- 1Division of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, Strasbourg, France
- 2Department of Radiology, Nouvel Hôpital Civil, Strasbourg University Hospital, Strasbourg, France
- 3UR 3074 Translational Cardiovascular Medicine CRBS, Strasbourg, France
Background: Senile transthyretin cardiac amyloidosis (AL) is an underdiagnosed infiltrative cardiomyopathy causing heart failure symptoms in elderly patients. It is associated with a higher incidence of atrial fibrillation (AF) and atrial flutter.
Case summary: A 75-year-old male patient with senile transthyretin cardiac AL presented with congestive heart failure [New York Heart Association (NYHA) IV] related to rapid perimitral atrial flutter, causing tachycardia-induced cardiomyopathy with a left ventricular ejection fraction (LVEF) of 25%. He underwent AF voltage-guided ablation with vein of Marshall (VOM) ethanol infusion to block the mitral isthmus. Left atrial bipolar voltage mapping revealed diffuse and severe left atrial low-voltage areas related to amyloid protein infiltration within the left atrium (LA). After a 48-month follow-up, no arrhythmia recurrence was observed. Heart failure symptoms improved significantly (NYHA I–II) with an improved LVEF of approximately 45%–50%.
Discussion: Diffuse and severe left atrial fibrosis related to amyloid protein infiltration within the LA is generally associated with worse catheter ablation outcomes in cardiac AL patients. This case demonstrated that the VOM ethanol infusion was critical to the success of the catheter ablation procedure, resulting in a better long-term outcome.
Introduction
Wild-type transthyretin (ATTRwt) cardiac amyloidosis (AL), also known as senile cardiac amyloidosis (SCA), is an underdiagnosed form of restrictive cardiomyopathy causing heart failure in elderly patients. Men over 65 years are predominantly affected (1). The diagnosis is increasing with the aging population and the development of non-invasive diagnostic imaging technologies. ATTRwt patients present a higher incidence of atrial fibrillation (AF) and atrial flutter compared with those with ATTRm and AL cardiac amyloidosis. Atrial arrhythmia can be the first manifestation at the time of diagnosis of cardiac amyloidosis (CA) (1, 2). The arrhythmic substrate is characterized by interstitial extracellular infiltration with amyloid protein in both ventricles and atria.
Case presentation
We report the case of a 75-year-old man referred to our institution for catheter ablation of persistent left atrial flutter. This arrhythmia had caused tachycardia-induced cardiomyopathy with a left ventricular ejection fraction (LVEF) of approximately 25% in April 2019. He experienced heart failure symptoms (NYHA IV) and had a dual-chamber pacemaker for high-degree atrioventricular block. His medical history included prostatic adenocarcinoma. The patient's medical history did not reveal any extracardiac manifestations, such as carpal tunnel syndrome, autonomic dysfunction, or lumbar canal stenosis.
Biological assessment revealed an elevated NT-proBNP level at 5,199 ng/L (normal <450 ng/L) and a normal troponin level at 11.5 ng/L (normal <57 ng/L). The electrocardiogram showed left atrial flutter at 87 bpm with a complete right bundle branch block (QRS duration of 179 ms) and a left anterior fascicular block (Supplementary Figure S1).
An external electric shock at 200 J was performed during the same hospitalization, with recurrence under amiodarone 30 days later.
Prior to the recurrence of atrial flutter, the transthoracic echocardiography demonstrated a recovery of LVEF at 35%, with a non-dilated left ventricle and diffuse hypokinesia. There was moderate left ventricular hypertrophy (posterior wall and septal thickness at 14 mm), and good right ventricular function. Cardiac magnetic resonance imaging on 9 July 2019 showed late gadolinium enhancement (LGE) extending subendocardially over both ventricles, the interatrial septum, and both atria, strongly suggesting the diagnosis of cardiac amyloidosis. Diffuse subendocardial LGE was observed in the left atrium (LA) at the level of superior pulmonary veins (Figure 1).
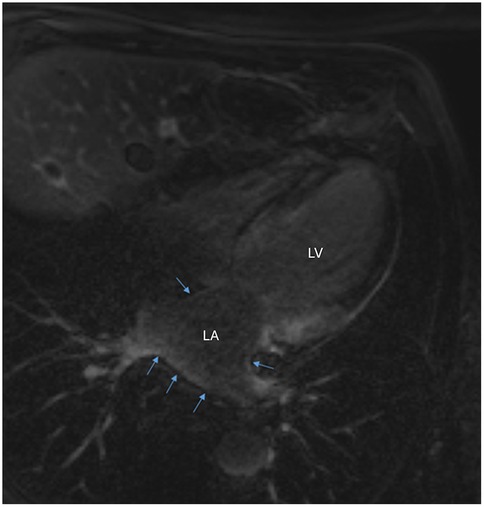
Figure 1. Cardiac magnetic resonance imaging demonstrating diffuse subendocardial late gadolinium enhancement in the left atrium at the level of superior pulmonary veins (blue arrows). LA, left atrium; LV, left ventricle.
Technetium 99m-labeled 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) scintigraphy showed no bone metastases but displayed significant cardiac uptake, suggesting senile transthyretin cardiac amyloidosis. The Perugini score was 2.
Protein electrophoresis and light chain assay were normal. Bence Jones protein analysis was negative, excluding the diagnosis of AL cardiac amyloidosis. The diagnosis of cardiac amyloidosis was strongly suggested by the findings on cardiac MRI and bone scintigraphy, which obviated the need for endomyocardial biopsy with Congo red staining. The most likely diagnosis was SCA in our patient. He underwent an electrophysiological study under local anesthesia, which confirmed left atrial flutter.
Three weeks later, a catheter ablation procedure with vein of Marshall (VOM) ethanol infusion was performed under general anesthesia, using a three-dimensional electroanatomic mapping system (CARTO3, Biosense Webster, Inc., CA, USA). Two transseptal punctures were made. A high-density catheter (PentaRay, Biosense Webster, Inc., CA, USA) was used to map the left atrium (LA). This catheter features 1 mm electrodes with interelectrode spacings of 2-6-2 mm. Tissue ablation was performed using a 4 mm irrigated contact-force ablation catheter (ThermoCool SmartTouch, Biosense Webster, Inc., CA, USA).
During the procedure, the activation map showed a counterclockwise perimitral macro-reentrant flutter with an atrial cycle length of 260 ms (Supplementary Video S1, Figure S2).
Entrainment maneuvers with post-pacing interval measurements were not performed because the activation map clearly indicated a perimitral flutter.
Endocardial linear ablation of the mitral isthmus slowed the atrial flutter cycle length to 290 ms (Supplementary Figure S3). The second activation map revealed a blocked endocardial mitral isthmus with an anticlockwise passage through the VOM (Supplementary Video S2). The VOM was identified by coronary sinus (CS) angiography using a sub-selector catheter with a ∼90° angle at the tip advanced through the CS sheath. It was then cannulated with an angioplasty wire and balloon (1.5 × 6 mm) (Figures 2A,B). A total of 9 ml of ethanol was delivered to the VOM. The first ethanol injection restored sinus rhythm, achieving bidirectional mitral isthmus block. While pacing from the left atrial appendage with the high-density catheter, the activation sequence in the CS was from proximal to distal, consistent with a complete mitral isthmus line block (Supplementary Figure S4). Differential pacing was also performed from the proximal CS to the line of block, with recordings taken on the lateral side of the line. LA geometry and endocardial bipolar voltage mapping were performed in sinus rhythm using the high-density catheter. The maps displayed diffuse low voltage in the LA, compatible with extensive scarring related to amyloid infiltration (Figures 3A,B). The overall LA median bipolar voltage amplitude was 0.1 mV, with LA volume measured at 139 ml. A linear LA ablation approach was chosen due to diffuse low voltage in the left atrium. Posterior left atrial wall isolation was performed using roof and inferior lines following pulmonary vein isolation. Additionally, an anteroseptal line was created from the septal mitral annulus to the right superior pulmonary vein to avoid septal atrial flutter (Figures 3C,D), achieving bidirectional block as confirmed by pacing from the left atrial appendage with the high-density catheter.
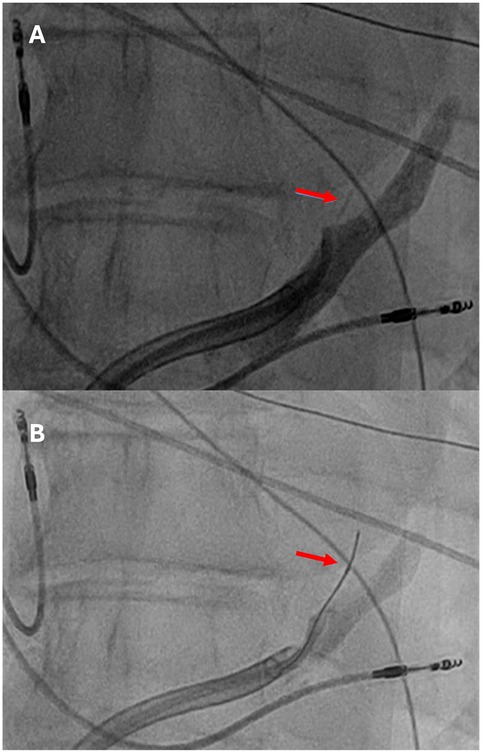
Figure 2. (A) Contrast injection identified the vein of Marshall (red arrow), which was subsequently cannulated with an angioplasty wire and a 1.5 mm × 6 mm angioplasty balloon (B).
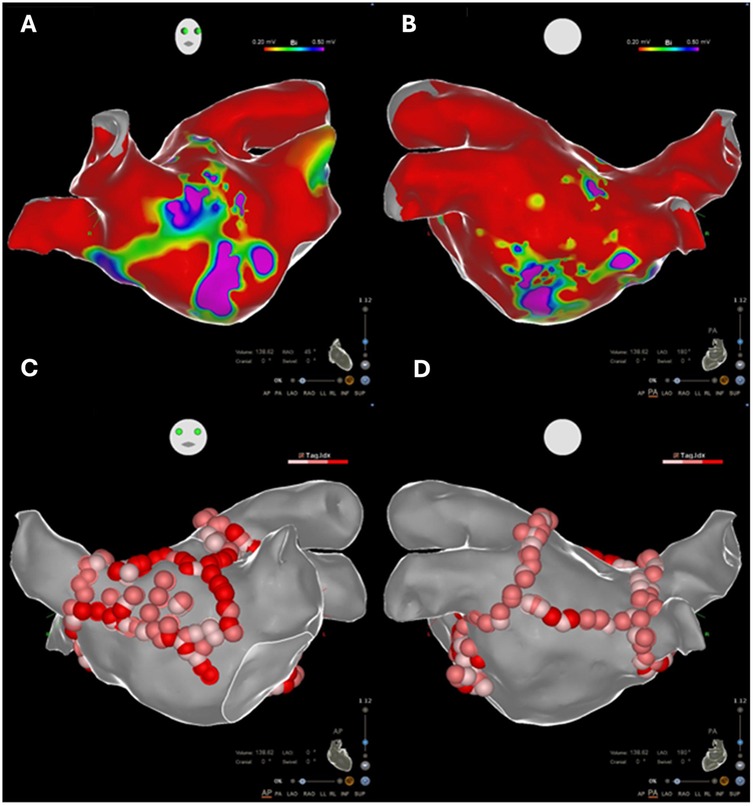
Figure 3. (A,B) Electroanatomic voltage maps demonstrating diffuse low-voltage areas in the left atrium (bipolar <0.5 mV), compatible with extensive scarring related to amyloid infiltration. (C,D) Linear ablative approach performed in the diffusely low-voltage left atrium.
No atrial arrhythmia was induced at the end of the procedure. During the 48-month follow-up, no arrhythmic events were observed in our patient without discontinuing amiodarone. Heart failure symptoms improved significantly (NYHA I–II). An improvement in LVEF to approximately 45%–50% was observed on transthoracic echocardiography, suggesting transthyretin amyloid cardiomyopathy with rhythmic involvement.
Discussion
We observed diffuse low-voltage areas (bipolar <0.5 mV) in LA voltage maps during sinus rhythm in cardiac amyloidosis patients undergoing ablation, as mentioned in several studies (3–5). This amyloid protein infiltration in the left atrium is characterized by diffuse subendocardial LGE on cardiac magnetic resonance imaging. Thus, extensive left atrial disease on voltage mapping should prompt physicians to consider cardiac amyloidosis. Severe left atrial fibrosis is known to be associated with frequent arrhythmia recurrences in AF patients undergoing catheter ablation (6). Previous studies have reported poor catheter ablation outcomes in cardiac amyloidosis patients, with 1-year recurrence rates of 60% (4) and 50% (5) without VOM ethanol infusion. The ablation procedure was performed without complications in our patient, although it is known that atrial fibrillation ablation in patients with cardiac amyloidosis may be associated with an increased risk of acute clinical adverse events in the short term (notably pericardial effusion and significant hemorrhagic events) and mortality, compared with patients with heart failure without cardiac amyloidosis (6).
In our patient, no atrial arrhythmic event was observed after 48 months of follow-up, and heart failure symptoms markedly improved, with no hospitalizations for acute decompensated heart failure during follow-up. This duration of follow-up remains insufficient, given the high risk of recurrence in this population. In cases of uncontrolled heart rate despite ablation, atrioventricular node ablation, with or without pacemaker implantation, should be considered.
The success of the catheter ablation procedure was also achieved thanks to the use of ethanol infusion into the VOM, particularly in patients with a severely dilated left atrium and advanced cardiac amyloidosis. The alcoholization of the VOM represents a promising complementary approach in the management of persistent atrial fibrillation, especially in refractory cases. This technique targets arrhythmogenic epicardial tissue that is often inaccessible to conventional endocardial ablation, thereby improving the outcomes of catheter ablation in patients with cardiac amyloidosis.
Conclusion
This case report demonstrates successful management of persistent left atrial flutter in a senile transthyretin cardiac amyloidosis patient using catheter ablation with VOM ethanol infusion. Despite the challenging substrate of diffuse low-voltage areas, the procedure achieved long-term arrhythmia control and significant improvement in heart failure symptoms. The use of VOM ethanol infusion was critical in achieving bidirectional mitral isthmus block and may have contributed to the favorable outcome. This report highlights the potential of advanced ablation techniques in managing atrial arrhythmias in CA patients, who typically have poor outcomes with conventional approaches. However, further research is needed to validate the efficacy of VOM ethanol infusion in larger cohorts and to establish optimal patient selection criteria for this approach in cardiac amyloidosis patients with refractory atrial arrhythmias.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SF: Writing – original draft, Writing – review & editing. MO: Writing – review & editing. TC: Writing – review & editing. LJ: Writing – review & editing. HM: Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by Groupe pour l'Enseignement, la prévention et la Recherche Cardiologique en Alsace (GERCA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1589051/full#supplementary-material
Supplementary Figure S1 | Twelve-lead electrocardiogram prior to ablation showing a left atrial flutter with complete right bundle branch block and left anterior fascicular block.
Supplementary Figure S2 | Intracardiac tracing demonstrating a counterclockwise perimitral macro-reentrant flutter with an atrial cycle length of 260 ms.
Supplementary Figure S3 | Endocardial linear ablation of the mitral isthmus slowed the atrial flutter cycle length to 290 ms.
Supplementary Figure S4 | Bidirectional mitral isthmus block confirmed by pacing from the left atrial appendage with the high-density catheter.
Supplementary Video S1 | Video loop of the activation map demonstrating a counterclockwise perimitral macro-reentrant atrial flutter with an atrial cycle length of 260 ms.
Supplementary Video S2 | Video loop of the activation map, after endocardial linear ablation of the mitral isthmus, demonstrating a blocked endocardial mitral isthmus with counterclockwise conduction through the vein of Marshall.
Abbreviations
ATTRwt, wild-type transthyretin; SCA, senile cardiac amyloidosis; ATTRm, mutant transthyretin amyloidosis; AL, light chain amyloidosis; CA, cardiac amyloidosis; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; 99mTc-DPD, technetium 99m-labeled 3,3-diphosphono-1,2-propanodicarboxylic acid; LGE, late gadolinium enhancement; VOM, vein of Marshall; LA, left atrial; CS, coronary sinus; AF, atrial fibrillation.
References
1. Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. (2016) 133:282–90. doi: 10.1161/CIRCULATIONAHA.115.018852
2. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. (2016) 68:1014–20. doi: 10.1016/j.jacc.2016.06.033
3. Schöppenthau D, Schatka I, Berger A, Pieske B, Hahn K, Knebel F, et al. Isolated atrial amyloidosis suspected by electrophysiological voltage mapping and diagnosed by 99 m Tc-DPD scintigraphy. ESC Heart Fail. (2020) 7:4305–10. doi: 10.1002/ehf2.12964
4. Black-Maier E, Rehorn M, Loungani R, Friedman DJ, Alenezi F, Geurink K, et al. Catheter ablation of atrial fibrillation in cardiac amyloidosis. Pacing Clin Electrophysiol. (2020) 43:913–21. doi: 10.1111/pace.13992
5. Tan NY, Mohsin Y, Hodge DO, Lacy MQ, Packer DL, Dispenzieri A, et al. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. (2016) 27:1167–73. doi: 10.1111/jce.13046
6. Lellouche N, Defaye P, Algalarrondo V, Gandjbakhch E, Fauchier L, Champ-Rigot L, et al. Management of conduction disease and arrhythmias in patients with cardiac amyloidosis: a position paper from the Working Group of Cardiac Pacing and Electrophysiology of the French Society of Cardiology. Arch Cardiovasc Dis. (2025) 118:63–74. doi: 10.1016/j.acvd.2024.10.323
Keywords: bipolar voltage map, vein of Marshall, cardiac amyloidosis, catheter ablation, atrial fibrillation
Citation: Fitouchi S, Ohana M, Cardi T, Jesel L and Marzak H (2025) Effect of catheter ablation with vein of Marshall ethanol infusion for perimitral flutter in a patient with senile transthyretin cardiac amyloidosis: a case report. Front. Cardiovasc. Med. 12:1589051. doi: 10.3389/fcvm.2025.1589051
Received: 6 March 2025; Accepted: 7 July 2025;
Published: 24 July 2025.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Gianluca Mirizzi, Toscana Gabriele Monasterio Foundation, ItalyLi-Da Wu, Nanjing Medical University, China
Copyright: © 2025 Fitouchi, Ohana, Cardi, Jesel and Marzak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon Fitouchi, c2ltb24uZml0b3VjaGlAY2hydS1zdHJhc2JvdXJnLmZy
 Simon Fitouchi
Simon Fitouchi Mickael Ohana2
Mickael Ohana2 Halim Marzak
Halim Marzak