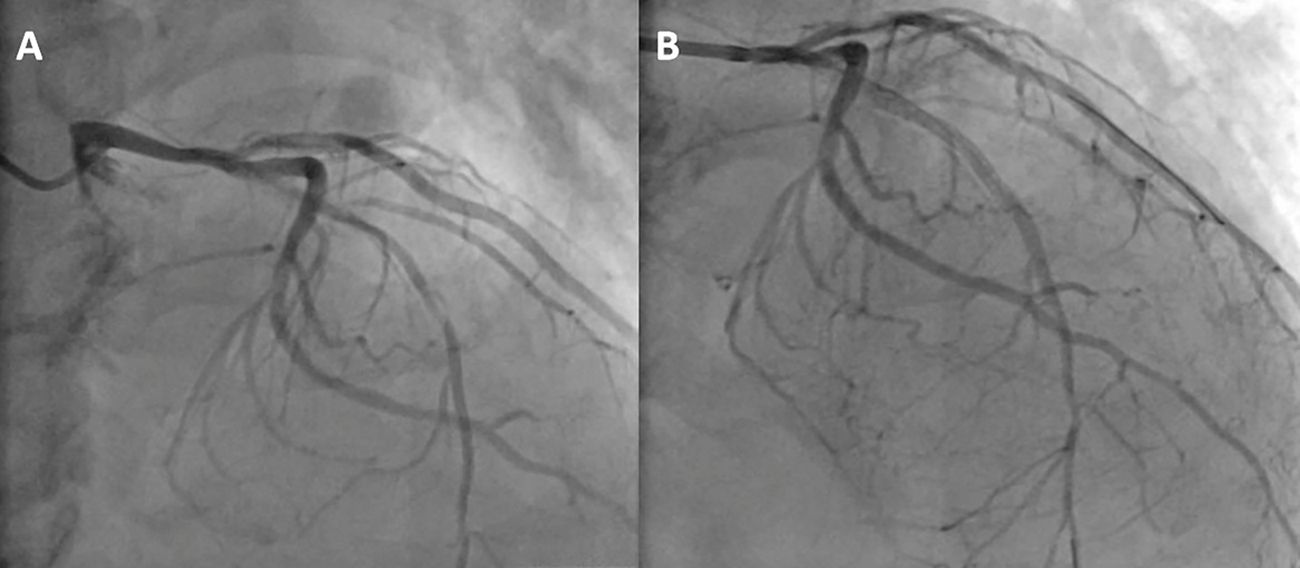
(A) CAG indicating a 90% diffuse stenosis in the proximal segment of the LAD artery. (B) the LAD artery after stent implantation).
After PCI, general anesthesia was administered for the TAVR procedure. A temporary pacemaker was inserted via the right internal jugular vein. The left internal carotid artery was selected as the primary access route, with the right radial artery as a secondary route. Through the right radial artery sheath, a pigtail catheter was advanced into the ascending aorta for aortography (Figure 3A). An AL2 catheter was introduced through the sheath of the left internal carotid artery into the ascending aorta. A straight guidewire was navigated across the aortic valve into the left ventricle, and the AL2 catheter was exchanged for a pigtail catheter to measure the left ventricular and aortic pressures, yielding a left ventricular systolic/diastolic pressure of 216/17 mmHg and an aortic pressure of 105/61 mmHg. With the stiffened guidewire positioned in the left ventricle, the pigtail catheter was exchanged for a delivery catheter, oriented appropriately for the procedure. A 23 mm × 4 cm NUMED balloon was used for pre-dilation of the aortic valve under rapid pacing at 180 bpm to stabilize the valve area during dilation(Figure 3B). Aortography was conducted to assess the risk of coronary obstruction and valve regurgitation. Based on preoperative CT scans, echocardiography measurements, and the results of the balloon dilation, a 29 mm Peijia TAVR prosthesis was selected and loaded onto the delivery system, then advanced over the stiffened guidewire into the left ventricle. Under guidance from digital subtraction angiography (DSA) and Transesophageal echocardiography (TEE), and while maintaining rapid pacing at 180 bpm, the prosthetic valve was gradually deployed (Figure 3C). After release, the delivery system was withdrawn. Post-procedure measurements indicated a peak gradient across the valve of 9 mmHg. Aortography of the aortic root showed no paravalvular leak or coronary obstruction. The depth of the implanted valve was measured at 3 mm on the non-coronary cusp and 6 mm on the left coronary cusp. TEE revealed mild central regurgitation with no paravalvular leak. Follow-up measurements demonstrated left ventricular and aortic pressures of 120/20 mmHg and 120/70 mmHg, respectively. Continuous cardiac monitoring showed sinus rhythm with no conduction block, QRS widening, or PR prolongation. The delivery system and arterial sheath were removed, and surgical closure of the left internal carotid artery was performed, along with the removal of the right radial artery sheath while maintaining the temporary pacemaker.
Figure 3
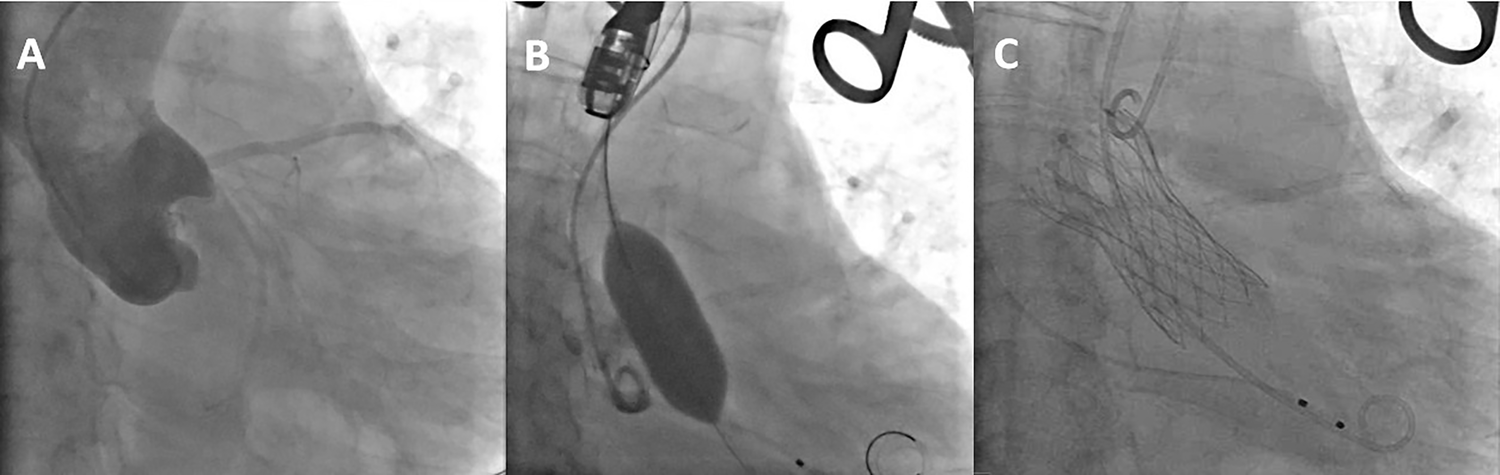
(A) the aortic root angiography. (B) balloon expansion (C) the completion of artificial valve deployment.
The patient's vital signs remained stable after surgery. Holter monitoring showed sinus rhythm with occasional episodes of atrial flutter and atrial fibrillation. TTE indicated normal function of the implanted valve and mildly reduced left ventricular systolic function. The patient was managed postoperatively with anticoagulants, antiplatelet agents, diuretics to improve cardiac function, lipid-lowering therapy, ventricular remodeling inhibitors, glucose-lowering agents, and renal protection. The patient was able to ambulate the following day and was discharged five days postoperatively. A one-month follow-up revealed no symptoms of chest tightness or palpitations. ECG showed sinus rhythm with frequent premature atrial contractions, and TTE confirmed normal function of the implanted valve with mildly reduced left ventricular systolic function. At three months and six months post-op, all follow-up evaluations were normal. The patient's hospitalization timeline is shown in Figure 4.
Figure 4
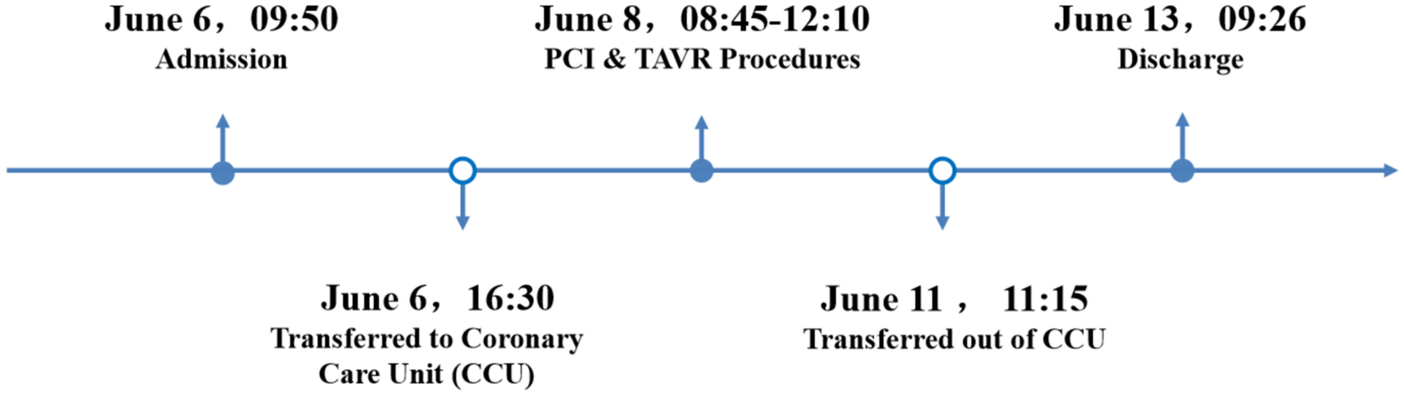
A timeline with relevant data from the episode of care.
Discussion
This case involved a patient with severe aortic stenosis complicated by chronic coronary artery disease who underwent Transcarotid TAVR combined with PCI due to a Z-shaped fold tortuosity of the aorta.
The patient exhibited pronounced tortuosity and angulation of the descending aorta. Although successful TAVR using femoral or axillary artery access has been reported for patients with tortuous aortas, these approaches require specialized equipment and techniques (10, 11). Along with significant curvature of the aortic arch. Based on our previous experience, transfemoral access would carry a high risk of intraoperative acute aortic injury, even with the use of longer sheaths or the placement of an additional stiff guidewire via an auxiliary access to enhance support. Although bilateral subclavian access could potentially minimize the impact on cerebral perfusion, anatomical limitations were present: the left subclavian artery was relatively small in diameter, and the right subclavian artery showed marked angulation. Following multidisciplinary team (MDT) consultation and evaluation of procedural feasibility, left carotid artery access was selected Transcarotid TAVR, as an alternative route to the transfemoral approach, has been confirmed in numerous clinical studies to safely and effectively perform TAVR with favorable outcomes, including lower rates of stroke and major vascular complications (12, 13). Preoperative CT angiography for this patient included evaluation of the aortic root, coronary ostial height, carotid artery access, and cerebrovascular assessment (including the integrity of the Willis circle). Intraoperatively, the left carotid artery was surgically exposed, and a 10F arterial sheath was initially inserted. Through this sheath, a guidewire was advanced across the native aortic valve and subsequently exchanged for a stiff guidewire positioned in the left ventricle. A 20F large-bore sheath was then introduced to facilitate subsequent balloon aortic valvuloplasty (BAV) and TAVR, with the goal of minimizing interruption of cerebral blood flow. The duration of left carotid artery occlusion by the 20F sheath was limited to 18 min. Throughout the procedure, continuous cerebral oximetry monitoring was performed by the anesthesia team to closely assess and ensure adequate cerebral perfusion, and there were no postoperative complications such as vascular bleeding, dissection, stroke, or atrioventricular conduction block.
Several studies have shown the safety and efficacy of one-stage treatment using TAVR combined with PCI. It is necessary, however, to monitor the risks of renal damage and bleeding carefully and to comprehensively consider the patient's overall condition, disease severity, possible complications, and surgical risk to develop an individualized treatment plan (3, 7, 8, 14). This patient presented with severe aortic stenosis and coronary artery disease. The PCI procedure was performed on the proximal left anterior descending (LAD) artery via the right radial artery, with intravascular ultrasound (IVUS) revealing severe calcification, which was treated with a shockwave balloon before implanting three drug-eluting stents. TAVR was then performed using the left internal carotid artery as the primary access and the right radial artery as an auxiliary route, with the Peijia TAV29 transcatheter valve being deployed. The patient was mobilized the next day and discharged on postoperative day 5, with follow-up examinations showing stable results, indicating successful surgery.
As of this writing, no related reports of combined transcarotid TAVR and PCI have been published. With the growing body of evidence supporting TAVR, improved surgical accessibility, and advances in valve technology, transcarotid TAVR combined with PCI may become a viable option for more patients with aortic valve disease complicated by chronic coronary artery disease who have difficulty with femoral access. A thorough preoperative examination, comprehensive assessment of the patient's condition, individualized treatment planning, and meticulous surgical strategy can further ensure favorable clinical outcomes for this procedure. However,the current single-patient experience at our institution limits definitive conclusions regarding this method's safety and efficacy. Additional studies are needed to establish indications, standardize the procedure, and enhance its effectiveness through iterative refinements.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study involving human participants was approved by the Ethics Committee of Wuhan Asia Heart Hospital (2025-B025). Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WC: Writing – original draft, Writing – review & editing. YB: Conceptualization, Writing – review & editing. HG: Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors acknowledge all who made contributions for the case diagnosis, therapy, and decision-making process.
Conflict of interest
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1522100/full#supplementary-material
References
1.
MackMJLeonMBThouraniVHMakkarRKodaliSKRussoMet alTranscatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380(18):1695–705. 10.1056/NEJMoa1814052
2.
PopmaJJDeebGMYakubovSJMumtazMGadaHO’HairDet alTranscatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380(18):1706–15. 10.1056/NEJMoa1816885
3.
FarouxLGuimaraesLWintzer-WehekindJJunqueraLFerreira-NetoANdel ValDet alCoronary artery disease and transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 74(3):362–72. 10.1016/j.jacc.2019.06.012
4.
OttoCMNishimuraRABonowROCarabelloBAErwinJPGentileFet al2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 143(5):e35–71. 10.1161/CIR.0000000000000932
5.
TarantiniGTangGNai FovinoLBlackmanDVan MieghemNMKimW-Ket alManagement of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European association of percutaneous cardiovascular interventions in collaboration with the ESC working group on cardiovascular surgery. EuroIntervention. (2023) 19(1):37–52. 10.4244/EIJ-D-22-00958
6.
XuCHuHSuX. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement for aortic stenosis complicated with acute STEMI: a case report and literature review. Front Cardiovasc Med. (2023) 10:1291089. 10.3389/fcvm.2023.1291089
7.
FischerJSteffenJArlartTHaumMGschwendtnerSDoldiPMet alConcomitant percutaneous coronary intervention in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. (2024) 103(1):186–93. 10.1002/ccd.30927
8.
ParkDYSimonatoMAhmadYBanksAZLowensternANannaMG. Insight into the optimal timing of percutaneous coronary intervention and transcatheter aortic valve replacement. Curr Probl Cardiol. (2024) 49(1 Pt A):102050. 10.1016/j.cpcardiol.2023.102050
9.
OchiaiTYoonS-HFlintNSharmaRChakravartyTKaewkesDet alTiming and outcomes of percutaneous coronary intervention in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. (2020) 125(9):1361–8. 10.1016/j.amjcard.2020.01.043
10.
YangYYangHPanJZhangG. Strategies to address extreme aortic tortuosity during transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2022) 15(7):791–2. 10.1016/j.jcin.2022.01.305
11.
HarloffMTPercyEDHirjiSAYazdchiFShimHChowdhuryMet alA step-by-step guide to trans-axillary transcatheter aortic valve replacement. Ann Cardiothorac Surg. (2020) 9(6):510–21. 10.21037/acs-2020-av-79
12.
Bob-ManuelTAlmusawiHRezanTKhairaHAkingbolaANasirAet alEfficacy and safety of transcarotid transcatheter aortic valve replacement: a systematic review. Cardiovasc Revasc Med. (2020) 21(7):917–26. 10.1016/j.carrev.2019.12.012
13.
ChamandiCAbi-AkarRRodés-CabauJBlanchardDDumontESpauldingCet alTranscarotid compared with other alternative access routes for transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2018) 11(11):e006388. 10.1161/CIRCINTERVENTIONS.118.006388
14.
LingMaJing-yaWangQian-weiXuXiao-linYu. Research progress on treatment strategies for simultaneous/staged percutaneous coronary intervention in patients with severe aortic valve stenosis and chronic coronary artery disease undergoing percutaneous aortic valve replacement surgery. Chin J Intervent Cardiol. (2024) 32(3):271–5. 10.3969/j.issn.1004-8812.2024.05.007
Summary
Keywords
transcatheter aortic valve replacement, percutaneous coronary intervention, transcarotid, aortic tortuosity, aortic stenosis
Citation
Chen W, Bao Y and Guo H (2025) Transcarotid transcatheter aortic valve replacement combined with percutaneous coronary intervention for severe aortic stenosis with coronary artery disease in a tortuous aortic arch: a Case Report. Front. Cardiovasc. Med. 12:1522100. doi: 10.3389/fcvm.2025.1522100
Received
03 November 2024
Accepted
30 April 2025
Published
16 May 2025
Volume
12 - 2025
Edited by
Xuchu Que, University of California, United States
Reviewed by
Alessandro Iadanza, Siena University Hospital, Italy
Sunny Goel, Mount Sinai Hospital, United States
Updates
Copyright
© 2025 Chen, Bao and Guo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Bao cooawf@163.com Hui Guo guohuiboy@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.