Abstract
Introduction:
Patients receiving thoracic radiotherapy (RT) have an increased risk of major adverse cardiac events (MACE) posttreatment. We utilized machine learning (ML) to discover novel predictors of MACE and validated them on an external cohort.
Methods:
This multi-institutional retrospective study included 984 patients [n = 803 non-small cell lung cancer (NSCLC), n = 181 breast cancer] treated with radiotherapy. Extreme gradient boosting was utilized to discover novel clinical, dosimetric, and anatomical features (CT-based cardiac substructure segmentations) associated with MACE in a cohort of locally advanced NSCLC patients. Fine–Gray regression was performed with non-cardiac death as a competing risk. External validation was performed utilizing independent cohorts of NSCLC or breast cancer patients.
Results:
In the discovery dataset (n = 701), 70 patients experienced MACE. ML modeling (training AUC, 0.68; testing AUC, 0.71) identified right and left atrial volume indices (RAVI and LAVI, respectively) as top predictors. After adjusting for baseline cardiovascular risk and known radiotherapy predictive factors, RAVI was associated with an increased risk of MACE [subdistribution hazard ratio (sHR) 1.02/unit, 95% confidence interval (CI): 1.00–1.04; p = 0.03]. In the validation cohorts (n = 102 NSCLC; n = 181 breast cancer), RAVI was associated with an increased risk of MACE (NSCLC: sHR 1.05, 95% CI: 1.001–1.106, p = 0.04; breast cancer: sHR 1.06, 95% CI: 1.01–1.11, p = 0.03). Similar findings were found for LAVI.
Discussion:
ML modeling identified right and left atrial enlargement as novel radiographic predictors for increased risk of MACE following chest radiotherapy, which was validated in independent breast and lung cancer datasets. Given that echocardiography studies have demonstrated the prognostic utility of atrial volume indices across cardiovascular risk groups, these findings warrant further study to identify additional strategies for upfront cardiovascular risk profiling.
Introduction
Radiotherapy (RT) forms the cornerstone of definitive treatment for many thoracic and chest malignancies (1–4); however, RT-associated cardiac toxicity remains a significant risk (2–5). Among primary chest malignancies, the highest rates of major adverse cardiac events (MACE) are observed in patients with lung cancer (6). For non-small cell lung cancer (NSCLC) patients undergoing definitive chemoradiation, RT-associated MACE occur early (median onset within 2 years) and are associated with increased mortality (3, 5).
Several studies have identified RT dose and volume metrics associated with increased risk of MACE following RT. These studies specifically outline cardiac substructure dose constraints for the left heart, left coronary arteries—left anterior descending (LAD) coronary artery and left circumflex (LCx) coronary artery—and left ventricle (LV) (2, 7). NSCLC patients are enriched for traditional patient-level cardiovascular risk factors, such as older age, smoking, and coronary heart disease (CHD) (8, 9). Indeed, 25%–40% of lung cancer patients have concomitant CHD (10, 11). Given elevated baseline cardiovascular risks, combined with known cancer therapy-related cardiovascular toxicities, NSCLC patients have an unmet clinical need for improved baseline cardiovascular risk stratification for MACE following chest radiotherapy.
Point-of-care interactions between patients and radiation oncologists represent informative time points for cardiovascular risk stratification. Mapping the contribution of predictors remains challenging because of complex interactions between the vast number of RT variables and patient-level cardiovascular risk factors. Tree-based machine learning (ML) captures imperceptible patterns from diverse inputs while limiting multicollinearity from high-dimensional data and offering reduced bias (omitted variable, confirmation, etc.). Given its continued applications (12–15), we utilized tree-based ML to identify MACE predictors from an expanded pool of features, including baseline cardiac health, cardiac substructure anatomy from volumetric CT segmentations, cancer-specific variables, and RT covariates including cardiac substructure dosimetry. These were identified in a cohort of locally advanced NSCLC patients. To evaluate generalizability, we validated a mixed cohort of patients who received chest RT for NSCLC or breast cancer at an external institution.
Materials and methods
Patient cohorts and treatment
This multi-institutional retrospective study included patients with locally advanced NSCLC or breast cancer treated with chest radiotherapy. ML modeling utilized 701 NSCLC patients treated between December 2003 and January 2014 at Brigham and Women's Hospital and Dana-Farber Cancer Institute, Boston, Massachusetts, denoted as the discovery dataset. External validation was performed on 273 patients treated between August 2005 and August 2021 at Cedars-Sinai Medical Center, Los Angeles, California, denoted as the validation dataset. To explore generalizability, the validation dataset included 181 breast cancer patients (16) and 102 NSCLC patients (17). Radiotherapy was delivered using 3D conformal RT (3D-CRT) or intensity-modulated RT (IMRT), excluding stereotactic body radiotherapy. For NSCLC patients, treatments were delivered free-breathing, typically based on internal-target volumes generated using four-dimensional CT scans (breath-hold or phase-based gating was not used). For the breast cancer validation cohort, deep-inspiration breath hold (typically for left-sided cancer) was utilized beginning in 2012. Other radiation planning specifics are previously described (2, 16, 17).
Clinical and radiotherapy features
Baseline clinical variables were curated from an in-depth medical record review, including CHD, congestive heart failure (CHF), arrhythmia, statin use, and cardiac risk factors (hyperlipidemia, hypertension (HTN), smoking, diabetes mellitus). CHD included coronary artery disease (CAD), heart failure (HF), or a CHD risk equivalent (peripheral vascular disease or stroke) (3). Cancer treatment-specific variables included chemotherapy, surgery, and RT. Cardiac substructure variables were generated (for the discovery cohort) by manual delineation of cardiac chambers and coronary arteries on non-electrocardiogram-gated radiotherapy planning CTs, as previously described (2, 17). For the validation cohort, an automated deep learning algorithm segmented cardiac substructures and was manually verified (CG) (18). RT dose was converted to an equivalent dose in 2 Gy fractions for tumor and normal tissue. The α/β ratios utilized for normal tissue (esophagus, lung, heart, and cardiac chambers) and NSCLC tumor were 3 and 10, respectively. Cardiac chamber volumes were indexed to body surface area (BSA), including right atrial volume index (RAVI) and left atrial volume index (LAVI). RT dosimetric variables, including mean (Gy), maximum (Gy), and volume (percent) receiving specific (X) gray dose [VX Gy (5 Gy increments)] were calculated for the lungs, esophagus, heart, and cardiac substructures (chambers and coronaries). For the training and test datasets, the primary endpoint was MACE (unstable angina, HF hospitalization or urgent visit, myocardial infarction, coronary revascularization, and cardiac death) following initiation of RT or after 30 days postoperatively, if applicable (19). For patients with preexisting cardiac comorbidity, MACE was recorded if the cardiac event was either greater in severity compared with the 6 months prior to radiotherapy or of a different MACE category (3, 20). Comprehensive, manual medical record review delineated cardiac events, as previously described (3).
Statistical analysis
Continuous variables were compared using the Wilcoxon rank sum test and categorical variables using the chi-square or Fisher exact test. Follow-up was calculated from RT start using the reverse Kaplan–Meier method. Extreme gradient boosting (XGBoost) (21) identified covariates related to developing MACE within the discovery cohort. The small proportion of missing data was binned into categorical unknown columns. The discovery dataset was split into training (75%) and test (25%) data (Supplementary Table S1). The training data constructed models, and the test dataset assessed model performance. XGBoost hyperparameters were bootstrap-tuned with a 50-round grid search during model training. The area under the receiver operator characteristic curve (AUC) evaluated model performance, and total gain ranked feature importance.
The Cedars-Sinai validation dataset was excluded from ML modeling and internal validation. Internal validation was performed on the top 15 ML-identified features utilizing the entire discovery dataset. Univariable and multivariable Fine–Gray regression models were utilized to evaluate the relationship between MACE and top predictors, with non-cardiac death as a competing risk. The multivariable analysis included ML-identified features that were significant in univariate analysis and variables with known prognostic value. Variance inflation factor and tolerance were used to assess multicollinearity. Given the multicollinearity between heart volume variables, when testing the multivariable association between MACE and a given substructure's volume, models were limited to a single cardiac volumetric parameter. External validation of the most predictive ML-identified features from the discovery dataset was performed using multivariable Fine–Gray regression on the Cedars-Sinai validation dataset. Analysis was performed utilizing R v4.2.2. and Stata SE, v17.0 (StataCorp LLC).
Results
Baseline characteristics
In the discovery cohort (n = 701), 345 (49.2%) were women, 252 (36.0%) had CHD, and 623 (88.9%) had clinical Stage III NSCLC. The median RT dose was 66.0 Gy (IQR, 56.0–66.0), with 539 (76.9%) receiving 3D-CRT. In the validation cohort of NSCLC patients (n = 102), 56 (54.9%) were women, 32 (31.4%) had CHD, and 74 (72.5%) had clinical Stage III disease. The median RT dose was 60.0 Gy (IQR, 55.8–60.0), with 20 (19.6%) receiving 3D-CRT. NSCLC patients from the validation cohort were generally older and had a lower prevalence of Stage III disease, lower 3D-CRT usage, and lower prescribed RT dose. Breast cancer patients from the validation cohort generally had lower rates of smoking and cardiac comorbidities (Table 1; Supplementary Table S2).
Table 1
| Characteristic | Discovery NSCLC cohort (n = 701) | Validation cohort | |||
|---|---|---|---|---|---|
| NSCLC (n = 102) | p (vs. discovery) | Breast cancer (n = 181) | p (vs. discovery) | ||
| Age median (IQR, years) | 65 (57, 73) | 71 (64, 77) | <0.0001 | 63 (53, 72) | 0.073 |
| Female sex | 345 (49.2%) | 56 (54.9%) | 0.46 | 181 (100%) | <0.001 |
| Tobacco | |||||
| Never | 56 (8.0%) | 24 (23.5%) | 114 (63.3%) | ||
| Current | 279 (39.8%) | 11 (10.8%) | 6 (3.3%) | ||
| Former | 366 (52.2%) | 67 (65.7%) | 60 (33.3%) | ||
| Unknown | 0 (0.0%) | 0 (0.0%) | <0.001 | 1 (0.6%) | <0.001 |
| Medical history | |||||
| HTN | 362 (51.6%) | 66 (64.7%) | 0.25 | 77 (42.5%) | 0.030 |
| HLD | 341 (48.6%) | 56 (54.9%) | 0.25 | 108 (59.7%) | 0.055 |
| DM | 97 (13.8%) | 30 (29.4%) | <0.001 | 25 (13.8%) | 1.0 |
| Stroke | 13 (1.9%) | 7 (6.9%) | 0.008 | 1 (0.6%) | 0.32 |
| CAD | 202 (28.8%) | 32 (31.4%) | 0.78 | 9 (5.0%) | <0.001 |
| CHF | 58 (8.3%) | 8 (7.8%) | 1.0 | 3 (1.7%) | 0.001 |
| Any CHDb | 252 (36.0%) | 32 (31.4%) | 0.44 | 97 (53.6%) | <0.001 |
| NSCLC clinical stage | |||||
| II III Unknown |
78 (11.1%) 623 (88.9%) 0 (0.0%) |
I–II: 19 (18.6%) III: 74 (72.5%) IV: 8 (7.8%) Unknown: 1 (0.9%) |
0.033 |
a
I-II: 137 (76.0%) III/IV: 44 (24.3%) Unknown: 0 (0.0%) |
NA |
| Tumor laterality | |||||
| Right | 392 (55.9%) | 68 (66.7%) | 84 (46.4%) | ||
| Left | 263 (37.5%) | 34 (33.3%) | 97 (53.6%) | ||
| NA/unknown | 46 (6.6%) | 0 (0.0%) | 0.23 | 0 (0.0%) | 0.002 |
| Treatment | |||||
| Definitive CRT | 405 (57.8%) | 63 (61.8%) | BCT: | ||
| RT alone | 56 (8.0%) | 4 (3.9%) | 122 (67.4%) | ||
| Neoadjuvant | 154 (22.0%) | 4 (3.9%) | Mastectomy: | ||
| Adjuvant | 86 (12.3%) | 31 (30.4%) | <0.001 | 59 (32.6%) | NA |
| RT technique | |||||
| 3D-CRT | 539 (76.9%) | 20 (19.6%) | 175 (96.7%) | ||
| IMRT | 162 (23.1%) | 82 (80.4%) | <0.001 | 6 (3.3%) | NA |
| RT dose | |||||
| Median (IQR, Gy) | 66.0 (56.0, 66.0) | 60.0 (55.8, 60.0) | <0.001 | 50.0 (42.7, 50.4) | NA |
Patient characteristics across discovery and external validation cohorts.
Individual values are listed to represent n (%) unless otherwise specified as median (IQR).
IQR, interquartile range; HTN, hypertension; HLD, hyperlipidemia; DM, diabetes mellitus; CAD, coronary artery disease; CHF, congestive heart failure; CHD, coronary heart disease; NSCLC, non-small cell lung cancer; CRT, chemoradiotherapy; BCT, breast-conserving treatment; Gy, Gray; 3D-CRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; NA, not applicable.
Pathological breast cancer stage.
CHD includes CAD, CHF, or CHD risk equivalent (stroke, peripheral artery disease).
ML identification of novel predictive features of MACE
In the discovery cohort, with over a median follow-up of 5.2 years (IQR, 3.4–7.8 years), there were 70 cases of MACE (10%) with a median time to MACE of 1.6 years (IQR, 0.5–2.8 years). The final model included 27 baseline characteristics, 164 cancer-specific or treatment-related variables, and 197 dose/volume variables (Supplementary Appendix). The training AUC for MACE was 0.68, and the testing AUC was 0.71 (Figure 1). The top predictive feature was RAVI, followed by lung V55Gy and CHD (Figure 2). Additional important predictors included cardiovascular risk factors (hypertension, CHF), RT dose (LCx V15Gy, LADV15Gy, lung V55Gy), and BSA-normalized cardiac substructure volumes (RAVI, LAVI, total heart volume, left main coronary artery volume). Multiple ML-identified features have been previously reported as predictors of MACE and/or cardiac toxicity (CHD, HTN, LCxV15Gy, and LADV15Gy). Novel features included RAVI, LAVI, and lung V55Gy.
Figure 1
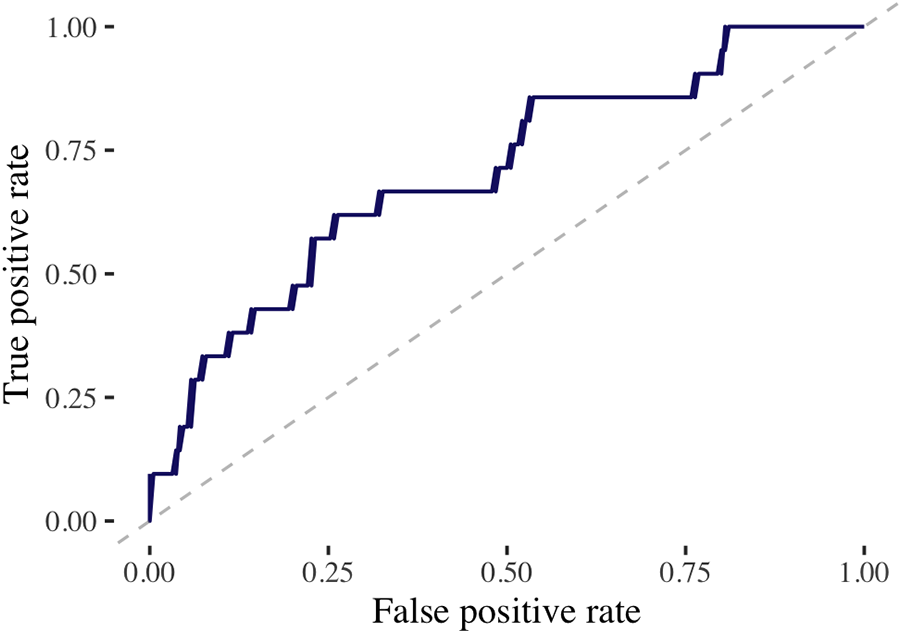
Performance of machine learning model on test dataset. This figure demonstrates the receiving operating characteristic curve for the extreme gradient boosting model to predict MACE in the test dataset utilizing the discovery cohort. The area under the curve (AUC) assesses the model’s accuracy. AUC, 0.71.
Figure 2
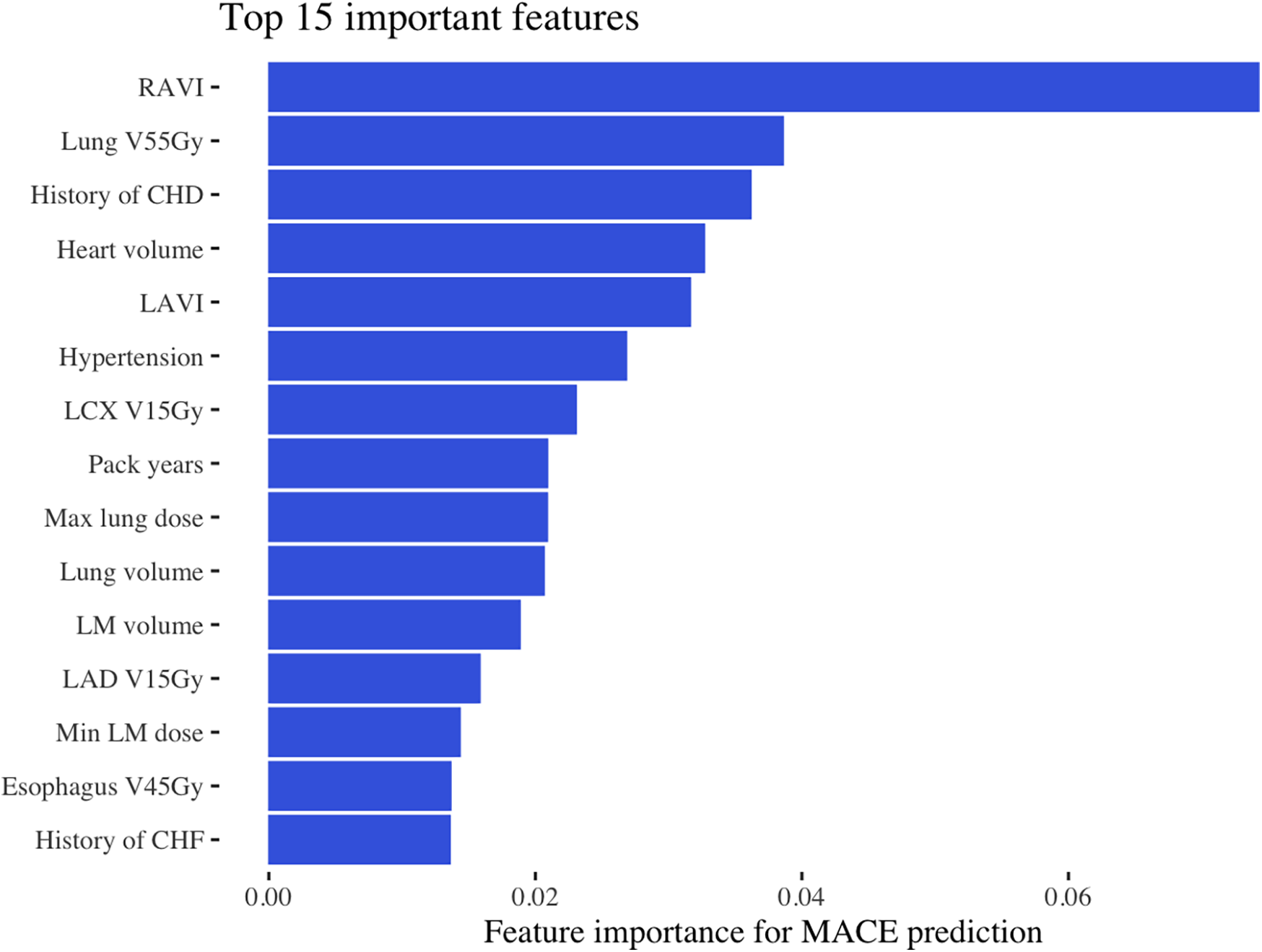
Most important features for MACE prediction. This figure shows the top 15 most important features identified by the machine learning model in the discovery dataset, ranked by total gain. RAVI, right atrial volume index; Gy, Gray; V55Gy, volume receiving 55 Gray; CHD, coronary heart disease LAVI, left atrial volume index; LCx, left circumflex artery; V15Gy, volume receiving 15 Gray; Dmin, dose minimum; V45Gy, volume receiving 45 Gray; CHF, congestive heart failure.
RAVI and LAVI predict MACE in the internal competing risk regression model
On univariate analysis, each unit (ml/m2) increase in RAVI was associated with a 2% increased risk of MACE [subdistribution hazard ratio (sHR) 1.02, 95% confidence interval (CI): 1.01–1.04; p = 0.001]. Additionally, each unit (ml/m2) increase in LAVI was associated with a 2% increased risk of MACE (sHR 1.02, 95% CI: 1.01–1.03; p = 0.001, Table 2). Lung V55Gy, lung volume and maximum dose, esophagus V45Gy, left main (LM) coronary artery minimum dose, and smoking pack-years were not significantly associated with MACE on univariable analysis. Notably, total heart volume and LM volume were significantly associated with MACE on univariable analysis. To limit multiple testing and collinearity, we focused exploration on RAVI and LAVI given RAVI's importance on ML analysis and the known impact of elevated atrial chamber volumes as clinical indicators for cardiac disease (22, 23). The median RAVI and LAVI values in the discovery cohort were 50.9 ml/m2 (IQR, 42.1–62.8 ml/m2) and 47.9 ml/m2 (IQR, 40.3–56.5 ml/m2), respectively. The cumulative incidence of MACE appeared similar for the first three RAVI quartiles but significantly increased for the highest quartile (RAVI ≥ 62.8 ml/m2; p = 0.02). LAVI showed similar results; the highest quartile (LAVI ≥ 56.5 ml/m2) trended toward significance (p = 0.052) (Figures 3A,B).
Table 2
| Covariable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | sHR (95% CI) | p-value | |
| Age | 1.03 (1.01–1.05) | 0 . 014 | 1.00 (0.97–1.03) | 0.99 |
| Sex, M (vs. F) | 1.11 (0.70–1.77) | 0.67 | – | |
| Smoking, pack-years | 1.01 (1.00–1.01) | 0.09 | – | |
| Hypertension | 3.61 (2.05–6.36) | <0.001 | 2.84 (1.54–5.23) | 0.001 |
| Hyperlipidemia | 1.19 (0.75–1.90) | 0.46 | – | |
| Diabetes | 2.09 (1.21–3.59) | 0.008 | 1.20 (0.66–2.16) | 0.55 |
| Arrhythmia | 2.09 (1.20–3.64) | 0.009 | 1.17 (0.61–2.26) | 0.63 |
| CHF | 4.04 (2.33–6.99) | <0.001 | ^ | |
| CHD | 3.68 (2.26–6.02) | <0.001 | 5.98 (2.99–11.94) | <0.001 |
| Surgery | 0.95 (0.58–1.55) | 0.83 | – | |
| Chemotherapy | 1.01 (0.37–2.78) | 0.98 | – | |
| 3D-CRT (vs. IMRT) | 2.63 (1.20–5.88) | 0.015 | 3.22 (1.42–7.14) | 0.005 |
| RAVI | 1.02 (1.01–1.04) | 0.001 | 1.02 (1.00–1.04) | 0.027 |
| Lung V55Gy | 0.99 (0.95–1.04) | 0.80 | – | |
| Heart volume | 1.01 (1.00–1.01) | <0.001 | # | |
| LAVI | 1.02 (1.01–1.03) | 0.001 | 1.00 (0.98–1.02) | 0.94 |
| LCx V15Gy | 1.01 (1.00–1.01) | 0.008 | # | |
| Lung Dmax | 1.01 (0.98–1.04) | 0.54 | – | |
| Lung volume | 1.00 (1.00–1.00) | 0.47 | – | |
| LM volume | 3.63 (1.75–7.54) | 0.001 | # | |
| LAD V15Gy | 1.01 (1.00–1.02) | 0.003 | 1.03 (1.02–1.04) | <0.001 |
| LM Dmin | 1.01 (1.00–1.02) | 0.10 | – | |
| Esophagus V45Gy | 1.01 (1.00–1.02) | 0.20 | – | |
| Interaction termsa | ||||
| CHD × RAVI | 1.02 (0.99–1.06) | 0.22 | – | |
| CHD × heart volume | 1.00 (0.99–1.01) | 0.74 | – | |
| CHD × LAVI | 1.00 (0.95–1.04) | 0.93 | – | |
| CHD × LCx V15Gy | 0.98 (0.97–0.99) | 0.003 | # | |
| CHD × LM volume | 0.59 (0.13–2.80) | 0.51 | – | |
| CHD × LAD V15Gy | 0.97 (0.96–0.99) | <0.001 | 0.97 (0.96–0.98) | <0.001 |
Fine–Gray regression model to predict MACE in the discovery cohort (n = 701).
M, male; F, female; CHF, congestive heart failure; CHD, coronary heart disease; 3D-CRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; RAVI, right atrial volume index; Gy, Gray; V55Gy, volume receiving 55 Gray; LAVI, left atrial volume index; Dmax, dose maximum; V15Gy, volume receiving 15 Gray; Dmin, dose minimum; V45Gy, volume receiving 45 Gray; LCx, left circumflex artery.
Bold indicates p < 0.05.
Interaction term between CHD as a dichotomous variable and ML-identified dose/volume or volume continuous variables (significant on univariable analysis).
Variable omitted due to being included within CHD variable.
Variable omitted due to collinearity and overfitting.
Figure 3
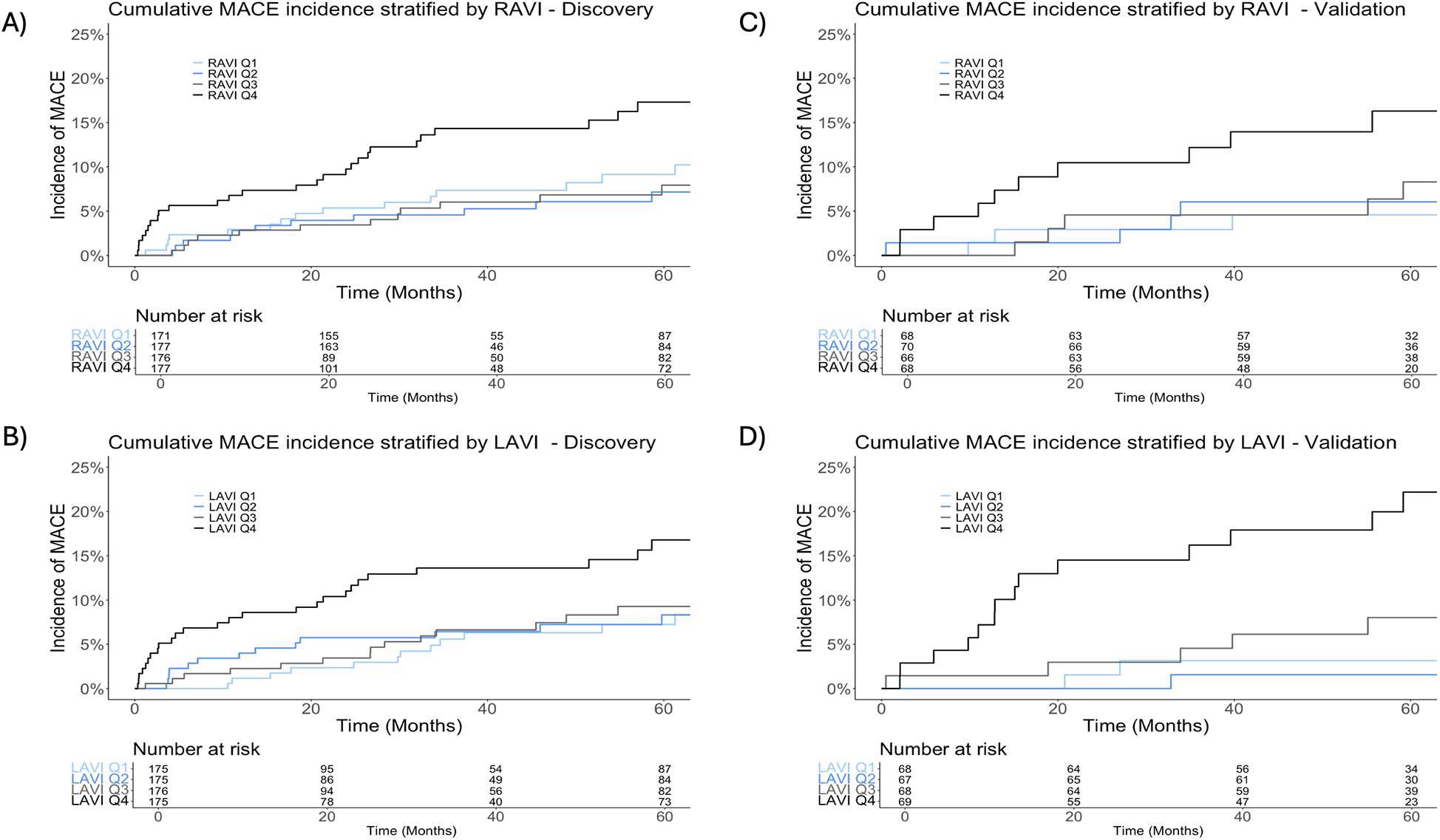
Cumulative incidence of major adverse cardiac events (MACE). Panels stratified by quartiles of right atrial volume indexed to body surface area (RAVI) and left atrial volume indexed to body surface area (LAVI) in the total (n = 701) discovery dataset (A,B) and the validation dataset (n = 283) (C,D).
After adjusting for age and ML-identified cardiovascular and cancer treatment factors, we observed similar results; each unit increase in RAVI was associated with a 2% increased risk of MACE (sHR 1.02, 95% CI: 1.01–1.04; p = 0.03). Among the baseline characteristics, CHD (sHR 5.98, 95% CI: 2.99–11.94; p < 0.001) and hypertension (sHR 2.84, 95% CI: 1.54–5.23; p = 0.001) significantly increased the risk of MACE. Among the RT and anatomical covariates, LADV15Gy (sHR 1.03, 95% CI: 1.02–1.04; p < 0.001) and utilization of 3D-CRT (vs. IMRT) (sHR 3.22, 95% CI: 1.42–7.14; p = 0.005) significantly increased risk of MACE (Table 2). LCxV15Gy also ranked highly but was excluded from the primary multivariable model due to its multicollinearity with LADV15Gy. A model substituting LADV15Gy with LCxV15Gy showed a significant relationship between MACE and RAVI (Supplementary Table S3). Given the collinearity between RAVI and LAVI, a separate analysis was performed using identical prognostic factors, which showed a significant relationship between LAVI and MACE. Each unit increase in LAVI was associated with a 1% increased risk of MACE (sHR 1.01, 95% CI: 1.00–1.03; p = 0.044), Supplementary Table S4.
External validation of RAVI and LAVI
With a median follow-up of 4.5 years (IQR, 2.3–8.0 years) in the NSCLC subset (n = 102) and 6.5 years (IQR, 5.1–7.7) in the breast cancer subset (n = 181), there were 26 cases of MACE (n = 11 NSCLC, n = 15 breast cancer). The median time to MACE was 1.8 years in the lung cohort (IQR, 1.3–4.3 years) and 5.7 years in the breast cancer cohort (IQR, 4.4–7.1 years). The median RAVI was 43.7 ml/m2 (IQR, 35.9–54.9 ml/m2) and 42.6 ml/m2 (IQR, 35.2–49.0 ml/m2) for the NSCLC and breast cancer subsets, respectively. The median LAVI was 41.9 ml/m2 (IQR, 35.4–51.9 ml/m2) and 40.9 ml/m2 (IQR, 34.9–48.0 ml/m2) for the NSCLC and breast cancer subsets, respectively. The 2-year cumulative incidence of MACE in the NSCLC subset was 10.3% (95% CI, 5.3%–17.3%). The 5-year cumulative incidence in the breast cancer subset was 7.3% (95% CI, 4.0%–12.0%). Stratifying by quartiles, the cumulative incidence was greatest among the highest RAVI quartile (≥49.9 ml/m2; p = 0.02) and highest LAVI quartile (≥49.6 ml/m2; p < 0.001) (Figures 3C,D).
After adjusting for age and baseline CHD, RAVI was associated with an increased risk of MACE (lung cancer subset: sHR 1.05, 95% CI: 1.00–1.11, p = 0.044; breast cancer subset, sHR 1.06, 95% CI 1.01–1.11; p = 0.025; Table 3). There was a significant interaction between CHD and RAVI in the breast cancer cohort (p = 0.041), such that the risk of MACE associated with RAVI was more pronounced in those without baseline CHD (sHR 1.07, 95% CI: 1.02–1.12; p = 0.009) than those with CHD (sHR 0.96, 95% CI: 0.86–1.06; p = 0.36). LAVI showed similar findings in the breast cancer and NSCLC subsets (Supplementary Table S5), but no significant interaction between CHD and LAVI was observed.
Table 3
| Covariable | Lung cancerc | Breast cancerc | ||
|---|---|---|---|---|
| sHR (95% CI) | p-value | sHR (95% CI) | p-value | |
| Age | 1.00 (0.94–1.06) | 0.98 | 1.06 (0.97–1.15) | 0.20 |
| Sex (M vs. F) | 1.90 (0.45–8.05) | 0.38 | Omitted | |
| Baseline CHD | 6.56 (0.08–536.76) | 0.40 | 402.90 (5.06–32,083.61) | 0.007 |
| RAVIa | 1.05 (1.00–1.11) | 0.044 | 1.06 (1.01–1.11) | 0.025 |
| CHD × RAVIb | 0.98 (0.90–1.06) | 0.63 | 0.91 (0.84–0.99) | 0.041 |
Multivariable Fine–Gray regression to predict MACE in external lung and breast cancer validation cohorts.
sHR, subdistribution hazard ratio; CHD, coronary heart disease; RAVI, right atrial volume index.
Continuous variable (ml/m2).
Interaction term between CHD as a dichotomous variable and RAVI as a continuous variable.
Of n = 94 (lung) and n = 178 (breast) with BSA available for volume normalization.
Discussion
In this multi-institutional retrospective study of nearly 1,000 patients with detailed cardiovascular and individual radiotherapy parameters, we utilized ML to analyze high-dimensionality clinical, anatomical, and dosimetric data. This allowed a less biased search through feature spaces to identify the most salient predictors. These data highlight readily available cardiovascular prognostic information acquired during radiation oncology point-of-care (routine CTs for RT planning). We report modest model performance for MACE prediction. Our ML framework not only identified risk factors consistent with previously reported (2) but also identified right and left atrial enlargement (RAVI, LAVI) as novel predictors of MACE following chest radiotherapy. Among these predictors, RAVI influences cardiac risk (24, 25) and ranked as the top predictive feature in our discovery cohort. LAVI—a physiologically related variable with known cardiac significance (26–28)—also ranked highly. The predictive value of RAVI and LAVI were externally validated on a mixed cohort of NSCLC and breast cancer patients, suggesting that both may offer distinct predictive value for MACE following chest radiotherapy. Deploying RAVI and LAVI estimation alongside existing cardiovascular risk assessments could provide a powerful tool during early point-of-care interventions and long-term surveillance of cancer survivors.
To our knowledge, this is the first study to demonstrate the association of indexed CT-derived cardiac chamber volumes with MACE following thoracic RT. In a recent study, Walls et al. (29) reported the association of left atrial volume with atrial arrhythmias utilizing the Northern Ireland Cardiovascular Health Events After Radiation Therapy (NI-HEART) study. However, their study did not index cardiac volumes to body habitus—an important distinction—since cardiac geometric dimensions vary by sex, body habitus, fitness, age, and ethnicity (30). Indeed, standard echocardiography practice involves indexing chamber volumes to body habitus (commonly BSA) and adjusting for sex and age (31). Furthermore, we utilized a single composite endpoint, the American Heart Association/American College of Cardiology-defined standard five-point MACE (19), which does not include atrial arrhythmias, whereas Walls et al. defined MACE as arrhythmias, acute HF, and myocardial infarction. These methodological differences and our larger cohort may explain our observed association between RAVI and LAVI with “MACE” compared with Walls et al.
Our findings are supported by several studies demonstrating the prognostic utility of atrial volume indices across the cardiovascular risk spectrum. Left atrial enlargement typically represents sequelae of chronic exposure to elevated cardiac filling pressures and is a well-established cardiac risk factor (23, 32). While classically evaluated from echocardiography, CT-derived LAVI is similarly associated with the risk of acute coronary syndrome (33). AI-based LAVI from lung cancer screening and coronary artery calcium CTs are associated with the risk of atrial fibrillation and MACE (34, 35). Fewer studies focus on RA enlargement, but a pathophysiological explanation for RAVI's pertinence in lung cancer could be the increased prevalence of comorbid cardiopulmonary diseases—obstructive and interstitial lung diseases—that drive increased right heart pressures (36–38). Growing research associates elevated RAVI with cardiac events, as RA remodeling is linked to arrhythmias and diastolic dysfunction (22, 39). RAVI may model risk conveyed by underlying cardiopulmonary disease given the potential for increased right heart pathology in these patients.
Given that multiple thoracic cancers demonstrate an increased risk of MACE after chest RT (3, 40–42), we evaluated the generalizability of RAVI and LAVI for MACE prediction by including both NSCLC and breast cancer patients during external validation. Among primary chest malignancies, posttreatment MACE rates are generally highest for lung cancer and lowest for breast cancer (6). Including both extremes suggests our results may generalize across broad baseline risks and cardiac radiation dose exposures. Moreover, patient populations and treatment paradigms for lung and breast cancer vary greatly, and our results may inform cardiovascular risk prediction across cancer histology and cardiovascular risk spectrums. Notably, new-onset HF and arrhythmias are increased within the first decade following breast RT (43, 44), possibly partially reflecting anthracycline exposure (45). Elevated RAVI/LAVI are plausible predictors for cardiac events in breast cancer given the mechanisms of treatment-related cardiotoxicity. Additional thoracic primaries demonstrate elevated MACE rates and share features with patients in this study. Lymphoma patients often receive multiple cycles of anthracyclines and consolidative thoracic RT (46). Risk factors for esophageal cancer overlap with patients with NSCLC and CHD, and these patients have elevated rates of concomitant cardiovascular disease and RT-related cardiac events (47). While our validation did not include all thoracic primaries, the shared risk and treatment factors suggest RAVI/LAVI could be applicable for risk stratification independent of cancer histology and is worthy of further investigation.
While typically quantified with echocardiograms in cardiovascular studies, atrial volumes estimated by CT are validated against echocardiograms (48–50). In the discovery cohort, median values for RAVI and LAVI were 51 ml/m2 and 48 ml/m2, respectively, compared with respective echocardiogram median estimates of 21 ml/m2 and 25 ml/m2 in healthy individuals (51, 52). Echocardiogram-based cutoffs of 35 ml/m2 for RAVI and 33–36 ml/m2 for LAVI show discriminatory power for cardiac dysfunction (53, 54). Standard-of-care chest or RT planning CTs lack cardiac gating and cannot fully account for dynamic changes in cardiac chamber volume when compared with ECG-gated CTs or echocardiograms. Measurement differences between these modalities may not translate to clinically meaningful discrepancies (50, 55), and cardiac chamber estimation via diagnostic CT appears feasible and relatively reproducible (56). Without established reference values for CT-based cardiac volume assessments, direct comparison of individual measurements is limited, and further investigation of specific cutoff values reflecting diverse cohorts would assist in translating these findings into clinical practice.
Studies consistently demonstrate underutilization of cardiac screening and medical optimization for cancer patients. For the NSCLC cohort, only half of the statin-eligible patients are on therapy (57), despite statins potentially decreasing the risk conveyed by higher heart RT dose and conferring a dose–response relationship with survival (58). Cardio-oncology guidelines recommend consideration of echocardiographic screening in patients with underlying cardiovascular disease before thoracic RT (59), but only 33% of our discovery cohort received an echocardiogram before RT. It is unclear if modern practice patterns are improved. Moreover, given the broad definitions and gaps in stratification for defining patients at high cardiovascular risk from RT in consensus guidelines, multiple studies have explored strategies to enhance upfront cardiovascular risk stratification. For instance, CT-based coronary artery calcification quantification shows promise in predicting MACE and mortality after chest RT (60–63). RAVI and LAVI show potential as radiologic markers to further inform CT-based risk stratification approaches. Our results support consensus guidelines that consider baseline echocardiographic screening. Advances in artificial intelligence-based approaches for automated segmentation of cardiac substructures (64–66) will provide opportunities for automation of RAVI and LAVI measurements on CT scans obtained at multiple time points during cancer care.
This study has limitations. Its retrospective nature is subject to sampling bias, misclassification, and follow-up bias. We recognize that systematic sampling bias cannot be fully accounted for. We believe the effect of follow-up bias on differences in observed rates of MACE to be low given the overall shorter time to MACE and relatively longer median follow-up within our cohort of patients entering longitudinal, routine cancer surveillance. Low MACE numbers within our validation cohort may increase the overfitting of our validation models. The concordance between estimates for RAVI/LAVI between discovery and validation modeling suggests that overfitting and bias were limited. Without established reference values for CT-based volumetric cardiac measurements and a lack of dynamic heart imaging, we were unable to assess atrial remodeling severity. Future work correlating CT-based atrial volume estimates with echocardiogram data and heart function, including analysis of atrial volume changes over time, is of interest. Our validation cohort was heterogenous compared with the discovery cohort, particularly with respect to treatment years and cancer type (inclusion of breast cancer in addition to NSCLC), but this could be considered a strength, since RAVI and LAVI remain significant predictors across time, primary tumor, and changes in cancer-directed therapies. Lastly, while the use of statins was included as a predictor, it was not ranked highly, and further studies should explore the impact of additional cardioprotective medications, such as beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Our study utilized ML to analyze high-dimensionality clinical, anatomical, and dosimetric data, identifying novel predictors of MACE and externally validating on a mixed cohort of NSCLC and breast cancer patients. Elevated RAVI and LAVI may convey a higher risk for MACE following chest radiotherapy. Overall, deploying RAVI and LAVI estimation alongside existing cardiovascular risk assessments could provide a powerful tool during early point-of-care interventions and long-term surveillance of cancer survivors. The utility of RAVI and LAVI for the identification of high-risk patients warrants further study in prospective trials.
Statements
Data availability statement
The datasets presented in this article are not readily available because of institutional review board restrictions regarding human subject research. The data that support the findings of this study are available upon reasonable by a qualified investigator under a data use agreement and with appropriate ethical oversight. Requests to access the datasets should be directed to katelyn.atkins@cshs.org.
Ethics statement
The studies involving humans were approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because of the study's retrospective design with minimal risk.
Author contributions
EQ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JH: Data curation, Writing – review & editing. KS: Writing – review & editing. JG: Writing – review & editing. PB: Writing – review & editing. DB: Writing – review & editing. EM: Writing – review & editing. JS: Writing – review & editing. CG: Writing – review & editing. AN: Writing – review & editing. ML: Writing – review & editing. HA: Writing – review & editing. APN: Writing – review & editing. RM: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. KA: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. KA reports funding from the Garber Award for Cancer Research. JH and RM report funding from NIH (NCI) Grant 5U01CA209414. DB reports funding from NIH (NCI) Grant U54CA274516-01A1 and NIH (NCI) Grant R01CA294033-01. HA reports funding from NIH-USA U01CA209414, NIH-USA R35CA22052, and the European Union - European Research Council (866504).
Conflict of interest
KA reports honoraria from OncLive. RM reports consulting with AstraZeneca, ViewRay, Novartis, Sio Capital Management, and Varian Medical Systems; advisory board with ViewRay and AstraZeneca; and grant funding from AstraZeneca, ViewRay, and Varian Medical Systems. AN reports research support from Bristol Myers Squibb and consulting fees from AstraZeneca, Bantam Pharmaceutical, and Takeda Oncology. DB reports advisory board with Mercurial AI. ML reports research support from AstraZeneca, Ionis, Johnson & Johnson Innovation, Kowa Pharmaceuticals America, MedImmune, the National Academy of Medicine, and the Risk Management Foundation of the Harvard Medical Institutions. HA reports consulting fees from Onc.AI, Love Health, and Sphera and stock from Onc.AI, Sphera, Love Health, Health AI, Ambient, and AstraZeneca, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1560922/full#supplementary-material
References
1.
Banfill K Giuliani M Aznar M Franks K McWilliam A Schmitt M et al Cardiac toxicity of thoracic radiotherapy: existing evidence and future directions. J Thorac Oncol. (2021) 16(2):216. 10.1016/j.jtho.2020.11.002
2.
Atkins KM Chaunzwa TL Lamba N Bitterman DS Rawal B Bredfeldt J et al Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol. (2021) 7(2):206–19. 10.1001/jamaoncol.2020.6332
3.
Atkins KM Rawal B Chaunzwa TL Lamba N Bitterman DS Williams CL et al Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. (2019) 73(23):2976–87. 10.1016/j.jacc.2019.03.500
4.
Wang K Eblan MJ Deal AM Lipner M Zagar TM Wang Y et al Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70–90 gy. J Clin Oncol. (2017) 35(13):1387–94. 10.1200/JCO.2016.70.0229
5.
Dess RT Sun Y Matuszak MM Sun G Soni PD Bazzi L et al Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol. (2017) 35(13):1395–402. 10.1200/JCO.2016.71.6142
6.
Mitchell JD Laurie M Xia Q Dreyfus B Jain N Jain A et al Risk profiles and incidence of cardiovascular events across different cancer types. ESMO Open. (2023) 8(6). 10.1016/j.esmoop.2023.101830
7.
No HJ Guo FB Park NJI Kastelowitz N Rhee JW Clark DE et al Predicting adverse cardiac events after radiotherapy for locally advanced non–small cell lung cancer. JACC Cardioncol. (2023) 5(6):775–87. 10.1016/j.jaccao.2023.08.007
8.
Tjong MC Bitterman DS Brantley K Nohria A Hoffmann U Atkins KM et al Major adverse cardiac event risk prediction model incorporating baseline cardiac disease, hypertension, and logarithmic left anterior descending coronary artery radiation dose in lung cancer (CHyLL). Radiother Oncol. (2022) 169:105–13. 10.1016/j.radonc.2022.02.010
9.
Wang X Wei C Fan W Sun L Zhang Y Sun Q et al Advanced lung cancer inflammation Index for predicting prognostic risk for patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Inflamm Res. (2023) 16:3631–41. 10.2147/JIR.S421021
10.
Janssen-Heijnen MLG Schipper RM Razenberg PPA Crommelin MA Coebergh JWW . Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. (1998) 21(2):105–13. 10.1016/S0169-5002(98)00039-7
11.
Sigel K Wisnivesky JP . Comorbidity profiles of patients with lung cancer: a new approach to risk stratification?Annals of the American Thoracic Society. American Thoracic Society. (2017) 14:1512–3. 10.1513/AnnalsATS.201706-442ED
12.
Nithya B Ilango V . Evaluation of machine learning based optimized feature selection approaches and classification methods for cervical cancer prediction. SN Appl Sci. (2019) 1(6):1–16. 10.1007/s42452-019-0645-7
13.
Sharma A Mishra PK . Performance analysis of machine learning based optimized feature selection approaches for breast cancer diagnosis. Int J Inf Technol. (2022) 14(4):1949–60. 10.1007/s41870-021-00671-5
14.
Hong JC Eclov NCW Dalal NH Thomas SM Stephens SJ Malicki M et al System for high-intensity evaluation during radiation therapy (SHIELD-RT): a prospective randomized study of machine learning–directed clinical evaluations during radiation and chemoradiation. J Clin Oncol. (2020) 38(31):3652–61. 10.1200/JCO.20.01688
15.
Qiao EM Voora RS Nalawade V Kotha NV Qian AS Nelson TJ et al Evaluating the clinical trends and benefits of low-dose computed tomography in lung cancer patients. Cancer Med. (2021) 10(20):7289–97. 10.1002/cam4.4229
16.
Gasho JO Silos K Guthier CV Zhang SC Burnison M Mirhadi AJ et al Association of left anterior descending coronary artery calcium progression and radiation dose with Major adverse cardiac events in breast cancer. Int J Radiat Oncol. (2023) 117(2):e175. 10.1016/j.ijrobp.2023.06.1020
17.
Tjong MC Zhang SC Gasho JO Silos KD Gay C McKenzie EM et al External validation of cardiac disease, hypertension, and logarithmic left anterior descending coronary artery radiation dose (CHyLL) for predicting major adverse cardiac events after lung cancer radiotherapy. Clin Transl Radiat Oncol. (2023) 42:100660. 10.1016/j.ctro.2023.100660
18.
Guthier CV McKenzie E Zeleznik R Bitterman DS Bredfeldt JS Aerts H et al Deep learning-based automated cardiac sub-structure contouring with dosimetric and clinical outcomes validation. Int J Radiat Oncol. (2022) 114(3):S46–7. 10.1016/j.ijrobp.2022.07.417
19.
Hicks KA Mahaffey KW Mehran R Nissen SE Wiviott SD Dunn B et al 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. (2018) 137(9):961–72. 10.1161/CIRCULATIONAHA.117.033502
20.
Kim KH Oh J Yang G Lee J Kim J Gwak SY et al Association of sinoatrial node radiation dose with atrial fibrillation and mortality in patients with lung cancer. JAMA Oncol. (2022) 8(11):1624–34. 10.1001/jamaoncol.2022.4202
21.
Chen T Guestrin C . XGBoost: a scalable tree boosting system. In: Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. New York, NY, USA: Association for Computing Machinery (2016). p. 785–94. 10.1145/2939672.2939785
22.
Borlaug BA Obokata M . The other atrium in heart failure∗. JACC Cardiovasc Imaging. (2019) 12(8P1):1471–3. 10.1016/j.jcmg.2018.08.019
23.
Benjamin EJ D’Agostino RB Belanger AJ Wolf PA Levy D . Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation. (1995) 92(4):835–41. 10.1161/01.CIR.92.4.835
24.
Schuster A Backhaus SJ Stiermaier T Navarra JL Uhlig J Rommel KP et al Impact of right atrial physiology on heart failure and adverse events after myocardial infarction. J Clin Med. (2020) 9(1):210. 10.3390/jcm9010210
25.
Darahim K . Usefulness of right atrial volume index in predicting outcome in chronic systolic heart failure. J Saudi Hear Assoc. (2014) 26(2):73–9. 10.1016/j.jsha.2013.09.002
26.
Laukkanen JA Kurl S Eränen J Huttunen M Salonen JT . Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. (2005) 165(15):1788–93. 10.1001/archinte.165.15.1788
27.
Gardin JM McClelland R Kitzman D Lima JAC Bommer W Klopfenstein HS et al M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the cardiovascular health study). Am J Cardiol. (2001) 87(9):1051–7. 10.1016/S0002-9149(01)01460-6
28.
Kizer JR Bella JN Palmieri V Liu JE Best LG Lee ET et al Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the strong heart study (SHS). Am Heart J. (2006) 151(2):412–8. 10.1016/j.ahj.2005.04.031
29.
Walls GM Hill N McMahon M Kearney BÓ McCann C McKavanagh P et al Baseline cardiac parameters as biomarkers of radiation cardiotoxicity in lung cancer: an NI-HEART analysis. Cardio Oncol. (2023) 6:529–40. 10.1016/j.jaccao.2024.05.009
30.
Davis EF Crousillat DR He W Andrews CT Hung JW Danik JS . Indexing left atrial volumes: alternative indexing methods better predict outcomes in overweight and obese populations. Cardiovasc Imaging. (2022) 15(6):989–97. 10.1016/j.jcmg.2022.02.006
31.
Aune E Baekkevar M Roislien J Rodevand O Otterstad JE . Normal reference ranges for left and right atrial volume indexes and ejection fractions obtained with real-time three-dimensional echocardiography. Eur J Echocardiogr. (2009) 10(6):738–44. 10.1093/ejechocard/jep054
32.
Khan MA Yang EY Zhan Y Judd RM Chan W Nabi F et al Association of left atrial volume index and all-cause mortality in patients referred for routine cardiovascular magnetic resonance: a multicenter study. J Cardiovasc Magn Reson. (2019) 21(1):1–12. 10.1186/s12968-018-0517-0
33.
Truong QA Bamberg F Mahabadi AA Toepker M Lee H Rogers IS et al Left atrial volume and index by multi-detector computed tomography: comprehensive analysis from predictors of enlargement to predictive value for acute coronary syndrome (ROMICAT study). Int J Cardiol. (2011) 146(2):171. 10.1016/j.ijcard.2009.06.029
34.
Naghavi M Yankelevitz D Reeves AP Budoff MJ Li D Atlas K et al AI-enabled left atrial volumetry in coronary artery calcium scans (AI-CACTM) predicts atrial fibrillation as early as one year, improves CHARGE-AF, and outperforms NT-proBNP: the multi-ethnic study of atherosclerosis. J Cardiovasc Comput Tomogr. (2024) 18(4):383–91. 10.1016/j.jcct.2024.04.005
35.
Aquino GJ Chamberlin J Mercer M Kocher M Kabakus I Akkaya S et al Deep learning model to quantify left atrium volume on routine non-contrast chest CT and predict adverse outcomes. J Cardiovasc Comput Tomogr. (2022) 16(3):245–53. 10.1016/j.jcct.2021.12.005
36.
Naccache JM Gibiot Q Monnet I Antoine M Wislez M Chouaid C et al Lung cancer and interstitial lung disease: a literature review. J Thorac Dis. (2018) 10:3829–44. 10.21037/jtd.2018.05.75
37.
Kolb TM Hassoun PM . Right ventricular dysfunction in chronic lung disease. Cardiol Clin. (2012) 30:243–56. 10.1016/j.ccl.2012.03.005
38.
Islam KMM Jiang X Anggondowati T Lin G Ganti AK . Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. (2015) 24(7):1079–85. 10.1158/1055-9965.EPI-15-0036
39.
Gunturiz-Beltrán C Nuñez-Garcia M Althoff TF Borràs R Ventura RMF Garre P et al Progressive and simultaneous right and left atrial remodeling uncovered by a comprehensive magnetic resonance assessment in atrial fibrillation. J Am Heart Assoc. (2022) 11(20):26028. 10.1161/JAHA.122.026028
40.
Darby SC Ewertz M McGale P Bennet AM Blom-Goldman U Brønnum D et al Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. (2013) 368(11):987–98. 10.1056/NEJMoa1209825
41.
Van Nimwegen FA Schaapveld M Cutter DJ Janus CPM Krol ADG Hauptmann M et al Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. (2016) 34(3):235–43. 10.1200/JCO.2015.63.4444
42.
Cai G Li C Li J Yang J Li C Sun L et al Cardiac substructures dosimetric predictors for cardiac toxicity after definitive radiotherapy in esophageal cancer. Int J Radiat Oncol Biol Phys. (2023) 115(2):366–81. 10.1016/j.ijrobp.2022.08.013
43.
Guha A Fradley MG Dent SF Weintraub NL Lustberg MB Alonso A et al Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-medicare analysis. Eur Heart J. (2022) 43(4):300–12. 10.1093/eurheartj/ehab745
44.
Jacobs JEJ L’Hoyes W Lauwens L Yu YL Brusselmans M Weltens C et al Mortality and major adverse cardiac events in patients with breast cancer receiving radiotherapy: the first decade. J Am Heart Assoc. (2023) 12(8):27855. 10.1161/JAHA.122.027855
45.
Boczar KE Aseyev O Sulpher J Johnson C Burwash IG Turek M et al Right heart function deteriorates in breast cancer patients undergoing anthracycline-based chemotherapy. Echo Res Pract. (2016) 3(3):79. 10.1530/ERP-16-0020
46.
Rihackova E Rihacek M Vyskocilova M Valik D Elbl L . Revisiting treatment-related cardiotoxicity in patients with malignant lymphoma—a review and prospects for the future. Front Cardiovasc Med. (2023) 10:1243531. 10.3389/fcvm.2023.1243531
47.
Søndergaard MMA Nordsmark M Nielsen KM Poulsen SH . Cardiovascular burden and adverse events in patients with esophageal cancer treated with chemoradiation for curative intent. JACC CardioOncology. (2021) 3(5):711. 10.1016/j.jaccao.2021.10.002
48.
Gopalan D Riley J Leong K Alsanjari S Ariff B Auger W et al Biatrial volumetric assessment by non-ECG-gated CT pulmonary angiography correlated with transthoracic echocardiography in patients with normal diastology. Tomography. (2022) 8(6):2761. 10.3390/tomography8060230
49.
Al-Mohaissen MA Kazmi MH Chan KL Chow BJW . Validation of two-dimensional methods for left atrial volume measurement: a comparison of echocardiography with cardiac computed tomography. Echocardiography. (2013) 30(10):1135–42. 10.1111/echo.12253
50.
Huckleberry J Haltom S Issac T Gabaldon J Ketai L . Accuracy of non-ECG-gated computed tomography angiography of the chest in assessment of left-sided cardiac chamber enlargement. J Thorac Imaging. (2012) 27(6):354–8. 10.1097/RTI.0b013e31822bddbb
51.
Soulat-Dufour L Addetia K Miyoshi T Citro R Daimon M Fajardo PG et al Normal values of right atrial size and function according to age, sex, and ethnicity: results of the world alliance societies of echocardiography study. J Am Soc Echocardiogr. (2021) 34(3):286–300. 10.1016/j.echo.2020.11.004
52.
Miyoshi T Addetia K Citro R Daimon M Desale S Fajardo PG et al Left ventricular diastolic function in healthy adult individuals: results of the world alliance societies of echocardiography normal values study. J Am Soc Echocardiogr. (2020) 33(10):1223–33. 10.1016/j.echo.2020.06.008
53.
Patel AR Alsheikh-Ali AA Mukherjee J Evangelista A Quraini D Ordway LJ et al 3D echocardiography to evaluate right atrial pressure in acutely decompensated heart failure: correlation with invasive hemodynamics. JACC Cardiovasc Imaging. (2011) 4(9):938–45. 10.1016/j.jcmg.2011.05.006
54.
Wu VCC Takeuchi M Kuwaki H Iwataki M Nagata Y Otani K et al Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. (2013) 6(10):1025–35. 10.1016/j.jcmg.2013.08.002
55.
Lu MT Cai T Ersoy H Whitmore AG Levit NA Goldhaber SZ et al Comparison of ECG-gated versus non-gated CT ventricular measurements in thirty patients with acute pulmonary embolism. Int J Cardiovasc Imaging. (2009) 25(1):101–7. 10.1007/s10554-008-9342-0
56.
Hota P Simpson S . Going beyond cardiomegaly: evaluation of cardiac chamber enlargement at non– electrocardiographically gated multidetector CT: current techniques, limitations, and clinical implications. Radiol Cardiothorac Imaging. (2019) 1(1). 10.1148/ryct.2019180024
57.
Atkins KM Bitterman DS Chaunzwa TL Williams CL Rahman R Kozono DE et al Statin use, heart radiation dose, and survival in locally advanced lung cancer. Pract Radiat Oncol. (2021) 11(5):e459–67. 10.1016/j.prro.2020.12.006
58.
Walls GM O’Connor J Harbinson M McCarron EP Duane F McCann C et al Association between statin therapy dose intensity and radiation cardiotoxicity in non-small cell lung cancer: results from the NI-HEART study. Radiother Oncol. (2023) 186. 10.1016/j.radonc.2023.109762
59.
Lyon AR López-Fernánde T Couch LS Asteggiano R Aznar MC Bergler-Klei J et al 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J. (2022) 43(41):4229–361. 10.1093/eurheartj/ehac244
60.
Ruparel M Quaife SL Dickson JL Horst C Burke S Taylor M et al Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. (2019) 74(12):1140. 10.1136/thoraxjnl-2018-212812
61.
Balata H Blandin Knight S Barber P Colligan D Crosbie EJ Duerden R et al Targeted lung cancer screening selects individuals at high risk of cardiovascular disease. Lung Cancer. (2018) 124:148–53. 10.1016/j.lungcan.2018.08.006
62.
Atkins KM Weiss J Zeleznik R Bitterman DS Chaunzwa TL Huynh E et al Elevated coronary artery calcium quantified by a validated deep learning model from lung cancer radiotherapy planning scans predicts mortality. JCO Clin Cancer Informatics. (2022) 6(6). 10.1200/CCI.21.00095
63.
Gal R Van Velzen SGM Hooning MJ Emaus MJ Van Der Leij F Gregorowitsch ML et al Identification of risk of cardiovascular disease by automatic quantification of coronary artery calcifications on radiotherapy planning CT scans in patients with breast cancer. JAMA Oncol. (2021) 7(7):1024–32. 10.1001/jamaoncol.2021.1144
64.
Guthier CV Kehayias CE Bitterman DS Atkins KM Mak RH . Deployment of a deep learning automated cardiac sub-structure contouring algorithm to measure coronary dose exposure trends in lung cancer radiation therapy. Int J Radiat Oncol. (2023) 117(2):S54–5. 10.1016/j.ijrobp.2023.06.345
65.
Kehayias CE Yan Y Bontempi D Quirk S Bitterman DS Bredfeldt JS et al Prospective deployment of an automated implementation solution for artificial intelligence translation to clinical radiation oncology. Front Oncol. (2023) 13:1305511. 10.3389/fonc.2023.1305511
66.
Walls GM Giacometti V Apte A Thor M McCann C Hanna GG et al Validation of an established deep learning auto-segmentation tool for cardiac substructures in 4D radiotherapy planning scans. Phys Imaging Radiat Oncol. (2022) 23:118–26. 10.1016/j.phro.2022.07.003
Summary
Keywords
oncology, radiotherapy, lung, breast, major adverse cardiac events, atrial volume, radiation oncology
Citation
Qiao EM, He J, Silos KD, Gasho JO, Belen P, Bitterman DS, McKenzie E, Steers J, Guthier C, Nohria A, Lu MT, Aerts HJWL, Nikolova AP, Mak RH and Atkins KM (2025) Baseline atrial volume indices and major adverse cardiac events following thoracic radiotherapy. Front. Cardiovasc. Med. 12:1560922. doi: 10.3389/fcvm.2025.1560922
Received
15 January 2025
Accepted
02 May 2025
Published
03 June 2025
Volume
12 - 2025
Edited by
Chiara Lestuzzi, Esperia Medical Center, Italy
Reviewed by
Sunil Krishnan, University of Texas Health Science Center at Houston, United States Aditya Murali, University of California, United States, in collaboration with reviewer SK
Lucía Deiros Bronte, University Hospital La Paz, Spain
Updates
Copyright
© 2025 Qiao, He, Silos, Gasho, Belen, Bitterman, McKenzie, Steers, Guthier, Nohria, Lu, Aerts, Nikolova, Mak and Atkins.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Katelyn M. Atkins katelyn.atkins@cshs.org Raymond H. Mak rmak@mgb.org
†These authors have contributed equally to this work and share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.