- 1Department of Cardiology, Fujian Medical Center for Cardiovascular Diseases, Fujian Institute of Coronary Heart Disease, Fujian Medical University Union Hospital, Fuzhou, China
- 2Department of Epidemiology, School of Public Health, Fujian Medical University, Fuzhou, China
Background: This study aims to investigate the relationship between estimated pulse wave velocity (ePWV) and all-cause mortality (ACM) and cardiovascular mortality (CVM) in patients with obstructive sleep apnea (OSA).
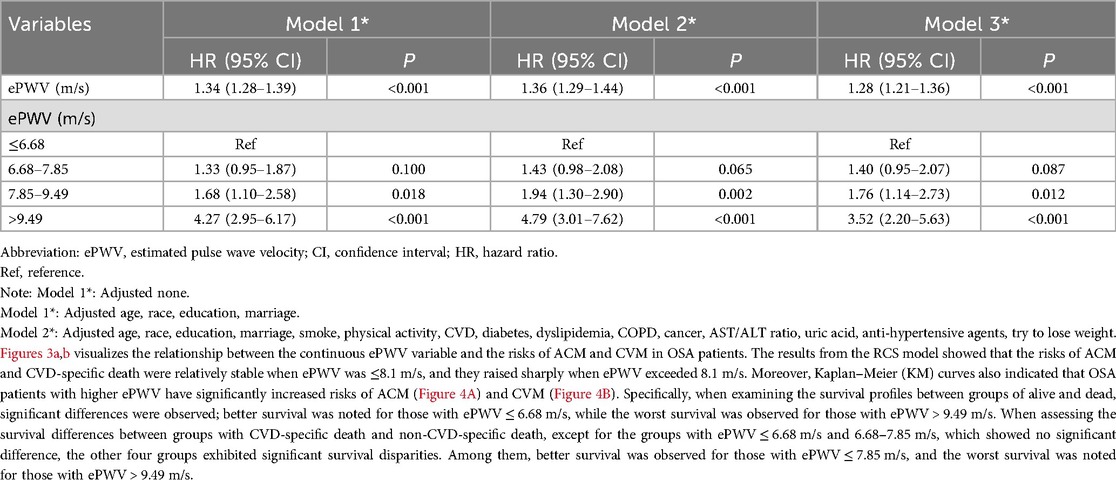
Method and results: A cohort study was conducted using data from the NHANES database (2005–2008, 2015–2018), focusing on adults with OSA. ePWV was calculated based on age and mean blood pressure. A weighted Cox regression model analyzed the association of ePWV with ACM and CVM, while restricted cubic spline (RCS) curves visualized this relationship. Kaplan–Meier (KM) survival curves assessed survival across different ePWV groups.The study involved 10,071 OSA patients with an average age of 48.46 years, with a follow-up period ending in December 2019 (average follow-up time: 102.10 ± 2.34 months). Results showed that increased ePWV correlated with higher ACM [hazard ratio [HR]: 1.25, 95% confidence interval [CI]: 1.20–1.31] and CVM (HR: 1.28, 95% CI: 1.21–1.36). RCS curves indicated stable mortality risks at ePWV ≤ 8.1 m/s, with rapid increases beyond this threshold. KM curves demonstrated poorer survival outcomes for OSA patients with elevated ePWV.
Conclusion: Elevated ePWV levels are linked to increased ACM and CVM in OSA patients, suggesting that monitoring ePWV could help mitigate these risks and promote healthier longevity in this population.
Introduction
Sleep apnea is a prevalent sleep disorder characterized by the recurrent cessation and initiation of breathing (apneas or hypopneas) during sleep (1, 2). The most common type of sleep-related breathing disorder is obstructive sleep apnea (OSA), with reports indicating that approximately one billion individuals worldwide are affected by OSA, and this number continues to escalate (3). The primary clinical hallmark of OSA is the repetitive collapse of the upper airway during sleep due to anatomical narrowing and increased pharyngeal compliance, leading to obstruction (4, 5). The most pronounced external impact is the loud, frequent, and bothersome snoring that occurs during sleep, which not only disrupts nocturnal rest and may even result in episodes of suffocation during sleep but also impairs daytime alertness (6, 7). Studies suggested that OSA may be a potential independent risk factor for sudden cardiac death (8). Besides, an increasing number of studies confirm that the risk of all-cause death was significantly increased in patients with moderate to severe or severe OSA (9, 10). Consequently, it is essential to pay attention to the health status of patients with OSA, strive to reduce the burden related to mortality, and ensure a better healthy lifespan.
Arterial stiffness (AS), also known as the loss of arterial elasticity, is a reliable indicator for assessing changes in arterial structure and function, and it is also an important cardiovascular risk factor (11, 12). AS is widely recognized as a significant predictor of cardiovascular events and mortality, including all-cause mortality (ACM) and cardiovascular mortality (CVM) (13, 14). Currently, carotid-femoral pulse wave velocity (cf-PWV) is a common method for assessing AS (15). However, the measurement of cf-PWV requires expensive specialized equipment and professional personnel (16). On the one hand, this leads to its limited use in clinical practice, and on the other hand, it restricts its widespread application in large-scale, large-sample population epidemiological studies (17). Researchers have proposed a new concept of estimating pulse wave velocity (ePWV). ePWV is a new indicator calculated based on mean blood pressure (MBP) and age (18). Previous studies have found that ePWV has good predictive value for stroke, myocardial infarction, CVM, and other outcomes (19, 20). Moreover, studies have shown a strong correlation between ePWV and measured cfPWV, suggesting that ePWV measurement can be used to monitor the severity of AS (21, 22).
Published studies have demonstrated a close association between OSA and AS (23–26), that OSA often promotes the development of AS. Patients with OSA often present with endothelial dysfunction, which contributes to the development of atherosclerosis (27). Additionally, the chronic intermittent hypoxia associated with OSA can activate signaling pathways that promote inflammation and vascular remodeling, leading to the progression of atherosclerotic plaques (28, 29). Tang et al. also found that compared to the general population, individuals with OSA have increased AS, which may rise significantly with the severity of OSA (30). The relationship between OSA and AS thus holds significant clinical implications, underscoring the need for OSA patients to be more vigilant about arteriosclerosis. Although several studies have investigated the relationship between ePWV and mortality in the general population, stroke patients, hypertensive population, and those with diabetes, to our knowledge, the association between ePWV and mortality among high-risk OSA patients remains uncharted (12, 17, 31, 32). Against this research backdrop, in the present study, we utilized the large multi-ethnic cohort data from the National Health and Nutrition Examination Survey (NHANES) to assess the relationship between AS, as indicated by ePWV, and ACM and CVM in patients with OSA.
Methods
Data source
The NHANES is a nationwide study conducted by the National Center for Health Statistics (NCHS) in the United States, focusing on the health and nutritional status of U.S. adult and pediatric populations. Using a complex multi-stage sampling design, the survey is conducted biennially to monitor the prevalence of diseases by examining the health and nutritional conditions of the aforementioned groups (33). Additionally, each participant provides the necessary written informed consent before starting investigations. All NHANES data is publicly available and can be freely downloaded through the following means: https://www.cdc.gov/nchs/nhanes/index.htm.
Study design and population selection
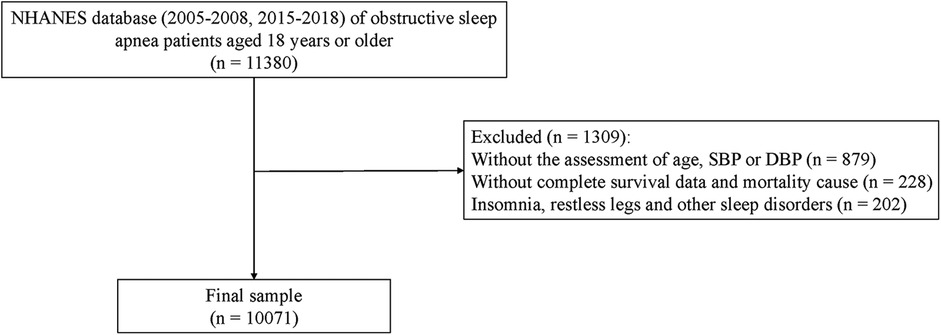
This study was a retrospective cohort study, with data sourced from the NHANES database for the years 2005–2008 and 2015–2018, which included specific sleep questionnaire information and blood pressure and survival and death information data. Patient information was collected through interviews and physical examinations during follow-up, which concluded in December 2019, lasting a total of 102.10 ± 2.34 months. Patient records meeting the following criteria were extracted from NHANES: (1) Age ≥ 18 years; (2) Patients with OSA. Records were excluded for patients who possessed the following status: (1) Missing data for age, systolic or diastolic blood pressure; (2) Lacking survival data; (3) With sleep disorders such as insomnia, restless legs syndrome, and other sleep disorders. Specific study population choices are displayed in Figure 1.
Definition of OSA
The definition of OSA was based on the following three conditions, and the presence of one or more of these conditions was considered diagnostic of OSA (6, 34): (1) Snoring on 3 or more nights per week: Select option 2 or 3 for sleep disorders (SLQ) 030; (2) Snoring, gasping, or experiencing episodes of breath cessation on at least 3 nights per week: Select option 2 or 3 for SLQ040; (3) Feeling extremely sleepy during the day on 16–30 days per month: Select option 4 for SLQ120.
Evaluation of ePWV
ePWV was calculated based on Equation (1), which was derived from the reference values of the Arterial Stiffness Collaboration (35).
Within this formula, MBP was computed according to Equation (2), where blood pressure was measured and recorded following the protocol published by the American Heart Association (36). Participants were required to remain seated for at least 5 min before testing, and the measurements were taken by trained medical staff. The blood pressure value was the average of three consecutive readings.
Study outcomes
The outcomes of this study were ACM and CVM. The mortality was determined based on the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (37). ACM referred to the sum of deaths from all causes. CVM was defined as deaths caused by a range of conditions related to the heart and vascular system. Specifically, in ICD-10, CVM codes included a range of codes related to conditions such as cardiomyopathy (I40–I42), myocarditis (I51.4–I51.6), heart failure (I50), cerebrovascular diseases (I60–I69), and adverse reactions from poisoning or effects on the cardiovascular system (T46).
Definition of potential variables
According to clinical practice guidelines and the relevant published literature, the following covariates were selected. The latent covariates for the ACM outcome were as follows: Age (years), race, education, marriage, poverty-to-income ratio, smoking (times/week), physical activity (met*minutes/week), total energy (kilocalorie), sleep duration (hours), cardiovascular disease (CVD), diabetes, dyslipidemia, chronic obstructive pulmonary disease (COPD), depression, cancer, aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio, anti-hypertensive agents, using barbiturates and benzodiazepines, trying to lose weight. The potential covariates for CVM were as follows: Age (years), race, education, marriage, smoking (times/week), physical activity (met*minutes/week), CVD, diabetes, dyslipidemia, COPD, cancer, AST/ALT ratio, uric acid (mg/dl), anti-hypertensive agents, trying to lose weight. According to the subsequent analysis, the final covariate most suitable for inclusion in each outcome was selected. In addition, a specific definition or criterion of each covariate is provided in the supporting material (Supplementary Table S1).
Statistical analysis
In accordance with the actual conditions of the original database, the existing presence of missing data values was observed, with specific missing variables and proportions detailed in (Supplementary Table S2). In this article, variables with a higher degree of missingness were categorized as unknown. For variables with fewer missing values, a multiple imputation method based on chained equations using a random forest approach was employed to handle the imputation of missing values. After imputation, in order to ensure the reliability of the imputation data, a sensitivity analysis was used to explore whether there was a difference between the missing variable data before and after imputation. The results of the analysis are shown in (Supplementary Table S3), which exhibited that there was no statistical difference between the data before and after imputation.
The study characterized continuous data with mean [SE] and categorical data with case numbers (percentage). Independent t-tests compared continuous data, chi-square tests for categorical, and rank sum tests for ordinal data between groups. A weighted univariate Cox regression identified ACM and CVM factors, detailed in (Supplementary Tables S4, S5). Significant factors were used in a weighted Cox regression to assess ePWV's association with ACM and CVM, both as a continuous and quartile variable. The Log-rank test and RCS analyzed survival differences and nonlinear relationships between ePWV and mortality risk. Stratified analyses were based on comorbidities: hypertension, diabetes, dyslipidemia, CVD, and depression.
All statistical tests were conducted using two-sided tests with a significance level of α = 0.05. Data cleaning and handling of missing values were performed using Python 3.9 (https://www.python.org/), while model statistical analysis was carried out using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the study population
This study included a total of 10,071 OSA patients surveyed by NHANES. Among them, 8,440 patients were alive and 1,631 were dead, with 513 experiencing CVD-specific death. The average age of all study population was 48.46 years old, with a male-female ratio close to 1:1. The weighted mean ePWV was 8.31 m/s. The ePWV was categorized into four quartiles as follows: the first quartile ≤ 6.68 m/s (2,457 individuals, 25%), the second quartile 6.68–7.85 m/s (2,127 individuals, 24.99%), the third quartile 7.85–9.49 m/s (2,313 individuals, 25.01%), and the fourth quartile > 9.49 m/s (3,174 individuals, 25.00%). The total population was stratified by survival status, with 8,440 individuals in the survival group and 1,631 in the dead group. Among the dead, there were 513 individuals attributed to CVD-specific death and 9,558 attributed to non-CVD-specific death. The baseline characteristics comparison according to survival status and CVD-specific death are detailed in Supplementary Table S6. The distribution of ePWV among OSA patients is shown in (Supplementary Figure S1), and the stratification of ePWV based on survival status and CVM is depicted in Figures 2a,b. The majority of dead OSA patients and those with CVD-specific death possessed higher ePWV values.
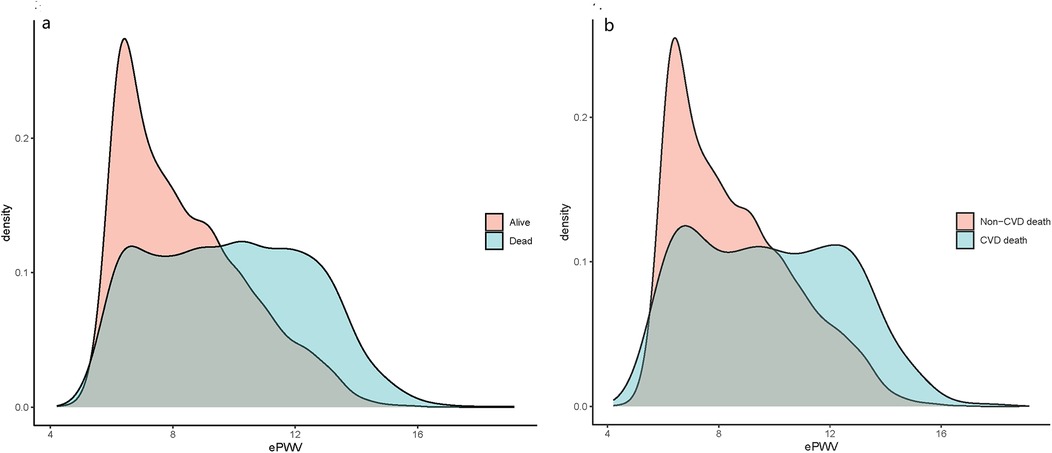
Figure 2. ePWV density distribution curves of OSA patients in different characteristic groups: (a) alive and dead population; (b) CVD deaths and non-CVD deaths.
Associations of ePWV with ACM and CVM
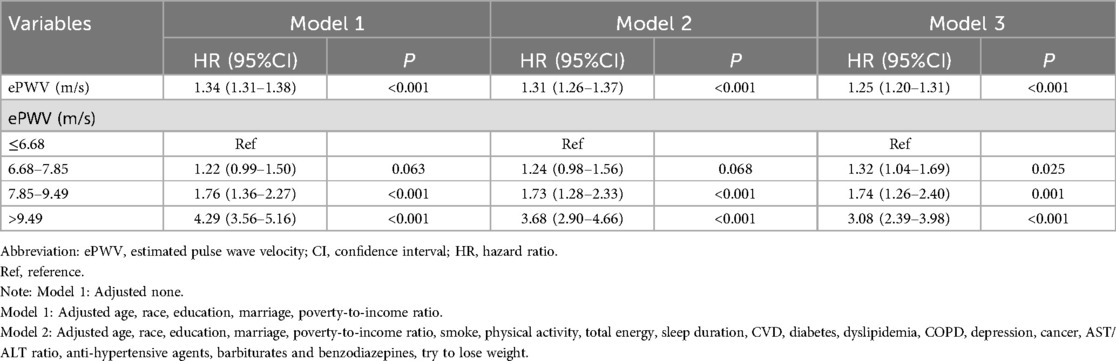
Table 1 and Table 2 showed the results of analyzing the relationship for ePWV with ACM and CVM, respectively. When ePWV was analyzed as a continuous variable, an increase of one unit in ePWV was associated with a 25% increase in the risk of ACM (HR: 1.25, 95% CI: 1.20–1.31) and a 28% increase in the risk of CVD-specific death (HR: 1.28, 95% CI: 1.21–1.36). Additionally, when ePWV was analyzed as a categorical variable, the risk of ACM significantly increased for individuals in the second, third, and fourth quartiles compared to those in the first quartile (ePWV ≤ 6.68 m/s) (all P < 0.05). The risks in the aforementioned three groups increased by 32% (HR: 1.32, 95% CI: 2.39–3.98), 74% (HR: 1.74, 95% CI: 1.26–2.40), and 208% (HR: 3.08, 95% CI: 2.39–3.98) compared to the reference group. In association with CVM, the risk of CVD-specific death increased by 76% (HR: 1.76, 95% CI: 1.14–2.73) and 252% (HR: 3.52, 95% CI: 2.20–5.63) for patients in the third and fourth quartiles, respectively, compared to those in the lowest quartile (all P < 0.05).
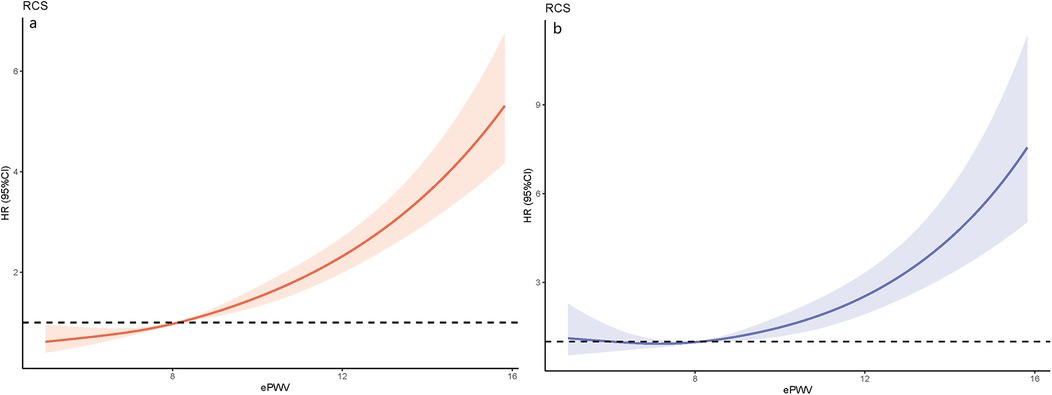
Figure 3. The results of RCS analysis: (a) association between ePWV and ACM using a RCS regression; (b) association between ePWV and CVM using a RCS regression.
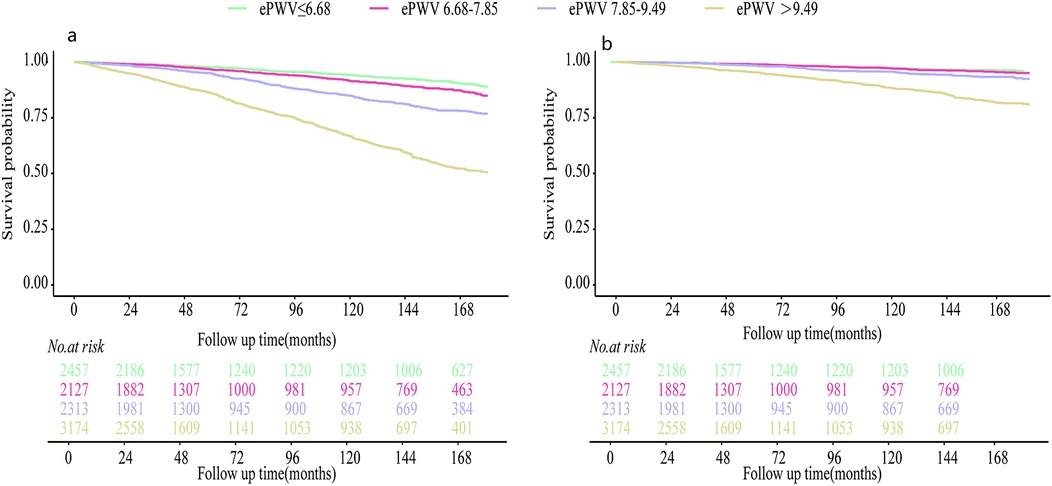
Figure 4. Kaplan–meier survival curves for patients with different degrees of ePWV: (a) the outcome variable was all-cause death; (b) the outcome variable was CVD-specific death.
Stratification analysis of ePWV association with ACM and CVM
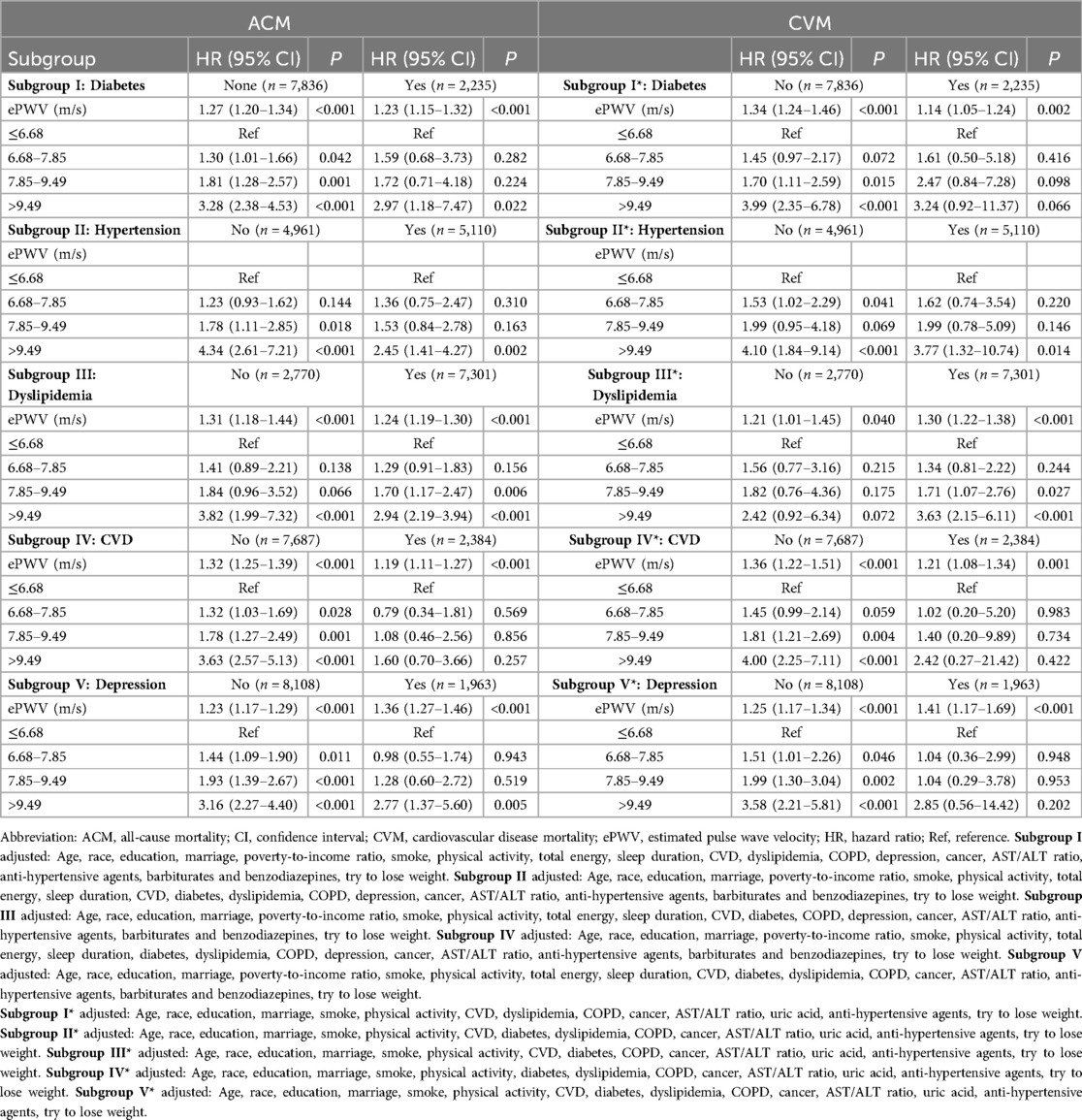
As shown in Table 3, the associations of ePWV with ACM and CVM were explored in subgroups defined by the presence of diabetes, hypertension, dyslipidemia, CVD, and depression. When ePWV was treated as a continuous variable, a statistically significant association between ePWV and both ACM and CVM was observed across all subgroups, indicating that ePWV was positively correlated with ACM and CVM. The analysis results of ePWV as a categorical variable in relation to ACM and CVM are specifically presented in Table 3.
Discussion
OSA is significantly associated with CVD occurrence and mortality (38). Arterial stiffness (AS), a known cardiovascular risk factor, is linked to OSA-related cardiovascular complications (39). Studies show a correlation between AS severity and OSA, suggesting OSA's role in AS development and associated CVD risks (40, 41). This study used ePWV, an indicator of AS, to explore its link with ACM and CVD-specific death in OSA patients. The results revealed a positive correlation between ePWV and both ACM and CVM, indicating that higher ePWV levels increase the risk of all-cause or CVD-related death in OSA patients (15).
Heffernan et al. (31) found ePWV linked to cardiovascular (CVD) and all-cause mortality (ACM) in a broad U.S. adult sample, suggesting it could provide insights into CVD and ACM risk beyond traditional risk factors. Huang et al. (12) and Wu et al. (17) found higher ePWV associated with increased ACM and cardio-cerebrovascular disease (CCD) mortality in stroke patients and diabetic patients, respectively, indicating ePWV as an independent risk factor. Similar results were seen in studies on hypertension and coronary artery disease patients (32, 42). In OSA patients, higher ePWV levels also correlated with increased ACM and CVM risks, regardless of confounding factors. Li's study (43) showed ePWV's strong predictive value for CVM and ACM in obese individuals, proposing it as a potential biomarker for mortality risk assessment. ePWV, derived from age and blood pressure, could be a valuable CVD risk indicator, especially in OSA patients, and its inclusion in routine health checks could aid in understanding and managing CVD risk across populations.
In patients with OSA, the intima-media thickness and carotid artery diameter are generally higher, and numerous studies have confirmed the association between OSA and vascular dysfunction (44, 45). The aforementioned linkage can be attributed to the intermittent hypoxia often experienced by OSA patients, leading to the occurrence of intermittent hypoxemia (46). Intermittent hypoxia tends to induce an increase in inflammatory substances such as high-sensitivity C-reactive protein and interleukin-6, causing an inflammatory response (47). These inflammatory substances are closely related to endothelial function and promote the occurrence of arteriosclerosis, leading to the development of CVD (30). Intermittent hypoxia also significantly increases the production of reactive oxygen species, leading to increased oxidative stress (48). Oxidative stress reduces the generation of nitric oxide, causing endothelial dysfunction and arteriosclerosis, ultimately increasing CVD in OSA patients (49, 50). This study used ePWV, a simple assessment indicator of arteriosclerosis, to observe the association between arteriosclerosis and ACM and CVM in OSA patients, finding a close positive correlation of ePWV with ACM and CVM. Therefore, early prevention of arteriosclerosis is very important for the OSA population. In summary, ePWV can serve as a widespread screening and self-monitoring indicator for the OSA population and even the general population. Early attention to arteriosclerosis may help the OSA population better prevent CVD, providing some assurance for the extension of healthy life expectancy in the OSA population.
Stratified analysis showed that in OSA patients with comorbidities like diabetes, dyslipidemia, hypertension, CVD, or depression, higher ePWV was linked to increased all-cause and CVD-specific mortality risks (51–54). This trend was also seen in OSA patients without these conditions, though the mechanisms are not fully understood. ePWV is a common indicator for assessing arterial stiffness (AS) (43), which can predict cardiovascular damage or prognosis by estimating the load on coronary, cerebral arteries, and aorta (55, 56). Epidemiological evidence supports arteriosclerosis's role in diseases like diabetes, dyslipidemia, hypertension, CVD, and depression (51–54). Possible reasons for subgroup analysis findings include: increased pulse pressure from arterial stiffness leading to vascular damage and mortality (12, 57); OSA patients with these diseases being more frail, obscuring the ePWV-mortality link; and early intervention reducing death risk, potentially diminishing ePWV's association with ACM and CVM (43). For instance, SGLT2 inhibitors like dapagliflozin can reduce PWV in diabetic patients (58), and their use is associated with lower cardiovascular event risks (59).
This study has certain strengths. Firstly, to our knowledge, this was the first study to explore the relationship between ePWV and ACM/CVM in patients with OSA, providing new perspectives for the screening and management of CVD prevention in this high-risk population. Secondly, the study population was drawn from the NHANES database, which employed a multi-stage complex sampling method, ensuring a good representation of the local population. Lastly, the large sample size and sufficient follow-up time of this survey ensured the occurrence of outcome events, providing robust statistical power. Despite some significant findings, the study possessed certain limitations. First, due to the limitations of the database, the study population was identified based on typical symptoms of OSA collected through questionnaires, lacking data related to polysomnography or sleep apnea tests, which may lead to selection bias and information bias. Therefore, future studies should use more objective measurement data to verify the aforementioned associations. Second, given that the database lacks follow-up data pertaining to ventilator treatment, this study did not delve into this aspect. This may impact the prognosis of OSA patients to a certain extent and thus should be taken into consideration when interpreting the conclusions. Third, although this study was a cohort study, it was also subject to inherent biases associated with this type of research, and future explorations could be conducted in studies with higher methodological rigor, such as randomized controlled trials. Fourth, Our study extends the application of the estimated pulse wave velocity (ePWV) equation to populations with cardiovascular disease (CVD), diabetes, and antihypertensive therapy—groups explicitly excluded from the original derivation cohort. While the generalizability of the ePWV formula in such populations may be questioned, our findings suggest that its predictive validity remains robust. This can be attributed to the pathophysiological primacy of age and blood pressure (BP) in driving arterial stiffness, which collectively explain over 80% of the variance in directly measured carotid-femoral PWV, even in high-risk populations. Importantly, the use of measured BP values (rather than hypothetical “untreated” BP) in ePWV calculation inherently accounts for the effects of antihypertensive therapy, reflecting the net arterial health status under real-world clinical management. Nevertheless, the original equation does not explicitly incorporate diabetes-specific pathways (e.g., advanced glycation end-product accumulation) or CVD-related vascular remodeling, which may partially attenuate its precision in these subgroups. Future studies integrating comorbidity-adjusted ePWV models with direct PWV measurements could further refine risk stratification in complex populations.Lastly, the study data came from the NHANES database of American adults, so caution should be exercised when generalizing the results to populations of different races or with different economic and geographical conditions, and further research is needed to interpret these results in other populations.
Conclusion
This study found that among 10,071 OSA patients surveyed by NHANES from 2005 to 2008 and 2015 to 2018, higher levels of ePWV during the follow-up period were associated with increased ACM and CVM. This research provides new scientific evidence for the future clinical use of ePWV as an initial assessment of AS and as a screening tool for the early identification of high-risk populations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Continuation of Protocol #2011-17. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: Writing – original draft. HZ: Writing – original draft. TL: Data curation, Methodology, Supervision, Writing – review & editing. LC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supporting funds of Fujian Medical Center for Cardiovascular Diseases (Grant N0: 0802701022100201) and Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2024Y9246).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1571610/full#supplementary-material
Abbreviations
ACM, all-cause mortality; ALT, alanine aminotransferase; AS, arterial stiffness; AST, aspartate aminotransferase; cf-PWV, carotid-femoral pulse wave velocity; CCD, cardio-cerebrovascular disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; CVM, cardiovascular mortality; ePWV, estimated pulse wave velocity; HR, hazard ratio; ICD, international classification of diseases; MBP, mean blood pressure; NCHS, national center for health statistics; NHANES, national health and nutrition examination survey; KM, Kaplan–Meier; OSA, obstructive sleep apnea; RCS, restricted cubic spline; SE, standard error; SGLT2, sodium-glucose cotransporter-2; SLQ, sleep disorders.
References
1. Montazeri Ghahjaverestan N, Aguiar C, Hummel R, Cao X, Yu J, Bradley TD. Sleep apnea detection by tracheal motion and sound, and oximetry via application of deep neural networks. Nat Sci Sleep. (2023) 15:423–32. doi: 10.2147/NSS.S397196
2. Yayan J, Rasche K. A systematic review of risk factors for sleep apnea. Prev Med Rep. (2024) 42:102750. doi: 10.1016/j.pmedr.2024.102750
3. Lyons MM, Bhatt NY, Pack AI, Magalang UJ. Global burden of sleep-disordered breathing and its implications. Respirology. (2020) 25(7):690–702. doi: 10.1111/resp.13838
4. Bazoukis G, Bollepalli SC, Chung CT, Li X, Tse G, Bartley BL, et al. Application of artificial intelligence in the diagnosis of sleep apnea. J Clin Sleep Med. (2023) 19(7):1337–63. doi: 10.5664/jcsm.10532
5. Menon T, Kalra DK. Sleep apnea and heart failure-current state-of-the-art. Int J Mol Sci. (2024) 25(10):5251. doi: 10.3390/ijms25105251
6. Gu X, Tang D, Xuan Y, Shen Y, Lu LQ. Association between obstructive sleep apnea symptoms and gout in US population, a cross-sectional study. Sci Rep. (2023) 13(1):10192. doi: 10.1038/s41598-023-36755-4
7. Patel SR. Obstructive sleep apnea. Ann Intern Med. (2019) 171(11):Itc81–itc96. doi: 10.7326/AITC201912030
8. Blackwell JN, Walker M, Stafford P, Estrada S, Adabag S, Kwon Y. Sleep apnea and sudden cardiac death. Circ Rep. (2019) 1(12):568–74. doi: 10.1253/circrep.CR-19-0085
9. Labarca G, Jorquera J, Dreyse J, Salas C, Letelier F. Hypoxemic features of obstructive sleep apnea and the risk of mortality: a cluster analysis. Sleep Breath. (2021) 25(1):95–103. doi: 10.1007/s11325-020-02064-7
10. Lin Y, Wu Y, Lin Q, Wing YK, Xu L, Ge J, et al. Objective sleep duration and all-cause mortality among people with obstructive sleep apnea. JAMA Netw Open. (2023) 6(12):e2346085. doi: 10.1001/jamanetworkopen.2023.46085
11. He Y, Niu Y, Li Z, Zhang R, Chen Y, Dong Z, et al. Arterial stiffness is associated with handgrip strength in relatively healthy Chinese older adults. Front Nutr. (2024) 11:1342411. doi: 10.3389/fnut.2024.1342411
12. Huang H, Bu X, Pan H, Yang S, Cheng W, Shubhra QTH, et al. Estimated pulse wave velocity is associated with all-cause and cardio-cerebrovascular disease mortality in stroke population: results from NHANES (2003–2014). Front Cardiovasc Med. (2023) 10:1140160. doi: 10.3389/fcvm.2023.1140160
13. Higaki Y, Fujie S, Yamato Y, Oshiden M, Iemitsu M. Four weeks of lower-limb static stretching reduces regional arterial stiffness in middle-aged and older women. Phys Act Nutr. (2022) 26(2):22–7. doi: 10.20463/pan.2022.0010
14. Trimarchi G, Pizzino F, Paradossi U, Gueli IA, Palazzini M, Gentile P, et al. Charting the unseen: how non-invasive imaging could redefine cardiovascular prevention. J Cardiovasc Dev Dis. (2024) 11(8):245. doi: 10.3390/jcdd11080245
15. Iaquinta FS, Grosso R, Di Napoli S, Cassano V, Naty S, Armentaro G, et al. Decreased pulse wave velocity in a systemic sclerosis population: preliminary results from a cross-sectional study. J Pers Med. (2022) 12(12):1952. doi: 10.3390/jpm12121952
16. Laugesen E, Olesen KKW, Peters CD, Buus NH, Maeng M, Botker HE, et al. Estimated pulse wave velocity is associated with all-cause mortality during 8.5 years follow-up in patients undergoing elective coronary angiography. J Am Heart Assoc. (2022) 11(10):e025173. doi: 10.1161/JAHA.121.025173
17. Wu LD, Chu P, Kong CH, Shi Y, Zhu MH, Xia YY, et al. Estimated pulse wave velocity is associated with all-cause mortality and cardiovascular mortality among adults with diabetes. Front Cardiovasc Med. (2023) 10:1157163. doi: 10.3389/fcvm.2023.1157163
18. Liu C, Pan H, Kong F, Yang S, Shubhra QTH, Li D, et al. Association of arterial stiffness with all-cause and cause-specific mortality in the diabetic population: a national cohort study. Front Endocrinol. (2023) 14:1145914. doi: 10.3389/fendo.2023.1145914
19. He XW, Park J, Huang WS, Leng LH, Yu Y, Pei YB, et al. Usefulness of estimated pulse wave velocity for identifying prevalent coronary heart disease: findings from a general Chinese population. BMC Cardiovasc Disord. (2022) 22(1):9. doi: 10.1186/s12872-022-02456-5
20. Ji C, Wang G, Huang Z, Zhu C, Liu Y. Estimated pulse wave velocity and risk of new-onset heart failure. ESC Heart Fail. (2024) 11(4):2120–8. doi: 10.1002/ehf2.14778
21. Chen H, Chen G, Zhang L, Wu W, Li W, Wang X, et al. Estimated pulse wave velocity can predict the incidence of new-onset atrial fibrillation: a 11-year prospective study in a Chinese population. Front Cardiovasc Med. (2022) 9:912573. doi: 10.3389/fcvm.2022.912573
22. Liu Y, Xu K, Wu S, Qin M, Liu X. Value of estimated pulse wave velocity to identify left ventricular hypertrophy prevalence: insights from a general population. BMC Cardiovasc Disord. (2022) 22(1):157. doi: 10.1186/s12872-022-02541-9
23. Zota IM, Stătescu C, Sascău RA, Roca M, Anghel L, Mitu O, et al. Arterial stiffness assessment using the arteriograph in patients with moderate-severe OSA and metabolic syndrome-A pilot study. J Clin Med. (2021) 10(18):4238. doi: 10.3390/jcm10184238
24. Gao CC, Kapoor E, Lipford MC, Miller VM, Schroeder DR, Mara KC, et al. Association of vasomotor symptoms and sleep apnea risk in midlife women. Menopause. (2018) 25(4):391–8. doi: 10.1097/GME.0000000000001020
25. Saeed S, Romarheim A, Mancia G, Saxvig IW, Gulati S, Lehmann S, et al. Characteristics of hypertension and arterial stiffness in obstructive sleep apnea: a Scandinavian experience from a prospective study of 6408 normotensive and hypertensive patients. J Clin Hypertens. (2022) 24(4):385–94. doi: 10.1111/jch.14425
26. Theofilis P, Kalaitzidis RG. Hypertension is the crucial link between obstructive sleep apnea and arterial stiffness. J Clin Hypertens. (2022) 24(4):398–400. doi: 10.1111/jch.14427
27. Sun H, Fang F, Li K, Zhang H, Zhang M, Zhang L, et al. Circulating ESM-1 levels are correlated with the presence of coronary artery disease in patients with obstructive sleep apnea. Respir Res. (2019) 20(1):188. doi: 10.1186/s12931-019-1143-6
28. Nagayoshi M, Lutsey PL, Benkeser D, Wassel CL, Folsom AR, Shahar E, et al. Association of sleep apnea and sleep duration with peripheral artery disease: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. (2016) 251:467–75. doi: 10.1016/j.atherosclerosis.2016.06.040
29. Sarfo FS, Jenkins C, Mensah NA, Saulson R, Sarfo-Kantanka O, Singh A, et al. Prevalence and predictors of sleep apnea risk among Ghanaian stroke survivors. J Stroke Cerebrovasc Dis. (2017) 26(7):1602–8. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.027
30. Tang B, Bai Y, Zhao J, Yang H, Avolio A, Zuo J. The severity of obstructive sleep apnea increases the risk of arteriosclerosis. Rev Cardiovasc Med. (2022) 23(3):94. doi: 10.31083/j.rcm2303094
31. Heffernan KS, SY J, Loprinzi PD. Association between estimated pulse wave velocity and mortality in U.S. Adults. J Am Coll Cardiol. (2020) 75(15):1862–4. doi: 10.1016/j.jacc.2020.02.035
32. Vlachopoulos C, Terentes-Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, et al. Association of estimated pulse wave velocity with survival: a secondary analysis of SPRINT. JAMA Netw Open. (2019) 2(10):e1912831. doi: 10.1001/jamanetworkopen.2019.12831
33. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2. (2013) (161):1–24.
34. Zhu H, Wu M. A cross-sectional study on the relationship between electronic cigarette and combustible cigarette use with obstructive sleep apnea among U.S. Adults: result from NHANES 2015–2018. Arch Public Health. (2023) 81(1):54. doi: 10.1186/s13690-023-01083-6
35. Reference Values for Arterial Stiffness’ Collaboration, Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. (2010) 31(19):2338–50. doi: 10.1093/eurheartj/ehq165
36. Wu LD, Kong CH, Shi Y, Zhang JX, Chen SL. Associations between novel anthropometric measures and the prevalence of hypertension among 45,853 adults: a cross-sectional study. Front Cardiovasc Med. (2022) 9:1050654. doi: 10.3389/fcvm.2022.1050654
37. Fulgoni VL 3rd, Drewnowski A. No association between low-calorie sweetener (LCS) use and overall cancer risk in the nationally representative database in the US: analyses of NHANES 1988–2018 data and 2019 public-use linked mortality files. Nutrients. (2022) 14(23):4957. doi: 10.3390/nu14234957
38. Henry O, Brito A, Lloyd MC, Miller R, Weaver E, Upender R. A model for sleep apnea management in underserved patient populations. J Prim Care Community Health. (2022) 13:21501319211068969. doi: 10.1177/21501319211068969
39. Sarinc Ulasli S, Sariaydin M, Ozkececi G, Gunay E, Halici B, Unlu M. Arterial stiffness in obstructive sleep apnoea: is there a difference between daytime and night-time? Respirology. (2016) 21(8):1480–5. doi: 10.1111/resp.12845
40. Ning Y, Zhang TS, Wen WW, Li K, Yang YX, Qin YW, et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Sleep Breath. (2019) 23(1):77–86. doi: 10.1007/s11325-018-1662-2
41. Njamnshi AK, Mengnjo MK, Mbong EN, Kingue S, Fonsah JY, Njoh AA, et al. Hypertension in Cameroon associated with high likelihood of obstructive sleep apnea: a pilot study. BMC Cardiovasc Disord. (2017) 17(1):112. doi: 10.1186/s12872-017-0542-1
42. Chen C, Bao W, Chen C, Chen L, Wang L, Gong H. Association between estimated pulse wave velocity and all-cause mortality in patients with coronary artery disease: a cohort study from NHANES 2005–2008. BMC Cardiovasc Disord. (2023) 23(1):412. doi: 10.1186/s12872-023-03435-0
43. Li D, Cao F, Cheng W, Xu Y, Yang C. Predictive value of estimated pulse wave velocity for cardiovascular and all-cause mortality in individuals with obesity. Diabetol Metab Syndr. (2023) 15(1):40. doi: 10.1186/s13098-023-01011-2
44. Zhou Y, Jin X, Liu X, Tang J, Song L, Zhu Y, et al. Correlation between obstructive sleep apnea and hypoperfusion in patients with acute cerebral infarction. Front Neurol. (2024) 15:1363053. doi: 10.3389/fneur.2024.1363053
45. Migacz E, Olejarz W, Głuszko A, Bednarek-Rajewska K, Proczka R, Smith DF, et al. Elevation of CD40/CD40l inflammatory pathway molecules in carotid plaques from moderate-and-severe obstructive sleep apnea patients. Diagnostics. (2021) 11(6):935. doi: 10.3390/diagnostics11060935
46. Flynn J, Boyd C, Yalamanchali S, Rouse D, Goodwin S, Penn J, et al. The effect of lateral pharyngeal collapse patterns on therapy response in upper airway stimulation surgery. Ann Otol Rhinol Laryngol. (2021) 130(9):985–9. doi: 10.1177/0003489420987979
47. Zhao X, Yu X, Xin S, Zhang W, Zhang X, Ji L. Correlation between OSAHS and early peripheral atherosclerosis indices in patients with type 2 diabetes Mellitus in China: a cross-sectional inpatient study. J Diabetes Res. (2021) 2021:6630020. doi: 10.1155/2021/6630020
48. Ikeda T, Kubota Y, Inami T, Seino Y, Asai K. Predicting heart failure symptoms from the apnoea-hypopnoea index determined by full- night polysomnography. ESC Heart Fail. (2024) 11(5):2789–97. doi: 10.1002/ehf2.14824
49. Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, et al. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: an updated meta-analysis and metaregression of 18 studies. J Am Heart Assoc. (2015) 4(11):e002454. doi: 10.1161/JAHA.115.002454
50. Huang ZX, Yuan S, Li D, Hao H, Liu Z, Lin J. A nomogram to predict lifestyle factors for recurrence of large-vessel ischemic stroke. Risk Manag Healthc Policy. (2021) 14:365–77. doi: 10.2147/RMHP.S289761
51. Cohen JB, Mitchell GF, Gill D, Burgess S, Rahman M, Hanff TC, et al. Arterial stiffness and diabetes risk in framingham heart study and UK Biobank. Circ Res. (2022) 131(6):545–54. doi: 10.1161/CIRCRESAHA.122.320796
52. Sırlıer Emir B, Yıldız S, Kazgan Kılıçaslan A, Kılıçarslan G, Kurt O, Korkmaz S, et al. Evaluation of arterial stiffness in depression patients. Alpha Psychiatry. (2023) 24(5):193–9. doi: 10.5152/alphapsychiatry.2023.221099
53. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. (2021) 119:154766. doi: 10.1016/j.metabol.2021.154766
54. Feola M. An update on the role of arterial stiffness in heart failure and the treatment of dyslipidemia. J Clin Med. (2023) 12(9):3507. doi: 10.3390/jcm12093270
55. Tanriover C, Copur S, Mutlu A, Peltek IB, Galassi A, Ciceri P, et al. Early aging and premature vascular aging in chronic kidney disease. Clin Kidney J. (2023) 16(11):1751–65. doi: 10.1093/ckj/sfad076
56. McNally RJ, Boguslavskyi A, Malek R, Floyd CN, Cecelja M, Douiri A, et al. Influence of blood pressure reduction on pulse wave velocity in primary hypertension: a meta-analysis and comparison with an acute modulation of transmural pressure. Hypertension. (2024) 81(7):1619–27. doi: 10.1161/HYPERTENSIONAHA.123.22436
57. Webb AJS, Werring DJ. New insights into cerebrovascular pathophysiology and hypertension. Stroke. (2022) 53(4):1054–64. doi: 10.1161/STROKEAHA.121.035850
58. Papadopoulou E, Loutradis C, Tzatzagou G, Kotsa K, Zografou I, Minopoulou I, et al. Dapagliflozin decreases ambulatory central blood pressure and pulse wave velocity in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. J Hypertens. (2021) 39(4):749–58. doi: 10.1097/HJH.0000000000002690
Keywords: estimated pulse wave velocity, obstructive sleep apnea, arterial stiffness, NHANES, all-cause mortality, cardiovascular mortality
Citation: Lin H, Zheng H, Lin T and Chen L (2025) Association of estimated pulse wave velocity with all-cause mortality and cardiovascular mortality in obstructive sleep apnea patients: results from NHANES. Front. Cardiovasc. Med. 12:1571610. doi: 10.3389/fcvm.2025.1571610
Received: 6 February 2025; Accepted: 29 May 2025;
Published: 12 June 2025.
Edited by:
DeLisa Fairweather, Mayo Clinic Florida, United StatesReviewed by:
Zhe Zhou, The First Affiliated Hospital of Sun Yat-sen University, ChinaMiaochan Lao, Guangdong Provincial People’s Hospital, China
Copyright: © 2025 Lin, Zheng, Lin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Lin, bGludGFvQG1haWwuZmptdS5lZHUuY24=; Lianglong Chen, NTY5Mjg0MTQyQHFxLmNvbQ==
†These authors have contributed equally to this work
 Huizhong Lin
Huizhong Lin Huiyun Zheng1,†
Huiyun Zheng1,†