- 1Department of Clinical Laboratory Sciences, Fukushima Medical University School of Health Sciences, Fukushima, Japan
- 2Department of Physical Therapy, Fukushima Medical University School of Health Sciences, Fukushima, Japan
- 3Department of Occupational Therapy, Fukushima Medical University School of Health Sciences, Fukushima, Japan
- 4Department of Cardiovascular Medicine, Fukushima Medical University School of Medicine, Fukushima, Japan
Aims: Cardio-ankle vascular index (CAVI) is a non-invasive method for evaluating arterial stiffness. In adults, CAVI has been reported to show negative correlation with body mass index (BMI) known as the “obesity paradox”; however, whether this also applies to children remains unclear. In addition, childhood obesity is a problem in developed countries, and the utility of CAVI in children has not yet been clarified. We here aimed to clarify the relationship between obesity parameters and CAVI in healthy children.
Methods: This was a cross-sectional study conducted in 2024. We evaluated CAVI and its associated factors in 590 children aged 6–15 years (mean age: 10.5 years, 51.0% female). Additionally, obesity parameters, including the Rohrer index, percentage of overweight (POW), body fat percentage and body fat mass determined by bioelectrical impedance analysis, and BMI were assessed. The participants were categorized into groups based on the obesity parameters.
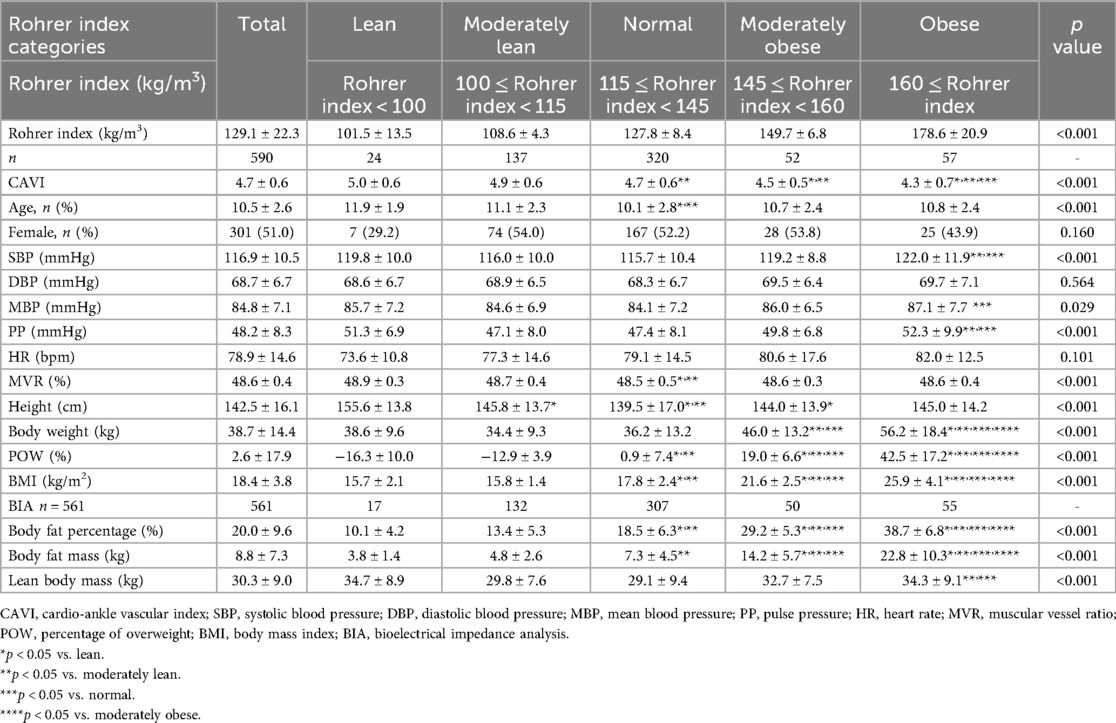
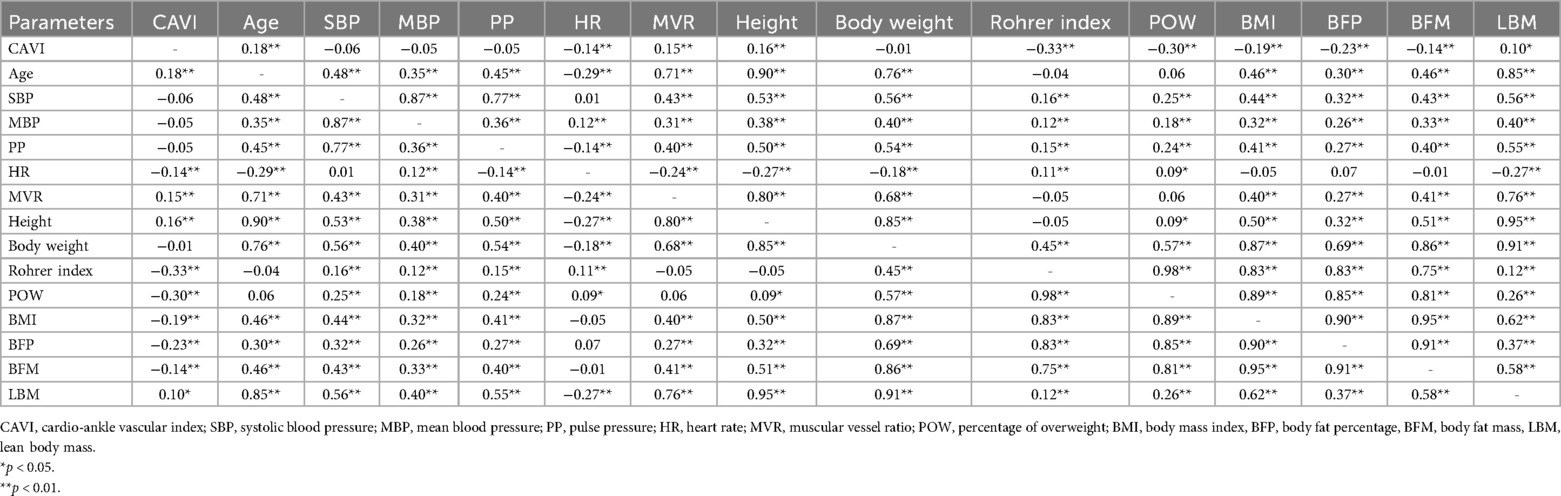
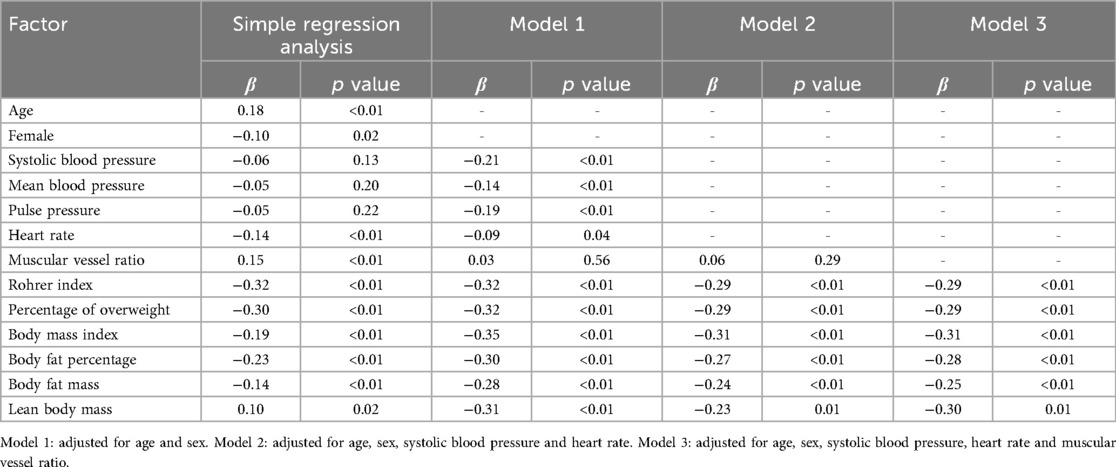
Results: CAVI decreased as obesity level increased, showing the lowest CAVI in the highest obesity category. CAVI was positively correlated with age (R = 0.18, p < 0.05), and was negatively correlated with Rohrer index, body fat percentage, body fat mass, POW, BMI, and heart rate, (R = −0.33, R = −0.23, R = −0.14, R = −0.30, R = −0.19, R = −0.14, respectively; p < 0.01 for all). In contrast, CAVI showed no significant correlation with blood pressure or body weight. Furthermore, multiple linear regression analyses after adjusting for possible obesity-related factors including age, sex, blood pressure, and heart rate, showed that all obesity parameters were independent predictors of CAVI.
Conclusion: Obesity parameters are negatively and independently associated with CAVI in healthy children.
Introduction
Cardio-ankle vascular index (CAVI) is a non-invasive and simple method for evaluating arterial stiffness and arteriosclerosis, and has been reported to be useful for predicting cardiovascular disease in adults (1–5). However, there is a negative correlation between CAVI and body mass index (BMI) in healthy adults, known as the “obesity paradox” (6, 7). Namely, CAVI tends to be lower in obese adults, who are at increased risk for hypertension, diabetes, dyslipidemia, and cardiovascular disease. However, since there are possible obesity-related factors (age, sex, blood pressure, heart rate, body fat mass, vascular characteristics, and etc.), it is not clear enough about the cause of “obesity paradox”.
Childhood obesity is a significant problem in developed countries (8, 9). Pathological arteriosclerosis such as intimal thickening has been reported to progress with age, starting from the first year of life (10). Since childhood obesity is likely to continue into adulthood, with associated increases in morbidity and mortality, early intervention is crucial. Evaluating arterial stiffness from childhood is important for preventing arteriosclerosis and reducing the risk of future cardiovascular diseases (11). However, there are only a few studies on CAVI and BMI in children (12, 13), and its validity and accuracy in these populations remain unclear. Additionally, BMI is not necessarily appropriate for assessing childhood obesity. BMI is calculated based on height and weight, varies greatly during growth for specific ages, and thus fixed cut-off values for obesity cannot be set for children (14, 15). Instead, Rohrer index is known to be appropriate for assessing childhood obesity (16, 17). In addition, percentage of overweight (POW) has reflects the actual condition related to the physical status of children with regard to fatness in puberty to a greater extent than BMI (14). Furthermore, the bioelectrical impedance analysis (BIA) method can classify childhood obesity in more accurate than BMI (18, 19).
Furthermore, CAVI measures the combined properties of the aorta, femoral artery, and tibial artery. The aorta is an elastic artery, whereas the femoral and tibial arteries are muscular vessels (20). The difference in the ratio of elastic and muscular blood vessels in the total length of the measured vessels may affect CAVI. To clarify impact of property of elastic and muscular blood vessels on CAVI, we newly tried to use muscular vessel ratio (MVR) in the study.
Therefore, the purpose of the present study was (A) to evaluate the validity and accuracy of CAVI measurement, (B) to clarify the relationships between CAVI and several obesity parameters (Rohrer index, POW, BMI, body fat percentage and body fat mass determined by BIA), and (C) to examine the factor which influence “obesity paradox” such as blood pressure, MVR etc., in healthy children and adolescents.
Methods
Study population
Study flow chart is presented in Figure 1. This was a cross-sectional study of 626 children and adolescents aged 6–15 years (mean 10.5 years; 51% females) who took part in a health promotion event held by Fukushima Medical University School of Health Sciences in 2024. We measured their CAVI, height, weight, Rohrer index, POW, and BMI, as well as their body fat percentage and body fat mass, assessed using BIA. The exclusion criteria were clear evidence of obliterative arterial disease; clear evidence of arrhythmia such as atrial fibrillation; and refusal to undergo measurement. There were no subjects who met the exclusion criteria. However, of the 626 study participants, 36 were excluded due to inability to remain still or irregular waveforms, resulting in a final analysis of 590 individuals. Out of the 590 participants, 561 were measured body fat percentage and body fat mass determined by BIA.
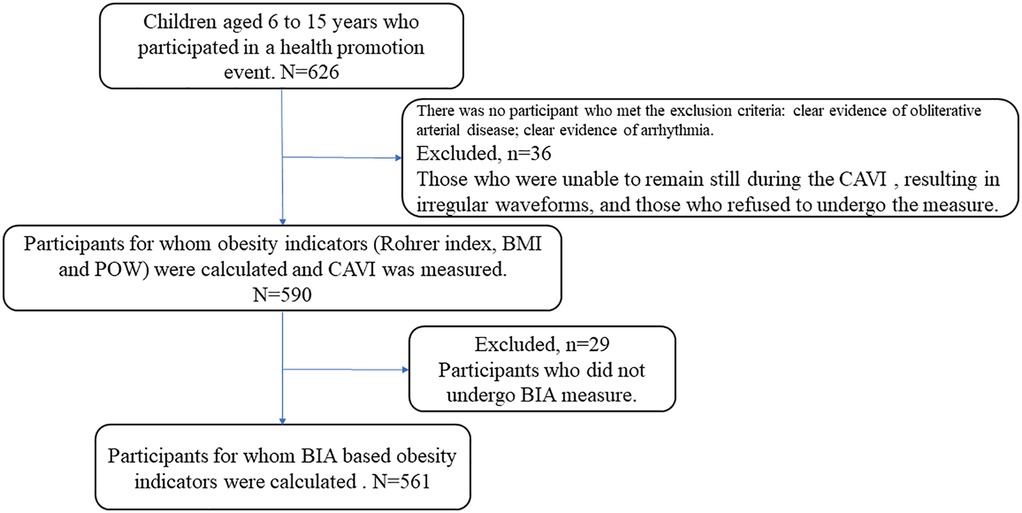
Figure 1. Study flow chart. This study included 626 children and adolescents aged 6–15 years. Of the 626 study participants, 36 were excluded because they were unable to maintain a resting state during CAVI measurement or showed irregular waveforms. Furthermore, 29 participants did not undergo BIA measurement. The analysis was ultimately conducted on 561 participants.
To evaluate the accuracy and validity of CAVI measurements, we measured the length of the participants' blood vessels over their clothing with a tape measure and compared it to the vessel length calculated from height based on CAVI measurements. CAVI is consisted of the combined properties of elastic vessel (i.e., aorta) and muscular vessel (i.e., femoral and tibial arteries) (20). To clarify the impact of property of elastic and muscular blood vessels on CAVI, we tried to assess MVR. Based on previous reports (20–22), vascular length was defined as follows: the distance from the second intercostal space to the base of the foot (L1), the distance from the base of the foot to the knee (L2), and the distance from the knee to the ankle (L3). The MVR was defined as (L2 + L3)/(L1 + L2 + L3).
We examined the relationship between each obesity parameter (Rohrer index, body fat percentage, POW, and BMI) and CAVI. The Rohrer index is used as an indicator of childhood obesity and is calculated as follows: weight (kg)/height (m3) (16, 17). Body fat percentage was measured using BIA. According to the criteria for childhood obesity adopted by the Ministry of Education, Culture, Sports, Science and Technology in Japan, a child was considered to be obese when the POW exceeded 20%. POW = 20% means that one's body weight is 120% of standard body weight, which is defined as the mean body weight corresponding to the height for that age obtained from the national statistics for Japanese school children in 2000. BMI is calculated as follows: weight (kg)/height (m2) (16, 18, 23, 24). The study participants were divided into groups based on Rohrer index (lean, moderately lean, normal, moderately obese, and obese) (17, 25), body fat percentage (underweight, −normal, +normal, overweight, obese), POW (underweight, normal, mild obesity, moderate obesity, severe obesity), and BMI (underweight, normal weight, and overweight-obesity) (15, 26–28). We compared CAVI, height, weight, Rohrer index, body fat percentage, POW, and BMI, blood pressure, heart rate (HR), and MVR among the groups. The study protocol was approved by the Research Ethics Committee of Fukushima Medical University (No.2021-186, REC2023-029). The participants were given a written explanation of the study, and consent for participation was obtained from both the participants and their guardians.
CAVI measurement
CAVI was measured automatically using VaSera VS-3000TE (Fukuda Denshi Co., Ltd., Tokyo, Japan) with the participant in the supine position (20). Cuffs were attached bilaterally to the upper arms and ankles. The cuff size for the upper arm was selected based on the measured circumference from the following three sizes: S (170–220 mm), M (220–300 mm), and L (300–390 mm). Electrocardiogram electrodes were placed on both wrists and a microphone was placed on the sternum. The average CAVI from both sides was used for analysis.
Measurements of body fat percentage and body fat mass
Body fat percentage and body fat mass were measured using MC-780A-N (TANITA Co., Ltd., Tokyo, Japan) with the participant in the standing position. Body composition was estimated to be using the multi-frequency bioelectrical impedance analysis method by measuring electrical resistance, which reflects the ease of current flow through the body (23).
Statistical analysis
Normality was confirmed using the Shapiro–Wilk test. Normally distributed data are expressed as mean ± standard deviation. Categorical variables are expressed as numbers (percentages), and the chi-square test was used for comparison. Comparison of parametric variables between groups was conducted using one-way analysis of variance, followed by Bonferroni post-hoc analysis. The associations between CAVI and obesity parameters (i.e., POW, Rohrer index, BMI, and body fat percentage) were examined using Pearson's correlation analysis for parametric variables. The difference between CAVI-based and manually measured vascular lengths was assessed with linear regression analysis and Bland-Altman analysis. Multiple linear regression analyses were performed to assess the associations between CAVI and obesity parameters, as well as variables commonly associated with CAVI (e.g., age, sex, and blood pressure, HR). A p value of <0.05 was considered statistically significant in all analyses. All analyses were conducted using IBM SPSS Statistics version 26 (IBM, Armonk, NY, USA).
Results
We firstly examined the accuracy and validity of CAVI measurements in children. Linear regression analysis revealed strong correlations between the manually measured and CAVI-based vessel lengths (R = 0.922, p < 0.001, Figure 2), and the Bland-Altman analysis showed a mean difference of 3.44 ± 5.36, indicating excellent concordance.
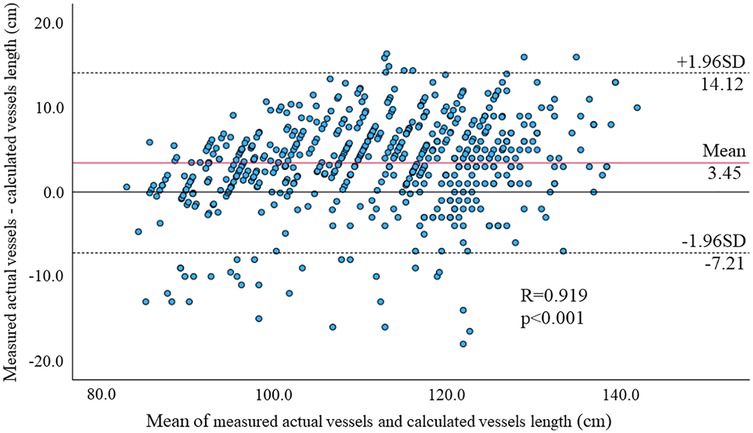
Figure 2. Bland-Altman plots of manually measured and CAVI-based vessel lengths for each parameter. The linear regression analysis revealed strong correlations between the vascular lengths measured by the two methods (R = 0.922, p < 0.001), and the Bland-Altman analysis showed mean differences were 3.45 ± 5.36. These results suggest excellent agreement between the two measurements.
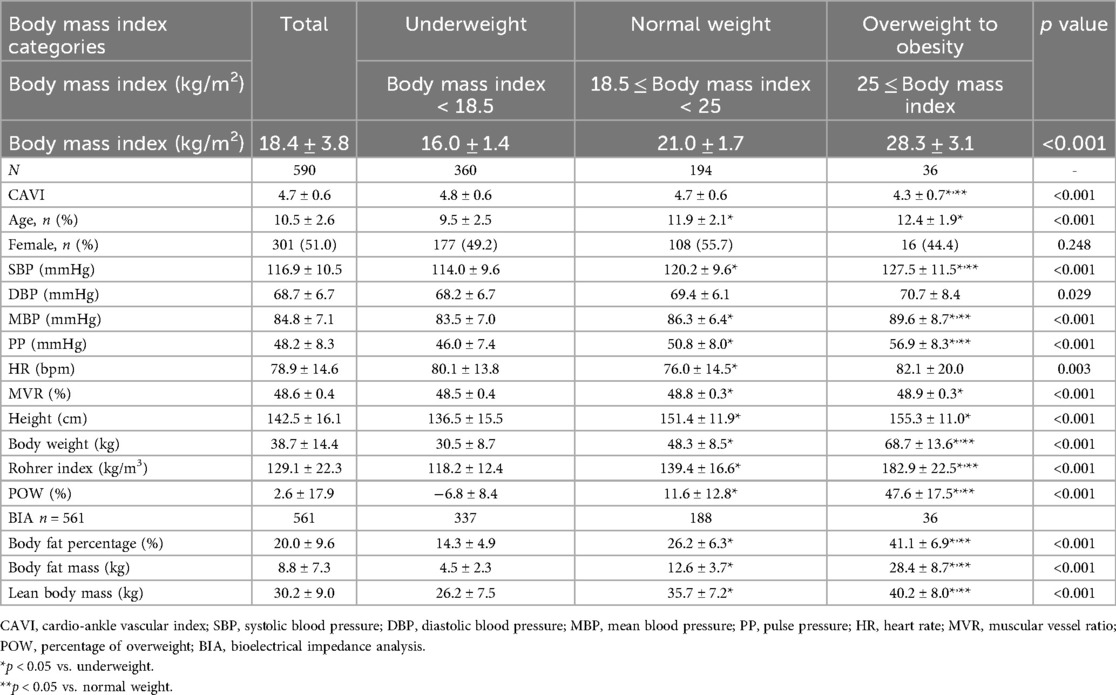
Next, we examined the relationship between each obesity parameter (Rohrer index, body fat percentage, POW, and BMI) and CAVI. The results of CAVI comparisons based on obesity classification using the Rohrer index are shown in Table 1. CAVI decreased as obesity levels increased, showing the lowest CAVI in the groups with the highest obesity (p < 0.001). Similarly, CAVI decreased as the degree of obesity increased for other obesity parameters (Table 2, body fat percentage based on BIA; Table 3, POW; and Table 4, BMI). Additionally, these highest obesity groups exhibited the highest SBP (p < 0.001; Tables 1–4). With respect to age and sex, except for the body fat percentage classification (Table 2), there were no differences in sex among the groups. Except for body fat percentage classification (Table 2), age showed significant differences without specific tendency. In both the male and female participants, CAVI was lowest in the group with the highest obesity (Figure 3). Comparisons of other parameters stratified by gender based on obesity classes are shown in Supplementary Tables (1a, Rohrer index-male; 1b, Rohrer index-female; 2a, body fat percentage based on BIA-male; 2b, body fat percentage based on BIA-female; 3a, POW-male; 3b, POW-female; 4a, BMI-male; 4b, BMI-female).
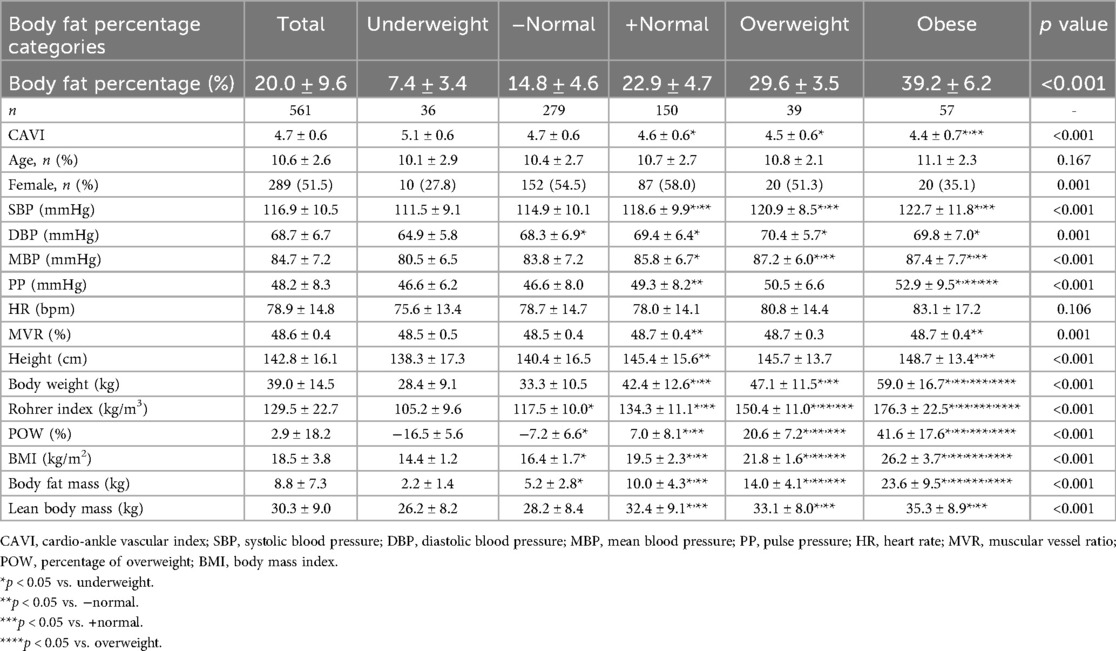
Table 2. Cardio-ankle vascular index of participants by body fat percentage classification (n = 561).
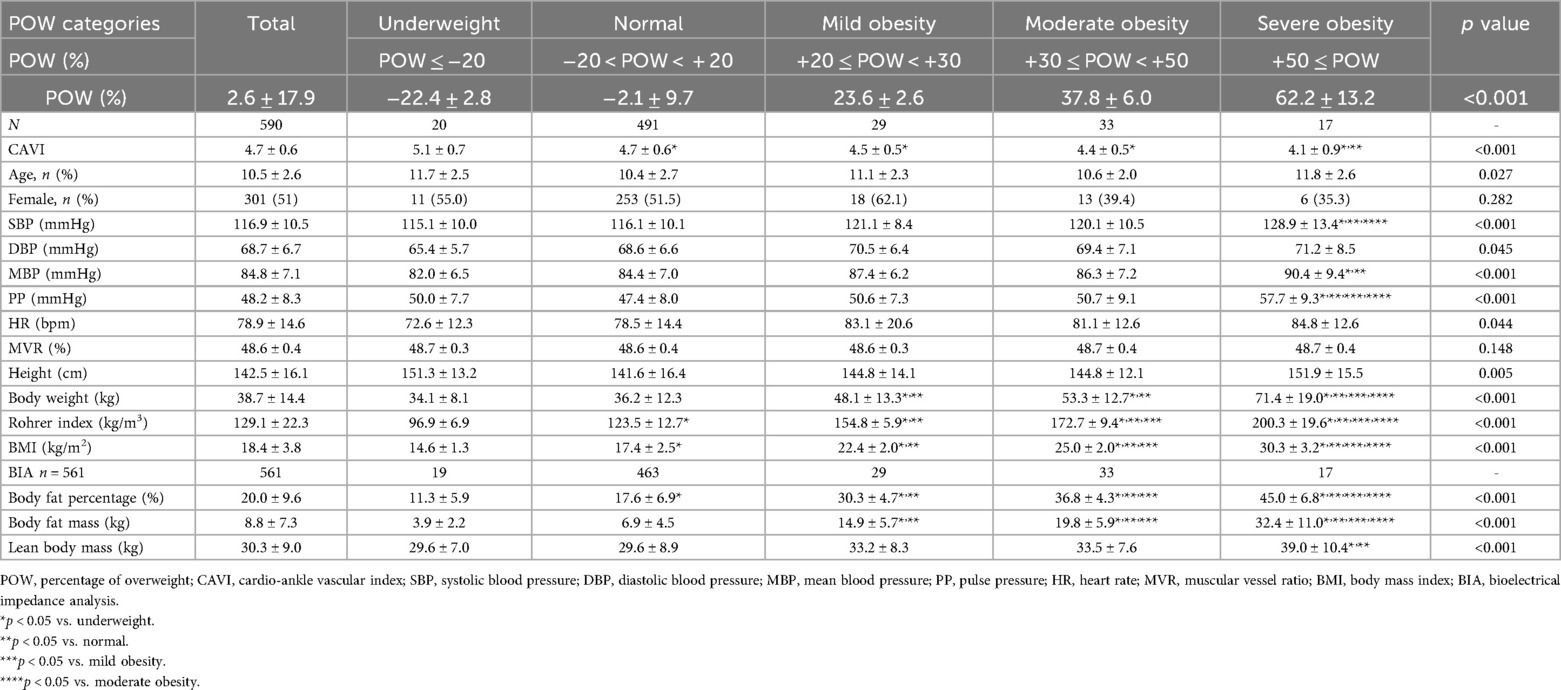
Table 3. Cardio-ankle vascular index of participants by percentage of overweight classification (n = 590).
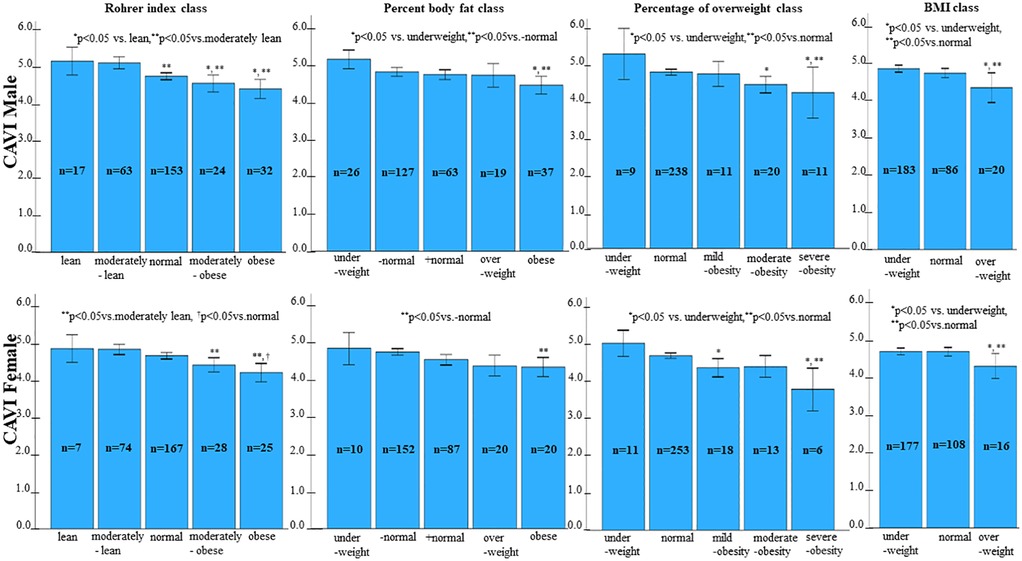
Figure 3. Comparison of CAVI by category of percentage of overweight. In both males and females, CAVI was lowest in the group with the highest obesity.
In addition, as shown in Table 5, CAVI was positively correlated with age, MVR, height, and lean body mass (R = 0.18, R = 0.15, R = 0.16, and R = 0.10, respectively; p < 0.05 for all), and was negatively correlated with HR, Rohrer index, POW, BMI, body fat percentage, and body fat mass (R = −0.14, R = −0.33, R = −0.30, R = −0.19, R = −0.23 and R = −0.14, respectively; p < 0.01, for all). In contrast, there were no significant correlations between CAVI and SBP or body weight.
Furthermore, multiple linear regression analyses adjusted for age, sex, blood pressure, HR and MVR showed that Rohrer index, POW, BMI, body fat percentage, body fat mass and lean body mass were independent predictors of CAVI (Table 6).
Discussion
To the best of our knowledge, the present study is the first to investigate the associations between multiple obesity parameters (Rohrer index, body fat percentage, body fat mass, POW, BMI) and CAVI in healthy children and adolescents. The main findings of this study were that: (A) manually measured and CAVI-based vessel lengths showed excellent concordance, suggesting the accuracy of CAVI; (B) CAVI was not only negatively correlated with obesity parameters, and but also positively correlated with age, height, and MVR, suggesting that CAVI reflects muscular vessel components; and (C) even after adjusting for possible obesity-related factors including age, sex, blood pressure and MVR, all obesity parameters itself remained independent predictors of CAVI in healthy children and adolescents.
CAVI measurement is a simple, non-invasive procedure that can be completed in a short time. Accurate measurements are possible if the child remains still and quiet to avoid interference with pulse waves and heart sound recordings. In children, it is important to select the appropriate cuff size, considering the individual variations in arm circumference. In addition, the length of the blood vessel is an important factor in determining the pulse wave velocity in CAVI measurement, and the current formula can be employed to calculate the length of the blood vessel using the height of the child. The present study demonstrated successful CAVI application in children and adolescents in terms of smooth measurements and accurate vessel length estimation.
Our study in children revealed a significant negative correlation between CAVI and multiple obesity parameters, including Rohrer index, body fat indices measured by BIA, POW, and BMI. Similar to our findings, CAVI has been reported to be negatively correlated with BMI in adults (6, 7, 29). Additionally, in children, both CAVI and pulse wave velocity have been shown to have a negative correlation with BMI (30–32). Although CAVI is an indicator that reflects arterial stiffness, most studies have shown a negative correlation between CAVI and BMI, indicating that lower CAVI is associated with obesity. Unlike previous CAVI studies which focused on BMI only (7, 30), our study also investigated the other obesity parameters (i.e., Rohrer index, POW, body fat percentage and body fat mass determined by BIA) in children. We firstly used the Rohrer index and POW in the present study, which is known to be appropriate for assessing childhood obesity (16, 17). We further measured body fat percentage base on the accurate BIA method, which is superior to BMI or Rohrer index to evaluate objective obesity (18, 19). As a result, not only BMI but also other obesity parameters showed a negative correlation with CAVI, and these results lead to establish a clear relationship between obesity and reduced CAVI.
We could not fully explain the reason for the “obesity paradox”; however, possible explanations are as follows: first, it has been reported that obesity induces adaptive physiological changes (e.g., vasodilation, increased blood volume and cardiac output, and changes in metabolic and hemodynamic demands) (30–32). Second, CAVI measures arterial properties across different vessel types, including the aorta, an elastic artery, and the femoral and tibial arteries, which are muscular vessels (20). Mileva et al. reported that the vasoreactivity for antihypertensive drugs was different in ascending aorta and descending aorta using computed tomography (33). These results suggest that there might be differences in response for antihypertensive drugs between the elastic and muscular arteries. We have firstly examined MVR, which is the ratio of elastic and muscular blood vessels, and found that CAVI especially reflects muscular vessel components.
Czippelova et al. reported that the diameter of the smaller peripheral resistance arteries is under the control of the sympathetic nervous system and sympatho-vagal balance. They also reported that obesity is associated with decreased sympathetic nervous activity and lower systemic vascular resistance, leading to low CAVI (32). CAVI was negatively correlated with heart rate, and might be affected by the impaired sympathetic nervous system in obese people. However, not only impaired sympathetic nerve activity (32, 34, 35), but also sympathetic nerve activity (36, 37) and shift in sympatho-vagal balance have previously been reported in obese people. In addition, it has been reported that arterial stiffness of muscular vessel (i.e., iliofemoral artery Beta) (38, 39) is lower in the Watanabe heritable hyperlipidemic rabbits than in normal rabbits (39, 40). The authors consider that arterial stiffness of muscular vessels might decrease at first stage where infiltration of lipids might soften the arterial wall. Third, perivascular fat in obese individuals may act as buffer, reducing pulse wave conduction and potentially lowering CAVI. In addition, in children during the growth period, fat content increases along with body development. Fourth, fat distribution and metabolic state may impact CAVI. Christakoudi et al. reported that obese individuals without abdominal fat can maintain a healthy metabolic state (41). These results support the hypothesis that the systemic accumulation of adipose tissue per se leads to decreased arterial stiffness in obese individuals without metabolic disorders; thus, adipose tissue in healthy children and adolescents might be associated with a healthy metabolic state.
Clinical implications
CAVI can be measured in children in the same way it is measured in adults. However, in cases of childhood obesity, caution is needed because arterial stiffness can be underestimated.
Study limitations
There are several limitations to our study. First, the present study was cross-sectional of healthy children, did not include participant with severe obesity, and did not follow-up. Thus, the relationship between childhood obesity and future cardiovascular and cerebrovascular disease is unknown. Second, we did not measure abdominal circumference, body shape index (42), laboratory data (such as blood glucose and lipid profile, which are components of metabolic syndrome), or other CAVI parameters (e.g., CAVI0) (43). Third, we manually evaluated vessel length using a tape measure, rather than using more precise methods like magnetic resonance imaging or computed tomography and did not measure vessel length and diameter. Fourth, we assessed only total body fat mass and body fat percentage, without considering the distribution of adipose tissue. Additionally, we did not measure visceral fat, epicardial fat, or subcutaneous fat. Fifth, our study was conducted with children and adolescents in the pubertal period. Given that development during this period significantly influences various physiological characteristics, including body composition and autonomic nervous system activity, another limitation is the lack of information on the stage of pubertal development (e.g., Tanner score) (32).
Conclusion
Even after consideration of possible obesity related factors (age, sex, blood pressure, and heart rate, MVR, and etc.), all obesity parameters (POW, Rohrer index, BMI, body fat percentage, and body fat mass) are negatively and independently associated with CAVI in healthy children. In cases of childhood obesity, caution is needed because arterial stiffness can be underestimated.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of Fukushima Medical University (No. 2021-186, REC2023-029). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH: Conceptualization, Validation, Methodology, Data curation, Investigation, Resources, Writing – review & editing, Funding acquisition, Software, Visualization, Project administration, Writing – original draft, Formal analysis. RS-H: Data curation, Project administration, Conceptualization, Writing – review & editing, Methodology, Resources, Investigation, Formal analysis, Funding acquisition. NS: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – review & editing. YI: Writing – review & editing, Formal analysis, Methodology, Data curation, Investigation, Conceptualization. MO: Formal analysis, Methodology, Writing – review & editing, Conceptualization, Data curation, Investigation. TK: Investigation, Conceptualization, Writing – review & editing, Methodology, Data curation, Formal analysis. TW: Methodology, Conceptualization, Data curation, Investigation, Writing – review & editing, Formal analysis. ET: Data curation, Investigation, Methodology, Project administration, Conceptualization, Resources, Formal analysis, Writing – review & editing. KO: Conceptualization, Methodology, Data curation, Investigation, Resources, Formal analysis, Project administration, Writing – review & editing. MH: Conceptualization, Data curation, Project administration, Methodology, Writing – review & editing, Investigation, Resources, Formal analysis. YE: Formal analysis, Project administration, Data curation, Resources, Investigation, Conceptualization, Methodology, Writing – review & editing. TN: Data curation, Project administration, Formal analysis, Methodology, Writing – review & editing, Investigation, Conceptualization, Resources. HA: Data curation, Methodology, Project administration, Conceptualization, Formal analysis, Investigation, Writing – review & editing, Resources. YK: Methodology, Project administration, Formal analysis, Resources, Data curation, Conceptualization, Investigation, Writing – review & editing, Funding acquisition. MY: Project administration, Conceptualization, Investigation, Writing – review & editing, Data curation, Resources, Methodology, Formal analysis. YS: Data curation, Investigation, Resources, Writing – review & editing, Methodology, Formal analysis, Project administration, Conceptualization. YTan: Data curation, Resources, Formal analysis, Conceptualization, Project administration, Methodology, Writing – review & editing, Investigation. AA: Resources, Investigation, Conceptualization, Data curation, Project administration, Formal analysis, Writing – review & editing, Methodology. SK: Project administration, Formal analysis, Methodology, Data curation, Writing – review & editing, Conceptualization, Investigation, Resources. YTak: Methodology, Writing – review & editing, Investigation, Resources, Conceptualization, Data curation, Formal analysis, Project administration. AY: Writing – original draft, Supervision, Funding acquisition, Writing – review & editing, Formal analysis, Project administration, Visualization, Data curation, Software, Investigation, Methodology, Validation, Conceptualization, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by JSPS KAKENHI Grant Number JP 24K18668 and 23K06870.
Acknowledgments
The authors thank Mr. Hideki Kanno, Ms. Atsuko Hattori, Mr. Sato Tetsuya, (Fukuda Denshi Co., Ltd., Tokyo, Japan) and the Ms. Rina Abe, Mr. Keisuke Sasaki, Ms. Kotomi Sato, Ms. Moemi Takahashi, Ms. Nanami Yamada, Ms. Rina Kashiwabara, Ms. Hinata Kase, Ms. Haruka Kuma, Ms. Momoka Nozaki, Ms. Rina Funada, and students of the “Physiomi” seminar of the Department of Clinical Laboratory Science at Fukushima Medical University School of Health Sciences, for their research cooperation and their technical assistance. We are deeply grateful for the advice of Mr. Shin-ichiro Katsuda.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1633849/full#supplementary-material
References
1. Tavolinejad H, Erten O, Maynard H, Chirinos JA. Prognostic value of cardio-ankle vascular index for cardiovascular and kidney outcomes: systematic review and meta-analysis. JACC Adv. (2024) 3(7):101019. doi: 10.1016/j.jacadv.2024.101019
2. Sato Y, Yoshihisa A, Ichijo Y, Watanabe K, Hotsuki Y, Kimishima Y, et al. Cardio-ankle vascular index predicts post-discharge stroke in patients with heart failure. J Atheroscler Thromb. (2021) 28(7):766–75. doi: 10.5551/jat.58727
3. Watanabe K, Yoshihisa A, Sato Y, Hotsuki Y, Anzai F, Ichijo Y, et al. Cardio-ankle vascular index reflects impaired exercise capacity and predicts adverse prognosis in patients with heart failure. Front Cardiovasc Med. (2021) 8:631807. doi: 10.3389/fcvm.2021.631807
4. Shimizu T, Sakuma Y, Muto Y, Anzai F, Kimishima Y, Sato Y, et al. Impact of cardio-ankle vascular index on future cancer in patients with coronary artery disease. Circ Rep. (2024) 6(9):372–80. doi: 10.1253/circrep.CR-24-0070
5. Okamoto Y, Miyoshi T, Ichikawa K, Takaya Y, Nakamura K, Ito H. Cardio-ankle vascular index as an arterial stiffness marker improves the prediction of cardiovascular events in patients without cardiovascular diseases. J Cardiovasc Dev Dis. (2022) 9(11):368. doi: 10.3390/jcdd9110368
6. Sugiura T, Dohi Y, Takagi Y, Yoshikane N, Ito M, Suzuki K, et al. Relationships of obesity-related indices and metabolic syndrome with subclinical atherosclerosis in middle-aged untreated Japanese workers. J Atheroscler Thromb. (2020) 27(4):342–52. doi: 10.5551/jat.50633
7. Nagayama D, Imamura H, Sato Y, Yamaguchi T, Ban N, Kawana H, et al. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. (2017) 13:1–9. doi: 10.2147/VHRM.S119646
8. Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. (2013) 62(15):1309–19. doi: 10.1016/j.jacc.2013.07.042
9. Maeda M, Maeda T, Ihara K. Secular trends in obesity and serum lipid values among children in Oita city, Japan, during a 27-year period. J Atheroscler Thromb. (2022) 29(12):1709–26. doi: 10.5551/jat.63056
10. Nakashima Y, Chen YX, Kinukawa N, Sueishi K. Distributions of diffuse intimal thickening in human arteries: preferential expression in atherosclerosis-prone arteries from an early age. Virchows Arch. (2002) 441(3):279–88. doi: 10.1007/s00428-002-0605-1
11. Brown CL, Halvorson EE, Cohen GM, Lazorick S, Skelton JA. Addressing childhood obesity: opportunities for prevention. Pediatr Clin North Am. (2015) 62(5):1241–61. doi: 10.1016/j.pcl.2015.05.013
12. Morita N, Kambayashi I, Okuda T, Oda S, Takada S, Nakajima T, et al. Inverse relationship between sleep duration and cardio-ankle vascular index in children. J Atheroscler Thromb. (2017) 24(8):819–26. doi: 10.5551/jat.36517
13. Nakagawa R, Kuwata S, Kurishima C, Saiki H, Iwamoto Y, Sugimoto M, et al. Arterial stiffness in patients after Kawasaki disease without coronary artery involvement: assessment by performing brachial ankle pulse wave velocity and cardio-ankle vascular index. J Cardiol. (2015) 66(2):130–4. doi: 10.1016/j.jjcc.2014.10.003
14. Sugiura R, Murata M. Problems with body mass index as an index to evaluate physical status of children in puberty. Pediatr Int. (2011) 53(5):634–42. doi: 10.1111/j.1442-200X.2010.03312.x
15. Dobashi K. Evaluation of obesity in school-age children. J Atheroscler Thromb. (2016) 23(1):32–8. doi: 10.5551/jat.29397
16. Kataoka K. Indices of obesity derived from body weight and height. Nihon Rinsho Jpn J Clin Med. (1995) 53(Suppl):147–53.
17. Nishida M, Funahashi T. Validity of indices (BMI, Rohrer index, Broca method) for assessment of obesity. Nihon Rinsho. (2009) 67(2):301–6.19202903
18. Messner A, Nairz J, Kiechl S, Winder B, Pechlaner R, Geiger R, et al. Comparison of body mass index and fat mass index to classify body composition in adolescents-the EVA4YOU study. Eur J Pediatr. (2024) 183(5):2203–14. doi: 10.1007/s00431-024-05474-x
19. Guida B, Pietrobelli A, Trio R, Laccetti R, Falconi C, Perrino NR, et al. Body mass index and bioelectrical vector distribution in 8-year-old children. Nutr Metab Cardiovasc Dis. (2008) 18(2):133–41. doi: 10.1016/j.numecd.2006.08.008
20. Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. (2006) 13(2):101–7. doi: 10.5551/jat.13.101
21. Horikoshi Y, Katsuda SI, Fujikura Y, Hazama A, Shimura H, Shimizu T, et al. Opposing responses of the calcium channel blocker nicardipine to vascular stiffness in the elastic and muscular arteries in rabbits. J Atheroscler Thromb. (2021) 28(12):1340–8. doi: 10.5551/jat.60848
22. Yamamoto T, Shimizu K, Takahashi M, Tatsuno I, Shirai K. The effect of nitroglycerin on arterial stiffness of the aorta and the femoral-tibial arteries. J Atheroscler Thromb. (2017) 24(10):1048–57. doi: 10.5551/jat.38646
23. Yamada Y, Nishizawa M, Uchiyama T, Kasahara Y, Shindo M, Miyachi M, et al. Developing and validating an age-independent equation using multi-frequency bioelectrical impedance analysis for estimation of appendicular skeletal muscle mass and establishing a cutoff for sarcopenia. Int J Environ Res Public Health. (2017) 14(7):809. doi: 10.3390/ijerph14070809
24. Dobashi K, Takahashi K, Nagahara K, Tanaka D, Itabashi K. Evaluation of hip/height(P) ratio as an index for adiposity and metabolic complications in obese children: comparison with waist-related indices. J Atheroscler Thromb. (2017) 24(1):47–54. doi: 10.5551/jat.35311
25. Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. (1995) 40(9):2002–9. doi: 10.1007/BF02208670
26. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3
27. Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. (2002) 66(11):987–92. doi: 10.1253/circj.66.987
28. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. (2012) 7(4):284–94. doi: 10.1111/j.2047-6310.2012.00064.x
29. Park HE, Choi SY, Kim HS, Kim MK, Cho SH, Oh BH. Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J Atheroscler Thromb. (2012) 19(6):570–6. doi: 10.5551/jat.12484
30. Philip R, Alpert BS, Schwingshackl A, Huang X, Blakely D, Rovnaghi CR, et al. Inverse relationship between cardio-ankle vascular index and body mass index in healthy children. J Pediatr. (2015) 167(2):361–5.e1. doi: 10.1016/j.jpeds.2015.04.042
31. Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. (2008) 28(5):287–93. doi: 10.1111/j.1475-097X.2008.00806.x
32. Czippelova B, Turianikova Z, Krohova J, Wiszt R, Lazarova Z, Pozorciakova K, et al. Arterial stiffness and endothelial function in young obese patients—vascular resistance matters. J Atheroscler Thromb. (2019) 26(11):1015–25. doi: 10.5551/jat.47530
33. Mileva N, Panayotov P, Hristova I, Koleva G, Georgieva D, Ivanova R, et al. Impact of renin-angiotensin system targeted therapy on aortic elastic properties assessed by computed tomography. Int J Cardiol Heart Vasc. (2024) 55:101562. doi: 10.1016/j.ijcha.2024.101562
34. Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Engl J Med. (1988) 318(17):1077–83. doi: 10.1056/NEJM198804283181701
35. Nagai N, Matsumoto T, Kita H, Moritani T. Autonomic nervous system activity and the state and development of obesity in Japanese school children. Obes Res. (2003) 11(1):25–32. doi: 10.1038/oby.2003.6
36. Cvijetic S, Macan J, Boschiero D, Ilich JZ. Body fat and muscle in relation to heart rate variability in young-to-middle age men: a cross sectional study. Ann Hum Biol. (2023) 50(1):108–16. doi: 10.1080/03014460.2023.2180089
37. Plaza-Florido A, Migueles JH, Mora-Gonzalez J, Molina-Garcia P, Rodriguez-Ayllon M, Cadenas-Sanchez C, et al. The role of heart rate on the associations between body composition and heart rate variability in children with overweight/obesity: the activebrains project. Front Physiol. (2019) 10:895. doi: 10.3389/fphys.2019.00895
38. Takahashi K, Yamamoto T, Tsuda S, Okabe F, Shimose T, Tsuji Y, et al. Coefficients in the CAVI equation and the comparison between CAVI with and without the coefficients using clinical data. J Atheroscler Thromb. (2019) 26(5):465–75. doi: 10.5551/jat.44834
39. Katsuda SI, Horikoshi Y, Shiomi M, Kitajima S, Ito T, Hazama A, et al. Arterial stiffness of the aorta and iliofemoral artery and their responses to nitroglycerin administration in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits. J Hypertens. (2024) 42(3):441–9. doi: 10.1097/HJH.0000000000003608
40. Miyata M. Basic research sheds light on the aspect of cardio-ankle vascular index (CAVI) including elastic and muscular arteries. J Atheroscler Thromb. (2021) 28(6):588–9. doi: 10.5551/jat.ED147
41. Christakoudi S, Tsilidis KK, Muller DC, Freisling H, Weiderpass E, Overvad K, et al. A body shape index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep. (2020) 10(1):14541. doi: 10.1038/s41598-020-71302-5
42. Nagayama D, Watanabe Y, Yamaguchi T, Maruyama M, Saiki A, Shirai K, et al. New index of abdominal obesity, a body shape index, is BMI-independently associated with systemic arterial stiffness in real-world Japanese population. Int J Clin Pharmacol Ther. (2020) 58(12):709–17. doi: 10.5414/CP203778
Keywords: cardio-ankle vascular index, Rohrer index, body fat percentage, percentage of overweight, body mass index, obesity paradox
Citation: Horikoshi Y, Sakuraba-Hirata R, Suzuki N, Inomata Y, Oikawa M, Kotani T, Watanabe T, Takahashi E, Okazaki K, Hoshi M, Endo Y, Nakanowatari T, Abe H, Kusumoto Y, Yokozuka M, Shiba Y, Tanaka Y, Asao A, Kurasawa S, Takeishi Y and Yoshihisa A (2025) Associations between obesity and arterial stiffness assessed by cardio-ankle vascular index in healthy children and adolescents. Front. Cardiovasc. Med. 12:1633849. doi: 10.3389/fcvm.2025.1633849
Received: 23 May 2025; Accepted: 29 July 2025;
Published: 9 September 2025.
Edited by:
Ryuichi Morishita, Osaka University, JapanReviewed by:
Niya Mileva, Aleksandrovska University Hospital, BulgariaPrakhar Gupta, Sagar Multi-speciality Hospital, India
Copyright: © 2025 Horikoshi, Sakuraba-Hirata, Suzuki, Inomata, Oikawa, Kotani, Watanabe, Takahashi, Okazaki, Hoshi, Endo, Nakanowatari, Abe, Kusumoto, Yokozuka, Shiba, Tanaka, Asao, Kurasawa, Takeishi and Yoshihisa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akiomi Yoshihisa, eW9zaGloaXNAZm11LmFjLmpw
†These authors have contributed equally to this work
 Yuko Horikoshi1,†
Yuko Horikoshi1,† Hiroaki Abe
Hiroaki Abe Yasuaki Kusumoto
Yasuaki Kusumoto Akihiko Asao
Akihiko Asao Yasuchika Takeishi
Yasuchika Takeishi Akiomi Yoshihisa
Akiomi Yoshihisa