Abstract
The study presents a case of INOCA attributed to CMVD in a 53-year-old male patient experiencing exertional angina, despite the absence of significant coronary artery stenosis on angiography. The patient presented with reversible myocardial ischemia detected by myocardial perfusion imaging, with an ischemic area accounting for 12% of the left ventricular wall. Diagnostic tests revealed an elevated index of microcirculatory resistance (IMR = 46.3) and a quantitative flow ratio (QFR = 0.94), confirming CMVD. Genetic testing identified a NOTCH1 c.3862G>A variant in the proband and some family members, suggesting a potential contribution to CMVD pathogenesis through impaired vascular remodeling and microcirculatory regulation. After six months of targeted treatment with nicorandil, coenzyme Q10, trimetazidine, and rosuvastatin, the patient's symptoms resolved, and myocardial ischemia reversed. While an MYH7 variant was also detected, its clinical relevance was ruled out due to the family's absence of associated cardiomyopathy phenotypes. The NOTCH1 gene may play a potential role in INOCA caused by CMVD, however, further research is needed to elucidate its underlying regulatory mechanisms. The findings provide a foundation for precise diagnosis and personalized management of INOCA.
1 Introduction
INOCA is defined as all coronary stenoses <50%, with the 2019 European Society of Cardiology guidelines noting that ∼45% of patients presenting with angina or ischemia showed no obstructive stable coronary artery disease based on angiography or stress testing (1, 2). CMVD may be a key cause of ischemia with non-obstructive coronary arteries (INOCA) (3). Coronary microvascular dysfunction (CMVD) refers to ischemic heart disease caused by abnormalities in the structure and function of the coronary microcirculation. Among the 112 million angina patients worldwide, approximately 70% of them show no abnormalities upon coronary angiography but experience symptoms of coronary artery disease (4). The typical symptoms of CMVD include effort-induced angina, and in resting conditions, patients may also experience atypical symptoms such as pressure-like discomfort or intermittent chest pain (5).
Although experimental studies show that CMVD can induce myocardial ischemia, its role in ischemic syndromes remains unclear in clinical practice. This is due to the inability of angiography to visualize small coronary artery abnormalities, the need for complex and time-consuming methods to assess microcirculation function invasively, and the lack of detectable signs of ischemia, such as stress-induced left ventricular contractile changes and ischemic metabolites, in patients suspected of having microvascular angina (6). The primary structural changes in coronary microvascular dysfunction include luminal narrowing of arterioles and capillaries, perivascular fibrosis, and capillary rarefaction, often associated with left ventricular hypertrophy, hypertrophic cardiomyopathy, and hypertensive heart disease, leading to impaired coronary blood flow (7). Over 50% of ischemic heart disease susceptibility is linked to genetic variants, primarily single-nucleotide polymorphisms identified through genome-wide association studies, which can predispose individuals to CMVD, leading to ischemia and myocardial infarction in non-obstructive coronary arteries by affecting proteins involved in coronary blood flow regulation (8).
This paper reported a case of INOCA caused by microcirculation disturbance, diagnosis, treatment and follow-up, and genetic study of the proband and her family members.
2 Case report
A 53-year-old male patient presented to our hospital in January 2024 with intermittent chest pain in the anterior chest region following exertion, radiating to the left shoulder, left arm, and back. The pain was accompanied by sensations of choking, chest tightness, and a feeling of obstruction. Upon inquiry, the symptoms were found to be activity-related, occurring after walking approximately 100 m, with episodes lasting 3–5 min and occurring frequently. The patient was admitted for further diagnosis and treatment.
Physical examination on admission revealed a heart rate of 78 bpm and blood pressure of 127/71 mmHg. An electrocardiogram showed sinus rhythm, ST-T wave changes, and left bundle branch block, indicating myocardial ischemia. Echocardiography revealed aortic sclerosis, mild regurgitation of the mitral and tricuspid valves, normal cardiac structure, and a normal left ventricular ejection fraction. The patient had significant angina symptoms classified as Grade III according to the Canadian Cardiovascular Society Angina Grading Scale (CCS). Coronary angiography indicated mild coronary stenosis <50% (Figure 1). Myocardial perfusion imaging showed mild to moderate reversible myocardial ischemia in a portion of the left ventricular apex and part of the interventricular septum, involving approximately 12% of the left ventricular wall. Based on coronary angiography and imaging results, the patient exhibited slight enlargement of the left ventricular cavity during stress imaging compared to rest imaging, with a decrease in the left ventricular ejection fraction, suggesting a possible association with microcirculatory dysfunction (Figure 2).
Figure 1
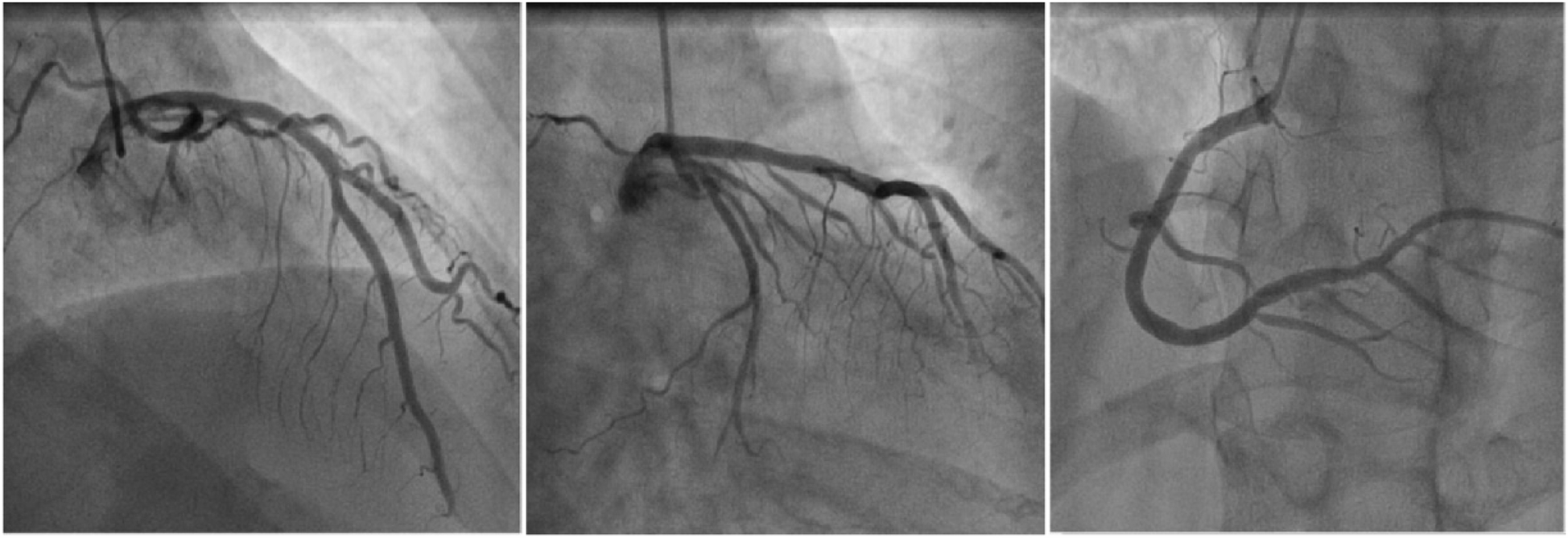
Schematic diagram of coronary angiography. From left to right are the left anterior descending artery, circumflex artery, and right coronary artery.
Figure 2
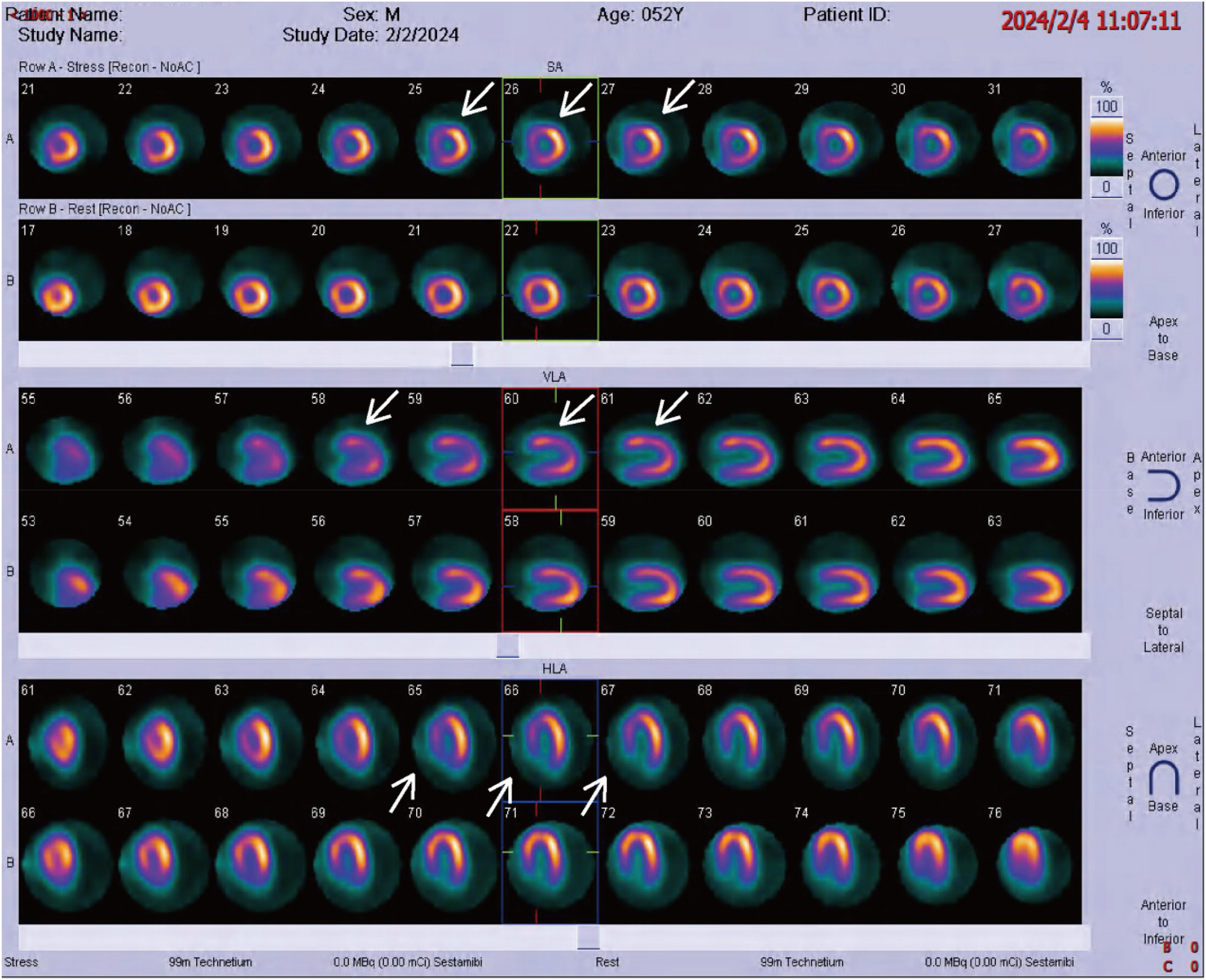
Radionuclide myocardial perfusion imaging, with white arrows indicating myocardial ischemia.
Subsequently, coronary microcirculatory resistance index (IMR) and quantitative flow ratio (QFR) testing were performed. The stenosis rate of the left anterior descending (LAD) artery ranged from 22.5% to 29.2%, all <50%. The QFR of the LAD was 0.94, and the IMR was 46.3, well above the threshold of 25, indicating coronary microvascular dysfunction (CMVD) (Figure 3).
Figure 3
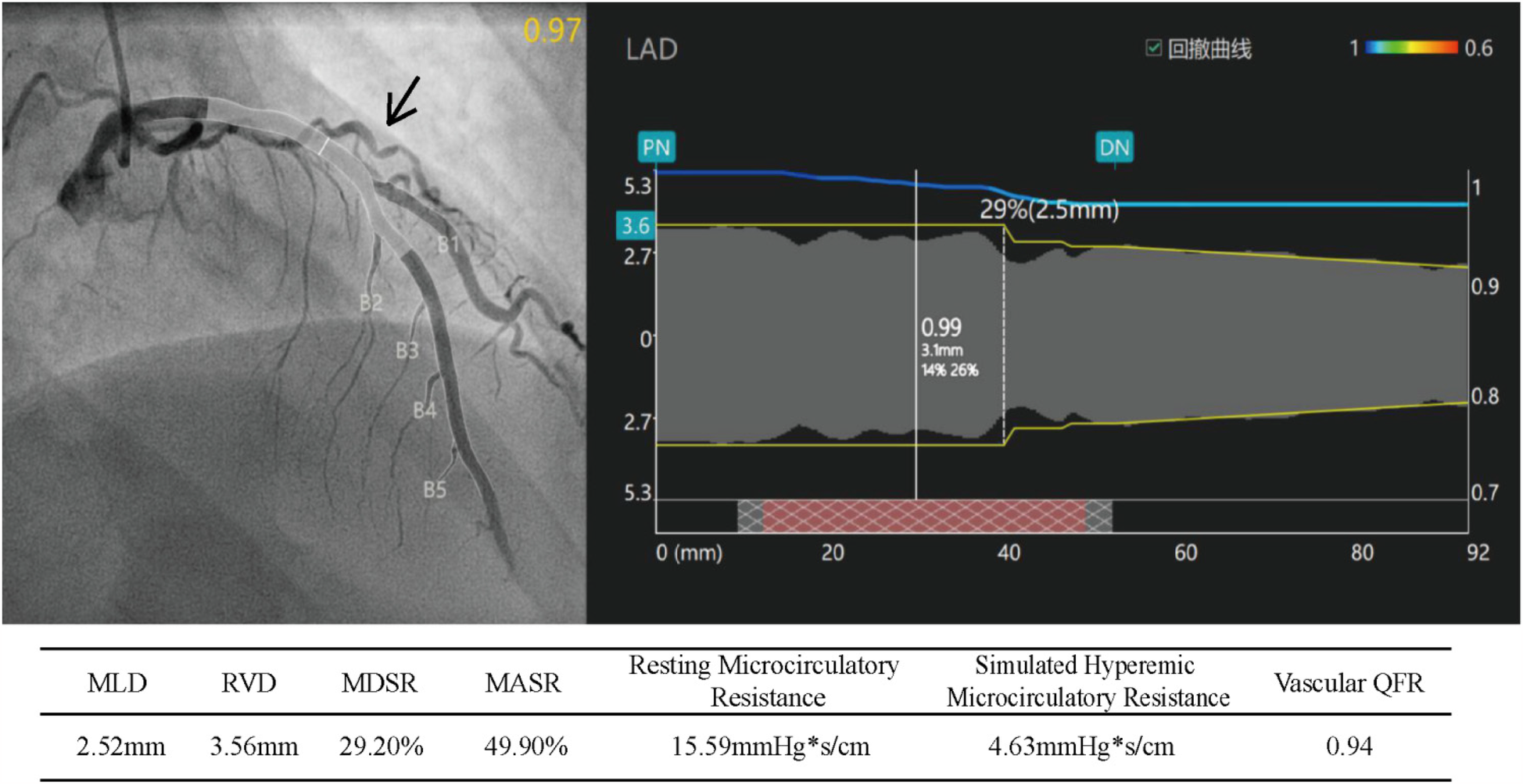
Index of microcirculatory resistance (IMR) and quantitative flow reserve (QFR) of the left anterior descending artery (IMR = simulated hyperemic microcirculatory resistance × 10). MLD, minimum lumen diameter; RVD, reference vessel diameter; MDSR, maximum diameter stenosis rate; MASR, maximum area stenosis rate.
Additionally, to rule out myocardial disease as the cause of the patient's chest tightness and other discomforts, cardiac MRI with contrast enhancement was performed. The left heart function and structure showed no significant abnormalities, no obvious myocardial fibrosis, and a low left ventricular T1 mapping value (1,172 ± 80 ms). The patient was treated with oral nicorandil (5 mg, three times daily), coenzyme Q10 (10 mg, three times daily), trimetazidine (20 mg, three times daily), and rosuvastatin (5 mg, once in the evening). The patient was prescribed 5 mg of rosuvastatin to provide minimal vascular protection while avoiding unnecessary risks, given their normal lipid levels, lack of significant coronary stenosis, absence of inflammatory or oxidative stress markers, and the need to minimize potential side effects. Additionally, aspirin was not administered to the patient due to the lack of a coronary artery disease diagnosis, the suspected presence of microvascular dysfunction based on the IMR index, and concerns regarding its potential side effects, such as platelet inhibition and an increased risk of bleeding. Six months later, the patient had no significant angina symptoms, and a follow-up myocardial perfusion scan showed no significant reversible ischemia (Supplementary Figure S1).
The proband's father (II-2) has a history of coronary artery disease and heart failure and was treated at our hospital's outpatient clinic. His electrocardiogram showed atrial arrhythmia, poor R-wave progression in the precordial leads, and ST-T changes. The patient has heart failure with New York Heart Association (NYHA) Functional Class IV, with suspected ischemic cardiomyopathy. Contrast-enhanced CTA revealed left main and three-vessel coronary artery disease, and ultrasound indicated aortic valve calcification with reduced ejection fraction.
I-1 (the proband's grandmother) died at age 60 from lung cancer, and I-2 (the proband's grandfather) died suddenly at age 62, possibly from a myocardial infarction. III-3 (the proband's brother) is asymptomatic, and his electrocardiogram showed no abnormalities. IV-1 (the proband's son) had a normal electrocardiogram (Supplementary Figure S2).
The proband's grandparents have already passed away, so blood samples could not be obtained and were therefore excluded from the analysis. Exome sequencing of family members revealed a novel heterozygous mutation (c.5647G>A p.Glu1883Lys) in exon 38 of the MYH7 gene on chromosome 14, which is a missense mutation, present in both II-2 and the proband. Additionally, a novel heterozygous mutation (c.3862G>A p.Val1288Ile) was found in exon 23 of the NOTCH1 gene, present in II-2, III-2, III-3, and IV-1, which is also a missense mutation (Figure 4). According to the ACMG guidelines, the MYH7 gene mutation was classified as PM2_Supporting + PP3, and the NOTCH1 mutation was classified as PM2_Supporting.
Figure 4
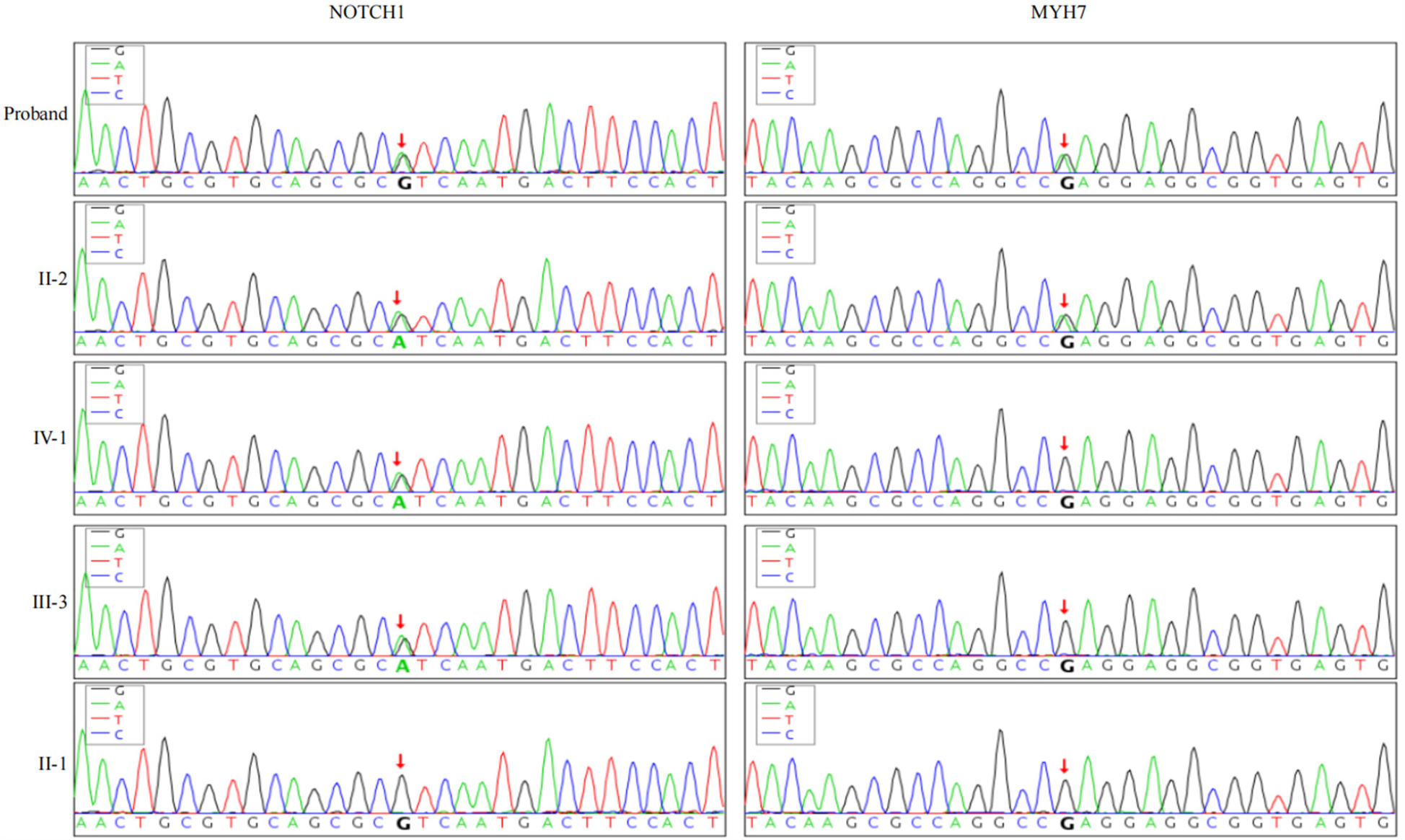
Genetic sequencing results of NOTCH1 and MYH7 genes in family members: the proband and his father carry heterozygous variants NOTCH1 c.3862G>A and MYH7 c.5647G>A. The proband's son and brother carry only the heterozygous NOTCH1 c.3862G>A variant, while the proband's mother has wild-type alleles for both genes.
3 Discussion
Ischemic heart disease is a leading cause of mortality and morbidity worldwide. In clinical practice, patients with coronary artery disease (CAD) may show normal or non-obstructive coronary arteries upon coronary angiography, which could be attributed to coronary microvascular dysfunction (CMVD) (9). According to the guidelines of the European Society of Cardiology (ESC), invasive methods such as Coronary Flow Reserve (CFR) and IMR play a crucial role in assessing coronary microvascular function, especially in patients with INOCA (10). Canu M. et al. highlighted that IMR and HMR are essential for the early detection of severe CMVD in STEMI patients, serving as important prognostic markers and potential therapeutic targets for clinical intervention (11). It is currently believed that patients with coronary microvascular dysfunction are at higher risk of developing hypertrophic cardiomyopathy, dilated cardiomyopathy, Fabry disease, aortic valve stenosis, and stable angina (12). Exogenous microvascular dysfunction caused by myocardial cell hypertrophy, cardiomyopathy, or left ventricular diastolic dysfunction may play a crucial role in the initiation of myocardial ischemia or necrosis (13). In our case, despite the presence of coronary artery stenosis, the stenosis rate was less than 50%, which could not explain the coronary ischemia and angina symptoms. However, based on myocardial scintigraphy, cardiac MRI, and stress imaging, we speculate that the symptoms may be due to a combination of microvascular dysfunction and coronary artery stenosis. The patient's left anterior descending (LAD) coronary artery QFR was 0.94, and the microvascular resistance index (IMR) was 46.3, much higher than the diagnostic threshold of IMR > 25 (14, 15), with increased microvascular resistance in the LAD territory corresponding to the myocardial ischemia seen on the scintigraphy. This suggests that the myocardial ischemia in this patient was caused by coronary microvascular dysfunction, which was significantly reversed after six months of treatment aimed at improving coronary microcirculation.
Although genetic testing identified MYH7 gene mutations in both the proband and his father, these mutations are primarily associated with hypertrophic cardiomyopathy and dilated cardiomyopathy (16, 17), and neither the proband nor his family members exhibited related clinical symptoms. Thus, we exclude the pathogenic effect of this mutation. Mutations in the NOTCH1 gene are known to cause aortic valve stenosis and calcific atrioventricular disease, which are important factors in the development of heart disease (18). Activation of NOTCH1 in myocardial cells is closely related to myocardial injury repair (19), and binding of NOTCH1 signaling proteins to Jagged1 reduces fibrotic tissue proliferation, increases microvascular density, and inhibits cardiac endothelial-mesenchymal transition (EMT), thereby preventing myocardial fibrosis (20). While activation of the Notch signaling pathway promotes Nrg-1 transcription and inhibits endothelial cell apoptosis by facilitating NICD binding to histone acetylase GCN5, forming a regulatory complex that enhances GCN5 recruitment to the Nrg-1 promoter, modulates histone acetylation via H3K9Ac, and regulates myocardial microvascular endothelial cell function to mitigate coronary microvascular dysfunction (21). The VEGFA-NOTCH1 signaling pathway can regulate trabecular blood vessel regeneration during EMT transformation, which helps prevent trabecular myocardial damage and promotes normal coronary microcirculation (22). Therefore, based on the phenotypes of the proband and his father, we infer that the heterozygous mutation of NOTCH1 c.3862G>A may be an important factor in the development of CMVD in the family. The differences in the copy number variation of the NOTCH1 gene and the resulting gene dosage effect (23, 24) may explain why the patient's brother and son have no symptoms yet. Although there are reports on the relationship between the NOTCH1 signaling pathway and aortic valve lesions, valve calcification, and atrioventricular fibrosis (25), as well as its close involvement in coronary artery development and migration (26), the relationship and regulatory mechanisms of the NOTCH1 signaling pathway in endothelial dysfunction are still unclear.
The findings of this study suggest that the NOTCH1 signaling pathway may play a potential role in regulating vascular network remodeling and warrants further basic research to clarify the exact regulatory mechanisms. Due to the limited number of family members, we need more cases and larger families to confirm the role of NOTCH1 gene variants in INOCA caused by CMVD.
Statements
Data availability statement
The datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by the study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Tianjin Chest Hospital (protocol code 2024YS-059-01 and date of approval: 13 Sep 2024). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WQ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YZ: Data curation, Methodology, Writing – review & editing, Formal analysis, Software. LW: Methodology, Software, Supervision, Writing – review & editing. KH: Investigation, Validation, Visualization, Writing – review & editing. TL: Data curation, Formal analysis, Writing – review & editing. JL: Supervision, Validation, Visualization, Writing – review & editing. HC: Supervision, Validation, Visualization, Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (grant No. 82200310) and the Tianjin Key Medical Discipline (Specialty) Construction Project (grant No. TJYXZDXK-055B).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1556064/full#supplementary-material
References
1.
Reynolds HR Diaz A Cyr DD Shaw LJ Mancini GBJ Leipsic J et al Ischemia with nonobstructive coronary arteries: insights from the ISCHEMIA trial. JACC Cardiovasc Imaging. (2023) 16(1):63–74. 10.1016/j.jcmg.2022.06.015
2.
Boden WE De Caterina R Kaski JC Bairey Merz N Berry C Marzilli M et al Myocardial ischemic syndromes: a new Nomenclature to harmonize evolving international clinical practice guidelines. Circulation. (2024) 150(20):1631–7. 10.1161/CIRCULATIONAHA.123.065656
3.
Benenati S Campo G Seitun S Caglioni S Leone AM Porto I . Ischemia with non-obstructive coronary artery (INOCA): non-invasive versus invasive techniques for diagnosis and the role of #FullPhysiology. Eur J Intern Med. (2024) 127:15–24. 10.1016/j.ejim.2024.07.017
4.
Kunadian V Chieffo A Camici PG Berry C Escaned J Maas AHEM et al An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European society of cardiology working group on coronary pathophysiology & microcirculation endorsed by coronary vasomotor disorders international study group. EuroIntervention. (2021) 16(13):1049–69. 10.4244/EIJY20M07_01
5.
Schindler TH Dilsizian V . Coronary microvascular dysfunction: clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging. (2020) 13(1 Pt 1):140–55. 10.1016/j.jcmg.2018.11.036
6.
Lanza GA Crea F . Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. (2010) 121(21):2317–25. 10.1161/CIRCULATIONAHA.109.900191
7.
Marano P Wei J Merz CNB . Coronary microvascular dysfunction: what clinicians and investigators should know. Curr Atheroscler Rep. (2023) 25(8):435–46. 10.1007/s11883-023-01116-z
8.
Severino P D'Amato A Prosperi S Myftari V Colombo L Tomarelli E et al Myocardial infarction with non-obstructive coronary arteries (MINOCA): focus on coronary microvascular dysfunction and genetic susceptibility. J Clin Med. (2023) 12(10):3586. 10.3390/jcm12103586
9.
Mileva N Nagumo S Mizukami T Sonck J Berry C Gallinoro E et al Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: systematic review and meta-analysis. J Am Heart Assoc. (2022) 11(7):e023207. 10.1161/JAHA.121.023207
10.
Crea F Camici PG Bairey Merz CN . Coronary microvascular dysfunction: an update. Eur Heart J. (2014) 35(17):1101–11. 10.1093/eurheartj/eht513
11.
Canu M Khouri C Marliere S Vautrin E Piliero N Ormezzano O et al Prognostic significance of severe coronary microvascular dysfunction post-PCI in patients with STEMI: a systematic review and meta-analysis. PLoS One. (2022) 17(5):e0268330. 10.1371/journal.pone.0268330
12.
Bradley C Berry C . Definition and epidemiology of coronary microvascular disease. J Nucl Cardiol. (2022) 29(4):1763–75. 10.1007/s12350-022-02974-x
13.
Smilowitz NR Toleva O Chieffo A Perera D Berry C . Coronary microvascular disease in contemporary clinical practice. Circ Cardiovasc Interv. (2023) 16(6):e012568. 10.1161/CIRCINTERVENTIONS.122.012568
14.
Rehan R Yong A Ng M Weaver J Puranik R . Coronary microvascular dysfunction: a review of recent progress and clinical implications. Front Cardiovasc Med. (2023) 10:1111721. 10.3389/fcvm.2023.1111721
15.
Ekenbäck C Jokhaji F Östlund-Papadogeorgos N Mir-Akbari H Linder R Witt N et al Changes in index of microcirculatory resistance during PCI in the left anterior descending coronary artery in relation to total length of implanted stents. J Interv Cardiol. (2019) 2019:1397895. 10.1155/2019/1397895
16.
Catrina BI Batar F Baltat G Bitea CI Puia A Stoia O et al A family with Myh7 mutation and different forms of cardiomyopathies. Biomedicines. (2023) 11(7):2065. 10.3390/biomedicines11072065
17.
Lee S Vander Roest AS Blair CA Kao K Bremner SB Childers MC et al Incomplete-penetrant hypertrophic cardiomyopathy MYH7 G256E mutation causes hypercontractility and elevated mitochondrial respiration. Proc Natl Acad Sci U S A. (2024) 121(19):e2318413121. 10.1073/pnas.2318413121
18.
Theodoris CV Zhou P Liu L Zhang Y Nishino T Huang Y et al Network-based screen in iPSC-derived cells reveals therapeutic candidate for heart valve disease. Science. (2021) 371(6530):eabd0724. 10.1126/science.abd0724
19.
Li Y Hiroi Y Liao JK . Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction. Trends Cardiovasc Med. (2010) 20(7):228–31. 10.1016/j.tcm.2011.11.006
20.
Zhou H Chen X Chen L Zhou X Zheng G Zhang H et al Anti-fibrosis effect of scutellarin via inhibition of endothelial–mesenchymal transition on isoprenaline-induced myocardial fibrosis in rats. Molecules. (2014) 19(10):15611–23. 10.3390/molecules191015611
21.
Qin XF Shan YG Dou M Li FX Guo YX . Notch1 signaling activation alleviates coronary microvascular dysfunction through histone modification of nrg-1 via the interaction between NICD and GCN5. Apoptosis. (2023) 28(1-2):124–35. 10.1007/s10495-022-01777-2
22.
Lu P Wu B Wang Y Russell M Liu Y Bernard DJ et al Prerequisite endocardial-mesenchymal transition for murine cardiac trabecular angiogenesis. Dev Cell. (2023) 58(9):791–805.e4. 10.1016/j.devcel.2023.03.009
23.
Arcaroli JJ Tai WM McWilliams R Bagby S Blatchford PJ Varella-Garcia M et al A NOTCH1 gene copy number gain is a prognostic indicator of worse survival and a predictive biomarker to a Notch1 targeting antibody in colorectal cancer. Int J Cancer. (2016) 138(1):195–205. 10.1002/ijc.29676
24.
Mase S Shitamukai A Wu Q Morimoto M Gridley T Matsuzaki F . Notch1 and Notch2 collaboratively maintain radial glial cells in mouse neurogenesis. Neurosci Res. (2021) 170:122–32. 10.1016/j.neures.2020.11.007
25.
Kraler S Blaser MC Aikawa E Camici GG Lüscher TF . Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J. (2022) 43(7):683–97. 10.1093/eurheartj/ehab757
26.
Moore P Murdock P Ramanathan A Sathyamoorthy M . A contemporary review of the genomic associations of coronary artery myocardial bridging. Genes (Basel). (2023) 14(12):2175. 10.3390/genes14122175
Summary
Keywords
coronary arteries, microcirculation dysfunction, myocardial ischemia, angina, variation
Citation
Qi W, Zhang Y, Wang L, Hou K, Li T, Lang J and Cong H (2025) Coronary microcirculation dysfunction causing ischemia with non-obstructive coronary arteries: a case report. Front. Cardiovasc. Med. 12:1556064. doi: 10.3389/fcvm.2025.1556064
Received
06 January 2025
Accepted
28 March 2025
Published
15 April 2025
Volume
12 - 2025
Edited by
Antonio Maria Calafiore, Henry Dunant Hospital, Greece
Reviewed by
Sun-Joo Jang, Yale University, United States
Antonio Totaro, University of Molise, Italy
Updates
Copyright
© 2025 Qi, Zhang, Wang, Hou, Li, Lang and Cong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Hongliang Cong conghl_hh@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.