- 1Jiangxi Medical College, Nanchang University, Nanchang, China
- 2Jiangxi Cardiovascular Research Institute, Jiangxi Provincial People's Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
- 3Department of Cardiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
- 4Department of Cardiovascular Surgery, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
Objective: Acute decompensated heart failure (ADHF) is the most common and severe type of HF. The aim of this study is to evaluate the impact and predictive value of a novel inflammatory marker, the inflammatory burden index (IBI), on the 30-day mortality and adverse prognosis in patients with ADHF.
Methods: This retrospective cohort study included 1,241 ADHF patients from Jiangxi Provincial People's Hospital between 2018 and 2024. The IBI was calculated as C-reactive protein × (neutrophil count/lymphocyte count). In the event analysis, the study outcome was defined as the 30-day mortality rate after hospital admission in ADHF patients. Multivariable Cox regression and receiver operating characteristic curve analysis were used to assess the impact and predictive value of the IBI on 30-day mortality. Additionally, subgroup analyses were performed to determine the risk dependency of the IBI within specific populations.
Results: During the 30-day observation period, a total of 108 death events (8.70%) were recorded. When the study population was stratified into tertiles based on the IBI, the 30-day mortality rates were 1.93%, 4.60%, and 19.57%, respectively. Multivariable Cox regression analysis revealed a significant positive association between the IBI and 30-day mortality in ADHF patients (HR per SD increase: 1.29, 95% CI: 1.15–1.46). Compared to ADHF patients with a low IBI (T1), those with a high IBI (T3) showed a 368% higher risk of 30-day mortality (HR: 4.68, 95% CI: 1.06–13.73). Subgroup analysis revealed a significant interaction between the IBI and 30-day mortality in ADHF patients across sex subgroups (P-interaction < 0.05). In particular, compared to male patients, female ADHF patients exhibited a significantly higher risk of IBI-related in-hospital all-cause mortality (HR: 1.52 vs. 1.33). Receiver operating characteristic analysis further demonstrated that the novel inflammatory marker IBI had the highest AUC value (0.80) compared to conventional inflammatory markers, including C-reactive protein, white blood cell count, neutrophil count, lymphocyte count, and monocyte count.
Conclusion: The cohort study conducted in Jiangxi, China, revealed that the novel inflammatory marker IBI is significantly positively associated with 30-day mortality in ADHF patients and demonstrated strong predictive value. Incorporating IBI into the clinical management of ADHF patients may hold significant potential for preventing further disease deterioration.
Graphical Abstract. Explanation: In this graphic abstract, we drew inspiration from characters in Journey to the West, a classic Chinese literary masterpiece. Here, the “White Bone Demon” (Bai Gu Jing; a shape-shifting demoness) symbolizes ADHF patients, while 'Sun Wukong' (the Monkey King) represents physicians. As depicted, the physician, akin to Sun Wukong, employs his fiery golden eyes (a metaphor for clinical acumen) to identify the IBI (represented by a skull icon) as the key risk factor in the ADHF patient.
Introduction
With the intensification of global aging, heart failure (HF) is becoming an increasingly serious public health issue. According to the Global Burden of Disease data report, the number of HF patients worldwide has exceeded 55 million as of 2021 (1). Among the various types of HF, Acute decompensated HF (ADHF) represents the most prevalent and severe form, characterized by new or worsening clinical symptoms and signs of HF (2–4). ADHF is not only one of the most frequent causes of hospitalization among the elderly population but is also associated with a significantly elevated risk of short-term adverse clinical outcomes. Studies have demonstrated that ADHF has an in-hospital mortality rate of approximately 5.3%–7.5% and a one-year mortality rate of around 25%, imposing a significant disease burden on both patients and society (4–8). Despite recent key advancements in the treatment of HF, the management of ADHF patients remains one of the greatest challenges for cardiologists (9, 10). Therefore, it is crucial to identify clinically useful biomarkers that can predict the prognosis of ADHF at an early stage, thereby optimizing clinical decision-making.
Inflammation plays a critical role in HF progression through multiple mechanisms. Compared to chronic HF, inflammatory activation is more pronounced in acute HF patients, and inflammatory levels are significantly associated with adverse outcomes (11–16). Therefore, early assessment of inflammation holds significant importance for ADHF patients. In recent years, a novel inflammatory indicator known as the inflammatory burden index (IBI), calculated based on C-reactive protein (CRP), neutrophil count, and lymphocyte count, has garnered the attention of numerous researchers. They have discovered that IBI may possess high application potential as an inflammatory indicator and holds significant value in the prognostic assessment of various chronic and oncological diseases (17–32). For chronic diseases, existing research evidence indicates that the IBI is applicable to the prognostic assessment of osteoarthritis, rheumatoid arthritis, inflammatory airway diseases, ischemic stroke, and intracerebral hemorrhage (28–32). IBI has also been identified as an independent risk factor for cardiovascular diseases and can be utilized for risk assessment in HF, angina pectoris, coronary heart disease (CHD), and stroke (33). Given that the progression of ADHF is significantly associated with the activation of inflammation (11–16), further elucidating the relationship between the IBI and ADHF prognosis may provide valuable insights for disease management. To address this issue, this study aims to evaluate the impact and predictive value of IBI on 30-day mortality prognosis in ADHF patients using the ADHF cohort from Jiangxi, China.
Methods
Study population and design
The data used in this survey comes from Jiangxi-acute decompensated heart failure study II. This is a cohort study initiated by Jiangxi Provincial People's Hospital, consecutively enrolling 3,484 patients with ADHF admitted to the Jiangxi Provincial People's Hospital from January 2018 to January 2024. The primary objective of this project is to establish a high-quality cohort of ADHF patients, effectively utilize their clinical record data during hospitalization, and explore new methods for early risk stratification to improve the adverse prognosis of ADHF patients. In this study, the diagnosis of ADHF was based on the ESC and ACC/AHA/HFSA Heart Failure Guidelines (2, 3), incorporating clinical symptoms, physical signs, and laboratory findings. The diagnostic criteria were as follows: The presence of at least one sign of HF: (a) Elevated N-terminal pro-brain natriuretic peptide (NT-proBNP); (b) Pulmonary edema detected by physical examination or chest x-ray; (c) Abnormal cardiac structure and/or function as indicated by echocardiography. The presence of at least one symptom of worsening HF: (a) Systemic venous congestion; (b) Dyspnea; (c) Insufficient tissue perfusion.
In the current study, we established the following exclusion criteria based on the research objectives: (i) To account for the potential impact of additional fluid and sodium retention, we excluded patients with uremia or a history of hemodialysis (n = 231) and those with liver cirrhosis (n = 42); (ii) Considering the potential influence on life expectancy, we excluded patients with malignant tumors (n = 160); (iii) Due to the significant role of reperfusion therapy in short-term prognosis, participants who had undergone percutaneous coronary intervention (PCI) within the past 3 months were excluded (n = 102); (iv) Participants under the age of 18 (n = 22); (v) Pregnant individuals (n = 4); (vi) Individuals with pacemaker-controlled heart rhythms, as their heart rates were not expected to be regulated by autonomic nervous control (n = 121). Additionally, we excluded participants with missing IBI data (n = 1,561). Ultimately, 1,241 patients with ADHF were included in the analysis. Given the high rate of missing IBI data in this study, we conducted a systematic evaluation of baseline characteristic differences between the complete-case group and the missing-data group prior to formal analysis. As shown in Supplementary Table S1, no statistically significant differences were observed between the two groups across most baseline characteristics (P > 0.05), suggesting that the missing data mechanism aligns with the missing at-random assumption. This finding provides methodological assurance regarding data quality for subsequent analyses. A detailed flowchart of the study population screening process is shown in Figure 1.
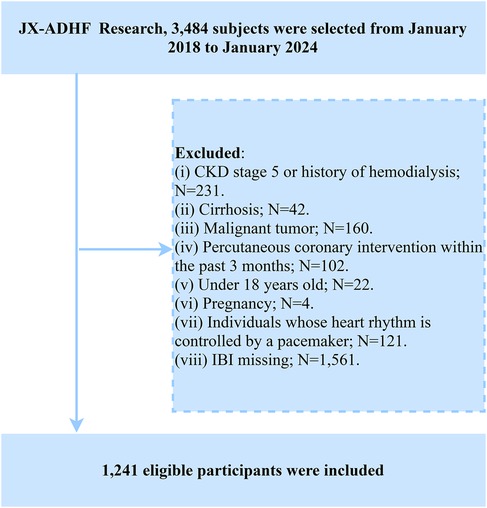
Figure 1. Flow chart for inclusion and exclusion of study participants. ADHF, acute decompensated heart failure; IBI, inflammatory burden index; CKD, chronic kidney disease.
Ethical approval
This study adhered to the ethical principles outlined in the Declaration of Helsinki. The use of research data strictly complied with ethical review requirements, and authorization was obtained from patients and their families. The study protocol was approved by the Ethics Committee of Jiangxi Provincial People's Hospital (IRB: 2024-01). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines to ensure transparency and scientific rigor of the findings (34).
Data collection
The baseline data for this study were collected by two trained researchers from the hospital's electronic medical record system, with cross-verification to ensure accuracy. The specific details are as follows: (i) Demographic and clinical data: sex, age, drinking status, smoking status, comorbidities [including hypertension, diabetes, stroke, and CHD], cardiac function (New York Heart Association classification: NYHA), blood pressure data [measured using an Omron automatic blood pressure monitor (HBP-1300) in a quiet environment or at the bedside] and medication information during hospitalization [Includes the use of beta-blockers, diuretics, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor inhibitors (ARB)/angiotensin receptor neprilysin inhibitors (ARNI), and vasopressor medications]. (ii) Echocardiographic examination: Left ventricular ejection fraction (LVEF). (iii) Laboratory test data: The biochemical indicators measured included albumin, alanine aminotransferase, aspartate aminotransferase (AST), creatinine (Cr), uric acid (UA), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and fasting plasma glucose (FPG). Additionally, other assessed parameters included white blood cell (WBC) count, red blood cell (RBC) count, platelet count, CRP, neutrophil count, lymphocyte count, and NT-proBNP. All blood samples were collected within 24 h of hospital admission, adhering strictly to the timing requirements for laboratory results. For liver enzymes, lipid profiles, and FPG, venous blood samples were collected either at admission under fasting conditions or on the morning of the second day after admission.
IBI calculation
IBI = CRP × (neutrophil count/lymphocyte count) (17).
Study outcomes
The primary endpoint of this study was all-cause mortality within 30 days after hospital admission in patients with ADHF. The 30-day survival status of all participants was tracked by trained medical staff through multiple methods, including text messages, phone calls, and face-to-face follow-ups during outpatient clinics or hospital admissions.
Statistical analysis
All statistical analyses in this study were performed using R software (version 4.2.1) and Empower® software (version 2.0). Statistical significance was defined as a two-sided p-value < 0.05.
First, we stratified ADHF patients into tertiles (low, moderate, and high) based on IBI, which were determined by calculating the 33.33% and 66.67% percentiles of IBI values. Baseline variables were described according to their type and distribution: categorical variables were expressed as counts (%), while continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range), as appropriate. Group differences were analyzed using chi-square tests, one-way ANOVA, or non-parametric tests, as appropriate.
To assess the association between IBI and 30-day all-cause mortality in ADHF patients, we performed Kaplan–Meier analysis to plot survival curves for the three IBI groups. The significance of differences in survival rates among the groups was assessed using the log-rank test. Subsequently, we developed three adjusted Cox proportional hazards regression models to evaluate the association between IBI and 30-day mortality. Model 1 adjusted for baseline information assessed at admission, including sex, age, hypertension, diabetes, stroke, and CHD. Model 2 added NYHA classification, drinking status, smoking status, and LVEF. Based on Model 2, Model 3 was further adjusted for monocyte count, RBC, platelet count, AST, Cr, UA, TC, TG, HDL-C, LDL-C, FPG, and NT-proBNP. The proportional hazards assumption was evaluated using Kaplan–Meier curves for IBI groups and Schoenfeld residual tests, revealing no evidence of violation of this assumption (Figure 2 and Supplementary Figure S1). Additionally, based on collinearity assessments, we confirmed the absence of multicollinearity among covariates in the multivariable regression models (Supplementary Table S2) (35).
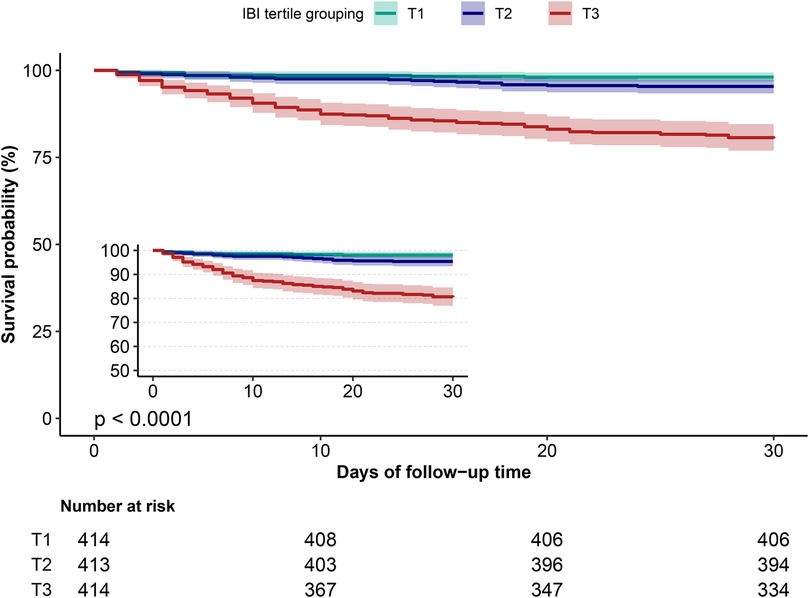
Figure 2. Cumulative survival rate curves of ADHF patients in IBI group. ADHF, acute decompensated heart failure; IBI, inflammatory burden index.
We also performed subgroup analyses to examine whether the association between IBI and 30-day mortality in ADHF patients was consistent across different subgroups. The subgroup variables and detailed stratification were as follows: age (<65 years vs. ≥65 years), sex (male vs. female), LVEF (<50% vs. ≥50%), NYHA classification (class III vs. class IV), hypertension (yes vs. no), diabetes (yes vs. no), stroke (yes vs. no), and CHD (yes vs. no). The significance of interaction effects was assessed using likelihood ratio tests.
To evaluate the predictive ability of IBI, we performed receiver operating characteristic curve analysis to assess the predictive performance of IBI and common inflammatory markers (CRP, neutrophil count, lymphocyte count, monocyte count, and WBC count) for 30-day mortality. The area under the curve (AUC), optimal threshold, sensitivity, and specificity were calculated for each indicator. Differences in AUCs were evaluated using the DeLong test. Additionally, we investigated the incremental predictive value of adding IBI to the established clinical risk model [Acute Decompensated Heart Failure National Registry (ADHFRE)] (36, 37) and calculated the C-index to quantify the improvement in predictive performance.
To ensure the robustness of the study findings, we conducted several sensitivity analyses: (i) Considering the potential impact of acute inflammation, we excluded patients with pulmonary infections at admission and repeated the primary analysis; (ii) To reduce the influence of reverse causality, we excluded participants who died within three days after admission; (iii) Given that hypertension, diabetes, stroke, and CHD are strong risk factors for adverse prognosis in ADHF patients, we excluded patients with these comorbidities and repeated the analysis (38, 39); (iv) For partially missing data (Supplementary Table S3), we performed multiple imputation to estimate missing values and repeated the primary analysis. (v) Medical treatment serves as the cornerstone of ADHF interventions. In subsequent models, we adjusted for ADHF treatment factors including beta-blockers, diuretics, ARB/ACEI/ARNI, and vasopressor agents. (vi) Considering that CHD patients undergoing PCI are typically a susceptible population for ADHF and do not interfere with the prognostic evaluation of IBI, we re-included this patient subgroup in further sensitivity analyses. (vii) To assess the generalizability of our findings, we utilized data from the United States National Health and Nutrition Examination Survey (1998–2018) to examine the association between IBI and all-cause mortality among participants diagnosed with congestive HF.
Results
Baseline characteristics
Among the 1,241 ADHF patients who met the study criteria, 720 were male and 522 were female, with a mean age of 68 years. The baseline characteristics of ADHF patients stratified by IBI tertiles are summarized in Table 1. Compared to patients in the low IBI group, those in the high IBI group were more likely to be male, older, and have a higher prevalence of diabetes, CHD, and NYHA Class IV. Additionally, they exhibited higher levels of CRP, WBC count, neutrophil count, monocyte count, AST, Cr, UA, FPG, and NT-proBNP, as well as lower levels of lymphocyte count, RBC count, TC, HDL-C, and LDL-C (All p < 0.05). Additionally, regarding treatment, compared to patients with low IBI, high IBI patients demonstrated a significantly lower proportion of ACEI/ARB/ARNI use (p = 0.015) and a markedly higher utilization rate of vasopressors (p < 0.001), while no significant differences were observed in diuretic or beta-blocker administration (both p > 0.05).
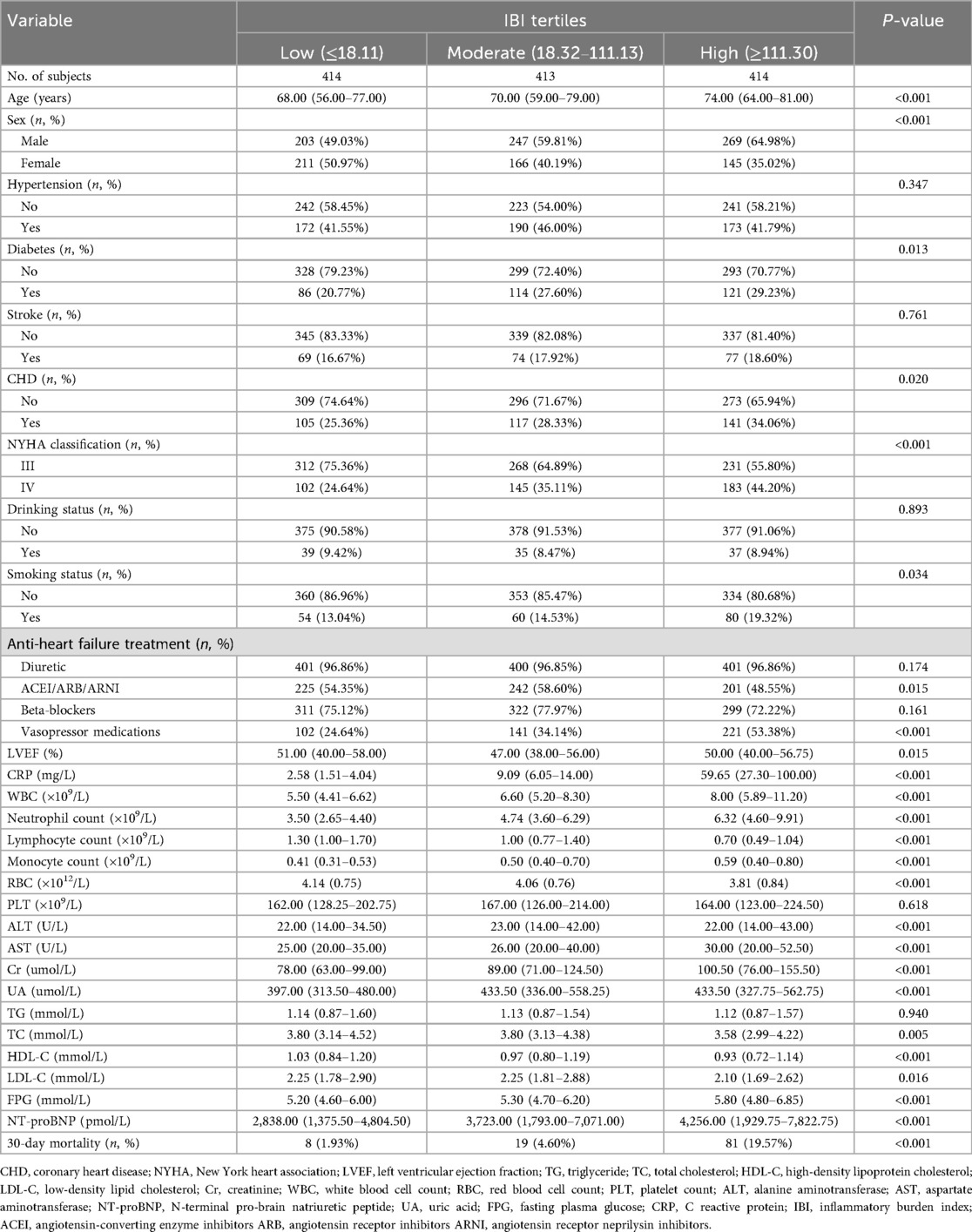
Table 1. Summary of baseline characteristics of the study population according to IBI tertiles group.
Follow-up
During the 30-day follow-up, 108 deaths occurred among the 1,241 ADHF patients. The mortality rates in the low, moderate, and high IBI groups were 1.93%, 4.60%, and 19.57%, respectively (Figure 3): as IBI increased, the 30-day mortality rate among ADHF patients demonstrated a progressive increase. Kaplan–Meier analysis showed that higher IBI was associated with increased all-cause mortality: the high IBI group had a significantly higher 30-day mortality rate compared to the low and moderate IBI groups (Figure 2: log-rank p < 0.0001).
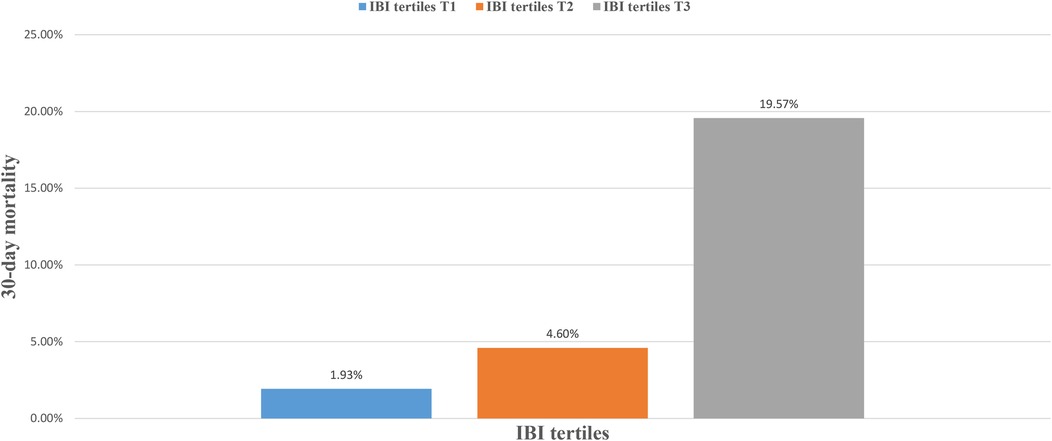
Figure 3. Bar chart showing 30-day mortality of ADHF patients stratified by IBI tertiles. ADHF, acute decompensated heart failure; IBI, inflammatory burden index.
Association between IBI and 30-day mortality in ADHF patients
Table 2 shows the hazard ratios (HRs) for the association between IBI, analyzed as both a continuous and categorical variable, and all-cause mortality. From Model 1 to Model 3, the HRs for the association between IBI and 30-day mortality in ADHF patients were 1.37, 1.40, and 1.29, respectively. Despite the attenuation of HRs with increasing levels of model adjustment, the positive association between IBI and 30-day mortality persisted across all models in ADHF patients. In the final model (Model 3), each SD increase in IBI was associated with a 29% increased risk of 30-day mortality (HR: 1.29, 95% CI: 1.15–1.46). Additionally, compared to the low IBI group, the high IBI group had a 368% higher risk of 30-day mortality (HR: 4.68, 95% CI: 1.06–13.73). Across all models, IBI showed a significant positive trend with 30-day mortality in ADHF patients (all p-trend < 0.001). These findings suggest that elevated IBI serves as an independent risk factor for poor short-term prognosis in ADHF patients.
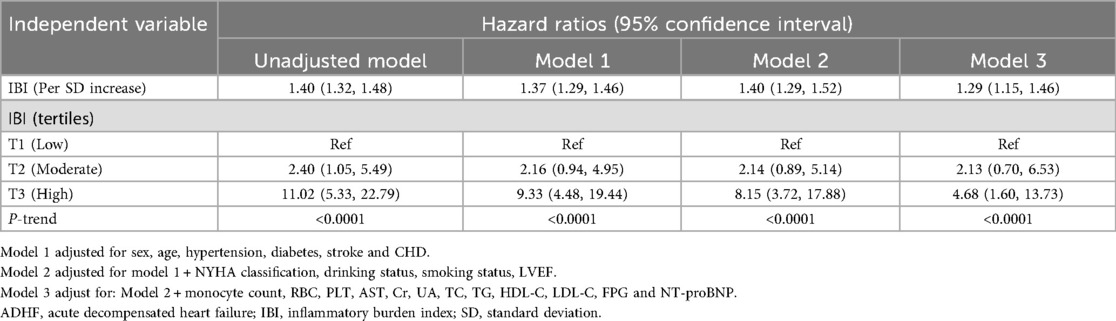
Table 2. Multivariable cox regression analysis of the association between IBI and 30-day mortality in patients with ADHF.
Subgroup analysis
Table 3 presents the results of subgroup analyses stratified by age, sex, LVEF, NYHA classification, and comorbidities (hypertension, diabetes, stroke, and CHD). After further likelihood ratio tests, we found no significant interaction between IBI and 30-day mortality in ADHF patients across subgroups (LVEF, NYHA classification, and comorbidities; All p-interaction >0.05), except for sex. These findings indicate that the association between IBI and short-term mortality prognosis in ADHF patients demonstrates robust stability across the majority of patient populations. In the sex subgroup, females had a significantly higher risk of IBI-related all-cause mortality compared to males (HR: female1.52 vs. male1.33, p-interaction = 0.0431). By contrast, female patients with IBI-related ADHF demonstrated a 1.14-fold higher 30-day mortality risk compared to males.
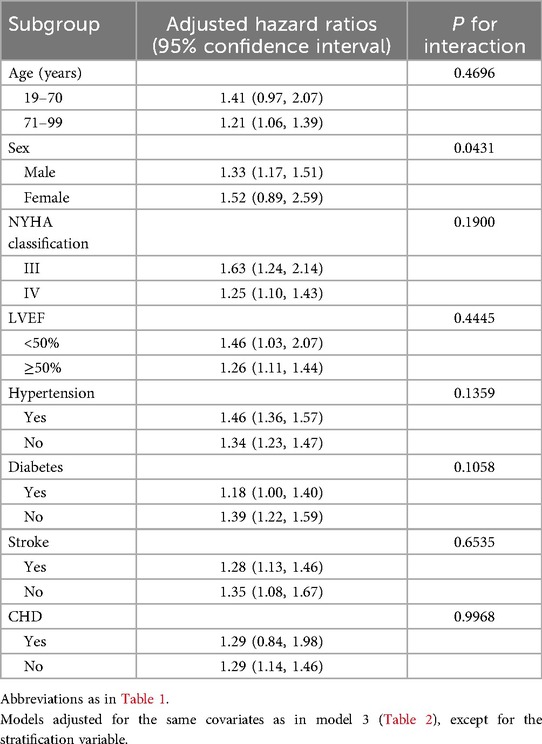
Table 3. Stratified analysis showed the relationship between IBI and 30-day mortality in patients with ADHF in different age, sex, NYHA classification, LVEF and whether combined with hypertension/diabetes/stroke/CHD.
To further explore the potential clinical explanations for gender-based differences in IBI-associated mortality risk among ADHF patients, we performed a gender-stratified analysis comparing baseline comorbidities and treatment factors between medium-to-high IBI subgroups (IBI ≥18.32): Our findings revealed that compared to male ADHF patients, female ADHF patients exhibited a higher prevalence of diabetes and stroke but lower rates of hypertension and CHD. Regarding treatment, women were less likely to receive ACEI/ARB/ARNI and diuretics but more likely to receive beta-blockers and vasopressors compared to men. However, despite these observed trends, no statistically significant differences were found between genders in comorbidities or medication use (Supplementary Table S4, all p > 0.05).
Predictive value of IBI and multiple common inflammatory markers for 30-day mortality
The results of the predictive value analysis for IBI and multiple common inflammatory markers for 30-day mortality in ADHF patients are shown in Table 4 and Figure 4. The study demonstrated that conventional inflammatory biomarkers— CRP, WBC, neutrophil count, lymphocyte count, and monocyte count—each exhibited predictive value for 30-day mortality in ADHF patients, with respective predictive accuracies of 74%, 66%, 70%, 68%, and 60%. Compared with these conventional inflammatory biomarkers, IBI demonstrated superior predictive performance for 30-day mortality in ADHF patients, achieving approximately 80% accuracy (all DeLong's test p < 0.0001). Additionally, the optimal threshold for IBI in predicting 30-day mortality in ADHF patients was calculated as 159.86, with a specificity of 0.76 and a sensitivity of 0.70. Collectively, as a novel inflammatory biomarker, IBI significantly enhances the predictive accuracy for short-term adverse outcomes in ADHF patients beyond conventional inflammatory indicators.
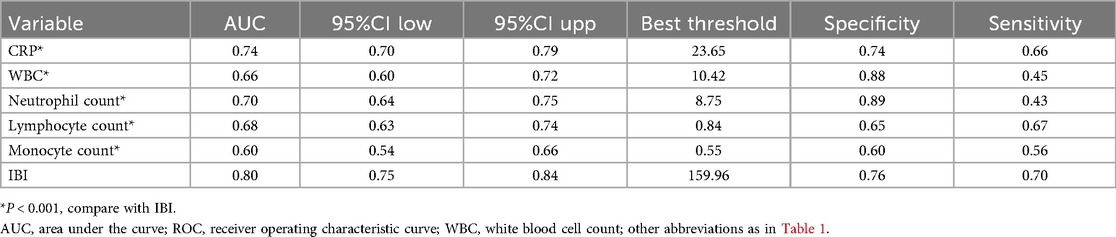
Table 4. ROC analysis of IBI and various commonly used inflammatory indicators on the predictive value of 30-day mortality in ADHF patients.
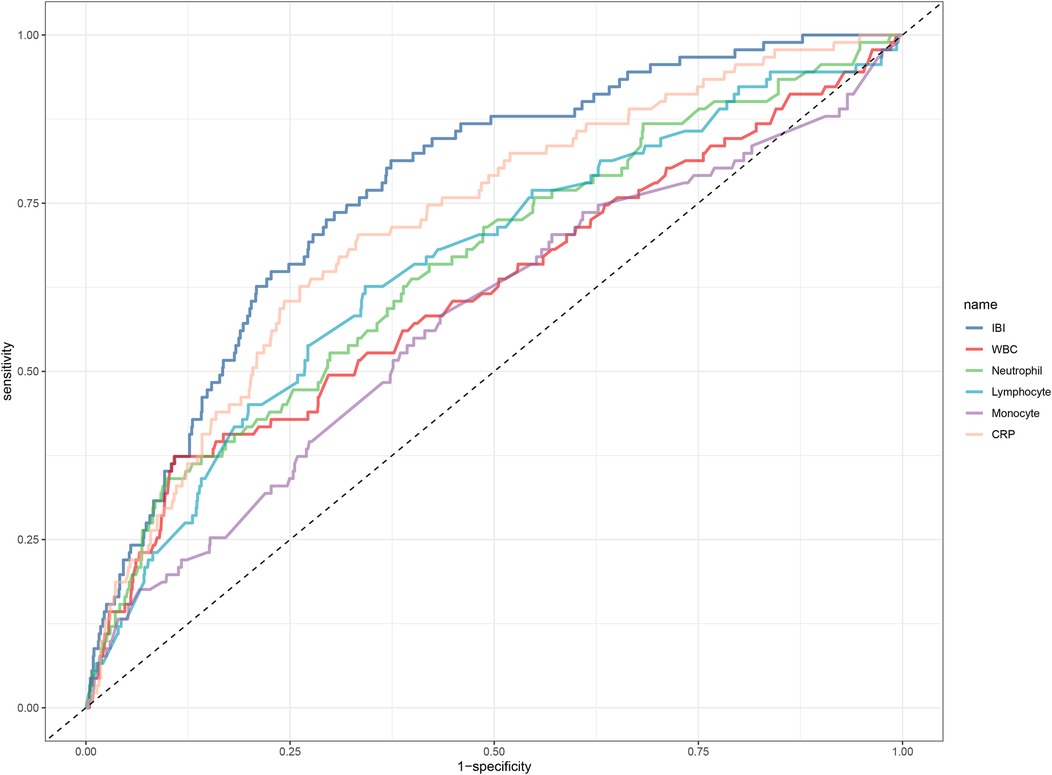
Figure 4. ROC analysis shows the predictive value of IBI and multiple common inflammatory markers on 30-day mortality in patients with ADHF. ROC, receiver operating characteristic curve; IBI, inflammatory burden index; WBC, white blood cell count; ADHF, acute decompensated heart failure.
Incremental predictive performance of IBI in mortality risk assessment
We further evaluated the incremental predictive value of adding IBI to the established clinical risk model (ADHFRE). The results demonstrated that incorporating IBI into the ADHFRE model for predicting 30-day mortality significantly improved its predictive performance: the C-index increased from 0.58 to 0.82 (P < 0.01). These findings highlight that the addition of IBI provides significant incremental value to the ADHFRE risk model for predicting short-term mortality.
Sensitivity analyses
In the sensitivity analysis, the association between IBI and 30-day mortality in ADHF patients remained significant after excluding those with pulmonary infection (Table 5: Sensitivity-1); Specifically, in ADHF patients without pulmonary infection, IBI remained positively associated with 30-day mortality, yielding a HR of 1.61 (95% CI: 1.08–2.41). Furthermore, the main results remained largely unchanged after further excluding patients with hypertension, diabetes, stroke, and CHD, or patients who died within three days of admission: IBI maintained a robust positive association with 30-day mortality across these subgroups (Table 5: Sensitivity Analyses 2 and 3). Repeating the primary analysis in the imputed complete dataset yielded robust results (Table 5: Sensitivity-4). After adjusting for treatment factors including β-blockers, diuretics, ARBs/ACEIs/ARNI, and vasopressors, the findings remained consistent with the primary analysis (Table 5, Sensitivity-5). Additionally, we repeated the analyses in the complete cohort including patients who underwent PCI within the last 3 months (n = 1,241 + 44; due to missing CRP data in 58 participants who received PCI in the past three months, 44 additional individuals were incorporated into the original cohort, yielding a total of 1,285 participants). The findings remained consistent with the primary results (Table 5, Sensitivity-6). Finally, analysis of the external United States cohort confirmed a positive association between IBI and mortality risk among individuals with congestive HF (Table 5, Sensitivity-7), which is consistent with the results reported in the present study.
Discussion
This study is the first to investigate the association between IBI and 30-day mortality in a cohort of ADHF patients. The results demonstrate a significant positive association between IBI and 30-day mortality, with multiple sensitivity analyses further supporting the robustness of these findings.
Although the exact pathophysiological mechanisms of ADHF have not been fully elucidated, its deterioration is closely associated with significant activation of the neurohormonal system and inflammatory pathways (40–45). Notably, in patients with HF, elevated levels of inflammatory markers often precede increases in neurohormonal biomarkers (12, 46). This temporal pattern suggests that inflammatory-related indicators may provide earlier prognostic warning information for ADHF patients. IBI is a recently developed inflammatory index calculated by combining CRP, neutrophil count, and lymphocyte count. Numerous previous studies have demonstrated its potential utility in assessing the progression of various chronic diseases and cancer, highlighting its significant clinical applicability (17–33). For example, Du et al. demonstrated that in ischemic stroke patients undergoing endovascular thrombectomy, each SD increase in IBI was associated with a 74% higher risk of poor prognosis within 90 days (31). Findings from the National Health and Nutrition Examination Survey revealed a positive correlation between IBI levels and the prevalence of cardiovascular disease: compared to the low IBI group (Q1), the high IBI group (Q4) had a 43% increased risk of cardiovascular disease (33). Overall, high IBI is an important risk factor for inflammation-related diseases and their prognosis. Further validation is needed to determine whether these findings extend to other inflammation-related conditions. Moreover, the association between IBI and the prognosis of ADHF remains unclear. In this study, we examined the association between IBI and 30-day mortality in ADHF patients based on the Jiangxi-ADHF cohort. Our results demonstrate that IBI is an independent predictor of 30-day mortality prognosis in ADHF patients. Compared to those with low IBI levels, ADHF patients with high IBI levels exhibited a 368% higher risk of death within 30 days. This finding aligns with previously reported studies on IBI (17–33), demonstrating that elevated IBI levels exert adverse effects on health. In contrast, our study further expands the application of IBI and identifies it as a significant risk assessment factor for short-term mortality prognosis in ADHF patients. The predictive value of IBI in mortality risk has been extensively discussed in recent years. Existing studies have shown that IBI's predictive accuracy for survival rates in patients with various types of cancer ranges from 0.62 to 0.70 (8, 19, 23, 25, 27). In chronic inflammatory airway disease patients, IBI's predictive accuracy for all-cause mortality was 0.70, 0.67, 0.65, and 0.63 at 3, 5, 10, and 15 years, respectively (30). It is worth noting that in the assessment of non-mortality prognosis, Du et al. reported that IBI predicted 90-day adverse outcomes in acute ischemic stroke patients undergoing endovascular thrombectomy with an accuracy of 0.66 (31). In the current study, we analyzed the predictive performance of IBI for 30-day mortality in ADHF patients. The results showed that IBI had a predictive accuracy of 0.80, significantly outperforming conventional inflammatory markers such as CRP, WBC count, neutrophil count, lymphocyte count, and monocyte count. Similar findings have been reported by Song and Du et al., where IBI demonstrated the best predictive value for mortality outcomes in cancer and stroke patients compared to conventional inflammatory markers (18, 31). Based on IBI-related studies, we conclude that IBI is a superior novel inflammatory marker compared to conventional markers and demonstrates high predictive accuracy for short-term prognosis in acute diseases.
The mechanism by which high IBI leads to poor outcomes in ADHF patients remains unclear. However, based on the calculation method of IBI, it is evident that a high IBI implies elevated CRP, increased neutrophil count, and decreased lymphocyte count. Based on this background and literature review, we conducted the following analysis, which may provide insights into the mechanisms by which high IBI contributes to adverse outcomes in ADHF patients: (1) CRP is the most representative clinical marker of acute systemic inflammation. In HF patients, the interleukin-6–hsCRP pathway is significantly activated, leading to increased expression of inducible nitric oxide synthase and reduced cardiac contractility, ultimately resulting in poor short-term prognosis (47, 48). (2) Activated neutrophils release various proteolytic enzymes, including acid phosphatase, myeloperoxidase, and elastase. These enzymes can damage cardiomyocytes, exacerbating cardiac dysfunction and inflammatory responses, thereby worsening the prognosis of ADHF patients (49, 50). (3) In HF patients, visceral congestion can lead to intestinal lymphocyte loss, further impairing cardiac function and creating a vicious cycle of increasingly severe visceral congestion and decreased lymphocyte counts (51, 52). Based on the above analysis, we propose that a high IBI reflects a combined state of elevated CRP, increased neutrophil count, and decreased lymphocyte count. This comprehensive measure provides a more holistic reflection of the body's inflammatory and immune status, offering valuable prognostic information for clinical practice.
In the subgroup analysis, we observed a sex-specific association between IBI and ADHF prognosis: female ADHF patients exhibited a higher mortality risk than males at the same IBI level, suggesting that the inflammatory response may be more detrimental to female ADHF patients. This finding aligns with the “female survival disadvantage in HF” phenomenon reported in multiple studies. For instance, a Swiss cohort study including 5,825 HF patients demonstrated that females had a higher overall mortality risk regardless of the LVEF category (53). Furthermore, a multicenter study from Turkey also indicated that female acute HF patients had a significantly higher risk of in-hospital mortality compared to males (54). Regarding the sex-dependent association between IBI and ADHF prognosis, we propose that differences in sex-related pathophysiological mechanisms may be the core driving factors. Previous studies have shown that HF in males is often caused by macrovascular diseases (e.g., myocardial infarction) and myocardial structural remodeling, whereas females are more susceptible to coronary microvascular dysfunction, endothelial inflammation, and fibrosis (55, 56). These differences may amplify the detrimental effects of inflammation in female ADHF patients: On one hand, females often exhibit a stronger pro-inflammatory response during the acute phase of ADHF (e.g., more pronounced increases in CRP and interleukin-6), and the decline in estrogen levels (the mean age of the current study population was 68 years) may further diminish its anti-inflammatory protective effects (57–60). On the other hand, inflammatory mediators can synergistically exacerbate damage by interacting with female-specific pathological mechanisms, such as aggravating microvascular endothelial dysfunction and promoting myocardial fibrosis (55, 56). It should be noted that systemic inflammation interacts intricately with renal function, contributing to impaired iron metabolism and attenuated erythropoietin production/responsiveness, ultimately leading to anemia and iron deficiency (61). This anemia phenotype is more pronounced in female HF patients and correlates with significantly worse clinical outcomes (61–63). Additionally, psychosocial factors cannot be overlooked: the high prevalence of depression and anxiety may further exacerbate inflammatory cascades through neuroendocrine pathways (64, 65). These findings have dual implications for clinical practice: First, IBI may serve as a sensitive indicator for risk stratification in female ADHF patients, with high-IBI females prioritized for close monitoring and management. Second, treatment strategies for female patients should emphasize anti-inflammatory interventions.
One of the central challenges in cardiovascular medicine remains the high incidence of short-term adverse clinical outcomes among ADHF patients (9, 10). Addressing this clinical dilemma, the present study investigated the predictive performance of the novel inflammatory biomarker IBI for 30-day mortality risk in ADHF patients. Our findings demonstrate that IBI serves as an independent risk factor for short-term mortality in ADHF, exhibiting superior predictive value (AUC = 0.80) when compared with conventional inflammatory biomarkers. Notably, the simplicity of IBI measurement significantly enhances its clinical utility in emergency or inpatient settings, enabling timely identification of ADHF patients at high risk of adverse outcomes and facilitating early targeted therapies. We advocate for integrating automated IBI calculation algorithms within hospital electronic health record systems to optimize its clinical application. Consistent with previous IBI validation studies across diverse clinical contexts (17–33), these findings collectively underscore IBI's potential as a robust inflammatory biomarker with high generalizability.
Strengths and limitations of the study
The strengths of this study lie in its novel findings and study population: (1) IBI showed excellent predictive value for 30-day mortality in ADHF patients (AUC: 0.80), which is promising news for ADHF patients as IBI can be obtained conveniently and effectively. (2) To our knowledge, this is the first study to evaluate the association between IBI and short-term mortality in ADHF patients, with results validated through multiple sensitivity analyses.
Some potential limitations should also be mentioned: (1) The participants in this study were primarily from Jiangxi, a southern city in China, which may restrict the applicability of our findings to northern China or other ethnic populations. (2) As a non-interventional study, it could not assess the impact of anti-inflammatory treatments on outcomes in ADHF patients after hospital admission. (3) This study primarily evaluated the predictive capability of IBI at admission for subsequent adverse events. The impact of IBI changes during hospitalization on prognosis is still unclear and warrants further investigation. (4) As with other observational studies, residual confounding cannot be eliminated. (5) A substantial proportion of ADHF patients lacked baseline CRP measurements at admission, resulting in missing IBI data. Although these missing values met the criteria for missing at random, the relative reduction in sample size may have influenced the findings to some extent, necessitating external validation in larger cohorts. (6) While 30-day follow-up effectively captures acute-phase events, it does not evaluate the longitudinal prognostic impact of IBI on ADHF patients. Future studies with extended follow-up are required to characterize the temporal trajectory of IBI's effects across short-, medium-, and long-term outcomes.
Conclusion
This cohort study in Jiangxi, China, revealed a significant positive association between IBI and 30-day mortality in ADHF patients, emphasizing its predictive value. Incorporating IBI into the clinical management of ADHF patients may significantly help in preventing further disease progression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jiangxi Provincial People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KJ: Investigation, Resources, Software, Visualization, Writing – original draft. GJ: Investigation, Resources, Visualization, Writing – original draft. ZL: Investigation, Visualization, Writing – original draft. SH: Investigation, Writing – review & editing. XH: Visualization, Writing – review & editing. LX: Investigation, Writing – review & editing. SZ: Investigation, Writing – review & editing. QW: Investigation, Writing – review & editing. HLu: Investigation, Writing – review & editing. ZX: Investigation, Writing – review & editing. ZW: Investigation, Writing – review & editing. GS: Data curation, Investigation, Writing – review & editing. YZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing. AX: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. HLa: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. WW: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China [82460091, 81670370 and 82360073]; the Natural Science Foundation of Jiangxi Province [20232BAB216004, 20224ACB206004 and 20224BAB216015]; and the Jiangxi Provincial Health Technology Project [No. 202410011].
Acknowledgments
We would like to thank the members of the JX-ADHF research group for their great efforts in data compilation. We thank the Home for Researchers editorial team (http://www.home-for-researchers.com) for Graphic Abstract editing service (USRTId12d3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1604094/full#supplementary-material
References
1. GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2100–32. doi: 10.1016/S0140-6736(24)00367-2
2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368. Erratum in: Eur Heart J (2021) 42(48):4901. doi: 10.1093/eurheartj/ehab670.34447992
3. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(17):e263–421. doi: 10.1016/j.jacc.2021.12.012
4. Masip J, Frank Peacok W, Arrigo M, Rossello X, Platz E, Cullen L, et al. Acute heart failure in the 2021 ESC heart failure guidelines: a scientific statement from the association for acute CardioVascular care (ACVC) of the European society of cardiology. Eur Heart J Acute Cardiovasc Care. (2022) 11(2):173–85. doi: 10.1093/ehjacc/zuab122
5. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. Euroheart failure survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. (2006) 27(22):2725–36. doi: 10.1093/eurheartj/ehl193
6. Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, et al. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA heart failure long-term registry. Eur J Heart Fail. (2019) 21(11):1338–52. doi: 10.1002/ejhf.1492
7. Miró Ò, García Sarasola A, Fuenzalida C, Calderón S, Jacob J, Aguirre A, et al. Departments involved during the first episode of acute heart failure and subsequent emergency department revisits and rehospitalisations: an outlook through the NOVICA cohort. Eur J Heart Fail. (2019) 21(10):1231–44. doi: 10.1002/ejhf.1567
8. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo-Leiro MG, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC heart failure long-term registry. Eur J Heart Fail. (2017) 19(10):1242–54. doi: 10.1002/ejhf.890
9. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the heart failure association of the European Society of Cardiology, the European society of emergency medicine and the society of academic emergency medicine. Eur J Heart Fail. (2015) 17(6):544–58. doi: 10.1002/ejhf.289
10. Njoroge JN, Teerlink JR. Pathophysiology and therapeutic approaches to acute decompensated heart failure. Circ Res. (2021) 128(10):1468–86. doi: 10.1161/CIRCRESAHA.121.318186
11. Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75(11):1324–40. doi: 10.1016/j.jacc.2020.01.014
12. Dutka M, Bobiński R, Ulman-Włodarz I, Hajduga M, Bujok J, Pająk C, et al. Various aspects of inflammation in heart failure. Heart Fail Rev. (2020) 25(3):537–48. doi: 10.1007/s10741-019-09875-1
13. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. (2002) 91(11):988–98. doi: 10.1161/01.res.0000043825.01705.1b
14. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. (2011) 365(1):32–43. doi: 10.1056/NEJMoa1100171
15. Zhu X, Cheang I, Xu F, Gao R, Liao S, Yao W, et al. Long-term prognostic value of inflammatory biomarkers for patients with acute heart failure: construction of an inflammatory prognostic scoring system. Front Immunol. (2022) 13:1005697. doi: 10.3389/fimmu.2022.1005697
16. Ye GL, Chen Q, Chen X, Liu YY, Yin TT, Meng QH, et al. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: a cohort study. Sci Rep. (2019) 9(1):10639. doi: 10.1038/s41598-019-47143-2
17. Xie H, Ruan G, Ge Y, Zhang Q, Zhang H, Lin S, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. (2022) 41(6):1236–43. doi: 10.1016/j.clnu.2022.04.019
18. Song R, Ni H, Huang J, Yang C, Qin S, Wei H, et al. Prognostic value of inflammation-immunity-nutrition score and inflammatory burden index for hepatocellular carcinoma patients after hepatectomy. J Inflamm Res. (2022) 15:6463–79. doi: 10.2147/JIR.S386407
19. Xie H, Ruan G, Wei L, Zhang H, Ge Y, Zhang Q, et al. Comprehensive comparative analysis of prognostic value of serum systemic inflammation biomarkers for colorectal cancer: results from a large multicenter collaboration. Front Immunol. (2023) 13:1092498. doi: 10.3389/fimmu.2022.1092498
20. Ding P, Wu H, Liu P, Sun C, Yang P, Tian Y, et al. The inflammatory burden index: a promising prognostic predictor in patients with locally advanced gastric cancer. Clin Nutr. (2023) 42(2):247–8. doi: 10.1016/j.clnu.2023.01.005
21. Xie H, Ruan G, Wei L, Deng L, Zhang Q, Ge Y, et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J Cachexia Sarcopenia Muscle. (2023) 14(2):869–78. doi: 10.1002/jcsm.13199
22. Yin C, Okugawa Y, Kitajima T, Shimura T, Ma R, Kawamura M, et al. Clinical significance of the preoperative inflammatory burden index in esophageal cancer. Oncology. (2024) 102(7):556–64. doi: 10.1159/000535727
23. Pelc Z, Sędłak K, Mlak R, Leśniewska M, Mielniczek K, Rola P, et al. Prognostic value of inflammatory burden Index in advanced gastric cancer patients undergoing multimodal treatment. Cancers (Basel). (2024) 16(4):828. doi: 10.3390/cancers16040828
24. Yamashita S, Okugawa Y, Mizuno N, Imaoka H, Shimura T, Kitajima T, et al. Inflammatory burden Index as a promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Gastroenterol Surg. (2024) 8(5):826–35. doi: 10.1002/ags3.12829
25. Deng J, Hua J, Zeng T, Que H, Zhang Q, Li Q, et al. Associations between inflammatory burden index, prostate cancer, and mortality among middle-aged and elderly individuals. World J Urol. (2024) 42(1):538. doi: 10.1007/s00345-024-05241-5
26. Aoyama T, Maezawa Y, Hashimoto I, Esashi R, Yamamoto S, Kazama K, et al. Inflammatory burden index is an independent prognostic factor for esophageal cancer patients who receive curative treatment. In Vivo. (2024) 38(6):2928–34. doi: 10.21873/invivo.13775
27. Qiu X, Zhang Y, Zhu Y, Yang M, Tao L. Association of the inflammatory burden index with increased mortality among cancer patients: insights from the NHANES study. Immun Inflamm Dis. (2024) 12(12):e70067. doi: 10.1002/iid3.70067
28. Xiong Z, Xu W, Wang Y, Cao S, Zeng X, Yang P. Inflammatory burden index: associations between osteoarthritis and all-cause mortality among individuals with osteoarthritis. BMC Public Health. (2024) 24(1):2203. doi: 10.1186/s12889-024-19632-1
29. Zhai J, Yuan B, Liu T, Mo L, Xie Y, Zhao Y, et al. Association between the inflammatory burden index and rheumatoid arthritis and its all-cause mortality: data from NHANES 1999–2018. Front Med (Lausanne). (2024) 11:1421497. doi: 10.3389/fmed.2024.1421497
30. Zhu N, Lin S, Wang L, Kong X, Huang W, Cao C. Elevated inflammatory burden index increases mortality in adults with chronic inflammatory airway diseases: a nationwide cohort study. BMC Pulm Med. (2024) 24(1):399. doi: 10.1186/s12890-024-03211-6
31. Du M, Xu L, Zhang X, Huang X, Cao H, Qiu F, et al. Association between inflammatory burden index and unfavorable prognosis after endovascular thrombectomy in acute ischemic stroke. J Inflamm Res. (2023) 16:3009–17. doi: 10.2147/JIR.S419087
32. Song Z, Lin F, Chen Y, Li T, Li R, Lu J, et al. Inflammatory burden Index: association between novel systemic inflammatory biomarkers and prognosis as well as in-hospital complications of patients with aneurysmal subarachnoid hemorrhage. J Inflamm Res. (2023) 16:3911–21. doi: 10.2147/JIR.S416295
33. Yu F, Peng J. Association between inflammatory burden index and cardiovascular disease in adult Americans: evidence from NHANES 2005–2010. Heliyon. (2024) 10(18):e38273. doi: 10.1016/j.heliyon.2024.e38273
34. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X
35. Elmståhl S, Gullberg B. Bias in diet assessment methods–consequences of collinearity and measurement errors on power and observed relative risks. Int J Epidemiol. (1997) 26(5):1071–9. doi: 10.1093/ije/26.5.1071
36. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the acute decompensated heart failure national registry (ADHERE). Am Heart J. (2005) 149(2):209–16. doi: 10.1016/j.ahj.2004.08.005
37. Available online at: https://www.mdcalc.com/calc/3828/acute-decompensated-heart-failure-national-registry-adhere-algorithm#when-to-use (Accessed September 01, 2025).
38. Chen Y, Zhang Y, Zhang M, Yang H, Wang Y. Consumption of coffee and tea with all-cause and cause-specific mortality: a prospective cohort study. BMC Med. (2022) 20(1):449. doi: 10.1186/s12916-022-02636-2
39. Ogaz-González R, Corpeleijn E, García-Chanes RE, Gutierréz-Robledo LM, Escamilla-Santiago RA, López-Cervantes M. Assessing the relationship between multimorbidity, NCD configurations, frailty phenotypes, and mortality risk in older adults. BMC Geriatr. (2024) 24(1):355. doi: 10.1186/s12877-024-04948-9
40. Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. (1992) 20(1):248–54. doi: 10.1016/0735-1097(92)90167-l
41. Packer M. Evolution of the neurohormonal hypothesis to explain the progression of chronic heart failure. Eur Heart J. (1995) 16(Suppl F):4–6. doi: 10.1093/eurheartj/16.suppl_f.4
42. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. (2017) 14(1):30–8. doi: 10.1038/nrcardio.2016.163
43. Ge Z, Li A, McNamara J, Dos Remedios C, Lal S. Pathogenesis and pathophysiology of heart failure with reduced ejection fraction: translation to human studies. Heart Fail Rev. (2019) 24(5):743–58. doi: 10.1007/s10741-019-09806-0
44. Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the studies of left ventricular dysfunction (SOLVD). J Am Coll Cardiol. (1996) 27(5):1201–6. doi: 10.1016/0735-1097(95)00589-7
45. Goonewardena SN, Stein AB, Tsuchida RE, Rattan R, Shah D, Hummel SL. Monocyte subsets and inflammatory cytokines in acute decompensated heart failure. J Card Fail. (2016) 22(5):358–65. doi: 10.1016/j.cardfail.2015.12.014
46. Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, et al. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. (2010) 13(7):1033–49. doi: 10.1089/ars.2009.2930
47. Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc Nutr Soc. (1989) 48(3):347–54. doi: 10.1079/pns19890050
48. Alonso-Martínez JL, Llorente-Diez B, Echegaray-Agara M, Olaz-Preciado F, Urbieta-Echezarreta M, González-Arencibia C. C-reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. (2002) 4(3):331–6. doi: 10.1016/s1388-9842(02)00021-1
49. Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. (2010) 56(6):944–51. doi: 10.1373/clinchem.2009.142257
50. Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. (2007) 49(24):2364–70. doi: 10.1016/j.jacc.2007.02.053
51. Yücel H, Refiker Ege M, Zorlu A, Kaya H, Beton O, Güngör H, et al. Lymphocytopenia is associated with poor NYHA functional class in chronic heart failure patients with reduced ejection fraction. Turk Kardiyol Dern Ars. (2015) 43(5):427–33. doi: 10.5543/tkda.2015.89439
52. Vaduganathan M, Ambrosy AP, Greene SJ, Mentz RJ, Subacius HP, Maggioni AP, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. (2012) 5(6):750–8. doi: 10.1161/CIRCHEARTFAILURE.112.970525
53. Fuentes Artiles R, Meçani R, Muka T, Hunziker L, Capék L. Investigation of left ventricular ejection fraction in a Swiss heart failure population: insights into mortality and sex differences. ESC Heart Fail. (2024) 12(3):1630–9. doi: 10.1002/ehf2.15174
54. Akçay FA, Sinan ÜY, Gürbüz DÇ, Şafak Ö, Kaya H, Yüksek Ü, et al. Gender-related differences in patients with acute heart failure: observation from the journey heart failure-Turkish population study. Anatol J Cardiol. (2023) 27(11):639–49. doi: 10.14744/AnatolJCardiol.2023.2971
55. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J. (2019) 40(47):3859–3868c. doi: 10.1093/eurheartj/ehz835
56. Masood MS, Hamid F. Sex-specific differences in heart failure: pathophysiology, risk factors, management, and outcomes. Can J Cardiol. (2022) 38(10):1615. doi: 10.1016/j.cjca.2022.06.015
57. Lau ES, Binek A, Parker SJ, Shah SH, Zanni MV, Van Eyk JE, et al. Sexual dimorphism in cardiovascular biomarkers: clinical and research implications. Circ Res. (2022) 130(4):578–92. doi: 10.1161/CIRCRESAHA.121.319916
58. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16(10):626–38. doi: 10.1038/nri.2016.90
59. Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. (2008) 8(9):737. doi: 10.1038/nri2394
60. Cignarella A, Bolego C, Barton M. Sex and sex steroids as determinants of cardiovascular risk. Steroids. (2024) 206:109423. doi: 10.1016/j.steroids.2024.109423
61. Arata A, Ricci F, Khanji MY, Mantini C, Angeli F, Aquilani R, et al. Sex differences in heart failure: what do we know? J Cardiovasc Dev Dis. (2023) 10(7):277. doi: 10.3390/jcdd10070277
62. Cohen-Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail. (2014) 16(9):984–91. doi: 10.1002/ejhf.139
63. Le Jemtel TH, Arain S. Mediators of anemia in chronic heart failure. Heart Fail Clin. (2010) 6(3):289–93. doi: 10.1016/j.hfc.2010.03.008
64. Patel N, Chakraborty S, Bandyopadhyay D, Amgai B, Hajra A, Atti V, et al. Association between depression and readmission of heart failure: a national representative database study. Prog Cardiovasc Dis. (2020) 63(5):585–90. doi: 10.1016/j.pcad.2020.03.014
Keywords: inflammatory burden index, acute decompensated heart failure, Chinese, prognosis, IBI
Citation: Jiang K, Jian G, Lu Z, He S, Huang X, Xie L, Zhang S, Wang Q, Lu H, Xiong Z, Wu Z, Sheng G, Zou Y, Xie A, Lai H and Wang W (2025) The impact of inflammatory burden index on the prognosis in acute decompensated heart failure: evidence from a cohort study in Jiangxi, China. Front. Cardiovasc. Med. 12:1604094. doi: 10.3389/fcvm.2025.1604094
Received: 23 April 2025; Accepted: 24 September 2025;
Published: 10 October 2025.
Edited by:
Marta Focardi, University of Siena, ItalyReviewed by:
Vinay Kumar, The Pennsylvania State University, United StatesGang-Yong Wu, 904th Hospital of PLA, China
Qi Zhang, Yale University, United States
Qingpeng Wang, Huazhong University of Science and Technology, China
Copyright: © 2025 Jiang, Jian, Lu, He, Huang, Xie, Zhang, Wang, Lu, Xiong, Wu, Sheng, Zou, Xie, Lai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Zou, anh5eHl6eUAxNjMuY29t; Aimin Xie, eGllYWltaW4xOTk4QDEyNi5jb20=; Hengli Lai, bGFpaGVuZ2xpQDE2My5jb20=; Wei Wang, d3dhbmdjdnJpQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Kun Jiang
Kun Jiang Guoan Jian
Guoan Jian Zihao Lu1,2,3,†
Zihao Lu1,2,3,† Shiming He
Shiming He Shuhua Zhang
Shuhua Zhang Guotai Sheng
Guotai Sheng Yang Zou
Yang Zou