- 1Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Nursing, Sichuan University, Chengdu, China
- 3First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 4The Department of Clinical Research, West China Hospital, Sichuan University, Chengdu, China
- 5Center of Excellence for Pancreatitis, Institute of Integrated Traditional Chinese and Western Medicine, West China Hospital, Sichuan University, Chengdu, China
Objective: To investigate the efficacy and safety of Glucagon-Like Peptide-1 Receptor Agonists(GLP-1RAs) (Liraglutide, Semaglutide, Exenatide, Dulaglutide, Lixisenatide, and Tirzepatide) in obese patients with chronic heart failure (CHF).
Method: A systematic search was performed in 3 databases (Pubmed, Embase, and Cochrane Library) for articles evaluating the effectiveness and safety of GLP-1RAs (Liraglutide, Semaglutide, Exenatide, Dulaglutide, Lixisenatide, and Tirzepatide) for the treatment of obese patients with CHF from the time the database was created until 5 January 2025. Meta-analyses were performed to evaluate: primary outcomes, including all-cause mortality, cardiovascular mortality, and worsening heart failure events; secondary outcomes, encompassing changes in body weight, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), 6-minute walk distance, B-type Natriuretic Peptide (BNP) level, high-sensitivity C-Reactive Protein (hs-CRP) level, and left ventricular ejection fraction (LVEF) level; and safety outcomes, specifically gastrointestinal adverse events and serious adverse events.
Results: A total of 6 papers were included for Meta-analysis. The primary clinical outcomes: all-cause mortality [OR=0.89, 95% confidence interval (CI): 0.40–2.00, p = 0.78], cardiovascular mortality (OR = 0.93, 95% CI: 0.22–4.00, p = 0.92) and worsening heart failure events (OR=0.43, 95% CI: 0.30–0.59, p < 0.00001); For secondary outcomes, change in body weight (MD = −7.90, 95% CI: −15.44 to −0.35, p = 0.04), change in the KCCQ-CSS (MD = 6.81, 95% CI: 6.62–6.99, p < 0.00001),change in the 6-minute walk distance (MD = 15.91, 95% CI: 15.36–16.47, p < 0.00001), change in the BNP level (MD = −0.13, 95% CI: −0.21 to −0.05, p = 0.001), changes in the hs-CRP level (MD = −16.61, 95% CI: −48.53 to 15.31, p = 0.31) and change in the LVEF level (MD = −0.91, 95% CI: −2.12 to 0.29, p = 0.14). For safety outcomes, gastrointestinal adverse events (OR=0.87, 95% CI: 0.11–7.05, p = 0.90) and serious adverse events (OR=0.63, 95% CI: 0.37–1.08, p = 0.09).
Conclusion: The study results show that GLP-1RAs significantly reduce the risk of worsening heart failure events and improve cardiac function, suggesting that GLP-1RAs are promising treatment options for obese patients with CHF.
Introduction
Heart failure (HF) is a progressive clinical syndrome that occurs when the heart is unable to pump blood effectively enough to meet the body's oxygen needs. People with a clear diagnosis or progressive onset of HF symptoms are called chronic heart failure (CHF) (1). The increasing incidence and prevalence of CHF is one of the most pressing therapeutic challenges in today's clinical medicine (2, 3). Characterized by diastolic dysfunction, dyspnoea, and reduced exercise tolerance, CHF poses a growing global healthcare burden due to its limited therapeutic options, and poor prognosis (4, 5).
Obesity is a growing health problem worldwide and a significant risk factor for cardiovascular disease, especially CHF (6). Inflammation is a key factor contributing to cardiovascular injury (7). Obesity-driven systemic inflammation can cause coronary microvascular dysfunction and increased epicardial adipose tissue, leading to myocardial fibrosis and molecular alterations in cardiomyocytes, which ultimately triggers myocardial stiffness and diastolic dysfunction (8). In addition, obesity induces insulin resistance, which elevates blood pressure and promotes atherosclerosis, thereby impairing ventricular-vascular coupling, reducing exercise tolerance, and ultimately contributing to CHF (9). Currently, first-line treatment for CHF includes SGLT 2 inhibitors, β Beta-blockers, Mineralocorticoid Receptor Antagonist (MRA) and so on. A comprehensive treatment strategy for obesity-related CHF is lacking.
Glucagon-Like Peptide-1 Receptor Agonists(GLP-1RAs) can exert glucose-lowering and weight-loss effects by activating GLP-1 receptors (10). It is worth noting that, tirzepatide is a dual Glucose-dependent Insulinotropic Polypeptide (GIP) and GLP-1 receptor agonist that exerts synergistic effects by simultaneously activating GIP and GLP-1 receptors.In this study, we included it in the GLP-1RA category. Liu et al. (11) demonstrated that GLP-1RAs reduced weight in a nonlinear dose-response manner in obese or overweight (without diabetes) patients in a Meta-analysis of a randomized controlled trial. By targeting endothelial dysfunction, GLP-1RAs can improve microvascular function and vascular endothelial function (12). In the mouse model of Heart Failure with Preserved Ejection Fraction(HFpEF), the administration of GLP-1 RA Lira can alleviate cardiometabolic dysregulation, and improve the state of fibrosis and inflammation (13). Wong et al. (14) reported in a meta-analysis that GLP-1RAs improved cardiac function in type 2 diabetes patients, with liraglutide specifically increasing LVEF and reducing LVESV. Liu et al. (15) found that GLP-1RAs brought about clinical benefits by improving ventricular diastolic function (e. g., reducing filling pressure and promoting ventricular relaxation), especially for people without a history of heart failure in a meta-analysis of randomized controlled trials.Although GLP-1RAs have demonstrated multiple cardiovascular benefits in patients with type 2 diabetes and obesity, clinical efficacy and safety data on GLP-1RAs in patients with obesity combined with CHF remain limited.
This study aims to evaluate the clinical efficacy and safety of GLP-1RAs (Liraglutide, Semaglutide, Exenatide, Dulaglutide, Lixisenatide, and Tirzepatide) in obese patients with CHF through a systematic review and meta-analysis.
Materials and methods
Search strategy
This meta-analysis followed the 2020 guidelines developed by Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA) (16). A comprehensive search was conducted in three databases, including PubMed, Embase, and Cochrane Library to retrieve the literature published as of January 5,2025. The search technique followed the PICOS principles and used a mixture of MeSH terms and unrestricted text phrases. The search method used was to combine the terms “Liraglutide”, “Semaglutide”, “Exenatide”, “Dulaglutide”, “Lixisenatide”, “Tirzepatide” and “chronic heart failure”. A detailed summary of the searched records is provided in Supplementary Material S1.
Inclusion and exclusion criteria
The inclusion criteria are as follows: (1) age ≥18 years; (2) Patients diagnosed with CHF (17) (including HFpEF and HFrEF); (3) Obesity (with a body mass index of at least 30); (4)At least one patient cohort was treated with GLP-1RAs (liraglutide, semaglutide, exenatide, dulaglutide, lixisenatide or tirzepatide), with or without other treatments;(5) At least one of the following results was recorded: all-cause mortality events, cardiovascular mortality, worsening heart failure events, gastrointestinal adverse events, serious adverse events, change in body weight, change in the KCCQ-CSS, change in the 6-minute walk distance, change in the BNP level, change in the hs-CRP level and change in the LVEF level; (6) Study types: randomized controlled trials.
Exclusion criteria were as follows: (1) other types of articles, such as review, letter, conference, case reports, protocols, meeting, proceeding, abstract, meta-analysis, etc; (2) other diseases; (3) unrelated; (4) missing data; (5) cohort of repeat patients;(6) acute heart failure.
Selection of studies
Literature screening, including elimination of duplicate entries, was performed using EndNote (version 20; Clarivate Analytics). Two independent reviewers separately conducted the first search, removed duplicates, evaluated the title and abstract to ensure their relevance, and finally read the full text to classify each study as inclusion or exclusion. The excluded studies and their potential bias introduced were discussed and consensus was reached through consultation. In the absence of consensus, a third reviewer acted as the mediator to determine the final number of literature included.
Data extraction
Data were extracted independently by the two reviewers. The retrieved data include the following data: (1) the basic information of the study, such as the first author, year of publication, study method, sample size, and follow-up time; (2) Basic characteristics of the individuals participating in the study, such as the number of patients, age, gender, and BMI; (3) Outcome measures: all-cause mortality, cardiovascular mortality, worsening heart-failure events, gastrointestinal adverse events, serious adverse events, change in body weight, change in the KCCQ-CSS, change in the 6-minute walk distance, change in the BNP level, change in the hs-CRP level, change in the LVEF level. In case of ambiguity, consensus was reached by consulting the third investigator. In the included studies, seven cohorts of patients received GLP-1RAs such as Liraglutide, Tirzepatide and Semaglutide, and seven additional cohorts received Placebo or Glimepiride. Based on this, a meta-analysis was performed to compare the efficacy and safety of GLP-1RAs in obese patients with CHF.
Quality assessment
Two independent reviewers assessed the quality of the included studies. Randomized controlled trials were assessed with the Cochrane Risk of Bias tool. Any discrepancies in the evaluations were resolved through group consensus.
Statistical analysis
Analyses were performed using Review Manager version 5.3. The study used mean difference (MD) and 95% CI for comparison of continuous variables and odds ratio (OR) and 95% CI for comparison of dichotomous variables. Median and interquartile spacing of the continuity variables were converted to mean and standard deviation. Statistical heterogeneity among the studies included in the analysis was evaluated using Cochrane's Q test and I2 index. Considering that the studies included in the analysis were derived from the open literature, it was generally more reasonable to choose a random effects model. A p-value below 0.05 was considered statistically significant.
Results
Search results
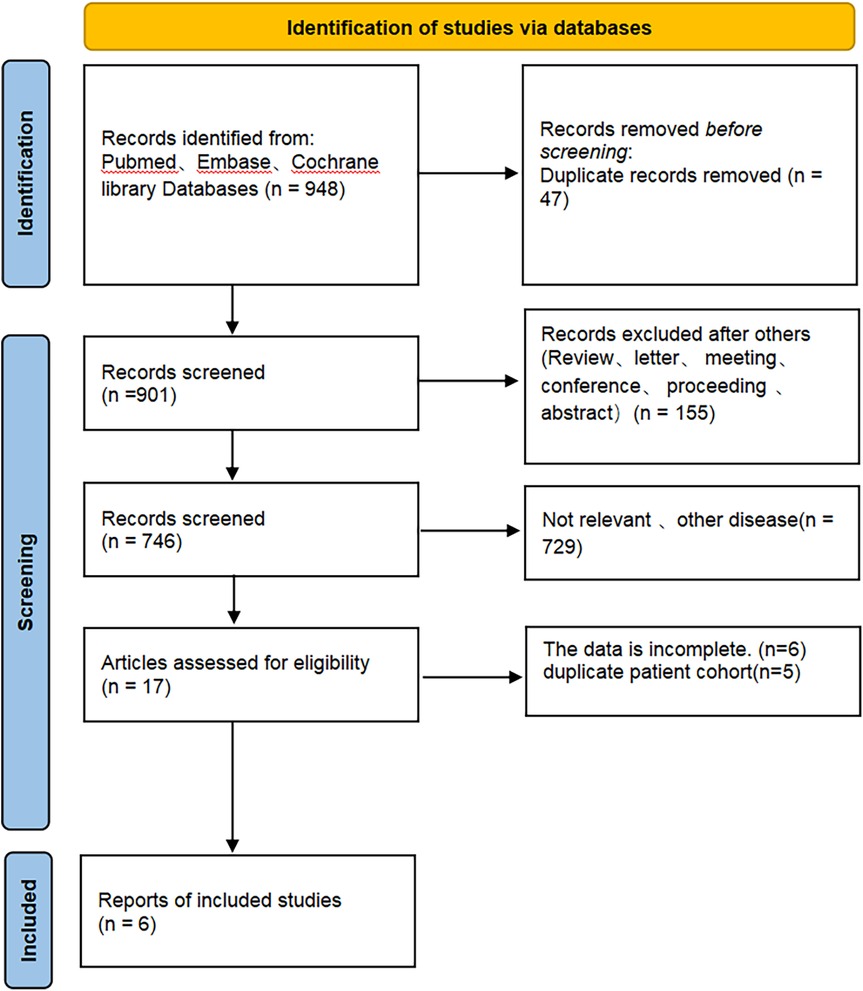
Figure 1 depicts the process of screening and integrating studies. A total of 948 studies were initially identified. A total of 901 articles remained after removing duplicate studies. A total of 884 articles were identified as unrelated when evaluating titles and abstracts. A total of 6 studies were selected for inclusion in this meta-analysis after a thorough examination of the full text.
Patient characteristics and quality assessment
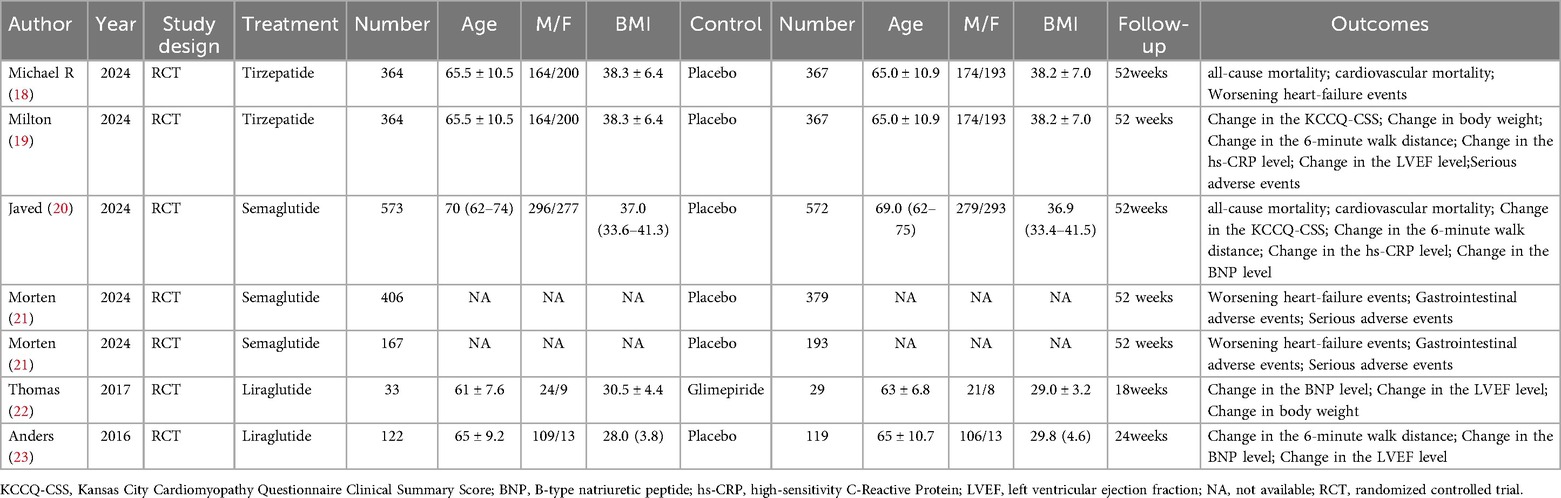
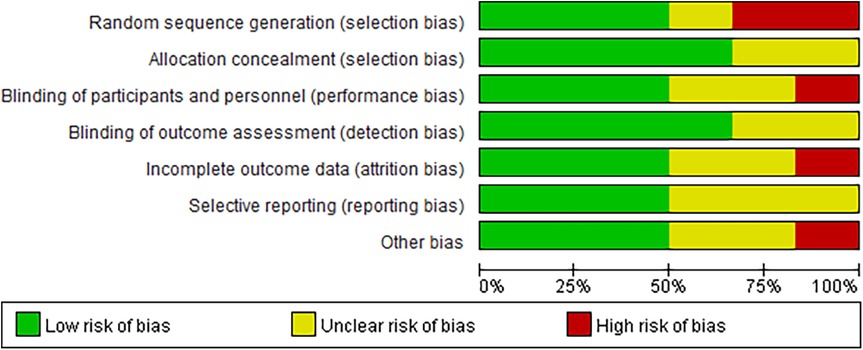
Six articles (18–23) were included in this meta-analysis, which consisted of seven randomized controlled trials. Detailed data on patient characteristics are presented in Table 1 and Figure 2 is the quality assessment results of the included studies.The meta-analysis focused on data from patients receiving GLP-1RAs to explore the efficacy and safety of GLP-1RAs in obese patients with CHF. More specifically, patients were divided into two groups according to their individual treatment regimen, one group received GLP-1RAs (Liraglutide, Tirzepatide, Semaglutide);the other group received placebo or Glimepiride. We performed a quality evaluation of included studies, and all articles were considered of good quality.
Primary clinical outcomes
Table 2 provides a brief overview of the Primary clinical outcomes. The primary clinical outcomes of patients receiving GLP-1RAs as treatment for obese patients with CHF were as follows: all-cause mortality (OR = 0.89, 95% CI:0.40–2.00, p = 0.78) (Figure 3A), cardiovascular mortality (OR = 0.93,95% CI: 0.22–4.00, p = 0.92) (Figure 3B)and worsening heart failure events (OR = 0.43,95% CI: 0.30–0.59, p < 0.00001) (Figure 3C).
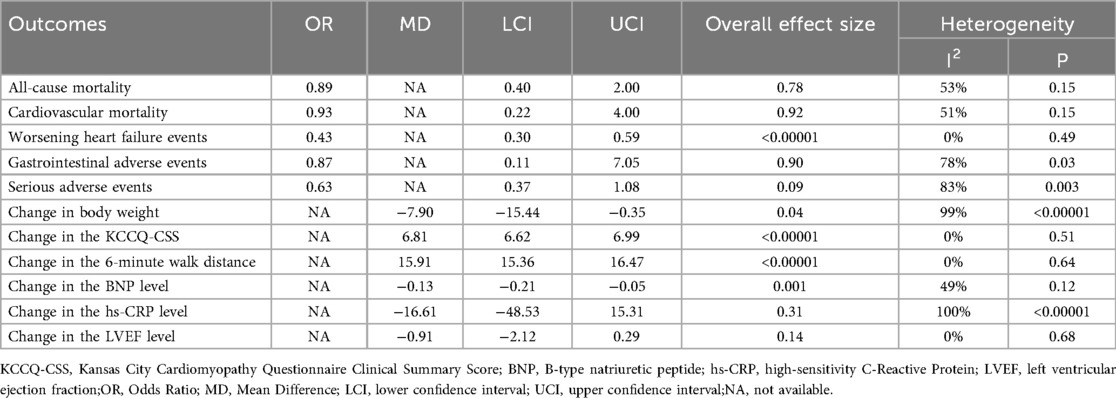
Table 2. The results of the meta-analysis for all-cause mortality,cardiovascular mortality, worsening heart-failure events, gastrointestinal adverse events, serious adverse events, change in body weight, change in the KCCQ-CSS,change in the 6-minute walk distance,change in the BNP level,change in the hs-CRP level,and change in the LVEF level.
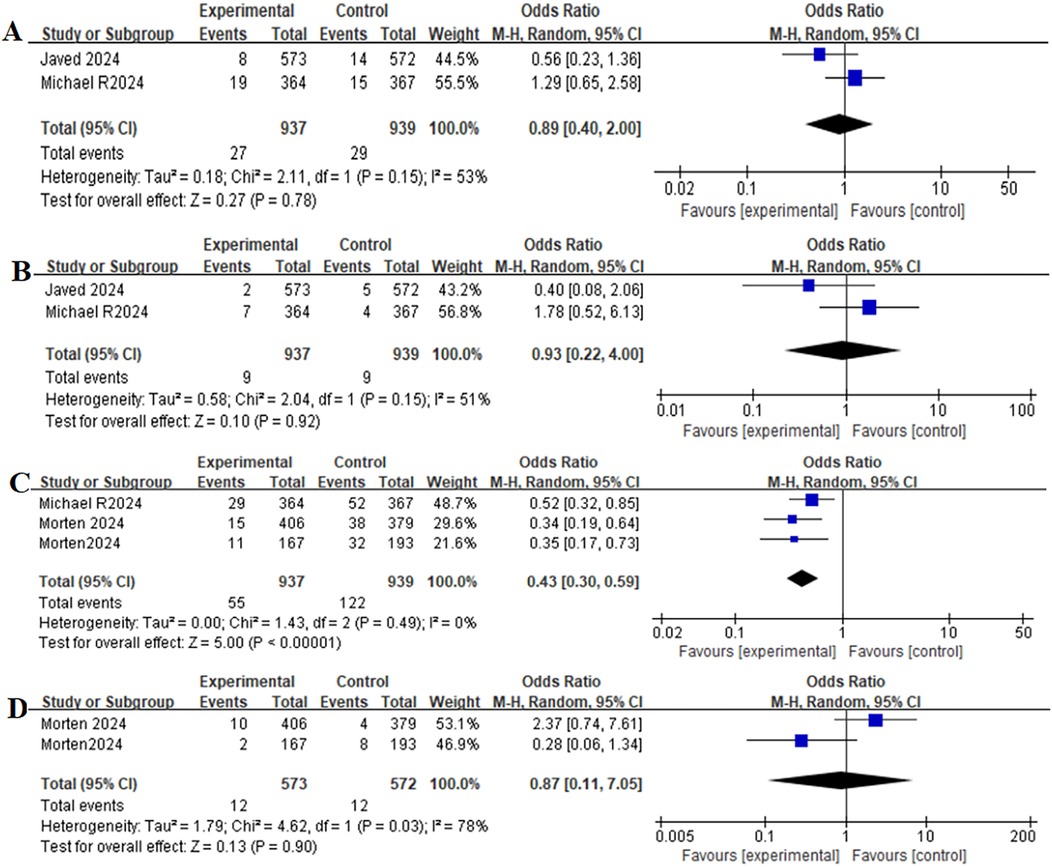
Figure 3. Forest plot of the meta-analysis for all-cause mortality-(A), death from cardiovascular causes-(B), worsening heart-failure events-(C), and gastrointestinal adverse events-(D) (Experimental group: Patients received GLP-1 receptor agonists; Control group: Patients received Placebo).
Safety outcomes
Table 2 provides a brief overview of the safety outcomes. Safety outcomes of patients receiving GLP-1RAs as treatment for obese patients with CHF were as follows: gastrointestinal adverse event (OR = 0.87,95% CI: 0.11–7.05, p = 0.90) (Figure 3D), serious adverse event (OR = 0.63,95% CI: 0.37–1.08, p = 0.09) (Figure 4A).
Secondary outcomes
Table 2 provides a brief overview of the secondary outcomes. Functional/biomarker/Imaging outcomes of patients receiving GLP-1RAs as treatment for obese patients with CHF were as follows:change in body weight (MD = −7.90,95% CI: −15.44 to −0.35, p = 0.04) (Figure 4B), change in the KCCQ-CSS (MD = 6.81,95% CI:6.62–6.99, p < 0.00001) (Figure 4C), change in the 6-minute walk distance (MD = 15.91,95% CI: 15.36–16.47,p < 0.00001) (Figure 4D), change in the BNP level (MD = −0.13,95% CI: −0.21 to−0.05,p = 0.001) (Figure 5A), change in the hs-CRP level (MD = −16.61, 95% CI:−48.53–15.31,p = 0.31) (Figure 5B), and change in the LVEF level (MD = −0.91,95% CI: −2.12–0.29, p = 0.14) (Figure 5C).
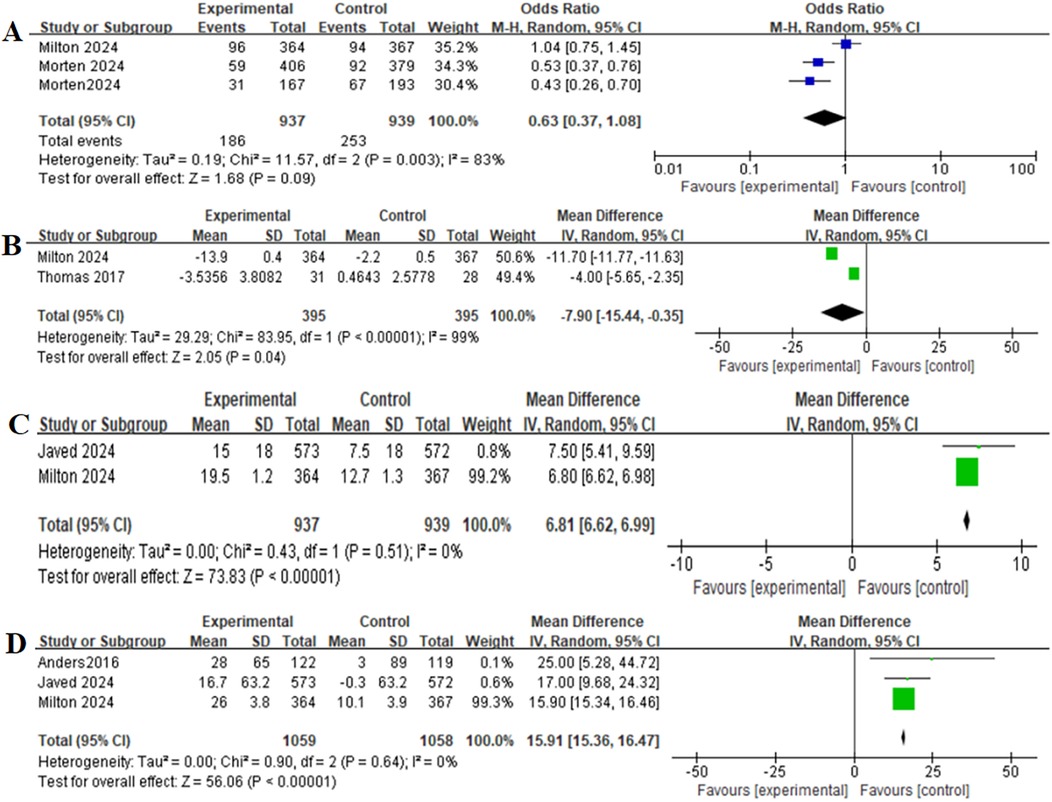
Figure 4. Forest plot of the meta-analysis for serious adverse events-(A), change in body weight-(B), change in the KCCQ-CSS-(C), and change in the 6-minute walk distance-(D) (experimental group: patients received GLP-1 receptor agonists; control group: patients received placebo or glimepiride).KCCQ-CSS, Kansas city cardiomyopathy questionnaire clinical summary score.
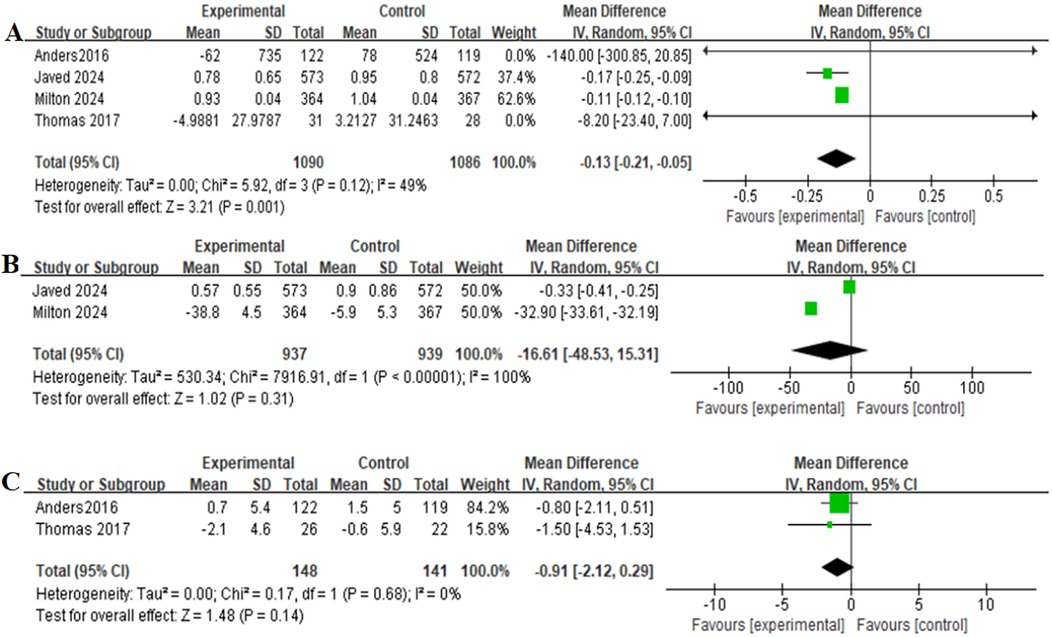
Figure 5. Forest plot of the meta-analysis for change in the BNP level-(A), change in the hs-CRP level-(B), and change in the LVEF level-(C) (experimental group: patients received GLP-1 receptor agonists; control group: patients received placebo or glimepiride).BNP, B-type natriuretic peptide; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction.
Discussion
The results of this meta-analysis provide important insights into the efficacy and safety of GLP-1RAs in the treatment of obese CHF patients. Our results show that GLP-1RAs such as Liraglutide, Semaglutide, and Tirzepatide showed significant improvements in key clinical outcomes such as weight loss, improved KCCQ-CSS, increased 6-min walk distance, and decreased BNP levels. In terms of primary clinical outcomes, GLP-1RAs significantly reduced the risk of worsening heart failure events notably, but no significant changes were observed in all-cause mortality, cardiovascular mortality.
The results showed that GLP-1RAs significantly reduced the risk of worsening heart failure events in the primary clinical outcome (worsening heart failure events: OR = 0.43, 95% CI: 0.30–0.59, p < 0.00001), but it had no effect on all-cause mortality and cardiovascular mortality (all-cause mortality: OR = 0.89, 95% CI: 0.40–2.00, p = 0.78; cardiovascular mortality: OR = 0.93, 95% CI: 0.22–4.00, p = 0.92). Shaylee and Arden (24) reported in their meta-analysis that GLP-1RAs significantly reduced all-cause mortality in patients with type 2 diabetes mellitus at high cardiovascular risk, but showed no significant effect on cardiovascular mortality. Arunkumar et al. (25), in a large population-matched cohort study, found that there was no significant difference between GLP-1RAs and SGLT2 inhibitors in reducing all-cause mortality in individuals with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes. Jo ˜ ao et al. (26) concluded in a analysis that liraglutide may increase the risk of adverse cardiovascular effects in patients with HFrEF. Other studies support the potential benefit of GLP-1RAs in reducing all-cause mortality, cardiovascular mortality, and worsening heart failure events (27–29). Future studies with larger samples and longer follow-up times are needed to further validate the effects of GLP-1RAs on all-cause mortality, cardiovascular mortality, and worsening heart failure events.
In secondary outcomes, GLP-1RAs significantly reduced patients' body weight (MD = −7.90, 95% CI: −15.44 to −0.35, p = 0.04), which is consistent with previous studies. Daniel et al. (30) found that among obese adolescents, a weekly 2.4 mg dose of semaglutide treatment plus a lifestyle intervention resulted in a greater reduction in BMI compared to lifestyle intervention alone in a double-blind, parallel-group, randomized, placebo-controlled trial. Similarly, Xie et al. (31) reported that GLP-1RAs exhibited significant glucose-lowering and weight-loss effects in patients with type 2 diabetes mellitus combined with a high BMI.GLP-1 agonists work by mimicking the physiological effects of GLP-1, delaying gastric emptying, increasing satiety, and reducing food intake; at the same time, they can directly stimulate the satiety centres in the hindbrain and hypothalamus, further suppressing appetite (32). In addition, liraglutide and semaglutide have been approved for weight loss treatment in overweight patients with comorbidities or obesity (33). GLP-1RAs also significantly improved KCCQ-CSS (MD = 6.81, 95% CI: 6.62–6.99, p < 0.00001) and 6-minute walk distance (MD = 15.91, 95% CI: 15.36–16.47, p < 0.00001).The significant increase in KCCQ-CSS and 6-min walking distance suggests that GLP-1RAs may improve cardiac function, reduce heart failure symptoms, and increase exercise tolerance in patients.GLP-1RAs may exert these benefits by reducing the generation of reactive oxygen species (ROS), decreasing systemic inflammation, and improving diastolic function (e. g., reducing diastolic filling pressure and ventricular load) (34). Amrit et al. (35) demonstrated that liraglutide not only improved myocardial perfusion and energy metabolism but also enhanced exercise tolerance (assessed by 6-minute walking distance) in patients compared to pioglitazone in their randomised crossover single-centre study.Melanie et al. (36) found that semaglutide significantly improved patients' symptoms of heart failure (e.g., dyspnea, fatigue) and physical function limitations (e.g., decreased mobility) in a prespecified analysis. In terms of biomarkers, GLP-1RAs significantly reduced BNP levels (MD = −0.13, 95% CI: −0.21 to −0.05, p = 0.001), which aligns with the results of a meta-analysis by Angelo et al., showing that GLP-1RAs significantly reduced N-terminal pro-BNP levels (37). However, GLP-1RAs did not improve hs-CRP levels (MD = −16.61, 95% CI: −48.53–15.31, p = 0.31), although Mohsen et al. demonstrated that GLP-1RAs significantly reduced serum CRP concentrations in patients with type 2 diabetes in their meta-analysis (38). The observed result of this study may be affected by high heterogeneity (I2 = 100%), and more large sample, high quality studies are needed for further verification. In addition, the improvement of LVEF by GLP-1RAs was not significant (MD = −0.91, 95% CI: −2.12–0.29, p = 0.14).Arif et al. (39) showed that GLP 1-RAs improved LVEF, significantly benefiting the management of HFpEF in patients with T2DM in a meta-analysis. Zhang et al. (40) concluded that GLP-1RAs may improve left ventricular function in HF patients in a single-center, prospective, interventional study.This result observed in the present study may be related to the specific characteristics of the study population and the relatively small sample size. Obese patients with CHF have complex pathophysiological mechanisms, often associated with metabolic disorders, chronic inflammation, and myocardial fibrosis, and these factors may influence the effects of GLP-1RAs on LVEF. Furthermore, it is crucial to recognize the limitations of LVEF as a cardiac function assessment tool. While primarily measuring myocardial contractility, LVEF shows limited sensitivity to subtle changes in diastolic function and ventricular remodeling, particularly in HFpEF where LVEF typically remains within normal ranges. The stability of LVEF suggests that the clinical benefits of GLP-1 receptor agonists (GLP-1RAs) may stem from non-positive inotropic mechanisms, such as systemic hemodynamic effects, improved myocardial metabolism, anti-inflammatory actions, and anti-fibrotic properties. Therefore, the lack of significant changes in LVEF does not diminish the potential value of GLP-1RAs in heart failure treatment, but rather distinguishes their mechanism of action from that of positive inotropic agents.
In the safety outcomes, GLP-1RAs had no significant effect on gastrointestinal adverse events (OR = 0.87, 95% CI: 0.11–7.05, p = 0.90). Nevertheless, Jamy et al. (41) noted that GLP-1RAs may increase the risk of gastrointestinal adverse events, but these adverse events are usually mild to moderate and transient. Future studies with larger samples and longer follow-up times are needed to further clarify the gastrointestinal safety of GLP-1RAs. On the other hand, there was no significant effect of GLP-1RAs group on the incidence of serious adverse events(OR = 0.63, 95% CI: 0.37–1.08, p = 0.09).Thomas et al. (42) noted that both tirzepatide and semaglutide did not increase the risk of serious adverse events in adults with type 2 diabetes in a network meta-analysis.This result of this study needs to be further confirmed in the future.
Our study outcomes differ from previous meta-analyses in certain endpoints (e.g., all-cause mortality, cardiovascular mortality, hs-CRP levels). We will delve into the potential reasons for these discrepancies. First, our chronic heart failure analysis included patients with various cardiac conditions, whereas other studies focused exclusively on HFpEF or HFrEF patients. This fundamental shift in patient composition constitutes the core reason for the outcome differences. Second, previous Meta-analyses likely relied more heavily on evidence from first-generation GLP-1 receptor agonists (e.g., liraglutide), while our analysis incorporates a higher proportion of newer, more potent drugs (e.g., semaglutide). Variations in drug molecular structures, half-lives, and receptor affinities may lead to differences in therapeutic efficacy and anti-inflammatory potency. Given the high heterogeneity in hs-CRP measurements (I2 = 100%), it's not surprising that our pooled estimates differ from previous Meta-analyses. These differences likely reflect significant variations in measurement methods, laboratory standards, or baseline inflammatory status rather than genuine differences in drug efficacy.
The study has its advantage. This study sets up a rich set of evaluation indicators, focusing not only on key primary outcomes such as all-cause mortality, cardiovascular mortality, and worsening heart failure events, but also on secondary outcomes like weight changes. It also considers safety metrics such as gastrointestinal adverse reactions and serious adverse events, providing a comprehensive assessment of the application of GLP-1RAs in obese CHF patients. Furthermore, it targets the specific group of obese patients with chronic heart failure, whose conditions are complex and who face unique treatment needs and challenges, making the research highly targeted.
The present meta-analysis has several limitations. First, the included studies varied in population characteristics, drug type、 dose, and follow-up time, leading to high heterogeneity of results (e.g., I2 = 100% for hs-CRP levels), which may affect the universality and reliability of the results. The heterogeneity of hs-CRP reached 100%, indicating that differences among studies almost entirely determined the variation in results rather than sampling errors. We speculate this may stem from three aspects: First, methodological heterogeneity. Although all studies used “hs-CRP”, variations in testing platforms and inconsistent quality control protocols across laboratories could introduce systematic measurement bias. Second, clinical heterogeneity. As a highly sensitive but nonspecific inflammatory marker, hs-CRP measurements may vary significantly between study participants' baseline conditions. Some studies permitted use of other anti-inflammatory drugs, which might confound the true effects of interventions on hs-CRP levels. Third, intervention impacts are not universal but heavily dependent on specific study contexts and population characteristics. Future research should define more homogeneous subgroups for analysis. Gastrointestinal adverse events, being subjective outcomes, exhibit complex heterogeneity due to inconsistent definitions across studies. Significant variations in baseline gastrointestinal health status and tolerance to intervention components lead to substantial response differences. The moderate-to-high heterogeneity (I2 = 78%) highlights that intervention-related gastrointestinal risks are not fixed, necessitating individualized risk assessment. In conclusion, these specific highly heterogeneous outcomes strongly suggest that the impact of our interventions on these indicators is context-dependent. Although the random effects model attempts to account for study-to-study variation, it does not eliminate the heterogeneity itself. Second, the small sample size of some studies, such as the study by Thomas et al. (2017) included only 62 patients, may not be sufficient to detect significant differences (22). In addition, no subgroup analysis (ie by drug type, dose) was performed due to the limited number of included studies, which limits the exploration of potential differences in different subgroups. Future research should prioritize this aspect, and can conduct head-to-head randomized controlled trials (RCTs), perform network meta-analysis (NMA), accumulate more studies for dose-response meta-analysis, and carry out meta-analysis using individual participant data (IPD). Third, the short duration of follow-up in most studies (e.g., 52 weeks) makes it difficult to assess the long-term effects and safety of GLP-1RAs, for example, whether weight regain will occur after discontinuation or the long-term effects on cardiovascular events are not known.Future studies should extend the follow-up time of existing RCTs, conduct longer prospective long-term RCTs, and perform patient-level Meta analysis. Furthermore,due to limited number of studies, subgroup analysis by ejection fraction (EF) was not conducted.The response of patients with heart failure in different ejection fraction ranges to treatment may vary. Future studies with larger sample sizes are needed to explore the possible efficacy differences based on the EF status. Finally, it should be noted that this study included patients in the overweight category. Specifically, as presented in Table 1, the mean BMI of one cohort was 28. While this may be perceived as a deviation from the conventional definition of obesity, we contend that it offers a unique and clinically relevant perspective. Grounded in the context of the “obesity paradox,” the applicability of traditional BMI classifications for heart failure patients remains debated. Both overweight and obesity share common pathophysiological underpinnings, such as insulin resistance and features of heart failure mechanisms like volume overload and impaired myocardial metabolism. Consequently, this study aimed to move beyond strict BMI cut-offs to evaluate the therapeutic value of GLP-1RAs not only in a “strictly defined obese” population but also in a “broader spectrum of heart failure patients who, while potentially benefiting from the obesity paradox, might still require active weight management” (encompassing the range from overweight to obesity). We acknowledge that employing broad BMI inclusion criteria may introduce heterogeneity; however, this approach better reflects the complexity of real-world clinical practice and provides more comprehensive evidence on the use of GLP-1RAs in heart failure patients with concomitant metabolic abnormalities.
Conclusion
In conclusion, GLP-1 RAs represent a promising treatment option for obese patients with CHF. Our findings show that these agents significantly reduce the risk of worsening heart failure events and confer substantial clinical benefits, including improved symptom burden (KCCQ-CSS), greater functional capacity (6-minute walk distance), and reduced levels of BNP, a key heart failure biomarker. These improvements occurred despite the absence of a significant change in LVEF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
AJ: Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft. MY: Methodology, Software, Supervision, Writing – review & editing. TW: Methodology, Software, Supervision, Writing – review & editing. YH: Writing – review & editing, Methodology, Software, Supervision. HL: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1633114/full#supplementary-material
References
1. Shrestha J, Done S. An overview of chronic heart failure. Nurs Stand. (2023) 38(8):43–9. doi: 10.7748/ns.2023.e12049
2. Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. (2023) 329(10):827–38. doi: 10.1001/jama.2023.2020
3. Rogers C, Bush N. Heart failure: pathophysiology, diagnosis, medical treatment guidelines, and nursing management. Nurs Clin North Am. (2015) 50(4):787–99. doi: 10.1016/j.cnur.2015.07.012
4. Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. (2023) 81(18):1810–34. doi: 10.1016/j.jacc.2023.01.049
5. Campbell P, Rutten FH, Lee MMY, Hawkins NM, Petrie MC. Heart failure with preserved ejection fraction: everything the clinician needs to know. Lancet. (2024) 403(10431):1083–92. doi: 10.1016/S0140-6736(23)02756-3
6. Alebna PL, Mehta A, Yehya A, daSilva-deAbreu A, Lavie CJ, Carbone S. Update on obesity, the obesity paradox, and obesity management in heart failure. Prog Cardiovasc Dis. (2024) 82:34–42. doi: 10.1016/j.pcad.2024.01.003
7. Bonfioli GB, Rodella L, Metra M, Vizzardi E. GLP-1 receptor agonists as promising anti-inflammatory agents in heart failure with preserved ejection fraction. Heart Fail Rev. (2025) 30(1):131–6. doi: 10.1007/s10741-024-10450-6
8. Abdin A, Böhm M, Shahim B, Karlström P, Kulenthiran S, Skouri H, et al. Heart failure with preserved ejection fraction epidemiology, pathophysiology, diagnosis and treatment strategies. Int J Cardiol. (2024) 412:132304. doi: 10.1016/j.ijcard.2024.132304
9. Ramirez MF, Lau ES, Parekh JK, Pan AS, Owunna N, Wang D, et al. Obesity-related biomarkers are associated with exercise intolerance and HFpEF. Circ Heart Fail. (2023) 16(11):e010618. doi: 10.1161/CIRCHEARTFAILURE.123.010618
10. Shaefer CF Jr, Kushner P, Aguilar R. User’s guide to mechanism of action and clinical use of GLP-1 receptor agonists. Postgrad Med. (2015) 127(8):818–26. doi: 10.1080/00325481.2015.1090295
11. Liu Y, Ruan B, Jiang H, Le S, Liu Y, Ao X, et al. The weight-loss effect of GLP-1RAs glucagon-like peptide-1 receptor agonists in non-diabetic individuals with overweight or obesity: a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Am J Clin Nutr. (2023) 118(3):614–26. doi: 10.1016/j.ajcnut.2023.04.017
12. Hullon D, Subeh GK, Volkova Y, Janiec K, Trach A, Mnevets R. The role of glucagon-like peptide-1 receptor (GLP-1R) agonists in enhancing endothelial function: a potential avenue for improving heart failure with preserved ejection fraction (HFpEF). Cardiovasc Diabetol. (2025) 24(1):70. doi: 10.1186/s12933-025-02607-w
13. Withaar C, Meems LMG, Markousis-Mavrogenis G, Boogerd CJ, Silljé HHW, Schouten EM, et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc Res. (2021) 117(9):2108–24. doi: 10.1093/cvr/cvaa256
14. Wong SY, Lee ARYB, Sia AHJ, Wo YJ, Teo YH, Teo YN, et al. Effects of glucagon-like peptide-1 receptor agonist (GLP-1RA) on cardiac structure and function: a systematic review and meta-analysis of randomized-controlled trials. Cardiovasc Drugs Ther. (2024) 38(2):371–89. doi: 10.1007/s10557-022-07360-w
15. Huixing L, Di F, Daoquan P. Effect of glucagon-like peptide-1 receptor agonists on prognosis of heart failure and cardiac function: a systematic review and meta-analysis of randomized controlled trials. Clin Ther. (2023) 45(1):17–30. doi: 10.1016/j.clinthera.2022.12.006
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Pavía-López AA, Magaña-Serrano JA, Cigarroa-López JA, Chávez-Mendoza A, Mayorga-Butrón JL, Araiza-Garaygordobil D, et al. Clinical practice guidelines for diagnostic and treatment of the chronic heart failure. Arch Cardiol Mex. (2024) 94(Supl 1):1–74. English. doi: 10.24875/ACM.M24000095
18. Zile MR, Borlaug BA, Kramer CM, Baum SJ, Litwin SE, Menon V, et al. Effects of tirzepatide on the clinical trajectory of patients with heart failure, preserved ejection fraction, and obesity. Circulation. (2025) 151(10):656–68. doi: 10.1161/CIRCULATIONAHA.124.072679
19. Packer M, Zile MR, Kramer CM, Baum SJ, Litwin SE, Menon V, et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med. (2025) 392(5):427–37. doi: 10.1056/NEJMoa2410027
20. Butler J, Shah SJ, Petrie MC, Borlaug BA, Abildstrøm SZ, Davies MJ, et al. Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomised trials. Lancet. (2024) 403(10437):1635–48. doi: 10.1016/S0140-6736(24)00469-0
21. Schou M, Petrie MC, Borlaug BA, Butler J, Davies MJ, Kitzman DW, et al. Semaglutide and NYHA functional class in obesity-related heart failure with preserved ejection fraction: the STEP-HFpEF program. J Am Coll Cardiol. (2024) 84(3):247–57. doi: 10.1016/j.jacc.2024.04.038
22. Nyström T, Santos-Pardo I, Hedberg F, Wardell J, Witt N, Cao Y, et al. Effects on subclinical heart failure in type 2 diabetic subjects on liraglutide treatment vs. glimepiride both in combination with metformin: a randomized open parallel-group study. Front Endocrinol (Lausanne). (2017) 8:325. doi: 10.3389/fendo.2017.00325. Erratum in: Front Endocrinol (Lausanne). (2018) 9:50. doi: 10.3389/fendo.2018.00050.
23. Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. (2017) 19(1):69–77. doi: 10.1002/ejhf.657
24. Peterson SC, Barry AR. Effect of glucagon-like peptide-1 receptor agonists on all-cause mortality and cardiovascular outcomes: a meta-analysis. Curr Diabetes Rev. (2018) 14(3):273–9. doi: 10.2174/1573399813666170414101450
25. Krishnan A, Schneider CV, Hadi Y, Mukherjee D, AlShehri B, Alqahtani SA. Cardiovascular and mortality outcomes with GLP-1 receptor agonists vs other glucose-lowering drugs in individuals with NAFLD and type 2 diabetes: a large population-based matched cohort study. Diabetologia. (2024) 67(3):483–93. doi: 10.1007/s00125-023-06057-5
26. Neves JS, Vasques-Nóvoa F, Borges-Canha M, Leite AR, Sharma A, Carvalho D, et al. Risk of adverse events with liraglutide in heart failure with reduced ejection fraction: a post hoc analysis of the FIGHT trial. Diabetes Obes Metab. (2023) 25(1):189–97. doi: 10.1111/dom.14862
27. Al-Sadawi MA, Aslam FM, Tao M, Alsaiqali M, Almasry IO, Fan R, et al. Effects of GLP-1 agonists on mortality and arrhythmias in patients with type II diabetes. Int J Cardiol Heart Vasc. (2023) 47:101218. doi: 10.1016/j.ijcha.2023.101218
28. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. (2021) 9(10):653–62. doi: 10.1016/S2213-8587(21)00203-5
29. Riley DR, Essa H, Austin P, Preston F, Kargbo I, Ibarburu GH, et al. All-cause mortality and cardiovascular outcomes with sodium-glucose co-transporter 2 inhibitors, glucagon-like peptide-1 receptor agonists and with combination therapy in people with type 2 diabetes. Diabetes Obes Metab. (2023) 25(10):2897–909. doi: 10.1111/dom.15185
30. Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. (2022) 387(24):2245–57. doi: 10.1056/NEJMoa2208601
31. Xie Z, Hu J, Gu H, Li M, Chen J. Comparison of the efficacy and safety of 10 glucagon-like peptide-1 receptor agonists as add-on to metformin in patients with type 2 diabetes: a systematic review. Front Endocrinol (Lausanne). (2023) 14:1244432. doi: 10.3389/fendo.2023.1244432
32. Raza FA, Altaf R, Bashir T, Asghar F, Altaf R, Tousif S, et al. Effect of GLP-1 receptor agonists on weight and cardiovascular outcomes: a review. Medicine (Baltimore). (2024) 103(44):e40364. doi: 10.1097/MD.0000000000040364
33. Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. (2023) 20(7):463–74. doi: 10.1038/s41569-023-00849-3
34. Cimino G, Vaduganathan M, Lombardi CM, Pagnesi M, Vizzardi E, Tomasoni D, et al. Obesity, heart failure with preserved ejection fraction, and the role of glucagon-like peptide-1 receptor agonists. ESC Heart Fail. (2024) 11(2):649–61. doi: 10.1002/ehf2.14560
35. Chowdhary A, Thirunavukarasu S, Joseph T, Jex N, Kotha S, Giannoudi M, et al. Liraglutide improves myocardial perfusion and energetics and exercise tolerance in patients with type 2 diabetes. J Am Coll Cardiol. (2024) 84(6):540–57. doi: 10.1016/j.jacc.2024.04.064
36. Davies MJ, van der Meer P, Verma S, Patel S, Chinnakondepalli KM, Borlaug BA, et al. Semaglutide in obesity-related heart failure with preserved ejection fraction and type 2 diabetes across baseline HbA1c levels (STEP-HFpEF DM): a prespecified analysis of heart failure and metabolic outcomes from a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. (2025) 13(3):196–209. doi: 10.1016/S2213-8587(24)00304-8
37. Avogaro A, Azzolina D, Gregori D, De Kreutzenberg S, Fadini GP, Mannucci E. The effect of GLP-1 receptor agonists on N-terminal pro-brain natriuretic peptide. A scoping review and metanalysis. Int J Cardiol. (2022) 357:123–7. doi: 10.1016/j.ijcard.2022.03.032
38. Mazidi M, Karimi E, Rezaie P, Ferns GA. Treatment with GLP1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications. (2017) 31(7):1237–42. doi: 10.1016/j.jdiacomp.2016.05.022
39. Albulushi A, Tanoh DB, Almustafa A, Al Matrooshi N, Zolty R, Lowes B. Comparative effects of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors on heart failure with preserved ejection fraction in diabetic patients: a meta-analysis. Cardiovasc Diabetol. (2024) 23(1):324. doi: 10.1186/s12933-024-02415-8
40. Zhang JY, Wang XY, Wang X. Effects of liraglutide on hemodynamic parameters in patients with heart failure. Oncotarget. (2017) 8(37):62693. doi: 10.18632/oncotarget.18570
41. Ard J, Fitch A, Fruh S, Herman L. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. (2021) 38(6):2821–39. doi: 10.1007/s12325-021-01710-0
42. Karagiannis T, Malandris K, Avgerinos I, Stamati A, Kakotrichi P, Liakos A, et al. Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Diabetologia. (2024) 67(7):1206–22. doi: 10.1007/s00125-024-06144-1
Keywords: GLP-1 receptor agonists, chronic heart failure, obesity, meta-analysis, semaglutide
Citation: Jia A, Yang M, Wang T, Hua Y and Lu H (2025) Efficacy and safety of GLP-1 receptor agonists in the treatment of obese patients with chronic heart failure: a meta-analysis. Front. Cardiovasc. Med. 12:1633114. doi: 10.3389/fcvm.2025.1633114
Received: 22 May 2025; Accepted: 8 September 2025;
Published: 3 October 2025.
Edited by:
Otilia Tica, Emergency County Clinical Hospital of Oradea, RomaniaReviewed by:
Kumar Ashish, CarolinaEast Medical Center, United StatesManeeth Mylavarapu, Independent Researcher, Birmingham, United States
Copyright: © 2025 Jia, Yang, Wang, Hua and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusi Hua, eXVzaWh1YUB3Y2hzY3UuY24=; Huimin Lu, aHVpbWlubHVoeEAxMjYuY29t
 Anna Jia1,2
Anna Jia1,2 Tianhong Wang
Tianhong Wang Yusi Hua
Yusi Hua