Abstract
Background:
Managing aortic paravalvular leak (PVL) regurgitation following multiple surgical aortic valve replacements (SAVRs) due to recurrent infective endocarditis (IE) presents significant clinical challenges.
Case summary:
A 46-year-old woman with a history of severe aortic regurgitation and an asymptomatic membranous ventricular septal defect underwent SAVR with a bioprosthetic aortic valve (Perimount 23 mm) in 2005. Concomitantly, a double-chambered right ventricle was diagnosed. Ten years later, due to recurrent IE, another bioprosthetic valve replaced the previous valve (Magna Ease #25). In 2018, she developed sepsis from Bordetella hinzii endocarditis, leading to a third SAVR in 2019, this time with a mechanical aortic valve (On-X® #23). In 2024, two-dimensional transesophageal echocardiography (TEE) revealed moderate-to-severe PVL regurgitation near the right coronary cusp. After a multidisciplinary evaluation, transcatheter PVL closure was planned. Under general anesthesia and TEE/angio-fluoroscopic guidance, the PVL was successfully crossed via the right femoral artery, and a 10 mm × 4 mm Occlutech paravalvular leak device was deployed. Post-procedural imaging confirmed effective PVL closure with a trace-mild residual leak.
Discussion:
This case highlights the feasibility of transcatheter PVL closure as a less invasive alternative for patients with multiple prior SAVRs and high surgical risk. Advanced imaging techniques were crucial in procedural success, ensuring precise device placement. A multidisciplinary heart team approach is essential for optimizing outcomes in complex valve pathology. Long-term follow-up is necessary to monitor the durability of the closure and potential complications.
Introduction
Paravalvular leaks (PVLs) are a known complication following surgical aortic (SAVR) and mitral (SMVR) valve replacements, as well as transcatheter aortic valve implantation (TAVI). They result from an incomplete seal between the prosthetic valve and native annulus due to tissue friability from infection (1) or calcification. PVLs are more common after SMVR (17%) than SAVR (10%) (2–5) and may appear intraoperatively or during follow-up. Late PVLs often stem from suture dehiscence due to prosthetic valve endocarditis (PVE) or residual annular calcifications (6). While 1%–5% of cases lead to heart failure (HF), hemolytic anemia (HA), or left ventricular enlargement (7), transcatheter PVL closure has become a less invasive alternative for high-risk surgical patients (8).
A double-chambered right ventricle (DCRV) is a rare congenital abnormality where hypertrophied muscle bundles divide the right ventricle into high- and low-pressure chambers. Typically diagnosed in childhood, a DCRV may persist into adulthood, often asymptomatically or mimicking other cardiovascular conditions, leading to misdiagnosis (9, 10).
Case summary
A 46-year-old woman with hypertension and dyslipidemia was admitted with congestive heart failure [New York Heart Association (NYHA) class III] in late March 2005. Her history included a silent membranous ventricular septal defect (pmVSD) and severe symptomatic aortic regurgitation. In April 2005, she underwent bioprosthetic aortic valve replacement (Perimount #23, Edwards Lifesciences, USA), with concurrent pmVSD closure using 2-0 Ti-Cron™ polyester sutures. She showed significant clinical improvement with optimal medical therapy and regular follow-up.
Ten years later, the aortic valve prosthesis was replaced with a second bioprosthetic valve (Magna Ease #25, Edwards Lifesciences, USA) due to degeneration. In December 2018, she was hospitalized for sepsis caused by Bordetella hinzii and Propionibacterium granulosum infective endocarditis. A 45-day antibiotic regimen led to the normalization of inflammatory markers. Persistent infection suggested an antibiotic-resistant biofilm on the prosthetic valve (11).
In January 2019, she underwent a third SAVR with a mechanical aortic valve (On-X® #23, Life Technologies, Inc.). Surgical inspection revealed significant paravalvular leakage at the right coronary cusp and fibrous tissue (pannus) extending into the prosthesis orifice. Cultures confirmed Acremonium species infection.
Subsequent imaging (2019–2021) showed mild residual PVL regurgitation with normal valve function. However, by 2024, two-dimensional transesophageal echocardiography (2D TEE) and multidetector computed tomography angiography (MDCTA) confirmed moderate-severe PVL regurgitation (vena contracta width 0.6 cm) near the right coronary cusp (Figures 1, 2). A coincidental DCRV was also identified (Figure 3, Supplementary Video 1). Given the prohibitive surgical risk, transcatheter PVL closure was planned.
Figure 1
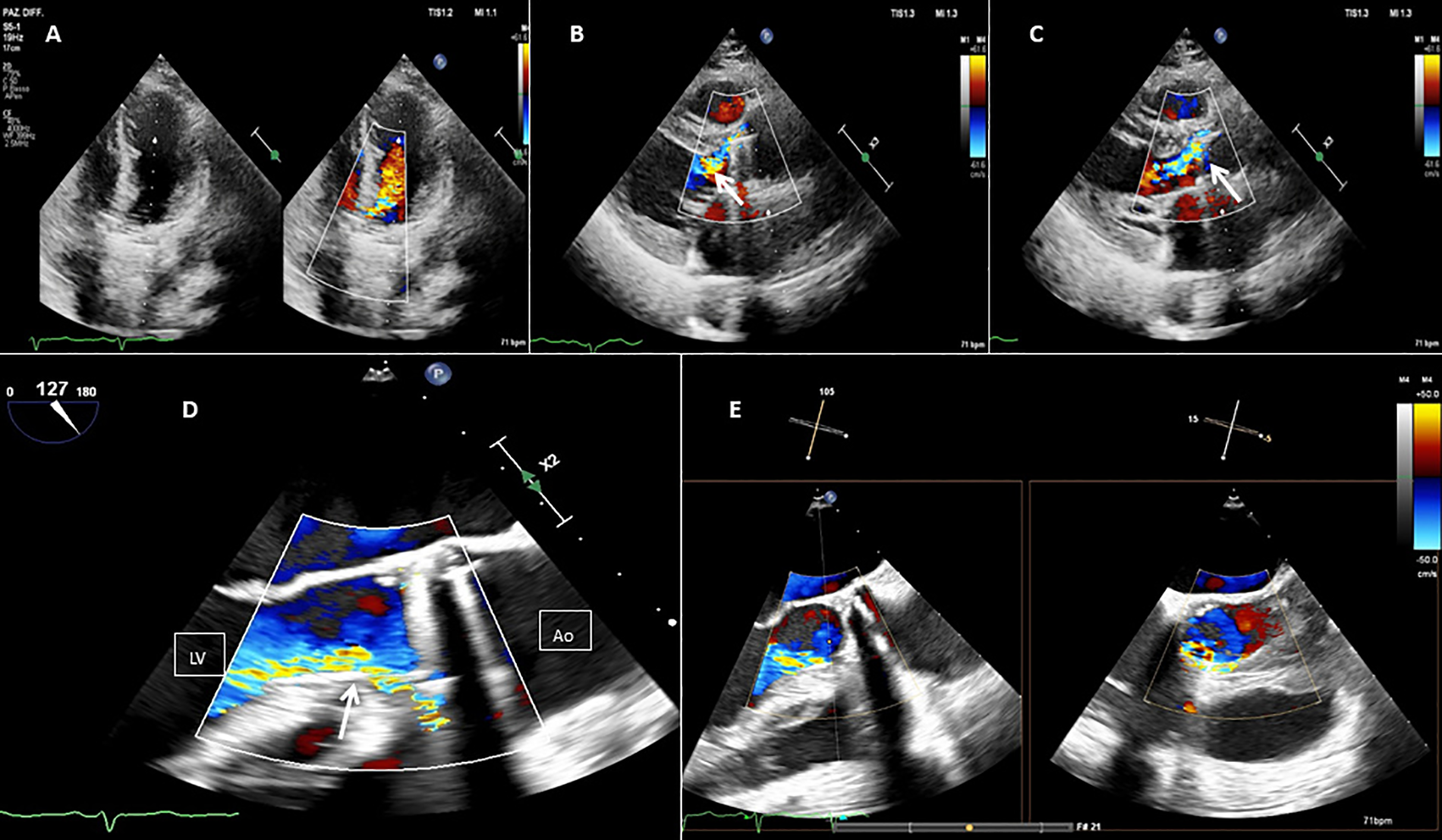
Baseline 2D TEE color Doppler at the apical five chamber view (A), at the parasternal long axis view (B,C), at the mid-esophageal (ME) long axis view (D), and at the ME long axis view with Xplane (E), showing an On-X® prosthetic aortic valve with moderate-to-severe regurgitation (orange arrow) through a long paravalvular leakage. Red line, PVL; LV, left ventricle; Ao, aortic valve.
Figure 2
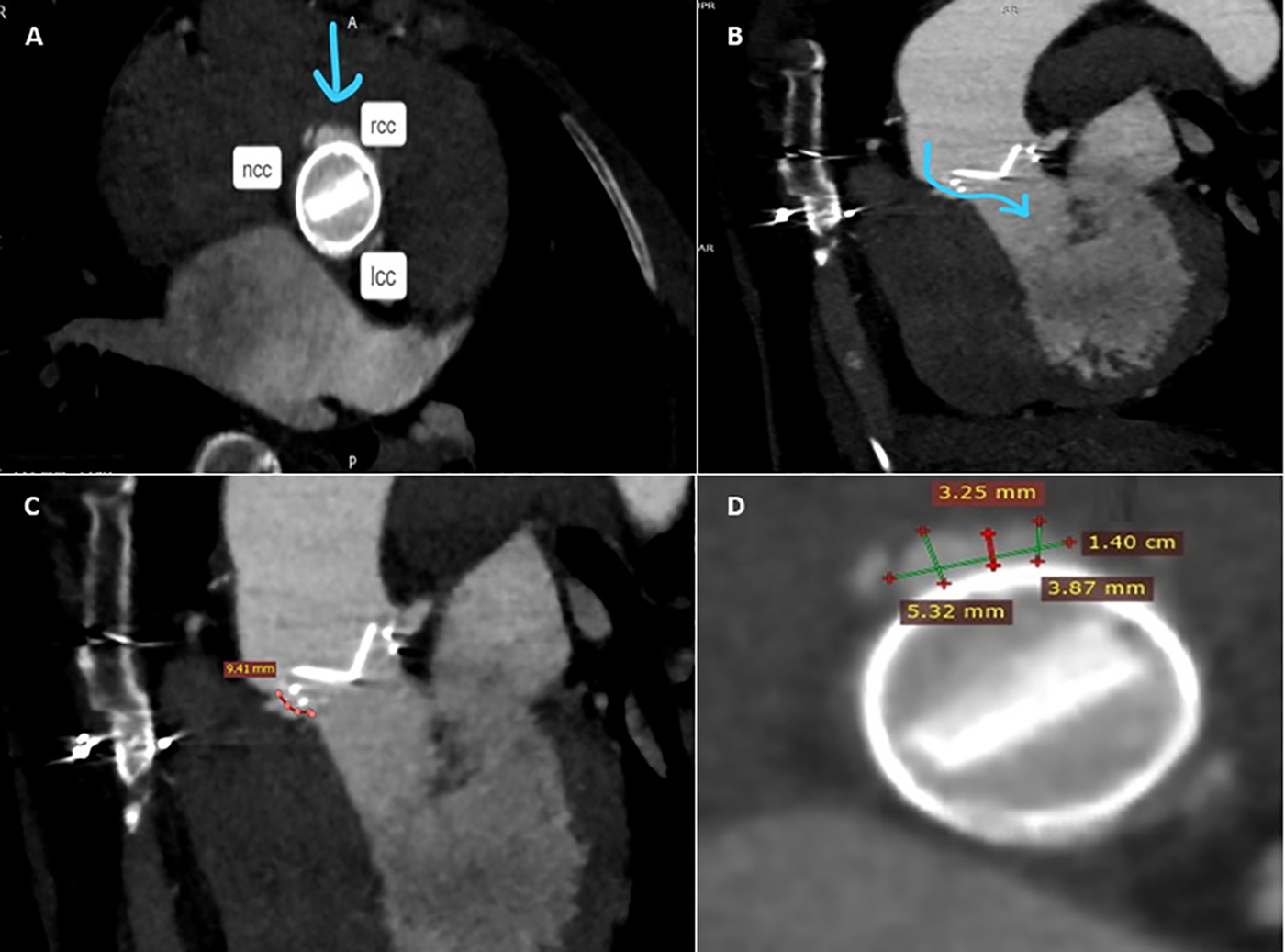
Pre-procedure MDCTA scans with acquired frames confirming the presence of the paravalvular leak, located in close proximity to the right coronary cusp (A), and its channel configuration and length (B,C). Short axis view of the aortic mechanical prosthetic valve showing multiple measurements of the long crescent-shaped PVL (D). rcc, right coronary cusp; lcc, left coronary cusp; ncc, non-coronary cusp.
Figure 3
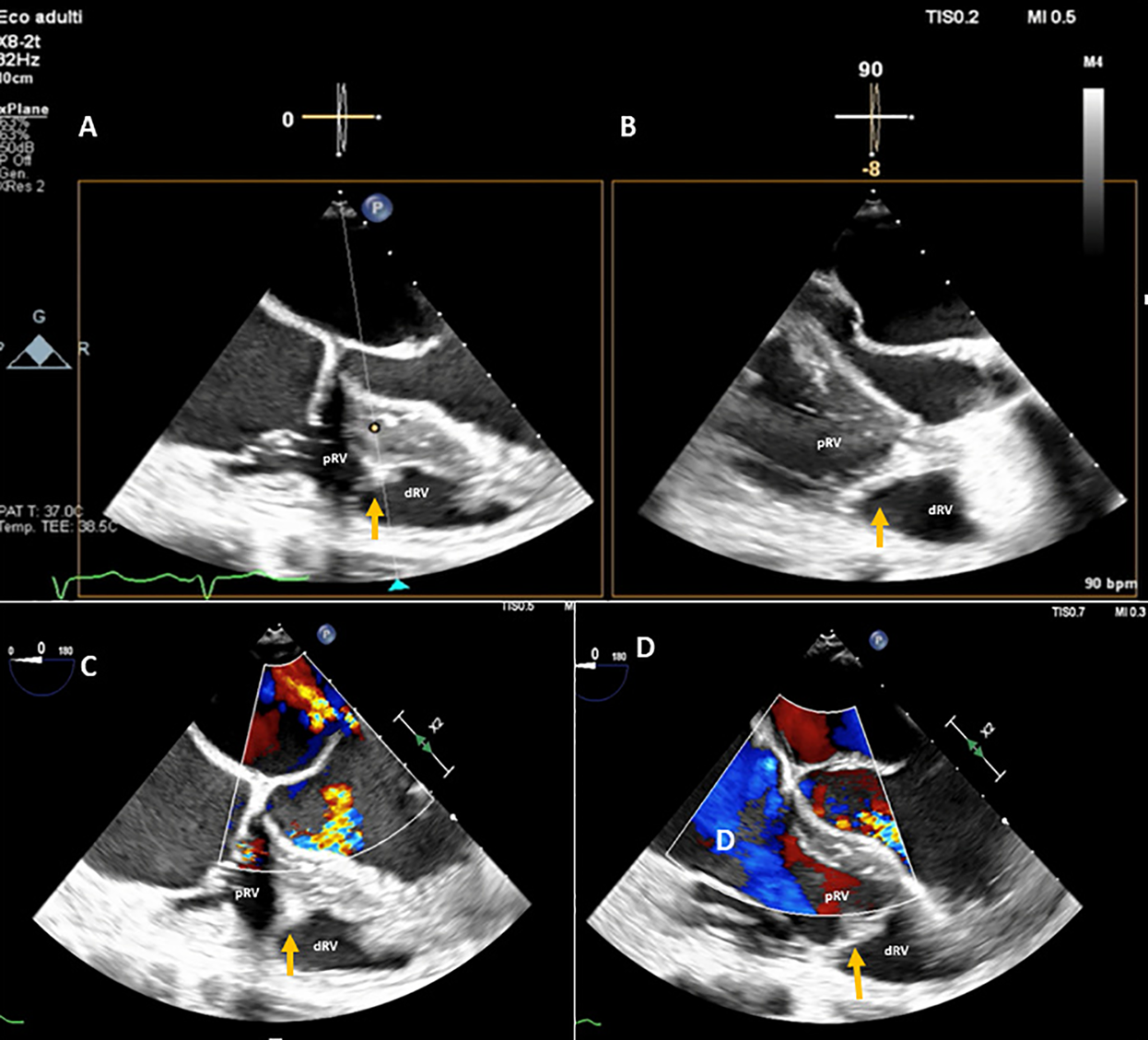
A DCRV created by an anomalous muscle band (orange arrow) diagnosed with TEE by the presence of a proximal high-pressure region under the tricuspid valve and a low-pressure distal region near the pulmonary valve outflow tract. (A,B) TEE transgastric RV inflow-outflow view (double orifice). (C,D) TEE color Doppler mid-esophageal four-chamber view focused on the RV. pRV, proximal right ventricle; dRV, distal right ventricle.
The procedure was performed in March 2024 under general anesthesia with TEE and angio-fluoroscopic guidance. A hydrophilic guidewire crossed the PVL and was replaced with an Amplatz Super Stiff™ wire. An 8-Fr delivery sheath was advanced, and a 10 mm × 4 mm Occlutech paravalvular leak device (PLD) was successfully deployed, achieving significant leak reduction without prosthetic interference (Figure 4, Supplementary Figure 1). Post-procedure imaging confirmed device stability with a trace-mild residual leak (Supplementary Figure 2). The patient was discharged on postoperative day 3 in better clinical conditions.
Figure 4
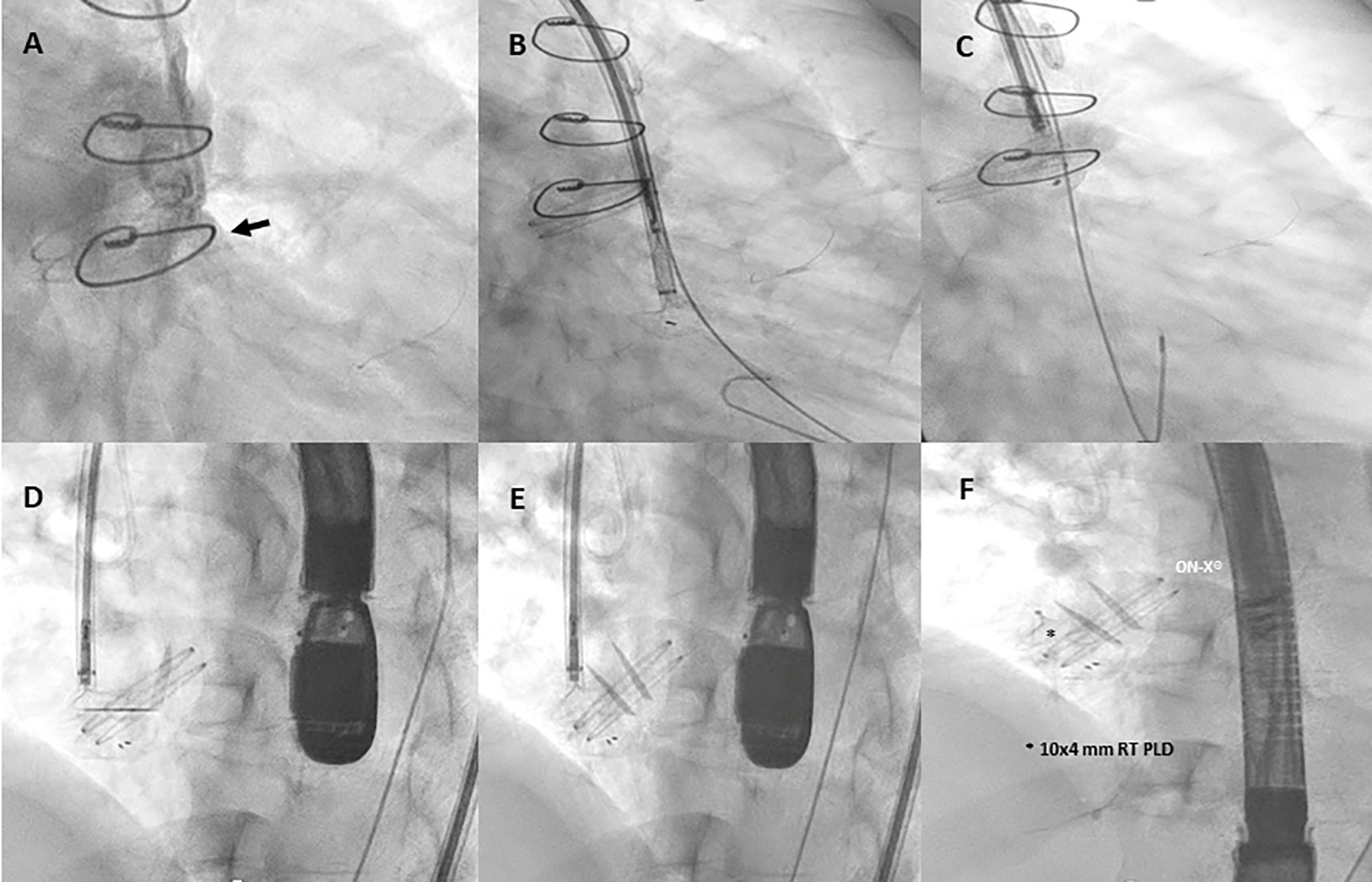
Fluoro-angiography procedural steps. (A) Right anterior oblique aortography by pigtail 6-Fr showing PVL regurgitation (black arrow). (B,C) The left disc of a 10 mm × 4 mm rectangular twist (RT) PLD (Occlutech, Helsingborg, Sweden) is opened into the left ventricle through an 8-Fr dedicated delivery sheath (Occlutech ODS III) across the leakage, with a buddy wire (black arrowhead) at its site; (D–F) 10 mm × 4 mm RT PLD successfully deployed and released within the leak without interfering with normal leaflet movement of the On-X® prosthetic aortic valve.
Finally, 3- and 12-month follow-ups confirmed clinical improvement, enhanced quality of life, and device stability with a persistent trace-mild residual leak.
Discussion
PVE accounts for 20% of infective endocarditis cases, leading to valve dysfunction, heart failure, and increased mortality (12, 13). PVE is more frequent in bioprosthetic valves, particularly porcine, due to susceptibility to bacterial colonization (14–16). Our case of B. hinzii and P. granulosum endocarditis is rare. B. hinzii, an emerging Gram-negative pathogen, is often resistant to antibiotics, while P. granulosum requires prolonged culture incubation for detection (17, 18).
These device-related microorganisms can be difficult to eradicate by antibiotic therapy alone. Bacterial biofilms are communities of various microorganisms that adhere to any surface and are enveloped within extracellular polymeric substances (19). Disruption of the bacterial biofilm plays an essential role in recovering the causative agent in the culture. Certain culture and nucleic acid amplification techniques are more accurate in guiding directed treatment regimens. It is clear that diagnostic testing will need to evolve beyond the standard current microbiological procedures to include nucleic acid detection, enhanced culture techniques, novel microbe imaging, and local immune response (20).
Transcatheter PVL closure is a minimally invasive alternative for high-risk patients who are often ineligible for redo surgery (21). PVLs are very heterogeneous in their location, size, and shape, and their track can be tortuous, serpiginous, or heavily calcified.
Undoubtedly, aortic PVL closure is a complex and challenging procedure with several periprocedural complications, such as device malposition or embolization and residual regurgitation, requiring optimal patient selection and comprehensive intraprocedural imaging guidance. Furthermore, a successful PVL reduction results in acute and long-lasting symptomatic improvements in clinical parameters and NYHA class, reducing the need for repeat cardiac surgeries and blood transfusions (22).
PVL closure has been performed in the past with multiple off-label devices that were not specifically designed for the purpose, which had inappropriate designs, shapes, and sizes. In previous studies with off-label devices, technical success rates varied between 62% (23) and 87% (5).
Notably, the Occlutech PLD, designed specifically for PVL closure, offers improved stability and sealing, achieving procedural success in over 90% of aortic PVL cases (24).
DCRV, a rare congenital defect (0.5%–2% of congenital heart disease), typically presents in childhood but can persist asymptomatically in adults (25–29). It results from hypertrophic muscle bands dividing the right ventricle into high- and low-pressure chambers. Though often misdiagnosed, our patient remained asymptomatic with a low-pressure gradient, requiring only medical follow-up.
Conclusion
This case highlights the effectiveness of transcatheter aortic PVL closure as a safe and less invasive alternative for high-risk patients with recurrent infections. The concomitant asymptomatic DCRV required no intervention.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because this was the standard of care practice in our hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
CS: Data curation, Investigation, Methodology, Writing – original draft. AT: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. GT: Data curation, Investigation, Methodology, Writing – original draft. AR: Data curation, Investigation, Methodology, Writing – original draft. PA: Data curation, Formal analysis, Software, Writing – original draft. CG: Data curation, Investigation, Methodology, Writing – original draft. EO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Francesco Paradisi, PhD Engineer, and all the Team at Occlutech Italia for their continuous logistic support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1558686/full#supplementary-material
Supplementary Figure 1Real-time three-dimensional transoesophageal echocardiography at aortic view showing the 10x4 mm rectangular twist (RT) PLD (Occlutech, Helsingborg, Sweden) in situ.
Supplementary Figure 2Post-procedure TTE color Doppler parasternal long axis view showing trace-mild residual leak. Ao, aorta; LV, left ventricle; LA, left atrium.
Supplementary Video 1Two-dimensional (2D) transesophageal echocardiography (TEE) color Doppler at transgastric RV inflow-outflow view (double orifice) (A) and TEE mid-esophageal (ME) short axis view (B) focused on right ventricle illustrating double-chambered right ventricle (DCRV) created by anomalous muscle band (orange arrow) with the presence of a proximal high-pressure region under the tricuspid valve and a low-pressure distal region near the pulmonary valve outflow tract. pRV, proximal right ventricle; dRV, distal right ventricle.
References
1.
FowlerVGDurackDTSelton-SutyCAthanEBayerASChamisALet alThe 2023 Duke–international society for cardiovascular infectious diseases criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis. (2023) 77:518–26. 10.1093/cid/ciad271
2.
KligerCEirosRIsastiGEinhornBKligerCEirosRet alReview of surgical prosthetic paravalvular leaks: diagnosis and catheter-based closure. Eur Heart J (2013) 34:638–49. 10.1093/eurheartj/ehs347
3.
Cruz-GonzalezIRama-MerchanJCRodríguez-ColladoJMartin-MoreirasJDiego-NietoABarreiro-PerezMet alTranscatheter closure of paravalvular leaks: state of the art. Neth Heart J. (2017) 25:116–24. 10.1007/s12471-016-0918-3
4.
RuizCECohenHValle-FernandezRDJelninVPerkGKronzonI. Closure of prosthetic paravalvular leaks: a long way to go. Eur Heart J Suppl. (2010) 12:E52–62. 10.1093/eurheartj/suq009
5.
GarcíaEArzamendiDJimenez-QuevedoPSarnagoFMartiGSanchez-RecaldeAet alOutcomes and predictors of success and complications for paravalvular leak closure: an analysis of the SpanisH realwOrld paravalvular LEaks closure (HOLE) registry. EuroIntervention. (2017) 12:1962–8. 10.4244/EIJ-D-16-00581
6.
BlackstoneEHKirklinJWKouchoukosNTDhasmanaJP. Factors associated with periprosthetic leakage following primary mitral valve replacement: with special consideration of the suture technique. Ann Thorac Surg. (1983) 35:170–8. 10.1016/S0003-4975(10)61456-7
7.
McCulloughPABarnardDClareREllisSJFlegJLFonarowGFet alAnemia and associated clinical outcomes in patients with heart failure due to reduced left ventricular systolic function. Clin Cardiol. (2013) 36(10):611–20. 10.1002/clc.22181
8.
OttoCMNishimuraRABonowROCarabelloBAErwinJPGentileFet al2020 ACC/AHA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. (2020) 77(4):e25–19. 10.1016/j.jacc.2020.11.018
9.
McElhinneyDBChatterjeeKMReddyVM. Double-chambered right ventricle presenting in adulthood. Ann Thorac Surg. (2000) 70:124–7. 10.1016/S0003-4975(00)01320-5
10.
NikolicAJovovicLIlisicTAntonicZ. An (in)significant ventricular septal defect and/or double-chambered right ventricle: are there any differences in diagnosis and prognosis in adult patients. Cardiology. (2016) 134:375–80. 10.1159/000444743
11.
SharmaSMohlerJMahajanSDSchwartzSABruggemannLAalinkeelR. Microbial biofilm: a review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms. (2023) 11:1614. 10.3390/microorganisms11061614
12.
MurdochDRCoreyGRHoenBMiroJMFowlerVGJrBayerASet alClinical presentation, etiology, and outcome of infective endocarditis in the 21stcentury: the international collaboration on endocarditis-prospective cohort study. Arch Intern Med. (2009) 169:463–73. 10.1001/archinternmed.2008.603
13.
GlaserNJacksonVHolzmannMJFranco-CerecedaASartipyU. Prosthetic valve endocarditis after surgical aortic valve replacement. Circulation. (2017) 136:329–31. 10.1161/CIRCULATIONAHA.117.028783
14.
BrennanJMEdwardsFHZhaoYO’BrienSBoothMDokholyanRet alLong-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation. (2013) 127:1647–55. 10.1161/CIRCULATIONAHA.113.002003
15.
GlaserNJacksonVHolzmannMJFranco-CerecedaASartipyU. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J. (2016) 37:2658–67. 10.1093/eurheartj/ehv580
16.
PerssonMGlaserNFranco-CerecedaANilssonJHolzmannMJSartipyU. Porcine vs bovine bioprosthetic aortic valves: long-term clinical results. Ann Thorac Surg. (2021) 111:529–35. 10.1016/j.athoracsur.2020.05.126
17.
GadeaICuenca-EstrellaMBenitoNBlancoAFernández-GuerreroMLValero-GuillénPLet alBordetella hinzii, a “new” opportunistic pathogen to think about. J Infect. (2000)40:298–9.
18.
GuíoLSarriáCde las CuevasCGamalloCDuartedJ. Chronic prosthetic valve endocarditis due to Propionibacterium acnes: an unexpected cause of prosthetic valve dysfunction. Rev Esp Cardiol. (2024) 77:976–84.
19.
ViolaGMDarouicheRO. Cardiovascular implantable device infections. Curr Infect Dis Rep. (2011) 13:333–42. 10.1007/s11908-011-0187-7
20.
KadirveluLSivaramalingamSSJothivelDChithiraiselvanDDKaraiyagowder GovindarajanDKandaswamyK. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr Res Microb Sci. (2024) 6:100231.
21.
TaramassoMMaisanoFDentiPGuidottiASticchiAPozolliAet alSurgical treatment of paravalvular leak: long-term results in a single-center experience (up to 14 years). J Thorac Cardiovasc Surg. (2015) 149:1270–5. 10.1016/j.jtcvs.2014.12.041
22.
Flores-UmanzorENogicJCepas-GuillénPHascoetSPyszPBazJAet alPercutaneous paravalvular leak closure after transcatheter aortic valve implantation: the international PLUGinTAVI registry. EuroIntervention. (2023) 19(5):e442–9. 10.4244/EIJ-D-22-01110
23.
AlkhouliMZackCJSarrafMEleidMFCabalkaAKReederGSet alSuccessful percutaneous mitral paravalvular leak closure is associated with improved midterm survival. Circ Cardiovasc Interv. (2017) 10:e005730. 10.1161/CIRCINTERVENTIONS.117.005730
24.
OnoratoEMAlamanniFMuratoriMSmolkaGWojakowskiWPyszPet alSafety, efficacy and long-term outcomes of patients treated with the Occlutech paravalvular leak device for significant paravalvular regurgitation. J Clin Med. (2022) 11:1978. 10.3390/jcm11071978
25.
HartmannAFTsifutisAAArvidssonHGoldringD. The two chambered right ventricle: report of nine cases. Circulation. (1962) 26:276–87. 10.1161/01.CIR.26.2.279
26.
GaliutoLO’LearyPWSewardJB. Double-chambered right ventricle: echocardiographic features. J Am Soc Echocardiogr. (1996) 9:300–3. 10.1016/S0894-7317(96)90144-3
27.
HoffmanPWojcikAWRozanskiJSiudalskaHJakubowskaEWodarskaEKet alThe role of echocardiography in diagnosing double chambered right ventricle in adults. Heart. (2004) 90:789–93. 10.1136/hrt.2003.017137
28.
LoukasMHousmanBBlaakCKralovicSTubbsRSAndersonRH. Double-chambered right ventricle: a review. Cardiovasc Pathol. (2013) 22:417–23. 10.1016/j.carpath.2013.03.004
29.
GargAAgrawalDSharmaGL. Isolated double-chambered right ventricle—a rare entity. J Cardiovasc Echogr. (2020) 30:162–4. 10.4103/jcecho.jcecho_36_20
Summary
Keywords
infective endocarditis, surgical aortic valve replacement, paravalvular leak regurgitation, paravalvular leak closure, double-chambered right ventricle, membranous ventricular septal defect
Citation
Sacra C, Totaro A, Triggiani G, Romano A, Astore P, Galluccio C and Onorato EM (2025) Case Report: Moderate-to-severe paravalvular leak regurgitation after recurrent prosthetic valve endocarditis in a patient with a double-chambered right ventricle associated with a restricted membranous ventricular septal defect. Front. Cardiovasc. Med. 12:1558686. doi: 10.3389/fcvm.2025.1558686
Received
10 January 2025
Accepted
22 April 2025
Published
14 May 2025
Volume
12 - 2025
Edited by
Badal Thakkar Sinai Hospital of Baltimore, United States
Reviewed by
Nagaraj Sanchitha Honganur, University of South Alabama, United States
Nismat Javed, Mount Sinai Morningside-BronxCare, United States
Amaraja Kanitkar, Henry Ford Hospital, United States
Ayush Arora, Cardiovascular Institute of the South, United States
Updates
Copyright
© 2025 Sacra, Totaro, Triggiani, Romano, Astore, Galluccio and Onorato.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eustaquio Maria Onorato eustaquio.onorato@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.