- 1Department of Orthopaedic Surgery and Orthopedic Research Institute, West China Hospital, Sichuan University, Chengdu, China
- 2Orthopaedics Department, The Sichuan Modern Hospital, Chengdu, China
- 3Anesthesia and Surgery Center, West China Hospital, Sichuan University, Chengdu, China
Background: Coronary artery calcium (CAC) is one of the main factors causing coronary stenosis and is often identified on ordinary chest computed tomography (CT). This study aims to explore the effectiveness of utilizing routine chest CT for preoperative coronary stenosis assessment in patients scheduled total hip or knee arthroplasty.
Materials and methods: Between July 2020 and July 2024, a retrospective analysis was conducted, and a total of 293 patients who intended to perform total hip or knee arthroplasty were included from 12,150 chest CT scans. Coronary artery calcium Score (CACS) was used to evaluate coronary stenosis based on the preoperative ordinary chest CT. Correlation between CACS and the degree of coronary artery stenosis determined via the coronary angiography (CAG) and the predictive ability of CACS for coronary artery stenosis ≥50% were analyzed.
Results: The number of patients with CACS scores ranging from 0 to 499 was the largest, with 139 patients (47.44%). There were 88 patients (30.03%) with coronary artery stenosis ≥50%, 40 patients (13.65%) with ≥70%, and left anterior descending artery stenosis was the most common, with a total of 72 patients (24.57%). A strong correlation (R = 0.891, p < 0.001) between the degree of coronary stenosis and CACS was observed. A CACS threshold of ≥1,500 demonstrated a specificity of 100% and a positive predictive value (PPV) of 100% for coronary stenosis of ≥50%.
Conclusion: Ordinary chest CT is highly effective in evaluating the risk of coronary artery stenosis before total hip or knee arthroplasty, introducing a novel approach to facilitate our surgical decision-making.
1 Introduction
It is well known that atherosclerotic cardiovascular disease (ASCVD) has a high prevalence and mortality rate worldwide (1, 2). In China, ASCVD alone caused 2.4 million deaths in 2016, accounting for approximately 25% of all mortality cases (3). Coronary artery calcium (CAC) is a component of atherosclerosis (4). Investigators have noted a close correlation between calcification and severity of clinical coronary disease. Numerous studies have shown a significant association between the presence and severity of CAC and future cardiovascular disease risk (5, 6). CAC detection is one of the most sensitive, reliable, and repeatable non-invasive methods to identify subclinical atherosclerosis (7). Imaging can be used to non-invasively quantify atherosclerosis, primarily through ECG-gated computed tomography (CT) to quantify CAC. In recent years, Coronary artery calcium Score (CACS) has emerged to quantify CAC and predict the presence of coronary artery stenosis (8).
The Agatston score, the most commonly used calcium quantification method on CT, relies on software to multiply the lesion area of CAC by a density weighting factor (DWF) that is derived from the maximal CT attenuation within a given calcified lesion (7). The score for all lesions in all coronary arteries is then summed, irrespective of location or coronary distribution, to determine the total Agatston score (9, 10). The CACS is more predictive of cardiovascular events than traditional risk scores (5, 11, 12). The severity of coronary artery stenosis increases with the increase of CACS, and its prognosis can also be predicted through CACS (9, 13). It has been reported that the 10-year incidence of cardiovascular adverse events with CACS values of 0, >100, and >300 is 5.6%, 7.5%, and 13.1%, respectively (14, 15). Additional studies have demonstrated the predictive value of CACS for death, non-fatal myocardial infarction, and future revascularization (16, 17).
Ordinary chest CT is a standard medical imaging examination method mainly used to observe and evaluate the internal structure and organ lesions of the chest. Moreover, CAC can be detected during chest CT scans. As the parameters of chest CT scans are adjusted and technology advances, both ordinary and low-dose chest CT scans now exhibit high-density resolution. This improvement enables the more accurate identification of smaller lesions, CAC included.
When the CACS is applied to ordinary non-ECG gated chest CT, the modified Agatston score, calculated using low-dose non-gated CT, has demonstrated consistency with the Agatston score derived from ECG-gated scans in lung cancer screening patients and the general population (15, 18, 19). Evaluating CAC on ordinary non-contrast chest CT is a cost-effective and low-radiation method for quantifying coronary artery calcification, and it is increasingly being applied in clinical practice, such as lung cancer screening, preoperative evaluation, health check-ups, etc (20, 21). Although CAC can be detected in these routine examinations, radiologists rarely report CAC systematically and routinely, and clinical doctors rarely pay attention to these potential lesions (22, 23). In a prior study encompassing 207 patients diagnosed with CAC, radiologists reported that only 44% of patients were found to have CAC. Furthermore, within this cohort of CAC-diagnosed patients, the proportion of cases where the specific affected arteries were precisely identified was a mere 1% (23).
Presently, the guidelines for coronary artery revascularization define significant stenosis as non-left main lesion diameter stenosis ≥70% and left main lesion diameter stenosis ≥50% (24). In previous studies, Palumbo et al. advocated the use of CACS as a preliminary screening tool for coronary computed tomography angiography (CCTA) (25). Alshamrani employed various CACS cutoff values to evaluate coronary artery stenosis ≥50% and ≥70%, showing that when CACS ≥250, all symptomatic patients had coronary artery stenosis ≥50% (26). However, it must be acknowledged that the high critical value of CACS may lead to overestimating the degree of coronary artery stenosis (27). Currently, there is no universally accepted standard for CACS cutoff values to define different degrees of coronary stenosis, leading to variability in clinical interpretation and risk stratification. Prior studies have not explored the relationship between CACS based on ordinary chest CT and CAG, nor the optimal selection strategy for diagnosing coronary stenosis after detecting CAC on CT. Preoperative cardiovascular risk assessment for orthopedic surgery follows guidelines like the 2014 ACC/AHA Perioperative Cardiac Guidelines (28), which focus on clinical risk factors but often overlook subclinical coronary artery disease (CAD) detected incidentally on chest CT. CAC, a marker of atherosclerosis frequently seen on chest CT, is rarely integrated into risk stratification. This study links CAC severity to obstructive stenosis (≥50%) to inform improved pre-op CAD screening in arthroplasty patients. This study employs CAG to diagnose coronary artery stenosis and the patient population comprises those who had been detected with CAC by ordinary chest CT before total hip or knee arthroplasty. The aim is to explore the effectiveness of ordinary chest CT for preoperative coronary stenosis assessment in these patients.
2 Materials and methods
2.1 Study design
This retrospective study has been approved by the Bioethics Review Committee of West China Hospital, Sichuan University. All research data was retrieved from the medical record system. Since anonymous patient data was used in the study without any medical intervention measures, the requirement for informed consent was waived. This Retrospective Cohort Study was conducted using the STROCSS 2019 Guideline (29).
2.2 Patient data
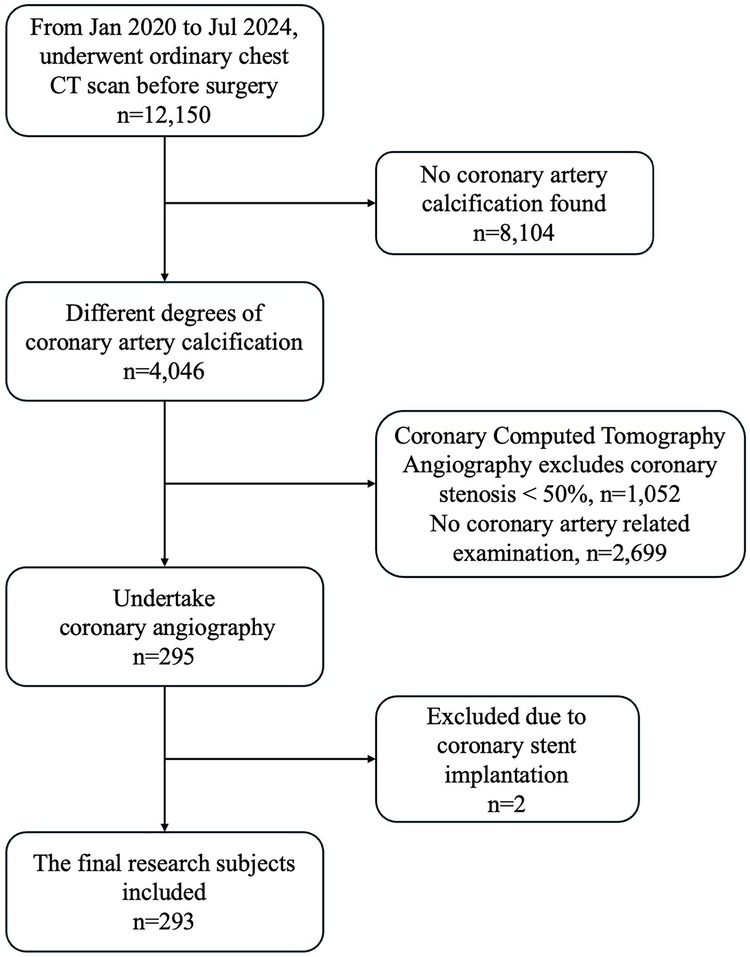
This retrospective study focused on patients who had been intending to undergo total hip or knee arthroplasty in our joint surgery department between July 2020 and July 2024. 12,150 patients underwent ordinary chest CT before total hip or knee arthroplasty. Among the total number of patients included in the study, no CAC was found in 8,104 patients (66.70%) and 4,046 (33.30%) were found to have varying degrees of CAC on chest CT. Patients were referred for further evaluation based on a multidisciplinary decision involving cardiologists and radiologists. The decision to proceed with CCTA or CAG was guided by: clinical symptoms (e.g., angina, dyspnea) and preferences, comorbidities and CAC severity on ordinary chest CT. 2,699 (22.21%) were excluded for not undergoing either CCTA or CAG primarily due to low clinical suspicion of Coronary Artery Disease. Additionally, 1,052 (8.66%) patients who underwent CCTA were excluded because the degree of coronary artery stenosis in these patients was <50%. 295 (2.43%) patients underwent CAG, of which two patients with a previously implanted coronary artery stent were excluded due to the potential interference of the stent with the evaluation of the current study. Consequently, a total of 293 patients were included in the final analysis (Figure 1).
The study population consisted of patients presenting with either symptomatic, including angina, dyspnea, syncope, and palpitation, or asymptomatic patients. The exclusion criteria were as follows: (1) Patients who had undergone percutaneous coronary intervention (PCI) and coronary artery bypass grafting surgery prior to admission; (2) Patients with incomplete key clinical data (such as missing preoperative imaging results). Baseline clinical data, including demographic characteristics, body mass index (BMI), laboratory tests, past medical history (including chronic diseases, medications, etc.) and surgical history, as well as the results of CACS and CAG, were collected. All diseases included in this study are defined by using the 10th revision of the International Classification of Diseases (ICD-10) diagnostic codes. The results of CACS were evaluated by three experienced radiologists blinded to the CAG results. All three cardiologists with cardiology specialist certification and over 10 years of experience in interventional treatment of ASCVD performed CAG and evaluated coronary artery stenosis severity in a blinded manner without prior knowledge of CACS results. Stenosis was graded into four categorical levels according to the 2011 ACCF/AHA/SCAI Guidelines: mild (0%–24%), slight-moderate (25%–49%), moderate (50%–69%), and severe (≥70%) (30). All doctors were blinded to each other's evaluation results. For statistical analysis, categorical grades were converted to numerical midpoints (12%, 37%, 60%, 85%), and the average of the three observers' scores was used. In cases of disagreements exceeding two categories, a fourth senior cardiologist arbitrated to reach consensus. Retrospective analysis of inter-observer agreement in a subset of 50 cases showed an intraclass correlation coefficient (ICC) of 0.82 (95% CI: 0.71–0.89) with a standard deviation (SD) of 8.7%, indicating excellent consistency across evaluations.
2.3 Ordinary chest CT scan and CACS
The Ordinary chest CT examinations were performed using Siemens CT scanners (SOMATOM Definition, Siemens Medical Solutions, Forchheim, Germany; and SOMATOM Definition FLASH, Siemens Medical Solutions, Forchheim, Germany) or a Revolution CT scanner (GE Healthcare, Waukesha, WI, USA) with patients in the supine position. The scan covered the area from the lung's apex down to the lower boundary of the costophrenic angle, with a slice thickness of 1 mm. No contrast agents or drugs were injected during the scanning period. Subsequently, the initial data set was reconstructed upon completion of the scan, and images were transferred to image-processing workstations for image analysis. The CACS was determined using Agatston's algorithm (10, 31). CACS was performed utilizing commercially available calcium scoring software (PHILIPS), which was used to identify and score any calcium in the four main coronary arteries based on established minimum attenuation values. Finally, the Agatston score was generated by summing the scores of all lesions, which were derived by multiplying the lesion area CAC by DWF.
2.4 Coronary angiography (CAG)
The cardiologists, blinded to the CACS results, performed a right radial artery puncture using Seldinger's technique and inserted a 6Fr sheath. Heparin (3,000 μ) was administered with a 6Fr Heartrail angiography catheter before coronary angiography. Subsequently, the severity of stenosis in the left main coronary artery (LM), left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery (RCA) was evaluated. Finally, the cardiologists provided relevant reports and images based on the operation.
2.5 Statistical analysis
All statistical analyses were performed using SPSS software (version 27.0, SPSS Inc., USA). Characteristics of the study population were presented as mean ± SD (standard deviation) for continuous variables that followed a normal distribution, while categorical variables were expressed in percentages. Additionally, continuous variables that were not normally distributed were described using the median and interquartile range. Differences in categorical variables were assessed using a Chi-square test. Then, the correlation between CACS and coronary stenosis severity was assessed using Pearson's correlation coefficient, with stenosis categories converted to numerical midpoints. Finally, the severity and location of coronary stenosis were evaluated by CAG. A receiver operating characteristic (ROC) curve analysis was performed, including calculations of specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), and area under the ROC curve (AUC) for evaluating the ability of CACS to predict coronary stenosis. The Youden index was used to identify the best cutoff point. The diagnostic performance of different CACS cutoff values based on routine chest CT was assessed to detect coronary stenosis with degrees of ≥50% and ≥70%. Moreover, the significance level was set at a bilateral P-value threshold of <0.05.
3 Results
3.1 Patient characteristics
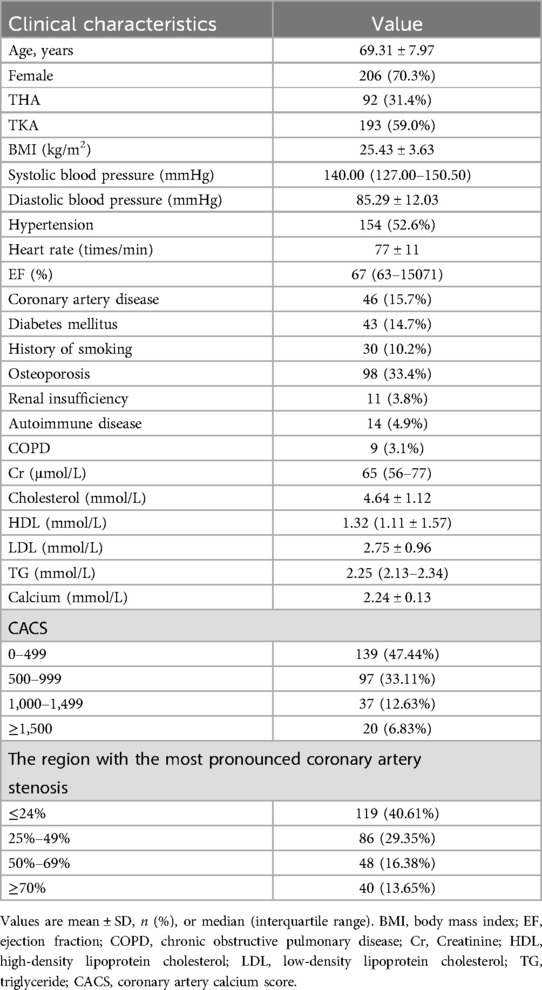
Among 293 patients, 87 were male and 206 were female. Ages ranged from 42 to 89, with a mean of 69.31 ± 7.97 years. Of them, 92 had total hip arthroplasty (THA), 193 had total knee arthroplasty (TKA), and 8 didn't proceed with the surgery. The median CACS was 632 (from 12 to 2016, with an interquartile range of 230.5–898.5). 205 (69.97%) patients did not have significant coronary artery stenosis (<50%), while 88 (30.0%) exhibited mild (50%–69%, n = 48) to severe (≥70%, n = 40) coronary stenosis (Table 1).
3.2 Predictive value of CACS in coronary stenosis evaluation
The distribution of CACS within the cohort was shown in Figure 2A, with the highest number of patients (n = 139, 47.44%) having scores between 0 and 499. The region of the most severe coronary artery stenosis ≥50% in CAG was shown in Figure 2B, where LAD stenosis was found to be the most common (n = 72, 24.57%). Meanwhile,a strong correlation between the coronary stenosis degree and CACS was also observed (Pearson correlation R = 0.891, p < 0.001).
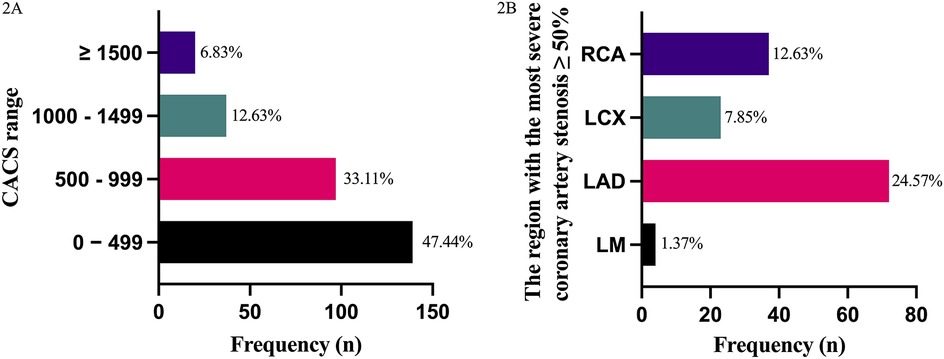
Figure 2. Predictive value of CACS in coronary stenosis evaluation. (A) Frequency of coronary artery calcium scores (CACS) categories in 293 patients scheduled for arthroplasty. X-axis: CACS groups (0–499, 500–999, 1,000–1,499, ≥1,500). Y-axis: Number of patients. (B) Frequency of severe coronary stenosis (≥50%) by artery location in coronary angiography (CAG). X-axis: Coronary artery segments (LM, left main coronary artery; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery). Y-axis: Number of patients.
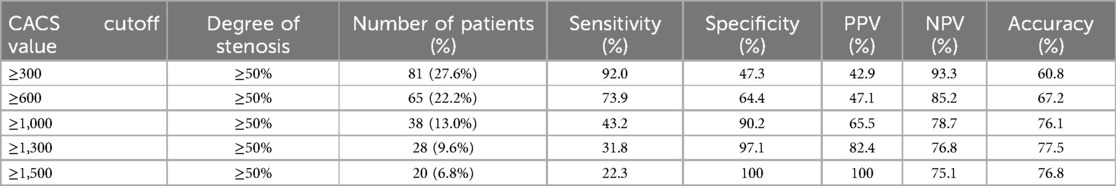
Based on the ROC curve analysis, for the detection of coronary stenosis with a severity of ≥50%, the optimal CACS cutoff point was 350.5. At this value, the sensitivity reached 92.0%, the specificity was 53.7%, the positive predictive value (PPV) was 42.9%, the negative predictive value (NPV) was 93.3%, and the accuracy was 60.8% (AUC = 0.79). For coronary stenosis ≥70%, the optimal CACS cutoff point was 856.5, resulting in a sensitivity of 67.5%, a specificity of 81.4%, a PPV of 17.3%, an NPV of 87.6%, and an accuracy of 69.6% (AUC = 0.82) (Figure 3). The diagnostic efficacy of various CACS cutoff values for coronary artery stenosis ≥50% was presented in Table 2. Specifically, when the CACS cutoff was set at ≥1,500, a sensitivity of 22.3% was exhibited, a specificity of 100% was shown, a PPV of 100% was demonstrated, an NPV of 75.1% was revealed, and a diagnostic accuracy of 76.8% was achieved. In contrast, when CACS ≤143, coronary stenosis ≥50% was not found in any patients.
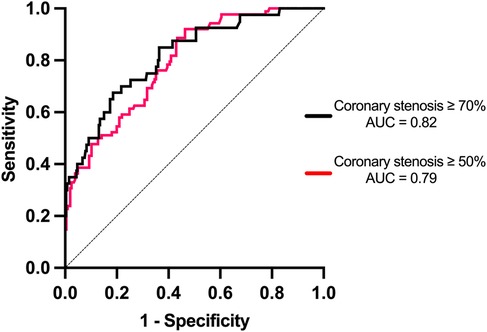
Figure 3. Receiver operating characteristic (ROC) curves evaluating the performance of CACS in detecting coronary stenosis of ≥50% and ≥70% on coronary angiography (CAG). For ≥50% stenosis, the optimal CACS cutoff was 350.5, yielding a sensitivity of 92.0%, specificity of 53.7%, PPV of 42.9%, NPV of 93.3%, and accuracy of 60.8% (AUC = 0.79, 95% CI: 0.73–0.85). For ≥70% stenosis, the optimal cutoff was 856.5, with sensitivity of 67.5%, specificity of 81.4%, PPV of 17.3%, NPV of 87.6%, and accuracy of 69.6% (AUC = 0.82, 95% CI: 0.75–0.89). Dashed line represents the reference line (AUC = 0.5).
4 Discussion
Total joint arthroplasty is an excellent solution for end-stage joint diseases, and its prevalence has increased yearly (32). The future demands for primary total hip and knee arthroplasties are estimated to grow by nearly 2-fold and 7-fold, respectively, with middle-aged and elderly patients accounting for the vast majority (33). The study highlights the clinical utility of chest CT-derived CACS in orthopedic preoperative screening for total hip/knee arthroplasty. Routine chest CT, primarily for pulmonary assessment, can secondarily identify unsuspected CAD via calcification detection. A high CACS (e.g., ≥1,500) correlates strongly with significant stenosis (≥50%), enabling risk stratification to guide further cardiac evaluation (e.g., CCTA/CAG) and optimize perioperative care. We found that CACS is ubiquitous in patients undergoing THA and TKA. Most patients exhibit a score between 0 and 499, accounting for 47.44%. Among patients with CAC, a total of 88 patients were found to have significant stenosis of the coronary arteries (≥50%), with LAD stenosis being the most common. Among the 88 patients, 72 had significant stenosis of LAD, accounting for 24.57%. In general, the results of this study indicated a strong correlation between CACS and the degree of coronary artery stenosis (Pearson correlation R = 0.891, p < 0.001), which is consistent with previous research findings that the CACS based on ordinary chest is a reliable cardiovascular risk prediction method (3, 18, 34).
We conducted an ROC curve analysis to evaluate the predictive ability of CACS for coronary artery stenosis, revealing that the AUC of coronary artery stenosis ≥70% was higher than that of coronary artery stenosis ≥50%. Besides, this study assessed multiple CACS cutoff values for diagnosing coronary stenosis and investigated the association between CACS and the presence of coronary stenosis. Since the evaluation of coronary artery stenosis is crucial for surgical decision-making in patients undergoing procedures like THA and TKA (where the presence of coronary artery disease may impact the surgical risk and outcome), accordingly, we focused on exploring the relationship between CACS values and coronary artery stenosis ≥50% and ≥70% (24).
Previous studies by De Agustin et al. showed that in patients with chest pain and CACS ≥400, the specificity and PPV for detecting coronary artery stenosis ≥70% were 93.5% and 85.8%, respectively (35). Therefore, it is recommended that patients with chest pain symptoms and CACS ≥400 should avoid undergoing CCTA examination and instead undergo CAG. Previous studies also have indicated a positive correlation between higher CACS and an increased likelihood of coronary stenosis (36–38). Since CCTA may offer little extra value for patients with a high CACS-indicating a high likelihood of ≥50% coronary stenosis, these patients can make an informed decision about foregoing CCTA and instead choose early CAG and decide whether to undergo percutaneous coronary revascularization based on the results. The ≥1,500 threshold was optimized for maximal specificity and PPV in our population, but its generalizability may be limited in cohorts with different prevalence of obstructive disease or imaging protocols. External validation in diverse datasets is essential to confirm its robustness.
The application of CCTA and the gold-standard CAG in coronary artery examination requires further study. Some patients skipped CAG as CCTA showed no significant stenosis. CCTA has advantages. Its high specificity helps rule out non-obvious stenosis in the initial screening, reducing unnecessary follow-up tests (39, 40). However, a high false-positive rate may lead to needless further checks (41). As a non-therapeutic tool of diagnosis, CCTA, when it reveals severe stenosis, indicates that patients still need CAG. This not only increases costs but also raises the risks of complications such as renal problems, thyroid-related issues, and hypersensitivity reactions caused by repeated contrast agent use (42, 43).
Our study also found that when the patient's CACS ≤143, there was no significant coronary artery stenosis. Hence, we advise that when the preoperative chest CT shows CACS ≤143, usually no special treatment or additional test needs to be performed unless the patient exhibits obvious chest pain symptoms or a long-term history of coronary heart disease. However, CACS cannot visualize coronary stenosis caused by non-calcified plaque. In patients with CACS = 0, most plaques were predominantly non-calcified (44). It is worth noting that the early stages of coronary atherosclerosis do not exhibit any calcification. Among the 12 patients with CACS = 0 and coronary artery stenosis ≥50%, non-calcified plaques were found in 10 cases (45). Therefore, we deem that for asymptomatic or chest pain patients, if the patient has multiple risk factors, such as age, family history, smoking, diabetes, hypertension, and abnormal blood lipid levels, even if ordinary chest CT shows no CAC, medical service providers can also consider CCTA as a non-invasive imaging method to assess the health status of coronary arteries preliminarily. For patients with significant stenosis on CCTA examination, early CAG to clarify the degree and location of the lesion and timely treatment is extremely necessary. In recent years, coronary magnetic resonance angiography (MRA), with its non-invasive nature, absence of ionizing radiation, option of non-contrast use, and low susceptibility to vascular calcification, has emerged as a promising new method for ASCVD screening and is nearly ready for clinical application (46–48). However, its complex imaging technique and long acquisition time currently impede large-scale implementation. Despite these challenges, the future of coronary MRA is bright.
There were some limitations in this study. Firstly, non-ECG-gated chest CT is prone to artifacts such as motion and respiratory artifacts, which complicate the calculation of CACS and may affect accuracy. In future studies, it is recommended to integrate ECG gating or phase-reconstruction algorithms, such as using motion-corrected deep learning models, to mitigate errors caused by artifacts. Secondly, a key limitation of our study is the use of the Agatston algorithm to calculate CACS. This method fails to account for calcium density within plaques, instead assigning higher scores to denser calcifications. Paradoxically, prior research shows that higher calcium density within a given plaque volume correlates inversely with cardiovascular events. As a result, CACS may overstate the risk of dense plaques, potentially causing misestimation of cardiovascular risk. Thirdly, this study did not explore the relationship between non-CAC and coronary stenosis. Additionally, the total CACS did not offer a distinct evaluation of calcification in individual coronary vessels, especially the situation of single-vessel involvement (such as the degree of CAC and CACS in single vessels). This will remind doctors of the presence of severe coronary artery disease. Another limitation of this study is that the cutoff values for predicting coronary artery stenosis were not adjusted for age and gender. As a result, the established cutoff values may not accurately reflect the risk of coronary artery stenosis across different age and gender subgroups. This study focused on patients with moderate-to-severe coronary stenosis, excluding those with <50% stenosis after CCTA. This exclusion criterion may overestimate the predicted value of CACS. Moreover, the lack of standardized protocols and scoring criteria for coronary calcium scoring, along with the potential for missed detection or underestimation of mild calcifications, remains a limitation. Future research should leverage advanced software algorithms (e.g., deep learning, image fusion) and standardized scanning/post-processing procedures to enhance the reliability of chest CT-based calcium scores comparable to those from coronary CT angiography.
Ultimately, these improvements will significantly enhance the precision and clinical relevance of risk predictions grounded in CACS. This cross-disciplinary approach aligns with trends in shared decision-making but requires validation in larger cohorts and consideration of radiation/cost for broader use. Future studies should assess whether CACS screening reduces perioperative cardiac events to support its integration into standardized pre-op protocols.
5 Conclusion
The application of CACS derived from ordinary chest CT proves advantageous for evaluating coronary stenosis risk before total hip and knee arthroplasties. High CACS cutoff values exhibit high specificity and PPV in diagnosing coronary stenosis ≥50% and ≥70%. When preoperative CACS ≥1,500, we suggest patients skip CCTA and directly have CAG for a definite diagnosis and possible concurrent treatment. In short, preoperative coronary stenosis risk assessment based on ordinary chest CT might represent a novel approach that assists us in making surgical decisions. However, further high-quality research is essential to refine criteria.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Ethics Review Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because anonymous patient data was used in the study without any medical intervention measures, the requirement for informed consent was waived.
Author contributions
YL: Methodology, Validation, Visualization, Writing – original draft. YW: Data curation, Formal analysis, Investigation, Writing – original draft. TM: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Writing – original draft. XD: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. HS: Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 81802210) and the Department of Science and Technology of Sichuan Province (grant number 2021YFS0122).
Acknowledgments
We want to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1582704/full#supplementary-material
References
1. Nedkoff L, Briffa T, Zemedikun D, Herrington S, Wright FL. Global trends in atherosclerotic cardiovascular disease. Clin Ther. (2023) 45(11):1087–91. doi: 10.1016/j.clinthera.2023.09.020
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Chen X, Zhao J, Cai Q, Chen R, Wu W, Wang P, et al. Relationship between coronary artery calcium score and coronary stenosis. Cardiol Res Pract. (2023) 2023:5538111. doi: 10.1155/2023/5538111
4. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16(4):203–12. doi: 10.1038/s41569-018-0119-4
5. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. Mar. (2008) 358(13):1336–45. doi: 10.1056/NEJMoa072100
6. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. (2018) 72(4):434–47. doi: 10.1016/j.jacc.2018.05.027
7. Khan SS, Navar AM. The potential and pitfalls of coronary artery calcium scoring. JAMA Cardiol. Jan. (1 2022) 7(1):11–2. doi: 10.1001/jamacardio.2021.4413
8. Nicoll R, Wiklund U, Zhao Y, Diederichsen A, Mickley H, Ovrehus K, et al. The coronary calcium score is a more accurate predictor of significant coronary stenosis than conventional risk factors in symptomatic patients: Euro-CCAD study. Int J Cardiol. (2016) 207:13–9. doi: 10.1016/j.ijcard.2016.01.056
9. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. (1990) 15(4):827–32. doi: 10.1016/0735-1097(90)90282-t
10. Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary artery calcium scoring: is it time for a change in methodology? JACC Cardiovasc Imaging. (2017) 10(8):923–37. doi: 10.1016/j.jcmg.2017.05.007
11. Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. (2009) 53(4):345–52. doi: 10.1016/j.jacc.2008.07.072
12. Elias-Smale SE, Wieberdink RG, Odink AE, Hofman A, Hunink MG, Koudstaal PJ, et al. Burden of atherosclerosis improves the prediction of coronary heart disease but not cerebrovascular events: the Rotterdam study. Eur Heart J. (2011) 32(16):2050–8. doi: 10.1093/eurheartj/ehr125
13. Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. (2018) 39(25):2401–8. doi: 10.1093/eurheartj/ehy217
14. Groen RA, van Dijkman PRM, Jukema JW, Bax JJ, Lamb HJ, de Graaf MA. Coronary calcifications as assessed on routine non-gated chest CT; a gatekeeper to tailor downstream additional imaging in patients with stable chest pain. Int J Cardiol Heart Vasc. (2024) 52:101418. doi: 10.1016/j.ijcha.2024.101418
15. Hughes-Austin JM, Dominguez A 3rd, Allison MA, Wassel CL, Rifkin DE, Morgan CG, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. (2016) 9(2):152–9. doi: 10.1016/j.jcmg.2015.06.030
16. Watts JR Jr, Sonavane SK, Snell-Bergeon J, Nath H. Visual scoring of coronary artery calcification in lung cancer screening computed tomography: association with all-cause and cardiovascular mortality risk. Coron Artery Dis. (2015) 26(2):157–62. doi: 10.1097/mca.0000000000000189
17. Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with framingham score for risk prediction in asymptomatic individuals. JAMA. (2004) 291(2):210–5. doi: 10.1001/jama.291.2.210
18. Wu MT, Yang P, Huang YL, Chen JS, Chuo CC, Yeh C, et al. Coronary arterial calcification on low-dose ungated MDCT for lung cancer screening: concordance study with dedicated cardiac CT. AJR Am J Roentgenol. (2008) 190(4):923–8. doi: 10.2214/ajr.07.2974
19. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. (2008) 190(4):917–22. doi: 10.2214/ajr.07.2979
20. Htwe Y, Cham MD, Henschke CI, Hecht H, Shemesh J, Liang M, et al. Coronary artery calcification on low-dose computed tomography: comparison of Agatston and ordinal scores. Clin Imaging. (2015) 39(5):799–802. doi: 10.1016/j.clinimag.2015.04.006
21. Chiles C, Paul NS. Beyond lung cancer: a strategic approach to interpreting screening computed tomography scans on the basis of mortality data from the national lung screening trial. J Thorac Imaging. (2013) 28(6):347–54. doi: 10.1097/rti.0000000000000052
22. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. (2013) 7(3):167–72. doi: 10.1016/j.jcct.2013.05.003
23. Uretsky S, Chokshi N, Kobrinski T, Agarwal SK, Po JR, Awan H, et al. The interplay of physician awareness and reporting of incidentally found coronary artery calcium on the clinical management of patients who underwent noncontrast chest computed tomography. Am J Cardiol. (2015) 115(11):1513–7. doi: 10.1016/j.amjcard.2015.02.051
24. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2022) 145(3):e4–17. doi: 10.1161/cir.0000000000001039
25. Palumbo AA, Maffei E, Martini C, Tarantini G, Di Tanna GL, Berti E, et al. Coronary calcium score as gatekeeper for 64-slice computed tomography coronary angiography in patients with chest pain: per-segment and per-patient analysis. Eur Radiol. (2009) 19(9):2127–35. doi: 10.1007/s00330-009-1398-2
26. Alshumrani GA. Coronary artery calcium score above 250 confirms the presence of significant stenosis in coronary CT angiography of symptomatic patients. Coron Artery Dis. (2022) 33(3):189–95. doi: 10.1097/mca.0000000000001082
27. Kwan AC, Gransar H, Tzolos E, Chen B, Otaki Y, Klein E, et al. The accuracy of coronary CT angiography in patients with coronary calcium score above 1000 Agatston units: comparison with quantitative coronary angiography. J Cardiovasc Comput Tomogr. (2021) 15(5):412–8. doi: 10.1016/j.jcct.2021.03.007
28. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. (2014) 130(24):e278–333. doi: 10.1161/cir.0000000000000106
29. Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. (2019) 72:156–65. doi: 10.1016/j.ijsu.2019.11.002
30. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. (2011) 124(23):e574–651. doi: 10.1161/CIR.0b013e31823ba622
31. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. (2017) 11(1):8–15. doi: 10.1016/j.jcct.2016.10.001
32. Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. (2015) 97(17):1386–97. doi: 10.2106/jbjs.N.01141
33. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. (2007) 89(4):780–5. doi: 10.2106/jbjs.F.00222
34. Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD gene cohort. J Cardiovasc Comput Tomogr. Mar. (2011) 5(2):113–8. doi: 10.1016/j.jcct.2010.11.002
35. de Agustin JA, Marcos-Alberca P, Fernández-Golfin C, Feltes G, Nuñez-Gil IJ, Almeria C, et al. Should computed tomography coronary angiography be aborted when the calcium score exceeds a certain threshold in patients with chest pain? Int J Cardiol. (2013) 167(5):2013–7. doi: 10.1016/j.ijcard.2012.05.041
36. Zlibut A, Orzan RI, Farah D, Cionca C, Muresan ID, Horvat D, et al. Predictive ability of coronary computed tomography angiography parameters in patients suspected of obstructive coronary artery disease: a single-center cross-sectional study. Eur Rev Med Pharmacol Sci. (2021) 25(11):4074–85. doi: 10.26355/eurrev_202106_26049
37. Kaur M, Rahimi R, Razali F, Mohd Noor N, Omar E, Abdul Manaf Z, et al. Association of coronary artery calcium score with calcification and degree of stenosis: an autopsy study. Malays J Pathol. (2019) 41(2):177–83.31427553
38. Moradi M, Nouri S, Nourozi A, Golbidi D. Prognostic value of coronary artery calcium score for determination of presence and severity of coronary artery disease. Pol J Radiol. (2017) 82:165–9. doi: 10.12659/pjr.900643
39. Herzog BA, Husmann L, Burkhard N, Gaemperli O, Valenta I, Tatsugami F, et al. Accuracy of low-dose computed tomography coronary angiography using prospective electrocardiogram-triggering: first clinical experience. Eur Heart J. (2008) 29(24):3037–42. doi: 10.1093/eurheartj/ehn485
40. Huang C, Wan WJ, Yao YH, Xia LM, Huang WH. Feasibility of subtraction coronary computed tomographic angiography and influencing factor analysis: a retrospective study. Curr Med Sci. (2021) 41(4):821–6. doi: 10.1007/s11596-021-2413-3
41. Alkadhi H, Scheffel H, Desbiolles L, Gaemperli O, Stolzmann P, Plass A, et al. Dual-source computed tomography coronary angiography: influence of obesity, calcium load, and heart rate on diagnostic accuracy. Eur Heart J. (2008) 29(6):766–76. doi: 10.1093/eurheartj/ehn044
42. Xu Y, Tang L, Zhu X, Xu H, Tang J, Yang Z, et al. Comparison of dual-source CT coronary angiography and conventional coronary angiography for detecting coronary artery disease. Int J Cardiovasc Imaging. (2010) 26(Suppl 1):75–81. doi: 10.1007/s10554-009-9568-5
43. Gurm HS, Smith D, Share D, Wohns D, Collins J, Madala M, et al. Impact of automated contrast injector systems on contrast use and contrast-associated complications in patients undergoing percutaneous coronary interventions. JACC Cardiovasc Interv. (2013) 6(4):399–405. doi: 10.1016/j.jcin.2012.11.008
44. Hollenberg EJ, Lin F, Blaha MJ, Budoff MJ, van den Hoogen IJ, Gianni U, et al. Relationship between coronary artery calcium and atherosclerosis progression among patients with suspected coronary artery disease. JACC Cardiovasc Imaging. (2022) 15(6):1063–74. doi: 10.1016/j.jcmg.2021.12.015
45. Kelly JL, Thickman D, Abramson SD, Chen PR, Smazal SF, Fleishman MJ, et al. Coronary CT angiography findings in patients without coronary calcification. AJR Am J Roentgenol. (2008) 191(1):50–5. doi: 10.2214/ajr.07.2954
46. Lin L, Wang L, Zhang XN, Li X, Wang J, Shen ZJ, et al. A clinical strategy to improve the diagnostic accuracy of 1.5-T non-contrast MR coronary angiography for detection of coronary artery disease: combination of whole-heart and volume-targeted imaging. Eur Radiol. (2021) 31(4):1894–904. doi: 10.1007/s00330-020-07135-7
47. Sato S, Matsumoto H, Li D, Ohya H, Mori H, Sakai K, et al. Coronary high-intensity plaques at T1-weighted MRI in stable coronary artery disease: comparison with near-infrared spectroscopy intravascular US. Radiology. (2022) 302(3):557–65. doi: 10.1148/radiol.211463
48. Fukase T, Dohi T, Fujimoto S, Nishio R, Nozaki YO, Kudo A, et al. Relationship between coronary high-intensity plaques on T1-weighted imaging by cardiovascular magnetic resonance and vulnerable plaque features by near-infrared spectroscopy and intravascular ultrasound: a prospective cohort study. J Cardiovasc Magn Reson. (2023) 25(1):4. doi: 10.1186/s12968-023-00916-1
Keywords: coronary artery calcium, arthroplasty, coronary stenosis, ordinary chest CT, coronary angiography
Citation: Li Y, Wang Y, Ma T, Duan X and Si H (2025) Preoperative assessment of coronary stenosis through ordinary chest CT for patients scheduled total hip or knee arthroplasty. Front. Cardiovasc. Med. 12:1582704. doi: 10.3389/fcvm.2025.1582704
Received: 24 February 2025; Accepted: 11 June 2025;
Published: 1 July 2025.
Edited by:
Nicolau Beckmann, Novartis Institutes for BioMedical Research, SwitzerlandReviewed by:
Lisa Theresa Dam, Independent Researcher, Vienna, AustriaMurat Çap, Barts Heart Centre, United Kingdom
Mena Ekladious, Ain Shams University, Egypt
Didier Laurent, Novartis, Switzerland
Copyright: © 2025 Li, Wang, Ma, Duan and Si. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Duan, ZHhiYWFsQGhvdG1haWwuY29t; Haibo Si, c2loYWlib0B3Y2hzY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yi Li1,†
Yi Li1,† Yang Wang
Yang Wang Xin Duan
Xin Duan Haibo Si
Haibo Si