Abstract
Background:
This study aimed to explore the relationship between the blood cell distribution width-to-albumin ratio (RAR) and in-hospital mortality in patients with congestive heart failure (CHF) combined with chronic kidney disease (CKD).
Methods:
The patients' information was collected from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. The association of the RAR and in-hospital mortality were assessed via logistic regression analysis with adjusting for different covariate, followed by visualization via the restricted cubic splines (RCS) analysis. Correlation analysis and machine learning algorithms were used to screen the clinical features associated with RAR. Mediation analysis was used to explore the function of RAR-related feature on their association. The receiver operating characteristic (ROC) curve and decision curve analysis (DCA) curves were used to explore the efficacy of RAR in predicting patient outcomes and the net clinical benefit, respectively.
Results:
A total of 1,243 patients were included in this study. Logistic regression analysis showed that RAR was a risk factor of in-hospital death. RCS showed a linear relationship between RAR and in-hospital mortality, which was not affected by confounding factors. Monocytes played a mediating role in the relationship between RAR and in-hospital mortality. RAR had a good efficacy and clinical net for predicting in-hospital mortality, half-year mortality, one-year mortality, and three-year mortality.
Conclusions:
We found that RAR was a risk factor for in-hospital death and could predict short-term and long-term outcomes in patients with CHF combined with CKD. Monocytes played a mediating role in their relationship.
1 Introduction
Congestive heart failure (CHF) is a complex clinical syndrome characterized by impaired cardiac function, leading to systemic congestion and multi-organ dysfunction. Among the comorbidities associated with CHF, renal dysfunction is particularly prevalent and up to 50% of patients with CHF exhibit some degree of renal impairment (1). The coexistence of CHF combined with chronic kidney disease (CKD), often referred to as cardiorenal syndrome, is associated with worse clinical outcomes, including increased morbidity and mortality (2). Specifically, patients with CHF and concurrent renal dysfunction are at a significantly higher risk of in-hospital mortality compared to those with CHF alone (3).
The pathophysiology linking CHF combined with CKD involves a vicious cycle of hemodynamic and neurohormonal alterations. Reduced cardiac output in CHF leads to decreased renal perfusion, activating the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system, which further exacerbates fluid retention and cardiac workload (4). This interplay not only accelerates the progression of both cardiac and renal dysfunction but also complicates the management of these patients, making early risk stratification crucial.
In recent years, there has been growing interest in identifying biomarkers that can predict adverse outcomes in patients with CHF combined with kidney disease. Among those patients, the red blood cell distribution width (RDW) has recently been proven to be an effective marker for determining the prognosis of heart failure (5, 6). RDW, a measure of variability in red blood cell size, was associated with increased mortality in various cardiovascular conditions, including CHF (7). Elevated RDW levels are thought to reflect underlying inflammation, oxidative stress, and nutritional deficiencies, all of which are prevalent in CHF patients (8).
Albumin, a marker of nutritional status and systemic inflammation, was also independently linked to outcomes in different diseases. Hypoalbuminemia is common in patients with atrial fibrillation and is associated with increased mortality (9). The combination of RDW and albumin into a single metric, the RDW-to-albumin ratio (RAR), has recently been proposed as a novel prognostic tool. Preliminary studies suggest that RAR may provide superior predictive value for adverse outcomes compared to either marker alone, particularly in high-risk populations with cardiovascular disease (10–12), sepsis (13), and aortic aneurysms (14).
Given the high in-hospital mortality risk in patients with CHF combined with CKD, there is a critical need for reliable and easily accessible biomarkers to aid in risk stratification. The RAR, which integrates information on both inflammation and nutritional status, holds promise as a predictor of in-hospital mortality in this vulnerable population. This study aims to retrospectively evaluate the prognostic value of RAR in predicting in-hospital mortality among patients with CHF and concurrent renal dysfunction, with the hypothesis that elevated RAR levels are associated with increased mortality risk.
2 Materials and methods
2.1 Data source and study population
All data for this study were obtained from the Medical Information Mart for Intensive Care IV(MIMIC-IV) database. The database is an open public database, approved by the institutional review boards of Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology, and collects information on patients hospitalized in the intensive care unit (ICU) from 2008–2019. In addition, the database was anonymized to protect patient privacy, thus informed consent was waived. The personnel who extracted the data for this study had passed the training program exam (CITI program) and received a license to use the MIMIC-IV database. PostgreSQL software was used to extract the data.
Inclusion criteria: (1) patients who were eligible for CHF with CKD, (2) age greater than or equal to 18 years. Exclusion criteria: (1) patients with missing albumin and RDW; (2) no record of in-hospital mortality, half-year, one-year, and three-year mortality follow-up. Based on the inclusion criteria, we collected 5,153 patients' data, excluded 2,790 cases with missing data RAR, and excluded 917 cases without follow-up data. Finally, 1,243 cases were included in the study. Notably, both CHF and CKD were identified exclusively through the International Classification of Diseases (ICD) code (Supplementary document 1).
2.2 Variables and outcomes
In this study, we collected basic demographic data of the patients: gender (male, female), race (white, non-white), marital status (single, married, divorced, widowed); hospitalization record: length of hospitalization, laboratory markers: albumin (g/dl), alanine aminotransferase (ALT, IU/L), anion gap (mEq/L), aspartate aminotransferase (AST, IU/L), bicarbonate (mEq/L), blood urea nitrogen (mg/dl), calcium (mg/dl), chloride (mEq/L), creatinine (mg/dl), glucose (mg/dl), hematocrit (%), hemoglobin (g/dl), international normalized ratio (INR), platelets (×109/L), potassium (mEq/L), prothrombin time (PT, sec), red blood cell (RBC, m/ul), RDW, (%), sodium, (mEq/L), total bilirubin, (mg/dl), white blood cell (WBC, K/ul), creatine phosphorylation kinase (CPK, IU/L), creatine kinase isoenzyme B (CKMB, ng/ml), urea nitrogen (mg/dl), monocyte (%), estimated glomerular filtration rate (EGFR, ml/min), RDW-to-albumin ratio (RAR); comorbidities: infarction, hypertension, stroke, diabetes mellitus(DM), atrial fibrillation (AF); and medication record: β-receptor blocker (Metoprolol, Atenolol, Carvedilol) using, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACE-I/ARB) using (Captopril, Enalapril, Benazepril, Valsartan, Losartan), calcium inhibitor (Nifedipine, Amlodipine) using, diuretic (Furosemide, Hydrochlorothiazide, Acetazolamide) using. Patients were classified as β-receptor blocker users if they received any agent within a given drug class (Metoprolol, Atenolol, Carvedilol). Similarly, users of the other three types of drugs were defined in this way. However, extensive missing dosage data precluded quantification of dose ranges and mean doses for non-adherent individuals. All of the above variables were covariates, which were all collected from the first hospitalization.
We calculated the RAR of the patients according to the following formula: RAR = RDW (%)/albumin (g/dl). RAR was the independent variable of this study. In-hospital mortality was the study's primary outcome, with half-year mortality, one-year mortality, and three-year mortality as secondary outcomes. The date of death was extracted from two sources: the hospital information system and the Massachusetts State Registry of Vital Records and Statistics. Individual patient records from MIMIC were matched to the vital records using a custom algorithm based on identifiers including name, social security number, and date of birth.
2.3 Statistical analysis
Patients were divided into 2 groups according to in-hospital mortality status, and continuous variables that conformed to normal distribution between the two groups were compared using the t-test and expressed as mean and standard deviation, while data that did not conform to normal distribution were compared using the U-test and expressed as median and quartiles. Categorical variables between the two groups were compared using the chi-square test and expressed as frequencies and percentages. Logistic regression analysis was used to analyze the relationship between RAR and in-hospital mortality adjusting for different laboratory index or clinical features between the alive group and the death group (model 1 adjusted for ALT, anion gap, AST, bicarbonate, calcium, glucose, INR, platelets, total bilirubin WBC, CPK, monocyte, EGFR; model 2 adjusted for sex, calcium inhibitor, race, smoking, stroke, AF) and their association was visualized using restricted cubic spline (RCS). The receiver operating characteristic (ROC) curve and decision curve analysis (DCA) curves were used to explore the efficacy of RAR in predicting patient outcomes and the net clinical benefit, respectively. Correlation analysis and machine learning algorithms were used to screen for clinical features most relevant to RAR. Mediation analysis was used to explore the mediating role of the clinical characteristics most relevant to RAR on the relationship between RAR and the in-hospital death. All analyses were performed using R software (version 4.2.2), with P < 0.05 as the criterion for a significant difference.
3 Results
3.1 Baseline characteristics of the participants
A total of 1,243 patients, comprising 1,023 survivors and 220 deaths were included. The patients' age ranged from 25–91 years, with a median age of 78. The proportion of males was 58.57%, and the proportion of females was 41.43%. There was a statistically significant difference in ALT, anion gap, AST, bicarbonate, calcium, glucose, INR, platelets, total bilirubin WBC, CPK, monocyte, EGFR, RDW, albumin, RAR, sex, calcium inhibitor, race, smoking, stroke, and AF between the survival and death groups (Tables 1, 2, P < 0.05). It is noteworthy that the RAR of the death group was significantly higher than that of the survival group (5.387 vs. 4.788) (Table 1, P < 0.05).
Table 1
| Variables | Alive (n = 1,023) | Death (n = 220) | z | P |
|---|---|---|---|---|
| Age, (years) | 78.000 (68.000, 85.000) | 79.000 (71.000, 84.000) | −0.241 | 0.810 |
| Length of stay, (day) | 7.895 (4.678, 13.099) | 8.888 (4.960, 15.819) | −1.819 | 0.069 |
| Albumin, (g/dl) | 3.300 (2.900, 3.700) | 3.000 (2.500, 3.500) | 5.499 | <0.001 |
| ALT, (IU/L) | 20.000 (14.000, 36.000) | 25.000 (14.000, 48.000) | −2.702 | 0.007 |
| Anion gap, (mEq/L) | 16.000 (13.000, 18.000) | 16.000 (14.000, 19.000) | −3.009 | 0.003 |
| AST, (IU/L) | 28.000 (19.000, 45.000) | 39.000 (23.000, 76.000) | −4.935 | <0.001 |
| Bicarbonate, (mEq/L) | 24.000 (21.000, 28.000) | 23.000 (20.000, 26.000) | 3.364 | <0.001 |
| Blood urea nitrogen, (mg/dl) | 40.000 (29.000, 58.000) | 44.000 (29.000, 68.000) | −1.847 | 0.065 |
| Calcium, (mg/dl) | 8.700 (8.100, 9.100) | 8.600 (8.000, 9.000) | 2.510 | 0.012 |
| Chloride, (mEq/L) | 102.000 (98.000, 106.000) | 101.000 (97.000, 107.000) | 1.069 | 0.284 |
| Creatinine, (mg/dl) | 2.000 (1.400, 3.100) | 2.000 (1.400, 2.900) | 0.304 | 0.761 |
| Glucose, (mg/dl) | 120.000 (96.000, 159.000) | 129.000 (102.000, 186.000) | −2.649 | 0.008 |
| Hematocrit, (%) | 31.800 (28.200, 35.700) | 31.300 (28.000, 35.500) | 0.296 | 0.768 |
| hemoglobin, (g/dl) | 10.300 (9.100, 11.600) | 10.200 (9.000, 11.500) | 0.670 | 0.503 |
| INR | 1.300 (1.100, 1.700) | 1.400 (1.200,1.800) | −2.307 | 0.020 |
| Platelets, (×109/L) | 203.000 (151.000, 264.000) | 186.000 (129.000, 256.000) | 2.276 | 0.023 |
| Potassium, (mEq/L) | 4.300 (3.900, 4.800) | 4.300 (3.900, 4.800) | −0.827 | 0.408 |
| PT, (sec) | 14.400 (12.700, 18.900) | 14.900 (13.000, 20.100) | −1.814 | 0.070 |
| RBC, (m/ul) | 3.450 (3.060, 3.920) | 3.340 (3.030, 3.900) | 0.953 | 0.340 |
| RDW, (%) | 15.500 (14.400, 17.100) | 16.200 (14.600, 17.600) | −2.729 | 0.006 |
| Sodium, (mEq/L) | 139.000 (136.000, 141.000) | 138.000 (134.000, 141.000) | 1.923 | 0.054 |
| Total bilirubin, (mg/dl) | 0.500 (0.300, 0.900) | 0.600 (0.400, 1.400) | −3.989 | <0.001 |
| WBC, (K/ul) | 8.400 (6.300, 11.600) | 10.000 (7.400, 14.700) | −4.630 | <0.001 |
| CPK, (IU/L) | 75.000 (42.000, 153.000) | 97.000 (43.000, 240.000) | −2.118 | 0.034 |
| CKMB, (ng/ml) | 91.000 (69.000, 132.000) | 99.000 (72.000, 138.000) | −1.542 | 0.123 |
| Urea nitrogen, (mg/dl) | 40.000 (29.000, 58.000) | 44.000 (29.000, 68.000) | −1.847 | 0.065 |
| Monocyte, (%) | 4.300 (2.700, 6.000) | 3.000 (1.800, 5.000) | 5.091 | <0.001 |
| EGFR, (ml/min) | 0.995 (0.788, 1.025) | 1.005 (0.796, 1.027) | −2.178 | 0.029 |
| RAR | 4.778(4.184, 5.727) | 5.387(4.622, 6.577) | −6.123 | <0.001 |
Clinical characteristics (continuous variable) of patients.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; PT, prothrombin time; RBC, red blood cell; RDW, red blood cell distribution width; WBC, white blood cell; CPK, creatine phosphorylation kinase; CKMB, creatine kinase isoenzyme B; EGFR, estimated glomerular filtration rate; RAR, red blood cell distribution width-to-albumin ratio.
Table 2
| Variables | Alive (n = 1,023) | Death (n = 220) | χ2 | P |
|---|---|---|---|---|
| Race, n (%) | ||||
| White | 740 (76.446) | 142 (84.024) | 4.748 | 0.029 |
| No-white | 228 (23.554) | 27 (15.976) | ||
| Marital status, n (%) | ||||
| Single | 183 (18.392) | 37 (18.137) | 0.533 | 0.912 |
| Married | 470 (47.236) | 97 (47.549) | ||
| Divorced | 79 (7.940) | 19 (9.314) | ||
| Widowed | 263 (26.432) | 51 (25.000) | ||
| Sex, n (%) | ||||
| Male | 582 (56.891) | 146 (66.364) | 6.695 | 0.010 |
| Female | 441 (43.109) | 74 (33.636) | ||
| Alcohol use, n (%) | ||||
| No | 955 (93.353) | 199 (90.455) | 2.288 | 0.13 |
| Yes | 68 (6.647) | 21 (9.545) | ||
| Smoking, n (%) | ||||
| No | 648 (63.343) | 168 (76.364) | 13.612 | <0.001 |
| Yes | 375 (36.657) | 52 (23.636) | ||
| MI, n (%) | ||||
| No | 832 (81.329) | 165 (75.000) | 4.569 | 0.033 |
| Yes | 191 (18.671) | 55 (25.000) | ||
| DM, n (%) | ||||
| No | 506 (49.462) | 109 (49.545) | 0.001 | 0.982 |
| Yes | 517 (50.538) | 111 (50.455) | ||
| Stroke, n (%) | ||||
| No | 905 (88.465) | 106 (48.182) | 193.546 | <0.001 |
| Yes | 118 (11.535) | 114 (51.818) | ||
| Hypertension, n (%) | ||||
| No | 952 (93.060) | 197 (89.545) | 3.199 | 0.074 |
| Yes | 71 (6.940) | 23 (10.455) | ||
| AF, n (%) | ||||
| No | 553 (54.057) | 99 (45.000) | 5.955 | 0.015 |
| Yes | 470 (45.943) | 121 (55.000) | ||
| β-receptor blocker using, n (%) | ||||
| No | 332 (32.454) | 83 (37.727) | 2.264 | 0.132 |
| Yes | 691 (67.546) | 137 (62.273) | ||
| ACE-I/ARB, n (%) | ||||
| No | 857 (83.773) | 195 (88.636) | 3.293 | 0.07 |
| Yes | 166 (16.227) | 25 (11.364) | ||
| Calcium inhibitor, n (%) | ||||
| No | 745 (72.825) | 192 (87.273) | 20.366 | <0.001 |
| Yes | 278 (27.175) | 28 (12.727) | ||
| Diuretic, n (%) | ||||
| No | 240 (23.460) | 50(22.727) | 0.054 | 0.816 |
| Yes | 783(76.540) | 170(77.273) | ||
Clinical characteristics (categorical variables) of patients.
MI, myocardial infarction; DM, diabetes; ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AF, atrial fibrillation.
3.2 Relationship between the RAR and in-hospital mortality and its clinical value
Logistic regression analysis showed that RAR was a risk factor of in-hospital death (Table 3, OR = 1.388, P < 0.05). After controlling for confounders, it was independenlty related to the in-hospital death in both model 1 and model 2 (Table 3, model 1 OR [95% CI]: 1.344[1.145,1.579], model 2, OR [95% CI]: 1.28[1.134,1.445], P < 0.05). Logistic regression analysis showed that when RAR was set as a categorical variable according to RAR quartiles, the OR increased with the RAR increasing. The increased trend was not affected by confounders and still held in adjusted model 1 and model 2 (Table 3, P for tend <0.05). Therefore, the relationship was further explored using RCS analysis, which showed a linear relationship between RAR and in-hospital deaths and was not affected by confounding factors (crude model: P-overall < 0.001, P-nonlinear = 0.555; model 1: P-overall = 0.001, P-nonlinear = 0.853; model 2: P-overall < 0.001, P-nonlinear = 0.144, Figures 1A–C). The results of ROC analysis showed that the AUC value of RAR in predicting patients' in-hospital mortality was: 0.631 [95% CI (0.587,0.665)], and the sensitivity was 0.636 [95% CI (0.358–0.798)], with an accuracy of 0.822 [95% CI (0.801–0.838)] (Figure 2A). The DCA results suggested a net benefit of RAR in predicting patient in-hospital deaths over a threshold probability range of 0.18–0.40 (Figure 2B). The above results suggested that RAR is a risk factor for in-hospital death and has certain value in predicting in-hospital death in patients, which is of significant clinical value.
Table 3
| Model | Crude model | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| RAR | 1.388 (1.256, 1.534) | <0.001 | 1.344 (1.145, 1.579) | <0.001 | 1.28 (1.134, 1.445) | <0.001 |
| Q1 | ref | ref | ref | ref | ref | ref |
| Q2 | 1.342 (0.827, 2.177) | 0.233 | 1.279 (0.658, 2.487) | 0.469 | 1.222 (0.731, 2.041) | 0.445 |
| Q3 | 2.049 (1.298, 3.235) | 0.002 | 1.202 (0.635, 2.275) | 0.572 | 1.576 (0.965, 2.575) | 0.069 |
| Q4 | 3.018 (1.944, 4.685) | <0.001 | 2.533 (1.320, 4.861) | 0.005 | 1.855 (1.147, 3.000) | 0.012 |
| p for trend | 0.014 | 0.022 | 0.034 |
Logistic regression analysis investigating the relationship between RAR/quartiles of RAR and in-hospital mortality.
Significant laboratory indices (alive vs. death) from Table 1 and significant clinical features (alive vs. death) from Table 2 were used as adjusted covariates in Model 1 and Model 2, respectively.
Model 1 adjusted for ALT, anion gap, AST, bicarbonate, calcium, glucose, INR, platelets, total bilirubin WBC, CPK, monocyte, EGFR.
Model 2 adjusted for sex, calcium inhibitor, race, smoking, stroke, AF.
RAR, red blood cell distribution width-to-albumin ratio; OR, odd ratio; CI, confidence interval; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; CPK, creatine phosphorylation kinase; EGFR, estimated glomerular filtration rate; AF, atrial fibrillation.
Figure 1
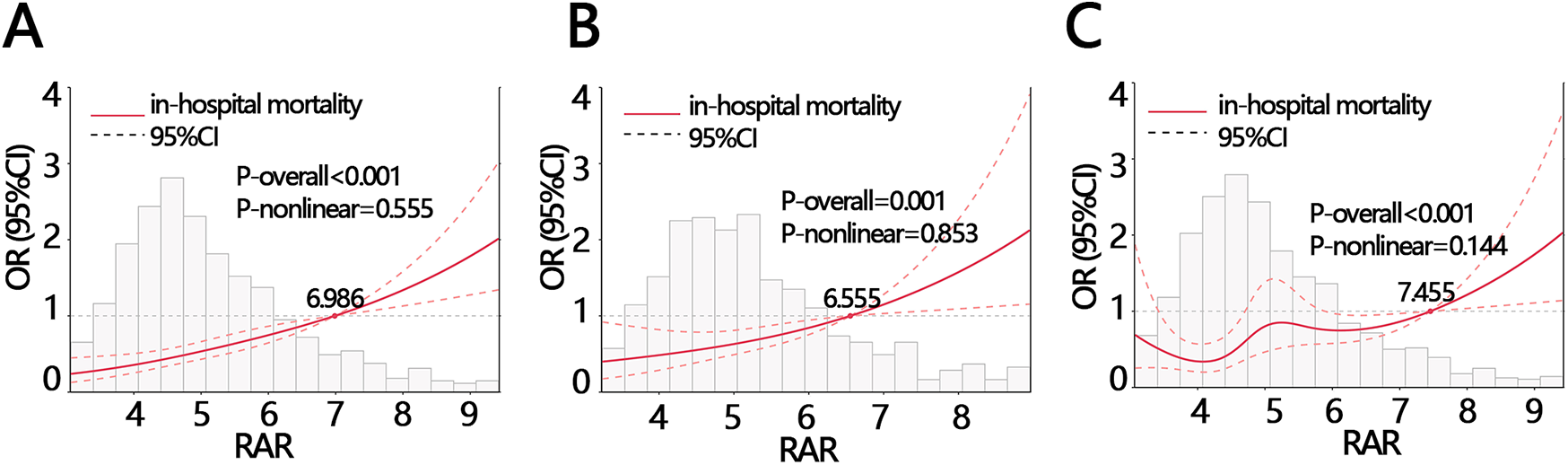
The RCS analysis between RAR and in-hospital mortality with different adjustments (A) crude model: without adjustment, (B) model 1: adjusting for ALT, anion gap, AST, bicarbonate, calcium, glucose, INR, platelets, total bilirubin WBC, CPK, monocyte, EGFR, (C) model 2: adjusting for sex, calcium inhibitor, race, smoking, stroke, AF. ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; WBC, white blood cell; CPK, creatine phosphorylation kinase; EGFR, estimated glomerular filtration rate; RAR, red blood cell distribution width-to-albumin ratio; AF, atrial fibrillation.
Figure 2
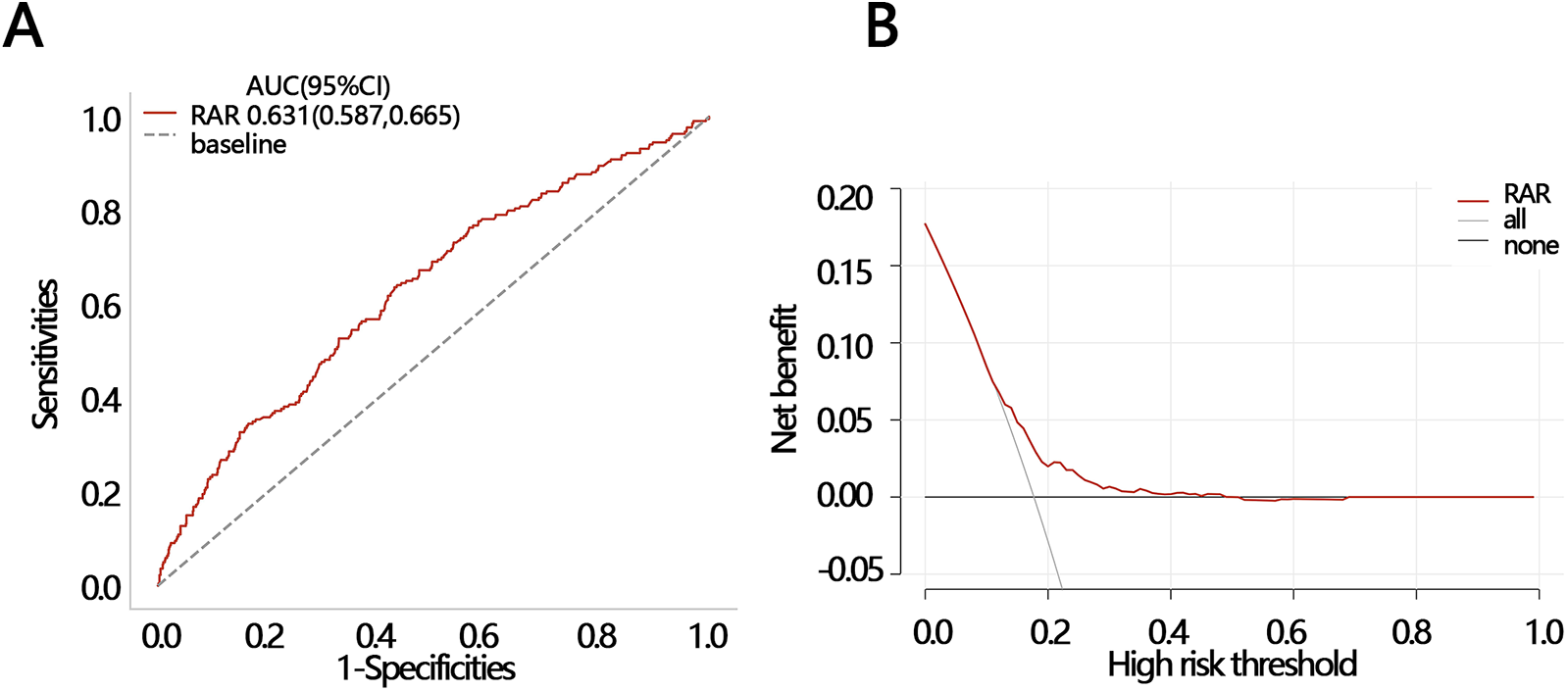
Two methods were used to evaluate the efficacy of RAR in predicting in-hospital death. (A) ROC and (B) DCA. ROC, receiver operating characteristic curve; DCA, decision curve analysis; AUC, areas under curve; CI, confidence interval.
3.3 The mediating role of clinical features significantly associated with RAR
To explore whether other factors mediated the relationship between RAR and in-hospital mortality, we obtained clinical characteristics associated with RAR based on the above indicators that were significantly different between the survival and death groups (except for RAR and RDW) by correlation analysis. The results of the correlation analysis showed that 12 variables, including calcium, CPK, monocyte, EGFR, anion gap, glucose, bicarbonate, INR, stroke, AF, smoking, and calcium inhibitor, were significantly correlated with RAR (Figures 3A,B). These 12 variables were then ranked for importance by three machine learning methods, and top five variables were found including calcium, CPK, anion gap, bicarbonate, monocyte (Figure 4A) for ababoost, a top five ranking of calcium, CPK, stroke, monocyte, INR (Figure 4B) obtained by the Xgboost algorithm, and a top five ranking of calcium, CPK, monocyte, EGFR, anion gap (Figure 4C) obtained by the Random Forest algorithm. The results of Venn showed that calcium, CPK, and monocyte were present in all three top five rankings (Figure 4D), indicating that these three variables were the most relevant clinical characteristics to RAR. Then mediation analysis was performed, and the results indicated that among the three variables, only monocytes mediated the relationship between RAR and in-hospital death (Table 4).
Figure 3
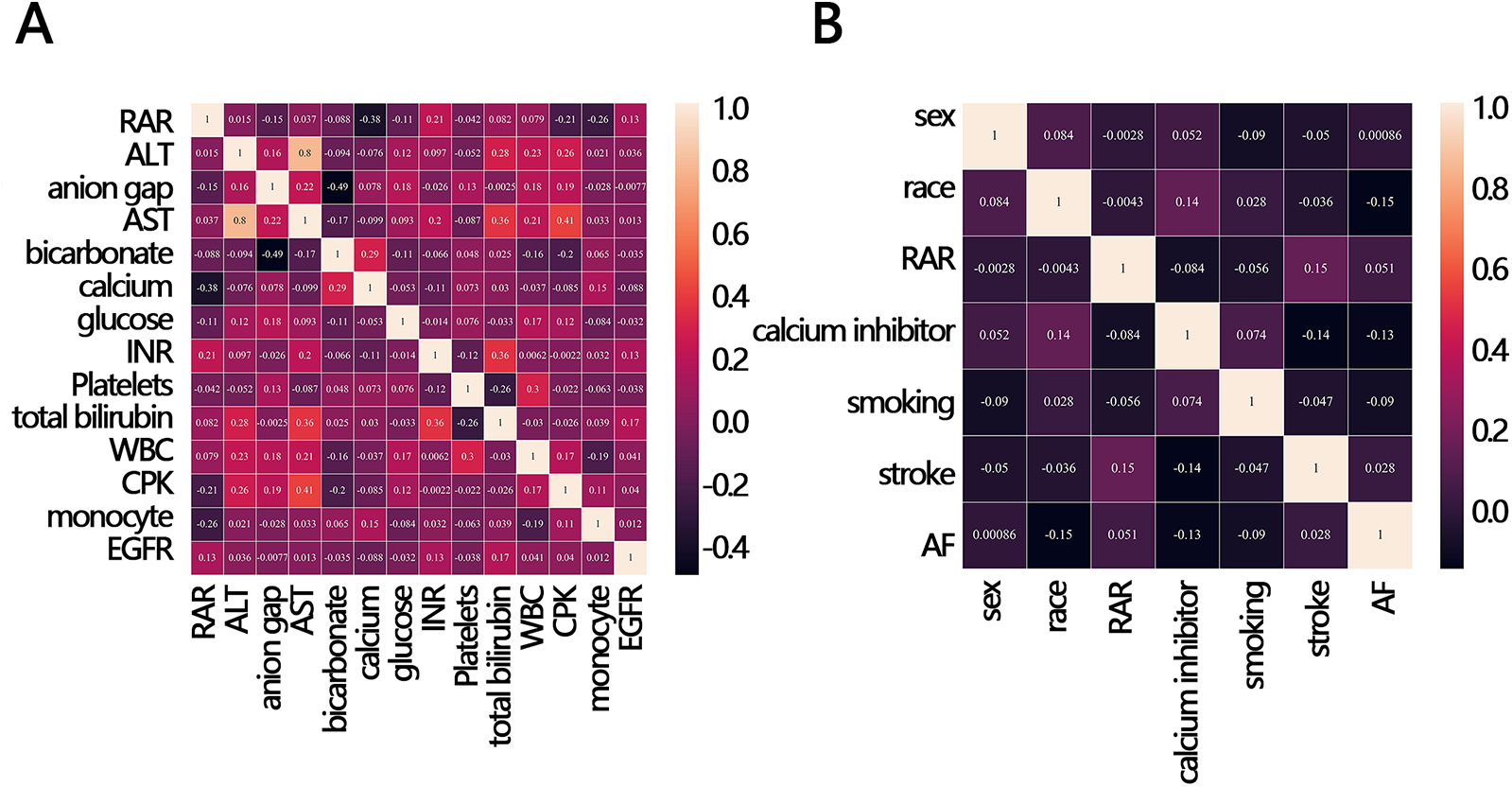
Correlation heat map between RAR and in-hospital mortality-related clinical variables. (A) continuous variable, (B) categorical variables. ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; WBC, white blood cell; CPK, creatine phosphorylation kinase; EGFR, estimated glomerular filtration rate; RAR, red blood cell distribution width-to-albumin ratio; AF, atrial fibrillation.
Figure 4
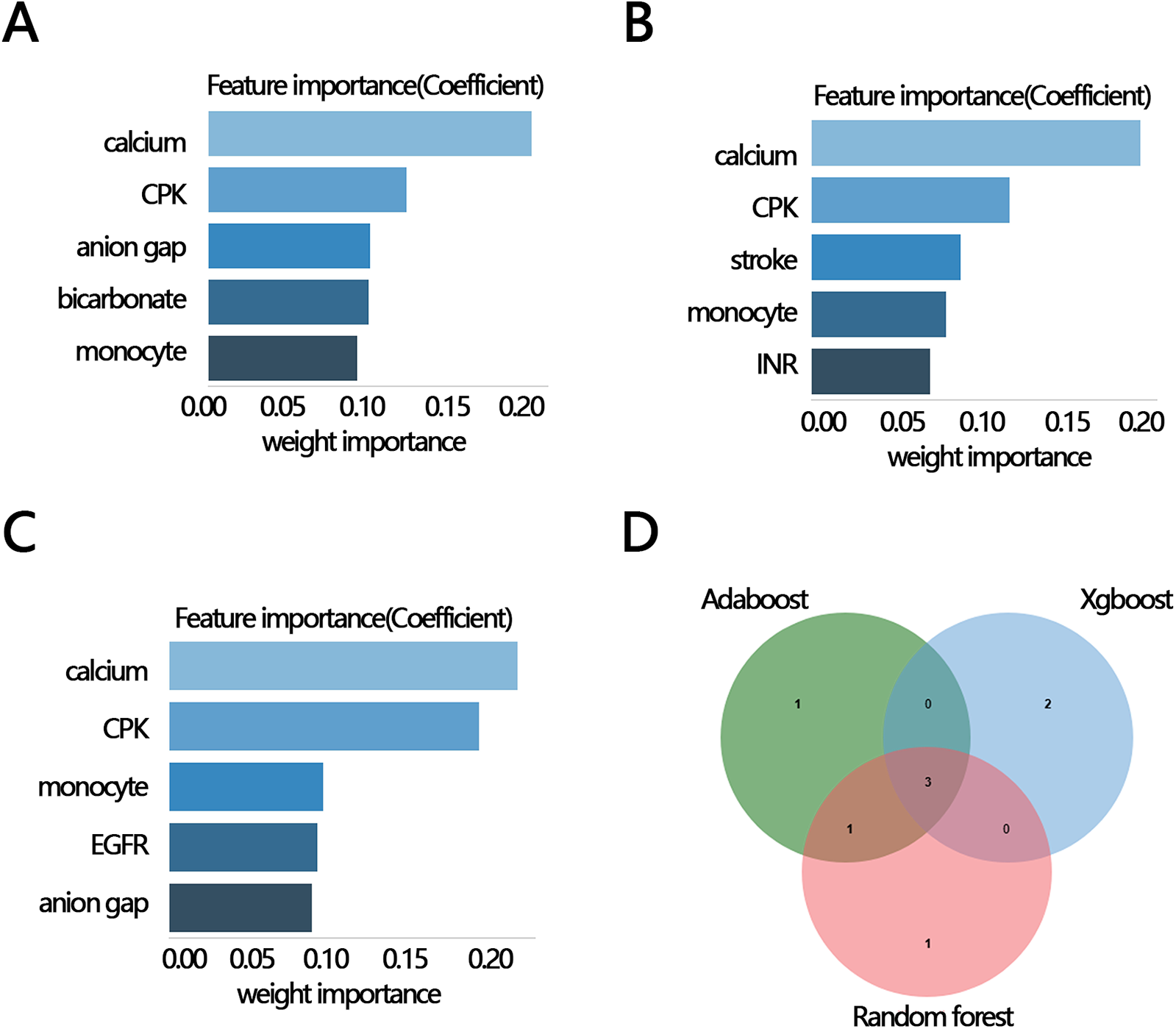
The importance ranking bar chart of the top five RAR-related factors via three methods. (A) AdaBoost, (B) Xgboost, (C) Random forest, (D) Venn plot of three kinds of rank variables. INR, international normalized ratio; CPK, creatine phosphorylation kinase; EGFR, estimated glomerular filtration rate; RAR, red blood cell distribution width-to-albumin ratio; AF, atrial fibrillation.
Table 4
| Variables | Coef (95% CI) | SE | P |
|---|---|---|---|
| Calcium | |||
| Calcium∼RAR | −0.221 (−0.259, −0.184) | 0.019 | <0.001 |
| In-hospital mortality ∼ calcium | −0.035 (−0.057, −0.012) | 0.011 | 0.003 |
| Total | 0.053 (0.038, 0.069) | 0.008 | <0.001 |
| Direct | 0.051 (0.034, 0.067) | 0.008 | <0.001 |
| Indirect | 0.003 (−0.002, 0.008) | 0.003 | 0.308 |
| Monocyte | |||
| Monocyte ∼ RAR | −0.332 (−0.476, −0.187) | 0.074 | <0.001 |
| In-hospital mortality ∼ monocyte | −0.020 (−0.029, −0.010) | 0.005 | <0.001 |
| Total | 0.055 (0.035, 0.076) | 0.010 | <0.001 |
| Direct | 0.050 (0.030, 0.070) | 0.010 | <0.001 |
| Indirect | 0.005 (0.002, 0.011) | 0.002 | <0.001 |
| CPK | |||
| CPK∼RAR | −14.873 (−59.723, 29.978) | 22.85 | 0.515 |
| In-hospital mortality ∼ CPK | 0.000 (0.000, 0.000) | 0.000 | 0.380 |
| Total | 0.056 (0.035, 0.077) | 0.011 | <0.001 |
| Direct | 0.056 (0.035, 0.077) | 0.011 | <0.001 |
| Indirect | 0.000 (−0.003, 0.000) | 0.001 | 0.464 |
Mediation analysis among the RAR, RAR-related key factors, and in-hospital mortality.
CPK, creatine phosphorylation kinase; RAR, red blood cell distribution width-to-albumin ratio; CI, confidence interval; SE, standard error.
3.4 The clinical value of RAR in secondary outcomes
To explore the clinical value of RAR in predicting death at follow-up, we analyzed the efficacy and net clinical benefit of RAR in predicting patients' half-year, one-year, and three-year mortality using ROC and DCA. The ROC results showed that the AUC of RAR in predicting patients' death within half-year, one-year, and three-year periods were: 0.640 [95% CI (0.613–0.671)], 0.637 [95% CI (0.609–0.672)], and 0.644 [95% CI (0.603–0.679)], respectively (Figures 5A–E). The sensitivities of the ROC analyses for the three secondary outcomes were 0.549 [95% CI (0.456–0.750)], 0.474 [95% CI (0.434–0.774)], and 0.446 [95% CI (0.369–0.783)]; and the accuracies of the ROC analyses for the three secondary outcomes were 0.444 [95% CI (0.424–0.472)], 0.302 [95% CI (0.283–0.323)], and 0.163 [95% CI (0.141–0.183)], respectively. The DCA results indicated that at a threshold probability of 0.42–0.81, RAR was of net benefit in predicting patient death within half-year (Figure 5B); at a threshold probability of 0.63–0.92, RAR was of net benefit in predicting one-year survival of patient (Figure 5D); at a threshold probability of threshold probabilities of 0.79–0.10, RAR had a net benefit in predicting three-year survival of patient (Figure 5F). These results suggest that RAR has significant value in predicting patient death at follow-up.
Figure 5
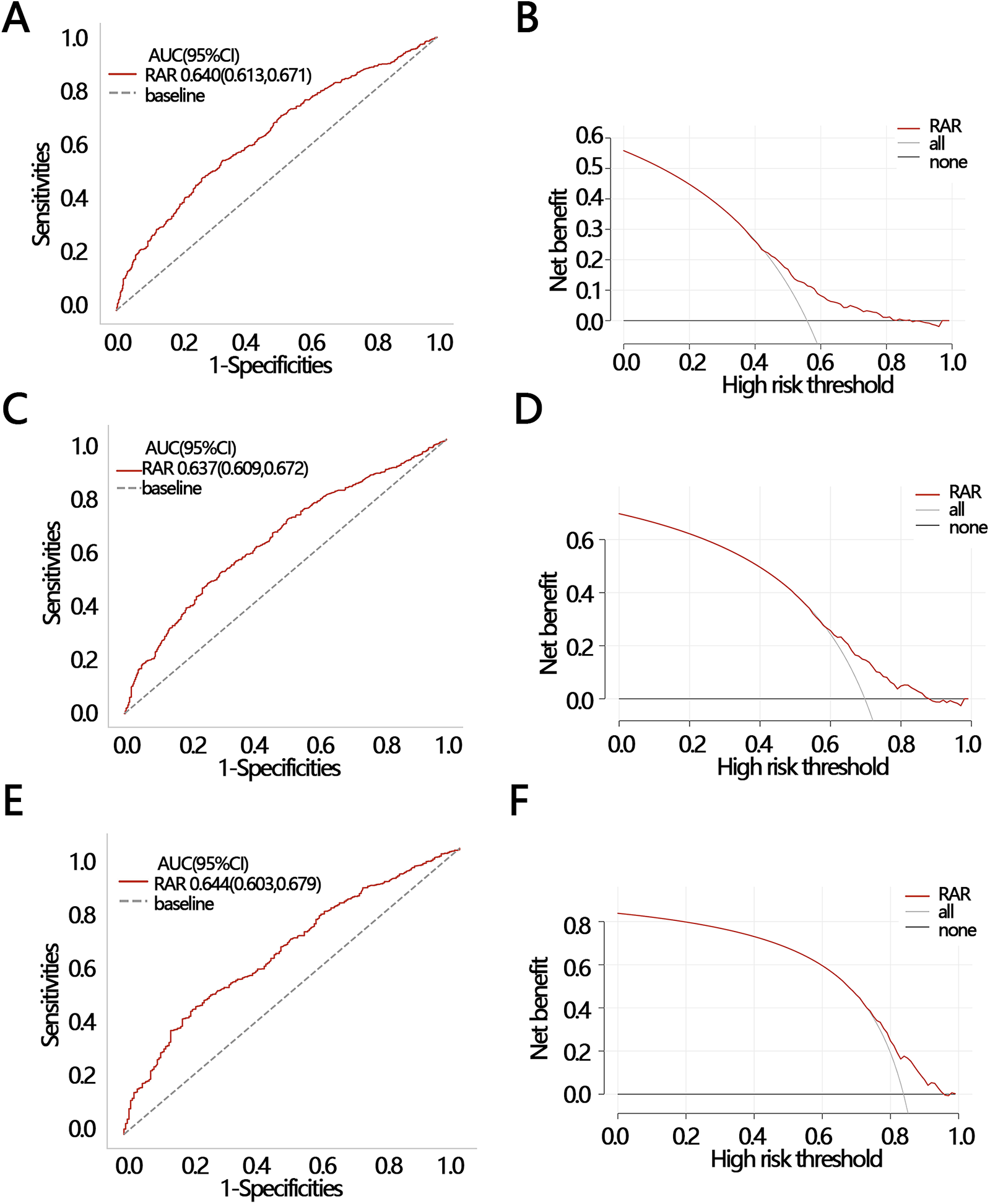
2 ROC and DCA were used to evaluate the clinical value of RAR in different outcomes. (A) ROC and (B) DCA for half-year mortality; (C) ROC and (D) DCA for one-year mortality; (E) ROC and (F) DCA for three-year mortality. ROC, receiver operating characteristic curve; DCA, decision curve analysis; AUC, areas under curve; CI, confidence interval.
4 Discussion
The pathogenesis of CHF combined with CKD is a complex condition, involving multiple mechanisms such as CKD resulting from heart failure, inflammatory responses, and neurohormonal activation (15, 16). RAR is a novel composite marker reflecting the body's immune and nutritional status (17). In this study, we found that RAR was significantly associated with the risk of in-hospital mortality, and this relationship was linear among patients with CHF combined with CKD. Furthermore, we also demonstrated that RAR serves as a strong predictor of patient survival, with good prognostic value for predicting 6-month, 1-year, and 3-year survival.
It had been suggested that RAR is associated with poor prognosis in various diseases, including coronary artery disease (10), heart failure (11), sepsis (13), aortic aneurysm (14), cancer (18), and autoimmune encephalitis (19). In our study, we also found that RAR was an independent risk factor of in-hospital mortality in patients. This may be related to inflammation and oxidative stress. In the context of CHF combined with CKD, CKD induces both local and systemic inflammatory responses. Cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are elevated, while oxidative stress can damage endothelial cells, increase vascular permeability, and further contribute to sodium and water retention and tubular damage. The interaction between induced oxidative stress and inflammation exacerbates the functional impairment of both the heart and kidneys (20). Furthermore, inflammation suppresses iron metabolism and erythropoietin production, leading to the release of more immature cells into the bloodstream, which results in an increase in RDW (21, 22). Additionally, studies have shown that inflammatory cytokines inhibit red blood cell maturation and promote the entry of immature red blood cells into peripheral circulation, thereby causing an increase in RDW (22).
Albumin is also a biomarker of inflammation and nutritional status. Inflammatory processes lower albumin levels, primarily due to the elevated expression of cytokines such as IL-6 and nitric oxide (NO), which induce the level of vascular endothelial growth factor (VEGF) to rise. This leads to increased microvascular permeability and angiogenesis, altering the distribution of albumin between the intravascular and extravascular spaces, thus lowering serum albumin concentration (23, 24). Moreover, most studies suggest that a decrease in RAR is associated with poor prognosis in various diseases (25). In summary, during inflammatory states, RAR is elevated, reflecting the degree of inflammation and is associated with poor patient prognosis. It is noteworthy that patients with concomitant CHF and CKD commonly present advanced age, volume overload (26, 27), and hepatic congestion. These characteristics collectively influence RAR levels and adversely impact prognosis. Advanced age is frequently associated with immunosenescence and impaired nutrient absorption (28), which may directly or indirectly elevate RAR. Volume overload potentially induces hemodilution and altered vascular permeability, both of which reduce serum albumin concentration and consequently increase RAR (29). Impaired cardiac function leads to systemic venous congestion, including compromised hepatic venous drainage, resulting in hepatic congestion [Liver abnormalities in heart failure]. Hepatic sinusoidal distention compresses hepatocytes, causing hepatocellular atrophy and mitochondrial dysfunction. This reduces ATP synthesis and impairs albumin production (30), thereby elevating RAR. Thus, the clinical significance of elevated RAR may lie in its integrative reflection of these intertwined prognostic risk factors, particularly the combination of inflammation and malnutrition.
Monocytes are key mediators of innate immunity (31). In this study, we also found that monocytes mediate the relationship between the RAR and in-hospital mortality. Patients with CHF often exhibit a systemic inflammatory state, with monocytes playing a central role in this process. By secreting cytokines such as TNF-α, interleukin-1 (IL-1), and IL-6, as well as chemokines, monocytes contribute to both local and systemic inflammation, leading to structural and functional damage to the heart and kidneys (32, 33). In the context of CHF combined with kidney disease, monocytes may exacerbate the interactions between the heart and kidney, affecting prognosis (34). RAR reflects the inflammatory and immune status within the patient's body. A higher RAR indicates a more severe stress response, with further activation of monocytes through their pro-inflammatory actions. This amplifies the deterioration of both heart and kidney functions, ultimately increasing the risk of in-hospital mortality.
Currently, several researchers have been focusing on the prediction of in-hospital mortality in patients with CHF and CKD. For example, Li et al. established a XGBoost model using 22 factors, including sequential organ failure assessment (SOFA) score, age, simplified acute physiology score II (SAPS II), and urine output, and achieved a prediction performance with AUC = 0.587 (35). The XGBoost model validated the potential of multi-metric federation. Chen et al. employed 13 variables and constructed a prognostic model, and the AUC of the model was 0.771 (36), which demonstrated the value of multidimensional assessment in specific populations. The AUC value of RAR identified in our study for predicting in-hospital mortality was 0.631, which was moderate. The core difference between our study and existing research is that, moderate predictive power is achieved with a minimalist metric (RDW/ALB ratio), while taking into account clinical operability and biological rationality. Compared with other studies, our research focused on the useful clinical routine indicators, and was designed to provide more practical tools for scenarios with limited resources or where rapid assessment is required. Furthermore, RAR demonstrates predictive capability not only for in-hospital mortality but also for half-year, one-year, and three-year mortality.
In other factors, our study has some strengths. First, this study is the first to reveal the relationship between the RAR and in-hospital mortality in patients with CHF combined with CKD Our study suggested the index simplicity and clinical operability. Only two routine laboratory indicators are required, eliminating the need for complex or expensive tests (e.g., genetic testing, dynamic imaging), significantly reducing the difficulty of data collection and the cost of clinical application. Second, RAR reflects the integration of multidimensional pathological information. RDW and albumin reflect different pathophysiological states, respectively, and their ratios may capture the core drivers of disease deterioration, while the multi-indicator model may mask key signals due to redundancy or correlation of indicators. Third, the black box nature of multi-metric models (such as XGBoost's complex algorithm) often makes it difficult for clinicians to understand the prediction logic, limiting its practical application. The RDW/ALB ratio is highly interpretable.
However, this study has certain limitations. First, the data used in this study were derived from the MIMIC database, which may have limitations related to racial diversity. Second, the study lacked measurements of RAR at multiple time points due to excessive missing data, preventing further exploration of the association between dynamic changes in RAR and mortality outcomes. Third, this study had several limitations regarding data availability. Important clinical indicators of heart failure severity-including NYHA functional classification, left ventricular function/dimensions, arrhythmia documentation, and B-type natriuretic peptide were not available in the MIMIC-IV database. C-reactive protein, NT-proBNP, and hs-C-reactive protein were excluded from analysis due to excessive missing data, rendering them unsuitable for meaningful statistical evaluation. Neutrophils, lymphocytes, and other immune-related indicators were also not available, leading to the inability to further explore the impact of immunity on the prognosis of patients.
5 Conclusion
This study released the positive linear relationship between RAR and in-hospital mortality, which provided a new biomarker for predicting the prognosis in patients with CHF combined with CKD. The study found that monocytes played a mediating role in the relationship between RAR and in-hospital mortality. In addition, the RAR had a good function for predicting the 6-month, 1-year, and 3-year survival.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
LQ: Conceptualization, Data curation, Formal analysis, Writing – original draft. LH: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1563512/full#supplementary-material
Abbreviations
CHF, congestive heart failure; CKD, chronic kidney disease; MIMIC-IV, medical information mart for intensive care IV; RCS, restricted cubic splines; ROC, receiver operating characteristic; DCA, decision curve analysis; RAAS, renin-angiotensin-aldosterone system; RDW, red blood cell distribution width; RAR, RDW-to-albumin ratio; ICU, intensive care unit; RBC, red blood cell; WBC, white blood cell; DM, diabetes mellitus; AF, atrial fibrillation.
References
1.
DammanKValenteMAVoorsAAO'ConnorCMvan VeldhuisenDJHillegeHL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. (2014) 35(7):455–69. 10.1093/eurheartj/eht386
2.
RoncoCHaapioMHouseAAAnavekarNBellomoR. Cardiorenal syndrome. J Am Coll Cardiol. (2008) 52(19):1527–39. 10.1016/j.jacc.2008.07.051
3.
HeywoodJTFonarowGCCostanzoMRMathurVSWigneswaranJRWynneJet alHigh prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. (2007) 13(6):422–30. 10.1016/j.cardfail.2007.03.011
4.
MullensWAbrahamsZFrancisGSSokosGTaylorDOStarlingRCet alImportance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. (2009) 53(7):589–96. 10.1016/j.jacc.2008.05.068
5.
DangHNNViet LuongTCaoMTTBuiVTTranTTNguyenHM. Assessing red blood cell distribution width in Vietnamese heart failure patients: a cross-sectional study. PLoS One. (2024) 19(7):e0301319. 10.1371/journal.pone.0301319
6.
JiXKeW. Red blood cell distribution width and all-cause mortality in congestive heart failure patients: a retrospective cohort study based on the Mimic-III database. Front Cardiovasc Med. (2023) 10:1126718. 10.3389/fcvm.2023.1126718
7.
FelkerGMAllenLAPocockSJShawLKMcMurrayJJPfefferMAet alRed cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the Duke Databank. J Am Coll Cardiol. (2007) 50(1):40–7. 10.1016/j.jacc.2007.02.067
8.
AllenLAFelkerGMMehraMRChiongJRDunlapSHGhaliJKet alValidation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. (2010) 16(3):230–8. 10.1016/j.cardfail.2009.11.003
9.
Management AIAFF-uIoR. Baseline characteristics of patients with atrial fibrillation: the AFFIRM study. Am Heart J. (2002) 143(6):991–1001. 10.1067/mhj.2002.122875
10.
HuangMLiuFLiZLiuYSuJMaMet alRelationship between red cell distribution width/albumin ratio and carotid plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. (2023) 22(1):39. 10.1186/s12933-023-01768-w
11.
NiQWangXWangJChenP. The red blood cell distribution width-albumin ratio: a promising predictor of mortality in heart failure patients—a cohort study. Clin Chim Acta. (2022) 527:38–46. 10.1016/j.cca.2021.12.027
12.
ZhouPTianPCZhaiMHuangYZhouQZhuangXFet alAssociation between red blood cell distribution width-to-albumin ratio and prognosis in non-ischaemic heart failure. ESC Heart Fail. (2024) 11(2):1110–20. 10.1002/ehf2.14628
13.
XuWHuoJChenGYangKHuangZPengLet alAssociation between red blood cell distribution width to albumin ratio and prognosis of patients with sepsis: a retrospective cohort study. Front Nutr. (2022) 9:1019502. 10.3389/fnut.2022.1019502
14.
LongJXieXXuDHuangCLiuYMengXet alAssociation between red blood cell distribution width-to-albumin ratio and prognosis of patients with aortic aneurysms. Int J Gen Med. (2021) 14:6287–94. 10.2147/IJGM.S328035
15.
SchefoldJCFilippatosGHasenfussGAnkerSDvon HaehlingS. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. (2016) 12(10):610–23. 10.1038/nrneph.2016.113
16.
BoorsmaEMTer MaatenJMVoorsAAvan VeldhuisenDJ. Renal compression in heart failure: the renal tamponade hypothesis. JACC Heart Fail. (2022) 10(3):175–83. 10.1016/j.jchf.2021.12.005
17.
HaoMJiangSTangJLiXWangSLiYet alRatio of red blood cell distribution width to albumin level and risk of mortality. JAMA Netw Open. (2024) 7(5):e2413213. 10.1001/jamanetworkopen.2024.13213
18.
LuCLongJLiuHXieXXuDFangXet alRed blood cell distribution width-to-albumin ratio is associated with all-cause mortality in cancer patients. J Clin Lab Anal. (2022) 36(5):e24423. 10.1002/jcla.24423
19.
LiDYangAXiaMMaKZhangJGuoYet alAssociation between red blood cell distribution width-to-albumin ratio and the prognosis in patients with autoimmune encephalitis: a retrospective cohort study. Front Neurol. (2023) 14:1276026. 10.3389/fneur.2023.1276026
20.
McCallumWSarnakMJ. Cardiorenal syndrome in the hospital. Clin J Am Soc Nephrol. (2023) 18(7):933–45. 10.2215/CJN.0000000000000064
21.
SalvagnoGLSanchis-GomarFPicanzaALippiG. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52(2):86–105. 10.3109/10408363.2014.992064
22.
WangHWangJHuangRXiaJZuoLYanXet alRed blood cell distribution width for predicting significant liver inflammation in patients with autoimmune hepatitis. Eur J Gastroenterol Hepatol. (2019) 31(12):1527–32. 10.1097/MEG.0000000000001447
23.
ShinoharaMMirakajVSerhanCN. Functional metabolomics reveals novel active products in the DHA metabolome. Front Immunol. (2012) 3:81. 10.3389/fimmu.2012.00081
24.
ArtigasAWernermanJArroyoVVincentJLLevyM. Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. (2016) 33:62–70. 10.1016/j.jcrc.2015.12.019
25.
SoetersPBWolfeRRShenkinA. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43(2):181–93. 10.1002/jpen.1451
26.
MillerWL. Assessment and management of volume overload and congestion in chronic heart failure: can measuring blood volume provide new insights?Kidney Dis (Basel). (2017) 2(4):164–9. 10.1159/000450526
27.
KhanYHSarriffAAdnanASKhanAHMallhiTH. Chronic kidney disease, fluid overload and diuretics: a complicated triangle. PLoS One. (2016) 11(7):e0159335. 10.1371/journal.pone.0159335
28.
CaldeiraMHRMello Almada-FilhoCBrunialtiMKCSalomaoRCendorogloM. Immune profile and body composition of independent oldest old: the longevous project. Gerontology. (2023) 69(6):660–70. 10.1159/000527485
29.
HungSCKuoKLPengCHWuCHLienYCWangYCet alVolume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. (2014) 85(3):703–9. 10.1038/ki.2013.336
30.
Castro-NarroGMoctezuma-VelazquezCMale-VelazquezRTrejo-EstradaRBosquesFJMoreno-AlcantarRet alPosition statement on the use of albumin in liver cirrhosis. Ann Hepatol. (2022) 27(4):100708. 10.1016/j.aohep.2022.100708
31.
FuMSDrummondRA. The diverse roles of monocytes in cryptococcosis. J Fungi (Basel). (2020) 6(3):111. 10.3390/jof6030111
32.
AdamoLRocha-ResendeCPrabhuSDMannDL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. (2020) 17(5):269–85. 10.1038/s41569-019-0315-x
33.
PeetCIveticABromageDIShahAM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. (2020) 116(6):1101–12. 10.1093/cvr/cvz336
34.
JohnsonDWJonesGRMathewTHLudlowMJChadbanSJUsherwoodTet alChronic kidney disease and measurement of albuminuria or proteinuria: a position statement. Med J Aust. (2012) 197(4):224–5. 10.5694/mja11.11468
35.
LiXWangZZhaoWShiRZhuYPanHet alMachine learning algorithm for predict the in-hospital mortality in critically ill patients with congestive heart failure combined with chronic kidney disease. Ren Fail. (2024) 46(1):2315298. 10.1080/0886022X.2024.2315298
36.
ChenJLiYLiuPWuHSuG. A nomogram to predict the in-hospital mortality of patients with congestive heart failure and chronic kidney disease. ESC Heart Fail. (2022) 9(5):3167–76. 10.1002/ehf2.14042
Summary
Keywords
congestive heart failure combined with chronic kidney disease, blood cell distribution width-to-albumin ratio, in-hospital mortality, machine learning algorithms, mediation analysis
Citation
Qian L-j and Hong L-x (2025) Association between red blood cell distribution width-to-albumin ratio and in-hospital mortality in patients with congestive heart failure combined with chronic kidney disease. Front. Cardiovasc. Med. 12:1563512. doi: 10.3389/fcvm.2025.1563512
Received
20 January 2025
Accepted
25 June 2025
Published
04 July 2025
Volume
12 - 2025
Edited by
Alexander E. Berezin, Paracelsus Medical University, Austria
Reviewed by
Vincenzo Santinelli, IRCCS San Donato Polyclinic, Italy
Tahir Yagdi, EGE University, Türkiye
Junping Tian, Capital Medical University, China
Updates
Copyright
© 2025 Qian and Hong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu-xi Hong hong5940@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.