- 1Department of Cardiology, Tianjin Chest Hospital, Tianjin, China
- 2Department of Cardiology, Tianjin Medical University General Hospital, Tianjin, China
- 3Department of Cardiology, Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Background: This study aimed to estimate the global burden, temporal trends, and geographic disparities of younger-onset atrial fibrillation (AF) in adults aged 30–45 years across 204 countries from 1990 to 2021, using data from the Global Burden of Disease Study. Focusing on this under-researched age group, we aimed to characterize the epidemiology of premature AF and its implications for prevention strategies.
Methods: We analyzed the global burden of younger-onset AF (30–45 years) from 204 countries using Global Burden of Disease Study 2021 data. The primary outcomes included age-standardized prevalence, mortality, disability-adjusted life years (DALYs), and average annual percentage change (AAPC).
Results: From 1990 to 2021, the age-standardized prevalence of AF among young and middle-aged adults decreased slightly from 57.81 to 57.48 cases per 100,000 population, with an AAPC of 0 (95% CI −0.05 to 0.05). Age-standardized mortality rates (ASMR) increased from 0.04 to 0.05 per 100,000 population, with an AAPC of 0.17% (95% CI 0.08 to 0.26). The age-standardized DALYs rate (ASDR) rate rose from 6.98 to 7.08 per 100,000 population (AAPC 0.05%, 95% CI −0.02 to 0.11). Men exhibited higher AF-related mortality, with an AAPC of 0.3% (95% CI 0.14 to 0.47). Higher Socio-demographic Index (SDI) countries showed higher AF prevalence (e.g., 111.54 per 100,000 in Australasia) but lower mortality rates, whereas low-SDI countries had higher mortality rates (AAPC 0.49% for low-middle SDI). Regional analysis revealed the highest prevalence increase in East Asia (AAPC 0.72%).
Conclusions: From 1990 to 2021, the global age-standardized prevalence and ASDR for AF in adults aged 30–45 remained stable, though ASMR slightly increased, particularly among men and in regions with lower SDI. These findings emphasize the need for targeted prevention strategies to reduce AF-related mortality in this population.
Introduction
Atrial Fibrillation (AF), a prevalent type of arrhythmia globally, significantly elevates the risk of major cardiovascular and cerebrovascular events. According to data from the Global Burden of Disease (GBD) study, the total number of AF patients has exceeded 59.7 million, accounting for 33% of all arrhythmia-related hospitalizations (1). Studies have projected that by 2060, the prevalence of AF among those aged 55 and above in Europe will increase from 8.8 million in 2010 to 17.9 million (2). In the United States, it is projected that the prevalence of AF will increase to 12.1 million by 2030 (3). This upward trend not only poses health challenges but is also accompanied by a significant economic burden. In the United States, direct-cost estimates per-patient-year ranged from $2,000 to $14,200, while in Europe, they ranged from €450 to €3,000 (4).
The pathological mechanism of atrial fibrillation (AF) is complex, and its core harm is mainly manifested as systemic embolism. Notably, AF in younger individuals exhibits distinct pathogenic mechanisms, with vagus-mediated pathways, high-intensity exercise, alcohol consumption, obesity, congenital heart disease, and genetic mutations serving as significant predisposing factors (5–8). Studies have shown that approximately 20%–30% of ischemic stroke events are caused by AF (9, 10). On the other hand, an increase in AF burden is associated with an increased risk of heart failure hospitalization and death (11). However, most countries and regions around the world lack comprehensive data on the burden of AF in young and middle-aged people. The disease burden of younger-onset AF may be severely underestimated. More seriously, due to a longer expected survival time, the lifetime cumulative risk of complications (such as dementia and kidney injury) in younger-onset AF patients may be higher than that in the elderly AF population (12, 13). From a socioeconomic perspective, AF patients may experience a decline in quality of life and occupational ability due to problems such as symptomatic palpitations and fatigue (14, 15). However, currently published clinical studies and guidelines focus more on the elderly population, resulting in a lack of evidence-based data and standardized intervention pathways for disease management in young and middle-aged people. Therefore, regular assessment of the epidemiological characteristics of AF in young and middle-aged people is crucial for formulating effective prevention and control strategies.
Therefore, our study analyzed the burden of AF among young and middle-aged adults from an epidemiological perspective. We investigated and analyzed the prevalence, mortality, and disability-adjusted life years (DALYs) of AF among adults aged 30–45 years at the global, regional, and national levels from 1990 to 2021, stratified by the level of social development, age, and gender of the young and middle-aged population.
Methods
Study population and data collection
This study utilized the 2021 GBD database—which employs three standardized tools: Cause of Death Ensemble Model (CODEm), Spatiotemporal Gaussian Process Regression (ST-GPR), and Disease Model-Bayesian Meta-regression (DisMod-MR)—to analyze burden trends of younger-onset AF from 1990 to 2021 (Supplementary Methods). The database is mainly used for disease burden analysis, risk factor research, comparison of regional health inequalities, time trend analysis, health policy evaluation, and assessment of the health status of specific populations. It covers 306 diseases and injuries as well as 87 risk factors. The study population consisted of AF patients aged 30–45 years. We extracted data on AF among individuals aged 30–45 years from the 2021 GBD study, including prevalence specific to location, age, and sex; mortality; numbers and rates of DALYs; and risk factor-attributable DALYs [with corresponding 95% uncertainty interval (UI)]. DALYs measure the health loss in a population over a specific time period due to disease or injury, mainly composed of years of life lost (YLL) and years lived with disability (YLD).
We evaluated the burden of AF at the global, regional (21 regions), and national (204 countries) levels, with a particular focus on the young and middle-aged population aged 30–45 years. The analysis divided this population into three age groups: 30–34 years, 35–40 years, and 41–45 years. In addition, this study conducted a stratified analysis based on the Socio-demographic Index (SDI). The evaluation criteria for the SDI include the per capita income level (reflecting material resources and medical accessibility), the average years of education of the population aged 15 and above (reflecting health literacy and disease prevention ability), and the total fertility rate of women under 25 years of age (representing population growth pressure and the burden of resource allocation). The SDI values range from 0 to 1, representing the economic development level from low to high. Countries and regions globally are divided into five levels according to the SDI: low SDI, low-middle SDI, middle SDI, high-middle SDI, and high SDI. This stratified design can help us reveal the association between the disease burden and the regional development level.
Statistical analysis
This study analyzed the burden of AF among young and middle-aged adults aged 30–45 years globally. Age-standardized prevalence, mortality, and DALY rates (per 100,000 population) were compared across age groups, genders, regions, and countries. To address age-structure heterogeneity, age-standardized rates with corresponding 95% confidence interval (CI) were calculated using weighted averages, enabling valid inter-regional comparisons. Prevalence represents the proportion of existing cases within a specified timeframe. Mortality directly quantifies AF-attributable deaths, serving as a critical severity indicator. Where AF does not cause direct fatality, it may substantially reduce quality of life and increase disability burden. Thus, DALYs comprehensively capture disease impact on health status. The average annual percentage change (AAPC) was estimated using joinpoint regression to assess temporal trends. AAPC represents the mean annualized change rate derived from weighted slope coefficients of the joinpoint model (1990–2021). An upward trend is established when both AAPC and the lower 95% CI limit exceed zero; conversely, a downward trend is confirmed when both AAPC and the upper 95% CI limit fall below zero. Calculate the cumulative absolute change (per 100,000) (ΔR) of age—standardized rates from 1990 to 2021 and its 95% confidence interval to evaluate the changes in age—standardized rates.
All analyses were performed using R v4.4.1, with trend analysis conducted in Joinpoint v5.1.0.0. Statistical significance was defined as P < 0.05.
Results
Global trends
Globally during 1990–2021, the number of young and middle-aged patients (aged 30–45 years) with AF increased from 565,407 to 952,298, representing a 68% increase. However, the age-standardized prevalence did not change significantly. The age-standardized prevalence decreased from 57.81 cases per 100,000 in 1990 to 57.48 cases per 100,000 in 2021. During these 31 years, the AAPC in prevalence was 0 (Table 1). In addition, compared with the total number of AF patients, the proportion of young and middle-aged AF patients showed a gradually increasing and then stabilizing trend during 1990–2000, and a gradually decreasing trend during 2001–2021 (Supplementary Figure S1A). The age-standardized mortality rate (ASMR) for AF in young and middle-aged populations increased from 0.04 cases per 100,000 in 1990 to 0.05 cases per 100,000 in 2021, with an AAPC of 0.17% (Supplementary Table S1). The age-standardized DALY rate (ASDR) in this population increased slightly from 6.98 cases per 100,000 in 1990 to 7.08 cases per 100,000 in 2021, and the AAPC change trend was not obvious (AAPC 0.05%, 95%CI −0.02 to 0.11) (ΔR 0.01, 95%CI −0.20 to 0.40) (Supplementary Table S2). However, compared with total AF cases, the proportions of deaths and DALYs related to AF among individuals aged 30–45 years both showed gradually decreasing trends (Supplementary Figures S1B, S1C).
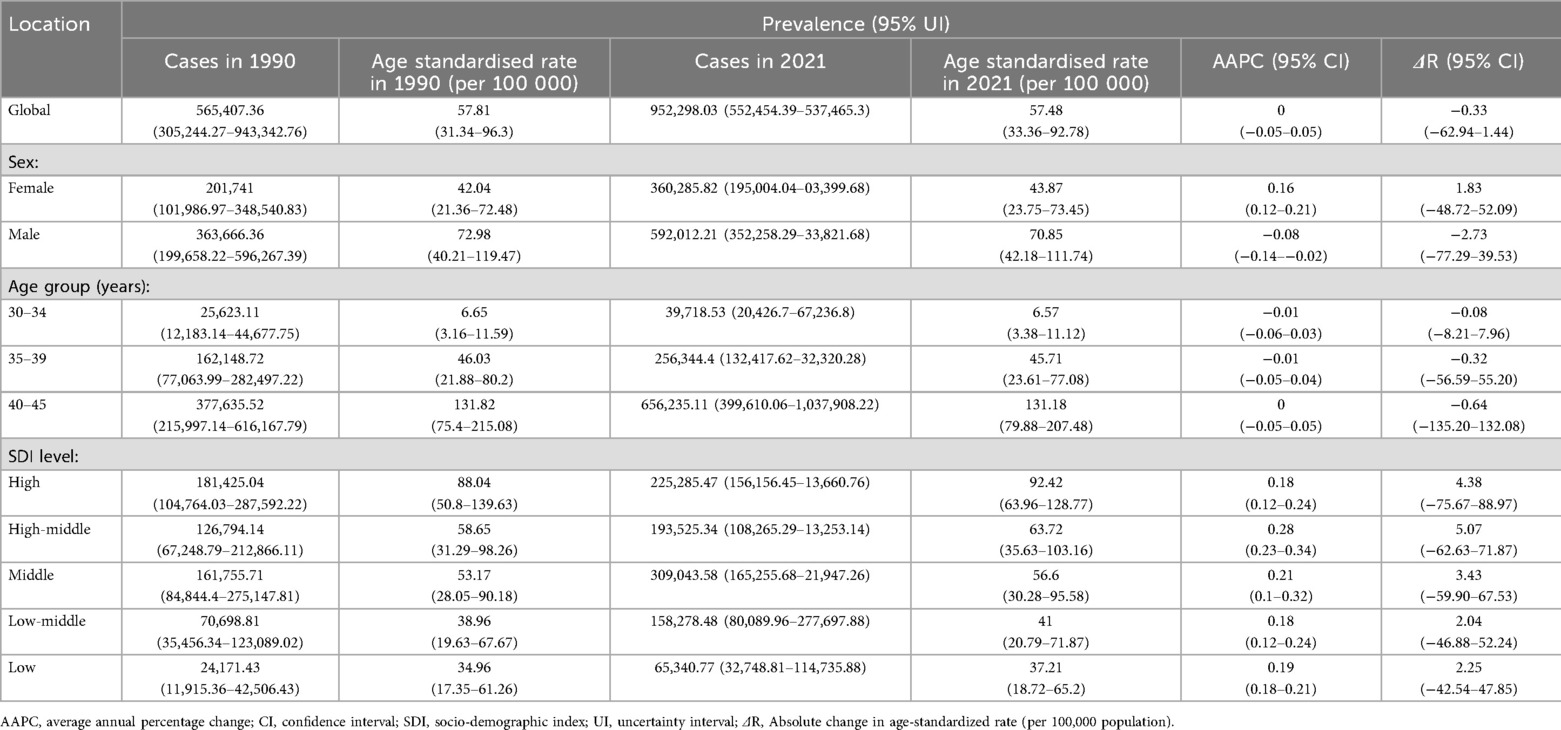
Table 1. Age-standardized prevalence and AAPC of AF among people aged 30-45 years at the global and regional levels from 1990 to 2021.
Global trends by sex
Globally during 1990–2021, among the young and middle-aged population aged 30–45 years, the age-standardized prevalence of AF increased in women (from 42.04 cases per 100,000 in 1990 to 43.87 cases per 100,000 in 2021, with an AAPC of 0.16%, 95%CI 0.12 to 0.21) (ΔR 1.83, 95%CI −48.72 to 52.09) (Table 1). In men, the age-standardized prevalence of AF decreased (from 72.98 cases per 100,000 in 1990 to 70.85 cases per 100,000 in 2021, with an AAPC of −0.08%, 95%CI −0.14 to −0.02) (ΔR −2.73, 95%CI−77.29 to 39.53) (Table 1). During the same period, the ASMR of AF in young and middle-aged men increased (AAPC 0.3%, 95%CI 0.14 to 0.47) (ΔR 0, 95%CI −0.03 to 0.03) (Supplementary Table S1), while the ASMR trend for women in this population was not significant (AAPC 0.03%, 95%CI −0.06 to 0.12) (ΔR 0, 95%CI −0.02 to 0.02) (Supplementary Table S1).
For DALYs, the increase was more pronounced in men (from 5.31 cases per 100,000 in 1990 to 5.48 cases per 100,000 in 2021, AAPC 0.11%, 95%CI 0.05 to 0.17) (ΔR 0.17, 95%CI −0.10 to 0.44) than in women (from 8.58 cases per 100,000 in 1990 to 8.65 cases per 100,000 in 2021, AAPC 0.02%, 95%CI −0.05 to 0.09) (ΔR 0.07, 95%CI −0.17 to 0.31) (Supplementary Table S2). This gender disparity persisted irrespective of SDI variations. Globally, the disease burden remained higher in men than in women, particularly in regions with middle or lower SDI, where the difference was most evident (Supplementary Figures S2, S3).
Global trends by age subgroup
Globally during 1990–2021, AF prevalence in all age subgroups (30–34 years, 35–39 years, 40–45 years) of young and middle-aged individuals showed an upward trend (30–34 years: 25,623 to 39,718; 35–39 years: 162,148 to 256,344; 40–45 years: 377,635 to 656,235). Notably, crude prevalence growth was higher in women than men across subgroups (Supplementary Figure S4). However, age-standardized AF prevalence trends remained non-significant (Table 1). During this period, ASMR increased in the 35–39 years and 40–45 years subgroups, most notably in 35–39 years ((AAPC 0.32%, 95%CI 0.24 to 0.39) vs. (AAPC 0.14%, 95%CI 0.03 to 0.25) in 40–45 years). The 30–34y subgroup showed no significant ASMR trend (Supplementary Table S1). In 2021, ASMR increased with age (30–34 years: 0.02 cases per 100,000; 40–45 years: 0.09 cases per 100,000) (Supplementary Table S1). Consistently, men showed rising mortality trends vs. women in all age subgroups (Supplementary Figure S4).
From 1990 to 2021, ASDR for AF in the 35–39 years age subgroup showed the most pronounced upward trend (AAPC 0.08%, 95%CI 0.02 to 0.14) (ΔR 0.12, 95%CI −0.11 to 0.35) compared to the 30–34 years and 40–45 years subgroups (Supplementary Table S2).
Global trends by sociodemographic index
Between 1990 and 2021, age-standardized AF prevalence among individuals aged 30–45 years increased across all SDI subgroups, particularly in high-middle SDI countries (AAPC 0.28%,95%CI 0.23 to 0.34) (ΔR 5.07, 95%CI −62.63 to 71.87). In 2021, high SDI countries had the highest AF prevalence (92.4 cases per 100 000) in this population (Table 1). During this period, AF-related mortality increased in low, low-middle, and middle SDI subgroups, with the largest increase in low-middle SDI (AAPC 0.49%, 95%CI 0.37 to 0.61) (ΔR 0.01 95%CI −0.02 to 0.04). Conversely, high-middle SDI subgroups showed declining mortality (AAPC −0.47%, 95%CI −0.74 to −0.19) (ΔR 0 95%CI −0.02 to 0.02) (Supplementary Table S1). When SDI exceeded 0.625, this declining trend became more pronounced (Supplementary Figure S5). In 2021, high-middle SDI countries had the lowest ASMR (0.03 cases per 100,000) (Figures 2, 3, Supplementary Table S1).
During the same period, ASDR showed significant increases across all SDI subgroups, most notably in low-middle SDI countries (AAPC 0.3%, 95%CI 0.18 to 0.42) (ΔR 0.52 95%CI −0.10 to 1.14). However, in 2021, high SDI countries had the highest ASDR (9.95 cases per 100,000) (Supplementary Table S2).
Regional trends
During 1990–2021, Australasia was the only region among 21 studied demonstrating a declining trend in AF prevalence among adults aged 30–45 years (AAPC −0.95%, 95%CI −1.09 to −0.81) (ΔR −33.27 95%CI −66.06 to −0.48) (Supplementary Table S3). The most rapid increases in age-standardized AF prevalence occurred in East Asia (AAPC 0.72%,95%CI 0.66 to 0.78) (ΔR 13.47 95%CI −0.40 to 27.34), High-income Asia Pacific (AAPC 0.59%, 95%CI 0.2 to 0.99) (ΔR 16.47 95%CI 1.88 to 31.61), and Central Europe (AAPC 0.41%, 95%CI 0.33 to 0.49) (ΔR 7.22 95%CI −0.61 to 15.05) (Supplementary Figure S6). In 2021, Australasia exhibited the highest age-standardized AF prevalence (111.54 cases per 100,000 population) (Supplementary Table S3). Gender-stratified analysis identified High-income Asia Pacific had the highest female prevalence (83.05 cases per 100,000), while Western Europe had the highest male prevalence (174.36 cases per 100,000) (Supplementary Table S4).
During 1990–2021, High-income North America showed the largest increase in ASMR of AF among adults aged 30–45 years (AAPC 1.82%, 95%CI 1.55–2.08) (ΔR 0.03 95%CI 0.02–0.03), while Central Europe exhibited the most pronounced decline (AAPC −1.71%,95%CI −1.97 to −1.45) (ΔR −0.02 95%CI −0.02 to −0.01) (Supplementary Table S5). Concurrently, the steepest rises in ASDR occurred in East Asia (AAPC 0.45%, 95%CI 0.41–0.5) (ΔR 0.86 95%CI −6.01 to 7.99) and High-income North America (AAPC 0.45%, 95%CI 0.34–0.56) (ΔR 1.41 95%CI −7.26 to 8.59), contrasting with Australasia's significant decrease (AAPC −0.79%, 95%CI −0.86 to −0.72) (ΔR −2.59 95%CI −14.22 to 7.67) (Supplementary Table S6, Figure S6). Sex stratification did not alter these trends (Supplementary Figure S7). In 2021, Oceania had both the highest ASMR (0.35 cases per 100,000) and ASDR (22.88 cases per 100,000) for AF in this cohort (Supplementary Tables S5, S6). No significant sex-based differences were observed (Supplementary Table S4).
National trends
At the national level (1990–2021), Belgium showed the steepest rise in age-standardized AF prevalence among adults aged 30–45 years (AAPC 1.95%, 95%CI 1.83–2.07), followed by the Republic of Korea (AAPC 1.67%, 95%CI 1.17–2.17) and Georgia (AAPC 1.64%, 95%CI 1.47–1.81). In contrast, Japan demonstrated the most significant decline in age-standardized AF prevalence (AAPC −1.19%, 95%CI −1.29 to −1.09). Concurrently, Georgia had the greatest increases in both ASMR (AAPC 3.04%, 95%CI 2.23–3.85) and ASDR (AAPC 2.04%, 95%CI 1.71–2.37), while Lebanon showed the sharpest ASMR reduction (AAPC −3.44%, 95%CI −3.79 to −3.08). In 2021, the Republic of Korea recorded the highest age-standardized AF prevalence (224.38 cases per 100,000). Nauru exhibited the highest ASMR (0.77 cases per 100,000) and ASDR (45.65 cases per 100,000) values (Figures 1–3 and Supplementary Table S7).
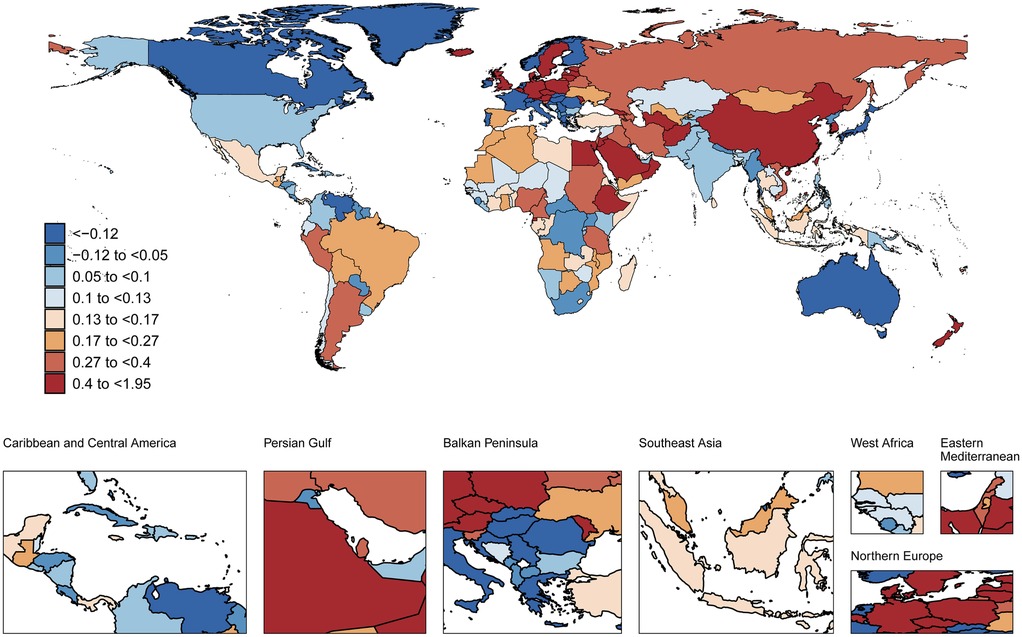
Figure 1. Global spatial distribution of atrial fibrillation prevalence trends: AAPC among adults aged 30-45 years, 1990-2021. World map adapted from: “Global country administrative boundary data”, Research and Environmental Science Data Platform (https://www.resdc.cn/).
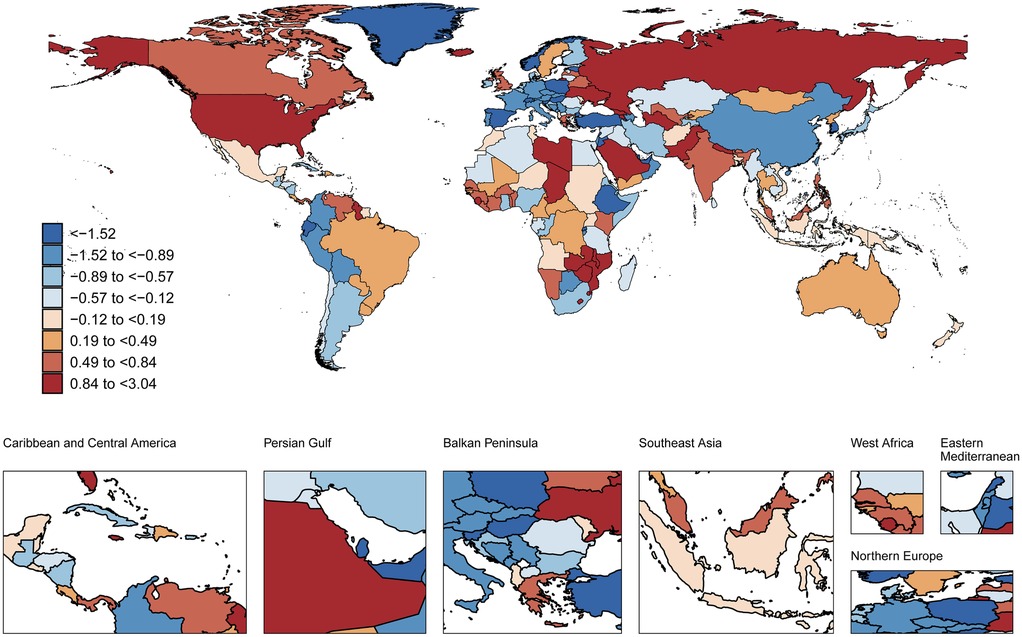
Figure 2. Global spatial distribution of atrial fibrillation ASMR trends: AAPC among adults aged 30-45 years, 1990-2021. World map adapted from: “Global country administrative boundary data”, Research and Environmental Science Data Platform (https://www.resdc.cn/).
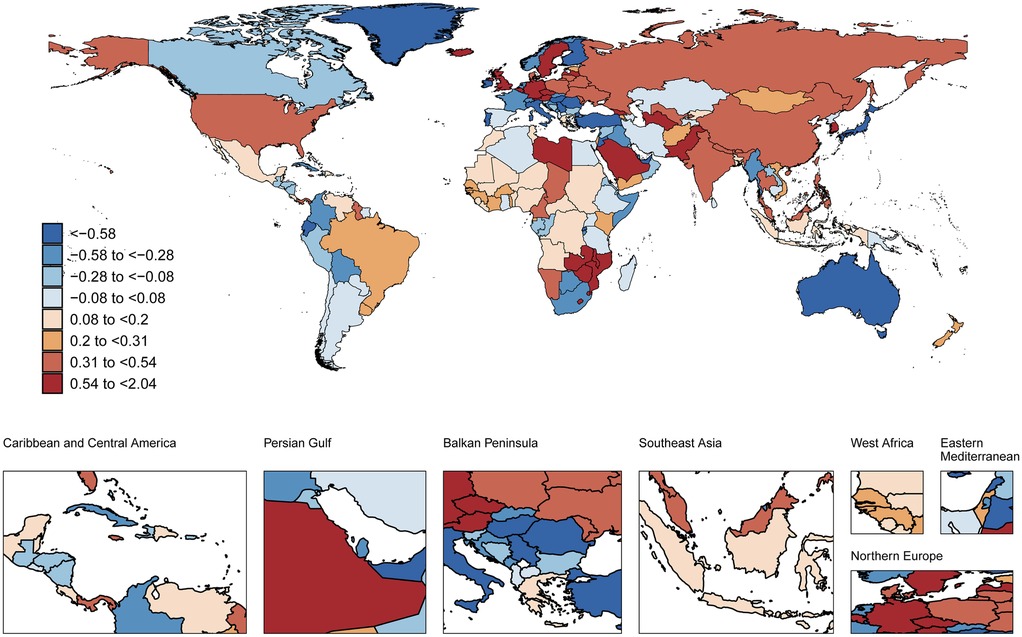
Figure 3. Global spatial distribution of atrial fibrillation ASDR trends: AAPC among adults aged 30-45 years, 1990-2021. World map adapted from: “Global country administrative boundary data”, Research and Environmental Science Data Platform (https://www.resdc.cn/).
Discussion
Globally during 1990–2021, AF cases among adults aged 30–45 years increased from 565,407 to 952,298 (68% rise). The age-standardized prevalence showed non-significant change, declining marginally from 57.81 to 57.48 per 100,000 population. Despite advances in cardiovascular risk management, the AF burden appeared to increase during the study period, as evidenced by positive annual percentage changes in both age-standardized mortality rate (AAPC 0.17%) and age-standardized disability-adjusted life years rate (AAPC 0.05%). Significant disparities in AF-related DALYs existed across SDI levels, with low-middle SDI countries exhibiting steeper ASDR increases (AAPC 0.30%). Effective rhythm control and complication prevention remain clinical challenges in this cohort. Our findings advance understanding of evolving global AF burdens by delineating incidence trends specifically in 30–45 years.
Current research reports show unequal prevalence rates of AF between men and women (16). Generally, the prevalence of AF is higher in men than in women (17). Our analysis reveals that although men with AF outnumbered women, age-standardized prevalence rose among women (AAPC 0.16%) but declined in men (AAPC −0.08%) during 1990–2021. Some research results suggest that reproductive factors have a causal effect on cardiovascular diseases in women. The higher the number of live births, the greater the risk of AF (18). Women are more affected by the impact of psychosocial stress on cardiovascular health than men, particularly during young and middle adulthood. Moreover, events such as menarche, pregnancy, and menopause may further amplify this stress (19, 20). However, ASMR and ASDR of AF in young and middle-aged men are significantly higher than in women, especially in low SDI regions. This sex-based difference may relate to physiological, lifestyle, and socioeconomic factors. Women may prioritize health management earlier in life and demonstrate stronger disease awareness and self-management capabilities, thereby reducing AF-related mortality. Conversely, men exhibit greater exposure to cardiovascular risk factors including smoking, alcohol consumption, and obesity, which may accelerate AF pathogenesis. Elevated body mass index (BMI) constitutes the strongest predictor of AF, with higher risk ratios in men vs. women. Moderate alcohol consumption elevates AF risk exclusively in men, whereas heavy intake increases risk in both sexes (21–23). Clinical studies have demonstrated that while estradiol reduces AF risk, sole administration of conjugated estrogens elevates this risk, suggesting complex interactions between hormone preparations and estrogen receptor specificity (24, 25). At the cellular level, cardiomyocytes express both estrogen and androgen receptors, indicating that sex hormones directly modulate ion channels and their expression profiles (26, 27). These electrophysiological alterations manifest as a net prolongation of action potential duration (APD) and QT interval by estrogen, whereas testosterone induces net APD/QT shortening—potentially explaining the divergent clinical outcomes (28). However, as current mechanistic evidence predominantly derives from ventricular cardiomyocyte studies, further confirmation is required regarding the translatability of these findings to atrial electrophysiological phenotypes. The precise mechanism underlying sex differences in AF patients remains incompletely characterized, though existing studies implicate cardiac structural, electrophysiological, and hormonal factors in AF pathogenesis (29).
Our analysis demonstrates rising AF prevalence across all age subgroups (30–45 years), with particularly pronounced increases in the 35–39 years and 40–45 years cohorts. Advancing age constitutes an independent risk factor for AF (30). Unlike elderly AF patients, younger individuals exhibit stronger risk factor associations with obesity and alcohol consumption (31). Notably, age-standardized prevalence changes remained non-significant across subgroups, potentially attributable to population aging and healthcare advancements. Mortality increased in both 35–39 years and 40–45 years subgroups, with the steepest rise observed in the 35–39 years cohort (AAPC 0.32%).
This indicates that the AF-related death risk among young and middle-aged people gradually increases with age, suggesting that we should strengthen the screening and intervention of AF in the early middle age.
The findings of this study reveal a substantial rise in the prevalence of AF in East Asia, with an AAPC of 0.72%. This increase may be linked to the accelerated aging process of the population and the growing prevalence of metabolic syndrome (32). Conversely, despite North America's leading position in global medical investment, the mortality rate of AF among individuals aged 30–45 shows an abnormal upward trend (AAPC 1.82%), further highlighting the “medical paradox” in regions with a high SDI. Specifically, the uneven distribution of social resources and over—intervention actually exacerbate the disease burden (33–35). In the future, targeted prevention and control strategies are required. East Asia should concentrate on metabolic intervention and utilize wearable devices for screening. Meanwhile, North America urgently needs to tackle medical inequality, establish a drug safety warning system, and standardize the comprehensive management process of AF.
Significant disparities exist in AF epidemiological characteristics across regions among adults aged 30–45 years. These variations may stem from genetic, environmental, lifestyle, and healthcare resource factors (36, 37). Our analysis demonstrates higher age-standardized AF prevalence but lower mortality in SDI regions, contrasting with lower prevalence yet higher mortality in low SDI areas. This pattern likely reflects superior healthcare resources and disease management capabilities in high SDI settings. In regions with a high SDI, the utilization rate of wearable intelligent detection devices among the population is high, such as smartwatches. Moreover, the population screening policies implemented by the universal healthcare systems in high-SDI regions (e.g., the UK's NHS) can detect more asymptomatic or occult subclinical AF patients. This detection gradient and systemic ascertainment bias may lead to an overestimation of the disease burden in high-SDI regions. These strategies—rigorous risk factor control, advanced diagnostics, and novel therapies—collectively improve quality of life and reduce AF mortality (20, 38, 39). Collectively, these observations highlight socioeconomic influences on early cardiovascular health, indicating that low SDI regions face challenges in healthcare access, health education, and cardiovascular risk prevention for younger populations (40).
Utilizing GBD study data, this analysis acknowledges inherent limitations despite the database's broad coverage. First, primary reliance on national reporting systems risks data omissions and ascertainment bias, with additional uncertainties arising from GBD model estimation methodologies. Second, substantial variability in primary data sources combined with geographic heterogeneity in AF diagnostic criteria and therapeutic approaches may compromise result accuracy. Third, temporal data latency reflecting time lags between clinical events and registry reporting potentially limits reflection of contemporary epidemiological profiles. Finally, this study considered the physiological changes and health risks of the population when stratifying age (such as, in the age group of 30–34 years, people in this stage begin to face work-life stress, and the proportion of anxiety increases, suggesting that both mental and physical health need to be concerned simultaneously. The risk of some chronic diseases (e.g., hypertension and hyperlipidemia) gradually emerges; in the age group of 40–45 years, women enter the perimenopausal period, with fluctuating estrogen levels and an increased risk of decreased bone density. The risk of cardiovascular and cerebrovascular diseases in men further rises, and the detection rates of chronic diseases such as arteriosclerosis and diabetes increase). However, there is currently a lack of specific literature or evidence to support this, and there may be grouping bias. There are limitations in calculating the ΔR. The measurement errors in the data of the first and last years may significantly widen the confidence interval of the absolute change. In addition, the study population consisted of young-onset AF patients aged 30–45 years, which is a small low-prevalence subgroup. It is possible that the AAPC is significant while the absolute change rate is not. Consequently, priority should be given to: (1) implementing real-time AF surveillance systems, (2) enhancing data collection from adults aged 30–45 years, and (3) refining statistical methodologies through approaches such as (a) sensitivity analysis, and (b) stratified exploration of sex-by-SDI and age-by-SDI differentials through decomposition analysis,. These measures will collectively reduce research uncertainty, improve result interpretability, and strengthen the validity of future studies.
This study constitutes critical evidence for developing targeted AF prevention strategies in adults aged 30–45 years. Implementation priorities include: (1) enhanced health education to improve AF disease literacy and self-management capacity; (2) early screening and intervention programs for high-risk subgroups (males and low sociodemographic index regions) to reduce AF incidence and mortality; (3) increased national investment in AF care infrastructure to enhance healthcare accessibility and quality; (4) strengthened international technical cooperation for resource-limited settings, and (5) For regions with different SDI levels, different public health strategies should be formulated. For example, in high-SDI areas, region-tailored strategies such as wearables screening can be implemented, while in low-SDI settings, the focus can be on hypertension detection. Our findings further necessitate personalized intervention protocols, optimized resource allocation, and age-specific clinical guidelines. These evidence-based recommendations hold significant implications for cardiovascular practice, epidemiological research, and management of younger-onset AF.
Conclusion
This systematic evaluation delineates global epidemiological patterns of AF among adults aged 30–45 years, quantifying sex-specific, age-stratified, and SDI-driven disparities in disease burden across geographic regions. These findings establish critical evidence for investigating AF pathogenesis and optimizing targeted interventions, informing substantial public health initiatives worldwide.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
JS: Data curation, Formal analysis, Methodology, Software, Writing – original draft. JD: Data curation, Formal analysis, Methodology, Software, Writing – original draft. LL: Data curation, Validation, Visualization, Writing – original draft. ZX: Conceptualization, Investigation, Software, Writing – original draft. JD: Conceptualization, Funding acquisition, Project administration, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funded by: Tianjin Key Medical Discipline (Specialty) Construction Project (Grant No. TJYXZDXK-055B).
Acknowledgments
This article is primarily supported by the 2021 Global Burden of Disease Database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1677005/full#supplementary-material
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. (2013) 34(35):2746–51. doi: 10.1093/eurheartj/eht280
3. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. Adult population. Am J Cardiol. (2013) 112(8):1142–7. doi: 10.1016/j.amjcard.2013.05.063
4. Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace. (2011) 13(10):1375–85. doi: 10.1093/europace/eur194
5. D'Elia NW, Kistler PM, Voskoboinik A. Atrial fibrillation in the young: pathogenesis and clinical implications. JACC Clin Electrophysiol. (2025) 11(5):1051–66. doi: 10.1016/j.jacep.2025.04.001
6. Jurgens SJ, Melloni GE, Kany S, Fabritz L, Rämö JT, Goette A, et al. Pathogenic cardiomyopathy-associated gene variants and prognosis in atrial fibrillation: results in 18,000 clinical trial participants. J Am Coll Cardiol. (2025) 86(10):738–53. doi: 10.1016/j.jacc.2025.06.052
7. Vermeer J, Greef TV, Broek M, Louw B, Steenbergen G, Veghel D, et al. Improving outcomes of atrial fibrillation ablation by integrated personalized lifestyle interventions: a randomized controlled trial. Eur Heart J. (2025) 00:1–11. doi: 10.1093/eurheartj/ehaf689
8. Ji Y, Chen LY, Zhang MJ. Association of genetically predicted problem alcohol use with risk of atrial fibrillation: a Mendelian randomization study. Eur J Prev Cardiol. (2024) 31(18):2138–40. doi: 10.1093/eurjpc/zwae211
9. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20(10):795–820. doi: 10.1016/s1474-4422(21)00252-0
10. Kotalczyk A, Lip GY, Calkins H. The 2020 ESC guidelines on the diagnosis and management of atrial fibrillation. Arrhythm Electrophysiol Rev. (2021) 10(2):65–7. doi: 10.15420/aer.2021.07
11. Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J, et al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol. (2018) 71(23):2603–11. doi: 10.1016/j.jacc.2018.03.519
12. Chen LY, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agarwal SK, et al. Association of atrial fibrillation with cognitive decline and dementia over 20 years: the ARIC-NCS (atherosclerosis risk in communities neurocognitive study). J Am Heart Assoc. (2018) 7(6):e007301. doi: 10.1161/jaha.117.007301
13. Krisai P, Eberl M, Coslovsky M, Rodondi N, Chocano-Bedoya P, Aeschbacher S, et al. Biomarker and cognitive decline in atrial fibrillation: a prospective cohort study. Sci Rep. (2025) 15(1):12921. doi: 10.1038/s41598-025-89800-9
14. Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, et al. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes. (2015) 8(4):393–402. doi: 10.1161/circoutcomes.114.001303
15. Yue B, Hou Q, Bredehorst J, Han Q, Zhang B, Zhang C, et al. Atrial fibrillation increases the risk of new-onset myocardial infarction amongst working-age population: a propensity-matched study. Herz. (2023) 48(5):408–12. doi: 10.1007/s00059-023-05181-7
16. Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. (2016) 13(6):321–32. doi: 10.1038/nrcardio.2016.45
17. Andrade JG, Deyell MW, Lee AYK, Macle L. Sex differences in atrial fibrillation. Can J Cardiol. (2018) 34(4):429–36. doi: 10.1016/j.cjca.2017.11.022
18. Ardissino M, Slob EAW, Carter P, Rogne T, Girling J, Burgess S, et al. Sex-specific reproductive factors augment cardiovascular disease risk in women: a Mendelian randomization study. J Am Heart Assoc. (2023) 12(5):e027933. doi: 10.1161/jaha.122.027933
19. Ebong IA, Quesada O, Fonkoue IT, Mattina D, Sullivan S, Oliveira GMM, et al. The role of psychosocial stress on cardiovascular disease in women: JACC state-of-the-art review. J Am Coll Cardiol. (2024) 84(3):298–314. doi: 10.1016/j.jacc.2024.05.016
20. Gleason KT, Dennison Himmelfarb CR, Ford DE, Lehmann H, Samuel L, Han HR, et al. Association of sex, age and education level with patient reported outcomes in atrial fibrillation. BMC Cardiovasc Disord. (2019) 19(1):85. doi: 10.1186/s12872-019-1059-6
21. Gallagher C, Hendriks JML, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, et al. Alcohol and incident atrial fibrillation—a systematic review and meta-analysis. Int J Cardiol. (2017) 246:46–52. doi: 10.1016/j.ijcard.2017.05.133
22. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. (2014) 64(3):281–9. doi: 10.1016/j.jacc.2014.03.048
23. Kim YG, Han KD, Choi JI, Choi YY, Choi HY, Boo KY, et al. Non-genetic risk factors for atrial fibrillation are equally important in both young and old age: a nationwide population-based study. Eur J Prev Cardiol. (2021) 28(6):666–76. doi: 10.1177/2047487320915664
24. Tsai WC, Haung YB, Kuo HF, Tang WH, Hsu PC, Su HM, et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: a nationwide cohort study. Sci Rep. (2016) 6:24132. doi: 10.1038/srep24132
25. Wong JA, Rexrode KM, Sandhu RK, Moorthy MV, Conen D, Albert CM. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart. (2017) 103:1954–61. doi: 10.1136/heartjnl-2016-311002
26. Goldstein J, Sites CK, Toth MJ. Progesterone stimulates cardiac muscle protein synthesis via receptor-dependent pathway. Fertil Steril. (2004) 82:430–6. doi: 10.1016/j.fertnstert.2004.03.018
27. Lizotte E, Grandy SA, Tremblay A, Allen BG, Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem. (2009) 23:75–86. doi: 10.1159/000204096
28. Odening KE, Koren G. How do sex hormones modify arrhythmogenesis in long QT syndrome? Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm. (2014) 11:2107–15. doi: 10.1016/j.hrthm.2014.06.023
29. Odening KE, Deiß S, Dilling-Boer D, Didenko M, Eriksson U, Nedios S, et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace. (2019) 21(3):366–76. doi: 10.1093/europace/euy215
30. Segev A, Maor E, Goldenfeld M, Itelman E, Grossman E, Beinart R, et al. Atrial fibrillation in young hospitalized patients: clinical characteristics, predictors of new onset, and outcomes. J Cardiol. (2023) 82(5):408–13. doi: 10.1016/j.jjcc.2023.04.013
31. Naderi S, Wang Y, Miller AL, Rodriguez F, Chung MK, Radford MJ, et al. The impact of age on the epidemiology of atrial fibrillation hospitalizations. Am J Med. (2014) 127(2):158.e1–7. doi: 10.1016/j.amjmed.2013.10.005
32. Wong CX, Tse HF, Choi EK, Chao TF, Inoue K, Poppe K, et al. The burden of atrial fibrillation in the Asia-Pacific region. Nat Rev Cardiol. (2024) 21(12):841–3. doi: 10.1038/s41569-024-01091-1
33. Hero JO, Zaslavsky AM, Blendon RJ. The United States leads other nations in differences by income in perceptions of health and health care. Health Aff (Millwood). (2017) 36(6):1032–40. doi: 10.1377/hlthaff.2017.0006
34. Dickman SL, Himmelstein DU, Woolhandler S. Inequality and the health-care system in the USA. Lancet. (2017) 389(10077):1431–41. doi: 10.1016/S0140-6736(17)30398-7
35. Gaver A, Much Healthcare T. The harmful combination of overdiagnosis and medical overuse, told and untold stories. Isr Med Assoc J. (2022) 24(6):399–402.35734840
36. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50(9):1234–39. doi: 10.1038/s41588-018-0171-3
37. Frederiksen TC, Christiansen MK, Benjamin EJ, Overvad K, Olsen A, Andersen MK, et al. Interaction of genetic risk and lifestyle on the incidence of atrial fibrillation. Heart (British Cardiac Society). (2024) 110(9):644–49. doi: 10.1136/heartjnl-2023-323333
38. Ceornodolea AD, Bal R, Severens JL. Epidemiology and management of atrial fibrillation and stroke: review of data from four European countries. Stroke Res Treat. (2017) 2017:8593207. doi: 10.1155/2017/8593207
39. Khatib R, McKee M, Shannon H, Chow C, Rangarajan S, Teo K, et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet (London, England). (2016) 387(10013):61–9. doi: 10.1016/s0140-6736(15)00469-9
40. GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet (London, England). (2018) 392(10159):1923–94. doi: 10.1016/s0140-6736(18)32225-6
Keywords: younger-onset atrial fibrillation, global burden of disease, temporal trends, geographic disparities, disability-adjusted life year
Citation: Sun J, Dong J, Liang L, Xue Z and Ding J (2025) Global burden, temporal trends and geographic disparities of younger-onset atrial fibrillation in adults aged 30–45 years, 1990–2021: a population-based study. Front. Cardiovasc. Med. 12:1677005. doi: 10.3389/fcvm.2025.1677005
Received: 31 July 2025; Accepted: 6 October 2025;
Published: 20 October 2025.
Edited by:
Rajeev Gupta, Spectrum Medical Center, United Arab EmiratesReviewed by:
Tanesh Ayyalu, Department of Cardiology, United StatesShekhar Vohra, Icahn School of Medicine at Mount Sinai, United States
Copyright: © 2025 Sun, Dong, Liang, Xue and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Ding, ZGoyMDAyMjA4OEBob3RtYWlsLmNvbQ==
 Jiayi Sun
Jiayi Sun Jiayang Dong2
Jiayang Dong2 Jun Ding
Jun Ding