- Department of Cardiology, St. James’s Hospital, Dublin, Ireland
Introduction: Heart failure (HF) hospitalizations are prognostically significant. Implantable hemodynamic monitors detect early congestion but are invasive and costly, with no clear mortality benefit. Wearable devices offer a non-invasive alternative for monitoring congestion. This meta-analysis examines the efficacy of wearable devices in reducing HF hospitalizations and mortality compared to standard care.
Methods: A systematic review and meta-analysis were conducted following PRISMA guidelines. PubMed, EMBASE, MEDLINE, and Cochrane databases were searched for trials comparing wearable device-guided care with standard HF treatment. Outcomes included hospitalisation for HF, worsening HF events (hospitalisation or emergency department visit for HF) and all-cause mortality. Total (first and recurrent) event meta-analyses were performed using random effect models.
Results: Four studies met inclusion criteria, including 958 patients who were enrolled either at the time of or within 10 days of discharge from a hospitalization for HF. Wearable device-guided care resulted in a 41% reduction in hospitalisations for HF (RR: 0.59, 95% CI: 0.41–0.87, p = 0.007) and a 40% reduction in HF events (RR: 0.60, 95% CI: 0.42–0.86, p = 0.005) compared to standard care. All-cause mortality was reduced by 26% in the wearable monitoring arm (RR: 0.74, 95% CI: 0.55–0.99, p = 0.04). The composite outcome of HF hospitalization and mortality was 37% lower with wearable monitoring (RR: 0.63, 95% CI: 0.44–0.91, p = 0.04). Treatment for HF, guided by wearable devices that measure pulmonary congestion, reduced hospitalisations for HF and all-cause mortality in recently hospitalised patients.
Conclusion: Wearable devices are a promising non-invasive strategy for managing high-risk patients, particularly when transitioning care from acute to community settings..
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024607770, identifier PROSPERO CRD42024607770.
1 Introduction
Hospitalisations for congestion are a hallmark of heart failure (HF) with each admission being of prognostic importance (1, 2). Implantable haemodynamic monitor (IHM) studies have demonstrated that subclinical alterations in physiology occur weeks before the development of overt clinical signs and symptoms that lead a patient to present to their care provider including a rise in intra-cardiopulmonary pressures and pulmonary congestion (3–5). Body weight is often monitored as an outpatient for the development of congestion but may not accurately predict decompensation (6, 7). IHMs such as pulmonary-artery sensors may reduce hospitalisations for HF but have yet to demonstrate a mortality benefit and are costly, requiring a dedicated invasive procedure (8). IHM-guided care received a modest class IIb recommendation in the 2021 European Society of Cardiology guidelines for HF (9). Wearable devices (wearables) offer a potential non-invasive alternative method of detecting pulmonary congestion and changes in physiological parameters (10, 11). Wearables could be applied readily by patients or carers with no requirement for an invasive implant and used by patients with HF across the range of ejection fraction (EF). In this meta-analysis, we examined the efficacy of these novel technologies that measure pulmonary congestion to reduce hospitalisations for HF, worsening HF events [hospitalisation for HF or emergency department (ED) visits for HF therapy] and mortality, compared with standard, unmonitored care.
Methods
Study design and search strategy
A systematic review of trials in patients with HF was performed, comparing wearable-guided care vs. standard treatment alone. This meta-analysis is registered on PROSPERO (CRD42024607770). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct the literature search, data extraction and reporting (12). A PRISMA checklist is included in the Supplementary Appendix Table S1. Bias was assessed using the Revised Cochrane Risk-of-Bias Tool for Randomised Trials V.2 (Supplementary Appendix Table S2) (13). Literature searches were performed on several databases (PubMed, EMBASE, MEDLINE and Cochrane) with the last search performed on November 1st 2024, using the terms “(HF OR congestive HF OR Acute HF) AND (wearable device* OR remote monitor* OR wearable sensor* OR lung fluid monitor OR lung impedance OR remote dielectric sensing) AND (hospitaliz* OR hospitalis* OR rehospitalis* OR rehospitaliz* OR admission* OR readmission* OR re-admission OR Mortality OR ED Presentations)”. Hand-searches of reference lists from the identified articles were performed. No restriction was placed on study size, language or country of publication. Titles and abstracts were screened according to pre-specified inclusion criteria using the population, intervention, comparator, outcomes and study (PICOS) framework:
• Population: patients with HF across ranges of EF
• Intervention: treatment for HF guided by wearable monitors that detect pulmonary congestion
• Comparator: standard (unmonitored) care for HF
• Outcomes:
○ Hospitalisation for HF
○ Worsening HF events (Hospitalisation for HF or ED visit for HF management)
○ All-cause mortality
○ All-cause mortality and hospitalisation for HF
• Study design: randomised controlled trials or non-randomised studies with a concurrently enrolled control arm
Full text articles of original studies were included. The Cochrane Collaboration's screening and data extraction tool, Covidence, was utilised to streamline data extraction and storage. Two researchers (CPM and APK) independently performed the literature searches and data collection including study characteristics for eligibility, participant and event numbers. Results were compared and differences were resolved with consensus from a third author (JPC). All authors reviewed the analysis and contributed to drafting the report. Each of the included studies in this meta-analysis were conducted with local institutional ethical approval.
Statistical analysis
The statistical analyses were performed using RevMan (version 5.4.1; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). As the included studies examined three different devices across two decades a random-effect [DerSimonian and Laird (D + L)] model (14) was used so that differences in study design and cohorts would be accounted for within the analysis. I2 statistic for percentage heterogeneity was computed with corresponding p values (15). Forest plots graphically report the pooled effect size estimates, the degree of heterogeneity and the weighted contribution each study made to the analyses. A p-value less than 0.05 was considered statistically significant.
Efficacy endpoints
The following clinical outcomes were examined according to study and device:
• Benefit of Microcor in Ambulatory Decompensated HF (BMAD) (16): (1) time-to-first hospitalisation for HF (2) time-to-first worsening HF event (hospitalisation for HF or ED visit for HF management) (3) all-cause mortality
• Bensimhon et al, Remote Dielectric Sensing system (ReDS) (Sensible Medical Innovations, Israel) (17): (1) total (first and recurrent) hospitalisations for HF
• Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients (IMPEDANCE-HF) (18): (1) total hospitalisations for HF (2) all-cause mortality
• Remote Dielectric Sensing Before and After Discharge in Patients With ADHF (ReDS-SAFE HF) (19): (1) total hospitalisations for HF (2) all-cause mortality
Numbers of clinical events and numbers of study patients in each study arm were extracted according to outcome. Risk ratios (RR) were calculated with 95% confidence intervals. Meta-analyses of the effect estimates were performed for (1) hospitalisation for HF (2) worsening HF event (hospitalisation for HF or ED visit for HF management) (3) all-cause mortality (4) hospitalisation for HF and all-cause mortality.
Results
Literature review and search results
3,865 articles were identified by searching electronic databases. After excluding 1,468 duplicates, the abstracts of 2,397 studies were assessed for potential inclusion. Full text review of 45 studies resulted in the identification of four published studies that met the inclusion criteria. These four studies were included in the analysis (16–19). Both 30-day and 90-day outcomes were reported in the ReDS trial with the 90-day results included in this meta-analysis. The search process and identification of relevant articles are summarised in a PRISMA flow chart (Supplementary Appendix Table S3).
Study and investigational device characteristics
Four eligible studies were identified (Table 1). BMAD was an international, prospective concurrent control trial studying the Zoll Heart Failure Monitoring System (HFMS) (Zoll, Pittsburgh, USA). The Zoll system uses a novel radiofrequency sensor to estimate thoracic fluid content. The trial compared a control arm (BMAD-HF) with an intervention arm (BMAD-TX) over a 12-month follow-up period. In the BMAD-TX arm, device-collected data were used to guide clinical HF management.
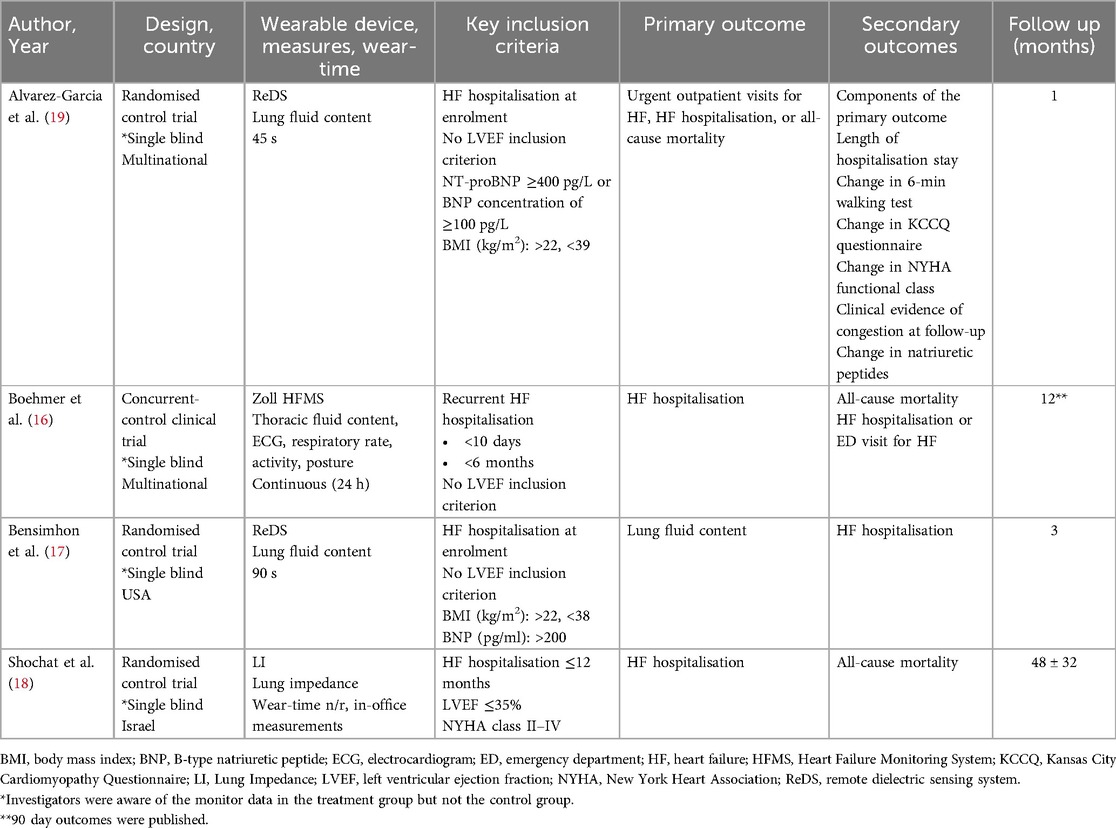
Table 1. Studies of HF management guided by wearable devices that detect pulmonary congestion compared with standard, unmonitored care.
Lung impedance (resistance) to an electric current passed across pulmonary tissue reduces as pulmonary congestion develops in people with HF (20–24). Thoracic impedance has been shown to correlate strongly with pulmonary capillary wedge pressure in invasive monitor studies (25). The IMPEDANCE-HF randomised controlled trial examined the effectiveness of the RS-205 wearable lung impedance (LI) monitor (RS Medical Monitoring, Jerusalem, Israel), to estimate pulmonary congestion and guide HF treatment compared with unmonitored HF care over a mean follow up period of 48 months (26).
Both of the studies by Bensimhon et al. and Alvarez-Garcia et al. were randomised trials examining the Remote Dielectric Sensing (ReDS) System (Sensible Medical Innovations, Israel). The ReDS device is a wearable vest that quantifies the percentage of lung fluid compared to lung volume by analysing the dielectric coefficient of the lung between the vest sensors (27, 28). The ReDS trials included in this meta-analysis examined clinical outcomes in people who received treatment guided by ReDS detected pulmonary congestion compared with standard, unmonitored care, over follow-up periods of 3 and 1 month, respectively (27, 28).
All four studies enrolled patients either during a hospitalisation for HF (ReDS, ReDS-SAFE and IMPEDANCE-HF) or during a hospitalisation or within 10 days of discharge (BMAD). All participants received wearable device readings on enrolment. Patients but not investigators were blinded to study data in the ReDS, ReDS-SAFE and IMPEDANCE-HF trials. In the BMAD trial, both investigators and patients in the monitored arm had access to the device data, whereas patients in the control arm were blinded. A total of 958 patients were included in the meta-analysis and baseline characteristics of the participants and the devices are summarised according to the individual trials in Table 2.
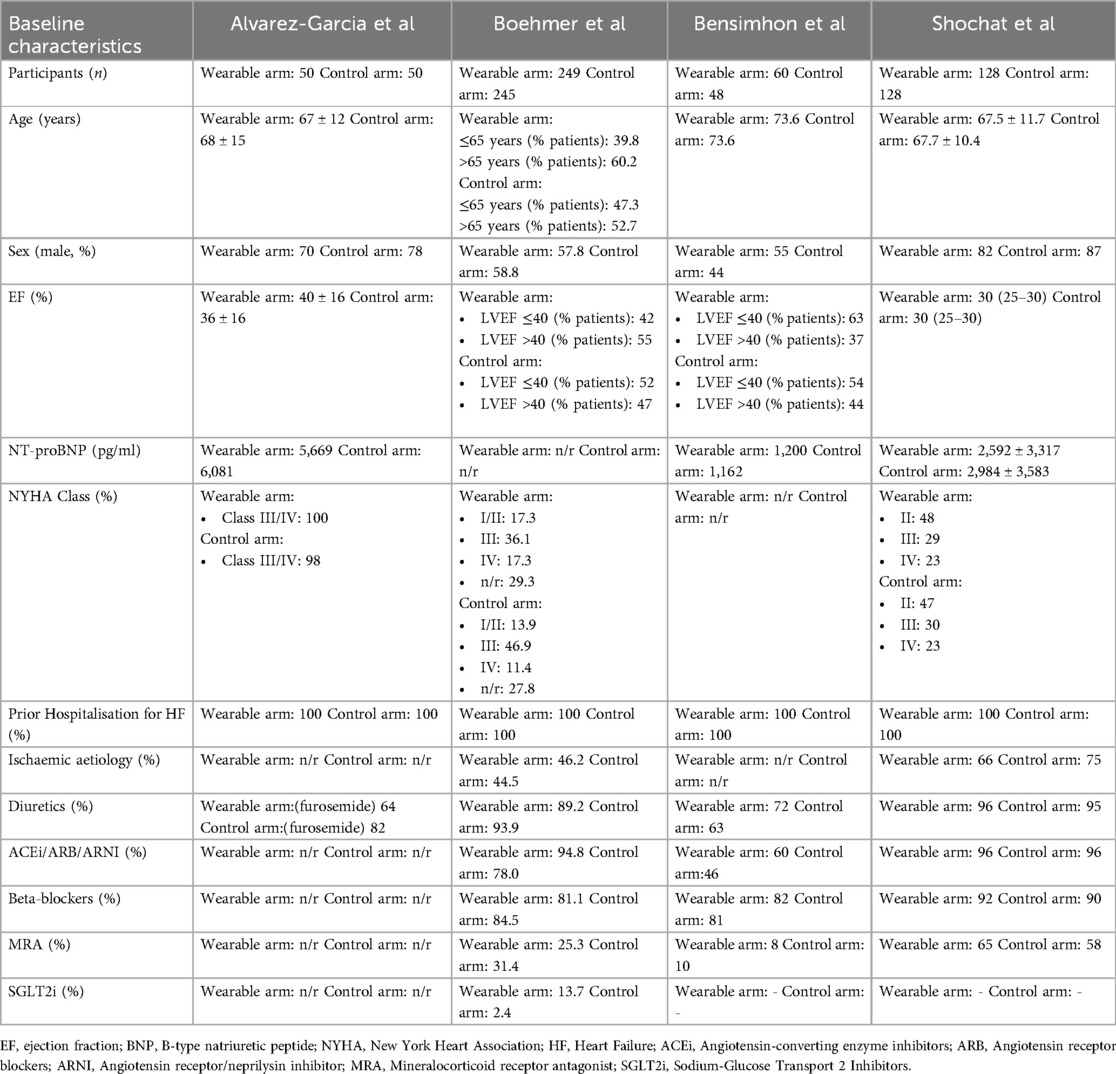
Table 2. Key baseline characteristics of patients enrolled in studies of wearable monitor-guided management of HF compared with standard care.
Hospitalisations for HF
There were 254 hospitalisations for HF among 487 patients receiving wearable-guided care compared with 451 hospitalisations in 471 patients who received standard unmonitored treatment. Hospitalisations for HF were reduced by 41% in the wearable monitored group [RR: 0.59, 95% CI: 0.41–0.87, p = 0.007; moderate heterogeneity (I2 42%)] (Figure 1).
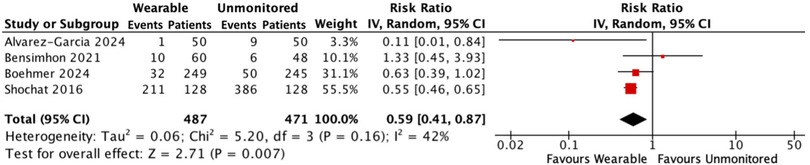
Figure 1. Forest plot displaying hospitalisations for HF in patients receiving wearable-guided care compared with standard care.
Worsening HF events
261 worsening HF events occurred in the wearable monitored group compared with 459 in the standard care group. Wearable monitored care reduced the composite outcome by 40% [RR: 0.60, 95% CI: 0.42–0.86, p = 0.005; moderate heterogeneity (I2 52%)] (Figure 2).
![Forest plot illustrating a meta-analysis comparing wearable versus unmonitored groups across four studies: Alvarez-Garcia 2024, Bensimhon 2021, Boehmer 2024, and Shochat 2016. Effect sizes and 95% confidence intervals are shown, with a pooled risk ratio of 0.60 [0.42, 0.86], indicating a significant favor towards wearables (Z = 2.78, P = 0.005). Heterogeneity is moderate (I² = 52%).](https://www.frontiersin.org/files/Articles/1612545/fcvm-12-1612545-HTML/image_m/fcvm-12-1612545-g002.jpg)
Figure 2. Forest plot displaying worsening HF events in patients receiving wearable-guided care compared with standard care.
All-cause mortality
Mortality was reported in the BMAD and IMPEDANCE-HF trials. Of 427 patients in the wearable arm, 53 (12.4%) died, compared with 71 of 423 (16.8%) people in the standard care group. Wearable guided care reduced the occurrence of all-cause death by 26%. [RR: 0.74, 95% CI: 0.55–0.99, p = 0.04; low heterogeneity (I2 0%)] (Figure 3).
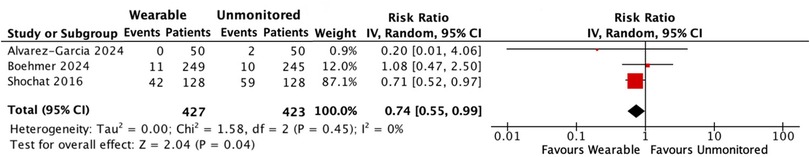
Figure 3. Forest plot displaying all-cause mortality in patients receiving wearable-guided care compared with standard care.
Hospitalisation for HF and all-cause mortality
In a combined analysis, there were 314 events in 487 people with HF receiving treatment guided by a wearable device compared with 530 events in 471 people receiving standard care without monitoring. Hospitalisations for HF and all-cause mortality were reduced by 37% in the monitored group [RR: 0.63; 95% CI: 0.44–0.91; p = 0.04; moderate heterogeneity (I2 55%)] (Figure 4).
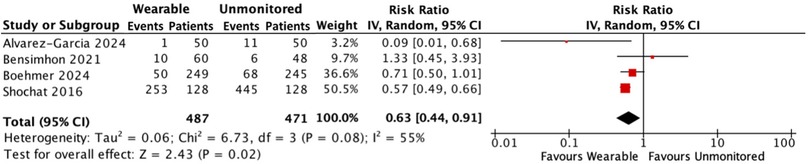
Figure 4. Forest plot displaying hospitalisations for HF and all-cause mortality in patients receiving wearable-guided care compared with standard care.
Discussion
This is the first meta-analysis to pool hospitalisation or mortality events from trials examining the effectiveness of wearable devices in HF. The main results support the use of wearable devices to guide care in patients with symptomatic HF irrespective of EF. Wearable device guided treatment provided a 41% reduction in the risk of hospitalisation for HF and a 26% reduction in all-cause mortality compared with standard care alone. Such substantial reductions in clinically important endpoints compare favourably alongside the benefits observed in randomised controlled trials of contemporary standards of HF care, including dapagliflozin (30% reduction in worsening HF events and 17% reduction in mortality) (29) and sacubitril-valsartan (21% reduction in hospitalisation for HF and 16% in mortality) (30). The findings of this meta-analysis also exceed the 28% reduction in hospitalisation for HF observed in the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III HF Patients (CHAMPION) (8) IHM trial. In the recent haemodynamic-GUIDEed management of Heart Failure (GUIDE-HF) randomised trial, neither hospitalisations for HF or mortality were reduced by IHM-guided care (31). Placed alongside CHAMPION and GUIDE-HF, there are some important differences between the IHM trials and the wearable studies included in this analysis. Firstly, CHAMPION and GUIDE-HF were multi-centre, randomised studies enrolling people with ambulatory HF (NYHA class III in CHAMPION, class II–IV in GUIDE-HF) and while prior hospitalisation for HF was an inclusion criterion in these trials, neither purposefully enrolled patients during an acute event. A formal comparison between an implanted and wearable device in a randomised trial is unlikely to ever happen. However, the lower risk option of a non-invasive wearable device that is proven to reduce clinically meaningful events would offer a sensible monitoring strategy to most clinicians.
The magnitude of relative benefit afforded to patients who were managed with wearable-based care compared with standard care alone is particularly notable given the high-risk profile of these people. In all four of the wearable studies included in this analysis, people were recruited either during a hospitalisation or within 10 days of discharge, a prognostically important period. In the three studies that reported NT-proBNP, arguably the most powerful risk predicting variable in HF, natriuretic peptide levels were markedly elevated. The rate of (re)hospitalisation in the control arms of the ReDS and IMPEDANCE-HF trials was between 50 and 94 per 100 person-years respectively, exceeding those rates observed in the same groups in the CHAMPION (68 per 100 person-years) and GUIDE-HF (49.7 per 100 person-years) trials, and considerably higher than in other contemporary trials such as the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, where the rate of hospitalisations for HF was 5–10 times lower (9.8 events per 100-person years) (29). The elevated event rate in this meta-analysis underscores how highly selected these patients were but also how effective a strategy of wearable-guided care may be to protect such a group at their most clinically vulnerable, a potentially valuable tool when transitioning care from the acute to community setting.
Several factors need to be considered to determine whether the wearable devices included in this analysis are practical monitoring options. Firstly, the duration a device was worn must be sufficient to capture meaningful amounts of data, balanced against patient comfort to ensure compliance using it. Two devices (the ReDS and LI systems) were worn in a healthcare setting for minutes at a time, minimising patient burden but healthcare providers were involved and the practicality of home use by patients was not explored. The Zoll HFMS device was used as intended within the BMAD study, for twenty-four hours a day and at-home. Data transmission rates were not reported for the BMAD trial but in wearable and IHM studies to date, adherence to data transmissions were approximately 90% of study days (8, 11). An indicator of whether a wearable device that is applied to a person's chest is likely to be adopted by patients is whether both men and women consented for the investigational study. The generalisability of wearable devices other than those included in this analysis has been reduced by near exclusive male enrolment in other studies (11). In this meta-analysis, a pooled average of 35% of participants were female, lower than real-world cohorts of people admitted to hospital with HF (32) but higher than the proportions of females recruited to other HF trials in which approximately a quarter were women (29, 30).
The included trials enrolled different populations of people with HF (patients in hospital, patients who were recently discharged, and routinely monitored ambulatory patients with HF). As the wearable devices were examined in patients in different settings and clinical status the generalisability of the meta-analysis findings are broadened. The IMPEDANCE-HF trial recruited high-risk patients with an EF ≤35%, whereas the BMAD, ReDS and ReDS SAFE trials enrolled patients with HF across the range of EF. A combined 46% of participants in the BMAD and ReDS studies had an EF >40%. The majority of patients with an EF >40% do not have an indication for an implantable device such as a defibrillator and so would not receive device-related HF diagnostics such as the COMPASS algorithm (Medtronic, Minneasota, USA). While sub-analyses of the CHAMPION study indicated that people with a preserved EF had a reduction in hospitalisation for HF, these data were limited by small numbers of patients and events (33). Among people with an EF > 40%, IHM-guided care did not reduce hospitalisations in the primary analysis in GUIDE-HF and a recent meta-analysis reported uncertainty regarding the benefits of IHM-guided care in people with an EF ≥50%1 (31, 34). Until wearable device studies report outcomes according to EF classification, it is difficult to conclude whether wearables that detect pulmonary congestion are an effective tool in caring for different HF populations. As people with preserved or milder impairment in left ventricular systolic function were participants in three of the four included studies, and given the ready application of these devices without an invasive procedure, future wearable trials should continue to actively recruit participants across the range of EF and subsequently report outcomes according to EF. Patients with both advanced and mild symptoms at baseline were recruited across the different trials. In the ReDS-SAFE HF trial, 99% of patients were NYHA class III/IV, compared with 52.5% in IMPEDANCE-HF. Rates of guideline-directed medical therapy for HF also differed across the trials, notably the use of sodium glucose co-transporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists (MRAs). Only the patients in BMAT study, which was conducted after the DAPA-HF (29) and Emperor-Reduced trials (35) were reported, were taking SGLT2 inhibitors at baseline. Rates of MRA use also differed substantially between studies [9.6% in ReDS (17) and 61.5% in IMPEDENCE-HF (18)]. Without individual patient-level data, which were not available for analysis, we were however unable to test for any interactions between major patient subgroups (e.g., symptoms severity, treatments, EF) and the effect of treatment guided by a wearable device or not.
This meta-analysis has several limitations. Notably, the four included studies assessed three different wearable devices for detecting pulmonary congestion, limiting direct comparability across studies. Additionally, only one study (BMAD) evaluated the device's effectiveness in a home setting, where patients used it for remote monitoring. Therefore, the feasibility of home use demonstrated in BMAD cannot be reliably extrapolated to the other devices. While both the ReDS and IMPEDANCE-HF studies were randomised controlled trials, both were conducted at single centres with expertise in the management of HF. The BMAD study was a non-randomised concurrent-control trial. Nonetheless, it is well established that patients hospitalized with HF are frequently discharged with residual congestion—a factor strongly associated with increased mortality and rehospitalization (36). Wearable technologies that detect pulmonary congestion in this vulnerable window offer an opportunity for timely intervention. Artificial intelligence (AI)-enabled devices offer a paradigm-shift in the capabilities of remote HF management. The HEARTFELT device, examined in the FOOT study, used AI to analyse 3-dimensional images of pedal oedema, presenting a novel non-invasive method to monitor for decompensation (36). The integration of AI into wearable devices and remote monitoring technologies in general is likely to develop rapidly, and as with any emerging technology should be examined in randomised clinical trials to confirm their role in the management of people with HF.
Conclusion
HF treatment guided by wearable devices that detect pulmonary congestion reduced hospitalisations for HF and mortality in patients with HF across the range of EF. This strategy was effective in patients who had features of adverse prognosis, including a recent hospitalisation, and indicate that wearable monitoring may provide greater protection against adverse events when discharging care from the acute to home setting.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CPM: Conceptualization, Methodology, Project administration, Writing – review & editing, Investigation, Writing – original draft, Formal analysis. APK: Investigation, Data curation, Writing – original draft. NJOS: Writing – original draft, Investigation, Formal analysis, Data curation. RTM: Writing – review & editing. JPC: Writing – original draft, Writing – review & editing, Supervision, Conceptualization, Formal analysis, Methodology, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1612545/full#supplementary-material
References
1. Lindmark K, Boman K, Stålhammar J, Olofsson M, Lahoz R, Studer R, et al. Recurrent heart failure hospitalizations increase the risk of cardiovascular and all-cause mortality in patients with heart failure in Sweden: a real-world study. ESC Heart Fail. (2021) 8(3):2144–53. doi: 10.1002/ehf2.13296
2. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJV, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. (2007) 116(13):1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906
3. Zile MR, Adamson PB, Cho YK, Bennett TD, Bourge RC, Aaron MF, et al. Hemodynamic factors associated with acute decompensated heart failure: part 1–insights into pathophysiology. J Card Fail. (2011) 17(4):282–91. doi: 10.1016/j.cardfail.2011.01.010
4. Zile MR, Bourge RC, Bennett TD, Stevenson LW, Cho YK, Adamson PB, et al. Application of implantable hemodynamic monitoring in the management of patients with diastolic heart failure: a subgroup analysis of the COMPASS-HF trial. J Card Fail. (2008) 14(10):816–23. doi: 10.1016/j.cardfail.2008.07.235
5. Zile MR, Bennett TD, St. John Sutton M, Cho YK, Adamson PB, Aaron MF, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. (2008) 118(14):1433–41. doi: 10.1161/CIRCULATIONAHA.108.783910
6. Mehta RH, Rogers JG, Hasselblad V, Tasissa G, Binanay C, Califf RM, et al. Association of weight change with subsequent outcomes in patients hospitalized with acute decompensated heart failure. Am J Cardiol. (2009) 103(1):76–81. doi: 10.1016/j.amjcard.2008.08.041
7. Lewin J, Ledwidge M, O'Loughlin C, McNally C, McDonald K. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail. (2005) 7(6):953–7. doi: 10.1016/j.ejheart.2005.06.003
8. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. (2011) 377(9766):658–66. doi: 10.1016/S0140-6736(11)60101-3
9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
10. Curtain JP, Talebi A, McIntosh A, McConnachie A, O'Donnell J, Welsh P, et al. Measuring congestion with a non-invasive monitoring device in heart failure and haemodialysis: CONGEST-HF. Eur J Heart Fail. (2024) 26(6):1383–92. doi: 10.1002/ejhf.3290
11. Stehlik J, Schmalfuss C, Bozkurt B, Nativi-Nicolau J, Wohlfahrt P, Wegerich S, et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF multicenter study. Circ Heart Fail. (2020) 13(3):e006513. doi: 10.1161/CIRCHEARTFAILURE.119.006513
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006
13. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
16. Boehmer JP, Cremer S, Abo-Auda WS, Stokes DR, Hadi A, McCann PJ, et al. Impact of a novel wearable sensor on heart failure rehospitalization: an open-label concurrent-control clinical trial. JACC Heart Fail. (2024) 12(12):2011–22. doi: 10.1016/j.jchf.2024.07.022
17. Bensimhon D, Alali SA, Curran L, Gelbart E, Garman DWV, Taylor R, et al. The use of the reds noninvasive lung fluid monitoring system to assess readiness for discharge in patients hospitalized with acute heart failure: a pilot study. Heart Lung. (2021) 50(1):59–64. doi: 10.1016/j.hrtlng.2020.07.003
18. Shochat MK, Shotan A, Blondheim DS, Kazatsker M, Dahan I, Asif A, et al. Non-Invasive lung IMPEDANCE-guided preemptive treatment in chronic heart failure patients: a randomized controlled trial (IMPEDANCE-HF trial). J Card Fail. (2016) 22(9):713–22. doi: 10.1016/j.cardfail.2016.03.015
19. Alvarez-Garcia J, Lala A, Rivas-Lasarte M, De Rueda C, Brunjes D, Lozano-Jimenez S, et al. Remote dielectric sensing before and after discharge in patients with ADHF: the ReDS-SAFE HF trial. JACC Heart Fail. (2024) 12(4):695–706. doi: 10.1016/j.jchf.2024.01.002
20. Pomerantz M, Baumgartner R, Lauridson J, Eiseman B. Transthoracic electrical impedance for the early detection of pulmonary edema. Surgery. (1969) 66(1):260–8.5788382
21. Luepker RV, Michael JR, Warbasse JR. Transthoracic electrical impedance; quantitative evaluation of a non-invasive measure of thoracic fluid volume. Am Heart J. (1973) 85(1):83–93. doi: 10.1016/0002-8703(73)90529-2
22. Baker LE, Denniston JC. Noninvasive measurement of intrathoracic fluids. Chest. (1974) 65(Suppl):35S–7. doi: 10.1378/chest.65.4_Supplement.35S
23. Sahalos JN, Nicolaidis A, Gotsis N. The electrical impedance of the human thorax as a guide in evaluation of intrathoracic fluid volume. Phys Med Biol. (1986) 31(4):425–39. doi: 10.1088/0031-9155/31/4/008
24. Van de Water JM, Mount BE, Barela JR, Schuster R, Leacock FS. Monitoring the chest with impedance. Chest. (1973) 64(5):597–603. doi: 10.1378/chest.64.5.597
25. Yu C-M, Wang L, Chau E, Chan RH-W, Kong S-L, Tang M-O, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. (2005) 112(6):841–8. doi: 10.1161/CIRCULATIONAHA.104.492207
26. Shochat M, Charach G, Meyler S, Kazatzker M, Mosseri M, Frimerman A, et al. Internal thoracic impedance monitoring: a novel method for the preclinical detection of acute heart failure. Cardiovasc Revasc Med. (2006) 7(1):41–5. doi: 10.1016/j.carrev.2005.10.005
27. Amir O, Azzam ZS, Gaspar T, Faranesh-Abboud S, Andria N, Burkhoff D, et al. Validation of remote dielectric sensing (ReDS™) technology for quantification of lung fluid status: comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int J Cardiol. (2016) 221:841–6. doi: 10.1016/j.ijcard.2016.06.323
28. Amir O, Ben-Gal T, Weinstein JM, Schliamser J, Burkhoff D, Abbo A, et al. Evaluation of remote dielectric sensing (ReDS) technology-guided therapy for decreasing heart failure re-hospitalizations. Int J Cardiol. (2017) 240:279–84. doi: 10.1016/j.ijcard.2017.02.120
29. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
30. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371(11):993–1004. doi: 10.1056/NEJMoa1409077
31. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. (2021) 398(10304):991–1001. doi: 10.1016/S0140-6736(21)01754-2
32. National Heart Failure Audit (NHFA). National Institute of Cardiovascular Outcomes Research (NICOR) (2023).
33. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. (2014) 7(6):935–44. doi: 10.1161/CIRCHEARTFAILURE.113.001229
34. Curtain JP, Lee MMY, McMurray JJ, Gardner RS, Petrie MC, Jhund PS. Efficacy of implantable haemodynamic monitoring in heart failure across ranges of ejection fraction: a systematic review and meta-analysis. Heart. (2023) 109(11):823–31. doi: 10.1136/heartjnl-2022-321885
35. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383(15):1413–24. doi: 10.1056/NEJMoa2022190
36. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. (2013) 34(11):835–43. doi: 10.1093/eurheartj/ehs444
Keywords: heart failure, wearable devices, remote monitoring, pulmonary congestion, hospitalisations
Citation: Murray CP, Kenny AP, O’Sullivan NJ, Murphy RT and Curtain JP (2025) Efficacy of wearable devices detecting pulmonary congestion in heart failure: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1612545. doi: 10.3389/fcvm.2025.1612545
Received: 15 April 2025; Accepted: 21 July 2025;
Published: 11 August 2025.
Edited by:
Ricardo Mourilhe-Rocha, Rio de Janeiro State University, BrazilReviewed by:
Hamayak Sisakian, Yerevan State Medical University, ArmeniaNiklas Lidströmer, Karolinska Institutet (KI), Sweden
Copyright: © 2025 Murray, Kenny, O’Sullivan, Murphy and Curtain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James P. Curtain, amN1cnRhaW5Ac3RqYW1lcy5pZQ==
 Cian P. Murray
Cian P. Murray Andrew P. Kenny
Andrew P. Kenny