Abstract
Background:
The prognostic value of coronary artery bypass grafting (CABG) may be suboptimal when guided solely by anatomical stenosis severity. Quantitative flow ratio (QFR), a computational angiography-derived hemodynamic assessment tool, offers functional insights; however, its prognostic interplay with lesion localization [proximal vs. mid-to-distal left anterior descending artery (LAD)] remains unclear. This study evaluates the impact of QFR-guided revascularization, stratified by LAD lesion location, on midterm clinical outcomes.
Methods:
A retrospective cohort of 481 patients undergoing left internal mammary artery (LIMA) to LAD grafting (2019–2023) was analyzed. Lesions were classified as proximal (Site 1) or mid-to-distal (Site 2) LAD and stratified by QFR thresholds (High: ≥0.80; Low: <0.80). The primary endpoint was 5-year major adverse cardiovascular and cerebrovascular events (MACCEs), assessed using Kaplan–Meier survival analysis and Cox regression.
Results:
High QFR patients (n = 139) exhibited lower diabetes (28.1% vs. 40.6%, p = 0.013), smoking rates (27.3% vs. 38.6%, p = 0.025), and 3-vessel disease (48.9% vs. 74.6%, p < 0.0001) compared to low QFR (n = 342). Proximal lesions with high QFR had markedly higher MACCEs risk (HR = 1.91, 95% CI: 1.18–3.10; Log-rank P = 0.0075), whereas mid-to-distal lesions showed no QFR-driven prognostic differences (p = 0.46). Lesion location alone did not independently influence survival (Log-rank P = 0.8).
Conclusion:
QFR-guided risk stratification is most prognostically impactful for proximal LAD lesions, where hemodynamic significance plays a critical role in clinical outcomes. In contrast, mid-to-distal lesions exhibit limited QFR utility, emphasizing anatomical-functional synergy in CABG planning. Despite comparable survival across lesion sites, proximal low QFR lesions warrant intensified surveillance.
1 Introduction
The use of left internal mammary artery (LIMA) in left anterior descending artery (LAD) bypass grafting is recognized as the gold standard in coronary artery bypass grafting (CABG) due to its superior long-term patency and well-documented survival benefits (1). However, the implantation of bypass conduits onto target vessels without significant stenosis may result in competitive flow between the native and grafted conduits, potentially compromising long-term graft patency by altering endothelial shear stress (2, 3). Furthermore, conventional coronary angiography solely identifies anatomical stenotic lesions but do not effectively clarify the physiological significance of such obstructions on myocardial perfusion territories. Consequently, relying solely on angiographic assessments may prove insufficient for optimal CABG decision-making.
Although the 2018 ESC/EACTS Guidelines on Myocardial Revascularization strongly advocate (Class I recommendation) for pressure wire-based physiological assessment, such as fractional flow reserve (FFR), for determining the hemodynamic significance of intermediate-grade stenoses (4), its clinical adoption remains limited. This is primarily attributed to procedural time constraints (typically >20 minutes per vessel), the risk of pressure wire-related complications (0.8%–2.1% incidence), the prevalence of adenosine-induced adverse effects (15%–30% prevalence), and the associated incremental healthcare costs (5, 6).
Quantitative flow ratio (QFR), an emerging angiography-derived index, utilizes three-dimensional (3D) coronary reconstruction—based on invasive angiograms—coupled with computational fluid dynamics (CFD) algorithms to compute virtual FFR values without requiring hyperemic agents or intracoronary pressure measurement. Multicenter validation trials (FAVOR II & III) (7) have demonstrated excellent diagnostic concordance between QFR and invasive FFR (89%–93% agreement rates), with the area under the curve (AUC) values exceeding 0.90 for detecting functionally significant stenoses. Additionally, QFR reduces procedural time by 7–12 minutes per case and eliminates the risks associated with pharmacological agents or pressure wire application.
Although accumulating evidence from multicenter registries supports the prognostic benefits of QFR-guided revascularization strategies in reducing the incidence of midterm major adverse cardiac events (MACE) (8–10), its applications in CABG surgical planning remain underexplored. Specifically, it remains unclear whether QFR-guided CABG significantly influences prognostic outcomes and long-term survival based on coronary lesion anatomic localization.
This study aims to evaluate the predictive value of QFR in midterm outcomes of LIMA grafts after CABG, focusing on stratification by lesion localization (proximal, mid, or distal segments) within the LAD.
2 Methods
2.1 Study design
This study used a retrospective analysis of adult patients who underwent preoperative coronary angiography evaluation and subsequent LIMA grafts to LAD at the Affiliated Hospital of Qingdao University between January 2019 and December 2023 (Figure 1). Outcomes were tracked via institutional Electronic Medical Records (EMR) for hospitalized patients and telephone interviews for others.
Figure 1
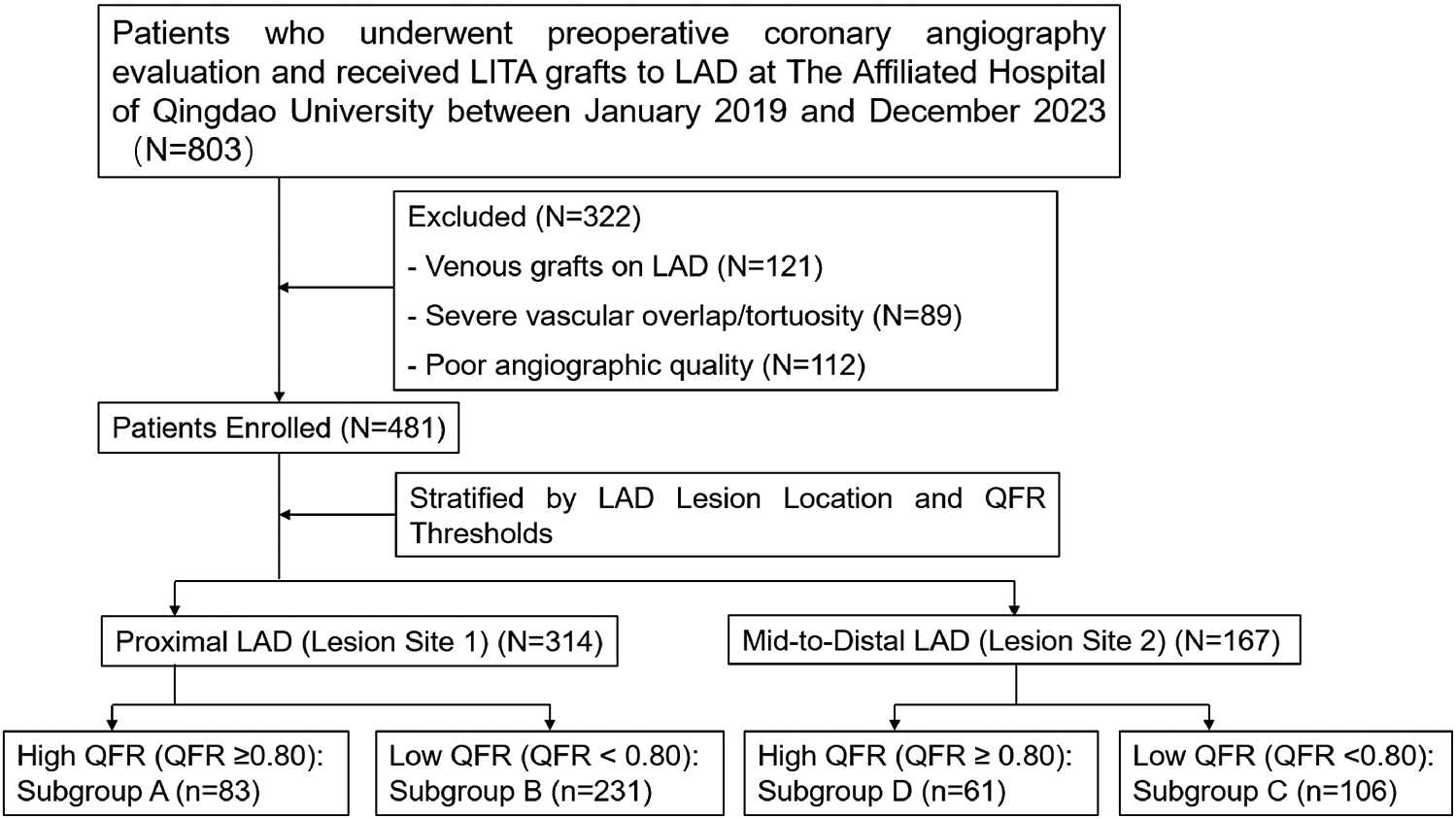
Enrollment diagram.
Inclusion criteria: (1) Patients who underwent coronary angiography at our center within 6 months before surgery; (2) Presence of LAD stenosis with severity ranging from 75%–90%.
Exclusion criteria: (1) Patients with venous grafts on the LAD artery; (2) Severe vascular overlap or tortuosity in the stenotic segment; (3) Poor angiographic image quality precluding accurate lumen contour detection.
Coronary lesion localization was classified according to the Society of Cardiovascular Computed Tomography (SCCT) guidelines (
11), grouping serial stenoses based on the originating segment of the most severe stenosis (≥70%) and stratifying LAD lesions into two anatomical subgroups:
- •
Lesion Site 1: Proximal LAD, defined as the segment extending from the ostium to the origin of the first major septal perforator.
- •
Lesion Site 2: Mid-to-distal LAD, comprising the segment distal to the first major septal perforator, including diagonal branch bifurcations.
Functional assessments were conducted using QFR, categorizing lesions into two hemodynamic groups:
- •
High QFR Group: QFR > 0.80 (indicative of functionally nonsignificant stenosis)
- •
Low QFR Group: QFR ≤ 0.80 (indicative of functionally significant stenosis)
A composite stratification was then performed by integrating anatomical and functional parameters, yielding four distinct clinical subgroups:
- •
Subgroup A: High QFR at Lesion Site 1(QFR > 0.80 with proximal LAD lesions)
- •
Subgroup B: High QFR at Lesion Site 2(QFR > 0.80 with mid-to-distal LAD lesions)
- •
Subgroup C: Low QFR at Lesion Site 1(QFR ≤ 0.80 with proximal LAD lesions)
- •
Subgroup D: Low QFR at Lesion Site 2(QFR ≤ 0.80 with mid-to-distal LAD lesions)
2.2 Coronary angiography
Coronary angiography was performed via radial or femoral access using 5-F/6-F sheaths. Angiographic imaging was acquired using Philips Allura Xper FD20 or GE Innova IGS520 systems at ≥15 frames/s. Intravenous unfractionated heparin (100 IU/kg) was administered before the procedure to prevent thrombotic complications.
2.3 QFR analysis
Lesions with a reference vessel diameter ≥1.5 mm were included in the QFR analysis. Two angiographic projections with angular separation >25° were processed using AngioPlus software version 2.1.3.0 (Pulse Medical Imaging Technology, Shanghai, China) for 3D QFR computation. Vessel contours were initially traced semi-automatically, with manual correction conducted if required. Key QFR parameters included minimal lumen diameter, area stenosis. All offline analyses were performed by two independent observers blinded to the clinical data, with discordant cases adjudicated by a third reviewer.
2.4 Coronary artery bypass surgery
All participants underwent coronary revascularization with the LIMA as the graft conduit for the LAD artery. The surgical approach, either off-pump or on-pump technique, was determined at the discretion of the operating surgeon based on patient-specific clinical characteristics.
2.5 Study end point
The primary endpoint was the cumulative incidence of 5-year MACCEs. The MACCEs were defined as a composite of all-cause mortality, cardiovascular mortality, myocardial infarction, stroke, repeat revascularization, or rehospitalization for angina pectoris during the 5-year follow-up period.
2.6 Statistical analysis
All statistical analyses were performed using R software (version 4.4.1). Continuous variables were expressed as mean ± standard deviation, while categorical variables were presented as frequencies (percentages). Group comparisons for continuous variables were assessed using Student's t-test, while Pearson's chi-squared test was used for categorical variables. Cumulative rates of MACCEs were estimated using Kaplan–Meier survival analysis, with comparisons between groups evaluated using the log-rank test. A two-tailed p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
The baseline demographic, clinical, and procedural characteristics of the study population, stratified according to the QFR thresholds and lesion locations, are summarized in Tables 1–5. The key findings are as follows:
Table 1
| Characteristic | High QFR | Low QFR | p value |
|---|---|---|---|
| (n = 144) | (n = 337) | ||
| Age (years) | 63.8 ± 0.6 | 63.0 ± 0.4 | 0.275 |
| Male | 106 (76.3%) | 254 (74.3%) | 0.734 |
| BMI (kg/m²) | 25.4 ± 0.2 | 26.4 ± 0.9 | 0.313 |
| Hypertension | 78 (56.1%) | 222 (64.9%) | 0.089 |
| Hypercholesterolemia | 2 (1.4%) | 10 (2.9%) | 0.533 |
| Diabetes mellitus | 39 (28.1%) | 139 (40.6%) | 0.013 |
| Prior MI | 12 (8.6%) | 50 (14.6%) | 0.104 |
| Smoking | 38 (27.3%) | 132 (38.6%) | 0.025 |
| Cerebrovascular diseases | 17 (12.2%) | 47 (13.7%) | 0.768 |
| Prior left coronary artery PCI | 11 (7.9%) | 15 (4.4%) | 0.184 |
| Preoperative LVEF,% | 57.5 ± 0.6 | 55.7 ± 0.5 | 0.025 |
| Concomitant surgery | 49 (35.3%) | 51 (14.9%) | <0.001 |
| Valvular surgery | 37 (26.6%) | 36 (10.5%) | <0.001 |
| Aortic surgery | 1 (0.7%) | 1 (0.3%) | >0.999 |
| Others | 6 (4.3%) | 6 (1.8%) | 0.190 |
| On-pump | 42 (30.2%) | 33 (9.6%) | <0.001 |
| 3-vessel disease | 68 (48.9%) | 255 (74.6%) | <0.001 |
| Hospital complications | 76 (54.7%) | 186 (54.4%) | >0.999 |
| Hospital stays | 27.6 ± 1.0 | 29.5 ± 0.8 | 0.110 |
| NYHA class | 0.962 | ||
| I | 3 (2.2%) | 6 (1.8%) | |
| II | 102 (73.3%) | 246 (71.9%) | |
| III | 27 (19.4%) | 73(21.3%) | |
| IV | 7(5.0%) | 17(5.0%) |
Comparison of baseline characteristics between high QFR and low QFR groups (all lesion sites combined).
BMI indicates body mass index; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York heart association; PCI, percutaneous coronary intervention. Other concomitant surgeries included left atrial appendectomy, ventricular aneurysmectomy, and arteriovenous fistula surgery. Hospital complications included infection, bleeding, heart failure, and reoperation.
Table 2
| Characteristic | Lesion Site 1 | Lesion Site 2 | p value |
|---|---|---|---|
| (n = 314) | (n = 167) | ||
| Age (years) | 63.1 ± 0.4 | 63.5 ± 0.6 | 0.613 |
| Male | 240 (76.4%) | 120 (71.9%) | 0.322 |
| BMI (kg/m²) | 25.6 ± 0.2 | 27.1 ± 1.8 | 0.395 |
| Hypertension | 203 (64.6%) | 97 (58.1%) | 0.188 |
| Hypercholesterolemia | 8 (2.5%) | 4 (3.4%) | >0.999 |
| Diabetes mellitus | 120 (38.2%) | 58 (34.7%) | 0.513 |
| Prior MI | 40 (12.7%) | 22 (13.1%) | >0.999 |
| Smoking | 116 (36.9%) | 54 (32.3%) | 0.365 |
| Cerebrovascular diseases | 43 (13.7%) | 21 (12.6%) | 0.839 |
| Prior left coronary artery PCI | 18 (5.7%) | 8 (4.8%) | 0.823 |
| Preoperative LVEF,% | 56.3 ± 0.5 | 56.2 ± 0.6 | 0.892 |
| Concomitant surgery | 50 (15.9%) | 50 (29.9%) | <0.001 |
| Valvular surgery | 33 (10.5%) | 40 (24.0%) | <0.001 |
| Aortic surgery | 1 (0.3%) | 1 (0.6%) | >0.999 |
| Others | 8 (2.5%) | 4 (2.4%) | >0.999 |
| On-pump | 32 (10.2%) | 43 (25.7%) | <0.001 |
| 3-vessel disease | 222 (70.7%) | 101 (60.5%) | 0.030 |
| Hospital complications | 161 (51.3%) | 101 (60.5%) | 0.067 |
| Hospital stays | 28.3 ± 0.7 | 30.1 ± 1.2 | 0.212 |
| NYHA class | 0.190 | ||
| I | 6 (1.9%) | 3 (1.8%) | |
| II | 237 (75.5%) | 111 (66.5%) | |
| III | 57 (18.2%) | 43(25.7%) | |
| IV | 14(4.5%) | 10(6.0%) |
Comparison of baseline characteristics between lesion site 1 and lesion site 2 (all QFR groups combined).
BMI indicates body mass index; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York heart association; PCI, percutaneous coronary intervention. Other concomitant surgeries included left atrial appendectomy, ventricular aneurysmectomy, and arteriovenous fistula surgery. Hospital complications included infection, bleeding, heart failure, and reoperation.
Table 3
| Characteristic | Lesion Site 1-High QFR | Lesion Site 1-Low QFR | p value |
|---|---|---|---|
| (n = 83) | (n = 231) | ||
| Age (years) | 63.1 ± 0.8 | 63.1 ± 0.5 | 0.946 |
| Male | 62 (79.5%) | 178 (75.4%) | 0.562 |
| BMI (kg/m²) | 25.5 ± 0.3 | 25.6 ± 0.2 | 0.834 |
| Hypertension | 47 (60.3%) | 156 (66.1%) | 0.424 |
| Hypercholesterolemia | 2 (2.6%) | 6 (2.5%) | 0.748 |
| Diabetes mellitus | 26 (33.3) | 94 (39.8) | 0.374 |
| Prior MI | 8 (10.3%) | 32 (13.6%) | 0.574 |
| Smoking | 23 (29.5%) | 93 (39.4%) | 0.150 |
| Cerebrovascular diseases | 12 (15.4%) | 31 (13.3%) | 0.756 |
| Prior left coronary artery PCI | 7 (9.0%) | 11 (4.7%) | 0.254 |
| Preoperative LVEF,% | 57.8 ± 0.8 | 55.8 ± 0.6 | 0.040 |
| Concomitant surgery | 18 (23.1%) | 32 (13.6%) | 0.070 |
| Valvular surgery | 12 (15.4%) | 21 (8.9%) | 0.160 |
| Aortic surgery | 0 (0%) | 1 (0.4%) | |
| Others | 4 (5.1%) | 4 (1.7%) | 0.210 |
| On-pump | 13 (16.7%) | 19 (8.1%) | 0.049 |
| 3-vessel disease | 46 (60.0%) | 176 (74.6%) | 0.013 |
| Hospital complications | 39 (50.0%) | 122 (51.7%) | 0.897 |
| Hospital stays | 26.2 ± 1.0 | 29.1 ± 0.8 | 0.835 |
| NYHA class | 0.278 | ||
| I | 2 (2.6%) | 4 (1.7%) | |
| II | 61 (78.2%) | 176 (74.6%) | |
| III | 12 (15.4%) | 45(19.1%) | |
| IV | 3(3.8%) | 11(4.7%) |
Comparison of baseline characteristics between high QFR and low QFR in lesion site 1 (proximal LAD).
BMI indicates body mass index; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention. Other concomitant surgeries included left atrial appendectomy, ventricular aneurysmectomy, and arteriovenous fistula surgery. Hospital complications included infection, bleeding, heart failure, and reoperation.
Table 4
| Characteristic | Lesion Site 1-High QFR | Lesion Site 2-Low QFR | p value |
|---|---|---|---|
| (n = 83) | (n = 106) | ||
| Age (years) | 63.1 ± 0.8 | 62.7 ± 0.8 | 0.778 |
| Male | 62 (79.5%) | 76 (71.7%) | 0.301 |
| BMI (kg/m²) | 25.5 ± 0.3 | 28.1 ± 2.9 | 0.361 |
| Hypertension | 47 (60.3%) | 66 (62.3%) | 0.901 |
| Hypercholesterolemia | 2 (2.6%) | 4 (3.8%) | 0.970 |
| Diabetes mellitus | 26 (33.3) | 45 (42.5%) | 0.270 |
| Prior MI | 8 (10.3%) | 18 (17.0%) | 0.280 |
| Smoking | 23 (29.5%) | 39 (36.8%) | 0.380 |
| Cerebrovascular diseases | 12 (15.4%) | 16 (15.1%) | >0.999 |
| Prior left coronary artery PCI | 7 (9.0%) | 4 (3.8%) | 0.248 |
| Preoperative LVEF,% | 57.8 ± 0.8 | 55.7 ± 0.8 | 0.066 |
| Concomitant surgery | 18 (23.1%) | 19 (17.9%) | 0.499 |
| Valvular surgery | 12 (15.4%) | 15 (14.2%) | 0.982 |
| Aortic surgery | 0 (0%) | 0 (0%) | |
| Others | 4 (5.1%) | 2 (1.9%) | 0.422 |
| On-pump | 13 (16.7%) | 14 (13.2%) | 0.657 |
| 3-vessel disease | 46 (60.0%) | 79 (74.5%) | 0.038 |
| Hospital complications | 39 (50.0%) | 64 (60.4%) | 0.210 |
| Hospital stays | 26.2 ± 1.0 | 30.5 ± 1.5 | 0.021 |
| NYHA class | 0.279 | ||
| I | 2 (2.6%) | 2 (1.9%) | |
| II | 61 (78.2%) | 70 (66.0%) | |
| III | 12 (15.4%) | 28(26.4%) | |
| IV | 3(3.8%) | 6(5.7%) |
Comparison of baseline characteristics between high QFR in lesion site 1 (proximal LAD) and low QFR in lesion site 2 (Mid-to-distal LAD).
BMI indicates body mass index; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York heart association; PCI, percutaneous coronary intervention. Other concomitant surgeries included left atrial appendectomy, ventricular aneurysmectomy, and arteriovenous fistula surgery. Hospital complications included infection, bleeding, heart failure, and reoperation.
Table 5
| Characteristic | Lesion Site 2-High QFR | Lesion Site 2-Low QFR | p value |
|---|---|---|---|
| (n = 61) | (n = 106) | ||
| Age (years) | 64.8 ± 0.9 | 62.7 ± 0.8 | 0.088 |
| Male | 44 (72.1%) | 76 (71.7%) | >0.999 |
| BMI (kg/m²) | 25.3 ± 0.4 | 28.1 ± 2.9 | 0.336 |
| Hypertension | 31 (50.8%) | 66 (62.3%) | 0.200 |
| Hypercholesterolemia | 34 (55.7%) | 4 (3.8%) | 0.321 |
| Diabetes mellitus | 13 (21.3%) | 45 (42.5%) | 0.009 |
| Prior MI | 4 (6.6%) | 18 (17.0%) | 0.093 |
| Smoking | 15 (24.6%) | 39 (36.8%) | 0.147 |
| Cerebrovascular diseases | 5 (8.2%) | 16 (15.1%) | 0.293 |
| Prior left coronary artery PCI | 4 (6.6%) | 4 (3.8%) | 0.664 |
| Preoperative LVEF,% | 57.0 ± 0.9 | 55.7 ± 0.8 | 0.279 |
| Concomitant surgery | 31 (50.8%) | 19 (17.9%) | <0.001 |
| Valvular surgery | 25 (41.0%) | 15 (14.2%) | <0.001 |
| Aortic surgery | 1 (1.6%) | 0 (0%) | |
| Others | 2 (3.3%) | 2 (1.9%) | 0.967 |
| On-pump | 29 (47.5%) | 14 (13.2%) | <0.001 |
| 3-vessel disease | 22 (36.1%) | 79 (74.5%) | <0.001 |
| Hospital complications | 37 (60.1%) | 64 (60.4%) | >0.999 |
| Hospital stays | 29.3 ± 1.8 | 30.5 ± 1.5 | 0.634 |
| NYHA class | 0.990 | ||
| I | 1 (1.6%) | 2 (1.9%) | |
| II | 41 (67.2%) | 70 (66.0%) | |
| III | 15 (24.6%) | 28(26.4%) | |
| IV | 4(6.6%) | 6(5.7%) |
Comparison of baseline characteristics between high QFR and low QFR in lesion site 2 (Mid-to-distal LAD).
BMI indicates body mass index; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York heart association; PCI, percutaneous coronary intervention. Other concomitant surgeries included left atrial appendectomy, ventricular aneurysmectomy, and arteriovenous fistula surgery. Hospital complications included infection, bleeding, heart failure, and reoperation.
3.1.1 High QFR vs. low QFR
Patients with high QFR (n = 139) demonstrated significantly lower rates of diabetes mellitus (28.1% vs. 40.6%, p = 0.013), smoking (27.3% vs. 38.6%, p = 0.025), and 3-vessel disease (48.9% vs. 74.6%, p < 0.0001) compared to low QFR patients (n = 342). Preoperative left ventricular ejection fraction (LVEF) was higher in the high QFR group (57.5% ± 0.6% vs. 55.7% ± 0.5%, p = 0.025) compared to the low QFR group. High QFR patients also underwent more concomitant surgeries (35.3% vs. 14.9%, p < 0.0001), valvular procedures (26.6% vs. 10.5%, p < 0.0001), and on-pump CABG (30.2% vs. 9.6%, p < 0.0001) (Table 1).
3.1.2 Lesion site 1 vs. lesion site 2
Lesion Site 2 was associated with greater procedural complexity, including elevated rates of concomitant surgery (29.9% vs. 15.9%, p = 0.0005), valvular surgery (24.0% vs. 10.5%, p = 0.0002), and on-pump utilization (25.7% vs. 10.2%, p < 0.0001). Conversely, 3-vessel disease was more prevalent in Site 1 (70.7% vs. 60.5%, p = 0.03). Baseline demographic characteristics and comorbidities were comparable between lesion sites (p > 0.05) (Table 2).
3.1.3 High vs. low QFR at lesion site 1
Patients with high QFR at Lesion Site 1 exhibited significantly high preoperative LVEF (57.8 ± 0.8% vs. 55.8 ± 0.6%, p = 0.04) and elevated on-pump surgery rates (16.7% vs. 8.1%, p = 0.049). All other baseline variables, including age, sex, BMI, and comorbidities, did not exhibit significant differences between the groups (p > 0.05) (Table 3).
3.1.4 High QFR at lesion site 1 vs. low QFR at lesion site 2
Significant differences were observed in 3-vessel disease prevalence (60.0% vs. 74.5%, p = 0.038) and hospital stay duration (26.2 ± 1.0 vs. 30.5 ± 1.5 days, p = 0.021). Other clinical parameters, including hypertension and prior myocardial infarction (MI), showed no statistical differences (p > 0.05) (Table 4).
3.1.5 High QFR vs. low QFR at lesion site 2
Compared to patients with low QFR at Site 2, those with high QFR exhibited lower diabetes prevalence (21.3% vs. 42.5%, p = 0.009) and reduced 3-vessel disease (36.1% vs. 74.5%, p < 0.0001). Additionally, high QFR patients exhibited higher rates of concomitant surgery (50.8% vs. 17.9%, p < 0.0001), valvular surgery (41.0% vs. 14.2%, p = 0.0002), and on-pump CABG (47.5% vs. 13.2%, p < 0.0001) (Table 5).
Baseline characteristics for the remaining groups are presented in the Supplementary Material.
3.2 Clinical outcomes
During 5 years of follow-up, 107 MACCE events (cumulative incidence 22.2%) occurred among 481 patients, comprising 26 all-cause deaths (5.4%), 18 cardiovascular deaths (3.7%), 4 myocardial infarctions (0.8%), 22 strokes (4.6%), 5 repeat revascularizations (1.0%), and 28 hospitalizations for angina pectoris (5.8%). Stratification revealed 71 events in proximal LAD lesions (Site 1; N = 314) vs. 36 in mid-to-distal LAD lesions (Site 2; N = 167). Functional assessment showed 66 events in the low QFR group (QFR < 0.80; n = 337) compared to 41 in the high QFR group (QFR ≥ 0.80; n = 144). The integrated subgroups demonstrated: Subgroup A (High QFR at Lesion Site 1; n = 83) had 26 events, Subgroup B (Low QFR at Lesion Site 1; n = 231) had 45 events, Subgroup D (High QFR at Lesion Site 2; n = 61) had 15 events, and Subgroup C (Low QFR at Lesion Site 2; n = 106) had 21 events.
3.2.1 High QFR vs. low QFR
Patients with high QFR exhibited significantly lower cumulative MACCEs compared to low QFR patients [Log-rank P = 0.013, HR = 1.053, 95% CI (0.705–1.572)]. The divergence in event rates emerged as early as 12 months post-CABG and continued to widen over the 60-month follow-up period (Figure 2).
Figure 2
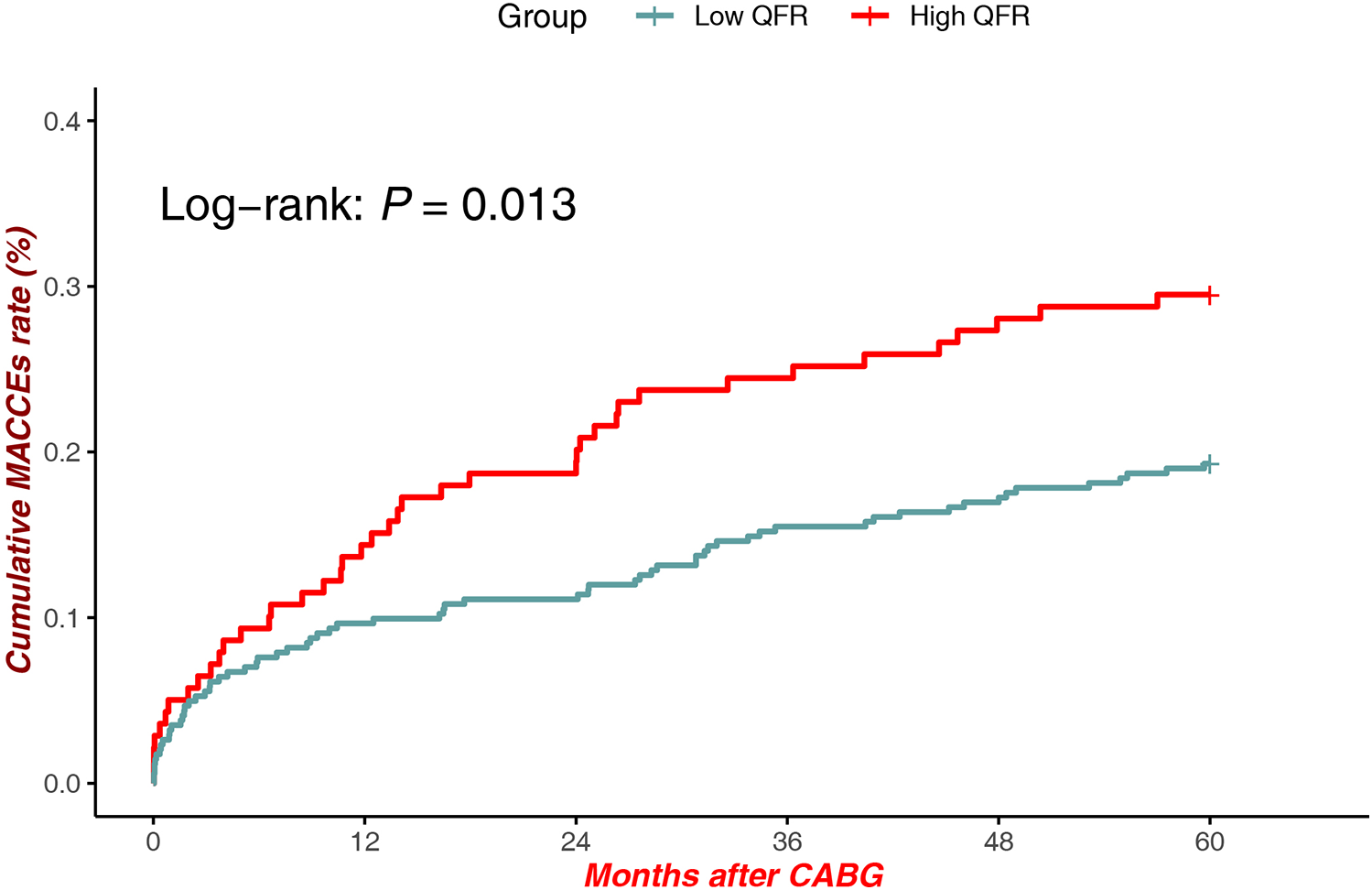
MACCEs in low QFR (all sites) vs. high QFR (All Sites).
3.2.2 Lesion site 1 vs. lesion site 2
There were no statistically significant differences in MACCEs between patients with lesions at Site 1 and Site 2 [Log-rank P = 0.8, HR = 1.634, 95% CI (1.106–2.413)], suggesting comparable long-term clinical outcomes regardless of anatomical lesion location (Figure 3).
Figure 3
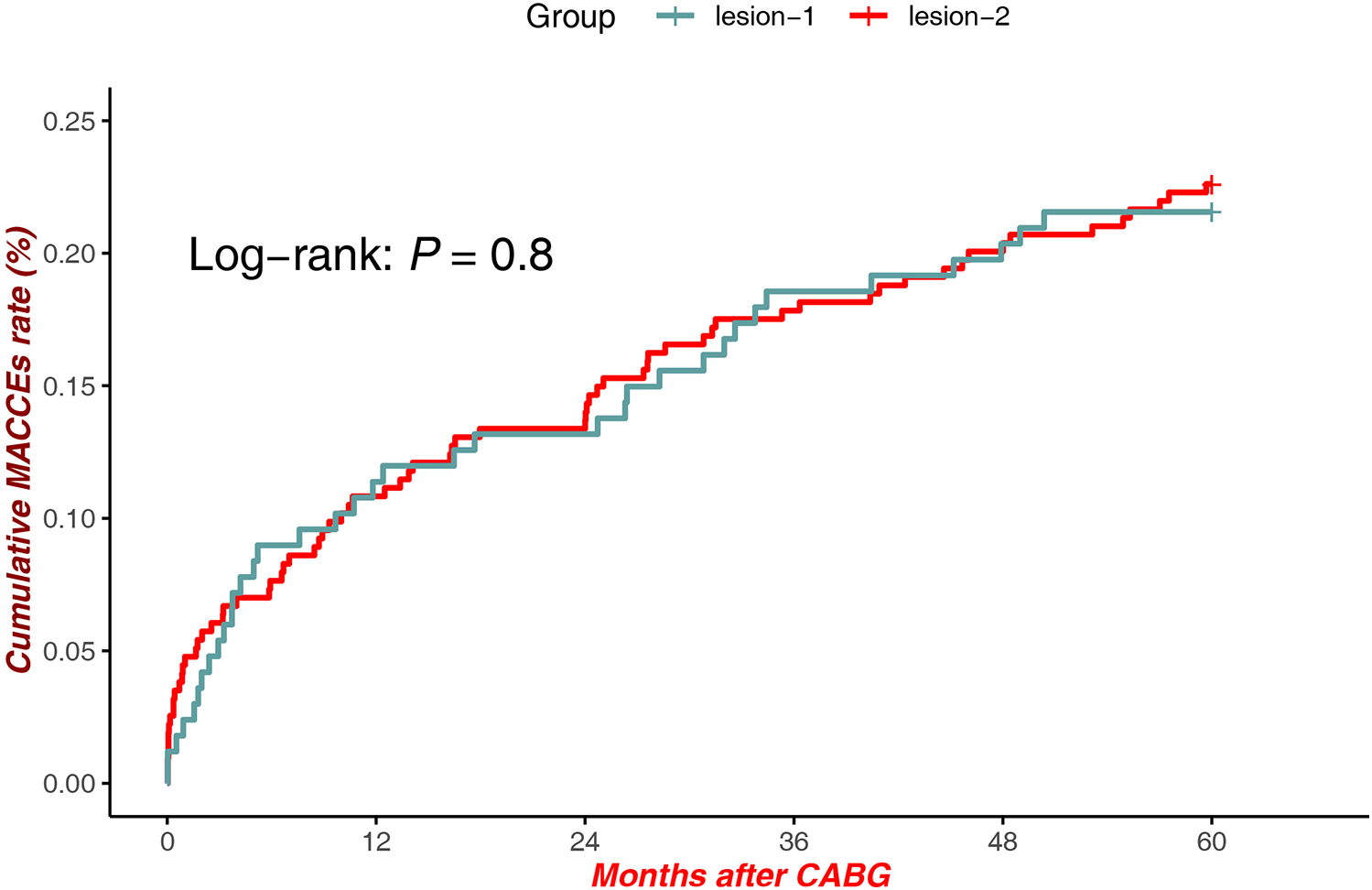
MACCEs in lesions (proximal LAD) vs. lesions (Mid-Distal LAD).
3.2.3 High QFR vs. low QFR at lesion site 1
Within lesions at Site 1, patients with high QFR demonstrated significantly reduced MACCEs compared to those with low QFR [Log-rank P = 0.0075, HR = 1.912, 95% CI (1.179–3.099)], underscoring the prognostic value of QFR stratification in this subgroup (Figure 4).
Figure 4
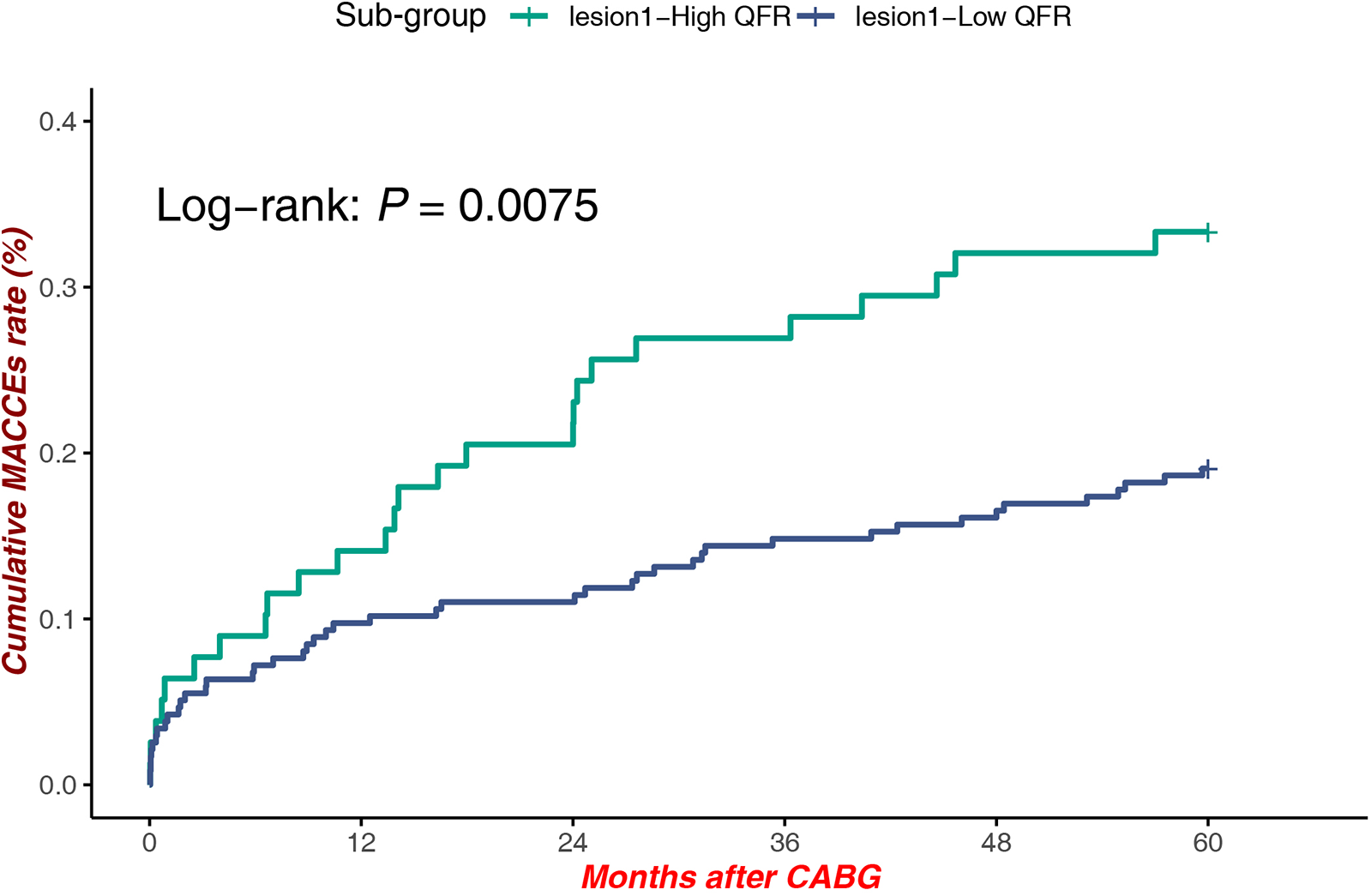
MACCEs in low QFR (proximal LAD) vs. high QFR (Proximal LAD).
3.2.4 High QFR at lesion site 1 vs. low QFR at lesion site 2
A significant survival benefit was observed in patients with high QFR at Site 1 compared to those with low QFR at Site 2 Low QFR patients [Log-rank P = 0.036, HR = 1.836, 95% CI (1.033–3.264)], highlighting the interplay between lesion location and hemodynamic significance in influencing clinical outcomes (Figure 5).
Figure 5
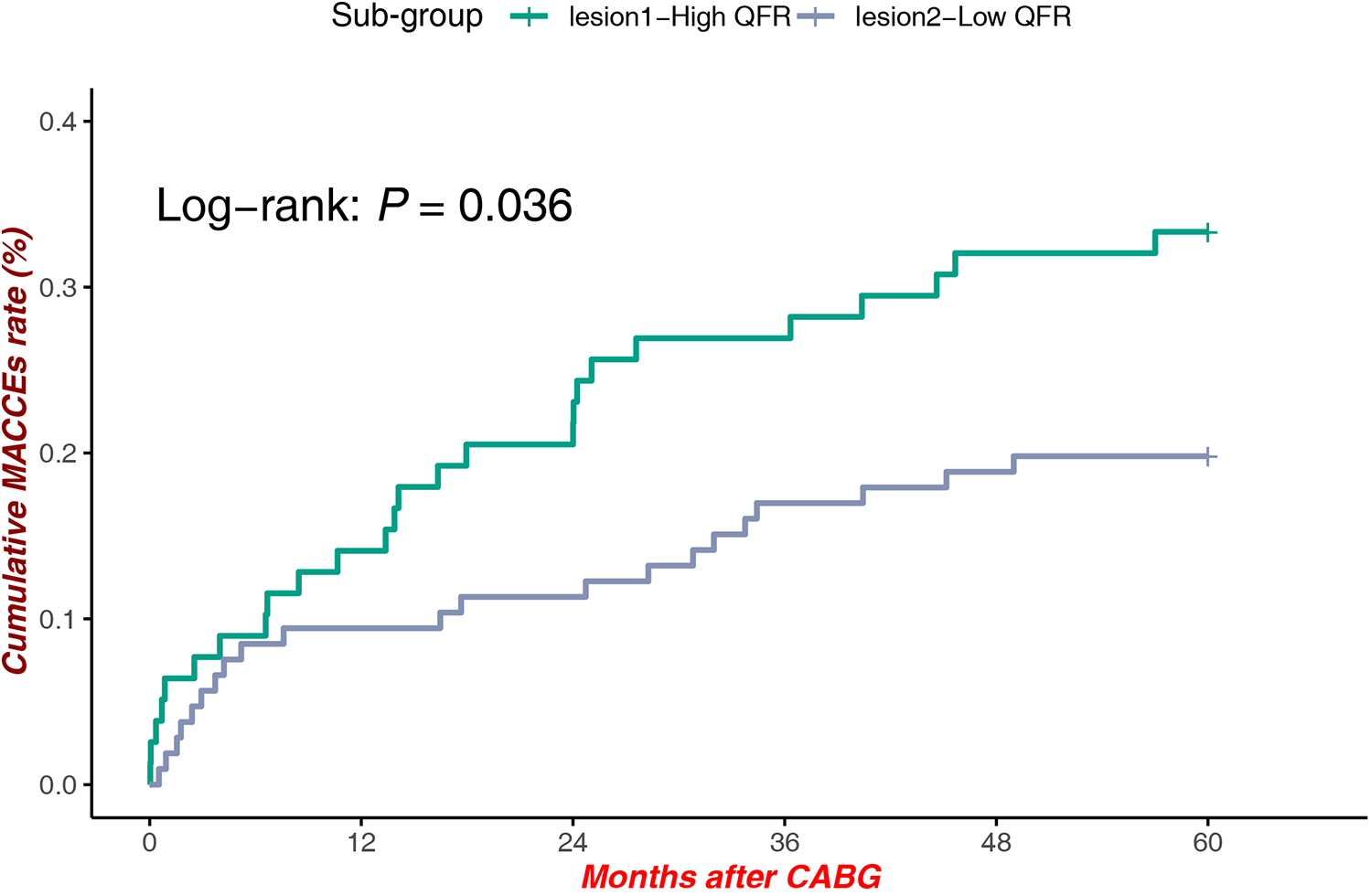
MACCEs in high QFR (proximal LAD) vs. low QFR (Mid-Distal LAD).
3.2.5 High QFR vs. low QFR at lesion site 2
Among patients with lesions at Site 2, QFR stratification was not associated with a statistically significantly difference in MACCEs rates [Log-rank P = 0.46, HR = 1.286, 95% CI (0.663–2.494)], indicating that anatomical and hemodynamic factors at this location may exert less impact on long-term outcomes (Figure 6).
Figure 6
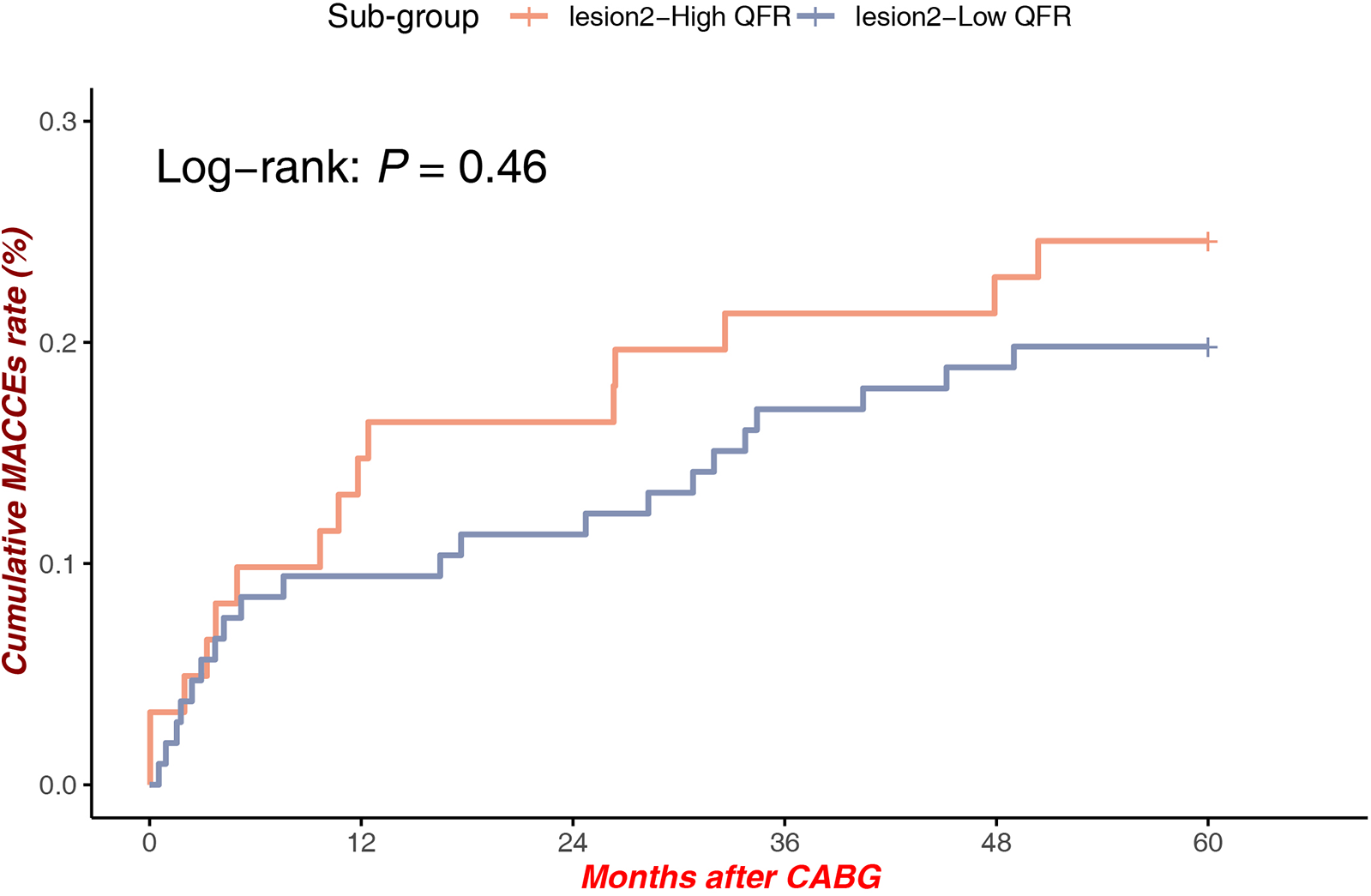
MACCEs in low QFR (Mid-distal LAD) vs. high QFR (Mid-Distal LAD).
Clinical outcomes for the remaining groups and multivariate cox regression for MACCEs with each individual event are presented in the Supplementary Material.
4 Discussion
In the current study, we found that LIMA grafting to quantitative flow ratio (QFR)-assessed functionally non-significant left anterior descending (LAD) arteries correlated with poor clinical outcomes compared with LIMA grafting to functionally significant LAD arteries. Notably, the anatomical location of coronary lesions did not significantly affect the long-term patient prognosis.
The principal objective of surgical coronary revascularization is to restore perfusion to the ischemic myocardial territories by deploying a low-resistance conduit in parallel alignment with the diseased coronary segment. Ideally, these adjunctive conduits must possess sufficient hemodynamic conductance to accommodate high-flow demands while maintaining minimal pressure attenuation throughout the distal implantation site. Competitive flow typically occurs when the resistance of the graft closely matches that of the stenosed native coronary artery (12). When analyzing the entire LAD using computational software, the functional significance of moderate stenosis localized to the mid-to-distal segment may be underestimated. This discrepancy arises from physiological pressure attenuation along the LAD. Which occurs naturally from its ostium to the distal segments under normal hemodynamics conditions (13). However, in QFR computation, the proximal pressure reference is typically derived from the vessel segment's origin, resulting in an overestimated baseline pressure compared to the actual pre-lesional pressure.
In this study, the analysis of QFR values across the entire LAD revealed that even when mid-to-distal segments were free of angiographically apparent proximal disease, the calculated QFR may be artifactually reduced. This phenomenon is attributed to coronary artery disease (CAD)-induced pathophysiological alterations (14), such as diffuse atherosclerosis or microvascular dysfunction (15), which may not manifest angiographically but contribute to distal pressure attenuation. Consequently, moderate stenosis in these segments may still induce myocardial ischemia due to impaired coronary flow reserve, potentially leading to angina pectoris despite the absence of hemodynamically significant proximal lesions.
Also, the lack of prognostic discrimination by QFR in mid-to-distal lesions may arises from: computational limitations in modeling serial stenoses. For improved assessment, we advocate combining invasive microcirculatory evaluation (IMR) with intravascular imaging (IVUS/OCT)-guided functional analysis to enable personalized ischemia risk stratification. Even when QFR values exceed the threshold 0.8, experienced cardiac surgeons may opt for LIMA grafting to the LAD following a multidisciplinary evaluation that integrates clinical manifestations, hemodynamic profiles, and ancillary diagnostic findings. This decision underscores the importance of individualized clinical judgment, which prioritizes anatomical complexity, ischemia burden, and long-term prognostic outcomes over isolated QFR thresholds alone in selected cases in select patients. Based on our findings, preoperative QFR assessment is recommended when feasible to optimize revascularization strategy selection and improve prognostic outcomes, without adding procedural risk. While QFR incurs higher costs, its integration should be guided by multidisciplinary evaluation and shared decision-making with patients, considering individual economic circumstances.
Conventionally, ostial and proximal coronary lesions are presumed to induce larger ischemic territories compared to mid-to-distal lesions, resulting in more severe clinical manifestations and poorer prognostic outcomes (16). However, the present study did not reveal significant differences in clinical outcomes between these lesion subtypes. This finding may be attributed to the effective surgical resolution of myocardial ischemia through comprehensive revascularization, as well as the high long-term graft patency rates associated with LIMA-LAD anastomosis (17). These factors collectively mitigate the impact of lesion location on ischemia-driven adverse events.
5 Limitation
This study has several limitations that warrant acknowledgment. First, its retrospective and observational design introduces inherent risks of selection bias, confounding by indication, and potential underreporting of adverse events, particularly given the limited sample size within certain subgroups. Second, the accuracy of QFR measurements is dependent on angiographic image quality and acquisition protocols, which could not be standardized in this retrospective analysis. Third, the number of clinical events was insufficient for robust multivariable adjustment of baseline differences between QFR groups (Supplementary Table S6), resulting in underpowered Cox regression analyses and residual confounding. Fourth, the follow-up duration (median 5 years) restricts the evaluation of long-term graft patency and late-term MACCEs dynamics, necessitating validation through longitudinal studies. Fifth, unmeasured confounders—such as microvascular resistance (18–20), plaque vulnerability, or genetic predispositions—were not adjusted for, potentially influencing QFR-outcome associations. Additionally, the single-center cohort limits the generalizability of these findings, underscoring the need for multicenter validation studies employing standardized angiographic acquisition protocols and adjudicated clinical endpoint reporting to enhance reproducibility and generalizability.
6 Conclusions
This study demonstrates that patients undergoing LIMA to LAD grafting with a preoperative LAD QFR > 0.80 exhibited poorer long-term prognoses, consistent with prior observational studies' evidence. Notably, in patients with mid-to-distal LAD lesions, QFR values exceeding 0.80 showed no significant association with adverse clinical outcomes. Furthermore, lesion location (proximal vs. mid-to-distal) did not independently influence long-term prognosis in the LIMA-LAD cohort.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethic committee of the Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZS: Data curation, Writing – original draft, Formal analysis, Investigation, Methodology, Software. YW: Writing – review & editing, Data curation. RJ: Data curation, Writing – review & editing. ZC: Writing – review & editing, Data curation. TW: Writing – review & editing, Data curation. WY: Validation, Resources, Formal analysis, Writing – review & editing, Project administration, Supervision. SY: Resources, Writing – review & editing, Validation, Project administration, Conceptualization, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1605573/full#supplementary-material
References
1.
LoopFDLytleBWCosgroveDMStewartRWGoormasticMWilliamsGWet alInfluence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. (1986) 314:1–6. 10.1056/NEJM198601023140101
2.
SpadaccioCGlineurDBarbatoEDi FrancoAOldroydKGBiondi-ZoccaiGet alFractional flow reserve–based coronary artery bypass surgery. JACC Cardiovasc Interv. (2020) 13(9):1086–96. 10.1016/j.jcin.2019.12.017
3.
WangCYHuZHouZHWangYSongLXuBet alImpact of preoperative quantitative flow ratio of the left anterior descending artery on internal mammary artery graft patency and midterm patient outcomes after coronary artery bypass grafting. J Am Heart Assoc. (2023) 12(11):e029134. 10.1161/JAHA.122.029134
4.
NeumannFJSousa-UvaMAhlssonAAlfonsoFBanningAPBenedettoUet alThe task force on myocardial revascularization of the European society of cardiology (ESC) and European association for cardio-thoracic surgery (EACTS). G Ital Cardiol (Rome). (2019) 20:1S–61. 10.1714/3203.31801
5.
TuSXBarbatoEKoszegiZYangJQSunZHHolmNRet alFractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv. (2014) 7:768–77. 10.1016/j.jcin.2014.03.004
6.
TuSXWestraJYangJQvon BirgelenCFerraraAPellicanoMet alDiagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR pilot study. JACC Cardiovasc Interv. (2016) 9:2024–35. 10.1016/j.jcin.2016.07.013
7.
XuBTuSXSongLJinZNYuBFuGSet alAngiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. (2021) 398(10317):2149–59. 10.1016/S0140-6736(21)02248-0
8.
FournierSCiccarelliGTothGGMilkasAXaplanterisPToninoPALet alAssociation of improvement in fractional flow reserve with outcomes, including symptomatic relief, after percutaneous coronary intervention. JAMA Cardiol. (2019) 4:370–4. 10.1001/jamacardio.2019.0175
9.
GötbergMBerntorpKRylanceRChristiansenEHYndigegnTGudmundsdottirIJet al5-year outcomes of PCI guided by measurement of instantaneous wave-free ratio versus fractional flow reserve. J Am Coll Cardiol. (2022) 79:965–74. 10.1016/j.jacc.2021.12.030
10.
ParikhRVLiuGPlomondonMESehestedTSGHlatkyMAWaldoSWet alUtilization and outcomes of measuring fractional flow reserve in patients with stable ischemic heart disease. J Am Coll Cardiol. (2020) 75:409–19. 10.1016/j.jacc.2019.10.060
11.
LeipsicJAbbaraSAchenbachSCuryREarlsJPManciniGJet alSCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr. (2014) 8(5):342–58. 10.1016/j.jcct.2014.07.003
12.
GlineurDHanetC. Competitive flow in coronary bypass surgery: is it a problem?Curr Opin Cardiol. (2012) 27(6):620–8. 10.1097/HCO.0b013e3283583000
13.
ChangHKimHKShinDLimKSKimSUJeonCYet alCoronary circulatory indexes before and after percutaneous coronary intervention in a porcine tandem stenoses model. J Am Heart Assoc. (2021) 10:e021824. 10.1161/JAHA.121.021824
14.
NomuraCHAssuncao-JrANGuimarãesPOLiberatoGMoraisTCFahelMGet alAssociation between perivascular inflammation and downstream myocardial perfusion in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging. (2020) 21(6):599–605. 10.1093/ehjci/jeaa023
15.
NgMKCYongASCHoMShahMGChawantanpipatCO’ConnellRet alThe index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv. (2012) 5(4):515–22. 10.1161/CIRCINTERVENTIONS.112.969048
16.
HyunJKimJHJeongYChoeKLeeJYangYJet alLong-term outcomes after PCI or CABG for left main coronary artery disease according to lesion location. JACC Cardiovasc Interv. (2020) 13(24):2825–36. 10.1016/j.jcin.2020.08.021
17.
HayashiYMaekawaASawakiSTokoroMYanagisawaJOzekiTet alLong-term patency of on- and off-pump coronary artery bypass grafting with bilateral internal thoracic arteries: the significance of late string sign development in the off-pump technique. Interact Cardiovasc Thorac Surg. (2017) 25:799–805. 10.1093/icvts/ivx214
18.
YongASCHoMShahMGNgMKCFearonWF. Coronary microcirculatory resistance is independent of epicardial stenosis. Circ Cardiovasc Interv. (2012) 5(1):103–8. 10.1161/CIRCINTERVENTIONS.111.966556
19.
LaylandJMacIsaacAIBurnsATSomaratneJBLeitlGWhitbournRJet alWhen collateral supply is accounted for epicardial stenosis does not increase microvascular resistance. Circ Cardiovasc Interv. (2012) 5(1):97–102. 10.1161/CIRCINTERVENTIONS.111.964718
20.
ChuJPLaiYYanWWYaoYLinHYuanDQet alImpact of radial wall strain on serial changes in vascular physiology in patients with intermediate coronary stenosis. Rev Cardiovasc Med. (2023) 24(8):245. 10.31083/j.rcm2408245
Summary
Keywords
quantitative flow ratio (QFR), coronary artery bypass grafting (CABG), left anterior descending artery (LAD), lesion localization, angiography
Citation
Sun Z, Wu Y, Jiang R, Chen Z, Wang T, Yan W and Yang S (2025) Impact of left anterior descending lesion location on midterm outcomes in patients undergoing left internal mammary artery grafting: a five-year cohort study integrating quantitative flow ratio assessment. Front. Cardiovasc. Med. 12:1605573. doi: 10.3389/fcvm.2025.1605573
Received
03 April 2025
Accepted
28 July 2025
Published
11 August 2025
Volume
12 - 2025
Edited by
Antonio Maria Calafiore, Henry Dunant Hospital, Greece
Reviewed by
Amr Arafat, Tanta University, Egypt
Konstantia-Paraskevi Gkini, National and Kapodistrian University of Athens, Greece
Updates
Copyright
© 2025 Sun, Wu, Jiang, Chen, Wang, Yan and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenlong Yan wenlong_yan@126.com Sumin Yang yangsumin@qdu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.