Abstract
Background:
Heart failure (HF) is a global health challenge with high morbidity and mortality. The serum creatinine/albumin ratio (CAR), a marker of renal dysfunction and malnutrition, has shown prognostic value in other critical illnesses but remains underexplored in HF patients.
Methods:
This retrospective cohort study included 1,893 HF patients hospitalized at the Fourth People's Hospital of Zigong, China, between December 2016 and June 2019. Cox proportional hazards models assessed the association between CAR and 28-day mortality. Dose-response relationship was assessed using restricted cubic spline analysis, Kaplan–Meier curves illustrated survival differences, and Receiver Operating Characteristic (ROC) analysis evaluated CAR's predictive performance.
Results:
Patients with CAR ≥ 3.5 were older, had worse cardiac function, and had more comorbidities than those with CAR < 3.5.A linear relationship was observed between CAR and 28-day mortality. Each 1-unit increase in CAR was associated with a 14% higher mortality risk (HR: 1.14, 95% CI: 1.07–1.21, p < 0.001). ROC analysis showed that CAR had an AUC of 77.1%, which was slightly higher than creatinine alone (76.2%) and markedly better than BNP (68.0%) and albumin alone (64.9%).
Conclusion:
In patients with HF, CAR may serve as an independent predictor of 28-day mortality. Its ability to simultaneously reflect renal dysfunction, malnutrition, and inflammation highlights its potential as a valuable biomarker for risk stratification. Further multicenter, prospective studies are needed to confirm its clinical utility and investigate its role alongside other biomarkers in guiding personalized treatment strategies and improving patient outcomes.
1 Introduction
Heart failure (HF) remains a significant global health concern, with an estimated 56.19 million cases reported worldwide by 2019 (1). Its prevalence increases markedly with age, affecting less than 1% of individuals under 55 years of age, but surpassing 10% in those aged 70 years and older (2, 3). Despite advances in treatment, HF remains associated with poor outcomes, including high mortality and frequent hospital readmissions (4–6). As populations age, the burden of HF is expected to intensify, making early identification of high-risk patients critical for optimizing treatment and improving prognosis.
The serum creatinine/albumin ratio (CAR) is an emerging biomarker that integrates two routinely measured clinical parameters—serum creatinine and albumin. It reflects both renal dysfunction and malnutrition, two interrelated conditions that are highly prevalent in HF and independently associated with adverse outcomes (7–10). Creatinine is a well-established indicator of renal function, and elevated levels often reflect impaired perfusion or chronic kidney disease in HF patients (9, 11). Albumin, on the other hand, is a marker of nutritional status and systemic inflammation. Hypoalbuminemia in HF has been linked to chronic inflammation and poor survival (10, 12).
Recent studies have demonstrated the prognostic value of CAR in several critical illnesses. For example, elevated CAR has been associated with increased mortality in patients with acute coronary syndrome (7), acute pancreatitis (13), and severe burn injuries (14). These findings suggest that CAR may serve as a composite biomarker that reflects multiple pathophysiological pathways—renal impairment, malnutrition, and inflammation—that contribute to poor outcomes.
However, the role of CAR in the context of heart failure remains underexplored. Given that HF commonly involves a combination of renal dysfunction, systemic inflammation, and nutritional decline, we hypothesize that CAR may be a useful predictor of short-term mortality in this population. In this study, we aim to investigate the association between CAR and 28-day mortality in Chinese patients hospitalized for HF, with the goal of providing a simple and effective risk stratification tool to inform early clinical decision-making.
2 Materials and methods
2.1 Study population
This retrospective cohort study analyzed data from patients hospitalized with HF at the Fourth People's Hospital of Zigong, Sichuan, China, between December 2016 and June 2019. The dataset, which undergoes regular updates, is maintained by the Massachusetts Institute of Technology (Cambridge, MA, USA; https://physionet.org/content/heart-failure-zigong/1.3/) (15). The collected data encompassed demographic information, baseline clinical indicators, comorbidities, laboratory findings, therapeutic interventions, and patient outcomes. HF was diagnosed according to the criteria established by the European Society of Cardiology (ESC) (16). Patients were included if they were hospitalized with a primary diagnosis of HF. The exclusion criteria were as follows: (1) patients with incomplete serum creatinine data and (2) those with incomplete serum albumin data. Ultimately, 1893 patients met the inclusion criteria for this study. Figure 1 provides a flowchart illustrating the selection of participants. The study protocol received approval from the ethics committee of Zigong Fourth People's Hospital (Approval Number 2020-010), with informed consent waived due to the retrospective study design. All procedures conformed to the principles outlined in the Declaration of Helsinki and adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (17).
Figure 1
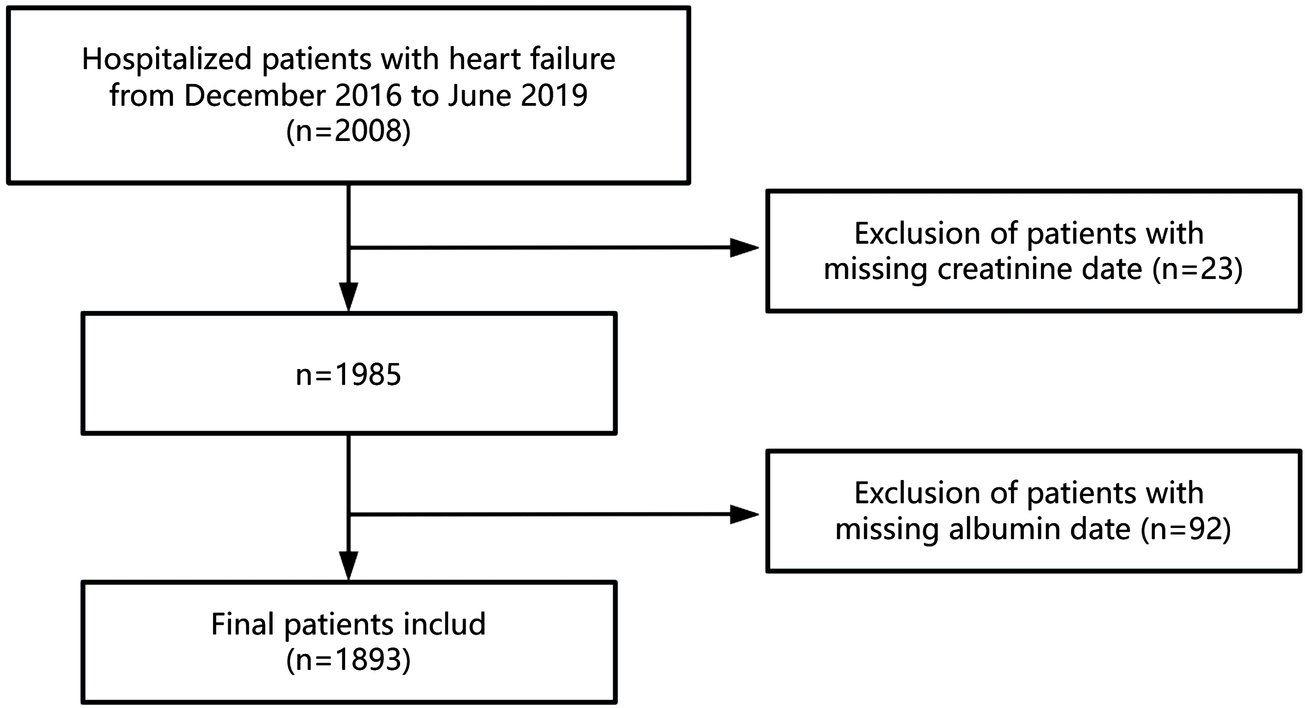
The flowchart of the participants selection.
2.2 Data collection
At admission, demographic data and clinical characteristics were comprehensively recorded. These included patient-specific factors such as age, gender, body mass index (BMI), mean arterial pressure (MAP), and the classification of cardiac function based on the New York Heart Association (NYHA) criteria. Additionally, comorbidities were evaluated, including histories of myocardial infarction, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), diabetes, and the presence of solid tumors. Laboratory investigations conducted within the initial 24 h post-admission included measurements of white blood cell (WBC) count, hemoglobin levels, platelet count, high-sensitivity cardiac troponin (hs-cTn), brain natriuretic peptide (BNP), potassium, sodium, serum creatinine, and albumin. Our data had missing values as follows: hs-cTn had a 2.9% missing rate (55 cases), BNP had a 1.2% missing rate (22 cases), hemoglobin had a 0.9% missing rate (17 cases), WBC and platelet count each had a 0.8% missing rate (16 cases), potassium and sodium each had a 0.2% missing rate (3 cases) (Supplementary Table S1). Missing data were imputed using a multivariate imputation method. To assess the robustness of our approach, we conducted a sensitivity analysis using complete-case data only, and the hazard ratios for CAR remained consistent in magnitude and direction across all models (Supplementary Table S2), supporting the reliability of our imputation-based results.
2.3 Measurement of CAR
The CAR was calculated as follows: CAR = serum creatinine (μmol/L)/albumin (g/L).
2.4 Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) for normally distributed data or as median with interquartile range (IQR) for skewed distributions; categorical variables were presented as percentages. Group comparisons were performed using one-way analysis of variance (ANOVA) for continuous variables with a normal distribution and the χ2 test for categorical variables. For continuous variables not normally distributed, the Kruskal–Wallis H test was employed.
The association between CAR and 28-day mortality was assessed using Cox proportional hazards regression models with progressive adjustment: Model 1 was unadjusted; Model 2 adjusted for age and gender; Model 3 further included NYHA cardiac function classification, platelet, BNP, and potassium. Covariates included in these models were selected based on their clinical significance and their potential to alter effect estimates by at least 10% (18).
The dose-response relationship between CAR and 28-day mortality was examined using restricted cubic spline analysis. Kaplan–Meier survival curves were generated to visualize cumulative 28-day mortality across CAR groups. Subgroup analyses were conducted to assess potential effect modification by age, gender, NYHA classification, myocardial infarction, and cerebrovascular disease. ROC analysis was used to evaluate the predictive performance of CAR, serum creatinine, albumin and BNP, with sensitivity, specificity, and area under the curve (AUC) reported. The optimal CAR cut-off was determined using the Youden index, and patients were categorized into two groups: CAR <3.5 and CAR ≥3.5. All analyses were performed using R software (version 3.3.2; http://www.R-project.org) and Free Statistics software (version 1.9). A p-value <0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of study population
The baseline characteristics of the patients are summarized in Table 1. A total of 1,893 patients were included in the analysis, categorized into two groups based on the CAR: CAR < 3.5 (n = 1,429) and CAR ≥ 3.5 (n = 464). Patients with CAR ≥ 3.5 were generally older (≥70 years: 85.6% vs. 68.9%, p < 0.001), and a greater proportion were male (54.5% vs. 37.8%, p < 0.001). Patients with higher CAR (≥3.5) had significantly lower mean arterial pressure, worse NYHA classification, and higher prevalence of myocardial infarction and diabetes compared to those with CAR < 3.5. Several laboratory parameters showed significant differences, with the CAR ≥ 3.5 group having higher WBC, BNP, and serum creatinine levels, but lower albumin levels, compared to the CAR < 3.5 group (all p < 0.05).
Table 1
| Covariates | Total | CAR < 3.5 | CAR ≥ 3.5 | P-value |
|---|---|---|---|---|
| (n = 1,893) | (n = 1,429) | (n = 464) | ||
| Age, years | <0.001 | |||
| <70 | 511 (27.0) | 444 (31.1) | 67 (14.4) | |
| ≥70 | 1,382 (73.0) | 985 (68.9) | 397 (85.6) | |
| Gender | <0.001 | |||
| Male | 793 (41.9) | 540 (37.8) | 253 (54.5) | |
| Female | 1,100 (58.1) | 889 (62.2) | 211 (45.5) | |
| BMI, kg/m2 | 20.8 (18.5, 23.4) | 20.8 (18.4, 23.4) | 20.7 (18.6, 23.4) | 0.845 |
| MAP, mmHg | 94.9 ± 16.2 | 95.7 ± 15.8 | 92.4 ± 17.2 | <0.001 |
| NYHA cardiac function classification, n (%) | <0.001 | |||
| II | 331 (17.5) | 262 (18.3) | 69 (14.9) | |
| III | 983 (51.9) | 768 (53.7) | 215 (46.3) | |
| IV | 579 (30.6) | 399 (27.9) | 180 (38.8) | |
| Myocardial infarction, n (%) | 136 (7.2) | 93 (6.5) | 43 (9.3) | 0.046 |
| Cerebrovascular disease, n (%) | 142 (7.5) | 110 (7.7) | 32 (6.9) | 0.569 |
| COPD, n (%) | 224 (11.8) | 176 (12.3) | 48 (10.3) | 0.253 |
| Diabetes, n (%) | 439 (23.2) | 307 (21.5) | 132 (28.4) | 0.002 |
| Solid tumor, n (%) | 36 (1.9) | 25 (1.7) | 11 (2.4) | 0.395 |
| WBC, 109/L | 7.3 ± 3.5 | 7.2 ± 3.2 | 7.8 ± 4.2 | <0.001 |
| Hemoglobin, g/L | 115.0 ± 24.3 | 119.5 ± 22.3 | 101.1 ± 25.0 | <0.001 |
| Platelet, 109/L | 144.3 ± 64.0 | 143.8 ± 61.6 | 146.0 ± 71.0 | 0.508 |
| Hs-cTn, pg/ml | 0.1 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.1 (0.0, 0.2) | <0.001 |
| BNP, pg/ml | 774.8 (319.4, 1,764.1) | 702.4 (271.8, 1,578.6) | 1,108.1 (461.1, 2,528.1) | <0.001 |
| Potassium, mmol/L | 4.0 ± 0.7 | 3.9 ± 0.6 | 4.4 ± 0.8 | <0.001 |
| Sodium, mmol/L | 138.3 ± 4.9 | 138.8 ± 4.7 | 136.7 ± 5.0 | <0.001 |
| Serum creatinine, μmol/L | 87.4 (65.0, 123.7) | 75.3 (61.2, 94.4) | 167.0 (140.0, 220.1) | <0.001 |
| Albumin, g/L | 36.5 ± 5.0 | 37.4 ± 4.7 | 34.0 ± 4.9 | <0.001 |
| CAR | 2.4 (1.8, 3.5) | 2.0 (1.6, 2.7) | 4.8 (4.0, 6.7) | <0.001 |
| Mortality, n (%) | 32 (1.7) | 9 (0.6) | 23 (5.0) | <0.001 |
Baseline characteristics of heart failure patients.
BMI, body mass index; MAP, mean arterial pressure; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; WBC, white blood cell; hs-cTn, high sensitivity cardiac troponin; BNP, brain natriuretic peptide; CAR, creatinine/albumin ratio.
3.2 Association between CAR and 28-day mortality in HF patients
Table 2 presents the findings from the multivariable Cox proportional hazards regression analysis. After adjusting for various clinical factors such as age, gender, NYHA classification, and other laboratory markers (Model 3), each 1-unit increase in CAR was associated with a 14% higher risk of mortality within 28 days (HR: 1.14, 95% CI: 1.07–1.21, P < 0.001).
Table 2
| Exposure | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| CAR | 1.15 (1.11∼1.20) | <0.001 | 1.17 (1.12∼1.23) | <0.001 | 1.14 (1.07∼1.21) | <0.001 |
Association of creatinine/albumin ratio and 28-day mortality in heart failure patients.
Model 1: no covariates were adjusted.
Model 2: adjusted for age and gender.
Model 3: adjusted for age, gender, NYHA cardiac function classification, platelet, BNP, and potassium.
CAR, creatinine/albumin ratio; NYHA, New York Heart Association; BNP, brain natriuretic peptide; HR, hazard ratio; CI, confidence interval.
3.3 Dose-response relationship between CAR and 28-day mortality
A dose-response relationship between CAR and 28-day mortality was observed in Figure 2. The analysis demonstrated a linear association, with an increasing CAR corresponding to a higher risk of 28-day mortality.
Figure 2
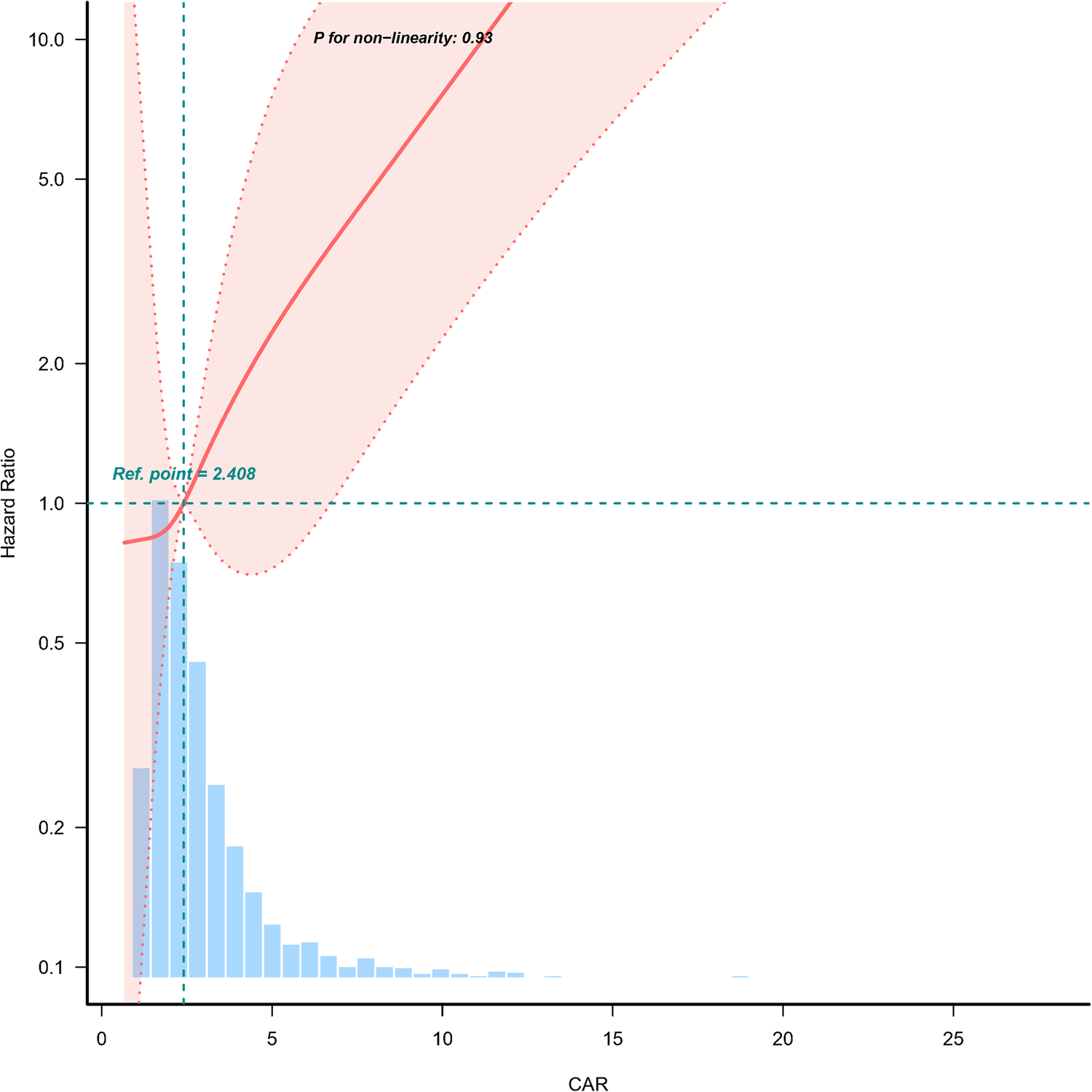
Dose-response relationship between creatinine/albumin ratio and 28-day mortality in heart failure patients. Adjustment factors included age, gender, NYHA cardiac function classification, platelet, BNP, and potassium. The red line and the area between the red dashed lines represents the estimated values and their corresponding 95% confidence intervals, respectively. CAR, creatinine/albumin ratio; NYHA, New York Heart Association; BNP, brain natriuretic peptide; HR, hazard ratio; CI, confidence interval.
3.4 Kaplan–Meier survival curve
Kaplan–Meier survival analysis was conducted to evaluate the 28-day survival probability stratified by CAR categories (Figure 3). Patients with CAR ≥ 3.5 had significantly lower survival rates compared to those with CAR < 3.5 (P < 0.001).
Figure 3
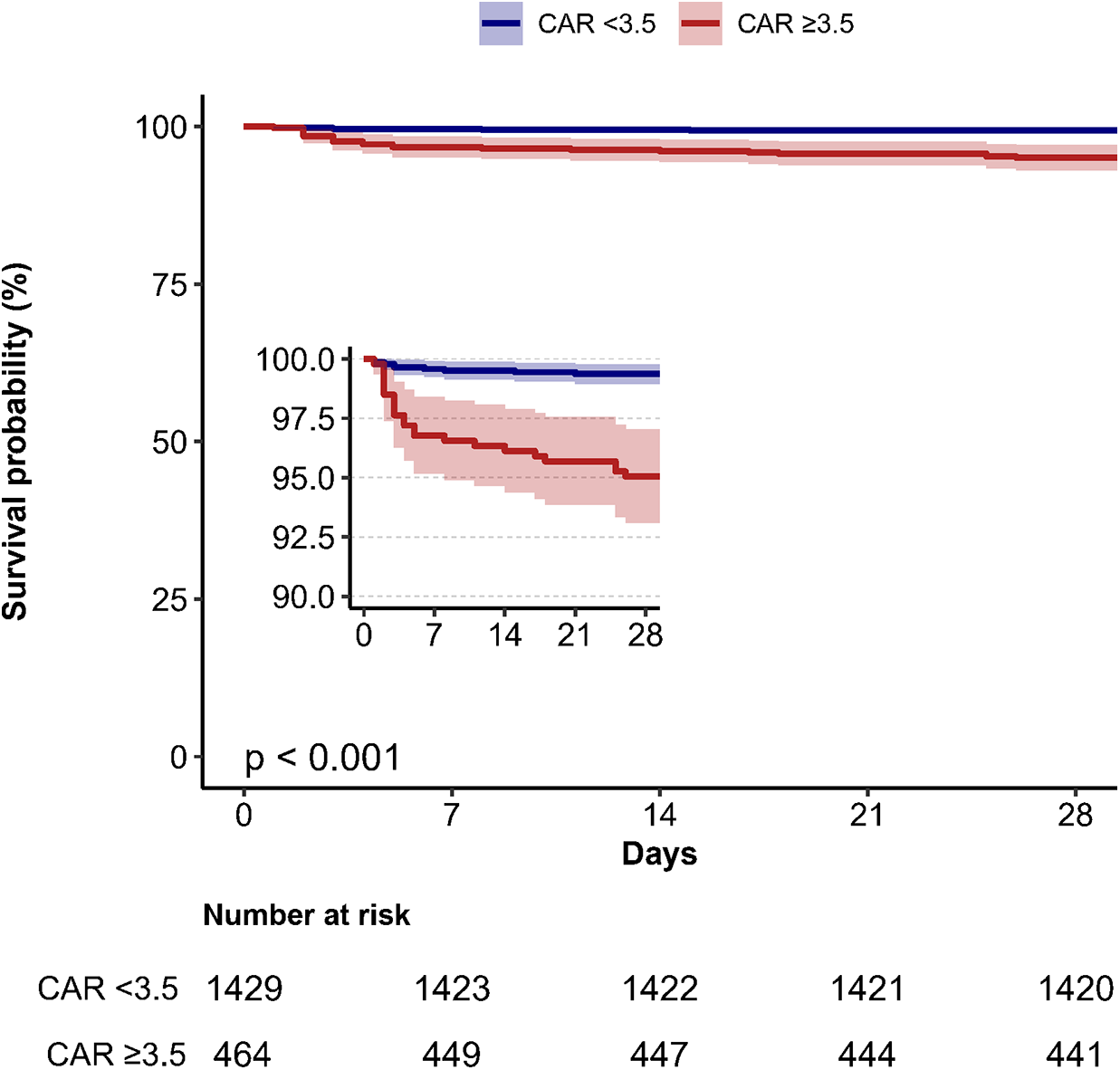
Kaplan–Meier survival curves depicting 28-day mortality stratified by creatinine/albumin ratio categories in heart failure patients. CAR, creatinine/albumin ratio.
3.5 ROC curve analysis
The ROC curve analysis was conducted to assess the predictive ability of CAR for 28-day mortality (Figure 4, Table 3). The AUC for CAR was 77.1% (95% CI: 67.6%-86.6%), indicating a good predictive performance. The optimal threshold for CAR was 3.548, with a sensitivity of 0.719 and specificity of 0.769. For comparison, BNP, creatinine and albumin alone had AUCs of 68.0%, 76.2% and 64.9%, respectively, suggesting that the predictive performance of CAR was slightly higher than that of creatinine and markedly better than that of BNP and albumin individually.
Figure 4
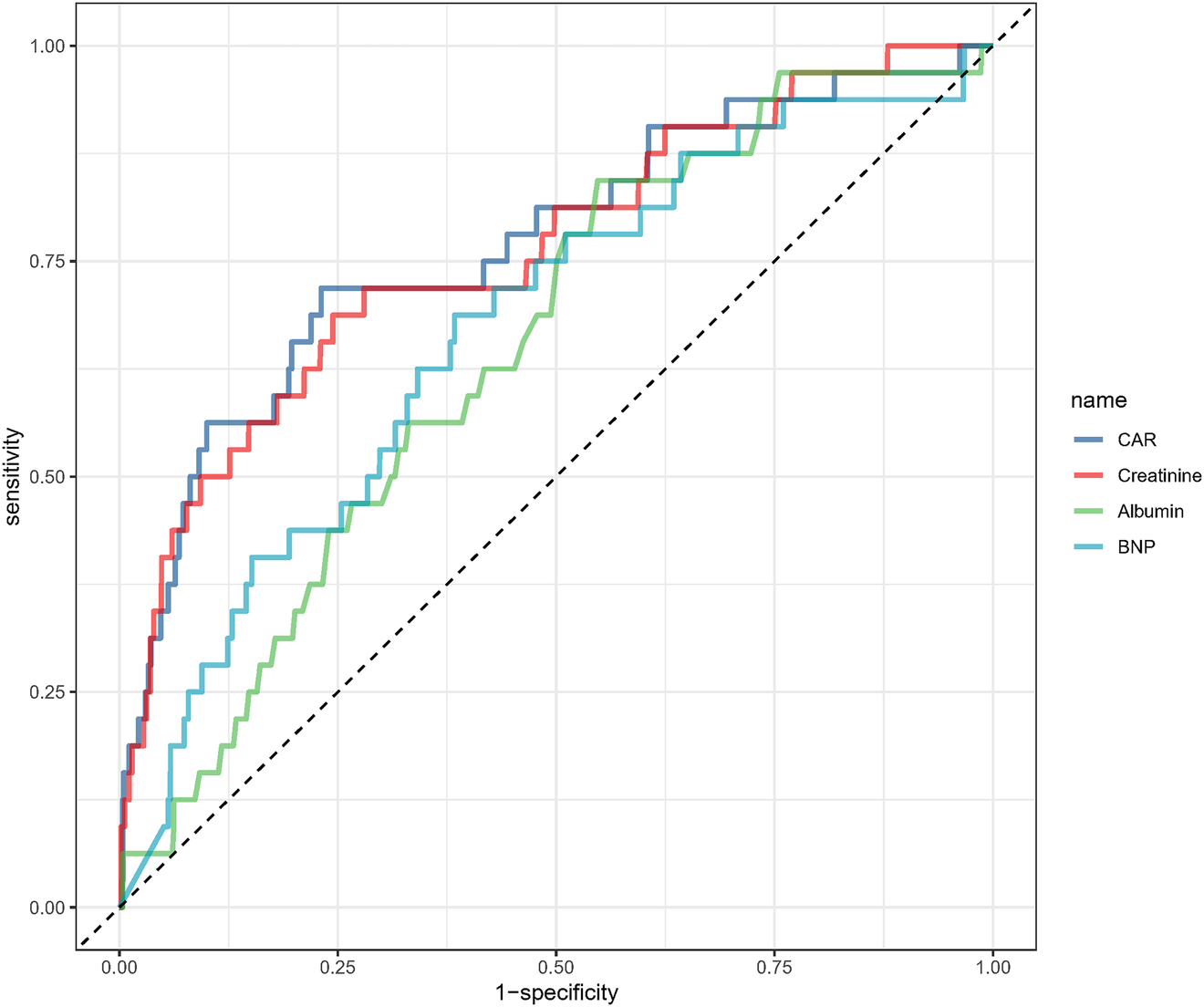
Receiver operating characteristic analysis of creatinine/albumin ratio in predicting 28-day mortality.
Table 3
| Variables | AUC | 95%CI | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|
| CAR | 77.072% | 67.591%∼86.554% | 3.548 | 0.719 | 0.769 |
| Creatinine | 76.177% | 66.788%∼85.566% | 123.05 | 0.687 | 0.756 |
| Albumin | 64.930% | 56.285%∼73.574% | 37.35 | 0.844 | 0.452 |
| BNP | 68.020% | 57.457%∼78.583% | 1,280.465 | 0.667 | 0.654 |
Information of receiver operating characteristic curve in figure 4.
CAR, creatinine/albumin ratio; BNP, brain natriuretic peptide; AUC, area under the curve; CI, confidence interval.
4 Discussion
In this retrospective cohort study, we found that CAR is a significant and independent predictor of 28-day mortality in patients with HF. The relationship between CAR and 28-day mortality was linear, as confirmed by dose-response analysis, and the predictive performance of CAR (AUC: 77.1%) surpassed that of BNP. Subgroup analyses showed consistent associations across most strata. These findings identify the CAR as a prognostic biomarker that simultaneously reflects renal dysfunction and nutritional status, providing critical insights for risk stratification in patients with HF.
The association between CAR and short-term mortality in HF patients remains underexplored, with previous studies primarily focusing on serum creatinine or albumin individually. Elevated serum creatinine has been widely associated with poor outcomes in HF due to its reflection of renal impairment (9, 11, 19), while hypoalbuminemia, indicative of malnutrition and systemic inflammation, has also been linked to higher mortality (10, 12). Additionally, inflammation plays a critical role in HF prognosis, as demonstrated by inflammatory markers such as the C-reactive protein to albumin ratio, which has been associated with long-term mortality in HF with reduced ejection fraction (20). Studies investigating CAR as a combined marker have shown its predictive value in acute coronary syndrome (7) and acute pancreatitis (13). These findings align with our results, further supporting the use of renal and nutritional biomarkers like CAR for risk stratification in HF patients. The relationship between CAR and mortality in HF can be explained by the combined effects of renal dysfunction and malnutrition, both of which are common in HF and contribute to worse outcomes. Renal dysfunction in HF is influenced by multiple interconnected mechanisms (21). Hemodynamic abnormalities, such as increased central venous pressure and reduced cardiac output, significantly impair renal function by altering renal preload and afterload. Sympathetic hyperactivity, characterized by increased renal sympathetic nerve activity, exacerbates sodium retention, reduces renal blood flow, and stimulates renin release. The renin–angiotensin–aldosterone system (RAAS) activation, initially compensatory, leads to long-term adverse effects including fibrosis, oxidative stress, and endothelial dysfunction. Hypoalbuminemia in HF results from a combination of interconnected mechanisms (22). Malnutrition and cardiac cachexia, stemming from reduced appetite, gastrointestinal dysfunction, and increased metabolic demands, diminish hepatic albumin synthesis. Chronic inflammation, marked by elevated cytokines like IL-6 and CRP, further suppresses albumin production and accelerates its breakdown. Hepatic congestion due to elevated venous pressures impairs liver function and albumin synthesis, while severe venous congestion can lead to protein-losing enteropathy, compounding albumin loss. These mechanisms likely interact synergistically, with CAR consolidating their combined impact into a unified predictive biomarker, reflecting a broader spectrum of pathophysiological processes and may provide additional predictive value beyond either marker individually.
This study employed several unique methodological approaches, such as multivariable Cox regression models with progressive adjustments and restricted cubic spline analyses, to elucidate the relationship between CAR and 28-day mortality. Additionally, ROC curve analysis was employed to identify an optimal CAR threshold, providing valuable insights for potential clinical application.
Despite these strengths, several limitations of the study must be acknowledged. First, the retrospective design is inherently prone to biases, including unmeasured confounding and incomplete data, even with the application of multivariate imputation techniques. Second, while the single-center setting ensures consistency in data collection and interpretation, it limits the extrapolation of the findings to broader, more diverse populations with different demographic and clinical characteristics. Third, the analysis was based solely on baseline CAR values, which restricts the ability to assess longitudinal changes in CAR, potentially limiting the understanding of its dynamic prognostic value over time. Fourth, left ventricular ejection fraction and other echocardiographic parameters were unavailable for most patients, preventing stratification by heart failure phenotype and constraining the interpretation of our results.
5 Conclusion
In patients with HF, CAR may serve as an independent predictor of 28-day mortality. Its ability to simultaneously reflect renal dysfunction, malnutrition, and inflammation highlights its potential as a valuable biomarker for risk stratification. Further multicenter, prospective studies are needed to confirm its clinical utility and investigate its role alongside other biomarkers in guiding personalized treatment strategies and improving patient outcomes.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://physionet.org/content/heart-failure-zigong/1.3/.
Ethics statement
The studies involving humans were approved by The ethics committee of Zigong Fourth People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective study design.
Author contributions
HL: Conceptualization, Data curation, Methodology, Software, Writing – original draft. XC: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1586327/full#supplementary-material
References
1.
YanTZhuSYinXXieCXueJZhuMet alBurden, trends, and inequalities of heart failure globally, 1990 to 2019: a secondary analysis based on the global burden of disease 2019 study. J Am Heart Assoc. (2023) 12(6):e027852. 10.1161/JAHA.122.027852
2.
van RietEEHoesAWWagenaarKPLimburgALandmanMARuttenFH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. (2016) 18(3):242–52. 10.1002/ejhf.483
3.
McDonaghTAMetraMAdamoMGardnerRSBaumbachABöhmMet al2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24(1):4–131. 10.1002/ejhf.2333
4.
SavareseGBecherPMLundLHSeferovicPRosanoGMCCoatsAJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. 10.1093/cvr/cvac013
5.
ChanDZLKerrAJDoughtyRN. Temporal trends in the burden of heart failure. Intern Med J. (2021) 51(8):1212–8. 10.1111/imj.15253
6.
DharmarajanKRichMW. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin. (2017) 13(3):417–26. 10.1016/j.hfc.2017.02.001
7.
YılmazMFKaragözAZerenGAvcıİITimurBSungurMAet alRelationship between in-hospital mortality and creatinine/albumin in patients with ST-elevation myocardial infarction without standard modifiable risk factors. Biomark Med. (2022) 16(14):1043–53. 10.2217/bmm-2022-0241
8.
KhanMSSamman TahhanAVaduganathanMGreeneSJAlrohaibaniAAnkerSDet alTrends in prevalence of comorbidities in heart failure clinical trials. Eur J Heart Fail. (2020) 22(6):1032–42. 10.1002/ejhf.1818
9.
SatoYYoshihisaAOikawaMNagaiTYoshikawaTSaitoYet alPrognostic impact of worsening renal function in hospitalized heart failure patients with preserved ejection fraction: a report from the JASPER registry. J Card Fail. (2019) 25(8):631–42. 10.1016/j.cardfail.2019.04.009
10.
AncionAAllepaertsSRobinetSOuryCPierardLALancellottiP. Serum albumin level and long-term outcome in acute heart failure. Acta Cardiol. (2019) 74(6):465–71. 10.1080/00015385.2018.1521557
11.
LlaugerLEspinosaBRafiqueZBooneSBeuhlerGMillán-SoriaJet alImpact of worsening renal function detected at emergency department arrival on acute heart failure short-term outcomes. Eur J Emerg Med. (2023) 30(2):91–101. 10.1097/MEJ.0000000000001016
12.
UthamalingamSKandalaJDaleyMPatvardhanECapodilupoRMooreSAet alSerum albumin and mortality in acutely decompensated heart failure. Am Heart J. (2010) 160(6):1149–55. 10.1016/j.ahj.2010.09.004
13.
WangJLiHLuoHShiRChenSHuJet alAssociation between serum creatinine to albumin ratio and short- and long-term all-cause mortality in patients with acute pancreatitis admitted to the intensive care unit: a retrospective analysis based on the MIMIC-IV database. Front Immunol. (2024) 15:1373371. 10.3389/fimmu.2024.1373371
14.
ChenWHYeHFWuYXDaiWTLingXWZhaoSet alAssociation of creatinine-albumin ratio with 28-day mortality in major burned patients: a retrospective cohort study. Burns. (2023) 49(7):1614–20. 10.1016/j.burns.2023.04.002
15.
ZhangZCaoLChenRZhaoYLvLXuZet alElectronic healthcare records and external outcome data for hospitalized patients with heart failure. Sci Data. (2021) 8(1):46. 10.1038/s41597-021-00835-9
16.
PonikowskiPVoorsAAAnkerSDBuenoHClelandJGFCoatsAJSet al2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2016) 18(8):891–975. 10.1002/ejhf.592
17.
von ElmEAltmanDGEggerMPocockSJGøtzschePCVandenbrouckeJP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147(8):573–7. 10.7326/0003-4819-147-8-200710160-00010
18.
QuXYangHYuZJiaBQiaoHZhengYet alSerum zinc levels and multiple health outcomes: implications for zinc-based biomaterials. Bioact Mater. (2020) 5(2):410–22. 10.1016/j.bioactmat.2020.03.006
19.
UngerEDDubinRFDeoRDaruwallaVFriedmanJLMedinaCet alAssociation of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. (2016) 18(1):103–12. 10.1002/ejhf.445
20.
TanıkVOAkdenizEÇınarTŞimşekBİnanDKıvrakAet alHigher C-reactive protein to albumin ratio portends long-term mortality in patients with chronic heart failure and reduced ejection fraction. Medicina (Kaunas, Lithuania). (2024) 60(3):441–52. 10.3390/medicina60030441
21.
MetraMCotterGGheorghiadeMDei CasLVoorsAA. The role of the kidney in heart failure. Eur Heart J. (2012) 33(17):2135–42. 10.1093/eurheartj/ehs205
22.
Bonilla-PalomasJLGámez-LópezALMoreno-CondeMLópez-IbáñezMCAnguita-SánchezMGallego de la SacristanaAet alHypoalbuminemia in acute heart failure patients: causes and its impact on hospital and long-term mortality. J Card Fail. (2014) 20(5):350–8. 10.1016/j.cardfail.2014.01.016
Summary
Keywords
heart failure, creatinine/albumin ratio, mortality, retrospective cohort study, biomarker
Citation
Luo H and Chen X (2025) Prognostic value of the serum creatinine/albumin ratio for 28-day mortality in heart failure: a retrospective cohort study. Front. Cardiovasc. Med. 12:1586327. doi: 10.3389/fcvm.2025.1586327
Received
02 March 2025
Accepted
26 June 2025
Published
09 July 2025
Volume
12 - 2025
Edited by
Andreas J. Rieth, Kerckhoff Clinic, Germany
Reviewed by
Lianyue Ma, Shandong University, China
Vladimir Đurović, University of Novi Sad, Serbia
Georgios Aletras, The General Hospital of Heraklion "Venizeleio-Pananio", Greece
Updates
Copyright
© 2025 Luo and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinqi Chen nydxc2010@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.