Abstract
Introduction:
Though numerous air pollutants have been mechanistically associated with inflammation and vascular morbidity, particulate matter (PM) is one of the pollutants with the strongest association. However, PM is generally categorized according to aerodynamic diameters between 2.5 and 10 μg m−3 (PM10) and less than 2.5 μg m−3 (PM2.5). Given their differential ability to enter the bloodstream, these sizes play a crucial role in the local or systemic inflammatory responses elicited after exposure. Given that vascular inflammation is a key marker of Kawasaki Disease (KD), this systematic review aims to summarize the available data on the association between KD and perinatal and early childhood PM exposure and identify any pathophysiological links.
Methods:
A systematic search was conducted using PubMed and EMBASE. Studies with PM included as a predictor, and Kawasaki disease as an outcome were included. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The risk of bias in the selected studies was assessed using the QUADAS-2 tool.
Results:
Eleven studies met the criteria for inclusion. All studies suggested an association between exposure to PM and increase risk or exacerbation of KD, though not all results reached statistical significance. Due to significant heterogeneity, pooled analyses were possible only in select studies for pre- and postnatal PM10 exposure and postnatal PM2.5 exposure. All studies identified immune-mediated inflammatory responses as a key pathophysiological link between exposure and KD, with PM10 noted as a significant risk factor for respiratory inflammation and poor maternal and child health, and PM2.5 for a wide range of adverse outcomes, especially in children and populations with preexisting inflammatory diseases. The role social, behavioral and environmental modifiers play in disease incidence was also highlighted.
Conclusion:
Particulate matter exposure is associated with an increased risk of developing and exacerbating KD, especially in populations experiencing temporary increased sensitivity and in populations with preexisting inflammatory diseases.
Systematic Review Registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD42023468937, PROSPERO CRD42023468937.
1 Introduction
Air pollution is associated with significant global morbidity and mortality, with 2021 estimates ranking it as the second leading risk factor for early death, contributing to 8.1 million deaths (1). Mechanistic studies have demonstrated the causative effect of exposure to numerous air pollutants. However, particulate matter, with aerodynamic diameters ranging from between 2.5 and 10 μg m−3 (PM10) and less than 2.5 μg m−3 (PM2.5) is one of the pollutants most strongly associated with adverse health effects. PM2.5 has notably demonstrated an ability to cross into the circulatory system, causing systemic toxic effects, including but not limited to respiratory, neurological, and cardiovascular dysfunction, typically driven by inflammatory and immunological responses (2–4). This systemic dysfunction is closely linked to higher rates of asthma, chronic lung disease, diabetes, cognitive decline, and various cardiovascular diseases (5–8). The impact of particulate matter exposures on the cardiovascular system is significant, given the global morbidity associated with cardiovascular diseases (9). Notably, cardiovascular diseases currently represent the top three leading causes of death, while air pollution ranks as the second leading risk factor (1, 9). Studies have demonstrated that exposure to PM, especially PM2.5, in pediatric populations is linked to an increase the risk of adverse cardiovascular outcomes, both in the short and long term (3, 10). Although various biological mechanisms play a role in these outcomes, they are generally the cumulative result of oxidative stress and endothelial dysfunction caused by vascular inflammation (8, 11).
Kawasaki disease (KD) is an example of an inflammatory vascular disease predominantly affecting pediatric populations under the age of five. It is characterized by inflammation of medium-sized arteries, especially the coronary arteries, and can lead to serious cardiovascular complications, including myocardial infarction, if not promptly treated (12). Although the etiology remains unclear, KD is believed to result from a dysregulated immune response triggered by environmental or infectious agents in genetically susceptible individuals (13). The burden of KD varies significantly across different countries, with the highest incidence found in North-East Asian countries. For example, in Japan, almost 1 in 100 children are affected by the disease by the age of five. In contrast, the lowest incidence is reported in sub-Saharan Africa (14). Globally, the incidence of KD has been increasing, particularly in East Asian countries and may partly be due to increased diagnostic awareness and environmental changes including increased prevalence of air pollutants (15, 16). Given that KD is characterized by inflammatory and immunological responses, the link between exposure to air pollutants, especially those that are causally linked to inflammatory outcomes, and the risk of development and exacerbation of KD is becoming clear (17–19). This justifies the focus on particulate matter, given its role as a trigger for immune-mediated inflammation in genetically susceptible children, which may lead to the onset or exacerbation of KD symptoms (20).
Thus, the objectives of this review are to: (1) summarize the available evidence on the association between perinatal and early childhood exposure to particulate matter and the risk and severity of Kawasaki disease, and (2) describe the likely pathophysiological connection between the disease and the pollutant in its varying aerodynamic diameters. Results from this review will add to the body of evidence on the influence of air pollution on the development and progression of Kawasaki disease, thus increasing indices of suspicion of these exposures during diagnosis and treatment.
2 Methods
2.1 Data sources and search strategy
A comprehensive search on PubMed and EMBASE was used to identify relevant studies, using the keywords (kawasaki) AND/OR (kawasaki disease) AND/OR mucocutaneous lymph node syndrome AND (particulate matter) AND/OR air pollution (Supplementary S1). Due to limited studies on this specific disease outcome, no date restrictions were applied to ensure all relevant studies were included.
2.2 Study selection and eligibility
This systematic review followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Citations retrieved from each database were imported into the reference manager Zotero v7.0.11, and duplicate entries were eliminated. Title and abstract screening by two independent reviewers (MK and TJ) determined article eligibility for inclusion. Subsequently, full texts of articles meeting established criteria were further assessed for relevance to this review. Discrepancies in eligibility assessments were resolved through discussion and arbitration by a third reviewer (CM). Studies were deemed eligible for inclusion in the review if they adhered to the following criteria: (1) employed a cohort, case-control, or cross-sectional design; (2) investigated or included the analysis of the prevalence or incidence of Kawasaki disease as an outcome, exposure to particulate matter as a predictor; and (3) incorporated a reference group. Excluded were (1) publications based on cell or animal model data and (2) reviews, commentaries, abstracts, and case reports (Supplementary S2). The protocol for this systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42023468937.
2.3 Data extraction and quality assessment
Two independent reviewers (MK and TJ) extracted the following information studies that met eligibility criteria: author, study site, design and sample size, objectives, and key findings. Disputes during bias assessments were resolved through discussion and arbitration by a third reviewer (CM). The risk of bias in selected studies was evaluated using the QUADAS-2 tool by NW and MR (Supplementary S3).
2.4 Statistical analysis
Statistical analysis was conducted to compare mean differences in PM10 and PM2.5 levels between individuals with Kawasaki disease and controls. Given the low heterogeneity among studies as indicated by the I2 statistic, fixed-effects (common-effects) meta-analyses were performed only for prenatal and postnatal PM10 levels, and postnatal PM2.5 levels. The mean difference (MD) was used as the summary measure, with 95% confidence intervals (CIs) calculated for each estimate. Forest plots were generated to visually summarize the meta-analysis results, showing individual study estimates with their weights, as well as the pooled MD and 95% CI. Analyses were conducted using the meta package in R, with the metacont function used to compute pooled effect estimates. Statistical significance was evaluated at p < 0.05. Publication bias was not assessed due to the small number of studies (n = 2). All statistical analyses were conducted using RStudio 2025.05.0 Build 496 (21).
3 Results
3.1 Study selection and quality assessment
A total of 121 papers met the initial search criteria. After removing duplicates and conducting an initial eligibility screening, 18 papers were selected for full-text review. This review resulted in a final selection of 11 papers that met the full eligibility criteria (Figure 1). These criteria included the use of observational study designs to quantitatively assess exposure to particulate matter (PM10 and PM2.5), regardless of source; measurable changes in risk, incidence, or prevalence of Kawasaki disease in prenatal and pediatric populations; and inclusion of a reference group (Figure 1).
Figure 1
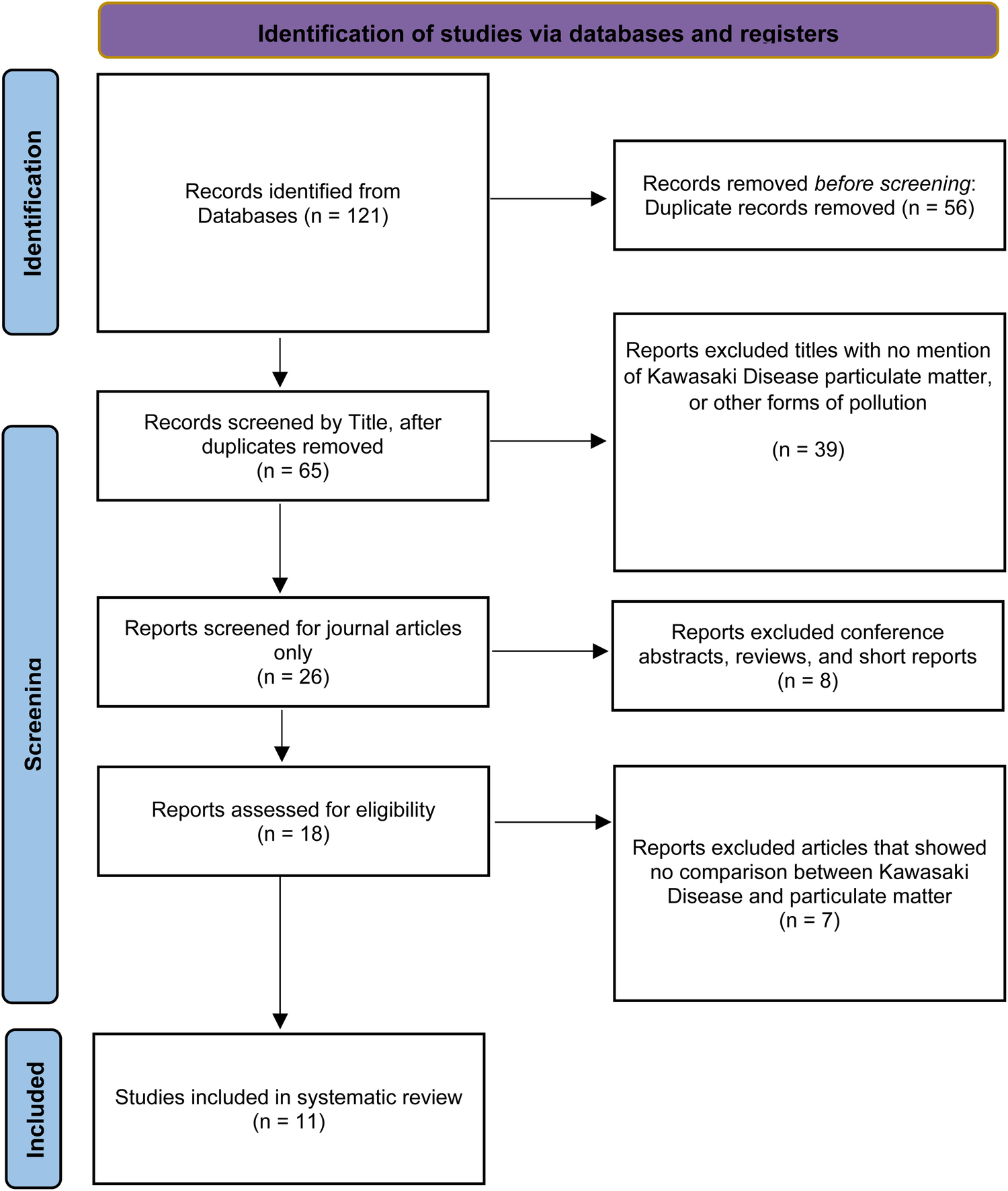
Flow diagram of the study selection process using the preferred reporting items for systematic reviews and meta-analyses.
The methodological quality of the selected studies was assessed using the QUADAS-2 tool. Despite a generally low risk of bias across the domains of subject selection, reference standard, and flow and timing, a high risk of bias was observed in the index test domain. This is partly due to the inherent limitations of environmental exposure studies, which rely on local or regional ambient particulate matter concentrations rather than a specific diagnostic test. Additionally, the selected studies did not specify a threshold but instead used post hoc statistical modeling (Supplementary S3).
3.2 Summary analysis of selected studies
A summary of the results is presented in Table 1. Although results varied across studies, positive associations were reported between exposure to particulate matter and increased KD risk. However, only cohort and case-control studies on PM2.5 showed statistically significant associations. These studies typically involved large sample sizes and longitudinal exposure assessments. In contrast, case-crossover studies assessing short-term exposure showed mixed results, despite similar directional trends.
Table 1
| Reference | Country affected | Study design | Sample number | Results | Findings |
|---|---|---|---|---|---|
| Buteau et al., (36) | Canada | Retrospective cohort Predictor: prenatal exposure; mean annual PM2.5 concentrations obtained from satellite estimates and regression models Outcome: KD incidence in children | 505,336 children, including 539 with KD | Ambient PM2.5 exposure associated with KD incidence: HR = 1.16 (95%CI: 0.96–1.39). Industrial PM2.5 exposure associated with KD: HR = 1.01 (95%CI: 0.97–1.05). | Observed association between PM2.5 and KD incidence. Findings not statistically significant. PM10 was not studied. |
| Jung et al., (31) | Taiwan | Case-crossover (time stratified) Predictor: hourly PM10 concentrations from fixed sites Outcome: Hospitalization of children with KD | 695 KD hospital admissions | IQR (40.60 μg m−3) increase in PM10 concentration associated with KD hospitalization: aOR 1.10 (95%CI: 0.94–1.30). | Observed association between PM10 and KD hospitalizations. Findings not statistically significant. PM2.5 was not studied. |
| Kim et al., (37) | South Korea | Cohort Predictor: monthly PM2.5 concentrations from an ensemble model Outcome: KD incidence in children | 134,634 individuals, including 1,220 that developed KD | Annual 5 μg m−3 increase in PM2.5 concentration associated with KD incidence: HR = 1.21 (95%CI: 1.05–1.39). | Observed association between PM2.5 and KD incidence. Location, income, sex, and age were important predictors of the HR of PM2.5 on KD. Findings statistically significant. |
| Kuo et al., (24) | Taiwan | Case-control Predictor: pre-and postnatal exposure; monthly composite pollutant standards index (PSI) concentrations from fixed sites Outcome: KD incidence in children | 16,768 without KD, 4,192 with KD | Prenatal exposure to PSI and KD incidence: OR = 1.01 (95%CI; 1.00–1.01). Postnatal exposure to PSI and KD incidence after pregnancy: 1.00 (95%CI; 1.00–1.01). | PSI included SO2, NO2, O3, CO, and PM10 were positively associated with KD incidence before and after pregnancy. Findings are borderline statistically significant. |
| Kwon et al., (30) | South Korea | Case-crossover (time stratified) Predictor: daily PM2.5 concentrations from fixed sites Outcome: KD incidence in children | 51,486 children that were treated for KD | At lag 0, IQR (14.67 μg m−3) increase PM2.5 concentration associated with KD incidence: aOR = 1.007 (95%CI: 0.994–1.020). At lag 0, IQR (28.68 μg m−3) increase PM10 concentration associated with KD incidence: aOR = 1.003 (95%CI: 0.992–1.015). At lag 1, IQR (14.67 μg m−3) increase PM2.5 concentration associated with KD incidence: aOR = 1.016 (95%CI: 1.004–1.029). At lag 0, IQR (28.68 μg m−3) increase PM10 concentration associated with KD incidence: aOR = 1.010 (95%CI: 0.999–1.022). | Observed association between PM10, PM2.5 and KD risk and lag 0 and lag 1. Only the findings for PM2.5 at lag 1 and KD risk were statistically significant. Lag 0 = day of fever onset Lag 1 = day preceding fever onset |
| Lin et al., (29) | China | Cohort Predictor: daily PM10 concentrations from fixed sites Outcome: KD incidence in children | 2,344 KD cases | At lag 0, PM10 exposure associated with percent increase in KD: 0.04% (95% CI: −1.34%, 1.34%) At lag 1, PM10 exposure associated with percent increase in KD: 1.05% (95% CI: −0.31%, 2.43%) At lag 2, PM10 exposure associated with percent increase in KD: 1.48% (95% CI: −1.07%, 1.89%) | Consistently positive associations between PM10 and daily Kawasaki disease (KD) incidence. Findings not statistically significant. PM2.5 was not studied. Lag 0 = current day of hospitalization Lag 1 = day preceding hospitalization Lag 2 = two days preceding hospitalization |
| Oh et al., (25) | South Korea | Case-crossover Predictor: atmospheric reanalysis Outcome: Children hospitalizations for KD | 771 KD cases | At lag 0–2, PM2.5 and KD hospitalization: OR = 1.00 (95% CI: 0.95, 1.06). At lag 7, PM2.5 and KD hospitalization: OR = 0.99 (95% CI: 0.91, 1.07). At lag 14, PM2.5 and KD hospitalization: OR = 0.93 (95% CI: 0.82, 1.05). Subgroup analyses (adjusting for multiple predictor pollutants): At lag 0–2, PM2.5 and KD hospitalization: OR = 1.01 (95% CI: 0.96, 1.06); adjusted for SO2. At lag 0–2, PM2.5 and KD hospitalization: OR = 1.03 (95% CI: 0.98, 1.10); adjusted for NO2. At lag 0–2, PM2.5 and KD hospitalization: OR = 1.02 (95% CI: 0.95, 1.09); adjusted for CO. At lag 0–2, PM2.5 and KD hospitalization: OR = 1.01 (95% CI: 0.96, 1.07); adjusted for O3. | No statistically significant association was found between KD and short-term PM2.5 exposure. PM10 was not studied in multi-pollutant models. Lag 0 -2 = two day moving average Lag 7 = seven day moving average Lag 14 = fourteen day moving average |
| Si et al., (19) | China | Case-crossover Predictor: monthly mean PM concentrations from fixed sites Outcome: KD incidence in children | 3,036 KD cases | Each 1 μg m−3 increase in PM2.5 concentration associated with increase in KD incidence: OR 0.22 (95% CI: 0.01, 0.42). Each 1 μg m−3 increase in PM10 concentration associated with decrease in KD incidence: OR −0.23 (95% CI: −0.67, 0.21). | Observed positive association between PM2.5 and KD incidence. Findings statistically significant. Observed negative association between PM10 and KD incidence. Findings not statistically significant. Statistical potentially limited due to use of seasonal statistics instead of daily statistics. |
| Yoneda et al., (17) | Japan | Retrospective cohort Predictor: monthly and annual PM concentration from fixed sites Outcome: KD incidence | 55,289 KD cases before COVID and 14,023 KD cases after | Before COVID-19 pandemic, each 1 μg m−3 annual PM2.5 exposure associated with KD incidence rate ratio: IRR = 1.03 (95%CI: 1.01–1.06) During COVID-19 pandemic, each 1 μg m−3 annual PM2.5 exposure associated with KD incidence rate ratio: IRR = 1.10 (95%CI: 1.04–1.17) | Observed link between annual exposure to PM2.5 and the onset of KD. Findings statistically significant. |
| Yorifuji et al., (23) | Japan | Cohort Predictor: pre-and postnatal; monthly SPM concentrations from fixed sites Outcome: Children hospitalizations for KD | 22,358 children, including 193 KD hospital admissions | Suspended particulate matter (SPM) associated with KD hospitalization: Prenatal Exposure to SPM > 25 μg/m3 vs. less than 20 μg m−3 with an OR = 1.59 (95%CI: 1.06–2.38) Postnatal exposure to SPM > 25 μg/m3 vs. less than 20 μg m−3 with an OR was 1.41 (95%CI: 0.82–2.41). | Stronger link between prenatal exposure and KD hospitalization compared to postnatal exposure. |
| Zeft et al., (47) | US and Canada | Case-crossover Predictor: daily PM2.5 concentration from fixed sites Outcome: KD onset in children | 3,009 KD cases | The OR for a 2-day moving average for KD association with an increase of 10 μg m−3 in PM2.5 exposure was 0.980 (95%CI: 0.915–1.050). | No evidence of an association between PM2.5 and KD was found when studying a 2-day moving average. |
Characteristics of eleven included studies on the association between PM exposure and Kawasaki disease.
Note: Studies arranged in alphabetical order.
3.3 Pooled analysis of selected studies
Due to significant methodological heterogeneity, a meta-analysis of all studies was not appropriate. This heterogeneity stemmed partly from differing outcome measures and regression results. Outcomes were assessed in various ways, with some studies using data from fixed-site monitoring stations and others relying on satellite-based estimations. Predictor variables also varied significantly, with some studies evaluating short-term exposure and others focusing on long-term exposure, leading to notable incomparability when discussing biological mechanisms. Finally, one study notably assessed exposure to suspended particulate matter (SPM) which corresponds to all particles with aerodynamic diameters < 10 µm, with a 50% cut-off at 7 µm, functionally categorizing it as PM10 (22). Nevertheless, comparisons with PM10 should acknowledge this source variability. However, meta-analyses were possible using subset data for pre- and postnatal PM10 exposure (23, 24) and postnatal PM2.5 exposure (24, 25) as they met criteria and provided data to estimate the mean difference in PM exposure between individuals with Kawasaki and controls.
The pooled analysis of prenatal PM10 exposure levels among children diagnosed with Kawasaki disease compared to controls, based on two eligible studies (n = 43,318), is shown in Figure 2. The pooled mean difference in PM10 concentration was 0.20 µg m−3 (95% CI: −0.01 to 0.42), indicating a modest elevation in prenatal exposure among KD cases. Heterogeneity was absent (I2 = 0, τ2 = 0), suggesting high consistency between studies. However, the result did not reach statistical significance. Similarly, the pooled analysis of postnatal PM10 exposure in Figure 3 shows a mean difference of 0.19 µg m−3 (95% CI: −0.04 to 0.46) between children diagnosed with Kawasaki disease and controls (n = 43,318). Heterogeneity was also absent, indicating consistent results. However, the findings were not statistically significant, suggesting that within the 0–10 µg/m3 exposure range, postnatal ambient PM10 levels do not differ meaningfully between cases and controls. Finally, the pooled analysis of two studies evaluating postnatal PM2.5 exposure between Kawasaki disease cases and controls (n = 24,360) also found no statistically significant difference (Figure 4). The pooled mean difference was 0.95 µg/m3 (95% CI: −0.58 to 2.49). While not statistically significant, the point estimate suggests higher exposure among KD cases.
Figure 2
![Forest plot comparing Kawasaki and Control groups in two studies: Yorifuji 2018 and Kuo 2022. Yorifuji shows a mean difference of 0.30 with a 95% confidence interval of [-0.35; 0.95] and weight of 11%. Kuo shows a mean difference of 0.19 with a 95% confidence interval of [-0.04; 0.42] and weight of 89%. The combined analysis indicates a mean difference of 0.20 with a 95% confidence interval of [-0.01; 0.42]. Heterogeneity is 0%. The plot suggests no significant overall effect, centered near zero.](https://www.frontiersin.org/files/Articles/1611757/xml-images/fcvm-12-1611757-g002.webp)
Pooled mean difference in prenatal PM10 exposure and children with Kawasaki disease and controls.
Figure 3
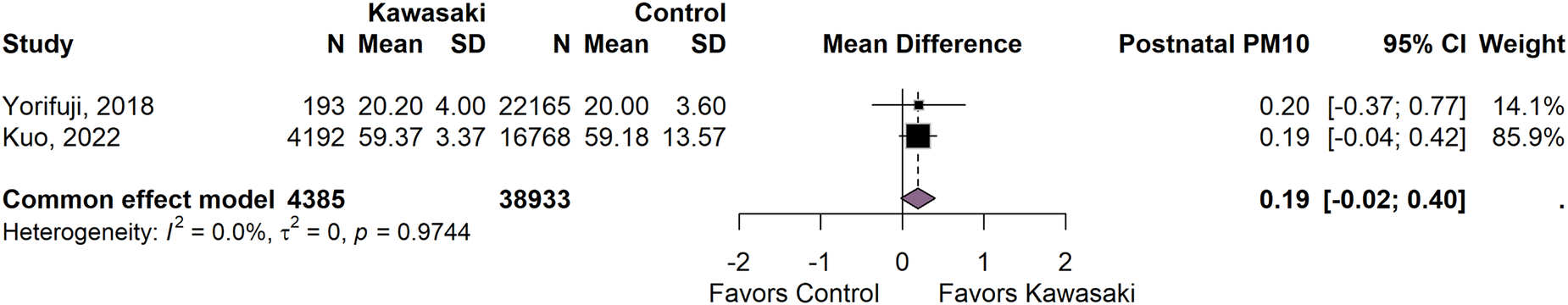
Pooled mean difference in postnatal PM10 exposure and children with Kawasaki disease and controls.
Figure 4
![Forest plot illustrating the mean difference in studies comparing Kawasaki and control groups, with data from Oh, 2021 and Kuo, 2022. Included are sample sizes, means, and standard deviations for both groups. The mean differences and 95% confidence intervals are shown, with postnatal PM2.5 values and weights. The common effect model shows a mean difference of 0.95 with a confidence interval of [-0.58, 2.49]. The plot indicates heterogeneity statistics: I^2 = 0.0%, τ^2 = 0, p = 0.7261. Results are displayed on a scale favoring control or Kawasaki.](https://www.frontiersin.org/files/Articles/1611757/xml-images/fcvm-12-1611757-g004.webp)
Pooled mean difference in postnatal PM2.5 exposure and children with Kawasaki disease and controls.
4 Discussion
4.1 PM10 and KD
Inhalation of PM10 particles leads to the penetration of the upper respiratory system and lungs, triggering an inflammatory cascade through oxidative stress and the activation of alveolar macrophages and dendritic cells (26). This activation results in the release of several pro-inflammatory cytokines, including IL-6, IL-1β, and TNF-α which promotes systemic inflammation and oxidative stress (27). Following penetration of the upper respiratory tract, PM10 disrupts tight junctions between endothelial cells leading to increased vascular permeability and in turn may be associated with the vascular inflammation characteristic of KD (28). Despite the biological plausibility, the epidemiological studies included in this review have yielded inconsistent findings on the association between PM10 and KD.
The nationwide longitudinal study in Japan performed by Yorifuji et al. on prenatal and postnatal exposure to suspended particulate matter (SPM) provided supporting evidence for the potential role of PM10 in the development of KD (23). Notably, the ORs for SPM exposure ≥25 µg m−3 compared with <20 µg m−3 was 1.59 (95% CI 1.06, 2.38). The authors also noted that SPM exposure during mid-to-late gestation appeared to significantly increase the risk of developing KD (23). Despite not reaching statistical significance, the meta-analysis suggests that prenatal exposure to PM10 increase susceptibility of exposed children to KD (23, 24), likely due to its ability to cross the placenta and alter immune system development (Figure 2). Postnatal exposure was also associated with a slight increase in KD risk (Figure 3). Lin et al. in Shanghai, China and Kwon et al. in Korea also showed an increase in KD incidence following exposure to PM10, also did not reach statistical significance (29, 30). Other studies that utilized population data from Taiwan by Jung et al. and Kuo et al. found no significant association between PM10 and KD entirely (24, 31). This suggests that while PM10 may contribute to KD pathogenesis, its impact may be weaker than that of other air pollutants and particulate matter with smaller aerodynamic diameters.
One potential explanation for the weak association between PM10 and KD is the larger size of the particles in comparison to finer particulate matter (PM2.5) and other air pollutants, which might limit their ability to penetrate deep in the alveolar system and get into systemic circulation (32). As a result, PM10 is generally associated with localized respiratory inflammation rather than the systematic immune response required to induce KD (33). Additionally, variations in study design may have contributed to the weaker association between PM10 and KD. Another crucial limitation across all studies is the lack of genetic stratification, despite KD's well-established link to genetic predisposition. Genetic factors, such as polymorphisms in the CXCL10/IP10 gene, which encodes a chemokine involved in immune function, have been linked to an increased risk of KD, supporting a causal relationship with susceptibility to environmental triggers such as PM10 (34, 35). This suggests that genetic susceptibility may act as a significant modifier, and if not accounted for, studies may underestimate the impact of PM10 on susceptible children.
4.2 PM2.5 and KD
PM2.5 is characterized by its ability to penetrate the alveoli and enter the bloodstream, triggering a significant inflammatory response (3, 4). In pooled analyses, postnatal exposure is associated with a slight but not statistically significant increase in KD risk (Figure 4). Despite the debate surrounding the precise etiology of KD, the associated vasculitis is known to involve inflammatory and immune responses, supporting the hypothesis that PM2.5 may be a significant environmental trigger (36). Buteau et al. found a positive association between ambient PM2.5 and KD at a Hazard Ratio for interquartile range increments of air pollutant exposure (HRIQR) of 1.16. Despite not reaching statistical significance, the results also suggested a greater susceptibility of those with preexisting diabetes for KD with respect to the increased inflammatory processes (37). Kim et al. corroborated these findings but with a statistically significant HR of 1.21 for each 5 μg/m3 increase in the annual PM2.5 concentration and a greater correlation when considering subpopulations (37). They specifically looked at biological sex and found that biological females could be affected more readily by oxidative stress, strengthening the idea that PM2.5 induces an immune response, which in turn induces KD. Their subgroup analysis showed that for biological females, the HR was 1.39 (95% CI: 1.12–1.73) for each 5 μg/m3 increase in PM2.5 concentration, while for biological males, the HR was 1.09 (95% CI: 0.90–1.31) (38). This may be partly due to the higher susceptibility of biological females to oxidative stress and greater immune response following exposure to air pollution (39). Additionally, biological females may be more sensitive to air pollutants in early life and more prone to inflammatory responses that could trigger the onset of KD (30).
Kwon et al. found a statistically significant effect of PM2.5 on KD at lag 1 (one day before the onset of KD), suggesting a possible short-term impact consistent with an immunological trigger (30, 37). Nevertheless, the statistical significance of the daily mean results observed by Kwon et al. and the annual mean results observed by Kim et al. suggest that while short-term PM2.5 exposure results in an acute inflammatory response, chronic exposure could also lead to prolonged inflammation and immune dysfunction (25). Oh et al. 2021, observed a similar outcome to Kwon et al., with the 2-day average of PM2.5 concentration showing an OR of 1.01 though no positive associations with the 7-day or 14-day averages (23, 25, 36). Si et al. and Yoneda et al. also found statistically significant increases in KD incidence during high vs. low PM2.5 seasons, with Yoneda et al. observing a consistent 3%–10% rise in KD incidence for each 1 μg/m3 increase in annual PM2.5 exposure (17, 19, 37).
Despite variations in statistical significance, all reviewed studies discussed systemic inflammation and immune dysregulation as the primary mechanisms involved in the pathogenesis of KD as a result of both direct inhalation of particulate matter and epigenetic effects. The authors suggest that inhalation of particulate matter is associated with changes in adaptive immune responses and induces cytokine-mediated endothelial cell damage, particularly in genetically predisposed populations (29). A link has also been suggested between perinatal exposure to air pollutants and maternal systemic inflammation, placental inflammatory disorders, and altered fetal immune responses, all of which are implicated in the disease's development. Although statistical significance was not achieved in subgroup analyses of PM exposure alone, findings from multipollutant models highlight the importance of evaluating both synergistic interactions and cumulative exposures when assessing risk (23).
4.3 Social, behavioral and environmental modifiers
In addition to evaluating multipollutant scenarios, it is also important to consider the social and environmental modifiers of KD incidence. Lin et al. evaluated the impact of high temperatures, described as the 99th percentile (32.4°C), and found a statistically significant increase in KD incidence with a cumulative relative risk of 1.91 (95% CI: 1.13, 3.23) compared to 10°C (37). On the other hand, Yorifuji et al. found that cold weather was also associated with an increased risk of hospitalization, with an odds ratio of 1.51 (95% CI: 1.07, 2.12) for a 20–25 µg/m3 exposure (36). The observed reduction in KD incidence during the COVID-19 pandemic years supports the role behavioral modifiers play in disease incidence. This decline coincided with decreased PM2.5 levels and the widespread implementation of non-pharmaceutical interventions, including mask mandates and social distancing measures, which ultimately reduced exposure to air pollutants, including particulate matter (37). A similar behavioral influence was observed when adjusting for maternal smoking during pregnancy. Buteau et al. reported a higher incidence rate among children of smoking mothers (19.8 cases per 100,000 person-years) compared to those of non-smoking mothers (12.3), again highlighting the role behavior can play in exposure levels and disease incidence (36). Socioeconomic modifiers also play a significant role in disease incidence. Populations from lower income settings were found to have higher KD incidence likely due to the cumulative burden of inadequate housing and healthcare access, as well as a limited ability to reduce exposure through solutions such as air purifiers (40). Buteau et al. specifically adjusted for neighborhood socioeconomic status and found higher incidence rates in areas of high material deprivation, at 13.4 cases per 100,000 person-years, compared to between 10.1 and 12.9 cases in areas with lower levels of material deprivation (41). These findings emphasize the importance of incorporating social, behavioral and environmental modifiers in future exposure assessment models, thus strengthening risk stratification and intervention targeting.
4.4 Study limitations and future perspectives
Although the studies in this review reported an overall positive association between particulate matter exposure and KD risk, several methodological limitations may affect the reliability of these findings. Firstly, the total number of reviewed studies was small, which highlights the need for additional primary research. Secondly, many studies relied on environmental exposure measurements, such as fixed-site or satellite observations, rather than individual-level assessments which may not accurately reflect personal exposure levels (42). Environmental data carries notable causal uncertainty, as generalized exposure measurements may underestimate the true effects (43). These challenges are especially pronounced in air pollution research, where the spatial resolution is often too broad to capture localized variability (44). Another important consideration is the mobility of participants during the study period, which can meaningfully impact analyses of perinatal outcomes, given variations in susceptibility throughout pregnancy and postpartum (24, 25, 31). Finally, while administrative health data help reduce recall bias, they often lack clinical detail and can inflate estimates. This particular challenge, combined with the absence of a gold standard diagnostic test for Kawasaki disease, increases the risk of misdiagnosis or outcome misclassification (45, 46).
Some of the results from the reviewed studies did not reach statistical significance, despite this overall trend of a positive association between increased particulate matter exposure and KD. Thus, as suggested by authors, future research should focus on validating this association across a range of study populations and geographical settings, and examine the biological and pathophysiological mechanisms involved (30). Addressing limitations in exposure assessment through individual-level exposure measurements, which are critical in air pollution research, may also strengthen the results (45, 46). Finally, as noted by Kwon et al., given the limitations of relying solely on clinical features for diagnosis, future studies may benefit from including objective inflammatory markers as intermediate indicators of Kawasaki disease (30).
5 Conclusion
This review explores the association between particulate matter (PM) exposure and the risk of developing and exacerbating Kawasaki disease (KD). Differentiation between aerodynamic sizes is particularly important. PM10, while linked to significant inflammatory responses, primarily affects the upper respiratory tract due to its limited ability to enter the bloodstream. Nevertheless, this inflammation, mediated by oxidative stress and cytokine production, may contribute to KD exacerbation, especially during mid-gestation with potential implications for maternal and child health. In contrast, PM2.5 penetrates the respiratory system and enters systemic circulation, triggering broader inflammatory and immune responses. Genetically predisposed children and individuals with preexisting inflammatory conditions, including metabolic syndromes, appear to be at increased risk due to heightened baseline inflammatory activity. Although not all reviewed studies reached statistical significance, the findings show a general positive association between exposure and disease and highlight the need for continued investigation into chronic air pollution exposure and its impact on KD. Future research should also examine the specific effects of these exposures on vulnerable populations to improve population health. In addition to these biological pathways, future research that integrates environmental, behavioral, and social modifiers into exposure assessment models will strengthen risk stratification, intervention targeting, and ultimately improve population health.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CM: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. MK: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. TJ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. NW: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. MR: Formal analysis, Writing – original draft, Writing – review & editing. KM: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Dr. Prakash Ramdass for his contribution to the meta-analysis component of this study, including statistical consultation and data synthesis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Microsoft Copilot, based on the GPT-4 architecture was used for spelling and grammar checks.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1611757/full#supplementary-material
References
1.
HEI. State of Global Air 2024 - A Special Report on Global Exposure to air Pollution and its Health Impacts, with a Focus on Children’s Health. Boston, MA: Health Effects Institute (2024).
2.
SchraufnagelDE. The health effects of ultrafine particles. Exp Mol Med. (2020) 52(3):311–7. 10.1038/s12276-020-0403-3
3.
BasithSManavalanBShinTHParkCBLeeW-SKimJet alThe impact of fine particulate matter 2.5 on the cardiovascular system: a review of the invisible killer. Nanomaterials (Basel). (2022) 12(15):1–28. 10.3390/nano12152656
4.
GarciaASanta-HelenaEDe FalcoAde Paula RibeiroJGiodaAGiodaCR. Toxicological effects of fine particulate matter (PM2.5): health risks and associated systemic injuries-systematic review. Water Air Soil Pollut. (2023) 234(6):346. 10.1007/s11270-023-06278-9
5.
ZhangXChenXZhangX. The impact of exposure to air pollution on cognitive performance. Proc Natl Acad Sci U S A. (2018) 115(37):9193–7. 10.1073/pnas.1809474115
6.
SukumaranKBotternhornKLSchwartzJGaudermanJCardenas-IniguezCMcConnellRet alAssociations between fine particulate matter components, their sources, and cognitive outcomes in children ages 9–10 years old from the United States. Environ Health Perspect. (2024) 132(10):107009-1–16. 10.1289/EHP14418
7.
PsistakiKRichardsonDAchilleosSRoantreeMPaschalidouAK. Assessing the impact of climatic factors and air pollutants on cardiovascular mortality in the eastern Mediterranean using machine learning models. Atmosphere (Basel). (2025) 16(3):325. 10.3390/atmos16030325
8.
KumarVSHHuligowdaLKDUmeshMChakrabortyPThazeemBet alEnvironmental pollutants as emerging concerns for cardiac diseases: a review on their impacts on cardiac health. Biomedicines. (2025) 13(1):1–25. 10.3390/biomedicines13010241
9.
BrauerMRothGAAravkinAYZhengPAbateKHAbateYHet alGlobal burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2162–203. 10.1016/S0140-6736(24)00933-4
10.
LiuYLiYXuHZhaoXZhuYZhaoBet alPre- and postnatal particulate matter exposure and blood pressure in children and adolescents: a systematic review and meta-analysis. Environ Res. (2023) 223:115373. 10.1016/j.envres.2023.115373
11.
PryorJTCowleyLOSimondsSE. The physiological effects of air pollution: particulate matter, physiology and disease. Front Public Health. (2022) 10:882569. 10.3389/fpubh.2022.882569
12.
McCrindleBWRowleyAHNewburgerJWBurnsJCBolgerAFGewitzMet alDiagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135(17):e927–99. 10.1161/CIR.0000000000000484
13.
LowTMcCrindleBWMuellerBFanC-PSSomersetEO’SheaSet alAssociations between the spatiotemporal distribution of Kawasaki disease and environmental factors: evidence supporting a multifactorial etiologic model. Sci Rep. (2021) 11(1):14617. 10.1038/s41598-021-93089-9
14.
ElakabawiKLinJJiaoFGuoNYuanZ. Kawasaki disease: global burden and genetic background. Cardiol Res. (2020) 11(1):9–14. 10.14740/cr993
15.
ZhuJYangFWangYWangZXiaoYWangLet alAccuracy of machine learning in discriminating Kawasaki disease and other febrile illnesses: systematic review and meta-analysis. J Med Internet Res. (2024) 26:e57641. 10.2196/57641
16.
AggarwalRPilaniaRKSharmaSKumarADhaliwalMRawatAet alKawasaki disease and the environment: an enigmatic interplay. Front Immunol. (2023) 14:1259094. 10.3389/fimmu.2023.1259094
17.
YonedaKShinjoDTakahashiNFushimiK. Spatiotemporal analysis of the association between Kawasaki disease incidence and PM2.5 exposure: a nationwide database study in Japan. BMJ Paediatr Open. (2024) 8(1):1–7. 10.1136/bmjpo-2024-002887
18.
FujiiFEgamiNInoueMKogaH. Weather condition, air pollutants, and epidemics as factors that potentially influence the development of Kawasaki disease. Sci Total Environ. (2020) 741:140469. 10.1016/j.scitotenv.2020.140469
19.
SiFZhouCYangYHuangL. Study of the relationship between occurrence of Kawasaki disease and air pollution in Chengdu by parametric and semi-parametric models. Environ Sci Pollut Res Int. (2023) 30(55):117706–14. 10.1007/s11356-023-30533-5
20.
HaraTYamamuraKSakaiY. The up-to-date pathophysiology of Kawasaki disease. Clin Transl Immunology. (2021) 10(5):e1284. 10.1002/cti2.1284
21.
RStudio Team. Integrated Development for R. RStudio (2024). Available online at: https://posit.co/ (Accessed March 01, 2025).
22.
TakeuchiANishiwakiYOkamuraTMilojevicAUedaKAsakuraKet alLong-term exposure to particulate matter and mortality from cardiovascular diseases in Japan: the Ibaraki prefectural health study (IPHS). J Atheroscler Thromb. (2021) 28(3):230–40. 10.5551/jat.54148
23.
YorifujiTTsukaharaHKashimaSDoiH. Intrauterine and early postnatal exposure to particulate air pollution and Kawasaki disease: a nationwide longitudinal survey in Japan. J Pediatr. (2018) 193:147–154.e2. 10.1016/j.jpeds.2017.10.012
24.
KuoN-CLinC-HLinM-C. Prenatal and early life exposure to air pollution and the incidence of Kawasaki disease. Sci Rep. (2022) 12(1):3415. 10.1038/s41598-022-07081-y
25.
OhJLeeJHKimEKimSKimHSHaE. Is short-term exposure to PM2.5 relevant to childhood Kawasaki disease?Int J Environ Res Public Health. (2021) 18(3):924. 10.3390/ijerph18030924
26.
LeeH-JKimD-K. Effect of airborne particulate matter on the immunologic characteristics of chronic rhinosinusitis with nasal polyps. Int J Mol Sci. (2022) 23(3):1018. 10.3390/ijms23031018
27.
Marín-PalmaDFernandezGJRuiz-SaenzJTabordaNARugelesMTHernandezJC. Particulate matter impairs immune system function by up-regulating inflammatory pathways and decreasing pathogen response gene expression. Sci Rep. (2023) 13(1):12773. 10.1038/s41598-023-39921-w
28.
LiuJChenXDouMHeHJuMJiSet alParticulate matter disrupts airway epithelial barrier via oxidative stress to promote pseudomonas aeruginosa infection. J Thorac Dis. (2019) 11(6):2617–27. 10.21037/jtd.2019.05.77
29.
LinZMengXChenRHuangGMaXChenJet alAmbient air pollution, temperature and Kawasaki disease in Shanghai, China. Chemosphere. (2017) 186:817–22. 10.1016/j.chemosphere.2017.08.054
30.
KwonDChoeYJKimS-YChunBCChoeS-A. Ambient air pollution and Kawasaki disease in Korean children: a study of the national health insurance claim data. J Am Heart Assoc. (2022) 11(9):e024092. 10.1161/JAHA.121.024092
31.
JungC-RChenW-TLinY-THwangB-F. Ambient air pollutant exposures and hospitalization for Kawasaki disease in Taiwan: a case-crossover study (2000–2010). Environ Health Perspect. (2017) 125(4):670–6. 10.1289/EHP137
32.
GoossensJJonckheereA-CDupontLJBullensDMA. Air pollution and the airways: lessons from a century of human urbanization. Atmosphere (Basel). (2021) 12(7):898. 10.3390/atmos12070898
33.
CipoliYAFurstLFelicianoMAlvesC. Respiratory deposition dose of PM2.5 and PM10 during night and day periods at an urban environment. Air Qual Atmos Health. (2023) 16(11):2269–83. 10.1007/s11869-023-01405-1
34.
HsuY-WLuH-FChouW-HKuoH-CChangW-C. Functional correlations between CXCL10/IP10 gene polymorphisms and risk of Kawasaki disease. Pediatr Allergy Immunol. (2021) 32(2):363–70. 10.1111/pai.13381
35.
KoT-MKuoH-CChangJ-SChenS-PLiuY-MChenH-Wet alCXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ Res. (2015) 116(5):876–83. 10.1161/CIRCRESAHA.116.305834
36.
ButeauSBelkaibechSBilodeau-BertrandMHatzopoulouMSmargiassiAAugerN. Association between Kawasaki disease and prenatal exposure to ambient and industrial air pollution: a population-based cohort study. Environ Health Perspect. (2020) 128(10):107006. 10.1289/EHP6920
37.
KimHJangHLeeWOhJLeeJ-YKimM-Het alAssociation between long-term PM2.5 exposure and risk of Kawasaki disease in children: a nationwide longitudinal cohort study. Environ Res. (2024) 244:117823. 10.1016/j.envres.2023.117823
38.
MoranCACollinsLFBeydounNMehtaPKFatadeYIsiadinsoIet alCardiovascular implications of immune disorders in women. Circ Res. (2022) 130(4):593–610. 10.1161/CIRCRESAHA.121.319877
39.
LiZ-HDaiS-WZhangS-YSunCLiJ-HHuangKet alAssociations between prenatal exposure to air pollution and infant growth trajectories in the first two years: exploring potential sexual dimorphism and sensitive windows. Environ Res. (2025) 285:122322. 10.1016/j.envres.2025.122322
40.
ZegerSLThomasDDominiciFSametJMSchwartzJDockeryDet alExposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. (2000) 108(5):419–26. 10.1289/ehp.00108419
41.
RhombergLRChandaliaJKLongCMGoodmanJE. Measurement error in environmental epidemiology and the shape of exposure-response curves. Crit Rev Toxicol. (2011) 41(8):651–71. 10.3109/10408444.2011.563420
42.
SafarovRShomanovaZNossenkoYKopishevEBexeitovaZKamatovR. Spatial analysis of air pollutants in an industrial city using GIS-based techniques: a case study of Pavlodar, Kazakhstan. Sustainability. (2024) 16(17):7834. 10.3390/su16177834
43.
BellMLBelangerK. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol. (2012) 22(5):429–38. 10.1038/jes.2012.42
44.
DuignanSDoyleSLMcMahonCJ. Refractory Kawasaki disease: diagnostic and management challenges. Pediatric Health Med Ther. (2019) 10:131–9. 10.2147/PHMT.S165935
45.
McCarronASempleSBrabanCFGillespieCSwansonVPriceHD. Personal exposure to fine particulate matter (PM2.5) and self-reported asthma-related health. Soc Sci Med. (2023) 337:116293. 10.1016/j.socscimed.2023.116293
46.
NovakRRobinsonJAKandučTSarigiannisDKocmanD. Assessment of individual-level exposure to airborne particulate matter during periods of atmospheric thermal inversion. Sensors (Basel). (2022) 22(19):7116. 10.3390/s22197116
47.
ZeftASBurnsJCYeungRSMcCrindleBWNewburgerJWDominguezSRet alKawasaki disease and exposure to fine particulate air pollution. J Pediatr. (2016) 177:179–83.e1. 10.1016/j.jpeds.2016.06.061
Summary
Keywords
Kawasaki disease, particulate matter, vascular disease, inflammation, PM10, PM2.5
Citation
Moncur C, Kamotho M, Jain T, Weslock N, Ragheb M and Mitchell K (2025) Early life exposure to ambient particulate matter and Kawasaki disease: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1611757. doi: 10.3389/fcvm.2025.1611757
Received
14 April 2025
Accepted
28 July 2025
Published
11 August 2025
Volume
12 - 2025
Edited by
Zenglei Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Reviewed by
Lei Huang, Southwest Jiaotong University, China
Prakash Thangavel, Gachon University, Republic of Korea
Updates
Copyright
© 2025 Moncur, Kamotho, Jain, Weslock, Ragheb and Mitchell.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerry Mitchell kmitche3@sgu.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.