- 1Division of Cardiovascular Medicine and Stanford Amyloid Center, Stanford University School of Medicine, Stanford, CA, United States
- 2Pfizer, New York, NY, United States
- 3Genesis Research Group, Hoboken, NJ, United States
- 4Clarify Health Solutions, New York, NY, United States
Background: Most patients with wild-type transthyretin amyloid cardiomyopathy (ATTRwt-CM) are diagnosed noninvasively, using nuclear imaging and monoclonal protein testing. However, concerns have been raised that some may receive an ATTRwt-CM diagnosis based on incomplete evaluations (not based on current consensus recommendations). Using a cohort of US Medicare Fee-for-Service patients, we aimed to examine the frequency and cadence of diagnostic testing for ATTRwt-CM.
Methods: In this retrospective observational cross-sectional study, administrative de-identified claims data from 2018 to 2022 were derived from patients aged ≥65 years who had at least one claim for ATTRwt-CM and heart failure or cardiomyopathy, and ≥2 years of continuous Medicare enrollment before the first ATTRwt-CM diagnosis. Patients with claims for any other form of amyloidosis, multiple myeloma, or plasma cell dyscrasias were excluded.
Results: Among 2,050 patients with ATTRwt-CM, mean (SD) age was 80.0 (6.9) years, and 75.5% were men. Annual new ATTRwt-CM diagnoses nearly tripled over the study period (2018, n = 198; 2022, n = 578). Technetium-99m pyrophosphate (PYP) scintigraphy use was performed in approximately half of diagnosed patients by the end of the study period (2018, 30%; 2022, 49%). Cardiac biopsy use declined from 14% in patients diagnosed in 2018 to 5% in those diagnosed in 2022. A small minority (14%) of patients underwent the recommended noninvasive diagnostic testing comprised of PYP scintigraphy and complete monoclonal protein testing.
Conclusions: Based on Medicare claims data, most patients diagnosed with ATTRwt-CM have not been diagnosed following consensus-recommended pathways.
1 Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a systemic amyloidosis characterized by misfolding of the transthyretin (TTR) protein, resulting in the formation and deposition of insoluble amyloid fibrils in the interstitial space of the myocardium and other organs (1). This progressive, debilitating disease may stem from either mutations in the TTR gene (variant/hereditary ATTR-CM) or from a genetically normal, aging-related process [wild-type ATTR-CM (ATTRwt-CM)] (2). The clinical manifestations of ATTR-CM can extend across cardiac and noncardiac systems and can often overlap with signs/symptoms of more prevalent cardiac conditions, such as hypertensive heart disease, aortic stenosis, and hypertrophic cardiomyopathy (3), which can be an obstacle to achieving an accurate diagnosis. Fewer than half of patients with the disease receive an ATTR-CM diagnosis within 6 months of symptom onset (4). And misdiagnosis or delayed diagnosis can lead to continued amyloid deposition and worsening prognosis (5, 6).
Important advances in ATTR-CM diagnosis have been achieved in recent years, due in part to the increased recognition of the clinical clues associated with ATTR-CM, its prevalence among older adults with heart failure, and the availability of a readily accessible and accurate noninvasive diagnostic approach to the disease. Beginning in 2016, a noninvasive pathway has been widely accepted for confirming a diagnosis of ATTR-CM in most patients, which requires a combination approach of bone scintigraphy and serum and urine studies to rule out the presence of a monoclonal protein (1, 5, 7, 8). However, concerns have been raised that some patients may receive a diagnosis of ATTR-CM based on incomplete evaluations rather than evaluations based on established consensus recommendations (9).
In the current study, conducted using a cohort of US Medicare Fee-for-Service (FFS) patients diagnosed with ATTR-CM, we explored the frequency and cadence of diagnostic testing that patients with ATTRwt-CM underwent in the 2 years prior to their diagnosis. In addition, we assessed the clinical comorbid conditions of patients with ATTR-CM in the prediagnosis period to help characterize the population. A plain language summary of this original research is included in the Supplementary Materials.
2 Methods
2.1 Study design
This noninterventional, retrospective cross-sectional study was conducted using administrative claims data from the Centers for Medicare & Medicaid Services Medicare FFS database for the study period of January 1, 2016, to December 31, 2022. Medicare is a federal health insurance program in the United States designed to primarily serve residents aged 65 years and older, along with younger individuals who have specific health conditions. Beneficiaries generally contribute to their healthcare and prescription drug costs through various financial mechanisms, including deductibles, coinsurance, and premiums.
2.2 Eligibility criteria
De-identified claims data were included for patients who had at least one International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code for ATTRwt-CM (E85.82) in the Medicare FFS database during the identification period between January 1, 2018 and December 31, 2022. Eligible patients also had at least one ICD-10-CM diagnosis code for heart failure (I50) or cardiomyopathy (I42 and I43) before the earliest date of ATTR-CM diagnosis code use during the identification period; were at least 65 years of age at diagnosis; lived in the United States; and had at least 2 years of continuous enrollment in Medicare Parts A, B, and D, with full medical and drug coverage prior to ATTR-CM diagnosis. Patients were excluded if they had an ICD-10 CM diagnosis code for amyloidosis other than wild-type ATTR-CM, including hereditary amyloidosis (E85.0, E85.1, E85.2), organ-limited amyloidosis (E85.4), other amyloidosis (E85.89), or unspecified amyloidosis (E85.9), in the database during the identification period. The other main exclusion criteria were designed to exclude patients with a diagnosis of light chain (AL) amyloidosis who may have been incorrectly coded with an ATTR amyloidosis code, including the presence of an ICD-10-CM code for AL amyloidosis (E85.81), multiple myeloma (C90.0), or a plasma cell dyscrasia (E88.09) during the study period, or a prescription claim for one or more of the following agents (identified using National Drug Codes) during the study period: bendamustine, bortezomib, carfilzomib, cyclophosphamide, daratumumab, elotuzumab, ixazomib, lenalidomide, melphalan, pomalidomide, thalidomide, or venetoclax.
The ICD-10-CM diagnosis codes used to identify diagnoses are listed in Supplementary Table S1.
2.3 Assessments
Patient demographics were assessed at the time of ATTRwt-CM diagnosis. The most frequently observed comorbidities were assessed during the 2-year period prior to ATTRwt-CM diagnosis; these conditions were identified using ICD-10-CM diagnosis codes (Supplementary Table S1). Diagnostic tests assessed at ATTR-CM diagnosis and during the 2-year period before diagnosis (defined as the baseline period) included technetium-99m pyrophosphate (99mTc-PYP) bone scintigraphy; cardiac, bone marrow, or abdominal fat pad biopsy; monoclonal protein testing with serum and urine protein electrophoresis with and without immunofixation, serum-free light chain assay, and urine-free light chain assay with and without serum-free light chain assay. Diagnostic imaging, laboratory tests, and diagnostic procedures were categorized based on the Healthcare Cost and Utilization Project Clinical Classifications Software Refined. Coding used to identify diagnostic tests in patient claims is summarized in Supplementary Table S2.
2.4 Statistical analysis
All patients satisfying eligibility criteria for ATTRwt-CM were included in analyses of demographics, comorbidities, and diagnostic tests. Data were handled and analyzed using SAS software, Version 9.4, or later (SAS Institute Inc., Cary, NC, USA). Data from all assessments were summarized using descriptive statistics. Diagnostic test data were also summarized for subgroups of patients stratified by the year of first diagnosis.
3 Results
3.1 Patient disposition and baseline characteristics
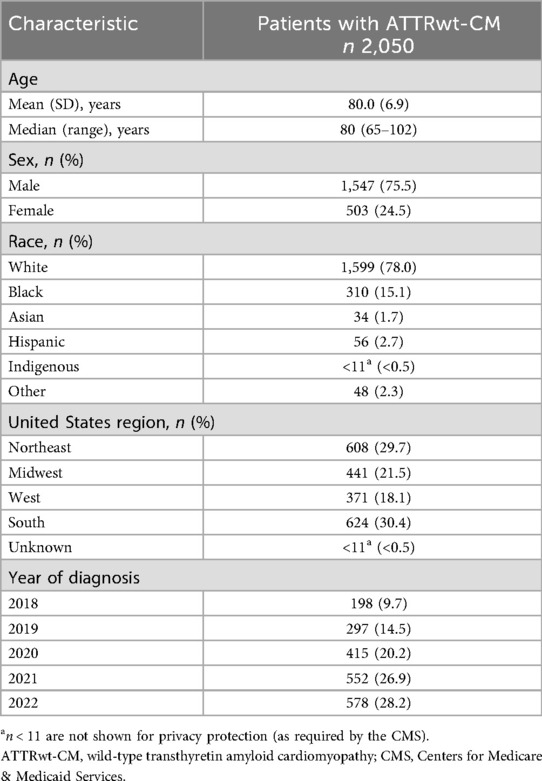
Of 10,470 screened patients, 2,050 patients with ATTRwt-CM were eligible for analysis (Figure 1). Among analyzed patients with ATTRwt-CM, the median age was 80 years and the majority were men and White (76% and 78%, respectively; Table 1). Most were from the Northeast and Southern regions of the United States (30% each).
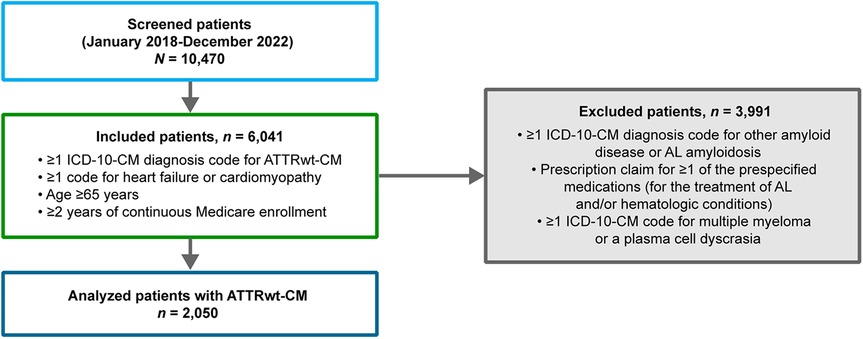
Figure 1. Disposition of patients With ATTRwt-CM. AL, light chain; ATTR-CM, transthyretin amyloid cardiomyopathy; ATTRwt-CM, wild-type transthyretin amyloid cardiomyopathy; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification.
3.2 Comorbid conditions in the baseline period
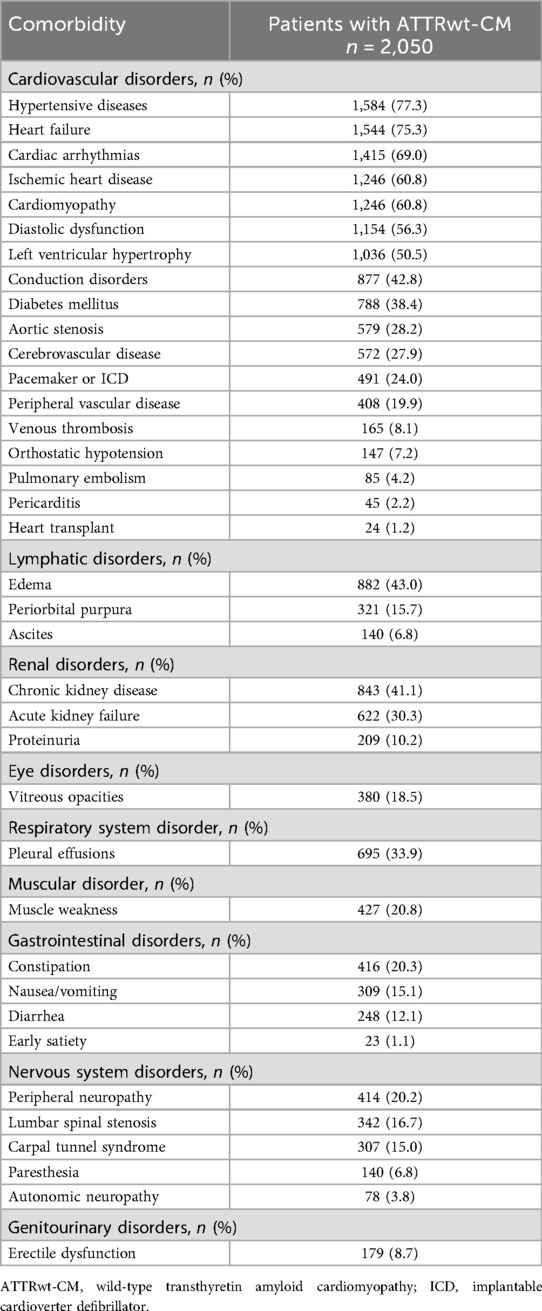
The prevalence of comorbid conditions associated with ATTR-CM during the 2-year period preceding ATTRwt-CM diagnosis is summarized in Table 2. The most common cardiovascular comorbidities occurring in at least 50% of patients were hypertensive diseases (77%), heart failure (75%), cardiac arrhythmias (69%), and ischemic heart disease (61%). The most common noncardiovascular comorbidities occurring in at least 30% of patients were edema (43%), chronic kidney disease (41%), cataracts (36%), pleural effusions (34%), and acute kidney failure (30%). Carpal tunnel syndrome, which can also occur as a consequence of ATTR-amyloid deposition, was present in 15% of patients.
3.3 Diagnostic testing in patients with ATTRwt-Cm
The total number of new ATTRwt-CM diagnoses per year increased from 198 in 2018 to 578 in 2022 (Table 1). Diagnostic imaging with 99mTc-PYP scintigraphy was performed in 30% of patients with ATTRwt-CM at the start of the study period (2018) and 49% at the end (2022) (Figure 2). Use of 99mTc-PYP scintigraphy was highest in 2020, when it was performed in 52% of patients. The performance of cardiac biopsies in patients with ATTRwt-CM declined from 14% in 2018 to 5% in 2022.
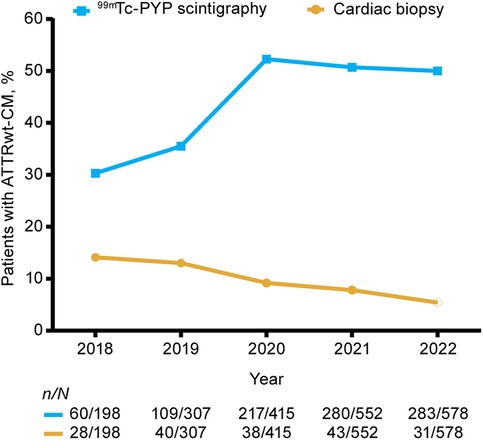
Figure 2. Proportions of patients undergoing 99mTc-PYP scintigraphy and cardiac biopsy for ATTRwt-CM in the 2-year period before ATTRwt-CM diagnosis by diagnosis year. 99mTc-PYP, technetium-99m pyrophosphate scintigraphy; ATTRwt-CM, wild-type transthyretin amyloid cardiomyopathy.
Across the study period, only 14% of patients with ATTRwt-CM underwent the recommended noninvasive diagnostic testing (1, 5, 7–9), comprised of 99mTc-PYP scintigraphy and complete monoclonal protein testing, defined as serum and urine protein electrophoresis with immunofixation and serum-free light chain assay. The recommended approach was followed in 9% and 16% of diagnosed patients in 2018 and 2022, respectively (Figure 3). 99mTc-PYP scintigraphy was performed with incomplete monoclonal protein testing in 13% and 24% in 2018 and 2022, respectively, and without any monoclonal protein testing in 8% and 9%.
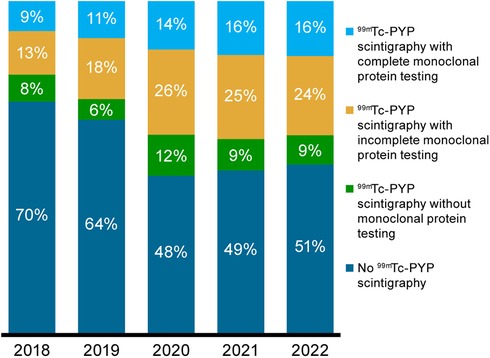
Figure 3. Proportions of patients without 99mTc-PYP scintigraphy and with 99mTc-PYP scintigraphy who had complete, incomplete, and no monoclonal protein testing for ATTRwt-CM in the 2-year period before ATTRwt-CM diagnosis by diagnosis year. 99mTc-PYP, technetium-99m pyrophosphate scintigraphy; ATTRwt-CM, wild-type transthyretin amyloid cardiomyopathy.
4 Discussion
Based on the Medicare claims data analyzed in this study, although the incidence of ATTRwt-CM diagnosis nearly tripled from 2018 to 2022, only a small minority of patients received an ATTRwt-CM diagnosis during this time based on consensus-recommended pathways (9). The proportion of patients diagnosed with ATTRwt-CM who underwent 99mTc-PYP scintigraphy increased from approximately 30% in 2018 to nearly 50% in 2020 to 2022; however, this imaging was conducted in combination with complete monoclonal protein testing in no more than 16% of patients per year during this period. This increase in PYP utilization is likely driven by multiple factors: greater awareness of ATTR-CM following the FDA approval of tafamidis in 2019, as well as increased education and dissemination of diagnostic algorithms (5). Given that cardiac biopsy was performed in only 5% of patients with ATTRwt-CM in 2022, an ATTRwt-CM diagnosis appears to have been reached based on accepted guidelines in only one in five Medicare patients with the disorder. While access to these diagnostic tools for Medicare patients may vary by region, the national increase in scan use observed in our data suggests that these services have become more routinely accessible over time. The availability of approved therapies may have also contributed to more widespread adoption of diagnostic imaging in clinical practice.
Accepted diagnostic tools, i.e., 99mTc-PYP scintigraphy (irrespective of monoclonal testing) and cardiac biopsy, were used in only 44% to 54% of patients with an ATTRwt-CM diagnosis between 2018 and 2022. We could not determine the basis for the diagnosis in the remaining patients, but these findings suggest a need for additional research and education regarding current diagnostic algorithms for ATTR-CM, to ensure appropriate and timely diagnosis and initiation of treatment for optimal outcomes.
Our findings also suggest insufficient understanding of the role of comprehensive monoclonal protein testing with cardiac scintigraphy in establishing a diagnosis of ATTR-CM. Among all the Medicare patients included in our study, a complete monoclonal protein screen was performed with 99mTc-PYP scintigraphy in only 16% of those diagnosed with ATTRwt-CM by the end of the study period. While monoclonal protein testing was incomplete in nearly 25% of patients in 2022, it was not conducted at all in almost 10%. Although cardiac scintigraphy is increasingly recognized as a cornerstone of noninvasive ATTR-CM diagnosis, cardiac uptake supporting a diagnosis of ATTR-CM (grade 2 or 3 uptake) may occur in 10% of patients with AL amyloidosis, with grade 1 uptake occurring in an additional 29% (10). Given that serum kappa and lambda-free light chain assays in combination with serum and urine immunofixation electrophoresis have >99% sensitivity for detecting AL amyloidosis (11–13), a complete monoclonal protein screen is an indispensable component of the nonbiopsy ATTR-CM diagnostic pathway (7). Notably, 16% of patients presented with periorbital purpura, a condition that can precede a diagnosis of amyloidosis (14–16).
Strengths of our study include the provision of insights into how patients with ATTRwt-CM are diagnosed in the real-world setting. Similar research was conducted by Bourque et al. using administrative claims from a Medicare FFS population (17) but was limited to a 2-year period ending in 2019; thus, examination of more recent patterns in diagnostic testing for ATTR-CM is needed, particularly given the growth of 99mTc-PYP scintigraphy as an accepted diagnostic tool in recent years. Secondary findings of our study have also allowed examination of the “real-world” demographics and comorbidities of a sizable population of patients with ATTRwt-CM. In addition, by limiting the population to patients with continuous Medicare A, B, and D coverage with full medical and drug coverage for at least 2 years prior to ATTRwt-CM diagnosis, it is likely that the vast majority of diagnostic laboratory and imaging studies and medication prescriptions were captured in the dataset.
Our study's limitations are primarily related to the use of administrative claims data. Claims data do not provide detailed clinical information and may contain inaccuracies in coding diagnoses, procedures, pharmacy claims, and other undetected confounders. Because ATTR-CM is uncommon, the disease and its subclassifications (wild type and variant) may not be well captured in administrative claims. Miscoding is also possible when using ICD-10-CM diagnosis and procedure codes to identify ATTR-CM diagnoses. For example, patients with AL amyloidosis may have been labeled as “other amyloidosis” or “ATTR amyloidosis”. However, to reduce the likelihood that patients with AL amyloidosis would be inadvertently included in the study, we excluded all patients with a concomitant diagnosis of plasma cell dyscrasia and who had received chemotherapy/immunotherapy commonly used to treat plasma cell dyscrasias. No data on biopsies following PYP test results are available in this dataset. In addition, the lack of a code for hereditary/variant disease may have resulted in patients with this subtype being included in the ATTRwt-CM cohort. Because only data from individuals with Medicare FFS health coverage were analyzed in this study, results may not be generalizable to patients with commercial health insurance or no health insurance coverage. Finally, because diagnostic testing patterns were examined only in patients who had been diagnosed with ATTR-CM, and not in patients who could have had ATTR-CM, the results of this study may not provide the full picture of the testing conducted in the underlying population.
5 Conclusions
Based on administrative claims data from 2018 to 2022, most Medicare patients with ATTRwt-CM did not receive a diagnosis based on accepted noninvasive diagnostic pathways. Improved understanding of these pathways, particularly pertaining to the requisite use of comprehensive monoclonal protein screens in combination with cardiac screening, is needed to improve ATTR-CM diagnosis in the clinical practice setting.
Data availability statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information. Please direct any further enquiries to the corresponding author.
Ethics statement
Institutional review board/independent ethics committee review was not required for this study because the study only used commercially available de-identified secondary data sources. As the data used in the study were in an anonymized structured format, which included no patient personal information, patient informed consent was also not required. The study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology (18), Good Practices for Outcomes Research of the International Society for Pharmacoeconomics and Outcomes Research (19), and Good Research for Comparative Effectiveness Principles (20).
Author contributions
RW: Methodology, Writing – review & editing, Investigation, Supervision, Conceptualization. HC: Resources, Visualization, Project administration, Methodology, Supervision, Writing – review & editing, Conceptualization. FD: Visualization, Formal analysis, Resources, Methodology, Supervision, Writing – review & editing. CG: Data curation, Supervision, Methodology, Software, Conceptualization, Formal analysis, Writing – review & editing. AR: Supervision, Methodology, Conceptualization, Writing – review & editing, Software, Formal analysis, Data curation. SS: Writing – review & editing, Software, Methodology, Formal analysis, Data curation. AE: Methodology, Supervision, Writing – review & editing, Visualization. CP: Writing – review & editing, Visualization, Conceptualization, Supervision, Funding acquisition, Formal analysis, Methodology, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by Pfizer, New York, New York, USA. Medical writing support was provided by Donna McGuire of Engage Scientific Solutions and funded by Pfizer.
Acknowledgments
The authors thank Jose Maria Jimenez Alvir, DrPH, of Pfizer Inc., and Danielle Sienko, MS, of Genesis Research Group, for their valuable contributions to this research. The authors thank Donna McGuire of Engage Scientific Solutions for her support in writing the manuscript and manuscript submission. All listed authors (Ronald M. Witteles, Haechung Chung, Feng Dai, Cynthia Gutierrez, Andrew Rava, Sameer Swarup, Abbas Ebrahim, Cindi Pankratova) confirm that they are responsible for the authenticity and academic quality of the current manuscript. They are all aware of and agree to the peer review and publication process, and take full responsibility for the entire content of the article.
Conflict of interest
RW has served as a paid adviser to Alexion, Alnylam, AstraZeneca, Bridge Bio, Novo Nordisk, and Pfizer. HC, FD, and CP are full-time employees of Pfizer and hold stock and/ or stock options. AE was an employee of Pfizer and held stock and/or stock options when the study was conducted and the manuscript developed. CG and AR are employees of Genesis Research Group. When the study was designed and conducted, SS was an employee of Clarify Health Solutions. The authors declare that this study received funding from Pfizer: Genesis Research Group received financial support from Pfizer for the design and conduct of this study. Clarify Health Solutions received financial support from Pfizer for the use of the Medicare claims database, consultation on study decisions, data collection, and data analysis. Medical writing support was funded by Pfizer. All authors were involved in the interpretation of data, the writing of this article, and the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1638380/full#supplementary-material
Abbreviations
AL, light chain; ATTR-CM, transthyretin amyloid cardiomyopathy; ATTRwt-CM, wild-type transthyretin amyloid cardiomyopathy; CMS, Centers for Medicare & Medicaid Services; FFS, Fee-for-Service; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; 99mTc-PYP, technetium-99m pyrophosphate; TTR, transthyretin.
References
1. Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, Clarke JO, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. (2023) 81:1076–126. doi: 10.1016/j.jacc.2022.11.022
2. Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, et al. Amyloid nomenclature 2020: update and recommendations by the international society of amyloidosis (ISA) nomenclature committee. Amyloid. (2020) 27:217–22. doi: 10.1080/13506129.2020.1835263
3. Nativi-Nicolau JN, Karam C, Khella S, Maurer MS. Screening for ATTR amyloidosis in the clinic: overlapping disorders, misdiagnosis, and multiorgan awareness. Heart Fail Rev. (2022) 27:785–93. doi: 10.1007/s10741-021-10080-2
4. Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Patient experience with hereditary and senile systemic amyloidoses: a survey from the amyloid research consortium. Orphanet J Rare Dis. (2015) 10:P22. doi: 10.1186/1750-1172-10-S1-P22
5. Witteles RM, Bokhari S, Damy T, Elliott PM, Falk RH, Fine NM, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. (2019) 7:709–16. doi: 10.1016/j.jchf.2019.04.010
6. Rozenbaum MH, Large S, Bhambri R, Stewart M, Whelan J, van Doornewaard A, et al. Impact of delayed diagnosis and misdiagnosis for patients with transthyretin amyloid cardiomyopathy (ATTR-CM): a targeted literature review. Cardiol Ther. (2021) 10:141–59. doi: 10.1007/s40119-021-00219-5
7. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. (2016) 133:2404–12. doi: 10.1161/circulationaha.116.021612
8. Witteles RM, Liedtke M. AL Amyloidosis for the cardiologist and oncologist: epidemiology, diagnosis, and management. J Am Coll Cardiol CardioOncol. (2019) 1:117–30. doi: 10.1016/j.jaccao.2019.08.002
9. Hanna M, Ruberg FL, Maurer MS, Dispenzieri A, Dorbala S, Falk RH, et al. Cardiac scintigraphy with technetium-99m-labeled bone-seeking tracers for suspected amyloidosis: JACC review topic of the week. J Am Coll Cardiol. (2020) 75:2851–62. doi: 10.1016/j.jacc.2020.04.022
10. Quarta CC, Zheng J, Hutt D, Grigore SF, Manwani R, Sachchithanantham S, et al. 99mTc-DPD Scintigraphy in immunoglobulin light chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. (2021) 22:1304–11. doi: 10.1093/ehjci/jeab095
11. Muchtar E, Gertz MA, Kyle RA, Lacy MQ, Dingli D, Leung N, et al. A modern primer on light chain amyloidosis in 592 patients with mass spectrometry-verified typing. Mayo Clin Proc. (2019) 94:472–83. doi: 10.1016/j.mayocp.2018.08.006
12. Kittleson MM, Maurer MS, Ambardekar AV, Bullock-Palmer RP, Chang PP, Eisen HJ, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. (2020) 142:e7–22. doi: 10.1161/cir.0000000000000792
13. Katzmann JA, Abraham RS, Dispenzieri A, Lust JA, Kyle RA. Diagnostic performance of quantitative κ and λ free light chain assays in clinical practice. Clin Chem. (2005) 51:878–81. doi: 10.1373/clinchem.2004.046870
14. Miyaue K, Isono H. Periorbital Purpura as a key diagnostic clue in immunoglobulin light chain amyloidosis. JMA J. (2025) 8:609–10. doi: 10.31662/jmaj.2024-0357
15. Wang XF, Li T, Yang M, Huang Y. Periorbital purpura can be the only initial symptom of primary light chain amyloidosis: a case report. World J Clin Cases. (2024) 12:5946–51. doi: 10.12998/wjcc.v12.i26.5946
16. Jhawar N, Reynolds J, Nakhleh R, Lyle M. Hereditary transthyretin amyloidosis presenting with spontaneous periorbital purpura: a case report. Eur Heart J Case Rep. (2023) 7:ytad108. doi: 10.1093/ehjcr/ytad108
17. Bourque JM, Schepart A, Bhambri R, Castaño A, O'Brien A, Chen Y, et al. Temporal trends in diagnostic testing patterns for wild-type transthyretin amyloid cardiomyopathy in the medicare fee-for-service population. Am J Cardiol. (2022) 167:98–103. doi: 10.1016/j.amjcard.2021.11.048
18. International Society for Pharmacoepidemiology. Guidelines for Good Pharmacoepidemiology Practices (GPP). (2015). Available online at: https://www.pharmacoepi.org/resources/policies/guidelines-08027/ (Accessed May 12, 2025).
19. Motheral B, Brooks J, Clark MA, Crown WH, Davey P, Hutchins D, et al. A checklist for retrospective database studies—report of the ISPOR task force on retrospective databases. Value Health. (2003) 6:90–7. doi: 10.1046/j.1524-4733.2003.00242.x
20. Dreyer NA, Schneeweiss S, McNeil BJ, Berger ML, Walker AM, Ollendorf DA, et al. GRACE Principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care. (2010) 16:467–71. Available online at: https://www.ajmc.com/ view/ajmc_10jundreyer_467to47120560690
Keywords: amyloidosis, cardiomyopathy, diagnosis, heart failure, identification, transthyretin
Citation: Witteles RM, Chung H, Dai F, Gutierrez C, Rava A, Swarup S, Ebrahim A and Pankratova C (2025) Trends in diagnostic testing in Medicare patients with wild-type transthyretin amyloid cardiomyopathy. Front. Cardiovasc. Med. 12:1638380. doi: 10.3389/fcvm.2025.1638380
Received: 30 May 2025; Accepted: 17 September 2025;
Published: 29 October 2025.
Edited by:
Ricardo Mourilhe-Rocha, Rio de Janeiro State University, BrazilReviewed by:
Fabio de Souza, Federal University of the State of Rio de Janeiro, BrazilMarcia Cruz, Federal University of Rio de Janeiro, Brazil
Copyright: © 2025 Witteles, Chung, Dai, Gutierrez, Rava, Swarup, Ebrahim and Pankratova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronald M. Witteles, d2l0dGVsZXNAc3RhbmZvcmQuZWR1
 Ronald M. Witteles1*
Ronald M. Witteles1* Feng Dai
Feng Dai Andrew Rava
Andrew Rava Sameer Swarup
Sameer Swarup