Abstract
Turner syndrome (TS) is the most common sex chromosome abnormality disorder, caused by complete or partial absence of the X chromosome, its clinical manifestations primarily include short stature, gonadal dysgenesis, and characteristic cardiovascular malformations, pediatric cardiologists pay particular attention to coarctation of the aorta (CoA), which occurs in 15%–30% of TS patients and represents a life-threatening condition requiring prioritized screening during the neonatal and childhood periods (1– 3). Furthermore, due to lymphatic system developmental abnormalities, TS patients also face elevated risks of aortic root dilation, bicuspid aortic valve, and vascular structural anomalies (3). Pulmonary arteriovenous malformation (PAVM) is a rare pulmonary vascular anomaly, with an estimated prevalence of approximately 1 in 50,000 in the general population (1–3). Although the exact prevalence of PAVM in TS patients remains unclear, case series suggest a significantly elevated risk compared to the general population (estimated risk ratio: 5- to 10-fold), this association may be attributed to defective vascular elastic fiber development and dysregulated angiogenic signaling pathways in TS patients (4, 5). Here, we report the first documented case of TS complicated by PAVM, aiming to enhance clinicians' awareness of this rare comorbidity and provide evidence-based diagnostic and therapeutic recommendations.
Introduction
Turner syndrome (TS) is the most common sex chromosome abnormality disorder, caused by complete or partial absence of the X chromosome, its clinical manifestations primarily include short stature, gonadal dysgenesis, and characteristic cardiovascular malformations, pediatric cardiologists pay particular attention to coarctation of the aorta (CoA), which occurs in 15%–30% of TS patients and represents a life-threatening condition requiring prioritized screening during the neonatal and childhood periods (1–3). Furthermore, due to lymphatic system developmental abnormalities, TS patients also face elevated risks of aortic root dilation, bicuspid aortic valve, and vascular structural anomalies (3).
Pulmonary arteriovenous malformation (PAVM) is a rare pulmonary vascular anomaly, with an estimated prevalence of approximately 1 in 50,000 in the general population, PAVM can reduce blood oxygen saturation, increase cardiac output, lead to embolism or hemorrhage, and in the long term, may result in hypoxemia and secondary polycythemia (1–3). Although the exact prevalence of PAVM in TS patients remains unclear, case series suggest a significantly elevated risk compared to the general population (estimated risk ratio: 5- to 10-fold), this association may be attributed to defective vascular elastic fiber development and dysregulated angiogenic signaling pathways in TS patients (4, 5).
Due to the unique circulatory and lymphatic system abnormalities in patients with Turner syndrome,they have an increased risk of arteriovenous malformations, which may manifest as more severe or more subtle clinical symptoms. Here we present a rare case of Turner Syndrome with pulmonary arteriovenous malformation, to date, no previous cases of this kind have been published, we share this case to raise clinical awareness of this rare variation and emphasize the importance of early diagnosis.
Case report
A 13-year-old female patient with Turner syndrome (TS), undergoing growth hormone and estrogen replacement therapy, was found to have a grade 3/6 systolic murmur at the left sternal border (second to third intercostal space) during routine physical examination. She had no prior history of cyanosis or exercise intolerance, with resting oxygen saturation (SpO2) of 96% (declining to 94% post-exertion). Transthoracic echocardiography revealed anomalous drainage of the left upper pulmonary vein, and computed tomography angiography (CTA) confirmed the presence of a left lower lobe pulmonary arteriovenous malformation (PAVM) with a feeding artery diameter of 5 mm (Figure 1, Supplementary Movie). According to current PAVM management guidelines (6), lesions <3 mm in diameter without symptoms may be managed conservatively with surveillance. Thus, a follow-up protocol was established, including CTA and transthoracic echocardiography (TTE) every 6 months. Notably, the patient showed no evidence of coarctation of the aorta or other aortic pathologies (normal aortic arch and root dimensions), ruling out the most common cardiovascular malformations associated with TS. The timing of murmur onset during puberty, coupled with CTA findings demonstrating congenital vascular malformation characteristics, suggests that this PAVM likely represents a congenital lesion that became hemodynamically significant due to adolescent circulatory changes.
Figure 1
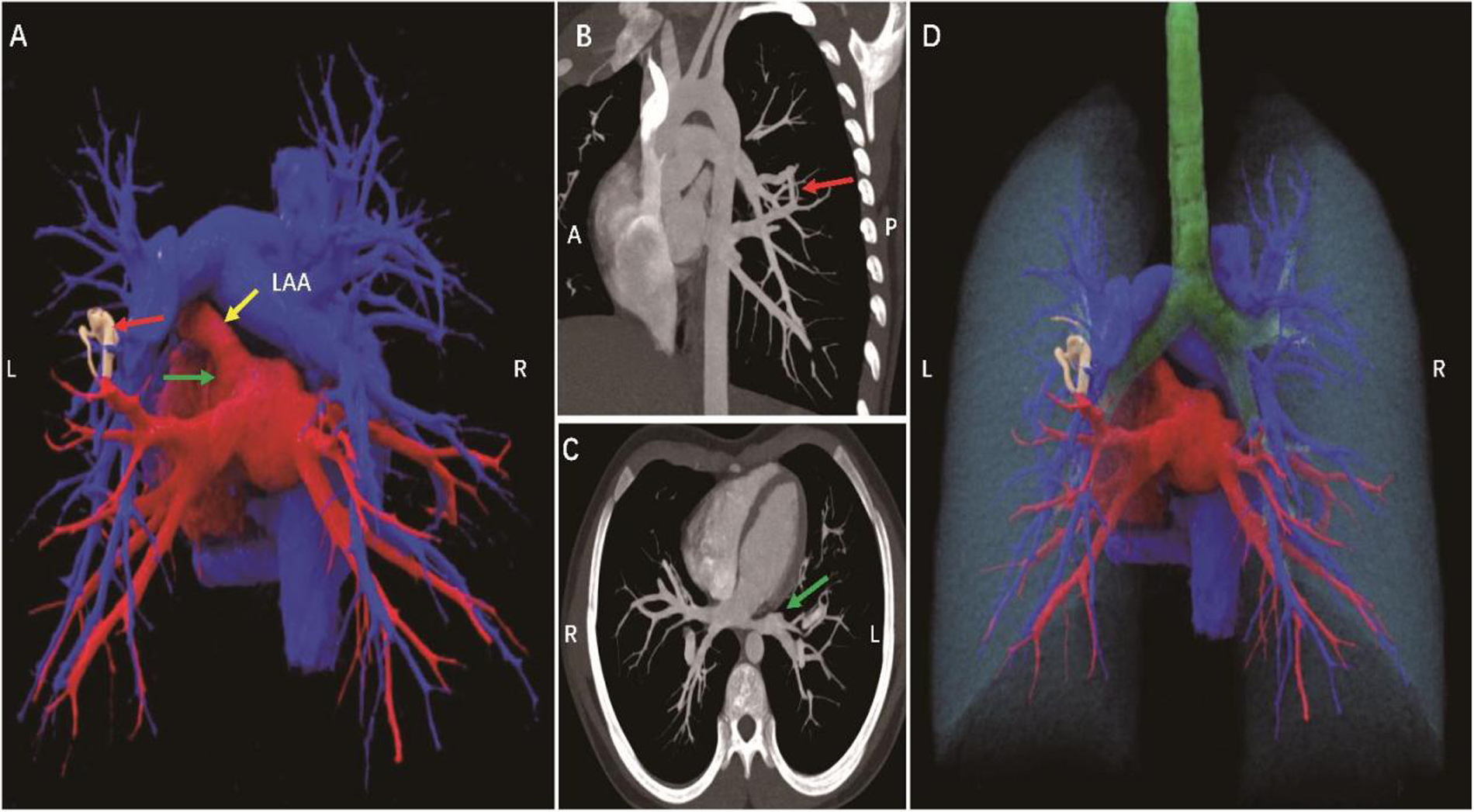
A 13-year-old female patient, pulmonary arteriovenous malformation (A). Cinematic rendering shows an abnormal vascular shadow with tortuous running and uneven thickness between the left lower pulmonary artery and the proximal left upper pulmonary vein (red arrow) (B,C). CT MIP images show that the pulmonary artery is directly connected to the vein, forming a traffic (red arrow), and the left upper pulmonary vein is not seen (green arrow). (D) Cinematic rendering clearly shows the spatial position relationship between the lung, pulmonary artery, pulmonary vein, trachea and the lesion site. A, anterior; P, posterior; L, left; R, right; LAA, left atrial appendage.
Discussion
This case highlights the need for clinicians to maintain vigilance for atypical vascular malformations when evaluating Turner syndrome (TS) patients, particularly in the presence of: (1) unexplained cardiac murmurs; (2) abnormal oxygen saturation; or (3) decreased exercise tolerance. Computed tomography angiography (CTA) represents the diagnostic gold standard for pulmonary arteriovenous malformations (PAVMs), while management decisions should integrate lesion size, symptomatology, and complication risks.
PAVMs carry significant mortality if untreated. Endovascular embolization serves as first-line therapy for all PAVMs with feeding vessels >3 mm in diameter. Current guidelines recommend proactive intervention for all technically amenable PAVMs, particularly symptomatic cases or those demonstrating right-to-left shunting, regardless of feeding artery diameter (7). Asymptomatic small lesions (such as in this case) warrant regular imaging surveillance to monitor progression.
Notably, the literature contains extremely few reports of TS coexisting with PAVM. This rarity underscores both the exceptional nature of our case and suggests potential underrecognition of pulmonary vascular anomalies in TS patients, whose vascular screening protocols may currently focus predominantly on aortic pathologies. Future large-scale cohort studies are needed to establish the true prevalence and natural history of PAVMs in TS populations.
This study has several limitations: As a single case report, it cannot establish the true association strength between TS and PAVM;the absence of long-term follow-up data precludes accurate assessment of lesion progression risk; genetic sequencing was not performed, leaving potential synergistic genetic mutations contributing to vascular maldevelopment undetermined. Future studies should accumulate additional cases and incorporate genetic analyses to elucidate the molecular mechanisms underlying rare vascular anomalies in TS patients.
Conclusion
This case represents the first confirmed report of pulmonary arteriovenous malformation (PAVM) co-occurring with Turner syndrome (TS), suggesting that PAVM should be included in the differential diagnosis of cardiovascular complications when evaluating TS patients. For patients presenting with cardiac murmurs or hypoxemia, contrast-enhanced computed tomography angiography (CTA) is recommended to rule out PAVM. In the absence of TS-specific evidence, therapeutic decisions should be based on existing PAVM management guidelines for the general population, while incorporating individualized treatment strategies tailored to each patient's clinical profile.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Research Ethics Committee of Children's Hospital Affiliated to Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
HG: Writing – original draft. HC: Writing – original draft. SC: Writing – original draft. ST: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1603250/full#supplementary-material
References
1.
BondyCBakalovVKChengCOlivieriLRosingDRAraiAE. Bicuspid aortic valve and aortic coarctation are linked to deletion of the X chromosome short arm in turner syndrome. J Med Genet. (2013) 50(10):662–5. 10.1136/jmedgenet-2013-101720
2.
SachdevVMaturaLASidenkoSHoVBAraiAERosingDRet alAortic valve disease in turner syndrome. J Am Coll Cardiol. (2008) 51(19):1904–9. 10.1016/j.jacc.2008.02.035
3.
OlivieriLJBabaRYAraiAEBandettiniWPRosingDRBakalovVet alSpectrum of aortic valve abnormalities associated with aortic dilation across age groups in turner syndrome. Circ Cardiovasc Imaging. (2013) 6(6):1018–23. 10.1161/CIRCIMAGING.113.000526
4.
LinAELippeBMGeffnerMEGomesALoisJFBartonCWet alAortic dilation, dissection, and rupture in patients with turner syndrome. J Pediatr. (1986) 109(5):820–6. 10.1016/S0022-3476(86)80700-4
5.
Kuiper-MakrisC. Dysregulation of angiogenic signaling and reduced elastic fibers are associated with impaired formation of microvessels in rat lungs after intrauterine growth retardation (doctoral dissertation). Universität zu Köln (2021).
6.
ShovlinCL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med. (2014) 190(11):1217–28. 10.1164/rccm.201407-1254CI
7.
ZhangJTongX-MWangX-MXingY. Sudden convulsion with multiple pulmonary nodules in a girl aged 15 years. Zhongguo Dangdai Erke Zazhi=Chin J Contemp Pediatr. (2021) 23(3):288–93. 10.7499/j.issn.1008-8830.2010094
Summary
Keywords
TS, PAVM, children, CTA, heart
Citation
Guo H, Chen H, Chen S and Tang S (2025) Turner syndrome with pulmonary arteriovenous malformation: a case report. Front. Cardiovasc. Med. 12:1603250. doi: 10.3389/fcvm.2025.1603250
Received
31 March 2025
Accepted
05 August 2025
Published
28 August 2025
Volume
12 - 2025
Edited by
DeLisa Fairweather, Mayo Clinic Florida, United States
Reviewed by
Almudena Ortiz Garrido, Regional University Hospital of Malaga, Spain
Bertrand Tchana, The University Hospital of Parma, Italy
Updates
Copyright
© 2025 Guo, Chen, Chen and Tang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shilong Tang sltang66@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.