- 1Epidemiology, Faculty of Medicine, University of Augsburg, Augsburg, Germany
- 2Department of Cardiology, Respiratory Medicine and Intensive Care, University Hospital Augsburg, Augsburg, Germany
- 3Medical Faculty, Ludwig-Maximilians-University München, Munich, Germany
- 4Institute for Medical Information Processing, Biometry and Epidemiology—IBE, LMU Munich, Munich, Germany
Introduction: The predictors and consequences of dyspnea after pulmonary embolism (PE) are only rarely investigated. The present study aimed to characterize dyspnea and its associated factors in patients with incident PE up to 2 years after hospital discharge.
Methods: Data from the German “Lungenembolie Augsburg (LEA)” cohort study were used. Baseline characteristics of adult patients with a first episode of acute PE were collected during hospital stay. Participants completed postal questionnaires 3, 6, 12, and 24 months after their PE. Dyspnea was assessed using the Chronic Respiratory Questionnaire (CRQ) and the Pulmonary Embolism Quality of Life Questionnaire (PEmb-QoL) was used to measure health-related quality of life (HRQOL). Linear mixed models were used to determine the variables associated with dyspnea.
Results: Out of 503 patients (55% male, mean age 62.8 ± 14.6 years), 45%–64% of the participants had dyspnea at any time point. No significant change of dyspnea over time was found. Body mass index (estimate −0.04, 95% CI −0.06 to −0.02, p = 0.0002), symptoms of depression (estimate −0.11, 95% CI −0.15 to −0.07, p < 0.0001), symptoms of anxiety (estimate −0.08, 95% CI −0.11 to −0.04, p < 0.001), and FEV1 values (estimate 0.35, 95% CI 0.10–0.61, p = 0.0060) were significantly associated with the CRQ dyspnea score. Furthermore, dyspnea had significant and strong adverse associations with all subscales of the PEmb-QOL (p < 0.0001).
Discussion: Dyspnea is a common and persisting complaint after PE. Symptoms of depression and anxiety are strongly related with dyspnea and dyspnea is significantly associated with impaired HRQOL.
1 Introduction
Acute pulmonary embolism (PE) is a serious clinical presentation of venous thromboembolism (VTE), and the third most common cardiovascular condition after myocardial infarction and stroke (1). While mortality rates in the acute phase are decreasing, incidence rates of PE are rising in the ageing societies (2–8). The long-term course of PE is characterized by a considerable proportion of patients who experience persisting dyspnea, limited exercise capacity, impaired functional status, or poor health-related quality of life (HRQOL) (9–15). This heterogenous condition is also termed “post-pulmonary embolism syndrome” (16, 17). In addition, mental problems such as depression or anxiety are common among survivors of acute PE (18, 19).
Dyspnea has been identified as a common long-term sequalae of PE affecting up to 50% of the patients (11, 14, 15, 20, 21, 22). It was shown to be significantly associated with impaired generic and disease-specific HRQOL in a few cross-sectional studies with samples of approximately 200 patients or less (11, 14, 23), whereas long-term investigations of this association are rare and are only conducted in smaller patient samples (21). Furthermore, it is still unclear what the risk factors of developing persisting dyspnea after acute PE are. Available studies indicated that female sex, advanced age, smoking history, cardiopulmonary comorbidity, higher BMI, depression, recurrent VTE, diffusion capacity of the lung for carbon monoxide, persistent perfusion defects, predicted VO2 peak <80%, pulmonary artery systolic pressure, chest pain, breathing variability and follow-up-time may play a role in the occurrence of persisting dyspnea (20, 21, 24–26), but the identified variables vary considerably across the studies. Other investigators were even not able to find any significant independent predictors of dyspnea (14).
Hence, the objective of the present study was to characterize dyspnea in patients with PE up to 2 years after hospital discharge, to identify factors associated with dyspnea and to determine the association between dyspnea and HRQOL.
2 Methods
2.1 Design and study population
The study sample consisted of participants of the “Lungenembolie Augsburg” (LEA) study. The LEA study is a long-term observational cohort study including adult patients with incident or recurrent confirmed PE diagnosis based on multidetector CT pulmonary angiography or ventilation–perfusion lung scanning who were hospitalized and treated at the University Hospital Augsburg, Germany. The study design has been described in detail by Meisinger et al. (27).
During their hospital stay, a baseline personal interview was conducted by study nurses. It covers information on socio-demographics, risk factors, and comorbidities. In addition, the patients completed a self-administered questionnaire on mental health. After discharge, study participants were requested to complete postal questionnaires after 3, 6, and 12 months in the first year, thereafter in yearly intervals up to 60 months.
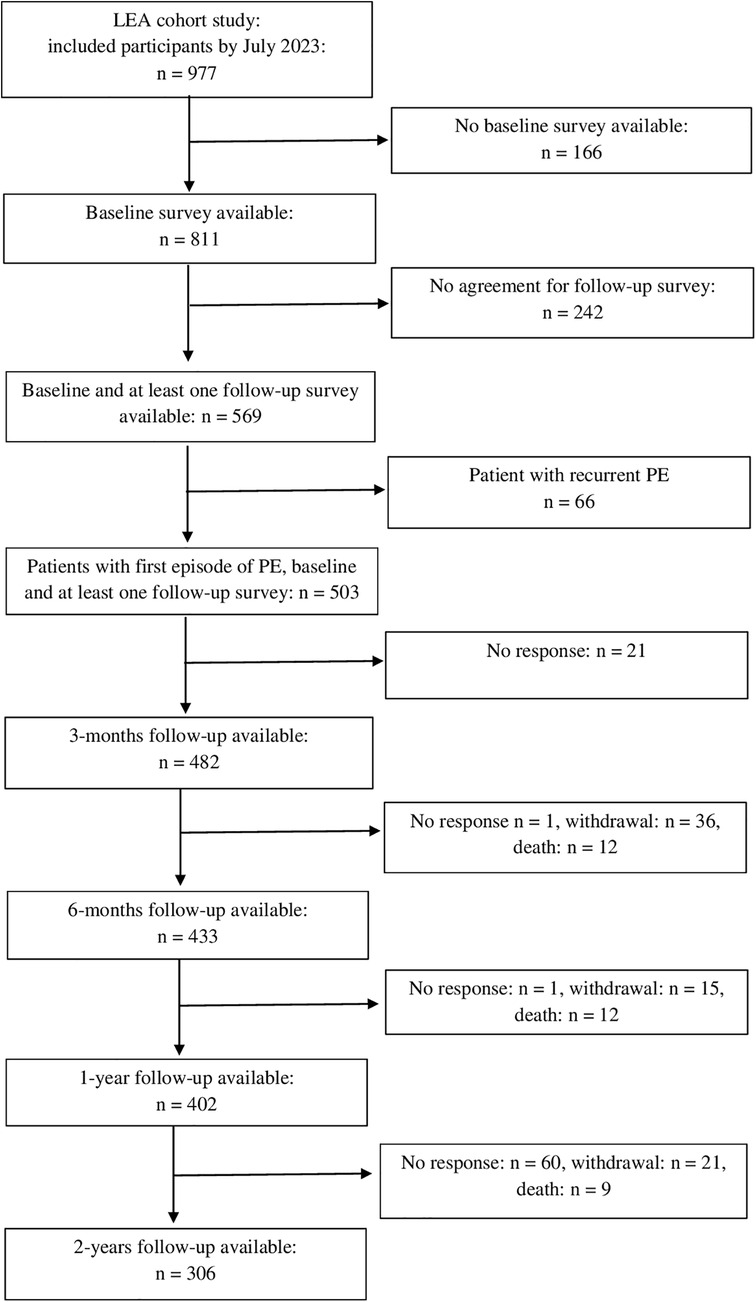
In total, 977 patients were included in the study between July 2017 and July 2023. The present analyses are based on data from 503 study participants with a first episode of PE who were followed up to two years post PE. Of these, 262 participants completed all four follow-up surveys. Figure 1 shows the flow chart of the study.
2.2 Clinical and socio-demographic characteristics
Clinical characteristics of the study participants were extracted from the medical records. Information on uni- versus bilateral PE presentation was collected. If a CT pulmonary angiography was available, information on the presence of central thrombi and infiltrates was noted. For patients who had a pulmonary function test during their hospital stay, the forced expiratory volume in 1 s (FEV1) was included in the data analysis. The Simplified Pulmonary Embolism Severity Index (sPESI) was calculated based on the patient's age, history of cancer, chronic cardiopulmonary disease, heart rate, systolic blood pressure and arterial oxygen saturation to classify patients into high (≥1) or low (0) PE-related risk of death (28). Furthermore, information on prior and current concomitant diseases, PE treatment and duration of hospitalization was extracted from the medical records.
Information on age, gender, marital status, school and professional education, risk factors such as smoking and body mass index, as well as prior and current diseases (e.g., asthma, COPD, cancer, depression) were obtained from the personal interview. School and professional education were classified according to the International Standard Classification of Education (ISCED) system into the levels 1 (lowest level) to 5 (highest level) (29). For the multivariable analyses ISCED categories 1 and 2 as well as the categories 4 and 5 were collapsed. For the analysis, self-reported information on comorbidities was only considered if the information was not available from the medical records.
2.3 Survey data
The postal follow-up assessments at 3 months, 6 months, 1 year and 2 years after the acute PE event included standardized questionnaires to assess dyspnea, HRQOL, and mental health.
Dyspnea was assessed in three different ways. First, the 5-item dyspnea subscale of the self-administered Chronic Respiratory Questionnaire (CRQ) (30) was used. These items request the intensity of dyspnea during the past 3 days on a scale with seven verbal response options (no dyspnea to extremely strong dyspnea) in five different situations, namely while walking, socializing, doing household chores, personal hygiene activities such as bathing, dressing or eating, and when having emotions (e.g., anger). The summary score of these items ranges between 1 and 6 with higher scores indicating less impairment.
Furthermore, two items from the PEmb-QoL questionnaire ask about the frequency of breathlessness during the past 4 weeks (never, less than once a week, about once a week, several times a week, every day) and its intensity (none, very slight, slight, quite a bit, serious, very serious). Higher scores indicate a higher frequency (range 1–5) or stronger intensity (range 1–6).
The PEmb-QoL questionnaire was also used to assess PE-specific HRQOL (31). It includes 38 items in six subscales (Frequency of complaints, intensity of complaints, activities of daily living limitations, work-related problems, social limitations, emotional complaints). Subscale scores were transformed into a scale ranging from 0–100 with higher scores indicating worse HRQOL (32, 33). Since questions about dyspnea were included in the PEmb-QoL subscales “Frequency of complaints” and “Intensity of complaints” these two subscales were not used as outcome measures.
Symptoms of depression and anxiety were assessed using the Hospital Anxiety and Depression Scale (HADS-D), a self-administered and validated German version of the HADS (34–36). The HADS-D consists of two subscales with seven items each which measure depression and anxiety. The subscale scores range between 0 and 21 with higher scores indicating more severe symptoms of depression or anxiety. The subscale scores can also be classified into four categories: 0–7 (no depression or anxiety), 8–10 (mild depression or anxiety), 11–14 (moderate depression or anxiety) and 15–21 (severe depression or anxiety).
2.4 Data analysis
Multivariable linear mixed models with random intercepts were used to investigate the association between the CRQ dyspnea score (dependent variable) and clinical, sociodemographic, health-related variables, and follow-up time point (independent variables). Interaction terms between follow-up time point and all other independent variables were tested in the full model but were not significant and therefore not included in the final model.
Similarly, the association between the PEmb-QOL subscales “Activities of daily living limitations”, “Work-related problems”, “Social limitations”, “Emotional complaints” (dependent variables), and the CRQ dyspnea score (independent variable), adjusted for confounding variables (e.g., clinical, sociodemographic, health-related variables, follow-up time point) was determined using multivariable linear mixed models with random intercepts. Interaction terms between CRQ dyspnea score and follow-up time were included in the full models but were not significant and therefore not considered in the final models.
As sensitivity analyses, the above-mentioned analyses were repeated using the PEmb-QOL items on dyspnea frequency and intensity instead of the CRQ dyspnea score.
In all multivariable analyses, potentially confounding variables were selected based on directed acyclic graphs (37). For statistical tests an alpha level of 0.05 was defined. Statistical analyses were performed using SAS Version 9.4.
3 Results
3.1 Sample characteristics
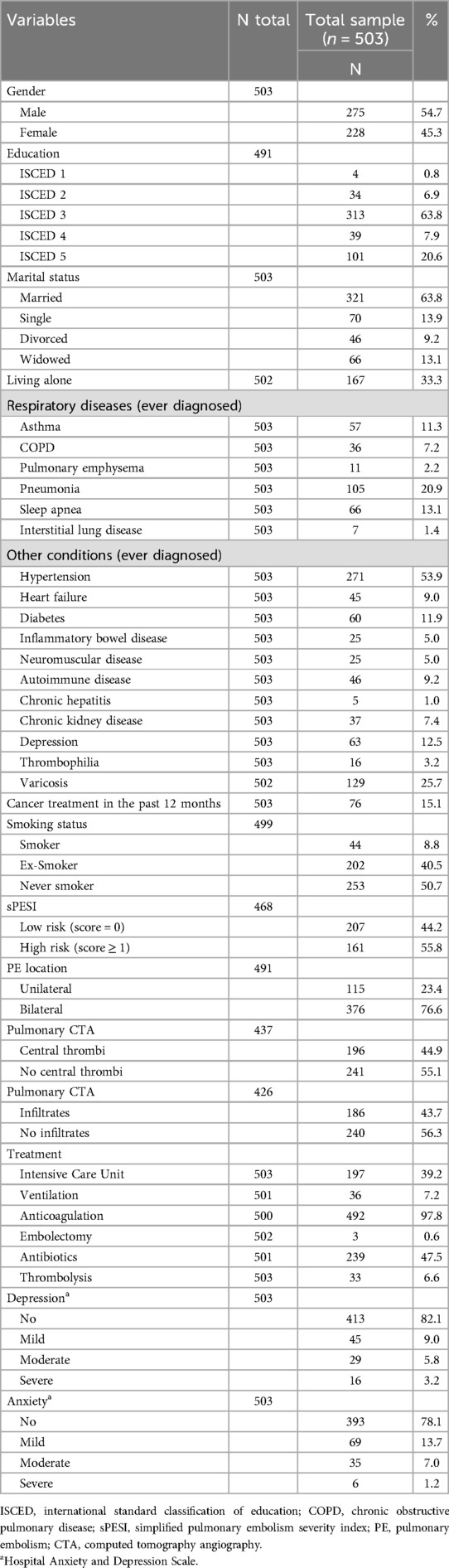
The demographic and clinical characteristics of the participants are shown in Table 1. The median age of the 503 enrolled participants at the time of the PE event was 64 years (25% quantile: 55 years, 75% quantile: 74 years) with 55% men and 45% women. The median duration of hospitalization was 9 days (25% quantile: 6 days, 75% quantile: 13 days), and the median body mass index was 28.3 kg/m2 (25% quantile: 24.7 kg/m2, 75% quantile: 33.0 kg/m2). The median FEV1 was 2.3 L (25% quantile: 1.8 L, 75% quantile: 3.1 L) in 188 participants who had a pulmonary function test.
Compared with participants who were excluded for missing follow-up, the analyzed sample consisted of significantly more men, had less often past diagnoses of cancer and depression, had lower PESI scores and a shorter hospitalization.
3.2 Dyspnea within 2 years after hospital discharge
The five single items of the CRQ dyspnea scale showed that dyspnea was most common when walking or doing household chores (see Supplementary Figure S1). The percentage of individuals who had any intensity of dyspnea in the past 3 days during the follow-up time course ranged between 44.9% (during social activities 6 months after acute PE) and 63.6% (during household chores 3 months after acute PE). The percentage of persons experiencing at least moderate dyspnea intensity ranged between 13.1% (during social activities 2 years after acute PE) and 32.1 (during household chores 6 months after acute PE).
The PEmb-QOL items on frequency and severity of dyspnea during the past four weeks showed that between 55.0% and 57.2% of the participants had dyspnea at least once a week during the 2 year-follow-up, with 5.5% to 13.9% having dyspnea every day (see Supplementary Figure S2). The percentage of participants who had at least very slight dyspnea ranged between 57.1% (2 years after acute PE) and 62.8% (6 months after acute PE). The proportion of individuals who experienced “quite a bit” to “very serious” dyspnea ranged between 16.6% (2 years after acute PE) and 22.2% (6 months after acute PE).
The median values of the CRQ dyspnea score and the PEmb-QOL items on frequency and severity of dyspnea did not differ significantly between the patient samples at each follow-up point (see Figures 2–4 and Supplementary Table S1).
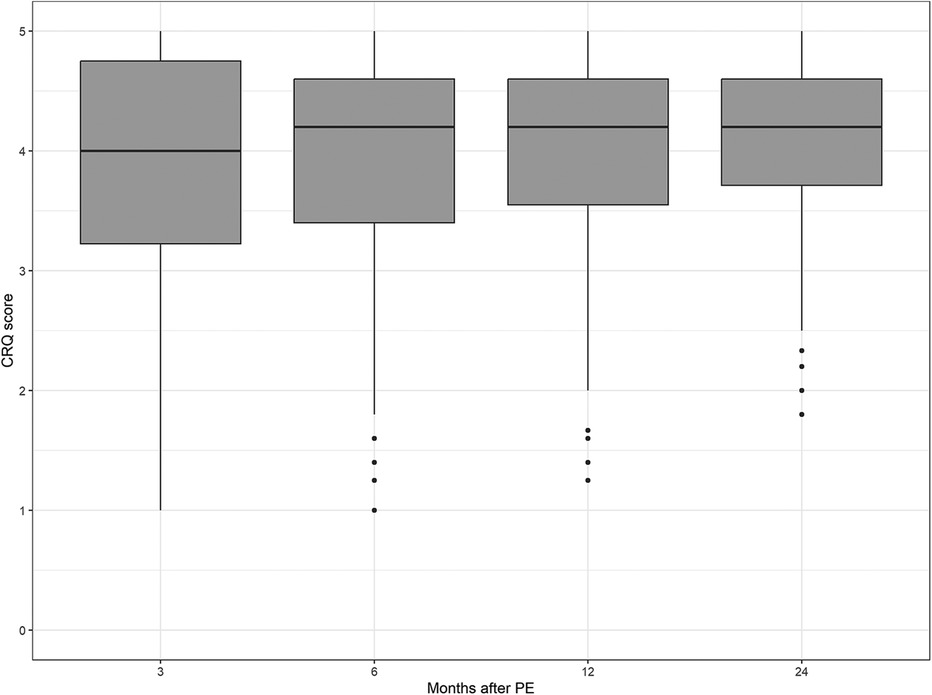
Figure 2. Chronic respiratory questionnaire scores over time. No significant differences between follow-up time points (p = 0.4986, Kruskal–Wallis test).
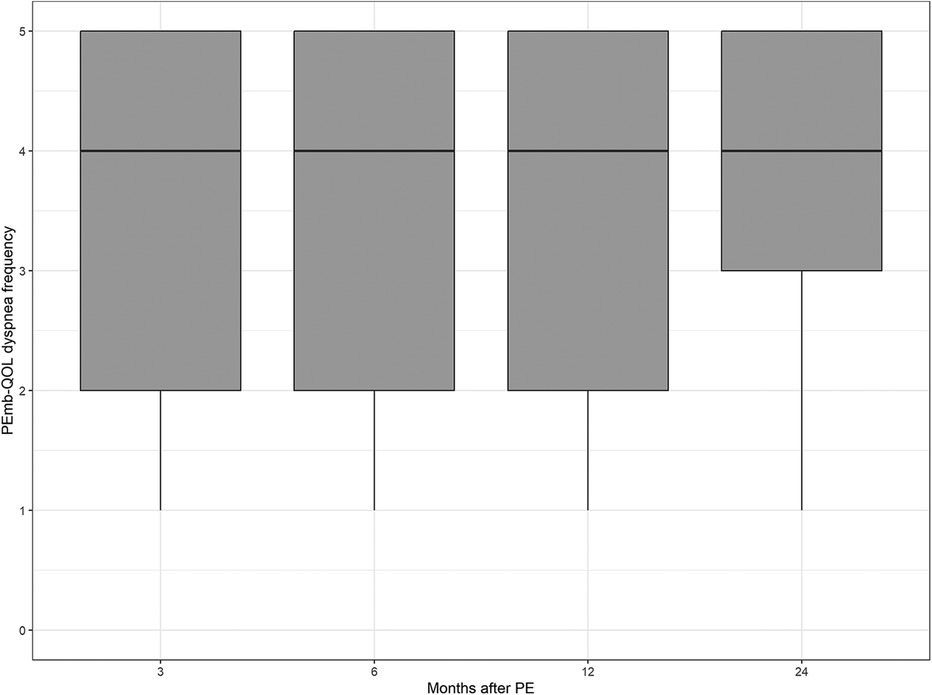
Figure 3. Dyspnea frequency according to the pulmonary embolism quality of life questionnaire over time. No significant differences between follow-up time points (p = 0.5767, Kruskal–Wallis test).
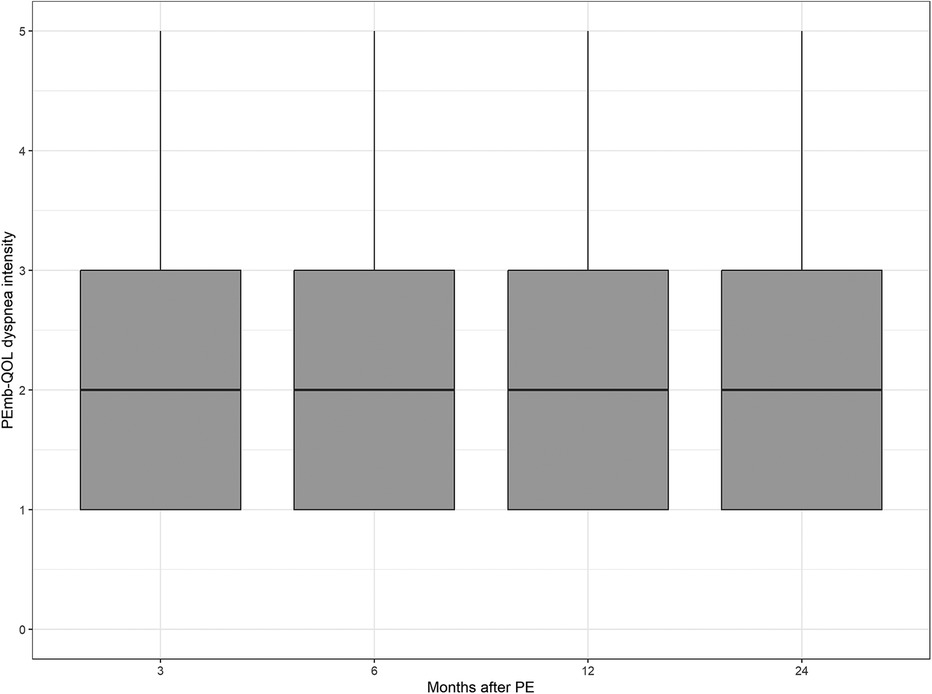
Figure 4. Dyspnea intensity according to the pulmonary embolism quality of life questionnaire over time. No significant differences between follow-up time points (p = 0.2732, Kruskal–Wallis test).
3.3 Factors associated with dyspnea
Since clinical characteristics may be relevant factors associated with dyspnea, the analysis included results of pulmonary function tests and CT pulmonary angiography which were not performed in all participants, leading to a reduced analysis sample of 156 patients. Compared with the total sample, this subgroup was characterized by a lower proportion of patients with a history of thrombophilia or cancer, and a higher proportion of patients with a history of heart failure or sleep apnea. Moreover, this subgroup had a significantly higher BMI and a longer duration of hospitalization.
The results of the mixed models showed that higher CRQ dyspnea scores (indicating less impairment by dyspnea) were significantly associated with low body mass index (estimate −0.04, 95% CI −0.06 to −0.02, p < 0.001), less symptoms of depression (estimate −0.11, 95% CI −0.15 to −0.07, p < 0.0001), less symptoms of anxiety (estimate −0.08, 95% CI −0.11 to −0.04, p < 0.001) and high FEV1 (estimate 0.35, 95% CI 0.10–0.61, p < 0.01) (see Table 2). The follow-up time points were not significantly related with the CRQ dyspnea scores, therefore a stability of dyspnea within the interval of 3 months to 2 years after the acute PE can be assumed.
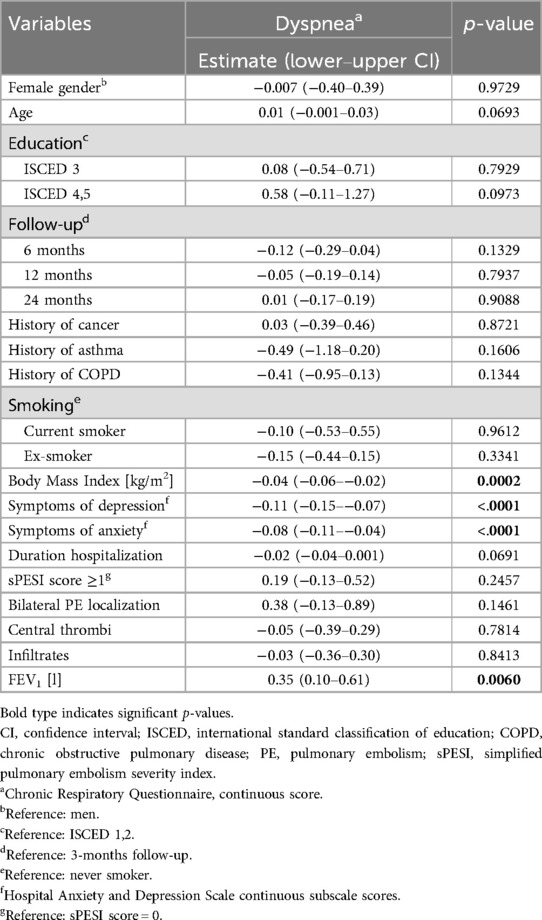
Table 2. Variables associated with dyspnea (chronic respiratory questionnaire): results of the mixed models (n = 156, 442 observations).
Additional analyses using the single PEmb-QOL items on dyspnea frequency and intensity confirmed the significant association of dyspnea with body mass index and symptoms of depression (see Supplementary Tables S2, S3), whereas FEV1 was not found to be significantly related with dyspnea frequency and intensity. History of asthma was significantly related with dyspnea frequency but not with intensity, and symptoms of anxiety were significantly related with dyspnea intensity, but not with dyspnea frequency.
3.4 Association between dyspnea and PE-specific HRQOL
The CRQ dyspnea score was independently and significantly associated with all PEmb-QOL subscales. High levels of dyspnea were associated with more “Activities of daily living limitations” (estimate −11.41, 95% CI −12.30 to −10.52, p < 0.0001), more “Work-related problems” (estimate −13.54, 95% CI −15.29 to −11.80, p < 0.0001), more “Social limitations” (estimate −10.13, 95% CI −11.13 to −9.14, p < 0.0001), and more “Emotional complaints” (estimate −4.02, 95% CI −4.61 to −3.44, p < 0.0001) (see Table 3). All models were adjusted for age, gender, education level, history of cancer, history of asthma, history of COPD, history of depression, smoking, body mass index, symptoms of anxiety and depression, duration of hospitalization, sPESI score, and follow-up time point.
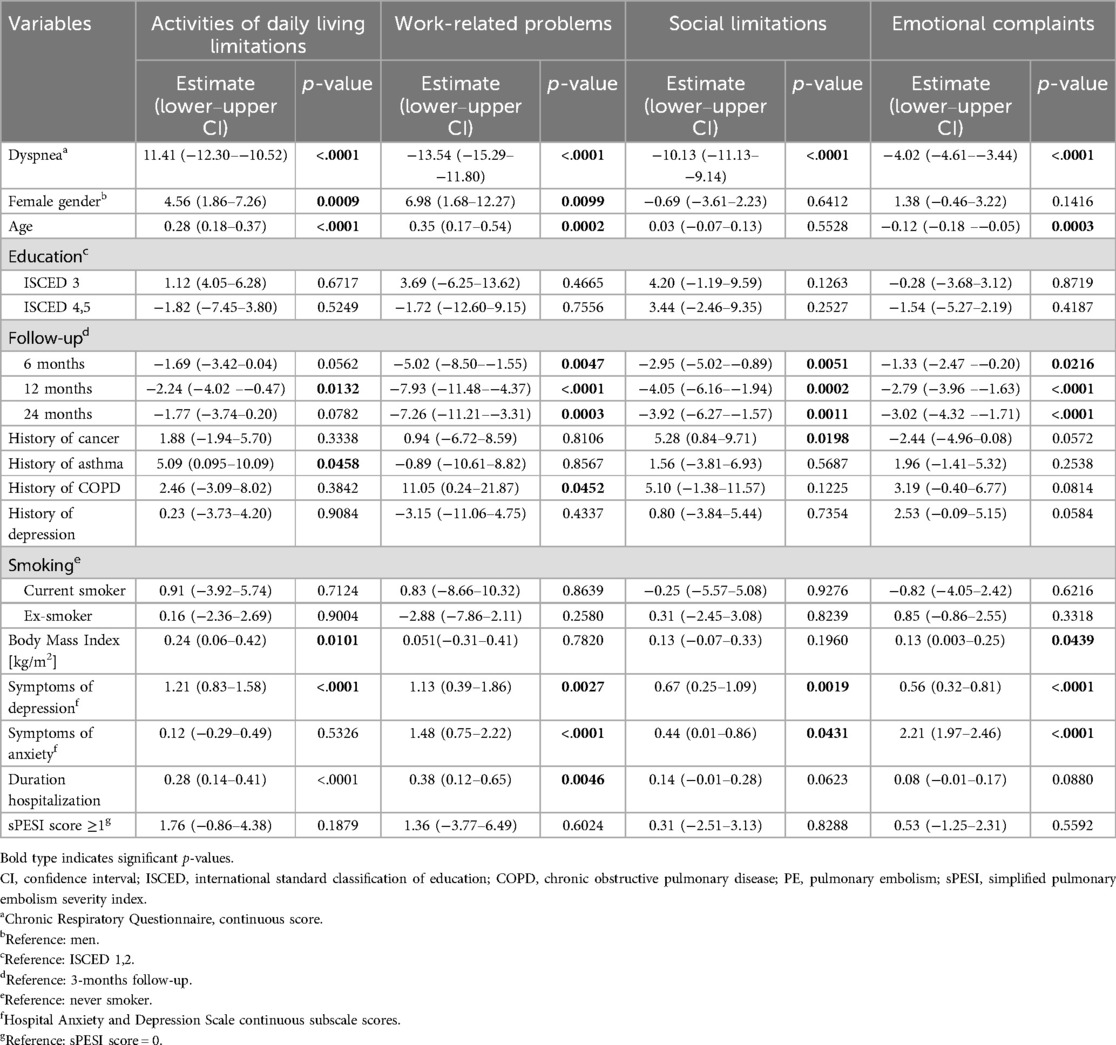
Table 3. Association between dyspnea (chronic respiratory questionnaire) and the PEmbQol subscales: results of the mixed models (n = 440).
When using the single PEmb-QOL items on dyspnea frequency and intensity as independent variables instead of the CRQ dyspnea score, the associations between dyspnea and all PEmb-QOL subscales remained significant (p < 0.0001).
4 Discussion
The present study showed that dyspnea—specifically dyspnea when walking or doing household chores—is a common problem in the time interval between 3 months and 2 years after acute PE. Even 2 years after acute PE only less than one half of the patients reported to be free of dyspnea. Furthermore, no significant change of dyspnea during the observation interval was found. High body mass index, symptoms of depression or anxiety, and FEV1 values were significantly associated with the CRQ dyspnea score. Furthermore, dyspnea was significantly and strongly associated with impairments of PE-specific HRQOL.
Depending on the measure used to assess dyspnea and the follow-up time, the present study showed that between 45% and 64% of the participants had dyspnea in some way with 13%–32% having at least moderate intensity of dyspnea. These results confirm findings from previous studies that dyspnea is a very common symptom in the post-acute phase of PE affecting up to 50% of the patients (11, 14, 15, 20, 21).
Besides the high prevalence of dyspnea, we also found no significant improvement of dyspnea over time. Consistent with these results, Farmakis et al. (15) found no significant improvement of the modified Medical Research Council dyspnea scale from 3–12 months after the acute PE event in 255 patients. Kahn at al (25). also reported that the strongest improvement of dyspnea happened in the first month after the acute PE. Farmakis et al. (15) supposed that the missing improvement of dyspnea despite an improvement of the 6-minute walking distance test among their study participants might have psychological reasons. This hypothesis may be supported by Fischer et al. (18) who found a significant association between dyspnea and symptoms of depression and anxiety in their analyses on the predictors of mental health problems after acute PE. However, so far no prior studies have investigated the contribution of symptoms of depression and anxiety to the persistence of dyspnea. Therefore, the results of the present study, where symptoms of depression and anxiety showed the strongest associations with dyspnea irrespective of other demographic and clinical variables are of utmost importance. The association between dyspnea and emotional or psychological states is not a new finding as a number of studies indicated that psychological states can cause or be caused by dyspnea (38, 39). Consequently, it seems reasonable to consider the multidimensional nature of dyspnea, involving the assessment of the sensory-perceptual domain, the affective distress and the symptom burden, in the post-PE patient management in the future (40). In addition, mental health problems such as anxiety and depression should be identified and treated early in the disease course (18).
In general, prior studies failed to identify a consistent set of clinical variables that predict dyspnea in patients with PE (20, 21, 25). Similarly, in the present study variables that indicate PE severity, such as sPESI score, duration of hospitalization, bilateral PE presentation, presence of central thrombi or infiltrates, showed no significant associations with dyspnea. Only low FEV1 values were significantly related with the CRQ dyspnea score, but not with the single items on dyspnea frequency and intensity, which may be a matter of small sample size or overadjustment in the statistical models.
High body mass index is the only variable that was consistently associated with dyspnea in several studies, including the present one (20, 21, 24, 25). Whether weight reduction could improve recovery and dyspnea after PE may be an interesting question for future research.
The present study showed that dyspnea was the strongest contributor to impaired HRQOL over the observation period of two years. Neither clinical factors nor socio-demographic characteristics or current mental health showed such a strong association with the subscales of the PEmb-QoL questionnaire. These results are in line with previous studies (11, 14, 23) and highlight the need for a comprehensive diagnosis and treatment of dyspnea in order to improve recovery and HRQOL, and to minimize the burden associated with acute PE (41).
4.1 Limitations
The main strengths of the present cohort study are the large sample of patients with a first episode of acute PE assessed four times between 3 months and 2 years after their acute PE with good response rates. Other available studies are mostly cross-sectional studies or follow-up studies with smaller sample sizes. Furthermore, the statistical models were adjusted for relevant confounders which were not considered in prior studies, for instance history of depression and current symptoms of depression and anxiety. Moreover, it is the first study which applied three measures of dyspnea in order to examine the validity of the statistical models.
However, some limitations should be considered. Since the instruments for the assessment of dyspnea and disease-specific HRQOL request information on the impairment in everyday life activities after the PE event, it was not appropriate to use them for an in-hospital baseline assessment. Consequently, a baseline assessment shortly after the acute PE is missing. Information about the presence of dyspnea prior to the acute PE event is also missing. However, such an effect might have been sufficiently considered by adjustment for history of asthma and COPD. Although many relevant confounders were included in the multivariable regression models, some potentially relevant confounders, e.g., pulmonary artery systolic pressure and diagnosis of chronic embolic pulmonary hypertension, were not assessed.
Despite excellent response rates above 90% in the first year of follow-up, the analyzed sample is not representative for the 977 patients initially included in the study. Many of those patients have disagreed in a follow-up and these are expected to be more severely ill than those patients who agreed to complete follow-up surveys. This selection bias may have also resulted in an underestimation of HRQOL impairments and dyspnea frequency and severity.
Patients were recruited from a single university hospital in southern Germany which may restrict external validity. Finally, due to the study design, no causal effects can be derived from the results.
4.2 Conclusions
In conclusion, the present study showed that dyspnea is a very common and persisting symptom that considerably impairs the patients' HRQOL and is strongly associated with symptoms of depression and anxiety. These results highlight the need for a comprehensive diagnostic and interventional strategy to improve dyspnea and the psychological factors, that may cause or maintain dyspnea.
Data availability statement
The data that support the findings of this study are available from the Chair of Epidemiology, Medical Faculty, University of Augsburg but restrictions apply to the availability of these data, which are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Chair of Epidemiology. Requests to access the datasets should be directed toQ2hyaXN0YS5NZWlzaW5nZXJAbWVkLnVuaS1hdWdzYnVyZy5kZQ==.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Ludwig-Maximilians-Universität München. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IK: Conceptualization, Formal analysis, Methodology, Writing – original draft. SF: Formal analysis, Methodology, Writing – review & editing. TB: Conceptualization, Funding acquisition, Investigation, Resources, Writing – review & editing. JL: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. CM: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the participants of the study and all members of the Chair of Epidemiology of the University of Augsburg and the Department of Cardiology, Respiratory Medicine and Intensive Care at the University Hospital Augsburg who were involved in conducting the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1595705/full#supplementary-material
References
1. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. (2014) 34:2363–71. doi: 10.1161/ATVBAHA.114.304488
2. Barco S, Mahmoudpour SH, Valerio L, Klok FA, Münzel T, Middeldorp S, et al. Trends in mortality related to pulmonary embolism in the European region, 2000–15: analysis of vital registration data from the WHO mortality database. Lancet Respir Med. (2020) 8(3):277–87. doi: 10.1016/S2213-2600(19)30354-6
3. Glazier CR, Baciewicz FA Jr. Epidemiology, etiology, and pathophysiology of pulmonary embolism. Int J Angiol. (2024) 33(2):76–81. doi: 10.1055/s-0044-1785487
4. Stein PD, Matta F, Hughes PG, Hughes MJ. Nineteen-Year trends in mortality of patients hospitalized in the United States with high-risk pulmonary embolism. Am J Med. (2021) 134(10):1260–4. doi: 10.1016/j.amjmed.2021.01.026
5. Hoskin S, Brieger D, Chow V, Kritharides L, Ng ACC. Trends in acute pulmonary embolism admission rates and mortality outcomes in Australia, 2002–2003 to 2017–2018: a retrospective cohort study. Thromb Haemost. (2021) 121(9):1237–45. doi: 10.1055/s-0041-1725932
6. Lehnert P, Lange T, Moller CH, Olsen PS, Carlsen J. Acute pulmonary embolism in a national Danish cohort: increasing incidence and decreasing mortality. Thromb Haemost. (2018) 118:539–46. doi: 10.1160/TH17-08-0531
7. Dentali F, Ageno W, Pomero F, Fenoglio L, Squizzato A, Bonzini M. Time trends and case fatality rate of in-hospital treated pulmonary embolism during 11 years of observation in northwestern Italy. Thromb Haemost. (2016) 115:399–405. doi: 10.1160/th15-02-0172
8. Keller K, Hobohm L, Ebner M, Kresoja KP, Munzel T, Konstantinides SV, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. (2020) 41:522–9. doi: 10.1093/eurheartj/ehz236
9. Bunte MC, Gosch K, Elkaryoni A, Noman A, Johnson E, Jones P, et al. Bleeding, death, and costs of care during hospitalization for acute pulmonary embolism: insights from the Saint Luke's outcomes of pulmonary embolism (SLOPE) study. Vasc Med. (2021) 26(1):28–37. doi: 10.1177/1358863X20967415
10. Nilsson LT, Andersson T, Larsen F, Lang IM, Liv P, Söderberg S. Dyspnea after pulmonary embolism: a nation-wide population-based case-control study. Pulm Circ. (2021) 11(4):20458940211046831. doi: 10.1177/20458940211046831
11. Keller K, Tesche C, Gerhold-Ay A, Nickels S, Klok FA, Rappold L, et al. Quality of life and functional limitations after pulmonary embolism and its prognostic relevance. J Thromb Haemost. (2019) 17(11):1923–34. doi: 10.1111/jth.14589
12. Luijten D, de Jong CMM, Ninaber MK, Spruit MA, Huisman MV, Klok FA. Post-pulmonary embolism syndrome and functional outcomes after acute pulmonary embolism. Semin Thromb Hemost. (2023) 49(8):848–60. doi: 10.1055/s-0042-1749659
13. Sista AK, Miller LE, Kahn SR, Kline JA. Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: systematic review with meta-analysis. Vasc Med. (2017) 22(1):37–43. doi: 10.1177/1358863X16670250
14. Tavoly M, Wik HS, Sirnes PA, Jelsness-Jørgensen LP, Ghanima JP, Klok FA, et al. The impact of post-pulmonary embolism syndrome and its possible determinants. Thromb Res. (2018) 171:84–91. doi: 10.1016/j.thromres.2018.09.048
15. Farmakis IT, Valerio L, Barco S, Christodoulou KC, Ewert R, Giannakoulas G, et al. Functional capacity and dyspnea during follow-up after acute pulmonary embolism. J Thromb Haemost. (2024) 22(1):163–71. doi: 10.1016/j.jtha.2023.08.024
16. Gonzalez-Hermosillo LM, Cueto-Robledo G, Navarro-Vergara DI, Garcia-Cesar M, Torres-Rojas MB, Graniel-Palafox LE, et al. Post-pulmonary embolism syndrome: a reminder for clinicians. Asian Cardiovasc Thorac Ann. (2024) 32(5):336–44. doi: 10.1177/02184923241272913
17. Fountain JH, Peck TJ, Furfaro D. Sequelae of acute pulmonary embolism: from post-pulmonary embolism functional impairment to chronic thromboembolic disease. J Clin Med. (2024) 13(21):6510. doi: 10.3390/jcm13216510
18. Fischer S, Meisinger C, Linseisen J, Berghaus TM, Kirchberger I. Depression and anxiety up to two years after acute pulmonary embolism: prevalence and predictors. Thromb Res. (2023) 222:68–74. doi: 10.1016/j.thromres.2022.12.013
19. Gkena N, Kirgou P, Gourgoulianis KI, Malli F. Mental health and quality of life in pulmonary embolism: a literature review. Adv Respir Med. (2023 20) 91(2):174–84. doi: 10.3390/arm91020015
20. Jervan Ø, Gleditsch J, Tavoly M, Klok FA, Rashid D, Holst R, et al. Pulmonary and cardiac variables associated with persistent dyspnea after pulmonary embolism. Thromb Res. (2021) 201:90–9. doi: 10.1016/j.thromres.2021.02.014
21. Alblas H, van Kan C, van Het Westeinde SC, Emmering J, Niezen A, Al Butaihi IAM, et al. Persistent dyspnea after acute pulmonary embolism is related to perfusion defects and lower long-term quality of life. Thromb Res. (2022) 219:89–94. doi: 10.1016/j.thromres.2022.09.008
22. Kirchberger I, Ruile S, Linseisen J, Haberl S, Meisinger C, Berghaus TM. The lived experience with pulmonary embolism: a qualitative study using focus groups. Respir Med. (2020) 167:105978. doi: 10.1016/j.rmed.2020.105978
23. Tavoly M, Utne KK, Jelsness-Jørgensen LP, Wik HS, Klok FA, Sandset PM, et al. Health-related quality of life after pulmonary embolism: a cross-sectional study. BMJ Open. (2016) 6(11):e013086. doi: 10.1136/bmjopen-2016-013086
24. Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Huisman MV. Prevalence and potential determinants of exertional dyspnea after acute pulmonary embolism. Respir Med. (2010 Nov) 104(11):1744–9. doi: 10.1016/j.rmed.2010.06.006
25. Kahn SR, Akaberi A, Granton JT, Anderson DR, Wells PS, Rodger MA, et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: results of the ELOPE cohort study. Am J Med. (2017) 130(8):990.e9–990.e21. doi: 10.1016/j.amjmed.2017.03.033
26. Sanchez O, Caumont-Prim A, Riant E, Plantier L, Dres M, Louis B, et al. Pathophysiology of dyspnoea in acute pulmonary embolism: a cross-sectional evaluation. Respirology. (2017) 22(4):771–7. doi: 10.1111/resp.12961
27. Meisinger C, Linseisen J, Kirchberger I, von Scheidt W, Berghaus TM. Long-term outcomes in patients with acute pulmonary embolism after in-hospital treatment: study protocol of the prospective Lungenembolie Augsburg Studie (LEA study). BMJ Open. (2019) 9(10):e031411. doi: 10.1136/bmjopen-2019-031411
28. Righini M, Roy PM, Meyer G, Verschuren F, Aujesky D, Le Gal G. The simplified pulmonary embolism severity Index (PESI): validation of a clinical prognostic model for pulmonary embolism. J Thromb Haemost. (2011) 9(10):2115–7. doi: 10.1111/j.1538-7836.2011.04469.x
29. UNESCO Institute for Statistics. International Classification of Education, ISCED 2011 (2012). Available online at: https://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf (Accessed June 30, 2025).
30. Puhan MA, Behnke M, Frey M, Grueter T, Brandli O, Lichtenschopf A, et al. Self-administration and interviewer-administration of the German chronic respiratory questionnaire: instrument development and assessment of validity and reliability in two randomised studies. Health Qual Life Outcomes. (2004) 2:1. doi: 10.1186/1477-7525-2-1
31. Gwozdz AM, de Jong CMM, Fialho LS, Likitabhorn T, Sossi F, Jaber PB, et al. Development of an international standard set of outcome measures for patients with venous thromboembolism: an international consortium for health outcomes measurement consensus recommendation. Lancet Haematol. (2022) 9(9):e698–706. doi: 10.1016/S2352-3026(22)00215-0
32. Frey PM, Méan M, Limacher A, Leiss W, Schwab N, Rochat M, et al. Quality of life after pulmonary embolism: prospective validation of the German version of the PEmb-QoL questionnaire. Thromb Res. (2015) 135(6):1087–92. doi: 10.1016/j.thromres.2015.03.031
33. Fischer S, Meisinger C, Linseisen J, von Scheidt W, Berghaus TM, Kirchberger I. The German version of the pulmonary embolism quality of life (PEmb-QoL) questionnaire: reliability, responsiveness and structural validity. Qual Life Res. (2022) 31(7):2235–45. doi: 10.1007/s11136-022-03120-3
34. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. (2002) 52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3
35. Herrmann C. International experiences with the hospital anxiety and depression scale–a review of validation data and clinical results. J Psychosom Res. (1997) 42(1):17–41. doi: 10.1016/s0022-3999(96)00216-4
36. Hermann-Lingen C, Buss U, Snaith RP. Hospital Anxiety and Depression Scale—deutsche Version (HADS-D). Bern: Verlag Hans Huber (2011).
37. Textor J, Hardt J. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. (2011) 22(5):745. doi: 10.1097/EDE.0b013e318225c2be
38. Basara L, Jokić Begić N, Popović Grle S, Jakopović M, SamarŽija M. Dyspnea from neuropsychyatric perspective: a narrative review. Psychiatr Danub. (2018) 30(1):11–20. doi: 10.24869/psyd.2018.11
39. Marines-Price R, Bernhardt V, Bhammar DM, Babb TG. Dyspnea on exertion provokes unpleasantness and negative emotions in women with obesity. Respir Physiol Neurobiol. (2019) 260:131–6. doi: 10.1016/j.resp.2018.11.008
40. Laveneziana P, Similowski T, Morelot-Panzini C. Multidimensional approach to dyspnea. Curr Opin Pulm Med. (2015) 21(2):127–32. doi: 10.1097/MCP.0000000000000134
41. Klok FA, Ageno W, Ay C, Bäck M, Barco S, Bertoletti L, et al. Optimal follow-up after acute pulmonary embolism: a position paper of the European society of cardiology working group on pulmonary circulation and right ventricular function, in collaboration with the European Society of Cardiology working group on atherosclerosis and vascular biology, endorsed by the European respiratory society. Eur Heart J. (2022) 43(3):183–9. doi: 10.1093/eurheartj/ehab816
Keywords: pulmonary embolism, dyspnea, quality of life, depression, anxiety
Citation: Kirchberger I, Fischer S, Berghaus TM, Linseisen J and Meisinger C (2025) Dyspnea after a first episode of pulmonary embolism: prevalence, predictors and long-term associations with health-related quality of life. Front. Cardiovasc. Med. 12:1595705. doi: 10.3389/fcvm.2025.1595705
Received: 18 March 2025; Accepted: 20 June 2025;
Published: 7 July 2025.
Edited by:
Hugo Hyung Bok Yoo, Sao Paulo State University, BrazilReviewed by:
Marcelo Gazzana, Hospital de Clinicas de Porto Alegre, BrazilFüsun Fakili, University of Gaziantep, Türkiye
Copyright: © 2025 Kirchberger, Fischer, Berghaus, Linseisen and Meisinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inge Kirchberger, aW5nZS5raXJjaGJlcmdlckBtZWQudW5pLWF1Z3NidXJnLmRl
 Inge Kirchberger
Inge Kirchberger Simone Fischer
Simone Fischer Thomas M. Berghaus2,3
Thomas M. Berghaus2,3 Jakob Linseisen
Jakob Linseisen