Abstract
Background:
Computed tomography (CT)-determined low skeletal muscle mass (SMM) has been reported to be associated with poor clinical outcomes in various diseases; however, limited information is available regarding patients with heart failure (HF). Our research aimed to investigate the prognostic significance of low SMM assessed by chest CT scans in hospitalized patients with acute heart failure (AHF).
Methods:
Skeletal muscle index (SMI) was assessed using chest CT scans at the twelfth thoracic vertebra (T12) level, and sex-specific optimal cutoff values for T12 SMI were determined using the X-tile program based on all-cause mortality. The outcomes of this study were all-cause death and cardiovascular death. Cox proportional hazards models were employed to identify the risk factors for mortality.
Results:
This study enrolled 305 inpatients with AHF (62.3% males). According to the optimum cutoff value (31.9 cm2/m2 for females and 40.6 cm2/m2 for males), a total of 154 patients (50.5%) had low SMM. Kaplan–Meier survival analysis showed that patients with low SMM had a higher likelihood of experiencing all-cause death (p < 0.001) and cardiovascular death (p < 0.001) compared to those with normal SMM. Furthermore, multivariable Cox regression analysis revealed that low SMM was independent risk factors associated with all-cause death [hazard ratio (HR) = 2.37, 95% CI: 1.51–3.73; p < 0.001] and cardiovascular death (HR = 3.06, 95% CI: 1.75–5.37; p < 0.001).
Conclusions:
Chest CT-determined low SMM has the potential to serve as a valuable imaging prognostic indicator for predicting adverse outcomes in AHF patients.
1 Introduction
Heart failure (HF) is a widespread global epidemic that represents an advanced stage or severe manifestation of various heart conditions. Its prevalence significantly increases with age progression. Despite substantial advancements in both pharmaceutical and non-pharmaceutical therapies, the prognosis remains unfavorable. It continues to be a primary cause of mortality and morbidity worldwide, imposing significant financial burdens on patients, families, and healthcare systems (1).
Sarcopenia is a progressive systemic skeletal muscle disease, characterized by the accelerated loss of muscle mass, strength, and function (2), which is often worsened by HF (3). This can lead to decreased physical performance, lower quality of life, and an increased risk of disability, falls, and mortality (2, 4). Therefore, it has been identified as a strong predictor of adverse outcomes in various clinical scenarios (5–7). The occurrence of sarcopenia in patients with HF has been reported to range from 10% to 69% in previous studies (8). Studies have indicated that sarcopenia is the primary indicator of poor clinical outcomes in patients with HF, including a poor quality of life, longer hospital stays, recurrent hospitalizations, as well as an increased risk for mortality (9, 10). Consequently, it is recommended that the assessment of sarcopenia should be integrated into standard care protocols for patients with HF in clinical settings (11). However, there is currently no standardized method tailored to HF for identifying muscle wasting, and there are no established normal reference values for this particular population (12). Therefore, it is crucial to develop a reliable and convenient approach for assessing the quality and quantity of skeletal muscles mass (SMM), allowing for early identification of sarcopenia in patients with HF.
CT imaging technology is widely regarded as the promising method for precise measurement of total body and regional SMM (13). Research has widely utilized single-slice CT scans at the third lumbar vertebra (L3) level to measurement SMM, which have shown correlations with adverse clinical outcomes in various diseases (14, 15). However, chest CT scans are the preferred tool in HF due to their more frequent utilization in routine clinical practice compared to abdominopelvic CT scans. Currently, single-slice CT scans at the twelfth thoracic vertebra (T12) level have been validated as a valuable tool for evaluating SMM. Furthermore, recent research has demonstrated a robust association between skeletal muscle index (SMI) at the T12 level (T12-SMI) and L3-SMI in clinical settings, with comparable prognostic relevance in clinical contexts (16–18).
Nevertheless, there is limited available information regarding the association between SMM determined by chest CT and clinical outcomes in patients with HF. Therefore, the objective of this study was to investigate the association between chest CT-determined low SMM and prognosis in AHF patients.
2 Materials and methods
2.1 Study population
The present study was a retrospective cross-sectional analysis of consecutive patients with AHF who were admitted to the Department of Cardiology, People's Hospital Affiliated to Jiangsu University between May 2020 and December 2021. AHF encompasses the onset of new HF as well as the acute worsening of pre-existing chronic HF. The inclusion criteria were as follows: (1) age ≥18 years; (2) diagnosis of AHF based on the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic HF (19). The patients were further excluded based on the following criteria: (1) presence of cancer; (2) presence of liver cirrhosis; (3) undergoing hemodialysis treatment; (4) in-hospital death; (5) absence of chest CT images or poor-quality CT images; (6) loss of follow-up. This study adhered to the Declaration of Helsinki and received approval from the Ethics Committee of The Affiliated People's Hospital of Jiangsu University (No. K-20240094-W). The requirement for written informed consent was eliminated due to the retrospective design.
2.2 Measurement of skeletal muscle mass and quality
The non-enhanced chest CT examinations were conducted within 48 h of hospital admission using the 64-layer spiral CT (SOMATON sensation64, SIEMENS Healthcare, Germany) or 256-layer spiral CT scanners (Brilliance iCT, ROYAL PHILIPS, Netherlands). A senior radiologist extracted axial CT slices of the T12 vertebra level for each patient from the medical imaging management system, followed by independent analysis of the images by two trained observers using the image analysis software Slice-O-matic (Tomovision, version 5.0, Magog, Quebec, Canada). They were blinded to the patients' clinical outcomes when reviewing all selected CT slices and determining the SMM area. The pre-validated CT threshold of −29 to +150 Hounsfield units (HU) was utilized for identification and quantification all skeletal muscles in the selected axial images (20). The cross-sectional area of skeletal muscle tissue (cm2) at T12 was automatically calculated by the software and standardized as T12 SMI (cm2/m2) by dividing skeletal muscle area by height squared (Supplementary Figure S1) (21). The skeletal muscle density (SMD) in HU was determined by calculating the average radiodensity of the entire muscle area at T12 cross-sectional levels.
The inter-observer variability was assessed in 30 randomly selected scan samples. The intraclass correlation coefficient values were employed to demonstrate the consistency among observers when evaluating the skeletal muscle area.
Interobserver variability was evaluated in 30 randomly selected samples from the scans. The intraclass correlation coefficient (ICC) was used to evaluate the consistency among observers when measuring the area of skeletal muscle. As the measurements were semi-automated, no assessment of intra-observer variability was performed. As shown in Supplementary Figure S2, the optimal cutoff value of T12 SMI for both male and female patients was determined using the X-tile program based on all-cause death (22). According to the sex-specific optimal cutoff value, patients were stratified into two groups: low SMM group and normal SMM group.
2.3 Data collection
We obtained patients' personal data from the electronic medical record system, including demographic information (sex and age), anthropometric measurements (height and weight), lifestyle factors (smoking and drinking history), medical history (hypertension, diabetes, coronary heart disease), clinical laboratory test results [hemoglobin, liver and kidney function tests, uric acid, fasting blood glucose and lipid, albumin and B-type natriuretic peptide (BNP) ], 12-lead electrocardiogram findings, thoracic echocardiography results, as well as medications used at discharge. Clinical laboratory results within 24 h of admission were collected. The BNP analysis was performed using a commercially available immunoassay (Alere Inc., Waltham, MA) on the Alere Triage MeterPro analyzer, following the manufacturer's instructions. BNP values were transformed into a logarithmic scale (log BNP) for statistical analysis. According to the left ventricular ejection fraction (LVEF), we further classified the patients into heart failure patients with reduced ejection fraction (HFrEF, LVEF <50%) and those with preserved ejection fraction (HFpEF, LVEF ≥50%). The estimated glomerular filtration rate (eGFR) [ml/min/1.73m2] can be calculated using the Cockcroft-Gault formula: for males and for females (23). The definition of anemia involves a hemoglobin level below 12 g/dl for males and below 11 g/dl for females. Hypoalbuminemia is characterized by a serum albumin level lower than 3.5 g/dl. Hyperuricemia is identified as a serum uric acid concentration exceeding 7.0 mg/dl.
2.4 Survival follow-up and endpoint events
All patients received standard heart failure treatment after discharge. The endpoint events of this study were all-cause mortality and cardiovascular mortality. Cardiovascular death primarily refers to mortality caused by congestive HF, malignant arrhythmia, myocardial infarction, sudden death, stroke, or another cardiac-related issue. The late follow-up was conducted in April 2024. Survival data were collected from the electronic medical record system by a clinician or through telephone contact with patients or their family members.
2.5 Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). The distribution of continuous variables was examined using the Kolmogorov–Smirnov test. Continuous data were presented as mean ± standard deviation (SD) or median and interquartile range (IQR) for normally and not normally distributed continuous variables, respectively. Group differences were compared using appropriate statistical tests such as Student's t-test or Mann–Whitney U tests. Categorical variables were described as numbers and frequencies (%), with Chi-squared tests applied for analysis. Spearman's rank correlation analysis (r) was used to investigate the association between SMI and clinical indicators. The Kaplan–Meier analysis with log-rank test was utilized to evaluate survival, while univariable and multivariable Cox regression analyses were further employed to identify risk factors associated with mortality. The presence of multicollinearity among variables was assessed using the Variance Inflation Factor (VIF), where variables with a VIF value ≥10 were subsequently excluded from further analysis. Hazard ratio (HR) was calculated and the results were presented as HR with a 95% confidence interval (CI). The subgroup analyses were conducted based on sex, age (<65 years or ≥65 years), BMI (<24 kg/m2 or ≥24 kg/m2), NYHA classification, hypertension, diabetes, ischemic etiology, HF phenotypes (HFrEF or HFpEF) and atrial fibrillation. The likelihood ratio test was employed to evaluate the interaction in these subgroups. Statistical significance was determined at a two-tailed p value <0.05 for all analyses conducted in this study.
3 Results
3.1 Patient characteristics
After implementing rigorous exclusion criteria, a total of 305 patients with AHF were included in this study (Figure 1). The demographic and clinical characteristics of the participants are shown in Table 1. Among them, there were 190 (62.3%) males and 115 (37.7%) females. The median age was 70 years (IQR: 61–77), and the median BMI was 23.66 kg/m2 (IQR: 21.97–25.89). The sex-specific cut-off values of T12 SMI associated with all-cause death, as determined by the X-tile program, were 40.6 cm2/m2 for males and 31.9 cm2/m2 for females, respectively. Based on these defined thresholds, 154 (50.5%) patients had low SMM. Patients with low SMM were older [74 (IQR: 68–79) vs. 66 (IQR: 54–73), p < 0.001], more likely to be male (70.1% vs. 54.3%, p = 0.004), had a greater proportion of ischemic etiology (52.0% vs. 33.8%, p = 0.001) and NYHA class III–IV (85.7% vs. 62.3%, p < 0.001), and a longer median LOS [8 (IQR: 7–11) days vs. 7 (IQR: 5–8) days, p < 0.001] compared to those with normal SMM. Additionally, these patients showed lower levels of body mass index (BMI), hemoglobin, eGFR, albumin, cholesterol and triglyceride levels, but higher levels of log BNP and uric acid (all p < 0.05). The phenotypic disparities classified according to LVEF indicated that 230 (75.4%) patients had HFrEF (LVEF < 50%), while 75 (24.6%) patients had HFpEF (LVEF ≥ 50%). No association was found between HF phenotype and low SMM.
Figure 1
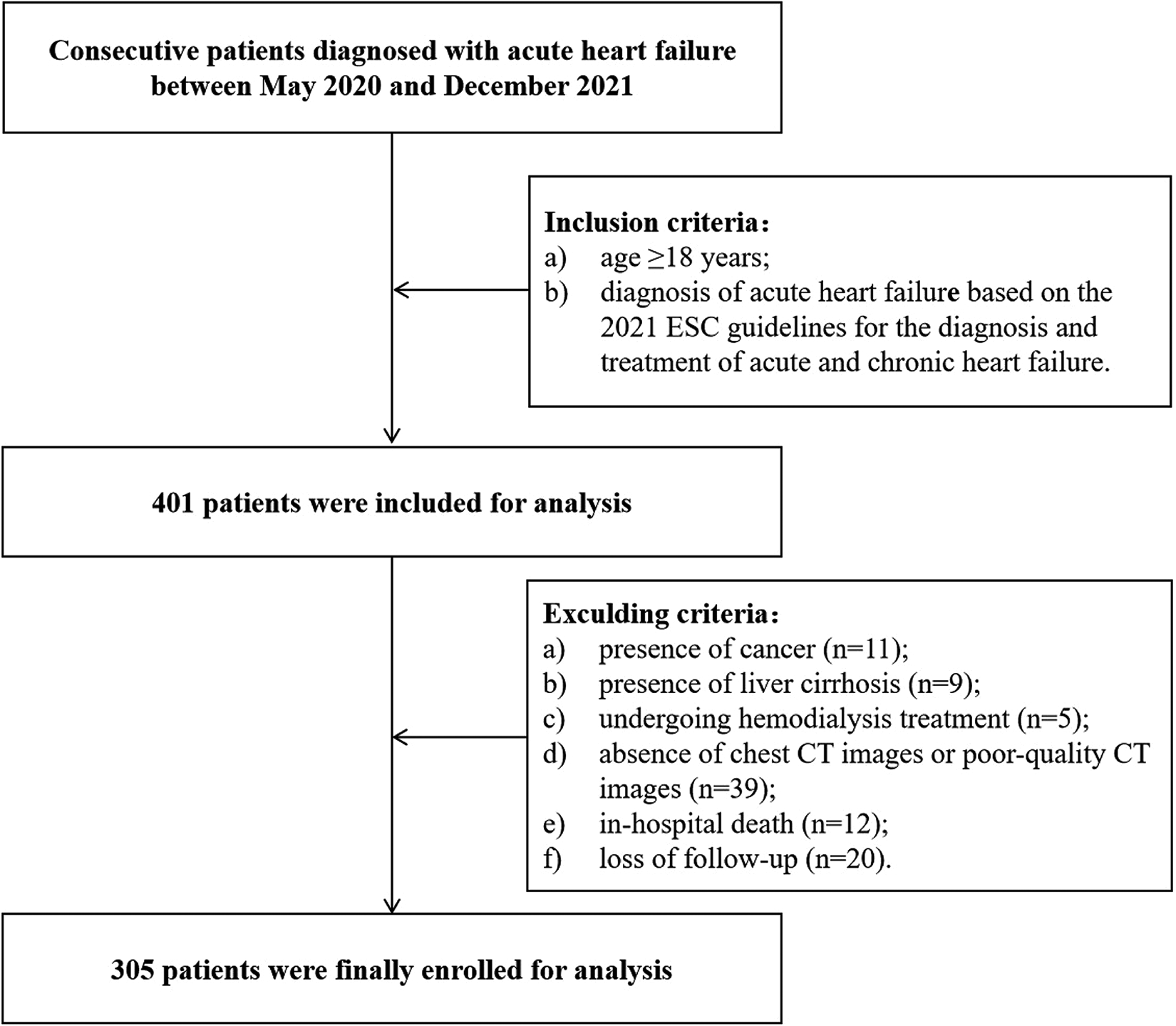
Flow diagram of the patient inclusion and exclusion process of this study. CT, computed tomography.
Table 1
| Characteristic | Total (n = 305) | Low SMM (n = 154) | Normal SMM (n = 151) | P-value |
|---|---|---|---|---|
| Age, year | 70 (61–77) | 74 (68–79) | 66 (54–73) | <0.001 |
| Sex, n (%) | 0.004 | |||
| Male, n (%) | 190 (62.3) | 108 (70.1) | 82 (54.3) | |
| Female, n (%) | 115 (37.7) | 46 (29.9) | 69 (45.7) | |
| BMI, kg/m2 | 23.7 (22.0–25.9) | 22.7 (20.9–24.2) | 25.0 (23.0–27.3) | <0.001 |
| SMD, HU | 29.1 ± 7.1 | 27.9 ± 6.8 | 30.3 ± 7.2 | 0.003 |
| Smoking, n (%) | 136 (44.6) | 72 (46.8) | 64 (42.4) | 0.443 |
| Drinking, n (%) | 59 (19.3) | 25 (16.2) | 34 (22.5) | 0.165 |
| Medical history, n (%) | ||||
| Hypertension | 186 (61.0) | 96 (62.3) | 90 (59.6) | 0.624 |
| Diabetes | 101 (33.1) | 47 (30.5) | 54 (35.8) | 0.331 |
| Atrial fibrillation | 126 (41.3) | 68 (44.2) | 58 (38.4) | 0.308 |
| Etiology of ischemic, n (%) | 131 (43.0) | 80 (51.9) | 51 (33.8) | 0.001 |
| Echocardiographic parameters | ||||
| LAD, mm | 46 (43–50) | 46 (42–50) | 47 (43–51) | 0.179 |
| LVEF, % | 37 (28–48) | 37 (28–48) | 35 (26.5–50) | 0.605 |
| LVEDD, mm | 58 (51–65) | 58 (50–63) | 58 (52–65) | 0.357 |
| LVESD, mm | 47 (37–55) | 46 (37–54) | 47 (37–56.5) | 0.455 |
| Heart failure phenotypes, n (%) | 0.619 | |||
| HFrEF (LVEF < 50%) | 230 (75.4) | 118 (76.6) | 112 (74.2) | |
| HFpEF (LVEF ≥ 50%) | 75 (24.6) | 36 (23.4) | 39 (25.8) | |
| NYHA functional class, n (%) | <0.001 | |||
| II | 79 (25.9) | 22 (14.3) | 57 (37.8) | |
| III | 134 (43.9) | 71 (46.1) | 63 (41.7) | |
| IV | 92 (30.2) | 61 (39.6) | 31 (20.5) | |
| Laboratory data | ||||
| BNP, ng/L | 744 (346–1,300) | 965 (489.5–1,582.5) | 545 (284–1,008.5) | <0.001 |
| Hemoglobin, g/dl | 12.6 (11.3–13.8) | 12.2 (10.8–13.6) | 12.9 (11.9–14.4) | 0.002 |
| Anemia, n (%) | 88 (28.9) | 59 (38.3) | 29 (19.2) | <0.001 |
| CRP, mg/L | 3.4 (1.1–8.7) | 3.6 (1.5–11.4) | 3.4 (0.7–7.2) | 0.055 |
| Albumin, g/dl | 3.6 (3.3–3.9) | 3.5 (3.2–3.8) | 3.7 (3.4–4.0) | <0.001 |
| Hypoalbuminemia, n (%) | 121 (39.7) | 75 (48.7) | 46 (30.5) | 0.001 |
| eGFR, ml/min/1.73 m2 | 59.8 (43.7–79.5) | 53.3 (38.1–73.2) | 66.1 (48.4–91.8) | <0.001 |
| Uric acid, mg/dl | 7.4 (5.8–9.2) | 7.9 (6.4–9.5) | 6.9 (5.6–9.1) | 0.010 |
| Hyperuricemia, n (%) | 163 (53.4) | 92 (59.7) | 71 (47.0) | 0.026 |
| Cholesterol, mmol/L | 3.7 (3.1–4.6) | 3.6 (3.1- 4.4) | 3.9 (3.3–4.7) | 0.014 |
| Triglyceride, mmol/L | 1.0 (0.8–1.4) | 0.9 (0.7–1.2) | 1.1 (0.8–1.5) | <0.001 |
| LDL-C, mmol/L | 2.0 (1.5–2.5) | 1.9 (1.4–2.4) | 2.0 (1.5–2.6) | 0.147 |
| Cardiovascular medications, n (%) | ||||
| ACE I/ARB/ARNI | 222 (72.8) | 107 (69.5) | 115 (76.2) | 0.190 |
| Beta-blocker | 224 (73.4) | 108 (70.6) | 116 (76.8) | 0.186 |
| Statin | 201 (65.9) | 106 (68.8) | 95 (62.9) | 0.276 |
| Diuretics | 290 (95.1) | 150 (97.4) | 140 (92.72) | 0.058 |
| MRA | 263 (86.2) | 135 (87.7) | 128 (84.8) | 0.463 |
| LOS, day | 7 (6–9) | 8 (7–11) | 7 (5–8) | <0.001 |
Baseline characteristics of the study population.
ACEI/ARB/ARNI, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor/neprilysin inhibitor; BMI, body mass index; BNP, B-type natriuretic peptide; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HU, hounsfield units; LAD, left atrium diameter; LDL-C, low-density lipoprotein cholesterol; LOS, length of hospital stay; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SMD, skeletal muscle density; SMM, skeletal muscle mass.
3.2 Consistency evaluation of skeletal muscle area measurement
The ICC for the inter-rater variability of skeletal muscle area measurements was 0.989 (95% CI: 0.965–0.998), demonstrating excellent consistency.
3.3 Correlation between T12 SMI and clinical parameters
Spearman's correlation analysis showed that T12 SMI was negatively correlated with age (r = −0.52, p < 0.001) and LVEF (r = −0.33, P < 0.001). Additionally, there is a strong positive correlation observed between T12 SMI and BMI (r = 0.49, p < 0.001) as well as hemoglobin (r = 0.43, p < 0.001). However, no significant correlations were found between T12 SMI and log BNP levels, albumin levels, cholesterol levels, triglyceride levels or CRP levels (Figure 2, all p > 0.05).
Figure 2
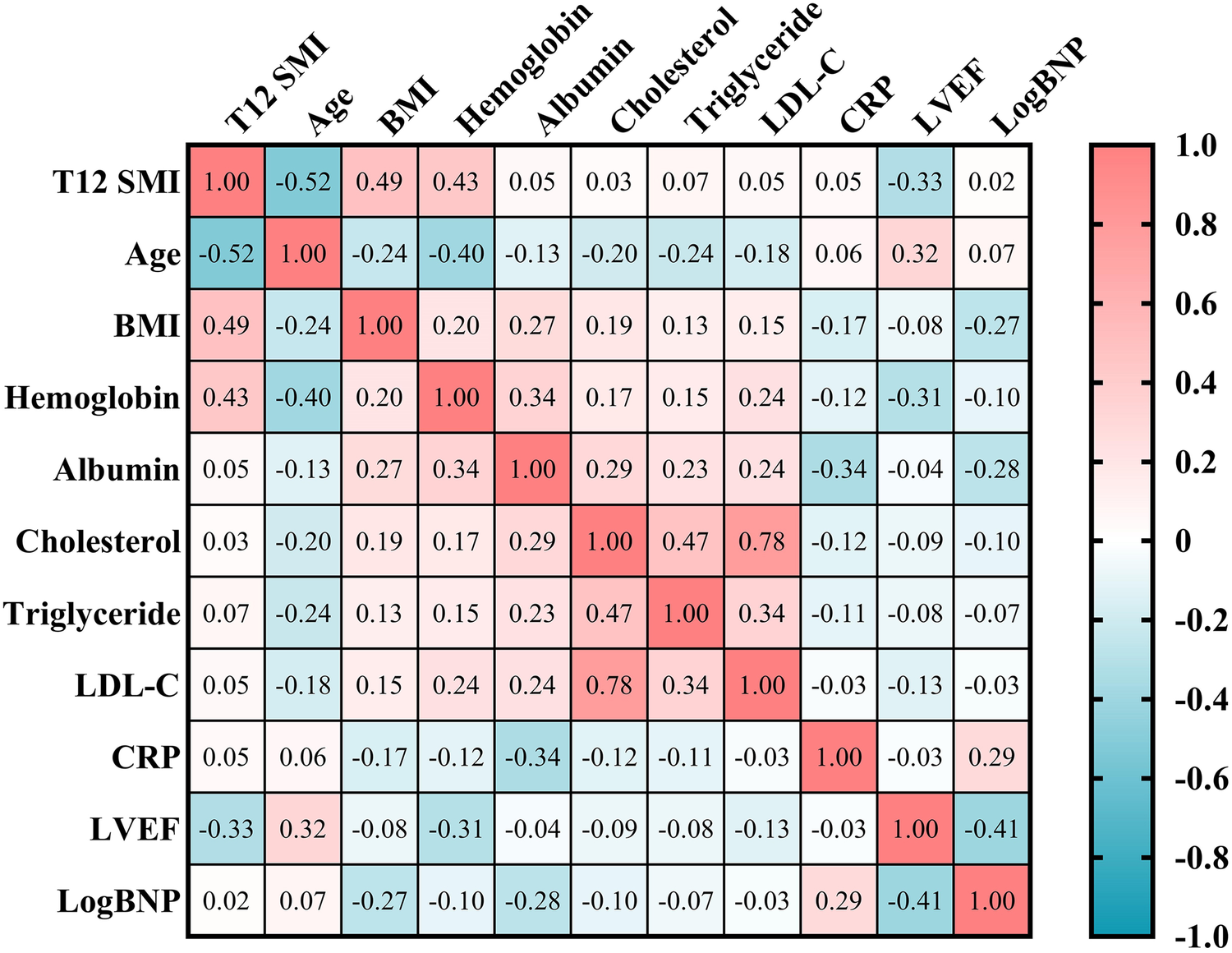
Spearman's correlation analysis of T12 SMI and clinical parameters. BMI, body mass index; BNP, brain natriuretic peptide; CRP, C-reactive protein; LDL-C, low-density lipoprotein cholesterol content; LVEF, left ventricular ejection fraction; T12 SMI, skeletal muscle index at the level of the twelfth thoracic vertebra.
3.4 Risk factors for survival outcomes
The median follow-up duration was 34 months (IQR: 27–39.5). Throughout the follow-up period, all-cause death occurred in 102 (33.4%) patients, while cardiovascular death was observed in 77 (25.3%) patients. According to Kaplan–Meier survival analysis, patients with low SMM had a higher likelihood of experiencing all-cause death (48.7% vs. 17.9%, log-rank p < 0.001; Figure 3A) and cardiovascular death (37.7% vs. 12.6%, log-rank p < 0.001; Figure 3B) compared to those with normal SMM. Based on the univariable Cox regression analysis presented in Supplementary Table S1, age, BMI, diabetes, atrial fibrillation, NYHA classification, log BNP, anemia, eGFR, hyperuricemia, and SMI (as both continuous and categorical variables) were identified as risk factors associated with all-cause death (p < 0.05). Meanwhile, BMI, atrial fibrillation, NYHA classification, log BNP, anemia, hypoproteinemia, eGFR, hyperuricemia, and SMI (as both continuous and categorical variables) were found to be risk factors for cardiovascular death (p < 0.05). In the multivariable Cox regression analysis, SMI as a continuous variable remained independently associated with all-cause death (HR = 0.94, 95% CI: 0.91–0.97, p < 0.001; Figure 4A, multivariate 1) and cardiovascular death (HR = 0.96, 95% CI: 0.93–0.99, p = 0.048; Figure 4B, multivariate 1). Furthermore, patients with low SMM exhibited a 2.37-fold increased risk of all-cause death (HR = 2.37, 95% CI: 1.51–3.73, p < 0.001; Figure 4A, multivariate 2) and a 3.06-fold increased risk of cardiovascular death (HR = 3.06, 95% CI: 1.75–5.37, p < 0.001; Figure 4B, multivariate 2) compared to those with normal SMM.
Figure 3
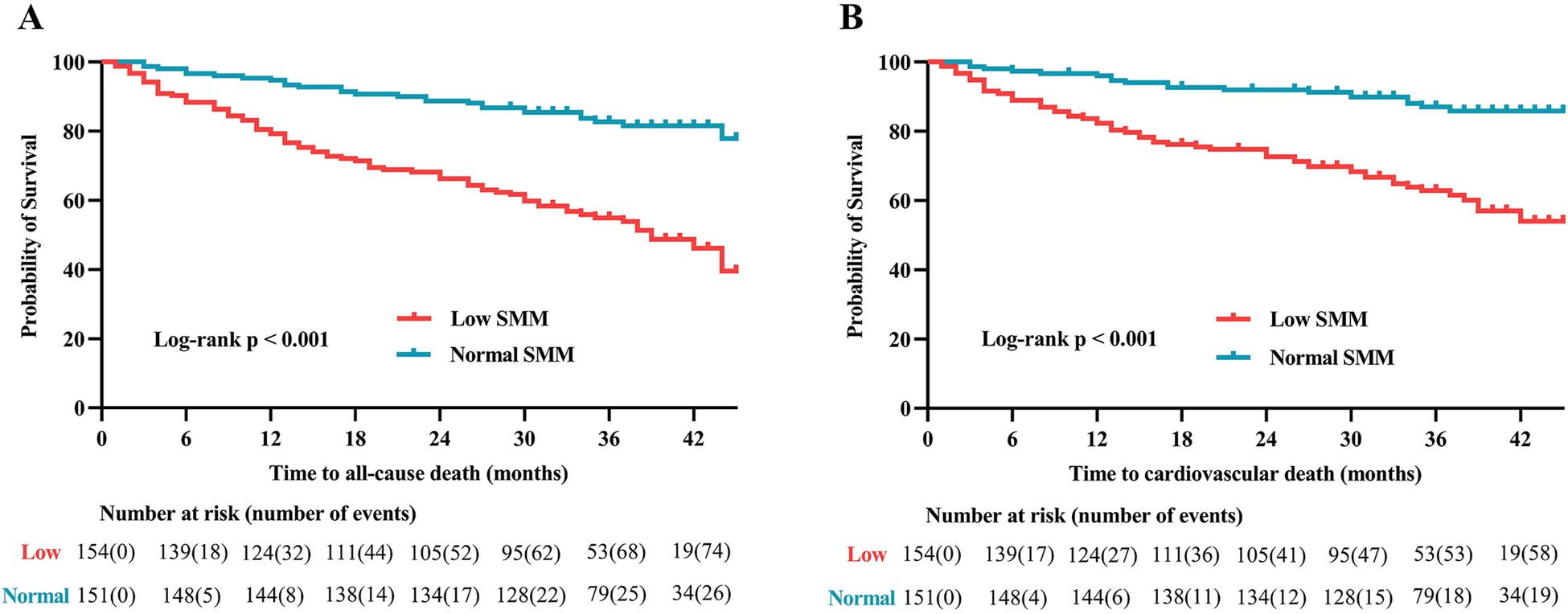
Kaplan–Meier analysis of (A) all-cause death and (B) cardiovascular death in patients with and without low SMM. SMM, skeletal muscle mass.
Figure 4
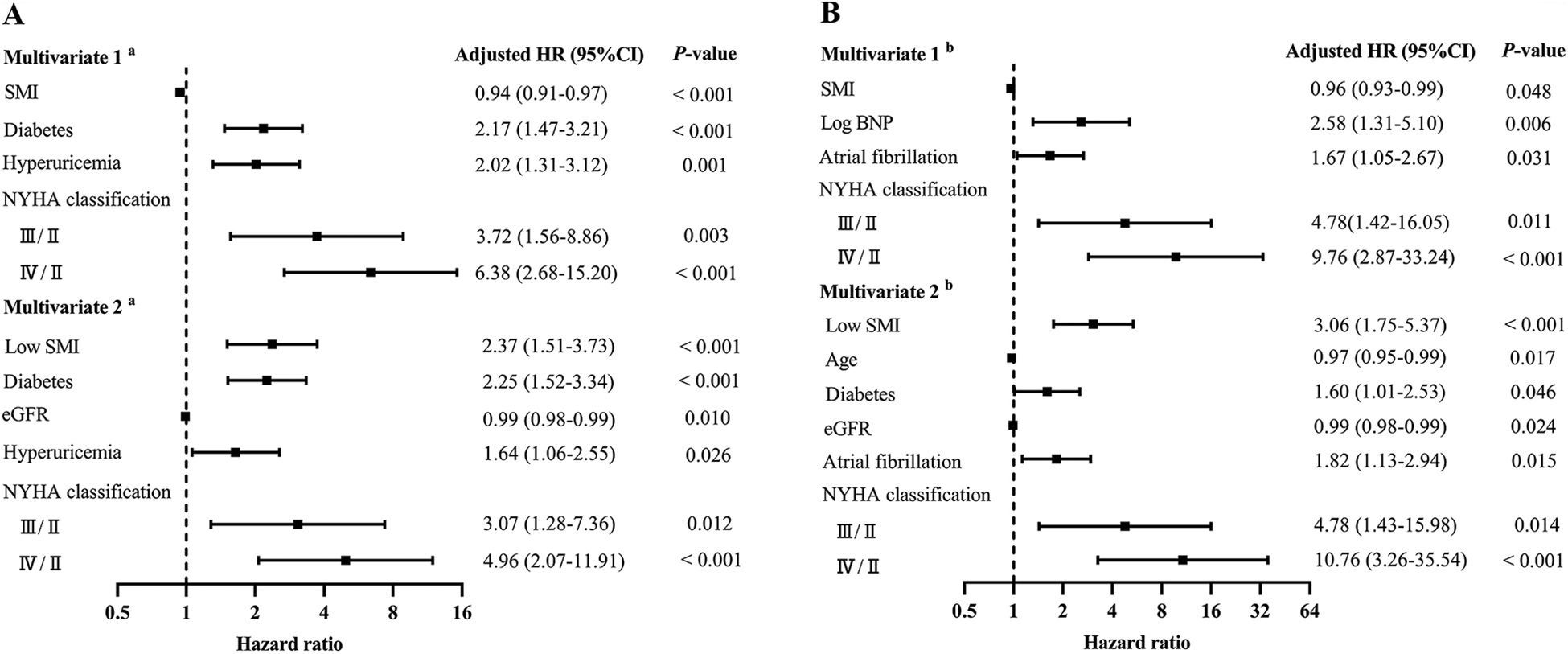
Forest plot of independent risk factors for all-cause death (A) and cardiovascular death (B). BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; SMI, skeletal muscle index. “III/II” and “IV/II” represent hazard ratios for NYHA class III and IV compared to class II (reference group).
3.5 Subgroup analyses
To further investigate the association of low SMM with all-cause death and cardiovascular death across diverse population, we further conducted subgroup analyses based on sex, age, BMI, NYHA classification, hypertension, diabetes, ischemic etiology, HF phenotypes and atrial fibrillation. As depicted in Figure 5, no statistically significant interaction was observed (all p for interaction >0.05), indicating that the association between low SMM and all-cause death as well as cardiovascular death remained unaffected by these factors.
Figure 5
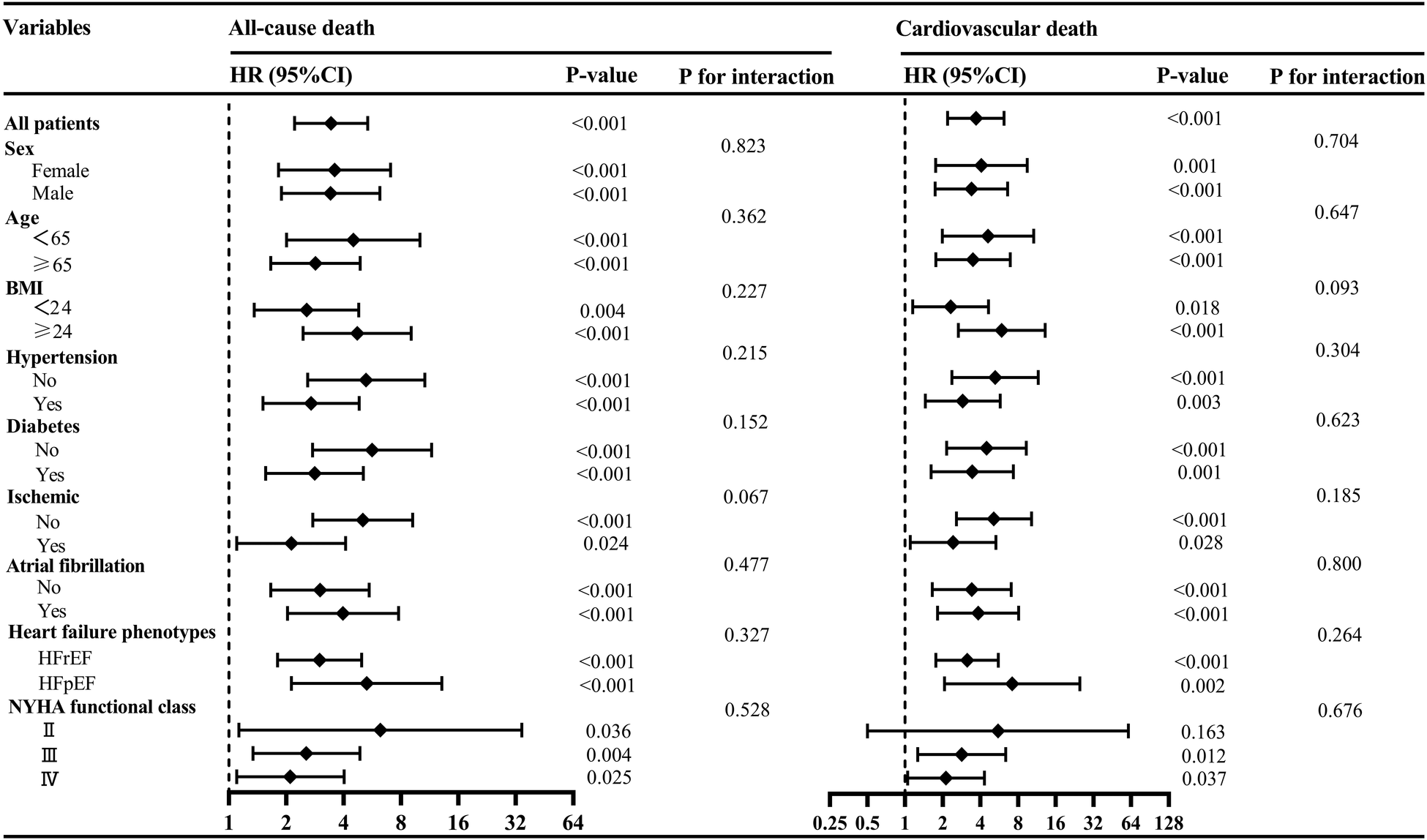
Subgroup analyses of the associations between low SMM and all-cause death and cardiovascular death. BMI, body mass index; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; SMM, skeletal muscle mass.
4 Discussion
Our study focused on utilizing clinically chest CT imaging to quantify SMM and investigate its prognostic implications in AHF patients. To the best of our knowledge, this is one of the few study that investigate the relationship between chest CT-determined SMM and survival outcomes in patient with AHF. In the present study, we found a significant and positive association between low SMM and increased all-cause mortality and cardiovascular mortality among AHF patients. Therefore, chest CT-determined low SMM may serve as a potentially reliable imaging biomarker for predicting adverse outcomes in AHF patients.
Muscle wasting is a significant concern not only for the elderly, but also a common complication of chronic diseases, independently associated with functional impairment, frailty, and mortality (2). A cross-sectional study utilizing the National Health and Nutrition Examination Survey database has demonstrated that low SMM is an independent risk factor for all-cause mortality and cardiovascular mortality in adults (24). Additionally, in certain specific populations such as those with chronic obstructive pulmonary disease, liver cirrhosis, and cancer, muscle wasting has been shown to be a stronger and independent predictor of adverse outcomes (6, 7, 25). Recent research has shown that muscle wasting is highly prevalent in patients with HF and has been recognized as a significant extracardiac factor that contributes to adverse clinical outcomes such as an increased likelihood of readmission for HF and higher mortality rates (12), despite variations in study populations and methodologies. In the studies investigating comorbidities aggravating heart failure, 200 stable outpatients with chronic HF and a disease duration of less than 3 months were included. SMM was assessed using dual-energy x-ray absorptiometry (DEXA), and muscle wasting was defined as a muscle mass index 2 SDs below the mean of a healthy young reference population aged 18–40 years. The results indicated that 19.5% of subjects had low SMM, which was associated with weaker muscle strength and reduced exercise capacity, as well as lower left ventricular ejection fraction (26). The prevalence of low SMM in our study cohort was significantly higher than that in this study, probably due to differences in the study population, as well as discrepancies in the measurement methods for SMM and the definition of low SMM. Another study conducted by Saito et al. investigated the prognostic significance of muscle mass in 226 elderly hospitalized patients with HF, using DEXA and bioelectrical impedance analysis (BIA). The study found poor consistency between the two methods of measuring SMM, and after adjusting for previous risk factors, only low SMM defined by DEXA, not BIA, was associated with all-cause mortality (27). This may be due to the susceptibility of BIA measurements to hydration status (28), thereby reducing the reliability of measurements in HF patients, which requires further investigation.
Currently, CT is a dependable technique favored by researchers for assessing muscle quantity and quality. Previous studies have fully confirmed that the muscle mass assessed by single-slice CT at L3 and T12 levels has predictive value for certain diseases (6, 15–18, 29). Chest CT has emerged as a valuable tool for diagnosing AHF due to its superior ability to detect subtle signs of congestion compared to chest x-rays and lung ultrasound (30). Its growing clinical utilization enables the acquisition of images at the T12 level in patients without additional expense. However, there remains a paucity of comprehensive research in the field of HF concerning the association between muscle mass evaluated through chest CT scans and clinical outcomes. Mirzai et al. conducted a retrospective study involving 385 patients with AHF and found that low SMM was independently associated with 1-year and 3-year all-cause mortality (31). Although the definition of low SMM in our study differs from theirs, the results are consistent with these observations, indicating that patients with low SMM have a higher risk of all-cause mortality and cardiovascular mortality. The potential reasons are as follows: low SMM may induce endothelial inflammation and exacerbate insulin resistance (32), thereby altering cardiac structure and function, ultimately leading to diastolic dysfunction (33). Moreover, low SMM is also associated with enhanced ergoreflex sensitization, which is considered an important contributor to typical symptoms of HF, such as exertional dyspnea, fatigue, and sympatho-vagal imbalance (34) Additionally, low SMM may also affect respiratory muscles, resulting in dyspnea, fatigue, and weakness, while worsening symptoms of exercise intolerance related to HF (35). This finding suggests that increasing muscle mass may be a feasible strategy to improve the prognosis of patients with AHF. Notably, exercise training, as an effective treatment for HF, can improve exercise tolerance by reducing ergoreflex sensitization and restoring the sympatho-vagal balance, further confirming the key role of ergoreflex sensitivity in the pathophysiology of HF in the context of low SMM (34). However, further comprehensive and rigorous intervention studies are required.
Serum albumin is a crucial biochemical marker for assessing nutritional status and metabolic function, with previous studies demonstrating their predictive value in HF (36). Armentaro et al. discovered that lower albumin levels in HF patients were linked to an increased risk of major adverse cardiac events, higher overall mortality, and a greater incidence of HF hospitalization (37). Similar findings were also reported by Ancion et al. (38). It is noteworthy that no correlation was observed between serum albumin levels and prognosis in this study cohort. This finding was incongruent with prior research results, possibly due to potential selection bias resulting from the inclusion criteria used in this study, thus limiting the generalizability of this finding.
This study has certain limitations: (1) Being a single-center cohort study, the relatively small sample size may introduce bias and confounding factors. Further validation through larger-scale multi-center studies is necessary to corroborate the results. (2) The X-tile program was employed to determine the optimal cut-off value based on all-cause death. While some subjectivity may exist in this process, there is currently no universally standardized cut-off value for CT-determined low SMM in patients with AHF. Nevertheless, we believe this method can provide valuable insights for future research. It is crucial to emphasize that the proposed SMM cut-offs are exploratory and require further validation in external cohorts. (3) While this study has indeed identified a new and promising alternative method for assessing sarcopenia, there is a lack of data on muscle strength and quality, such as grip strength and physical performance, which are essential for stratifying sarcopenia. Therefore, further research is necessary to address these gaps in the current understanding of the condition.
In conclusion, chest CT-determined low SMM at T12 level was independently associated with poor prognosis in patients with AHF. Utilizing chest CT for assessing muscle mass enables physicians to identify high-risk AHF patients and implement timely and effective intervention measures, guiding the selection of personalized treatment strategies to enhance patients' prognosis. Further research with larger prospective is urgently necessary to validate its efficacy and reliability.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Affiliated People's Hospital of Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective design of the study.
Author contributions
YH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. YW: Data curation, Investigation, Writing – original draft. JG: Data curation, Investigation, Writing – review & editing. PF: Data curation, Investigation, Writing – review & editing. BK: Validation, Writing – review & editing. ZW: Validation, Writing – review & editing. JZ: Conceptualization, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Zhenjiang First People’s Hospital Scientific Research Fund (KFB2024004) and Science and Technology Planning Social Development Project of Zhenjiang City (SH2024023).
Acknowledgments
The authors express their gratitude to all staff members for their support in data collection and to the participants who contributed their data. All authors approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1569681/full#supplementary-material
References
1.
SavareseGBecherPMLundLHSeferovicPRosanoGMCCoatsAJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. 10.1093/cvr/cvac013
2.
Cruz-JentoftAJSayerAA. Sarcopenia. Lancet. (2019) 393(10191):2636–46. 10.1016/S0140-6736(19)31138-9
3.
DamlujiAAAlfaraidhyMAlHajriNRohantNNKumarMAl MaloufCet alSarcopenia and cardiovascular diseases. Circulation. (2023) 147(20):1534–53. 10.1161/CIRCULATIONAHA.123.064071
4.
BeaudartCZaariaMPasleauFReginsterJYBruyèreO. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. (2017) 12(1):e0169548. 10.1371/journal.pone.0169548
5.
WilliamsGRDunneRFGiriSShacharSSCaanBJ. Sarcopenia in the older adult with cancer. J Clin Oncol. (2021) 39(19):2068–78. 10.1200/JCO.21.00102
6.
ZhouDZhangDZengCZhangLGaoXWangX. Impact of sarcopenia on the survival of patients undergoing liver transplantation for decompensated liver cirrhosis. J Cachexia Sarcopenia Muscle. (2023) 14(6):2602–12. 10.1002/jcsm.13334
7.
ChoiYJKimTParkHJChoJHByunMK. Long-term clinical outcomes of patients with chronic obstructive pulmonary disease with sarcopenia. Life (Basel, Switzerland). (2023) 13(8):1628. 10.3390/life13081628
8.
ZhangYZhangJNiWYuanXZhangHLiPet alSarcopenia in heart failure: a systematic review and meta-analysis. ESC heart Failure. (2021) 8(2):1007–17. 10.1002/ehf2.13255
9.
Bielecka-DabrowaAEbnerNDos SantosMRIshidaJHasenfussGvon HaehlingS. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur J Heart Fail. (2020) 22(12):2314–26. 10.1002/ejhf.2011
10.
KonishiMKagiyamaNKamiyaKSaitoHSaitoKOgasaharaYet alImpact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur J Prev Cardiol. (2021) 28(9):1022–9. 10.1093/eurjpc/zwaa117
11.
MirzaiSEckBLChenPHEstepJDTangWHW. Current approach to the diagnosis of sarcopenia in heart failure: a narrative review on the role of clinical and imaging assessments. Circ Heart Fail. (2022) 15(10):e009322. 10.1161/CIRCHEARTFAILURE.121.009322
12.
LenaAAnkerMSSpringerJ. Muscle wasting and sarcopenia in heart failure-the current state of science. Int J Mol Sci. (2020) 21(18):6549. 10.3390/ijms21186549
13.
Cruz-JentoftAJBahatGBauerJBoirieYBruyereOCederholmTet alSarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48(1):16–31. 10.1093/ageing/afy169
14.
RinninellaECintoniMRaoulPPozzoCStrippoliABriaEet alMuscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: a systematic review and meta-analysis. Clin Nutr. (2020) 39(7):2045–54. 10.1016/j.clnu.2019.10.021
15.
RaoulPCintoniMCoppolaAAlfieriSTortoraGGasbarriniAet alPreoperative low skeletal muscle mass index assessed using L3-CT as a prognostic marker of clinical outcomes in pancreatic cancer patients undergoing surgery: a systematic review and meta-analysis. Int J Surg. (2023) 110(10):6126–34. 10.1097/JS9.0000000000000989
16.
GrigioniSLvovschiVETamionFJolyLMCoeffierMVan ElslandeHet alLow thoracic skeletal muscle index is associated with negative outcomes in 244 patients with respiratory COVID-19. Clin Nutr. (2023) 42(2):102–7. 10.1016/j.clnu.2022.11.011
17.
ShenYLuoLFuHXieLZhangWLuJet alChest computed tomography-derived muscle mass and quality indicators, in-hospital outcomes, and costs in older inpatients. J Cachexia Sarcopenia Muscle. (2022) 13(2):966–75. 10.1002/jcsm.12948
18.
NemecUHeidingerBSokasCChuLEisenbergRL. Diagnosing sarcopenia on thoracic computed tomography: quantitative assessment of skeletal muscle mass in patients undergoing transcatheter aortic valve replacement. Acad Radiol. (2017) 24(9):1154–61. 10.1016/j.acra.2017.02.008
19.
AdamoMGardnerRSMcDonaghTAMetraM. The ‘ten Commandments’ of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2022) 43(6):440–1. 10.1093/eurheartj/ehab853
20.
AminiBBoyleSPBoutinRDLenchikL. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol Ser A Biol Sci Med Sci. (2019) 74(10):1671–8. 10.1093/gerona/glz034
21.
KimEHKimKWShinYLeeJKoYKimYJet alReference data and T-scores of lumbar skeletal muscle area and its skeletal muscle indices measured by CT scan in a healthy Korean population. J Gerontol A Biol Sci Med Sci. (2021) 76(2):265–71. 10.1093/gerona/glaa065
22.
CampRLDolled-FilhartMRimmDL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. (2004) 10(21):7252–9. 10.1158/1078-0432.CCR-04-0713
23.
CockcroftDWGaultMH. Prediction of creatinine clearance from serum creatinine. Nephron. (1976) 16(1):31–41. 10.1159/000180580
24.
KimDLeeJParkROhCMMoonS. Association of low muscle mass and obesity with increased all-cause and cardiovascular disease mortality in US adults. J Cachexia Sarcopenia Muscle. (2024) 15(1):240–54. 10.1002/jcsm.13397
25.
AnsariEGanryLVan CannEMde BreeR. Impact of low skeletal muscle mass on postoperative complications in head and neck cancer patients undergoing free flap reconstructive surgery—a systematic review and meta-analysis. Oral Oncol. (2023) 147:106598. 10.1016/j.oraloncology.2023.106598
26.
FulsterSTackeMSandekAEbnerNTschopeCDoehnerWet alMuscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. (2013) 34(7):512–9. 10.1093/eurheartj/ehs381
27.
SaitoHMatsueYMaedaDKasaiTKagiyamaNEndoYet alPrognostic values of muscle mass assessed by dual-energy x-ray absorptiometry and bioelectrical impedance analysis in older patients with heart failure. Geriatr Gerontol Int. (2022) 22(8):610–5. 10.1111/ggi.14424
28.
ZuoJZhouDZhangLZhouXGaoXHouWet alComparison of bioelectrical impedance analysis and computed tomography for the assessment of muscle mass in patients with gastric cancer. Nutrition. (2024) 121:112363. 10.1016/j.nut.2024.112363
29.
JinYMaXYangZZhangN. Low L3 skeletal muscle index associated with the clinicopathological characteristics and prognosis of ovarian cancer: a meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14(2):697–705. 10.1002/jcsm.13175
30.
CardinaleLPriolaAMMorettiFVolpicelliG. Effectiveness of chest radiography, lung ultrasound and thoracic computed tomography in the diagnosis of congestive heart failure. World J Radiol. (2014) 6(6):230–7. 10.4329/wjr.v6.i6.230
31.
MirzaiSSarnaikKSPersitsIMartensPEstepJDChenPHet alCombined prognostic impact of low muscle mass and hypoalbuminemia in patients hospitalized for heart failure: a retrospective cohort study. J Am Heart Assoc. (2024) 13(3):e030991. 10.1161/JAHA.123.030991
32.
HongSHChoiKM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. (2020) 21(2):494. 10.3390/ijms21020494
33.
YooJHParkSWJunJEJinSMHurKYLeeMKet alRelationship between low skeletal muscle mass, sarcopenic obesity and left ventricular diastolic dysfunction in Korean adults. Diabetes Metab Res Rev. (2021) 37(1):e3363. 10.1002/dmrr.3363
34.
AimoASaccaroLFBorrelliCFabianiIGentileFPassinoCet alThe ergoreflex: how the skeletal muscle modulates ventilation and cardiovascular function in health and disease. Eur J Heart Fail. (2021) 23(9):1458–67. 10.1002/ejhf.2298
35.
EmekliEBostanci CanEZ. Prognostic value of diaphragm diameter, muscle volume, and bone mineral density in critically ill COVID-19 patients. J Intensive Care Med. (2023) 38(9):847–55. 10.1177/08850666231169494
36.
BiancucciMBarbieroRPennellaBCannataAAgenoWTangianuFet alHypoalbuminaemia and heart failure: a practical review of current evidence. Eur J Heart Fail. (2024) 27(2):293–306. 10.1002/ejhf.3363
37.
ArmentaroGCondoleoVPasturaCAGrassoMFrascaAMartireDet alPrognostic role of serum albumin levels in patients with chronic heart failure. Intern Emerg Med. (2024) 19(5):1323–33. 10.1007/s11739-024-03612-9
38.
AncionAAllepaertsSRobinetSOuryCPierardLALancellottiP. Serum albumin level and long-term outcome in acute heart failure. Acta Cardiol. (2019) 74(6):465–71. 10.1080/00015385.2018.1521557
Summary
Keywords
computed tomography, acute heart failure, skeletal muscle mass, skeletal muscle index, prognosis
Citation
Huang Y, Wang Y, Guo J, Fang P, Kong B, Wang Z and Zuo J (2025) Impact of chest computed tomography-determined low skeletal muscle mass on the survival of patients with acute heart failure. Front. Cardiovasc. Med. 12:1569681. doi: 10.3389/fcvm.2025.1569681
Received
01 February 2025
Accepted
21 July 2025
Published
04 August 2025
Volume
12 - 2025
Edited by
Alberto Giannoni, Sant'Anna School of Advanced Studies, Italy
Reviewed by
Paolo Sciarrone, Sant'Anna School of Advanced Studies, Italy
Emre Emekli, Eskişehir Osmangazi University, Türkiye
Updates
Copyright
© 2025 Huang, Wang, Guo, Fang, Kong, Wang and Zuo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junbo Zuo zuojunbo88@ujs.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.