- 1Department of Cardiology, Jintang County First People’s Hospital, Chengdu, China
- 2Department of Cardiology, Sichuan Science City Hospital, Mianyang, China
Background: Inflammation has been widely recognized as a key contributor to the pathogenesis of numerous diseases, including cardiovascular disorders. This study aims to investigate the associations between different novel inflammatory markers and adverse outcomes within one year in patients with HF, and to identify the most effective predictor.
Methods: Three inflammatory markers—Systemic Inflammatory Response Index (SIRI), Systemic Immune-Inflammatory Index (SII), and Neutrophil-to-Lymphocyte Ratio (NLR)—were evaluated. Cox regression analysis was performed to examine their associations with adverse outcomes within one year. Kaplan–Meier (KM) survival analysis was used to estimate the cumulative risk of adverse events. Additionally, receiver operating characteristic (ROC) curves, time-dependent ROC curves, and C statistics were applied to compare the predictive performance of these markers.
Results: All three inflammatory markers were significantly associated with adverse one-year outcomes in HF patients. For each one standard deviation increase in SIRI, SII, and NLR, the risk of re-hospitalization increased by 45.5%, 54.9%, and 63.7%, respectively, while the risk of death increased by 63.8%, 70.1%, and 92.9%, respectively. ROC analysis indicated that SIRI had superior predictive performance compared with SII and NLR, and time-dependent ROC results further confirmed its stronger prognostic value.
Conclusion: This study demonstrate that several novel inflammatory markers are strongly associated with adverse one-year outcomes in HF patients. Comparative analysis revealed that SIRI provides the most robust predictive performance, highlighting its potential as a valuable clinical tool for monitoring and risk stratification in HF management.
1 Introduction
Heart Failure (HF) is a condition characterized by impaired cardiac function, preventing the heart from effectively pumping blood to meet the body's needs. This dysfunction leads not only to multi-organ failure but also significantly diminishes patients' quality of life (1, 2). As epidemiological data continues to evolve, the prevalence of HF has steadily increased, particularly among the elderly, making it a growing public health concern worldwide (3). More importantly, HF patients not only face a high mortality risk but also experience frequent hospitalizations and long-term health complications, which place a substantial strain on healthcare systems (4, 5). Consequently, early identification of high-risk individuals, improving long-term outcomes, and reducing readmission and mortality rates have become key priorities in current clinical research.
In the pathogenesis of HF, inflammation plays a crucial role. Inflammation is a protective response of the body to injury or infection, and it is not only involved in the initiation and progression of HF but is also strongly associated with the poor clinical prognosis of various diseases (6–8). This is especially true in cardiovascular diseases (CVD), where inflammation contributes to the development and maintenance of hypertension, atherosclerosis, and the rupture of arterial plaques (9–11). These findings suggest that controlling inflammation could offer a new strategy for preventing CVDs and their adverse outcomes.
Traditional inflammation assessment primarily relies on single indicators such as white blood cell count, C-reactive protein (CRP), and procalcitonin. However, these markers often fail to provide a comprehensive view of the body's inflammatory state. In contrast, newer inflammatory indices based on blood cell counts have gained attention due to their low cost and ease of use (12–15). For example, ratios such as the monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR) have demonstrated independent predictive value in assessing the prognosis of various diseases (14, 16, 17). More comprehensive markers, such as the systemic immune-inflammation index (SII) and systemic inflammatory response index (SIRI), offer a more accurate assessment of systemic inflammation by integrating data from multiple immune pathways. Studies have shown that SIRI not only predicts the course of inflammatory diseases but also shows promise in predicting the progression of cardiovascular diseases, metabolic disorders, and stroke (9, 12, 14). Notably, PLR is closely linked to the onset of hypertension (17), while elevated SII and SIRI levels are significantly associated with an increased risk of stroke, osteoporosis, and kidney disease progression (14, 18). Additionally, these indices have been shown to correlate with the occurrence of fatty liver disease and liver fibrosis (9). These innovative inflammatory markers, derived from routine blood cell counts, reflect the body's multi-pathway inflammatory and immune status, offering new avenues for early disease detection and prognosis assessment.
Building on this research background, the aim of this study is to systematically investigate the association between novel inflammatory markers and adverse outcomes in HF patients within one year. The study will focus on identifying the most predictive inflammatory indicators, thereby providing a scientific basis for early clinical monitoring and intervention, and optimizing treatment strategies for HF patients.
2 Material and methods
2.1 Screening of the study population
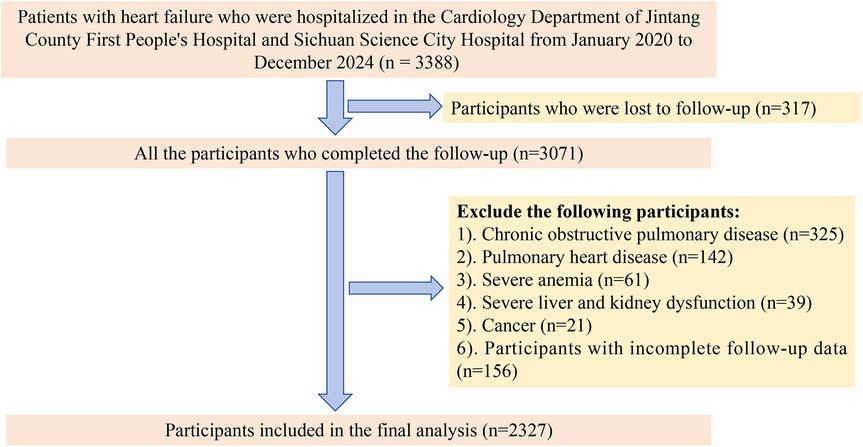
This study adopted a dual-center longitudinal cohort design and included patients diagnosed with HF who were hospitalized at the First People's Hospital of Jintang County and at Sichuan Province Science City Hospital between January 2020 and December 2024. The inclusion criteria for the study were as follows: (1) reduced ejection fraction HF, defined as a left ventricular ejection fraction (LVEF) < 40%; (2) a heart function classification of III to IV, representing varying degrees of HF severity; and (3) completion of the follow-up. To account for the potential impact of other underlying conditions on the outcomes, we excluded patients with chronic obstructive pulmonary disease, pulmonary heart disease, severe anemia, severe liver or kidney dysfunction, or cancer. Additionally, patients with incomplete follow-up data were also excluded. After applying these inclusion and exclusion criteria, a total of 2,327 patients were eligible for the study (Figure 1).
This study was conducted in accordance with the principles of the Helsinki Declaration and received approval from the Ethics Committee of the First People's Hospital of Jintang County (No. 20190913001). All participants provided written informed consent.
2.2 Data collection and definitions
The participants' basic information, physical examination results, medical history, medication usage, and laboratory test data were collected from the hospital's electronic medical records and telephone follow-up records. Basic information, such as height, weight, blood pressure, and body mass index (BMI), is detailed in the Supplementary Materials. Laboratory test data included blood routine tests, alanine aminotransferase (ALT), aspartate aminotransferase (AST), brain natriuretic peptide (BNP), total cholesterol (TC), triglycerides (TG), fasting blood glucose (FBG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and albumin, all measured using an automatic biochemical analyzer. LVEF was assessed by experienced ultrasound technicians following a standardized protocol. The definitions of diseases such as coronary heart disease (CHD), diabetes mellitus (DM), and dyslipidemia were based on current diagnostic criteria, which are described in more detail in the Supplementary Materials.
2.3 Calculation of new inflammatory markers
Based on the results of relevant blood routine tests, new inflammatory markers are calculated. The specific calculation method is as follows:
2.4 Study outcome
The primary outcome measure of this study is the occurrence of adverse events within one year during the follow-up period. These events include re-hospitalization and death within one year.
The clinical outcomes of the patients will be monitored continuously for 12 months after discharge through telephone follow-ups and medical records. In cases where multiple adverse events occur, the time of the first occurrence will be considered the endpoint to ensure data accuracy and consistency.
2.5 Statistical analysis
Participants were divided into three groups (T1, T2, and T3) based on the tertiles of various novel inflammatory markers. Univariate and multivariate Cox regression analyses were performed, and trend tests were conducted to evaluate the relationship between these markers and adverse outcomes within one year for patients with HF. Additionally, Kaplan–Meier (KM) curves were used to assess the risk of adverse outcomes among the different groups based on the tertiles of inflammatory markers, with log-rank tests performed for comparison. To further investigate the dose-response relationship between inflammatory marker levels and adverse outcomes, the restricted cubic spline (RCS) model was applied. To compare the predictive efficacy of different inflammatory indicators, we conducted a series of analyses, including receiver operating characteristic (ROC) curve analysis, time-dependent ROC analysis, and C statistics. These analyses were used to comprehensively evaluate the predictive performance of each marker for adverse outcomes.
All statistical analyses were performed using R software version 4.2.2, with a two-sided P value of less than 0.05 considered statistically significant.
3 Results
3.1 Baseline characteristics of the study population
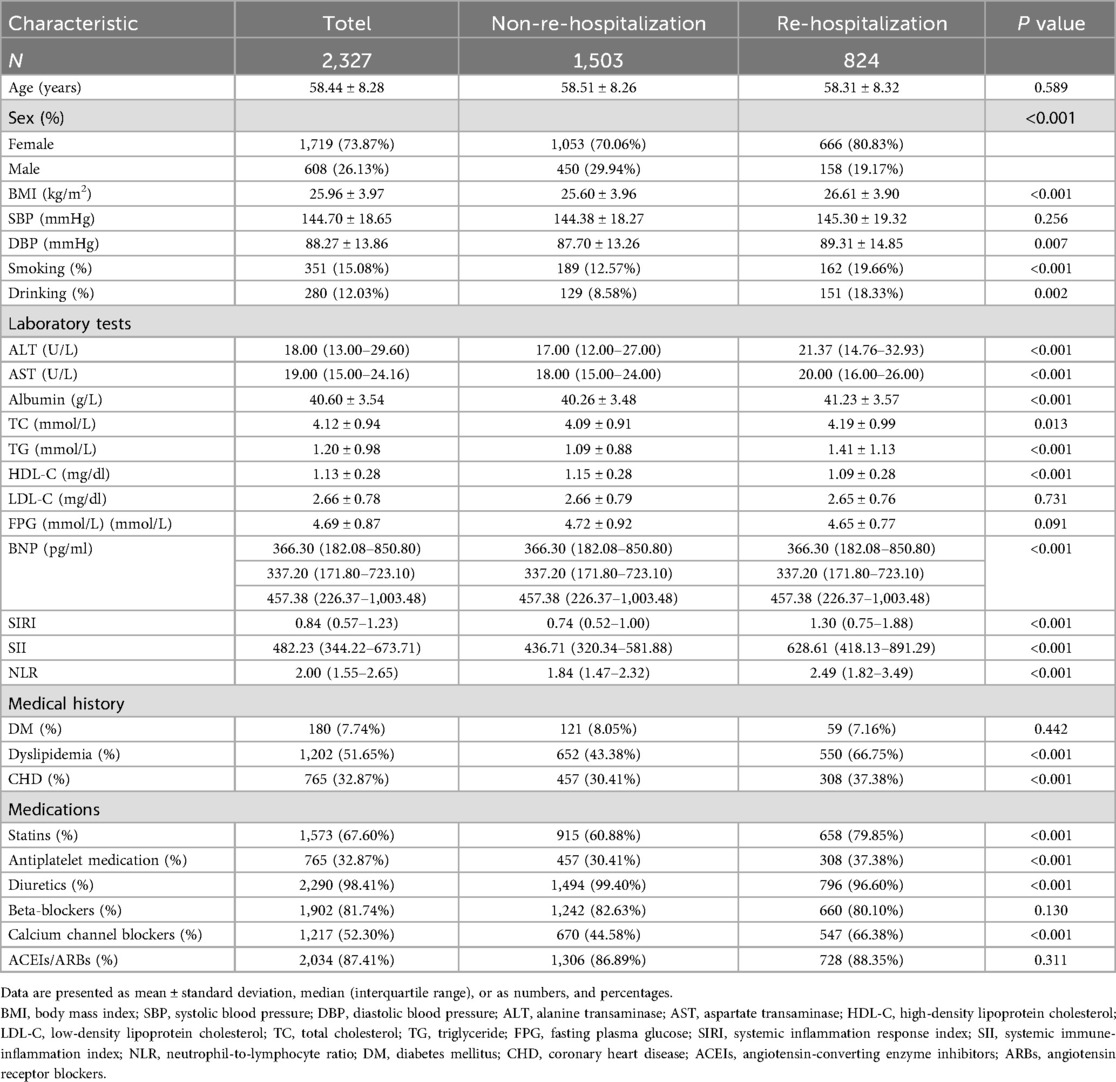
A total of 2,327 patients with HF were included in this study. Based on whether they were readmitted within one year, the patients were divided into two groups, and their baseline characteristics were compared. The results are shown in Table 1.
The average age of the study population was 58.44 ± 8.28 years, with 73.87% of participants being female, a relatively high proportion. When comparing the two groups, patients who were readmitted had significantly higher BMI and DBP, and were more likely to be current smokers and drinkers. Biochemically, patients who were readmitted had higher levels of ALT, AST, albumin, TC, and BNP, while their HDL-C was lower. In terms of medical history, the prevalence of CHD and dyslipidemia was significantly higher in the readmitted group. Additionally, these patients were more likely to be on medications such as lipid-lowering drugs, antiplatelet agents, diuretics, and other drugs aimed at improving ventricular remodeling. Notably, various new inflammatory markers were also significantly elevated in the readmitted group. Apart from these factors, no significant differences were found in other variables.
3.2 Incidence of adverse outcomes across different groups
The participants were divided into three groups based on the tertiles of various inflammatory markers. The results showed that, compared to the T1 group, both the T2 and T3 groups had significantly higher rates of rehospitalization and death within one year, with this trend continuing to increase (P for trend <0.001). Figure 2 illustrates the rehospitalization rates within one year for the three inflammatory markers across the three groups, while Figure 3 shows the death rates within one year for these markers.
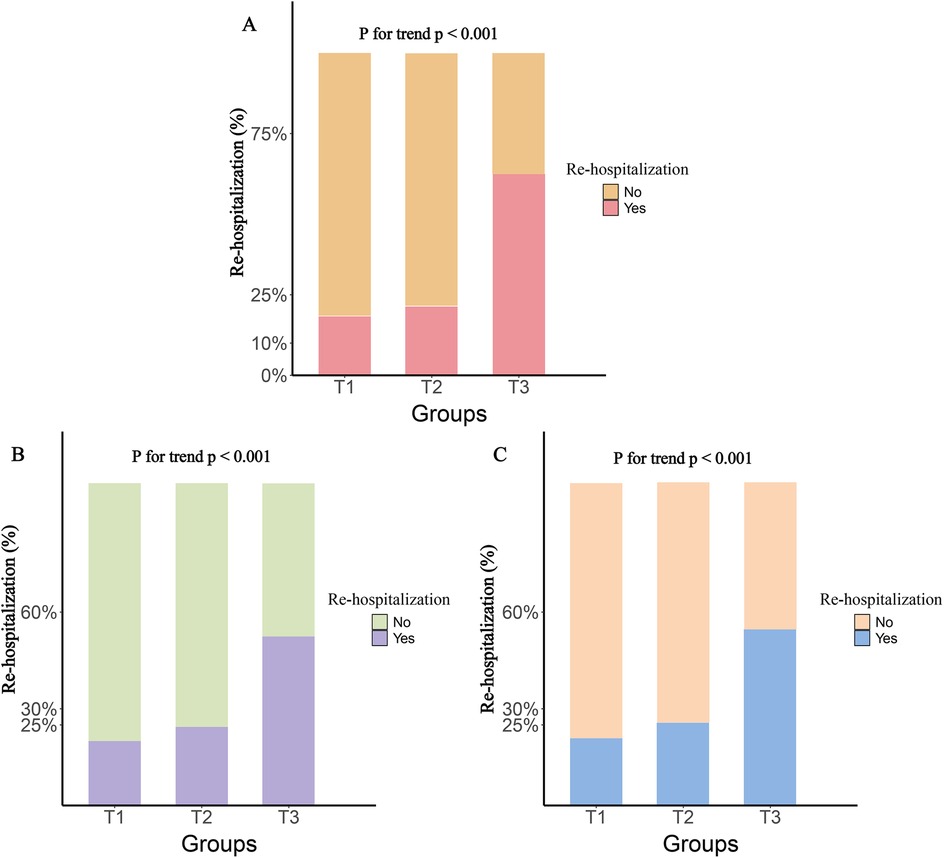
Figure 2. One-year rehospitalization rates by tertile groups of different inflammatory markers. (A), SIRI; (B), SII; (C), NLR.
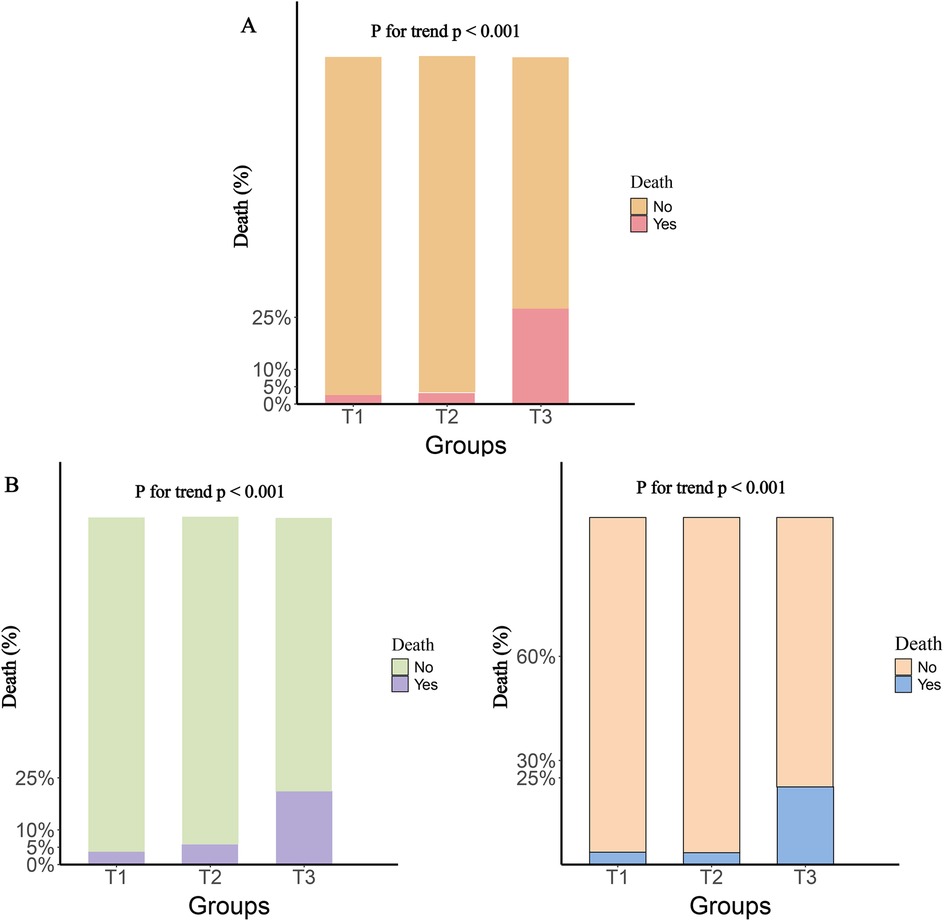
Figure 3. One-Year death rates by tertile groups of different inflammatory markers. (A), SIRI; (B), SII; (C), NLR.
3.3 Relationship between various new inflammatory markers and re-hospitalization within one year
To investigate the relationship between different novel inflammatory markers and re-hospitalization in patients with HF within one year, we conducted a multivariate Cox regression analysis. The results revealed that as the levels of inflammatory markers increased, the risk of re-hospitalization also significantly increased. Specifically, in Model 1, for each one standard deviation (SD) increase in SIRI, SII, and NLR, the corresponding hazard ratios (HRs) were 1.240 [95% confidence interval (CI): 1.207–1.273], 1.456 (95% CI: 1.401–1.513), and 1.433 (95% CI: 1.374–1.495), respectively. These findings remained consistent in the fully adjusted Model 5.
Furthermore, after categorizing the three inflammatory markers into T1, T2, and T3 groups, we found that compared to the T1 group, the re-hospitalization risk was significantly higher in both the T2 and T3 groups. The trend test was statistically significant, demonstrating a clear increasing trend (Table 2). To present these relationships more intuitively, we plotted the cumulative risk curves for the three groups. The results showed that, regardless of the inflammatory marker used, the re-hospitalization risk was significantly higher in the T3 group compared to the T1 and T2 groups, with log-rank test results being statistically significant (Figure 4). Finally, considering the gender differences, after stratification by gender, it is completely consistent with the overall results (Supplementary Table S1).
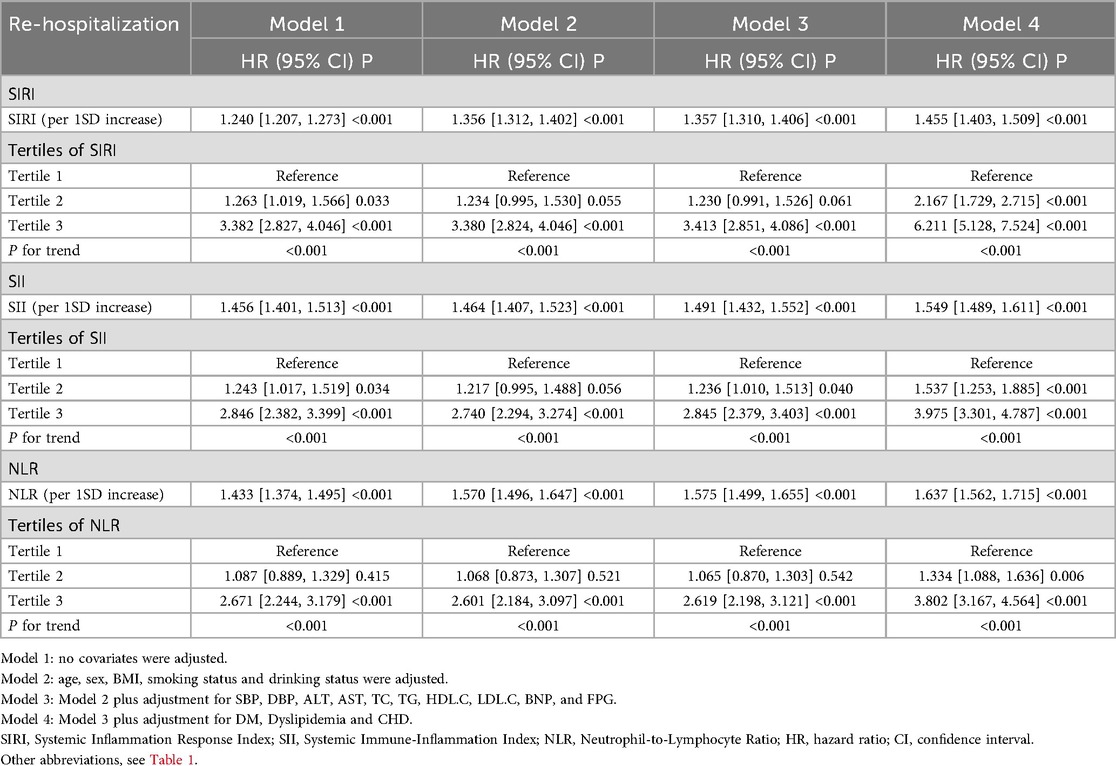
Table 2. Relationship between various novel inflammatory markers and re-hospitalization within one year in patients with heart failure.
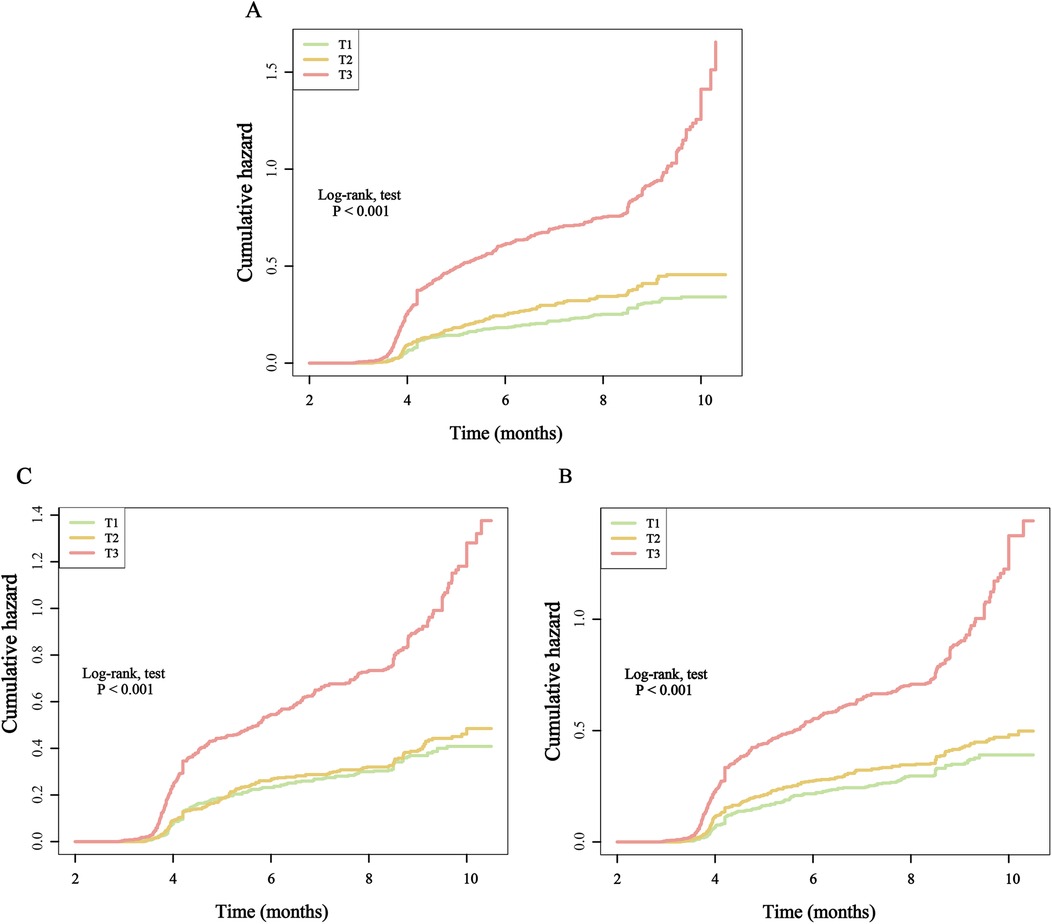
Figure 4. Kaplan–Meier cumulative risk curve of re-hospitalization within one year based on the tertiles of different inflammatory markers. (A), SIRI; (B), SII; (C), NLR.
Finally, using RCS analysis, we further examined the dose-response relationship between inflammatory markers and re-hospitalization risk. The results showed a significant increasing trend, indicating that higher levels of inflammatory markers are closely associated with an increased risk of future re-hospitalization (Figure 5).
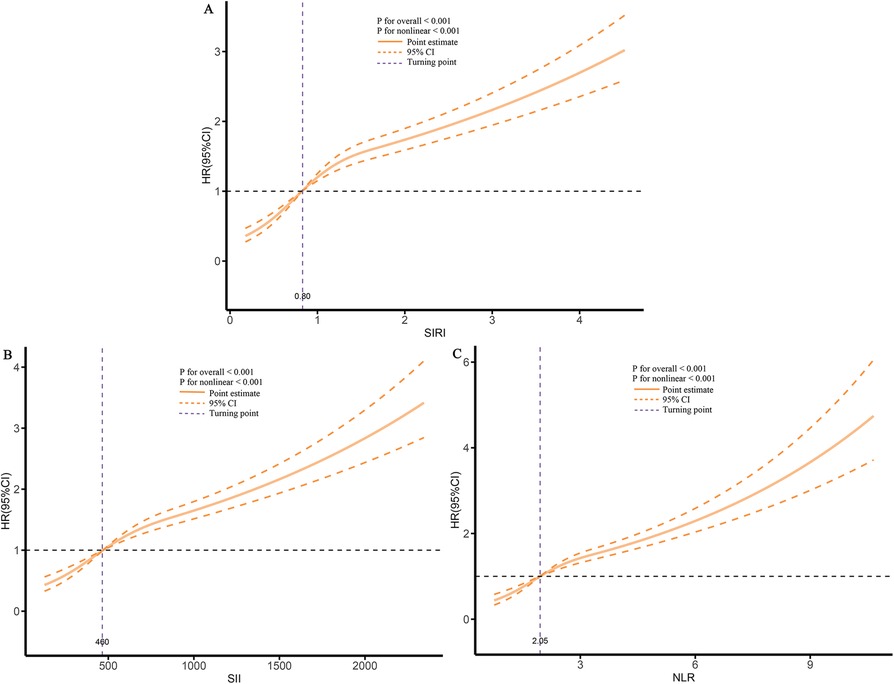
Figure 5. Dose-response association between different inflammatory markers and the risk of re-hospitalization. (A), SIRI; (B), SII; (C), NLR.
3.4 Relationship between various new inflammatory markers and the risk of death within one year
We also analyzed the relationship between different novel inflammatory markers and the risk of death within one year in patients with HF. The results showed that, in the fully adjusted Model 5, for every 1 SD increase in SIRI, SII, and NLR, the corresponding risk of death increased by 63.8%, 70.1%, and 92.9%, respectively.
When these markers were converted into categorical variables, the risk of death continued to show an increasing trend (Table 3). Additionally, the KM curve demonstrated that the risk of death in the T3 group was significantly higher than that in the T1 and T2 groups (Figure 6). Furthermore, the dose-response relationship based on RCS analysis confirmed these findings (Figure 7). Of course, even after stratification by gender, the results remained unchanged (Supplementary Table S2).
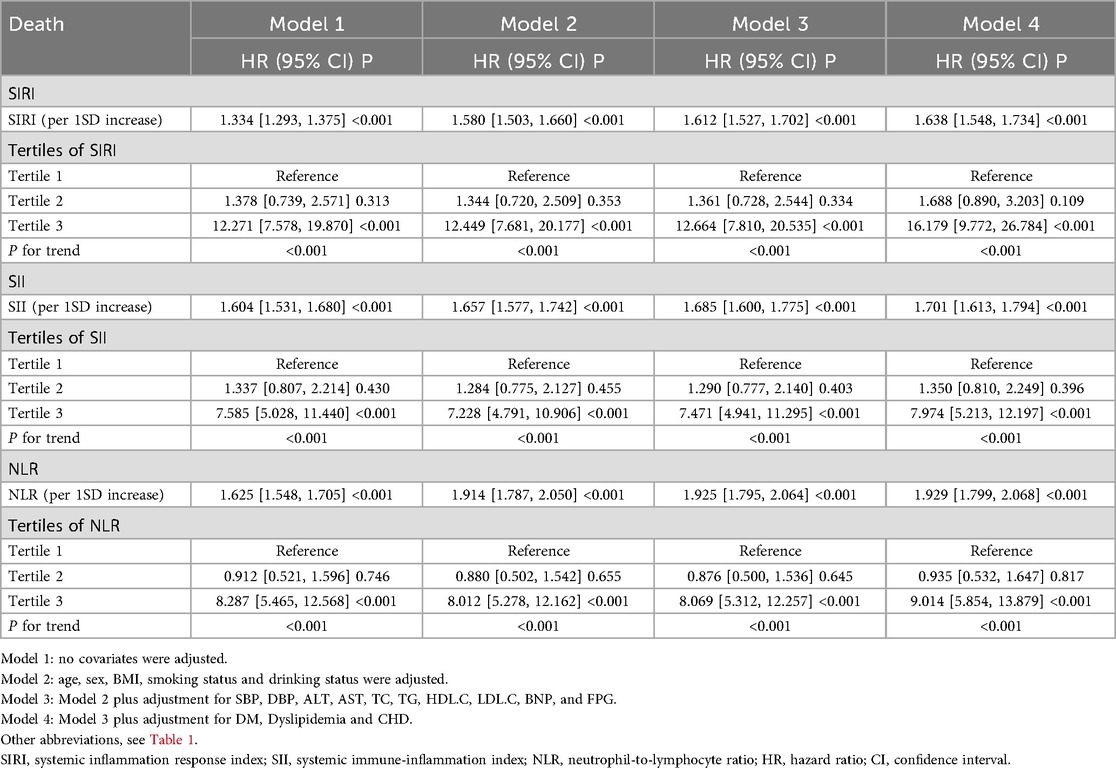
Table 3. Relationship between various novel inflammatory markers and the 1-year death risk of patients with heart failure.
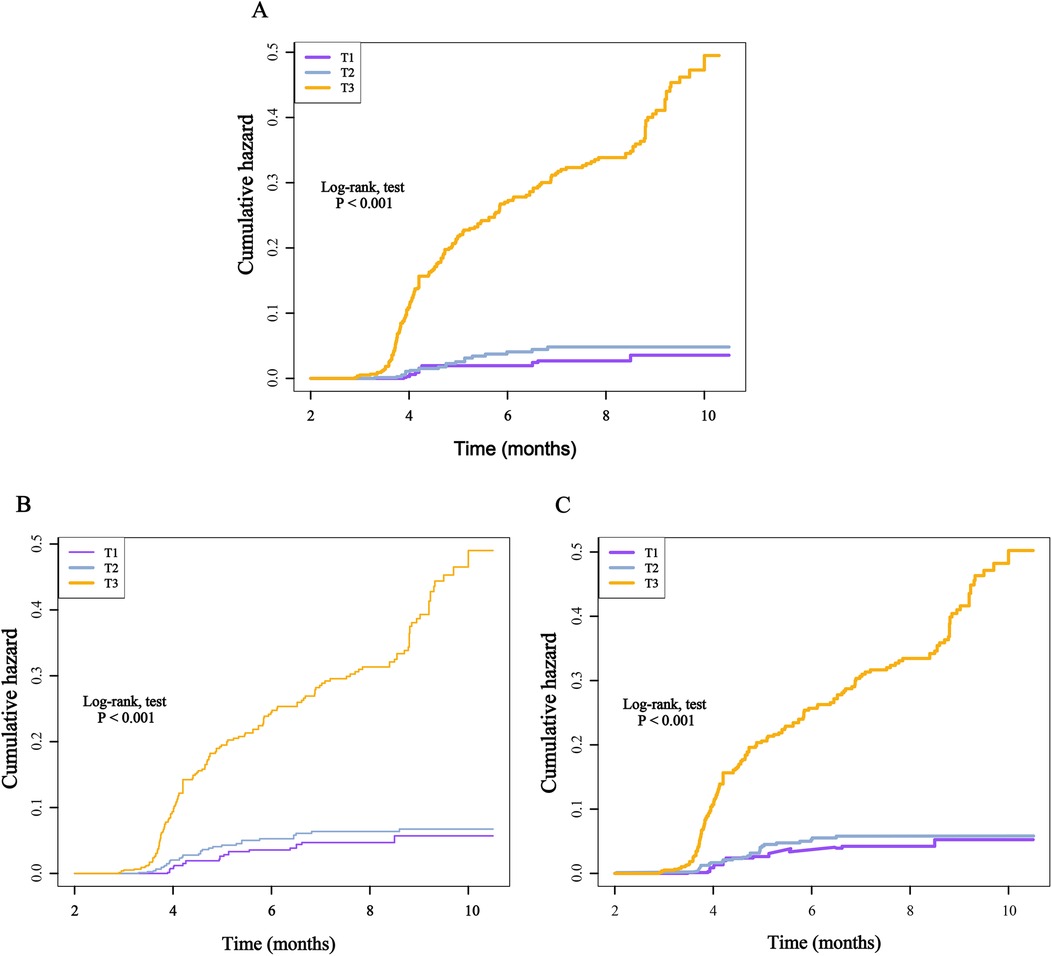
Figure 6. Kaplan–Meier cumulative risk curve of death within one year based on the tertiles of different inflammatory markers. (A), SIRI; (B), SII; (C), NLR.
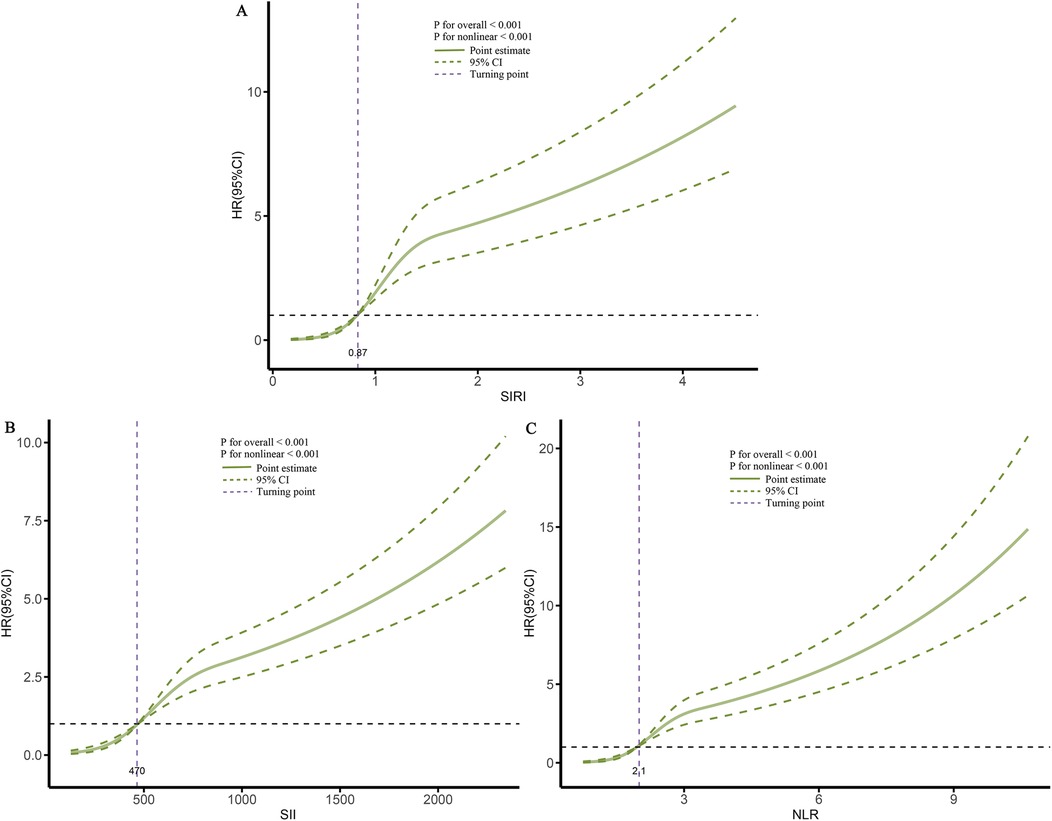
Figure 7. Dose-response association between different inflammatory markers and the risk of death. (A), SIRI; (B), SII; (C), NLR.
In summary, these results reinforce the conclusion that higher levels of inflammatory markers are associated with an increased risk of adverse outcomes. This suggests that controlling inflammatory levels could potentially prevent and reduce adverse outcomes in patients with HF, thereby improving their quality of life and prognosis.
3.5 Comparative analysis of the predictive performance of various novel inflammatory markers for adverse outcomes
To compare the predictive capabilities of different inflammatory markers for adverse outcomes, we first analyzed the area under the curve (AUC) of each indicator. The results showed that all three markers exhibited good predictive performance. Specifically, for predicting the risk of re-hospitalization within one year, the AUCs of SIRI, SII, and NLR were 0.747, 0.693, and 0.698, respectively, with SIRI demonstrating relatively better predictive ability (Table 4). For predicting death risk, the AUC of SIRI reached 0.866, which was significantly higher than that of the other inflammatory markers (Table 4). In the time-dependent ROC analysis, SIRI consistently outperformed the other indicators, showing superior predictive performance for both readmission and death risk (Figure 8).
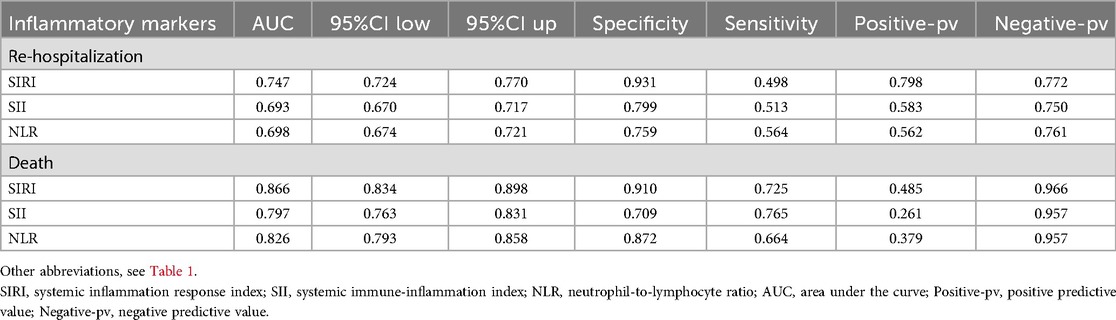
Table 4. Comparative analysis of ROC curves for various novel inflammatory markers in predicting adverse outcomes within one year in patients with heart failure.
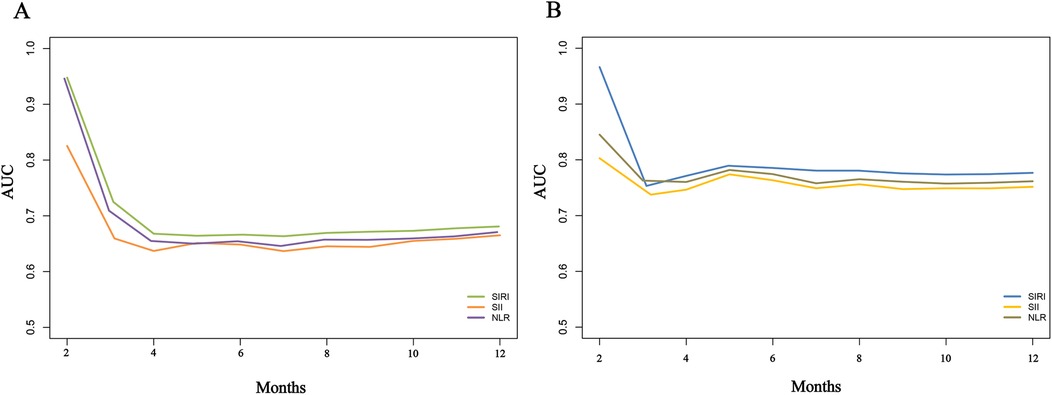
Figure 8. Time-dependent ROC curves for comparing the predictive value of different inflammatory markers for adverse outcomes in heart failure patients. (A), Re-hospitalization; (B), death.
Finally, based on the fully adjusted Model 4, we conducted a C-statistic analysis and added SIRI, SII, and NLR to the model sequentially. The C-statistics for one-year readmission outcomes were 0.751, 0.737, and 0.740, respectively, while the C-statistics for one-year death outcomes were 0.805, 0.791, and 0.801. These results indicated that while all markers demonstrated good predictive capabilities, SIRI still showed the best performance (Table 5).
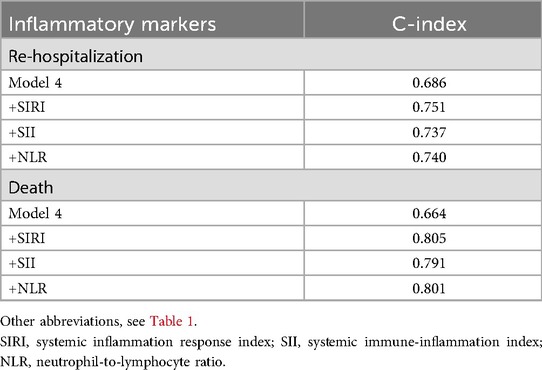
Table 5. Comparative analysis of various novel inflammatory markers in predicting adverse outcomes within one year for patients with heart failure.
In conclusion, these comparative analyses further suggest that SIRI, as a systemic inflammatory response index, holds significant clinical value as an indicator for assessing future adverse outcomes in patients with HF.
4 Discussion
In this longitudinal cohort study, we investigated the relationship between three novel inflammatory markers (SIRI, SII, NLR) and adverse outcomes in HF patients within one year. The Cox regression analysis showed that elevated levels of these inflammatory markers were significantly associated with an increased risk of adverse outcomes. Even after fully adjusting for various confounding factors, the results remained consistent. Additionally, the dose-response relationship, established using RCS, confirmed this trend. The KM survival curve demonstrated that the T3 group, with higher inflammatory levels, had a significantly greater risk of adverse outcomes compared to the T1 and T2 groups, which had lower levels of inflammation. Furthermore, we compared the predictive performance of the three inflammatory markers using single ROC curves, time-dependent ROC curves, and C-statistics. The results showed that the SIRI exhibited the best predictive ability, further confirming its reliability as a predictor of adverse outcomes in HF patients. Based on these results, positioning SIRI as a useful biomarker for assessing the risk of adverse outcomes in HF patients holds significant clinical value. Moreover, early control of inflammatory levels may help prevent or delay the onset of adverse outcomes, offering potential intervention strategies for clinical practice.
HF is a condition caused by the dysfunction of the heart's pumping ability, preventing it from supplying the body with adequate blood, which leads to a range of clinical symptoms (1). Due to its high prevalence and mortality rate, HF has become a major public health concern. Global epidemiological studies indicate that the incidence of HF is rising annually, particularly in the context of an aging population, resulting in an increasing disease burden (3, 19, 20). According to the World Health Organization (WHO), HF holds a significant position among all CVD, with its prevalence reaching 20%–30% in the elderly population. This figure continues to rise, especially in underdeveloped regions and developing countries (21, 22). Furthermore, HF patients often have multiple comorbidities, including hypertension, diabetes, chronic obstructive pulmonary disease, and chronic kidney disease. These comorbidities not only increase the complexity of the disease but also significantly affect treatment outcomes (19, 23–25). Given the high risk and complexity associated with HF, identifying effective predictive markers and implementing early preventive measures are crucial to delaying or preventing adverse outcomes.
Inflammation, as a protective response of the body to injury, infection, or stimulation, plays a crucial role in the development of various diseases. Studies have shown that the inflammatory response can damage vascular endothelial cells, increasing the risk of cardiovascular and cerebrovascular diseases (6, 26). Chronic inflammation, in particular, can stimulate the immune and humoral regulatory systems, leading to immune imbalance or overactivation, which increases the risk of autoimmune diseases (27–29). Additionally, inflammation is commonly observed across multiple organ systems, such as in liver cirrhosis, pulmonary fibrosis, and myocardial fibrosis, all of which are closely associated with inflammation (6, 9, 29).
Given the critical role of inflammation in these diseases, novel inflammatory markers derived from whole blood cells—such as SIRI, SII, PLR, NLR, and MLR—have gained widespread attention. These markers are not only easy to calculate and obtain but also demonstrate superior predictive performance (9, 12, 13, 30). For example, a large-scale study by Shen et al. highlighted the significant role of SIRI, SII, and NLR in diseases such as hypertension and fatty liver, promoting liver degeneration and even cirrhosis (9). Similarly, inflammation plays an essential role in bone loss and osteoporosis. A study from Xinjiang, China, confirmed that elevated SIRI levels are closely linked to the development of osteoporosis, suggesting that controlling inflammation could help prevent bone loss and damage (12). Inflammation is also crucial in CVD. A cohort study demonstrated that SIRI outperforms other indicators in predicting the risk of stroke in patients with hypertension, showing excellent predictive ability (14). Additionally, in sepsis research, SIRI was found to be superior to traditional indicators, such as white blood cells, procalcitonin, and CRP, in predicting patient mortality (31). Our findings are consistent with these results: as the levels of inflammatory markers increase, the risk of adverse outcomes in HF patients rises significantly. Among the three inflammatory markers, SIRI demonstrates particularly strong predictive ability. In summary, these studies collectively demonstrate that new inflammatory markers offer a more comprehensive reflection of the body's inflammatory state compared to traditional indicators. This discovery has significant clinical implications: it provides a valuable tool for monitoring inflammation levels in patients, enabling early prevention and intervention in disease progression, and reducing the occurrence of adverse outcomes.
Inflammation plays a complex and diverse role in the onset and progression of HF, with its mechanism involving multiple interacting aspects. First, inflammation promotes myocardial injury and remodeling: the inflammatory response induces cardiomyocyte apoptosis and fibrosis. This process accelerates ventricular remodeling, impairs the heart's pumping capacity, and thus drives disease progression (32–34). Second, elevated inflammatory mediators cause myocardial cell injury and necrosis (34, 35). In HF, multiple inflammatory factors—such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-1β, and CRP—are increased. These factors participate in the inflammatory process and also affect myocardial cell function and survival, contributing to enhanced cell death (35–38). Moreover, the inflammatory response can worsen coronary microcirculation disorders through oxidative stress and endothelial dysfunction, aggravating myocardial ischemia and energy metabolism disturbances (39, 40). In acute decompensated HF, higher levels of inflammatory markers (e.g., CRP, galectin-3) correlate with clinical deterioration, possibly by activating systemic immune responses and promoting fluid retention and insufficient organ perfusion (39–43). Studies indicate that persistently elevated inflammatory factors independently predict HF readmission and all-cause mortality and may relate to the progression of multiorgan failure and cachexia (44, 45). Additionally, inflammation activates the immune system: HF patients often exhibit systemic inflammatory activation, which can affect other organs (such as the kidneys and liver) and lead to multisystem dysfunction (45–47). In advanced HF, the role of inflammation becomes even more crucial. Inflammation can amplify cardiomyocyte apoptosis and necrosis, promote fibrosis, and reduce cardiac elasticity, thereby exacerbating endothelial dysfunction and hemodynamic instability (48–51). Collectively, these interconnected mechanisms contribute to adverse outcomes and increased risk of death.
The advantage of this study is that it is the first to perform a comprehensive statistical analysis based on a large sample and rich clinical data, enabling a deep exploration of the relationship between different novel inflammatory markers and the risk of adverse outcomes in patients with HF. In the comparative analysis, we identified the most effective clinical predictive indicators, which hold significant implications for future clinical monitoring of inflammatory conditions in HF patients as well as for early prevention and intervention of adverse outcomes. Of course, despite these advantages, the study has some limitations. First, we only collected baseline blood test information, and laboratory values may fluctuate due to various factors; future studies should collect multiple blood test data over time to validate and refine these findings. Second, the study population was drawn entirely from southwest China, and the gender distribution was skewed toward females; caution is needed when generalizing the results to other regions or to different gender compositions. Finally, we did not collect data on the use of anti-inflammatory drugs and some novel therapies for HF, as this could affect the study results; future research should address this gap.
5 Conclusion
This study is the first to systematically clarify the close correlation between multiple novel inflammatory markers and adverse outcomes in HF patients within one year. The results show that increases in inflammatory marker levels are significantly associated with a higher risk of adverse outcomes. Moreover, comparative analysis shows that SIRI has the best predictive performance in this study's data analysis. This finding highlights the potential value of SIRI for clinical monitoring of HF patients and for the early identification of high-risk individuals. At the same time, the findings suggest that actively managing inflammation in clinical practice may help prevent or slow the progression of adverse outcomes and thereby improve patients' prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was conducted in accordance with the principles of the Helsinki Declaration and received approval from the Ethics Committee of the First People's Hospital of Jintang County (No. 20190913001). All participants provided written informed consent to participate in this study.
Author contributions
ZZ: Data curation, Writing – original draft, Methodology, Investigation, Software, Writing – review & editing, Formal analysis, Conceptualization. CY: Methodology, Data curation, Writing – review & editing, Conceptualization. TL: Writing – review & editing, Methodology, Data curation, Conceptualization. FL: Formal analysis, Writing – review & editing, Conceptualization, Software. GL: Software, Investigation, Writing – review & editing, Data curation. YH: Data curation, Writing – review & editing, Investigation. JL: Investigation, Conceptualization, Writing – review & editing, Methodology, Software, Data curation. XL: Project administration, Conceptualization, Investigation, Methodology, Writing – review & editing, Data curation, Software.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1683273/full#supplementary-material
References
1. Boorsma EM, Ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol. (2020) 17(10):641–55. doi: 10.1038/s41569-020-0379-7
2. Greene SJ, Fonarow GC, Butler J. Risk profiles in heart failure: baseline, residual, worsening, and advanced heart failure risk. Circ Heart Fail. (2020) 13(6):e007132. doi: 10.1161/circheartfailure.120.007132
3. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. (2007) 93(9):1137–46. doi: 10.1136/hrt.2003.025270
4. Mbanze I, Spracklen TF, Jessen N, Damasceno A, Sliwa K. Heart failure in low-income and middle-income countries. Heart. (2025) 111(8):341–51. doi: 10.1136/heartjnl-2024-324176
5. Joseph P, Roy A, Lonn E, Störk S, Floras J, Mielniczuk L, et al. Global variations in heart failure etiology, management, and outcomes. JAMA. (2023) 329(19):1650–61. doi: 10.1001/jama.2023.5942
6. Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. (2020) 17(5):269–85. doi: 10.1038/s41569-019-0315-x
7. Thorp EB, Filipp M. Contributions of inflammation to cardiometabolic heart failure with preserved ejection fraction. Annu Rev Pathol. (2025) 20(1):143–67. doi: 10.1146/annurev-pathmechdis-111523-023405
8. Sinha G. Doubt cast on inflammation’s stop signals. Science. (2022) 376(6593):565–6. doi: 10.1126/science.abq8310
9. Shen D, Cai X, Hu J, Song S, Zhu Q, Ma H, et al. Inflammatory indices and MAFLD prevalence in hypertensive patients: a large-scale cross-sectional analysis from China. J Inflamm Res. (2025) 18:1623–38. doi: 10.2147/jir.S503648
10. Chen R, Zhang H, Tang B, Luo Y, Yang Y, Zhong X, et al. Macrophages in cardiovascular diseases: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. (2024) 9(1):130. doi: 10.1038/s41392-024-01840-1
11. Libby P, Kobold S. Inflammation: a common contributor to cancer, aging, and cardiovascular diseases-expanding the concept of cardio-oncology. Cardiovasc Res. (2019) 115(5):824–9. doi: 10.1093/cvr/cvz058
12. Ma H, Cai X, Hu J, Song S, Zhu Q, Zhang Y, et al. Association of systemic inflammatory response index with bone mineral density, osteoporosis, and future fracture risk in elderly hypertensive patients. Postgrad Med. (2024) 136(4):406–16. doi: 10.1080/00325481.2024.2354158
13. Tuzimek A, Dziedzic EA, Beck J, Kochman W. Correlations between acute coronary syndrome and novel inflammatory markers (systemic immune-inflammation index, systemic inflammation response index, and aggregate index of systemic inflammation) in patients with and without diabetes or prediabetes. J Inflamm Res. (2024) 17:2623–32. doi: 10.2147/jir.S454117
14. Cai X, Song S, Hu J, Wang L, Shen D, Zhu Q, et al. Systemic inflammation response Index as a predictor of stroke risk in elderly patients with hypertension: a cohort study. J Inflamm Res. (2023) 16:4821–32. doi: 10.2147/jir.S433190
15. Mutailifu S, Zhu Q, Wang M, Zhang D, Song S, Li N. Association between lactate dehydrogenase/albumin ratio and in-hospital mortality in patients with acute aortic dissection. J Inflamm Res. (2025) 18:6281–92. doi: 10.2147/jir.S515010
16. Chen G, Tan C, Liu X, Chen Y. Association between the neutrophil-to-lymphocyte ratio and diabetes secondary to exocrine pancreatic disorders. Front Endocrinol (Lausanne). (2022) 13:957129. doi: 10.3389/fendo.2022.957129
17. Xu JP, Zeng RX, Zhang YZ, Lin SS, Tan JW, Zhu HY, et al. Systemic inflammation markers and the prevalence of hypertension: a NHANES cross-sectional study. Hypertens Res. (2023) 46(4):1009–19. doi: 10.1038/s41440-023-01195-0
18. Liu X, Li X, Chen Y, Liu X, Liu Y, Wei H, et al. Systemic immune-inflammation Index is associated with chronic kidney disease in the U.S. population: insights from NHANES 2007–2018. Front Immunol. (2024) 15:1331610. doi: 10.3389/fimmu.2024.1331610
20. Osmanska J, Jhund PS. Contemporary management of heart failure in the elderly. Drugs Aging. (2019) 36(2):137–46. doi: 10.1007/s40266-018-0625-4
21. Khan MS, Shahid I, Bennis A, Rakisheva A, Metra M, Butler J. Global epidemiology of heart failure. Nat Rev Cardiol. (2024) 21(10):717–34. doi: 10.1038/s41569-024-01046-6
22. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. doi: 10.1093/cvr/cvac013
23. Sudevan R. Socioeconomic determinants of heart failure mortality: lessons from Denmark. Eur J Prev Cardiol. (2021) 28(1):76–7. doi: 10.1093/eurjpc/zwaa042
24. Böhm M, Butler J, Coats A, Lauder L, Mahfoud F, Filippatos G, et al. Empagliflozin in resistant hypertension and heart failure with preserved ejection fraction: the EMPEROR-preserved trial. Eur Heart J. (2025) 46(14):1304–17. doi: 10.1093/eurheartj/ehae938
25. Vivodtzev I, Lacasse Y. Stimulating advances in chronic heart failure and COPD. Chest. (2009) 136(1):5–6. doi: 10.1378/chest.09-0408
26. Hall CJ, Wicker SM, Chien AT, Tromp A, Lawrence LM, Sun X, et al. Repositioning drugs for inflammatory disease—fishing for new anti-inflammatory agents. Dis Model Mech. (2014) 7(9):1069–81. doi: 10.1242/dmm.016873
27. Zarrin AA, Bao K, Lupardus P, Vucic D. Kinase inhibition in autoimmunity and inflammation. Nat Rev Drug Discovery. (2021) 20(1):39–63. doi: 10.1038/s41573-020-0082-8
28. Krainer J, Siebenhandl S, Weinhäusel A. Systemic autoinflammatory diseases. J Autoimmun. (2020) 109:102421. doi: 10.1016/j.jaut.2020.102421
29. Behr J, Salisbury ML, Walsh SLF, Podolanczuk AJ, Hariri LP, Hunninghake GM, et al. The role of inflammation and fibrosis in interstitial lung disease treatment decisions. Am J Respir Crit Care Med. (2024) 210(4):392–400. doi: 10.1164/rccm.202401-0048PP
30. Fan W, Wei C, Liu Y, Sun Q, Tian Y, Wang X, et al. The prognostic value of hematologic inflammatory markers in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost. (2022) 28:10760296221146183. doi: 10.1177/10760296221146183
31. Xu T, Song S, Zhu K, Yang Y, Wu C, Wang N, et al. Systemic inflammatory response index improves prognostic predictive value in intensive care unit patients with sepsis. Sci Rep. (2025) 15(1):1908. doi: 10.1038/s41598-024-81860-7
32. Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res. (2020) 126(6):789–806. doi: 10.1161/circresaha.119.312321
33. Glinton KE, Ma W, Lantz C, Grigoryeva LS, DeBerge M, Liu X, et al. Macrophage-produced VEGFC is induced by efferocytosis to ameliorate cardiac injury and inflammation. J Clin Invest. (2022) 132(9):140685. doi: 10.1172/jci140685
34. Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther. (2020) 34(6):849–63. doi: 10.1007/s10557-020-07071-0
35. Li Z, Ding Y, Peng Y, Yu J, Pan C, Cai Y, et al. Effects of IL-38 on macrophages and myocardial ischemic injury. Front Immunol. (2022) 13:894002. doi: 10.3389/fimmu.2022.894002
36. Zhang L, Zhao S, Wang Y. Diannexin alleviates myocardial ischemia-reperfusion injury by orchestrating cardiomyocyte oxidative damage, macrophage polarization and fibrotic process by TLR4-NF-kB-mediated inactivation of NLRP3 inflammasome. Int Immunopharmacol. (2024) 130:111668. doi: 10.1016/j.intimp.2024.111668
37. Chen L, Mao LS, Xue JY, Jian YH, Deng ZW, Mazhar M, et al. Myocardial ischemia-reperfusion injury: the balance mechanism between mitophagy and NLRP3 inflammasome. Life Sci. (2024) 355:122998. doi: 10.1016/j.lfs.2024.122998
38. Zhang D, He Y, Ye X, Cai Y, Xu J, Zhang L, et al. Activation of autophagy inhibits nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation and attenuates myocardial ischemia-reperfusion injury in diabetic rats. J Diabetes Investig. (2020) 11(5):1126–36. doi: 10.1111/jdi.13235
39. Kinoshita D, Suzuki K, Yuki H, Niida T, Fujimoto D, Minami Y, et al. Sex-specific association between perivascular inflammation and plaque vulnerability. Circ Cardiovasc Imaging. (2024) 17(2):e016178. doi: 10.1161/circimaging.123.016178
40. Nomura CH, Assuncao-Jr AN, Guimarães PO, Liberato G, Morais TC, Fahel MG, et al. Association between perivascular inflammation and downstream myocardial perfusion in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging. (2020) 21(6):599–605. doi: 10.1093/ehjci/jeaa023
41. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352(16):1685–95. doi: 10.1056/NEJMra043430
42. Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, et al. Inflammation, immunity, and infection in atherothrombosis. JACC Review Topic of the Week. J Am Coll Cardiol. (2018) 72(17):2071–81. doi: 10.1016/j.jacc.2018.08.1043
43. Dai C, Zheng J, Qi L, Deng P, Wu M, Li L, et al. Chronic stress boosts systemic inflammation and compromises antiviral innate immunity in Carassius gibel. Front Immunol. (2023) 14:1105156. doi: 10.3389/fimmu.2023.1105156
44. Iwasaki M, Saito J, Zhao H, Sakamoto A, Hirota K, Ma D. Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: molecular mechanisms and implications. Inflammation. (2021) 44(1):13–34. doi: 10.1007/s10753-020-01337-3
45. Polcz VE, Barrios EL, Larson SD, Efron PA, Rincon JC. Charting the course for improved outcomes in chronic critical illness: therapeutic strategies for persistent inflammation, immunosuppression, and catabolism syndrome (PICS). Br J Anaesth. (2024) 133(2):260–3. doi: 10.1016/j.bja.2024.05.005
46. Dharap SB, Ekhande SV. An observational study of incidence, risk factors & outcome of systemic inflammatory response & organ dysfunction following major trauma. Indian J Med Res. (2017) 146(3):346–53. doi: 10.4103/ijmr.IJMR_1538_15
47. Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. (1996) 109(4):1033–7. doi: 10.1378/chest.109.4.1033
48. Palomer X, Román-Azcona MS, Pizarro-Delgado J, Planavila A, Villarroya F, Valenzuela-Alcaraz B, et al. SIRT3-mediated Inhibition of FOS through histone H3 deacetylation prevents cardiac fibrosis and inflammation. Signal Transduct Target Ther. (2020) 5(1):14. doi: 10.1038/s41392-020-0114-1
49. Zhang X, Ji R, Liao X, Castillero E, Kennel PJ, Brunjes DL, et al. MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation. (2018) 137(19):2052–67. doi: 10.1161/circulationaha.117.030486
50. Xiao H, Li H, Wang JJ, Zhang JS, Shen J, An XB, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J. (2018) 39(1):60–9. doi: 10.1093/eurheartj/ehx261
Keywords: heartfailure, inflammatoryMarkers, re-hospitalization, death, cardiovascular diseases
Citation: Zhao Z, Yuan C, Lan T, Liu F, Liu G, He Y, Li J and Liu X (2025) Elevated novel inflammatory markers in heart failure patients are associated with increased risk of adverse outcomes within one year: insights from a longitudinal study. Front. Cardiovasc. Med. 12:1683273. doi: 10.3389/fcvm.2025.1683273
Received: 15 August 2025; Accepted: 11 September 2025;
Published: 25 September 2025.
Edited by:
Emir Begagic, Cantonal Hospital Zenica, Bosnia and HerzegovinaReviewed by:
Emir Becirovic, University Clinical Center Tuzla, Bosnia and HerzegovinaEsra Polat, Gaziantep City Hospital, Türkiye
Copyright: © 2025 Zhao, Yuan, Lan, Liu, Liu, He, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, MTc2OTA1NzExNjFAMTYzLmNvbQ==; Xingjun Liu, bHhqcnVuQDE2My5jb20=
 Zeng Zhao
Zeng Zhao Chunmei Yuan1
Chunmei Yuan1