- 1Department of Anthropology, University of California, San Diego, La Jolla, CA, United States
- 2Archaeology Stable Isotope Laboratory, University of Kiel, Kiel, Germany
- 3Institute for Prehistoric and Protohistoric Archaeology, University of Kiel, Kiel, Germany
- 4Department of Anthropology, University of Wisconsin, Madison, WI, United States
- 5Department of Anthropology, University of Washington, Seattle, WA, United States
- 6Department of Archaeological Sciences, School of Archaeology and Maritime Cultures, University of Haifa, Haifa, Israel
The stable carbon (δ13C) and nitrogen (δ15N) isotope analysis of charred archaeological grains provides a remarkably precise scale of information: the growing conditions under which a plant was cultivated in a single field and season. Here we investigate how the measurement of single individual grains or aggregate “bulk” samples for carbon and nitrogen isotopes impacts how we characterize variation and, consequently, our interpretations of ancient cultivation practices. Using experimentally grown barley (Hordeum vulgare var. nudum), this work investigates δ13C and δ15N intra-panicle variation between both uncharred and charred individual grains from four plants. We found limited intra- and inter-panicle isotopic variation in single-grain isotope values, ca. 0.5‰ in δ13C and ca. 1‰ in δ15N, reemphasizing the degree to which grains are representative of their local growing conditions. To explore the interpretive impact of aggregate versus single-grain isotopic sampling, we measured charred barley recovered from a single storage context excavated from Trench 42 (ca. 1,900 BCE) at Harappa. Aggregate samples of a random selection of Trench 42 barley demonstrated remarkable inter-sample homogeneity, with a < 0.5‰ difference in δ13C and δ15N values, demonstrating aggregate samples capture well a representative isotopic average of a single depositional context. However, the measurement of single grains revealed moderate 2–3‰ variation in δ13C and an outstandingly wide isotopic variation of ca. 8‰ in δ15N values, indicating the degree to which growing conditions varied beyond what the isotope ratios from aggregate samples indicated. These results highlight how decisions in the selection and measurement of archaeological charred grains for isotopic analysis impact data resolution, with profound consequences for understanding past agricultural diversity.
1 Introduction
Over the past 10 years, stable carbon (δ13C) and nitrogen (δ15N) isotope analyses of charred seed remains have become an increasingly important analytical tool used to understand ancient cultivation practices and agricultural systems (Styring et al., 2017a; Bogaard et al., 2019; Ferrio et al., 2020; Li et al., 2022; Nayak et al., 2022). Carbon and nitrogen stable isotope ratios measured from cereal grains, pulses, and other plant parts have revealed the ways in which people managed their plant resources while also providing insights into local environmental conditions that further shaped their plant management practices (Riehl et al., 2008; Riehl, 2012). Isotope analyses of ancient charred seeds have provided crucial insights into how small-scale watering practices, dedicated irrigation, and the application of manure to fields as fertilizer have shaped the cultivation systems developed by hunter-gatherer-cultivators, fisher-farmers, and full-time farmers alike (Ferrio et al., 2005; Fraser et al., 2011; Kanstrup et al., 2011; Bogaard et al., 2013; Stroud et al., 2021). In turn, these essential plant husbandry practices have revealed new insights into how people sustained and increased crop yields (Bogaard et al., 2013; Yang et al., 2022), took up and incorporated novel crops into extant agricultural systems (Li et al., 2022), promoted agricultural intensification and extensification (Styring et al., 2017a), and fostered social inequality and emerging social complexity (Bogaard et al., 2019).
These crucial perspectives into ancient agricultural practices rely on information derived from the isotope analysis of a relatively limited number of carbonized seeds recovered from archaeological deposits. Analyses of ancient carbonized seeds typically involve the selection of ~5 to 25 specimens, each representing the same taxon, recovered from a single archaeological depositional context (Fraser et al., 2011), followed by the aggregation and homogenization of these seeds to form a single pooled sample that is then measured for carbon and nitrogen isotope ratios (Nitsch et al., 2015; Styring et al., 2017b). The rationale underlying this approach is that any isotopic differences between single grains would be effectively averaged to obtain a more robust and representative stable isotope ratio that fully encompasses growing conditions represented by the plant remains recovered from that context (Nitsch et al., 2015; Styring et al., 2017b). The recommendation to aggregate specimens also reflects an analytical choice to ensure the isotope ratio mass spectrometer has an adequate amount of nitrogen (%N) to reliably obtain nitrogen isotope values (Vaiglova et al., 2023). However, ongoing developments in mass spectrometry and higher sensitivity in instrumentation have reduced the amount of nitrogen (%N) required in a carbonized sample, increasing the reliability of carbonized single grain measurements. Despite the increasing viability of conducting stable isotope measurements on single grains, relatively few studies have explored how different sampling strategies in stable isotope analysis impact data output and interpretation (Gavériaux et al., 2022; Gron et al., 2021; Larsson et al., 2019; Lightfoot and Stevens, 2012).
Stable isotope ratios measured from aggregated sets of charred grains effectively trace temporal shifts in agricultural practices that alter water availability and nitrogen levels in soils (Styring et al., 2017b; Bogaard et al., 2019; Riehl, 2020). However, the extraction of isotope values measured from individual grains, rather than a single isotope value that a pooled aggregate “bulk” sample would provide, has considerable potential to more closely explore the diversity between and within ancient agricultural systems and better understand how cultivation practices changed over time in concert with or independently of broader shifts in environmental conditions, socio-cultural dynamics, and modes of economic production. As such, isotopic differences between individual carbonized grains in archaeobotanical assemblages can reveal variation in cultivar watering and manuring practices, differences that may be otherwise obscured in aggregated samples due to the conflation of specimens that may have been farmed at different times or in different fields under dissimilar growing conditions (Lightfoot and Stevens, 2012; Larsson et al., 2019). Recent discussion centered on single-grain and aggregate sampling notes that single-grain measurements are useful for understanding intra-context variability, while aggregate samples are more suitable for primary contexts where archaeobotanical assemblages represent a single year's harvest (Vaiglova et al., 2023). A single-grain isotope value represents highly specific, spatially bounded anthropogenic and environmental inputs that impact the growth of a plant (Bogaard et al., 2007). In contrast, aggregate samples in essence create an isotope average (IA) composed of multiple charred grains, which, even if recovered from the same archaeological context, may have originated from different agricultural fields subjected to widely different cultivation practices or localized growing conditions (Vaiglova et al., 2023). Furthermore, the resulting aggregated IA value would likely erase isotopic variation caused by, for example, differences in water availability between annual harvests (Flohr et al., 2011) or manuring priorities (Styring et al., 2016b), collapsing spatial variation in growing conditions (Lightfoot and Stevens, 2012). Alternatively, an IA may obscure cases where there is, in actuality, very little carbon or nitrogen isotope variation across grains reflecting homogeneity in cultivation practices and growing conditions (Gavériaux et al., 2022). In addition, long-term challenges in consistent reporting of results and scientific reproducibility can arise due to inter-aggregate sample dissimilarities in the numbers of grains (between 5 and 25) that comprise a single aggregate sample (Nitsch et al., 2015; Gron et al., 2021). The isotope value representing an aggregated sample is a mean value without a standard of deviation, standard of error, median, or variance, erasing measures of uncertainty before they can be assessed (Cowgill, 2015; Calin-Jageman and Cumming, 2019; Drennan, 2009; Shennan, 2006). In practice, these pitfalls might be overcome through analysis of large datasets composed of hundreds of aggregate samples, which may reasonably record wide regional and temporal ranges of variation (e.g., Styring et al., 2017a). Alternatively, they may be avoided by sampling depositional contexts that represent a very short formation period, based on the assumption that the recovered archaeobotanical remains would reflect a temporally constrained assemblage, and thus represent a narrow range of growing conditions (Styring et al., 2016b).
Here, we investigate how carbon and nitrogen isotope values measured from single-grains or aggregated sample sets impact the average isotope values and variation represented in a cohesive dataset, and, as such, our interpretations of ancient plant management practices detectable through the stable isotopic record. We first analyze modern barley (Hordeum vulgare var. nudum) grown from an uncontrolled experimental plot, presenting the first analysis of intra-panicle variation of both carbon and nitrogen values from the same panicle. This expands the empirical references for single-grain isotope variation available to archaeologists. These results are then compared with the carbon and nitrogen composition of both charred and uncharred modern grains to understand how representative the isotope values of a single grain may be of its proximate growing conditions, as well as how those values might be transformed through charring. We expand our analysis by directly comparing isotope values measured from ancient single-grain and aggregate samples recovered from the same archaeological context in order to evaluate the variation represented in single-grain and aggregate samples. To this end, we measured carbon and nitrogen isotope ratios of charred single grains and aggregate samples consisting of multiple individual specimens of hulled barley (Hordeum vulgare) from late urban contexts at the Indus civilization site of Harappa, Pakistan (Periods 4/5, 1900–1700 BCE). We examine the results from the single-grain and aggregate samples to compare potential interpretations of each sample set, discussing the implications for understanding past agricultural organization and land use.
2 Isotopic variation in aggregate samples and single grains
Single-grain isotope analyses are increasingly used to make use of limited archaeobotanical material or to understand variation between single archaeological contexts or sites (Riehl et al., 2008; Larsson et al., 2019; Vaiglova et al., 2020; Gron et al., 2021; Li et al., 2022). Isotope data derived from archaeological grains provide invaluable information on anthropogenic and environmental conditions present during crop cultivation. The relationships between watering or manuring conditions and cultivar δ13C and δ15N values have been established through the isotopic analyses of grains collected from experimentally grown plots of wheat, barley, and other crops (Flohr et al., 2011; Fraser et al., 2011; Kanstrup et al., 2011; Wallace et al., 2013; Styring et al., 2016a). In general, the relationship between these inputs and isotope values and variation has been established using aggregate samples of modern grains from such experimental fields, and the interpretative frameworks developed by this work is applied to both single-grain and aggregate archaeological samples, and there remains little direct discussion of the use of single grains over aggregate samples in archaeological contexts (i.e., Vaiglova et al., 2023).
2.1 Carbon variation in aggregate sample and single-grain experimental studies
To investigate the impact of irrigation and aridity on crop carbon isotope values, studies have relied on outdoor growing experiments that correlate broad categories of water availability with δ13C and carbon isotope discrimination (Δ13C) values in aggregate samples (Wallace et al., 2013; Styring et al., 2016a). Crop carbon stable isotope values are primarily influenced by photosynthetic pathway and water availability (Araus et al., 1997a; Cappers and Neef, 2012; Farquhar et al., 1989; Tieszen, 1991; Vogel, 1993), although field proximity to forests where light availability may be lower would also impact crop δ13C values (Van Der Merwe, 1982; van der Merwe and Medina, 1991; Bonafini et al., 2013). Internal stomatal conductance of CO2 also further modifies the carbon isotope discrimination of plants, with water-efficient flora typically exhibiting lower stomatal conductance and/or higher photosynthetic capacity that consequently impart a 13C-enrichment in plant tissues (Ma et al., 2021). This is reflected in the carbon isotopic composition of wheat and barley grown under the same conditions, with barley δ13C and Δ13C values ~1‰ higher than wheat (Flohr et al., 2019; Styring et al., 2016a; Wallace et al., 2013).
Modern aggregate samples show limited carbon isotope variation in crop plants grown in the same field, whereas more significant differences could exist between crops grown in different fields under similar watering conditions (Ferrio et al., 2005; Wallace et al., 2013; Jones et al., 2021). Research establishing the initial frameworks for assessing water availability drew on 168 sample sets, each consisting of ~25 to 50 aggregated grains of wheat and barley grown in arid and semi-arid fields in Span and Syria, that were collected from a mix of rainfed and irrigated water regimes. Aggregated samples of barley harvested in Spain in 2007 and 2008 demonstrated overall low variation in mean Δ13C values. In unirrigated (n = 2) and moderately irrigated (n = 3) barley from a single annual harvest, these differences were extremely limited (2008: 18.9 ± 0.2‰ vs. 18.7 ± 0.5‰), but fully irrigated fields (2007: n = 6, 2008: n = 3) exhibited higher mean Δ13C values as well as variation (2007: 18.8 ± 0.6‰, 2008: 19.6 ± 1.1‰; Wallace et al., 2013). This higher variation in fully irrigated wheat mean Δ13C values in different years (2007: n = 5, 2008: n = 3) was not as pronounced (2007: 17.81 ± 0.81‰, 2008: 18.14 ± 0.56‰; Wallace et al., 2013). Similar ranges of limited intra-field variation were also observed in aggregate samples (naggregate = 36, each of 50 grains) in plants subjected to three distinct regions with different watering in semi-arid fields in Morocco (Styring et al., 2016a). Two of the regions, Rainfed North and Rainfed South, reflect the overall water availability and precipitation exhibiting average carbon isotope values of −27.6 ± 0.5 ‰ (703 mm) and −23.6 ± 0.6‰ (272 mm), respectively. The irrigated and flood-cultivated Oasis crops exhibited a mean δ13C value of −26.3 ± 0.6‰ with similar watering conditions to Rainfed North. There is extremely limited overall variation between aggregate samples within each region of ± 0.6‰ (Styring et al., 2016a).
Notably, the Δ13C values of aggregate barley (n = 10 grains) grown on unirrigated rainfed fields located in Jordan showed greater carbon isotope differences between harvests carried in separate years but from the same field (n = 3 per year, 2005–2006: 15.4 ± 0.1‰, 2006–2007: 16.5 ± 0.2‰, 2007–8: 17.3 ± 0.1‰; Flohr et al., 2019). In addition to these temporal differences, Jones et al. (2021) documented spatial differences in Δ13C values, up to 2‰, between barley grown in different fields but under similar watering conditions across Northwest India. For 124 aggregate samples (ngrain = 20–30 grains each) from uncontrolled flooded (nsample = 101, 18.0 ± 1.5‰), sprinkler-irrigated (nsample = 19, 16.8 ± 1.4‰), and rainfed fields (nsample = 4, 17.2 ± 2.8‰), this study found that while water availability was the primary driver of carbon isotope variation, the variation between fields with the same watering conditions could be significant (Jones et al., 2021).
Single-grain isotope analysis has found limited intra-panicle and intra-field carbon isotope variation (Heaton et al., 2009). In two bread wheat (Triticum aestivum ssp. vulgare) plants grown in the same uncontrolled field in Nottingham (UK; ngrain = 18), single-grain δ13C values ranged between −26.5 and −27.5‰. In corresponding aggregate samples from the same fields, each consisting of ~300 uncarbonized grains from 6 panicles, yielded δ13C standard deviations between ± 0.37 and ± 0.83‰ (Heaton et al., 2009). These studies have found consistent limited ranges of δ13C and Δ13C variation found in aggregate and single-grain samples of grains grown in the same field and year (Araus et al., 1997b; Styring et al., 2016a; Flohr et al., 2019; Wallace et al., 2013; Araus et al., 2003). However, more notable variation in carbon isotope values is possible between inter-annual harvests from the same fields, as well as spatial variation independent of watering conditions (Wallace et al., 2013; Flohr et al., 2019; Jones et al., 2021). These patterns of carbon isotope variation indicate that information of watering practices may be lost in aggregate samples if grains originate from different fields or years.
2.2 Nitrogen variation in aggregate and single-grain experimental studies
Crop nitrogen isotopic composition is driven by plant uptake of bioavailable nitrogen through either nitrate () or ammonium (; Denk et al., 2017), both of which are affected by manuring and soil moisture (Craine et al., 2015; Szpak et al., 2017). Grain δ15N values reflect nutrient availability during grain-filling, and practices such as manuring enrich soil 15N through the direct addition of ammonia to soils, driving floral nitrogen isotope values up, sometimes considerably (up to 10‰; Fraser et al., 2011; Kanstrup et al., 2011; Szpak, 2014; Craine et al., 2015). In addition to marked 15N enrichment in grain values visible in cultivars grown under manured conditions (Bogaard et al., 2007; Chadwick et al., 2000; Aguilera et al., 2008; Fraser et al., 2013; Styring et al., 2016a), there can be differences in the amplitude of nitrogen isotopic variation for grains from manured plots compared to those from unmanured plots, but this appears to be dependent in part on regional environmental conditions. In controlled experimental plots located in temperate environments in Hertfordshire (UK), aggregate unmanured and manured samples yield widely different δ15N values but similar ranges of variation (δ15Nmanured = 1.9 ± 1.4‰, range = 0.6 to 4.0‰; δ15Nmanured 7.4 ± 1.1‰; Bogaard et al., 2007; Fraser et al., 2013). In contrast, aggregate samples from Morocco exhibited dramatic differences in nitrogen isotope values between manured and unmanured fields, but low nitrogen isotope variation within both unmanured fields (Rainfed North δ15Nmean 0.8 ± 0.6‰) and irrigated manured fields (Oasis δ15Nmean 14.0 ± 1.0‰), but wide variation in rainfed manured fields (Rainfed South δ15Nmean = 7.8 ± 2.1‰).
Analyses of single grains also found slightly greater variation in manured versus unmanured grains (Bogaard et al., 2007; Larsson et al., 2019). In plants from the same fields, single-grain intra-panicle variation in unmanured wheat δ15N values was limited to ± 0.1‰ (range = −0.5 to 0.5‰) while manured grains varied by ± 1.3‰ (range = 5 to 7.5‰; Bogaard et al., 2007). A similar pattern was identified in manured and unmanured experimental plots in Borgeby, Sweden, where intra-panicle variation in δ15N values measured from the single grains of unmanured 2-row hulled barley (Hordeum vulgare ssp. distichon) averaged 5.4 ± 0.6‰ (range = 4.1 to 6.2‰), but in manured grains 8.9 ± 1.6‰ (range = 6.4 to 11.8‰; Larsson et al., 2019). The variation observed between individual grains from the same fields (ca. 1–2‰), and the relatively high variation present between some aggregate sample wheat and barley δ15N mean values, suggests that important information on fertilization practices may be heavily obscured in these pooled samples.
2.3 The impact of charring on charred seed isotope values
Archaeobotanical assemblages are in most cases made up of plant material preserved through charring. There has been much research documenting the impact of carbonization on grain morphology and taphonomic processes that impact preservation (Boardman and Jones, 1990; Charles et al., 2015; Hillman et al., 1993; Pearsall, 2015; van der Veen, 2007). With increasing interest in the use of stable isotope analysis to understand ancient agricultural systems, this work has extended to explore how charring influences original in vivo carbon and nitrogen isotope values through experimental research on modern grains (DeNiro and Hastorf, 1985; Aguilera et al., 2008; Kanstrup et al., 2012; Fraser et al., 2013; Nitsch et al., 2015; Stroud et al., 2023a).
Grains exposed to temperatures above 200°C undergo Maillard reactions that volatilize carbon (C-) and nitrogen (N-) containing compounds as seed starches convert to dextrin (Pazola and Cieslak, 1979; Styring et al., 2013). Grain %C and %N rise by ca. 20 and 2.5%, respectively, while grains lose 20 to 40% of their mass, proportional to temperature (Czimczik et al., 2002; Braadbaart et al., 2004; Kanstrup et al., 2012). Between 230 and 300°C, these reactions lead to some loss of 14N and a corresponding increase in δ15N values (Bogaard et al., 2007; Kanstrup et al., 2012; Styring et al., 2013), while δ13C values remain largely unchanged (Aguilera et al., 2008; Fraser et al., 2011; Nitsch et al., 2015). At temperatures above 300°C, gross deformation in grain morphology occurs, with mass loss over 50% (Boardman and Jones, 1990; Braadbaart et al., 2004; Charles et al., 2015). This is accompanied by significant deviations from in vivo isotope values, with observed shifts of ≥1‰ in δ13C and ≥2‰ in δ15N values (Czimczik et al., 2002; Kanstrup et al., 2012; Fraser et al., 2013; Stroud et al., 2023a).
Many charring studies have compared the mean carbon and nitrogen isotope values between aggregate charred and uncharred samples. In grains charred between 200°C and 300°C, a mean increase of +0.6‰ in the δ15N values of 5 samples each composed of 15 grains was found when compared with uncharred aggregate samples (Kanstrup et al., 2011, 2012). Fraser et al. (2013) charred aggregate samples of 25 to 50 grains at 230°C from plots in Syria, Germany, and the United Kingdom; charred grains yielded higher δ15N values up to +0.8‰ compared to uncharred samples but no notable differences in δ13C values (Bogaard et al., 2007; Fraser et al., 2011).
Other research has assessed charring effects on grain isotope values using statistical correlations or linear regression models to predict isotopic offsets for archaeobotanical grain isotope samples. Aguilera et al. (2008) compared samples of five aggregate wheat or barley grains charred at 250°C with uncharred samples, testing correlations between both to suggest a δ15N offset of +0.68‰ to compensate for charring impacts. Other charring experiments on modern grains applied multiple linear regression models to isotope data sets to predict how time and temperature impact grain δ13C and δ15N values (Nitsch et al., 2015; Stroud et al., 2023a). In unmanured samples of eight taxa, including bread wheat (Triticum aestivum) and hulled barley (Hordeum vulgare), samples of 10 grains were fired between 230 and 300°C between 4 and 24 h and compared with uncharred samples. For every 15°C above 200°C, δ13C values increased by +0.05‰ and for δ15N by 0.12‰, whereas every 4 h of charring resulted in a 0.016‰ increase δ13C, and 0.04‰ for δ15N values (Nitsch et al., 2015; Stroud et al., 2023a). Nitsch et al. (2015) predicted overall isotopic offsets of +0.31‰ in δ15N, and−0.11‰ in δ13C for grains carbonized between 230 and 260°C. Stroud et al. (2023a), expanding and reanalyzing this data, argued for offsets of +0.32‰ in δ15N, and −0.16‰ in δ13C to be subtracted from archaeological wheat and barley grain isotope values (Stroud et al., 2023b). Notably, these valuable charring studies were based on grains randomly selected from multiple plants from a single field, carbonized and aggregated into a single sample, then compared with a corresponding aggregate sample of randomly selected sample of uncharred grains. To date, no analyses have compared the isotopic variation of single grains—either charred or uncharred, within a single panicle.
2.4 Aggregate sampling strategies in archaeobotanical material
Experimental studies conducted on modern uncharred and charred grains have repeatedly demonstrated the viability of correlating carbon and nitrogen stable isotope ratios measured in seeds to cultivation practices and environmental conditions (Ferrio et al., 2005; Bogaard et al., 2013; Wallace et al., 2013; Nitsch et al., 2015; Flohr et al., 2019; Jones et al., 2021). However, most experimental research has either assessed isotopic variability between fields or examined the impact of charring on aggregated samples using modern grains grown under known conditions. Archaeobotanical grains complicate the use of aggregate samples as they cannot be assumed to derive from a single field or set of growing conditions.
The clearest argument making the case for aggregating archaeological grains derives from work applying a multiple linear regression model calculating potential charring offsets (Nitsch et al., 2015; Stroud et al., 2023a). These studies used this model to define the range of carbon and nitrogen isotope variation within a single modern growing context (i.e., a field), and define adequate sample sizes for archaeobotanical stable isotope analysis (Nitsch et al., 2015; Stroud et al., 2023a). The analysis, based on 70 aggregate samples consisting of 10 grains representing eight taxa from unmanured fields, calculated a residual standard error (SE) of ca. 0.25‰ in δ13C and 0.5‰ in δ15N. From this calculation, this work argued that within a 95% confidence interval, any expected variation in grain δ13C and δ15N values from a single field would be ~± 0.5‰ in δ13C and ± 1.0‰ in δ15N (1.96 × SE; Nitsch et al., 2015; Stroud et al., 2023a). Nitsch et al. (2015) hypothesized that decreasing the number of grains homogenized together within a single aggregate sample would in turn increase the standard error, increasing the variation and uncertainty of a single measurement, thereby rendering individual grain isotope values too variable to be interpreted (Stroud et al., 2023a). From these findings drawing on aggregate samples from modern growing contexts, Nitsch et al. (2015) recommended the inclusion of ten (10) archaeological grains from a single archaeological context to create a single aggregated sample. This recommendation was based on their observation that uncertainty at the 95% confidence level decreased with the inclusion of 10 specimens grown under uniform conditions; increasing the sample size beyond this point would unnecessarily consume additional archaeobotanical specimens without further reducing the inherent isotopic variability within an aggregate sample (Nitsch et al., 2015; Stroud et al., 2023b). However, averaged isotope values from aggregated samples from a single field grown in a single season may not be representative of archaeobotanical assemblages of charred seeds that potentially span multiple unknown temporal and spatial scales. This underscores the necessity of single-grain isotope analysis to capture the isotopic variation present in past cultivation practices.
3 Materials and methods
3.1 Barley grains from an experimental plot at Steinzeitpark Dithmarschen
We performed carbon (δ13C) and nitrogen (δ15N) isotope analyses on single grains from the same panicle to better define the range of intra-panicle isotopic variation for unmanured crops growing in a well-watered C3 environment and assess how charring might impact that range in variation. Four panicles of naked barley (Hordeum vulgare var. nudum) were selected from legacy collections generated by growing experiments conducted by Institute of Prehistoric and Protohistoric Archaeology, University of Kiel at Steinzeitpark Dithmarschen, Albersdorf, Germany (Figure 1). The ca. 5 m × 5 m plot lies on the grounds of the Steinzeitpark Dithmarschen, with sandy clay soils that have not been manured or received fertilizer since at least 2005, when the park was founded (Burbaum et al., 2019; Beuker, 2020). The barley was sown by hand in April 2017, left to grow with no intervention until harvest, and received no manure or additional water beyond environmental precipitation of ca. 315 mm (Deutscher Wetterdienst., 2024), with mature ripe plants harvested in August 2017. Each panicle was assigned a letter (A, B, D, E). Grains on each panicle were sequentially numbered in ascending order, from the base to the apex, recording their relative position (Figure 2). All grains from two panicles, D (n = 33) and E (n = 30), were individually measured for carbon and nitrogen isotopes in order to assess intra-panicle isotopic variation within and between single inflorescences. These uncarbonized grains were ground individually into powder using an agate mortar and pestle and then weighed (2.5 mg) into tin capsules for carbon and nitrogen isotope ratio mass spectrometry.

Figure 2. Example grain numbering of Hordeum vulgare var nudum Panicle A from Steinzeitpark Dithmarschen.
Plant remains recovered from archaeological contexts are typically preserved through exposure to fire (Gallagher, 2015). Studies of charring impacts on grain stable isotope values using aggregate samples have found minimal increases to grains charred between 230 and 300°C (Fraser et al., 2013; Vaiglova et al., 2014). To assess how charring potentially impacts the range of intra-panicle isotopic variation, we measured individual grains subjected to a range of charring conditions. Four unaltered grains each from panicles A and B were selected for mass spectrometry and set aside. Additional 26 grains from panicles A and B were individually carbonized in both anoxic and oxidizing conditions at 230°C (n = 8), 300°C (n = 16), and 400°C (n = 4) in a muffle furnace (Table 1, Supplementary material 1). Grains carbonized in anoxic conditions were individually wrapped in aluminum foil, with each foil packet then placed together in an enclosed ceramic crucible. Grains carbonized in oxidizing conditions were placed individually in an open ceramic crucible. All uncarbonized and carbonized grains were ground individually into powder using an agate mortar and pestle and then weighed (2.5 mg) into tin capsules for carbon and nitrogen isotope ratio mass spectrometry.
3.2 Archaeological grains from a single context at Harappa, Trench 42
We expand our exploration of single-grain isotope analysis by examining the degree to which carbon and nitrogen stable isotope ratios derived from an aggregated pool of single grains encompass the range of isotopic variation exhibited by those single grains. This is done through the stable isotope analysis of aggregated and single-grain sample sets all obtained from the same primary archaeological context. Charred samples of hulled barley (Hordeum vulgare) were collected from a burned storage bin made of wattle and daub discovered by the Harappa Archaeological Project (HARP) in the eroded upper levels of Trench 42, on Mound AB at the Indus site of Harappa, Pakistan (Figure 1, ca. 1900–1700 BCE; Meadow and Kenoyer, 2001). This charred bin and associated wash layers contained thousands of charred barley grains (Meadow and Kenoyer, 2001). Cemetery H ceramics associated with the context and two samples of charred barley that have been radiocarbon dated confirm that these samples date to Periods 4/5 ca. 1900–1700 BCE (Meadow and Kenoyer, 2001).
Covering over 150 ha in size, Harappa was a large urban center that may have supported a population of over ca. 40,000 people (Kenoyer, 2008; Kenoyer and Meadow, 2016). Harappa was linked with other Indus cities by trade and a regionally integrated material culture that included similar ceramic traditions and systems of weights (Kenoyer, 1997; Possehl, 2002), set within a mosaic of dense rural settlements spanning a wide range of environmental conditions (Weber et al., 2010a; Petrie and Bates, 2017). Harappa is located adjacent to a paleochannel of the Ravi River and today receives ca. 250 mm annual precipitation with ca. 90 mm falling between November to April and ca. 160 mm between May to October (Pendall et al., 1990). Like other Indus sites, Harappa agricultural systems were structured by the South Asian monsoon (Bates, 2022; Clift and d'Alpoim Guedes, 2021; Weber, 2003; Fuller, 2006). Traditional farming practices for the region take advantage of seasonal precipitation that support two cropping seasons: the summer (kharif) season when crops such as millets and grams are grown during the June to October monsoonal season, and the winter (rabi) seasons when barley, wheat, and lentils are grown between November to April (Cappers et al., 2016; Miller, 2006). Harappan agriculture relied heavily on Rabi cropping, suggested by the high ubiquity, counts, and weight of charred barley, followed by winter grown wheat and lentil grains found in the archaeobotanical assemblage (James et al., 2024; Weber, 2003; Weber et al., 2010a).
Large-grained domesticated barley, the primary crop cultivated throughout the duration of occupation at Harappa, was intensively exploited (James et al., 2024), although millets and grams increased in importance by 2,600 BCE (Weber et al., 2010a,b). Floodplain cultivation or some form of localized irrigation potentially provided barley fields with an additional water source required to meet barley watering requirements (Miller, 2006, 2015; You, 2019). Crop production at Harappa may have been further enhanced through the use of zebu cattle and water buffalo (Meadow, 1996; Patel and Meadow, 2017), which served as a source of manure (Lancelotti, 2018; James et al., 2024), as well as traction, suggested by pathologies on their skeletal extremities (Miller, 2004), and terracotta models of cattle pulling carts and plows (Kenoyer, 2004). Secondary products formed a substantial component of Harappa agricultural systems, with cattle, sheep, and goats also husbanded for their meat and milk (Meadow, 1996; Miller, 2003; Patel and Meadow, 2017).
3.3 Archaeological materials: Trench 42 barley
A total of 46 carbonized grains were selected from the Trench 42 burnt storage feature. Grains were randomly selected and placed into two sample groups, the first representing single grains (S) each individually analyzed for carbon and nitrogen isotope ratios, and the second of aggregated samples (A), representing multiple seeds mechanically homogenized for subsequent isotope analyses. To assess if the range of isotopic variation measured from randomly selected single grains (S1) matched a paired sample of aggregated seeds (A1), 20 grains were collected from the Trench 42 barley.
Ten (S1; grain n = 10) of these grains were each individually ground into a fine powder using an agate mortar with ~2.5 mg of the resulting powder weighed into a tin capsule for single-grain measurements. The remaining 10 grains (total number of grains in sample A1, grain n = 10) were collectively homogenized into a single aggregate sample A1 (total number of grains in sample A1, n = 10). The powder from A1 was measured six times, and weighed into tin capsules (A1; n = 6).
Carbon (δ13C) and nitrogen (δ15N) isotope values and range of isotopic variation exhibited by an aggregated sample, representing multiple barley seeds, were then compared to the isotopic composition of single grains also used to create the aggregate sample sets. Twenty-six single grains (S2, n = 26) were individually homogenized with ~2.5 mg of each grain's powder separately weighed into tin capsules for single-grain stable isotope measurements. The remaining powders from the S1 individual grains were retained and homogenized together into the aggregate sample A2 (total number of grains in sample, n = 26). The powder from A2 was measured six times, with each sample weighed into tin capsules (A2; n = 6).
All archaeological powders were assessed by Attenuated Total Reflectance—Fourier-transform infrared spectroscopy (ATR-FTIR) for contamination and diagenetic alteration (Styring et al., 2013; Vaiglova et al., 2014). All ATR-FTIR data was preprocessed within OPUS software ver 8.1, utilizing the software's built-in baseline corrections and vector normalization. Existing screening protocols rely on observable wavelength peaks indicating high concentrations of contaminating nitrate (1,085 cm−1, 1,450 cm−1, 3,300 cm−1), carbonate (720 cm−1, 870 cm−1), or humic contamination (1,010 cm−1, 1,080 cm−1; Vaiglova et al., 2014; Brinkkemper et al., 2018). No peaks indicating contamination were found, so no pretreatment was conducted.
3.4 Mass spectrometry
Carbon and nitrogen isotope analyses were undertaken at the Archaeology Stable Isotope Laboratory (ASIL), Institute for Prehistoric and Protohistoric Archaeology, University of Kiel. Samples were measured using an isoprime visION continuous flow isotope ratio mass spectrometer coupled to a vario PYRO cube elemental analyzer (Elementar Analysesysteme GmbH, Langenselbold, Germany). Stable carbon and nitrogen isotope values were calibrated relative to the VPDB (δ13C) and AIR (δ15N) scales using the glutamic acid standards USGS40 (δ13C −26.39 ± 0.04‰, δ15N −4.52 ± 0.06‰) and USGS41a (δ13C 36.55 ± 0.08‰, δ15N 47.55 ± 0.15‰; Qi et al., 2003, 2016). Samples were measured in 14 analytical runs. Measurement uncertainty was monitored using wheat and millet internal standards. The isotope values reported here for internal standards represent long-term averages calibrated to VPDB and AIR with USGS40 and USGS41a (Supplementary material 1). Precision [u(Rw)] was ± 0.07‰ for δ13C and ± 0.16‰ for δ15N on the basis of repeated measurements of calibration standards, check standards, and sample replicates. Accuracy [u(bias)] was determined to be ± 0.12‰ for δ13C and ± 0.13‰ for δ15N on the basis of the difference between the observed and known δ values of the check standards and the long-term standard deviations of these check standards. The total analytical uncertainty was ± 0.19‰ for δ13C and ± 0.11 for δ15N (after Szpak et al., 2017).
Carbon isotopes in archaeological grains are most often expressed in analysis as carbon isotope discrimination (Δ13C) to accommodate for differences in past and modern atmospheric carbon dioxide (CO2) concentrations and atmospheric δ13C (Farquhar et al., 1989; Araus et al., 1997a; Wallace et al., 2015; Rosen et al., 2019). Here, all δ13C values measured from archaeological seeds are presented as Δ13C calculated using an estimated δ13Cair of 6.4‰ (Farquhar et al., 1989; Francey et al., 1999; Ferrio et al., 2005). This calculation indicates the carbon isotope values of the archaeological seed in relation to the source of atmospheric CO2 i.e., Δ reflects the difference in δ13C values between the air and plant tissue (Farquhar et al., 1989).
4 Results
4.1 Intra-panicle carbon and nitrogen variation and composition of uncarbonized and carbonized modern barley from a c3 environment
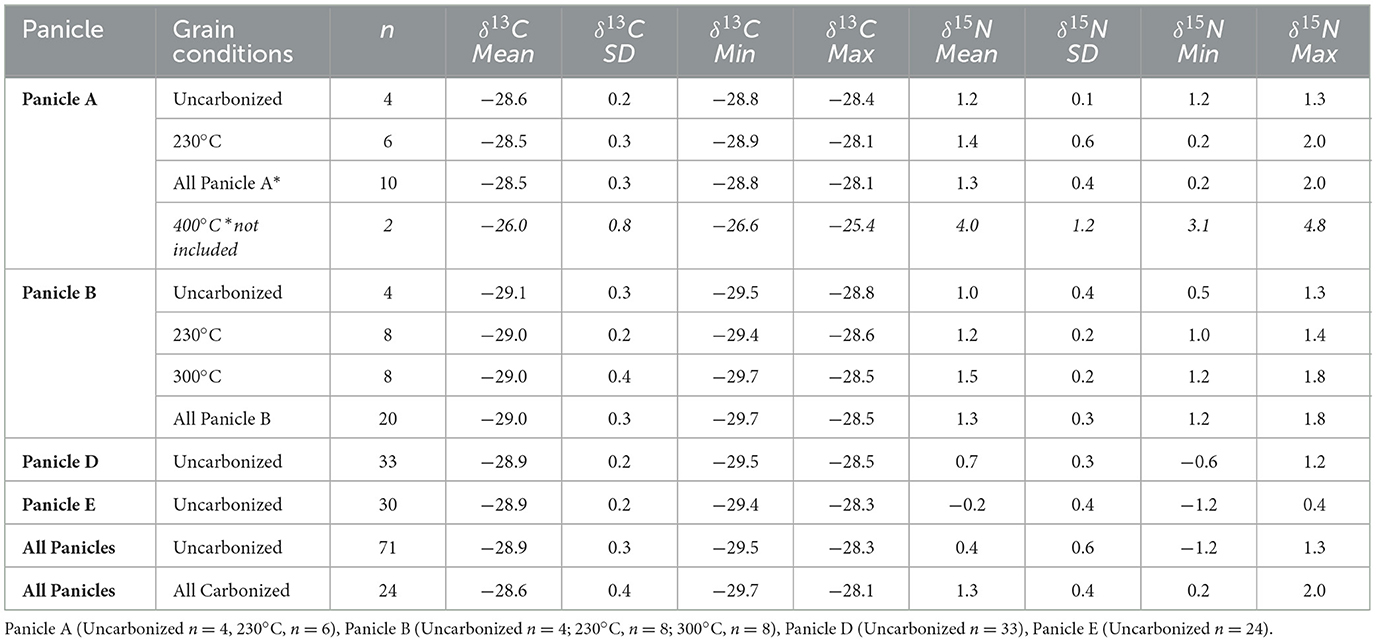
Uncarbonized grains (n = 71) from the four panicles of modern, experimentally grown barley exhibited low variation in δ13C averaging −28.9 ± 0.3‰ (range = −29.5 to −28.5‰; Table 1, Figure 3). The number of grains sampled per panicle influenced the isotopic variation expressed within each panicle. Panicle A (n = 4) yielded on average a δ13C value of −28.7 ± 0.2‰ (range = −28.8 to −28.4‰), Panicle B −29.1 ± 0.3‰ (n = 4, range = −29.5 to −28.8‰), Panicle D (n = 33) −28.9 ± 0.2‰ (n = 33, range = −29.5 to −28.5‰), and Panicle E −28.9 ± 0.2‰ (n = 30, range = −29.4 to −28.3‰).
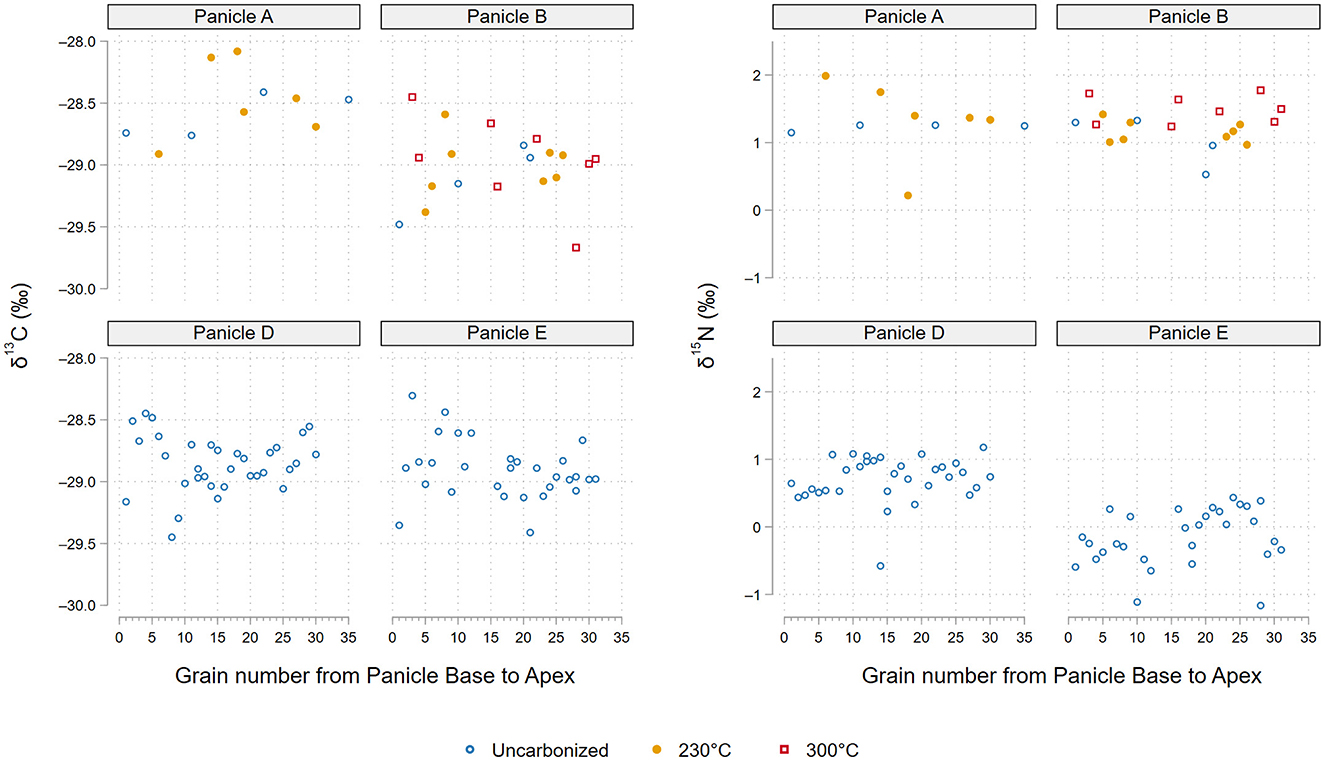
Figure 3. Steinzeitpark Dithmarschen single grain δ13C and δ15N values by panicle. Grains numbered from panicle base to apex.
For nitrogen isotopes, uncarbonized grains yielded an average δ15N value of 0.4 ± 0.6‰ (range = −1.2 to 1.3‰). Panicle A exhibited a mean δ15N value of 1.2 ± 0.1‰ (range = 1.2 to 1.3‰), Panicle B 1.0 ± 0.3‰ (range = 0.6 to 1.0‰), Panicle D 0.7 ± 0.3‰ (range = −0.6 to +1.2‰), and Panicle E −0.2 ± 0.4‰ (range = −1.2 to 0.4‰). No differences in δ13C or δ15N values based on the position of each grain on its respective panicle were observed (Figure 3).
Panicle A grains carbonized at 230°C (n = 6) averaging −28.5 ± 0.3‰ in δ13C (range = −28.9‰ to −28.1‰) were similar in their isotopic composition as uncarbonized grains (Table 1, Figure 4). Panicle B grains carbonized at 230°C (n = 8) also yielded similar carbon isotope values as uncarbonized grains from the same panicle, with an average of −29.0 ± 0.1‰ (−29.4 to −28.6‰). Panicle B grains carbonized at 300°C (n = 8) also yielded similar carbon isotope values as uncarbonized grains averaging −29.0 ± 0.1‰ in δ13C (range = −29.7 to −28.5‰). In nitrogen isotopes, grains from Panicle B carbonized at 230°C (n = 8), seeds averaged 1.2 ± 0.2‰ in δ15N (range =1.0 to 1.4‰), similar to uncharred grains from the same panicle. Panicle B seeds carbonized at 300°C (n = 8) exhibited on average 0.5‰ in δ15N relative to uncarbonized seeds from the same panicle with an average of 1.5 ± 0.2‰ (range = 1.2 to 1.8‰).
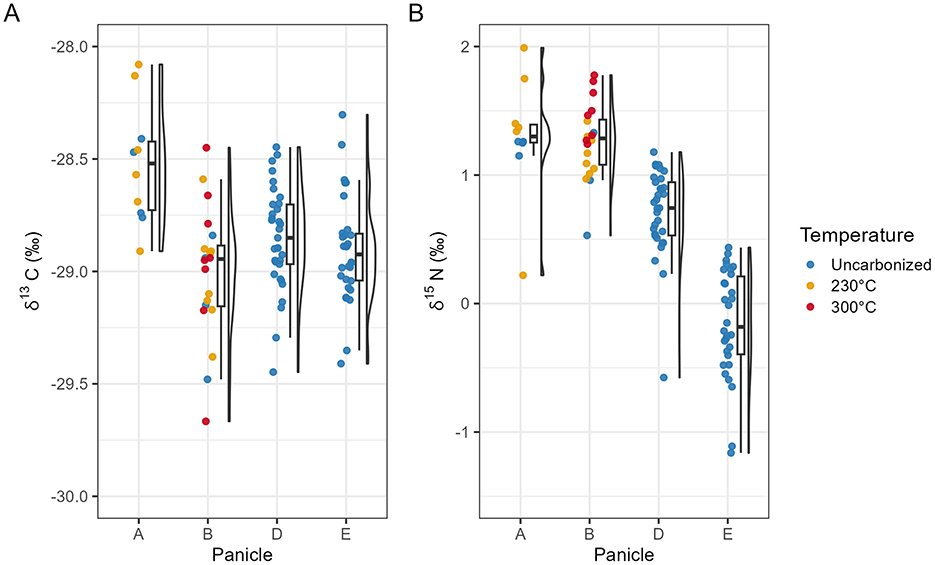
Figure 4. Boxplot of Steinzeitpark Dithmarschen barley isotope data. δ13C (A) and δ15N (B) values by panicle and temperature. Panicle A (Uncarbonized n = 4, 230°C n = 6), Panicle B (Uncarbonized n = 4, 230°C n = 8, 300°C = 8), Panicle D (Uncarbonized n = 33), Panicle E (Uncarbonized n = 24).
Grains carbonized at 400°C (n = 4) from Panicle A exhibited strongly different carbon and nitrogen isotope values with two specimens ashed during the firing process. The grains (n = 2) yielded an average δ13C of −26.0 ± 0.6‰ (range = −26.6 to −25.4‰) and an average δ15N of 4.0 ± 1.2‰ (range = 3.1 to 4.8‰).
Steinzeitpark barley from Panicles A and B carbonized at 230°C and 300°C showed overlapping ranges of carbon and nitrogen isotope variation with the corresponding uncarbonized grains from those panicles. Inter-panicle differences in δ13C were low, but more pronounced in δ15N regardless of charring condition; this is particularly clear in the diverging nitrogen values of Panicle E (Table 1, Figure 4).
4.2 Carbon discrimination (Δ13C) and nitrogen (δ15N) variation in Harappa aggregate and single-grain samples
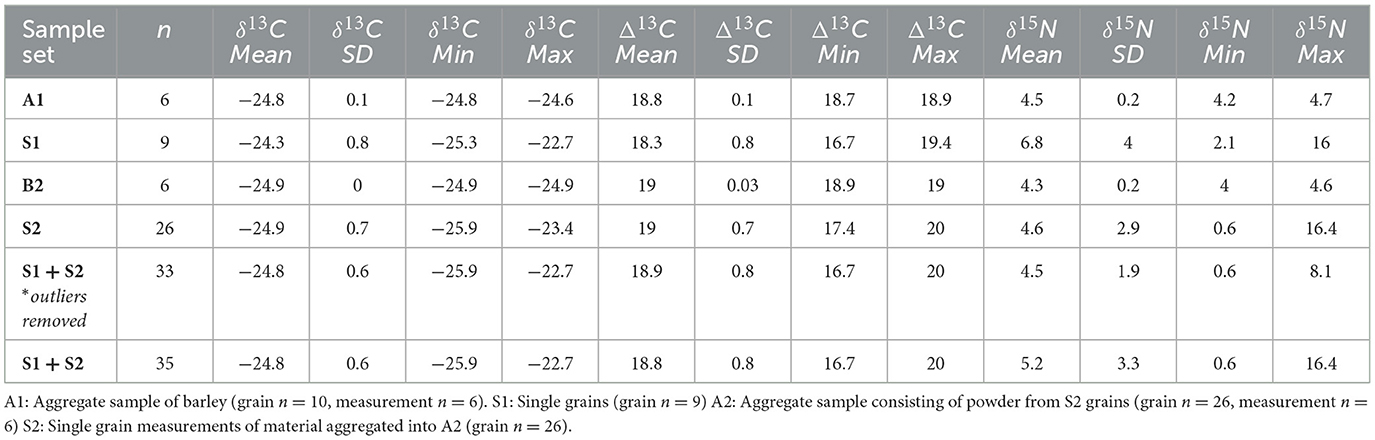
Single-grain barley from Harappa Trench 42 yielded extremely wide variation in single-grain isotope values but notable overlap with isotope values measured from aggregate samples (Table 2, Figure 5). Across the 46 individually sampled grains, one sample failed due to low %N (Supplementary material 1). Single grain measurements (n = 35) averaged 18.3 ± 0.8‰ in Δ13C (range = 16.7 to 20‰) and 5.2 ± 0.6‰ in δ15N. Two grains yielded outstandingly high δ15N values of 16.0 and 16.4‰, respectively; removal of these outliers results for an average δ15N value of 4.6 ± 1.9‰ for single grains (range = 0.6 to 8.1‰; Table 1, Figure 5).
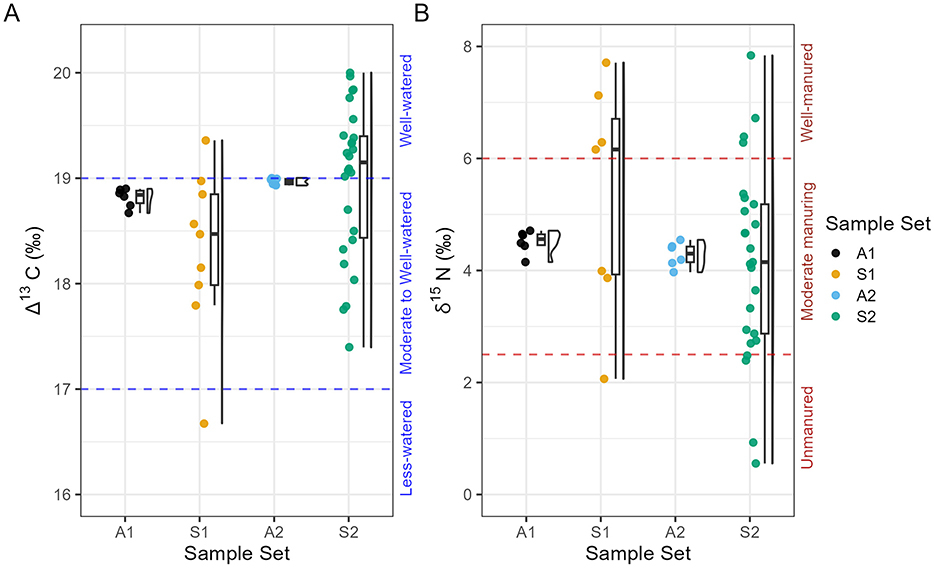
Figure 5. Boxplot of Harappa single-grain and aggregate isotope data. (A) Δ13C values (B) δ15N values. Grains with δ15N values ≥ 8‰ not shown here (n = 2). A1: Aggregate sample of barley (grain n = 10, measurement n = 6). S1: Single grains (grain n = 9). A2: Aggregate sample consisting of powder from S2 grains (grain n = 26, measurement n = 6). S2: Single grain measurements of material aggregated into A2 (grain n = 26).
S1 grains show average Δ13C values of 18.3 ± 0.8‰ (n = 9; range =16.7 to 19.4‰) and δ15N of 6.8 ± 4‰. The corresponding aggregate sample A1 (grain n = 10) shows a mean Δ13C value of 18.8 ± 0.1‰, and δ15N of 4.5 ± 0.2‰, enriched on average 2.3‰ in 15N relative to the S1 seeds. The average carbon discrimination values of S2 and A2 are effectively identical, with slight differences between their average nitrogen isotope values. S2 (n = 26) grains average Δ13C value is 19.0 ± 0.7‰ (range =17.4 to 20‰) and S2 mean δ15N values are 4.6 ± 2.9‰ (range = 0.6 to 16.4‰). The corresponding aggregate sample A2 (grain n = 26) yielded a mean Δ13C of 19.0 ± 0.03‰ and 4.3 ± 0.2‰ in δ15N. There are no significant differences between the single grain or aggregate samples in either Δ13C [Kruskal-Wallis: H (1, 43) = 7.61, p = 0.643] or δ15N values [Kruskal-Wallis: H (1, 43) = 3.59, p = 0.742] (Table 2, Figure 6).
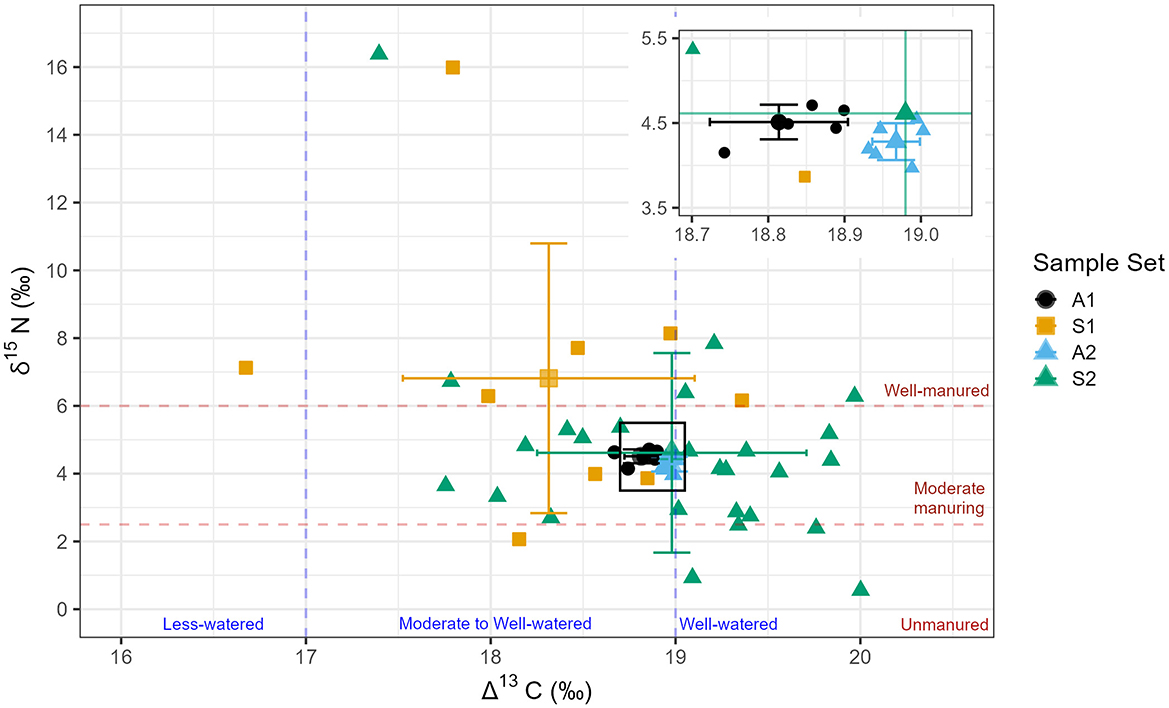
Figure 6. Summary biplot of Harappa single-grain and aggregate Δ13C and δ15N data. Error boundaries are one standard of deviation from each sample set mean. Inset shows A1 and A2 mean value and standard of deviation. A1: Aggregate sample of random grains (grain n = 10, measurement n = 6). S1: Single grains (grain = 9). A2: Aggregate sample consisting of powder from S2 grains (grain n = 26, measurement n = 6). S2: Single grain measurements of material aggregated into A2 (grain n = 26).
5 Discussion
5.1 Single-grain δ13C and δ15N values record their local growing conditions in a temperate environment
The Steinzeitpark Dithmarschen (SD) barley exhibited low variation in both δ13C and δ15N values as expected for C3 crops grown in a temperate environment with ample water and no manuring inputs (Figures 2, 3). For C3 grasses such as wheat and barley, grain δ13C values reflect water availability during crop grain-filling, whether mediated through precipitation, environmental humidity, soil moisture content, or irrigation (Araus et al., 1997a; Cappers and Neef, 2012; Farquhar et al., 1989; Tieszen, 1991; Vogel, 1993). The average δ13C value of Steinzeitpark uncarbonized grains (−28.9 ± 0.3‰) corresponds to crops grown in temperate conditions with ample water (“well-watered” = ≤ −26‰; Kohn, 2010; Wallace et al., 2013). There is also low carbon isotope variation (ca. 0.5‰) between inter-panicle mean δ13C values. Panicle D and E grains exhibit slightly greater ranges in δ13C values than uncarbonized grains from A and B, which is likely due to differences in the sample sizes of uncarbonized grains (Table 1).
The SD single-grain δ13C values display extremely low variation across all four panicles. The limited carbon isotope variation displayed in naked barley is consistent with previous studies examining single-grain intra-panicle variation in temperate grown wheat, which show ca. ± 0.5‰ variation in grains from a single panicle and grains between panicles (Heaton et al., 2009). This homogeneity is also consistent with studies showing ca. ± 0.5‰ δ13C variation between aggregate samples comprised of grains from crop plants from the same field (Nitsch et al., 2015; Wallace et al., 2015; Jones et al., 2021).
Crop nitrogen stable isotope values are influenced by soil conditions, aridity, and manuring intensity. The frameworks used for interpreting crop δ15N values derive from growing experiments where known amounts of manure were applied to fields in arid, semi-arid, and temperate environments δ15N values in England, Germany, Morocco, and Syria. Rather than correlating specific amounts of manure to grain nitrogen values, these frameworks rely on estimated benchmarks that gauge the relationship between grain δ15N values and manuring practices. Unmanured δ15N values fall below ≤ 2.5‰, moderately manured crops range between 2.5 and 6.0‰, and heavily manured crops are ~≥6.0‰ (Bogaard et al., 2007; Fraser et al., 2011, 2013; Kanstrup et al., 2014; Styring et al., 2016a).
The absence of fertilizer in the SD experimental plot in the form of manure or other amendments means bioavailable nitrogen in the low-nutrient sandy soils are driving plant tissue δ15N values (Craine et al., 2015; Szpak, 2014; Denk et al., 2017; Larsson et al., 2024). The low average δ15N value of 0.4 ± 0.6‰ for all uncarbonized grain from the SD plot reflects the lack of direct soil amendments through manuring and low bioavailable soil N (unmanured δ15N = ≤ 2.5‰; Fraser et al., 2011; Styring et al., 2016a; Larsson et al., 2019). This may be due to increased nitrate leaching in the sandy soils, and result in soil nitrogen depleted in 15N (Craine et al., 2015; Takebayashi et al., 2010), possibly leading to the low nitrogen isotope values seen here. The uncarbonized grain mean δ15N values from Panicles A and B exhibit greater similarity (A, n = 4, 1.2 ± 0.1‰; B n = 4, 1.0 ± 0.4‰) to each other than with Panicles D (n = 30, 0.7 ± 1.8‰) or E (n = 33, 0.2 ± 0.4‰) from which a larger number of grains were measured. Grain δ15N values from Panicle D and E display slightly more variation compared to A and B. In particular, Panicle E displays the lowest mean δ15N values, with 16 grains falling below −0.2‰ (Figure 4). The lower nitrogen isotope values of Panicle D and E may be due to slight spatial variation in soil conditions across the cultivation plot, with D and E root systems reflecting particularly low bioavailable nitrogen accessible by each plants root system (Amundson et al., 2003; Craine et al., 2009, 2015). The nitrogen isotope values displayed by Panicle D and E barley notably overlap with archaeological barley δ15N values from the Funnel Beaker Neolithic site Oldenburg LA77, located ca. 100 km away, with δ15N values of 0.1‰ (#264 grain n = 10, δ15N 0.1‰, #10284 grain n = 15, δ15N 0.6‰; Filipović et al., 2019).
These results underscore the minimal variation in carbon and nitrogen isotope ratios among grains from the same field. Aggregate samples of grain from arid rainfed fields in Jordan, Syria, and Spain have demonstrated an ± 1‰ difference in aggregate grain δ13C values from the same field but harvested in different years, attributed to annual differences in precipitation in arid environments (Flohr et al., 2011, 2019; Wallace et al., 2013). These studies highlight that intra-seasonal variation in precipitation, especially in semi-arid environments, in conjunction with anthropogenic watering practices, significantly influence carbon isotopic variation in charred seeds. In addition, they demonstrate how archaeological grains from contexts that were formed over multiple years, if combined into an aggregate sample, may yield a single carbon isotope ratio that erases key information of annual precipitation or water availability. Nitrogen isotope variation between unmanured grains harvested from single fields in England, Germany, Sweden, and Morocco averages ca. ± 1‰, and increases to ca. ± 2‰ in both manured single-grain (Bogaard et al., 2007; Larsson et al., 2019), and manured aggregate samples (Kanstrup et al., 2011; Fraser et al., 2013).
Archaeological studies using single grains have interpreted differences in δ15N values of greater than ≥2‰ between depositional contexts as either grains from crop plants that experienced different growing conditions in different fields (Lightfoot and Stevens, 2012; Larsson et al., 2019), or disparate access to and application of manure (Larsson et al., 2019). The consistent intra-panicle and inter-panicle isotopic differences found in grains from the same field, as shown here and in other studies, demonstrate the interpretive promise of single-grain isotope analysis in archaeological contexts.
5.2 Charring has little impact on carbon and nitrogen isotope values
The minimal impact of charring on the carbon and nitrogen isotopic composition of modern seeds from SD confirms previous research demonstrating that charring between 200 and 300°C leaves grains morphology intact and results in a slight ca. 0.1‰ enrichment in 13C and ca. 0.3‰ in 15N (Fraser et al., 2013; Charles et al., 2015; Nitsch et al., 2015; Stroud et al., 2023a). Grains carbonized at 230 and 300°C from Panicles A and B exhibit nominally more variation in their nitrogen isotopic compositions than uncarbonized grains (Table 1, Figure 4). There were slight incremental increases in the mean δ15N values of grains carbonized at 230 and 300°C in both Panicle A (230°C, n = 4, 1.0 ± 0.2‰; 300°C n = 8, 1.5 ± 0.2‰) and Panicle B (230°C, n = 8, 1.2 ± 0.2‰; 300°C, n = 8, 1.5 ± 0.2‰), but no changes in the overall variation (Table 1, Figure 4). In the four grains from Panicle A carbonized at 400°C, only two yielded enough material unrecognizable as grains, for analysis, and these specimens displayed clear deviations from the mean δ13C values (n = 2, 26.0 ± 0.8‰) and δ15N (n = 2, 4.0 ± 1.2‰). Critically, while there are indications of slightly increased nitrogen isotope variation between grains at higher temperatures, these differences are less than the limited variation displayed between different panicles (Table 1).
5.3 Isotope values measured from aggregate sample sets and individual grains reveal different aspects of Harappa agricultural systems
The aggregate and single-grain data from Trench 42 provides two slightly different interpretations of cultivation practices at Harappa ca. 1900 BCE. The high mean aggregate Δ13C values (A1 18.8 ± 1.5‰; A2 18.9 ± 1.5‰) for Harappa seeds fall within expectations for well-watered barley (≥ 18.5‰; Jones et al., 2021; Styring et al., 2016a; Wallace et al., 2013). Single-grain Δ13C values show only moderate variation (n = 35, 18.8 ± 0.8‰, range = 16.7 to 20.0‰), with most falling within the approximate range corresponding to medium-to-well-watered barley (Δ13C ≥ 17‰), with the exception of a single grain falling into the narrow “medium-to-low” (Δ13C 16 to 17‰) watering threshold (Wallace et al., 2013; Flohr et al., 2019; Jones et al., 2021). These values are similar to δ13C values of aggregated modern barley from Northwest India grown in flooded fields (n = 101, 18.0 ± 1.5‰), as opposed to rainfed (n = 4, 17.2 ± 2.8‰) or sprinkler-irrigated fields (n = 19, 16.8 ± 1.4‰; Jones et al., 2021). The high mean Δ13C values exhibited by single-grain samples are accompanied by relatively limited carbon isotopic variation, indicating that not only were many of the grains from well-watered fields, but also that this water availability was consistently managed (i.e., Styring et al., 2016a). This suggests that at Harappa, water access during the winter growing season was not restricted.
The aggregate mean δ15N values (A1: 4.5 ± 0.2‰; A2: 4.3 ± 0.2‰) fall within the benchmarks of low to medium levels of manuring (2.5 to 6.0‰; Bogaard et al., 2007; Fraser et al., 2011; Styring et al., 2016a), implying that the agricultural soils around Harappa received some manuring amendments, but not in large quantities. In contrast, single-grain δ15N values exhibit wide variation (n = 35, 5.2 ± 3.3‰, 0.55 to 16.38‰). Grain nitrogen isotope values are distributed across unmanured (n = 5, ≤ 2.5‰), low-to-medium (n = 19, 2.5 to 6.0‰), and heavily-manured (n = 11 ≥ 6.0‰) thresholds (Figure 5, Supplementary material; Fraser et al., 2011, 2013; Larsson et al., 2024; Styring et al., 2016a). While many of the grains span low to moderate manuring conditions, there is also the presence of some grains indicating no manuring, whereas a substantial number of grains indicate highly manured fields. This suggests either the ranges of δ15N variation from a single field are greater than might be expected, or that this storage feature held grains from multiple fields subjected to varying manuring practices or growing conditions.
5.4 Aggregate or single-grain stable isotope analysis impacts interpretation of plant management practices
The relationships between crop stable carbon and nitrogen values and agricultural organization hinges on the correlation of manuring and watering inputs to varying labor inputs and land use (Bogaard, 2004; Styring et al., 2017a; Bogaard et al., 2019). The interpretative framework for archaeobotanical stable isotope data guiding this correlation relies on models of labor- and land-limited agriculture derived from ethnographic studies of traditional smallholder farmers in Africa and Europe (Netting, 1993; Halstead, 2014). Labor-limited intensive practices are focused on small households dependent solely on scarce household labor for field management (Bogaard, 2004, 2005; Halstead, 2014). The expectation is that an increase in production under labor-limited conditions would entail high watering and high manuring inputs concentrated into small plots by nuclear households (Bogaard, 2005). These agricultural practices would be reflected in the isotopic composition of cultivated plants visible as high crop Δ13C and δ15N values (Wallace et al., 2013; Styring et al., 2016a). Land-limited extensive practices are associated with the use of plow agriculture, increasing available labor through traction to bring more land under cultivation, but the attendant spatial extensive land use means there are fewer watering, manuring, and labor inputs per field (Styring et al., 2017a). These less intensive applications of water and manuring would translate to a decrease in Δ13C and δ15N values (Styring et al., 2016a).
This framework traces connections between carbon and nitrogen isotope values that may indicate changing agricultural production encompassing a shift from intensive to extensive practices (Bogaard et al., 2013, 2019; Styring et al., 2017b,a; Yang et al., 2022). To date, exploration of land vs. labor-limited agriculture in ancient cultivation systems has relied on isotope values derived from aggregate grain samples. As archaeological specimens could span multiple growing seasons and thus different growing conditions (Riehl, 2020), aggregate samples containing large numbers of seeds attempt to ensure that samples encompass the entire range of variation of growing conditions in a field (Nitsch et al., 2015; Bogaard et al., 2016). Thus, aggregate studies attempt to endeavor to render comparison viable by averaging the isotopic information of all potential cultivation practices or growing conditions represented at a given site (Kanstrup et al., 2012; Styring et al., 2017a).
The Trench 42 aggregate samples (A1 and A2) mean Δ13C values correspond to those expected for moderately to well-watered crops (Jones et al., 2021), while the mean δ15N values fall within the benchmarks of medium to potentially low levels of manuring (Bogaard et al., 2007; Fraser et al., 2011; Styring et al., 2016a). Within the framework of labor- and land-limited agriculture, in conjunction with the widespread evidence of traction and plow agriculture at Harappa, this data suggests limitations on available land as opposed to available labor. It may be that labor was abundant and the cultivation of additional fields gave rise to land becoming more scarce and increasingly valuable (Bogaard, 2005). Therefore, within this framework, the isotope values from aggregate samples broadly correspond to evidence for land-limited extensive agricultural practices at Harappa (Bogaard, 2004; Bogaard et al., 2019).
The overlap between the mean Δ13C and δ15N values of single-grain S1 and S2 and aggregates A1 and A2 indicate that an aggregated sample pool captures a representative signature of cultivation. However, the variation displayed in S2 compared to A2 illustrates how isotope values measured from aggregate samples do not fully describe the full range of isotopic variation expressed in archaeological grains. Along these lines, the carbon and nitrogen isotopic variation shown by individual barley grains (S1 and S2) from Trench 42 potentially complicates an interpretation of land-limited production (Figures 5, 6). The mean Δ13C and δ15N values produced by S1 and S2 are similar to A1 and A2, indicating that the aggregate samples capture an average isotopic variation from a primary context (Figure 5). However, the variation in all single-grain δ15N values from all Trench 42 barley (5.2 ± 3.3‰, range = 0.6 to 16.4‰; Figure 6), is greater than observed intra-field variation in modern manured barley single-grain δ15N values (8.9 ± 1.64‰, range = 6.4 to 11.8‰; Larsson et al., 2019). At the same time, the limited variation in single-grain Δ13C values indicates the consistent watering of cultivars by farmers, or environmental conditions leading to well-watered barley (Δ13C 18.8 ± 0.8‰).
This pattern of constrained Δ13C values and wide δ15N variation at Harappa might be explained in two ways. First, grains from moderately manured fields may express a wider variation in δ15N values than so far documented in experimental plots and archaeological studies, as the irregular distribution of manure and patchy release of nitrogen across a field may be responsible for increased δ15N variation in manured plants (Larsson et al., 2019; Styring et al., 2024). If this is the case, S1 and S2 could represent a single well-watered field, but with varying application of soil amendments leading to wide nitrogen isotope variation in measured grains. Alternatively, this pattern in barley cultivation might indicate active water management which was accompanied with both low- and high-input manuring. Wide ranges of carbon and nitrogen isotope variation resulting from different field conditions might be expected from extensive agricultural practices that bring more land under cultivation (Styring et al., 2017a).
These results underscore the need to further evaluate the isotopic variation expressed in single grains from crops grown in intensive and extensive agricultural systems In particular, additional research exploring single-grain δ15N values for both low manure input fields and intensively manured fields is needed to better characterize how these differences in practice might impact the amplitude of nitrogen isotopic variation observed in charred seeds. What is clear is that single-grain isotope analysis increases the visibility of environmental and anthropogenic sources of uncertainty embedded in archaeobotanical isotope data and has additional implications for sample selection that should be investigated further (Calin-Jageman and Cumming, 2019). It potentially allows for the detection of smaller isotopic effect sizes (i.e., meaningful variation) that might be lost in an aggregate sample. In contrast, aggregate samples, while containing large numbers of grains, effectively create a single average with fewer statistical degrees of freedom, in turn increasing potential uncertainty, and lesser statistical precision. The use of single grains could in turn allow better quantification and interpretation of this uncertainty (Styring et al., 2024).
6 Conclusion: promise and limitations for single-grain isotope analyses
This comparison of single-grain and aggregate isotope values has key implications for archaeobotanical stable isotope analysis. In cases where grain recovered from an archaeological context might represent crops from multiple fields, a wide geographic area, or multiple depositional events spanning more than a single season of growth, an aggregate sample of 10 grains will capture higher isotopic variation than would be expected for seeds grown under a single set of growing conditions (Nitsch et al., 2015; Stroud et al., 2023a). Previous recommendations suggest aggregating samples for the analysis of material from primary depositional contexts (i.e., storage bins), as such contexts are more likely to represent a single year's harvest or set of growing conditions, and thereby are less likely to collapse isotopic variation (Vaiglova et al., 2023). In the case of a secondary depositional context that may represent multiple fields or harvests (i.e., a midden), these recommendations suggest 2–3 aggregate samples of ~10 grains each should be selected for stable isotope analysis to compensate for the potential loss of isotopic variation (Vaiglova et al., 2023). By analyzing charred grain from a depositional storage context, this study suggests that an average isotope value generated from aggregate samples may not encompass the ranges of variation present in archaeobotanical assemblages. By sampling single-grain isotope values researchers can, as with an aggregate sample, generate a representative isotopic average from a single context, in addition to assessing the full range of isotopic variation present in an archaeobotanical assemblage.
The sampling of individual grains for stable isotope analysis is subject to several clear restraints. Limited funding resources, time, or labor may restrict researchers' abilities to measure larger number of individual grains that would fully document the range of isotopic variation represented in an assemblage. Sample screening protocols also impact sampling strategy. Establishing if grain specimens contain humic or fulvic acids, carbonates, or other contaminants from the burial environment, and subsequent determination of wet chemistry pre-treatment protocols, requires for Attenuated Total Reflectance Fourier Transform Infrared Spectrometry (ATR-FTIR) ~1–2 mg of charred sample (Vaiglova et al., 2014; Brinkkemper et al., 2018). Although the amount of sample demands for ATR-FTIR instruments are low, requiring a very small volume of powder to cover the ATR window, single small grains may be consumed by this process. Larger specimens, even if sub-sampled for pre-screening with sufficient material for wet chemistry, may experience more extensive sample loss during the pretreatment phase. For example, mass loss of aggregated powers (ngrain = 5) exposed to 0.5 HCl at 80°C for 30 min followed by three rinses with Milli-U water to remove nitrate, humic, or carbonate contaminates, results in ca. 60% sample loss (Vaiglova et al., 2014).
Despite the promise of the high-resolution isotopic information gained through single-seed analyses, whether or not it is mandatory to analyze single seeds for carbon and nitrogen isotopes depends on the research question at hand (Gron et al., 2021; Gavériaux et al., 2022; Vaiglova et al., 2023). Studies that rely on large, regional-scale comparative datasets to address diachronic change in agricultural extensification/intensification in relation to landscape urbanization, emergent staple finance systems, or climatic change might be best served by an aggregate sampling strategy that would establish broad, settlement level agricultural systems. Tracing intra-settlement diversity in cultivation practices at the household or neighborhood level, or at urban sites such as Harappa, might be better achieved through a single-grain sampling strategy that closely queries intra-contextual variability. Environmental context can also play into decisions whether to employ an aggregate or single-seed sample strategy. The measurement of individual grains for δ13C is especially important in water-limited environments where variation in watering practices and irrigation systems, crop emergence and ripening time, and landscape-dependent soil moisture levels in agricultural plots would impart marked differences in the carbon isotopic composition of cereal crops (Wallace et al., 2013; Styring et al., 2016a; Flohr et al., 2019). Single-grain sampling may be less important in well-watered, temperate environments where carbon isotope variation at the floral base of the food web is less pronounced (Heaton, 1999; Kohn, 2010; Diao et al., 2023).
To further test the viability of single-grain stable isotope analysis, future studies should assess the extent of isotopic variation present across experimentally grown fields, and for archaeobotanical stable isotope data, further query between different sites, context types, and through time.
The overlap in single-grain and aggregate sample mean isotope values demonstrates the effectiveness of previous studies investigations into adequate sample sizes for archaeobotanical isotope analysis (Stroud et al., 2023b; Nitsch et al., 2015). This study presents a path toward exploring the isotopic variation present in archaeobotanical assemblages, and thus expands our understanding of farmers' decision-making within dynamic environmental conditions
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
NJ: Conceptualization, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. CW-S: Data curation, Formal analysis, Writing – review & editing. JK: Resources, Writing – review & editing. JD: Supervision, Writing – review & editing. CM: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, Project Number 390870439–EXC 2150; ROOTS: Social, Environmental, and Cultural Connectivity in Past Societies).
Acknowledgments
We thank Yasmin Dannath for facilitating access to the archived modern grain samples from Steinzeitpark Dithmarschen. We thank Fiona Walker-Friedrichs for laboratory assistance and brainstorming, Damini Pant for her initial comments, and Steve Weber for his insights into the Harappa archaeobotanical collection. This manuscript is available as a preprint on BioRxiv 10.1101/2024.08.15.607704.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fearc.2025.1510394/full#supplementary-material
References
Aguilera, M., Araus, J. L., Voltas, J., Rodríguez-Ariza, M. O., Molina, F., Rovira, N., et al. (2008). Stable carbon and nitrogen isotopes and quality traits of fossil cereal grains provide clues on sustainability at the beginnings of Mediterranean agriculture. Rapid Commun. Mass Spectrom. 22, 1653–1663. doi: 10.1002/rcm.3501
Amundson, R., Austin, A. T., Schuur, E. a. G., Yoo, K., Matzek, V., Kendall, C., et al. (2003). Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 17, 1–5. doi: 10.1029/2002GB001903
Araus, J. l, Febrero, A., Buxo, R., Camalich, M. D., Martín, D., Molina, F., et al. (1997a). Changes in carbon isotope discrimination in grain cereals from different regions of the western Mediterranean Basin during the past seven millennia. Palaeoenvironmental evidence of a differential change in aridity during the late Holocene. Glob. Change Biol. 3, 107–118. doi: 10.1046/j.1365-2486.1997.00056.x
Araus, J. L., Febrero, A., Buxó, R., Rodriguez-Ariza, M. O., Molina, F., Camalich, M. D., et al. (1997b). Identification of ancient irrigation practices based on the carbon isotope discrimination of plant seeds: a case study from the South-East Iberian Peninsula. J. Archaeol. Sci. 24, 729–740. doi: 10.1006/jasc.1997.0154
Araus, J. L., Villegas, D., Aparicio, N., del Moral, L. F. G., El Hani, S., Rharrabti, Y., et al. (2003). Environmental factors determining carbon isotope discrimination and yield in durum wheat under mediterranean conditions. Crop Sci. 43, 170–180. doi: 10.2135/cropsci2003.1700
Bates, J. (2022). “The origins and development of agriculture in South Asia,” in Oxford Research Encyclopedia of Anthropology (Oxford: Oxford University Press). doi: 10.1093/acrefore/9780190854584.013.553
Beuker, J. (2020). “Von Schiffsbohrwurm zum Hunebed Highway. Schutz und touristische Erschließung der niederländischen Megalithgräber,” in Nachrichten des Marschenrates (Wilhelmshaven: Marschenrat zur Förderung der Forschung im Küstengebiet der Nordsee). Available online at: https://d-nb.info/124182388X/34#page=58 (accessed July 6, 2024).
Boardman, S., and Jones, G. (1990). Experiments on the effects of charring on cereal plant components. J. Archaeol. Sci. 17, 1–11. doi: 10.1016/0305-4403(90)90012-T
Bogaard, A. (2004). Neolithic Farming in Central Europe, 1st Edn. London; New York, NY: Routledge. doi: 10.4324/9780203358009
Bogaard, A. (2005). “Garden agriculture” and the nature of early farming in Europe and the near East. World Archaeol. 37, 177–196. doi: 10.1080/00438240500094572
Bogaard, A., Fochesato, M., and Bowles, S. (2019). The farming-inequality nexus: new insights from ancient Western Eurasia. Antiquity 93, 1129–1143. doi: 10.15184/aqy.2019.105
Bogaard, A., Fraser, R., Heaton, T. H. E., Wallace, M., Vaiglova, P., Charles, M., et al. (2013). Crop manuring and intensive land management by Europe's first farmers. Proc. Natl. Acad. Sci. U.S.A. 110, 12589–12594. doi: 10.1073/pnas.1305918110
Bogaard, A., Heaton, T. H. E., Poulton, P., and Merbach, I. (2007). The impact of manuring on nitrogen isotope ratios in cereals: archaeological implications for reconstruction of diet and crop management practices. J. Archaeol. Sci. 34, 335–343. doi: 10.1016/j.jas.2006.04.009
Bogaard, A., Hodgson, J., Nitsch, E., Jones, G., Styring, A., Diffey, C., et al. (2016). Combining functional weed ecology and crop stable isotope ratios to identify cultivation intensity: a comparison of cereal production regimes in Haute Provence, France and Asturias, Spain. Veg. Hist. Archaeobot. 25, 57–73. doi: 10.1007/s00334-015-0524-0
Bonafini, M., Pellegrini, M., Ditchfield, P., and Pollard, A. M. (2013). Investigation of the ‘canopy effect' in the isotope ecology of temperate woodlands. J. Archaeol. Sci. 40, 3926–3935. doi: 10.1016/j.jas.2013.03.028
Braadbaart, F., van der Horst, J., Boon, J. J., and van Bergen, P. F. (2004). Laboratory simulations of the transformation of emmer wheat as a result of heating. J. Therm. Anal. Calorim. 77, 957–973. doi: 10.1023/B:JTAN.0000041672.45140.e9
Brinkkemper, O., Braadbaart, F., van Os, B., van Hoesel, A., van Brussel, A. A. N., and Fernandes, R. (2018). Effectiveness of different pre-treatments in recovering pre-burial isotopic ratios of charred plants. Rapid Commun. Mass Spectrom 32, 251–261. doi: 10.1002/rcm.8033
Burbaum, B., Filipinski, M., and Krienke, K. (2019). Die Böden Schleswig-Holsteins: mit Erläuterungen zur Bodenübersichtskarte 1:250.000. Flintbek: Landesamt für Landwirtschaft, Umwelt und ländliche Räume des Landes Schleswig-Holstein.
Calin-Jageman, R. J., and Cumming, G. (2019). The new statistics for better science: ask how much, how uncertain, and what else is known. Am. Stat. 73, 271–280. doi: 10.1080/00031305.2018.1518266
Cappers, R. T. J., and Neef, R. (2012). Handbook of Plant Palaeoecology. Groningen: Barkhuis. doi: 10.2307/j.ctt20p56g8
Cappers, R. T. J., Neef, R., Bekker, R. M., Fantone, F., and Okur, Y. (2016). Digital Atlas of Traditional Agricultural Practices and Food Processing., 1st Edn. Groningen: Barkhuis.
Chadwick, D. R., John, F., Pain, B. F., Chambers, B. J., and Williams, J. (2000). Plant uptake of nitrogen from the organic nitrogen fraction of animal manures: a laboratory experiment. J. Agric. Sci. 134, 159–168. doi: 10.1017/S0021859699007510
Charles, M., Forster, E., Wallace, M., and Jones, G. (2015). “Nor ever lightning char thy grain”1: establishing archaeologically relevant charring conditions and their effect on glume wheat grain morphology. STAR Sci. Technol. Archaeol. Res. 1, 1–6. doi: 10.1179/2054892315Y.0000000008
Clift, P. D., and d'Alpoim Guedes, J. (2021). Monsoon Rains, Great Rivers and the Development of Farming Civilisations in Asia, 1st Edn. Cambridge: Cambridge University Press. doi: 10.1017/9781139342889
Cowgill, G. L. (2015). Some things I hope you will find useful even if statistics isn't your thing. Annu. Rev. Anthropol. 44, 1–14. doi: 10.1146/annurev-anthro-102214-013814
Craine, J. M., Brookshire, E. N. J., Cramer, M. D., Hasselquist, N. J., Koba, K., Marin-Spiotta, E., et al. (2015). Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396, 1–26. doi: 10.1007/s11104-015-2542-1
Craine, J. M., Elmore, A. J., Aidar, M. P. M., Bustamante, M., Dawson, T. E., Hobbie, E. A., et al. (2009). Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 183, 980–992. doi: 10.1111/j.1469-8137.2009.02917.x
Czimczik, C. I., Preston, C. M., Schmidt, M. W. I., Werner, R. A., and Schulze, E.-D. (2002). Effects of charring on mass, organic carbon, and stable carbon isotope composition of wood. Org. Geochem. 33, 1207–1223. doi: 10.1016/S0146-6380(02)00137-7
DeNiro, M. J., and Hastorf, C. A. (1985). Alteration of 15N14N and 13C12C ratios of plant matter during the initial stages of diagenesis: studies utilizing archaeological specimens from Peru. Geochim. Cosmochim. Acta 49, 97–115. doi: 10.1016/0016-7037(85)90194-2
Denk, T. R. A., Mohn, J., Decock, C., Lewicka-Szczebak, D., Harris, E., Butterbach-Bahl, K., et al. (2017). The nitrogen cycle: a review of isotope effects and isotope modeling approaches. Soil Biol. Biochem. 105, 121–137. doi: 10.1016/j.soilbio.2016.11.015
Deutscher Wetterdienst. (2024). Available online at: https://www.dwd.de/EN/climate_environment/climatemonitoring/germany/germany_node.html (accessed June 11, 2024).
Diao, H., Wang, A., Yuan, F., Guan, D., and Wu, J. (2023). Changes in tree leaf δ13C along climatic and geographical gradients in China. Trees 37, 671–682. doi: 10.1007/s00468-022-02374-1
Drennan, R. D. (2009). Statistics for Archaeologists, 2nd Edition. Boston, MA: Springer US. doi: 10.1007/978-1-4419-0413-3
Farquhar, G. D., Ehlringer, J. R., and Hubick, K. T. (1989). Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537. doi: 10.1146/annurev.pp.40.060189.002443
Ferrio, J. P., Aguilera, M., Voltas, J., and Araus, J. L. (2020). “Stable carbon isotopes in archaeological plant remains,” in Stratigraphy and Timescales, ed. M. Montenari (Cambridge, MA: Elsevier), 107–145. doi: 10.1016/bs.sats.2020.08.008
Ferrio, J. P., Araus, J. L., Buxó, R., Voltas, J., and Bort, J. (2005). Water management practices and climate in ancient agriculture: inferences from the stable isotope composition of archaeobotanical remains. Veget. Hist. Archaeobot. 14, 510–517. doi: 10.1007/s00334-005-0062-2
Filipović, D., Brozio, J. P., Ditchfield, P., Klooß, S., Müller, J., and Kirleis, W. (2019). Middle-neolithic agricultural practices in the oldenburger graben wetlands, northern germany: first results of the analysis of arable weeds and stable isotopes. Holocene 29, 1587–1595. doi: 10.1177/0959683619857224
Flohr, P., Jenkins, E., Williams, H. R. S., Jamjoum, K., Nuimat, S., and Müldner, G. (2019). What can crop stable isotopes ever do for us? An experimental perspective on using cereal carbon stable isotope values for reconstructing water availability in semi-arid and arid environments. Veget. Hist. Archaeobot. 28, 497–512. doi: 10.1007/s00334-018-0708-5
Flohr, P., Müldner, G., and Jenkins, E. (2011). Carbon stable isotope analysis of cereal remains as a way to reconstruct water availability: preliminary results. Water Hist. 3, 121–144. doi: 10.1007/s12685-011-0036-5
Francey, R. J., Allison, C. E., Etheridge, D. M., Trudinger, C. M., Enting, I. G., Leuenberger, M., et al. (1999). A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B Chem. Phys. Meteorol. 51, 170–193. doi: 10.3402/tellusb.v51i2.16269
Fraser, R. A., Bogaard, A., Charles, M., Styring, A. K., Wallace, M., Jones, G., et al. (2013). Assessing natural variation and the effects of charring, burial and pre-treatment on the stable carbon and nitrogen isotope values of archaeobotanical cereals and pulses. J. Archaeol. Sci. 40, 4754–4766. doi: 10.1016/j.jas.2013.01.032
Fraser, R. A., Bogaard, A., Heaton, T., Charles, M., Jones, G., Christensen, B. T., et al. (2011). Manuring and stable nitrogen isotope ratios in cereals and pulses: towards a new archaeobotanical approach to the inference of land use and dietary practices. J. Archaeol. Sci. 38, 2790–2804. doi: 10.1016/j.jas.2011.06.024
Fuller, D. Q. (2006). Agricultural origins and frontiers in South Asia: a working synthesis. J. World Prehist. 20, 1–86. doi: 10.1007/s10963-006-9006-8
Gallagher, D. (2015). “Formation Processes of the Macrobotanical Record,” in Method and Theory in Paleoethnobotany, (Boulder, United States: University Press of Colorado). Available online at: http://ebookcentral.proquest.com/lib/ucsd/detail.action?docID=3039859 (accessed May 19, 2024). doi: 10.5876/9781607323167.c002
Gavériaux, F., Motta, L., Bailey, P., Brilli, M., and Sadori, L. (2022). Crop husbandry at gabii during the iron age and archaic period: the archaeobotanical and stable isotope evidence. Environ. Archaeol. 2022, 1–14. doi: 10.1080/14614103.2022.2101281
Gron, K. J., Larsson, M., Gröcke, D. R., Andersen, N. H., Andreasen, M. H., Bech, J.-H., et al. (2021). Archaeological cereals as an isotope record of long-term soil health and anthropogenic amendment in southern Scandinavia. Quat. Sci. Rev. 253:106762. doi: 10.1016/j.quascirev.2020.106762
Halstead, P. (2014). Two Oxen Ahead: Pre-Mechanized Farming in the Mediterranean. Hoboken, New Jersey: Wiley-Blackwell. doi: 10.1002/9781118819333
Heaton, T. H. E. (1999). Spatial, species, and temporal variations in the13C/12C ratios of C3Plants: implications for palaeodiet studies. J. Archaeol. Sci. 26, 637–649. doi: 10.1006/jasc.1998.0381
Heaton, T. H. E., Jones, G., Halstead, P., and Tsipropoulos, T. (2009). Variations in the 13C/12C ratios of modern wheat grain, and implications for interpreting data from Bronze Age Assiros Toumba, Greece. J. Archaeol. Sci. 36, 2224–2233. doi: 10.1016/j.jas.2009.06.007
Hillman, G., Wales, S., McLaren, F., Evans, J., and Butler, A. (1993). Identifying problematic remains of ancient plant foods: a comparison of the role of chemical, histological and morphological criteria. World Archaeol. 25, 94–121. doi: 10.1080/00438243.1993.9980230
James, N., Decaix, A., Villasana, I., Kenoyer, J. M., Wright, R., Meadow, R. H., et al. (2024). Taphonomy and labor at the indus site of Harappa, Pakistan (3700-1300 BCE). Antiquity 99, 1–18. doi: 10.15184/aqy.2024.196
Jones, P. J., O'Connell, T. C., Jones, M. K., Singh, R., and Petrie, C. A. (2021). Crop water status from plant stable carbon isotope values: a test case for monsoonal climates. Holocene 31, 993–1004. doi: 10.1177/0959683621994649
Kanstrup, M., Holst, M. K., Jensen, P. M., Thomsen, I. K., and Christensen, B. T. (2014). Searching for long-term trends in prehistoric manuring practice. δ15N analyses of charred cereal grains from the 4th to the 1st millennium BC. J. Archaeol. Sci. 51, 115–125. doi: 10.1016/j.jas.2013.04.018
Kanstrup, M., Thomsen, I. K., Andersen, A. J., Bogaard, A., and Christensen, B. T. (2011). Abundance of 13C and 15N in emmer, spelt and naked barley grown on differently manured soils: towards a method for identifying past manuring practice: method for identifying past manuring practice. Rapid Commun. Mass Spectrom. 25, 2879–2887. doi: 10.1002/rcm.5176
Kanstrup, M., Thomsen, I. K., Mikkelsen, P. H., and Christensen, B. T. (2012). Impact of charring on cereal grain characteristics: linking prehistoric manuring practice to δ15N signatures in archaeobotanical material. J. Archaeol. Sci. 39, 2533–2540. doi: 10.1016/j.jas.2012.03.007
Kenoyer, J. M. (1997). Trade and technology of the indus valley : new insights from Harappa, Pakistan trade and technology of the indus valley : new insights from Harappa, Pakistan. World Archaeol. 29, 262–280. doi: 10.1080/00438243.1997.9980377
Kenoyer, J. M. (2004). “Wheeled vehicles of the indus valley civilization of Pakistan and India,” in Bad unil Wagen: Der Ursprung einer Innovation Wagen im Vorderen Orient und Europa, eds. M. Fansa and S. Burmeister (Mainz am Rhein,), 87–106. Available online at: https://www.harappa.com/sites/default/files/pdf/Kenoyer2004_Wheeled Vehicles of the Indus Valley Civilizatio.pdf
Kenoyer, J. M. (2008). “The origin and character of Indus urbanism: new perspectives and challenges,” in The Ancient City: New Perspectives on Urbanism in the Old and New World, (Santa Fe: The Ancient City: New Perspectives on Urbanism in the Old and New World), 183–208.
Kenoyer, J. M., and Meadow, R. H. (2016). “Excavations at Harappa, 1986–2010,” in A Companion to South Asia in the Past (Hoboken, NJ: John Wiley & Sons, Ltd.), 145–168. doi: 10.1002/9781119055280.ch10
Kohn, M. J. (2010). Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. U.S.A. 107, 19691–19695. doi: 10.1073/pnas.1004933107
Lancelotti, C. (2018). “Not all that burns is wood”. A social perspective on fuel exploitation and use during the Indus urban period (2600-1900 BC). PLoS ONE 13:e0192364. doi: 10.1371/journal.pone.0192364
Larsson, M., Bergman, J., and Lagerås, P. (2019). Manuring practices in the first millennium AD in southern Sweden inferred from isotopic analysis of crop remains. PLoS ONE 14:e0215578. doi: 10.1371/journal.pone.0215578
Larsson, M., Bergman, J., and Olsson, P. A. (2024). Soil, fertilizer and plant density: exploring the influence of environmental factors to stable nitrogen and carbon isotope composition in cereal grain. J. Archaeol. Sci. 163:105935. doi: 10.1016/j.jas.2024.105935
Li, H., Sun, Y., Yang, Y., Cui, Y., Ren, L., Li, H., et al. (2022). Water and soil management strategies and the introduction of wheat and barley to northern China: an isotopic analysis of cultivation on the Loess Plateau. Antiquity 96, 1–17. doi: 10.15184/aqy.2022.138
Lightfoot, E., and Stevens, R. E. (2012). Stable isotope investigations of charred barley (Hordeum vulgare) and wheat (Triticum spelta) grains from Danebury Hillfort: implications for palaeodietary reconstructions. J. Archaeol. Sci. 39, 656–662. doi: 10.1016/j.jas.2011.10.026
Ma, W. T., Tcherkez, G., Wang, X. M., Schäufele, R., Schnyder, H., Yang, Y., et al. (2021). Accounting for mesophyll conductance substantially improves 13 C-based estimates of intrinsic water-use efficiency. New Phytol. 229, 1326–1338. doi: 10.1111/nph.16958
Meadow, R. H. (1996). “The origins and spread of agriculture and pastoralism in northwestern South Asia,” in The Origins and Spread of Agriculture and Pastoralism in Eurasia, ed. D. Harris (London: UCL Press), 608–608.
Meadow, R. H., and Kenoyer, J. M. (2001). Recent discoveries and highlights from excavations at Harappa: 1998–2000. Indo-Koko-Kenkyu 22, 19−36.
Miller, H. M.-L. (2006). “Water supply, labor organization and land ownership in indus floodplain agricultural systems,” in Agricultural Strategies (Los Angeles, CA: Cotsen Institute of Archaeology), 92–128. doi: 10.2307/j.ctvdjrr1w.10
Miller, H. M.-L. (2015). “Surplus in the indus civilization,” in Surplus: The Politics of Production and the Strategies of Everyday Life, eds. C. T. Morehart and K. De Lucia (Boulder: University Press of Colorado), 97–97. doi: 10.5876/9781607323808.c004
Miller, L. J. (2003). “Secondary products and urbanism in South Asia: the evidence for traction at Harappa,” in Ethnobiology and the Indus Civilization (Oxford: Lexington Books), 251–326.
Nayak, A., Basa, K. K., Boivin, N. L., Fuller, D. Q., Mohanty, R. K., Kingwell-Banham, E., et al. (2022). A stable isotope perspective on archaeological agricultural variability and Neolithic experimentation in India. J. Archaeol. Sci.141:105591. doi: 10.1016/j.jas.2022.105591
Netting, R. M. (1993). Smallholders, Householders: Farm Families and the Ecology of Intensive, Sustainable Agriculture. Stanford, CA: Stanford University Press.
Nitsch, E. K., Charles, M., and Bogaard, A. (2015). Calculating a statistically robust δ13C and δ15N offset for charred cereal and pulse seeds. STAR Sci. Technol. Archaeol. Res. 1, 1–8. doi: 10.1179/2054892315Y.0000000001
Patel, A. K., and Meadow, R. H. (2017). “South Asian contributions to animal domestication and pastoralism: bones, genes, and archaeology,” in The Oxford Handbook of Zooarchaeology, eds. U. Albarella, M. Rizzetto, H. Russ, K. Vickers, and S. Viner-Daniels (Oxford: Oxford University Press). doi: 10.1093/oxfordhb/9780199686476.013.19
Pazola, Z., and Cieslak, J. (1979). Changes in carbohydrates during the production of coffee substitute extracts, especially in the roasting process. Food Chem. 4, 41–52. doi: 10.1016/0308-8146(79)90029-3
Pearsall, D. M. (2015). Paleoethnobotany: A Handbook of Procedures, Third edition. Walnut Creek, California: Left Coast Press Inc.
Pendall, E., and Amundson, R. (1990). Soil/landform relationships surrounding the Harappa archaeological site, Pakistan. Geoarchaeology 5, 301–322. doi: 10.1002/gea.3340050402
Petrie, C. A., and Bates, J. (2017). ‘Multi-cropping', intercropping and adaptation to variable environments in indus South Asia. J. World Prehist. 30, 81–130. doi: 10.1007/s10963-017-9101-z
Possehl, G. (2002). The Indus Civilization: A Contemporary Perspective, 1st Edn. Walnut Creek, CA: AltaMira Press.
Qi, H., Coplen, T. B., Geilmann, H., Brand, W. A., and Böhlke, J. K. (2003). Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Commun. Mass. Spectrom. 17, 2483–2487. doi: 10.1002/rcm.1219
Qi, H., Coplen, T. B., Mroczkowski, S. J., Brand, W. A., Brandes, L., Geilmann, H., et al. (2016). A new organic reference material, l-glutamic acid, USGS41a, for δ13C and δ15N measurements – a replacement for USGS41. Rapid Commun. Mass. Spectrom. 30, 859–866. doi: 10.1002/rcm.7510
Riehl, S. (2012). Variability in ancient near eastern environmental and agricultural development. J. Arid Environ. 86, 113–121. doi: 10.1016/j.jaridenv.2011.09.014
Riehl, S. (2020). “Stable Isotopes in Ancient Agriculture,” in A Companion to Ancient Agriculture (Hoboken, NJ: John Wiley & Sons Ltd.), 55–81. doi: 10.1002/9781118970959.ch4
Riehl, S., Bryson, R., and Pustovoytov, K. (2008). Changing growing conditions for crops during the near eastern bronze age (3000–1200 BC): the stable carbon isotope evidence. J. Archaeol. Sci. 35, 1011–1022. doi: 10.1016/j.jas.2007.07.003
Rosen, A. M., Hart, T. C., Farquhar, J., Schneider, J. S., and Yadmaa, T. (2019). Holocene vegetation cycles, land-use, and human adaptations to desertification in the gobi desert of Mongolia. Veget. Hist. Archaeobot. 28, 295–309. doi: 10.1007/s00334-018-0710-y
Stroud, E., Bogaard, A., and Charles, M. (2021). A stable isotope and functional weed ecology investigation into Chalcolithic cultivation practices in Central Anatolia: Çatalhöyük, Çamlibel Tarlasi and Kuruçay. J. Archaeol. Sci. 38:103010. doi: 10.1016/j.jasrep.2021.103010
Stroud, E., Charles, M., Bogaard, A., and Hamerow, H. (2023a). Turning up the heat: assessing the impact of charring regime on the morphology and stable isotopic values of cereal grains. J. Archaeol. Sci. 153:105754. doi: 10.1016/j.jas.2023.105754
Stroud, E., Charles, M., Bogaard, A., Nitsch, E., and Hamerow, H. (2023b). The experimental heating of rye, oat, spelt, wheat and barley between 215 and 300 °C: the stable carbon and nitrogen isotope data and the photographic evidence of changes to the morphology of the grains. Data Brief 50:109544. doi: 10.1016/j.dib.2023.109544
Styring, A., Maier, U., Stephan, E., Schlichtherle, H., and Bogaard, A. (2016b). Cultivation of choice: new insights into farming practices at Neolithic lakeshore sites. Antiquity 90, 95–110. doi: 10.15184/aqy.2015.192
Styring, A., Rösch, M., Stephan, E., Stika, H.-P., Fischer, E., Sillmann, M., et al. (2017b). Centralisation and long-term change in farming regimes: comparing agricultural practices in neolithic and iron age south-west Germany. Proc. Prehist. Soc. 83, 357–381. doi: 10.1017/ppr.2017.3
Styring, A. K., Ater, M., Hmimsa, Y., Fraser, R., Miller, H., Neef, R., et al. (2016a). Disentangling the effect of farming practice from aridity on crop stable isotope values: a present-day model from Morocco and its application to early farming sites in the eastern mediterranean. Anthr. Rev. 3, 2–22. doi: 10.1177/2053019616630762
Styring, A. K., Charles, M., Fantone, F., Hald, M. M., McMahon, A., Meadow, R. H., et al. (2017a). Isotope evidence for agricultural extensification reveals how the world's first cities were fed. Nat. Plants 3:17076. doi: 10.1038/nplants.2017.76
Styring, A. K., Manning, H., Fraser, R. A., Wallace, M., Jones, G., Charles, M., et al. (2013). The effect of charring and burial on the biochemical composition of cereal grains: investigating the integrity of archaeological plant material. J. Archaeol. Sci. 40, 4767–4779. doi: 10.1016/j.jas.2013.03.024
Styring, A. K., Vaiglova, P., Bogaard, A., Church, M. J., Gröcke, D. R., Larsson, M., et al. (2024). Recommendations for stable isotope analysis of charred archaeological crop remains. Front. Environ. Archaeol. 3:1470375. doi: 10.3389/fearc.2024.1470375
Szpak, P. (2014). Complexities of nitrogen isotope biogeochemistry in plant-soil systems: implications for the study of ancient agricultural and animal management practices. Front. Plant Sci. 5:288. doi: 10.3389/fpls.2014.00288
Szpak, P., Metcalfe, J. Z., and Macdonald, R. A. (2017). Best practices for calibrating and reporting stable isotope measurements in archaeology. J. Archaeol. Sci. Rep. 13, 609–616. doi: 10.1016/j.jasrep.2017.05.007
Takebayashi, Y., Koba, K., Sasaki, Y., Fang, Y., and Yoh, M. (2010). The natural abundance of 15 N in plant and soil-available N indicates a shift of main plant N resources to NO from NH along the N leaching gradient. Rapid Comm. Mass Spectrometry 24, 1001–1008. doi: 10.1002/rcm.4469
Tieszen, L. L. (1991). Natural variations in the carbon isotope values of plants: implications for archaeology, ecology, and paleoecology. J. Archaeol. Sci. 18, 227–248. doi: 10.1016/0305-4403(91)90063-U
Vaiglova, P., Gardeisen, A., Buckley, M., Cavanagh, W., Renard, J., Lee-Thorp, J., et al. (2020). Further insight into Neolithic agricultural management at Kouphovouno, southern Greece: expanding the isotopic approach. Archaeol. Anthropol. Sci. 12:43. doi: 10.1007/s12520-019-00960-y
Vaiglova, P., Lazar, N. A., Stroud, E. A., Loftus, E., and Makarewicz, C. A. (2023). Best practices for selecting samples, analyzing data, and publishing results in isotope archaeology. Quat. Int. 650, 86–100. doi: 10.1016/j.quaint.2022.02.027
Vaiglova, P., Snoeck, C., Nitsch, E., Bogaard, A., and Lee-Thorp, J. (2014). Impact of contamination and pre-treatment on stable carbon and nitrogen isotopic composition of charred plant remains. Rapid Commun. Mass Spectrom. 28, 2497–2510. doi: 10.1002/rcm.7044
Van Der Merwe, N. J. (1982). Carbon isotopes, photosynthesis, and archaeology: different pathways of photosynthesis. Am. Sci. 70, 596–606.
van der Merwe, N. J., and Medina, E. (1991). The canopy effect, carbon isotope ratios and foodwebs in amazonia. J. Archaeol. Sci. 18, 249–259. doi: 10.1016/0305-4403(91)90064-V
van der Veen, M. (2007). Formation processes of desiccated and carbonized plant remains – the identification of routine practice. J. Archaeol. Sci. 34, 968–990. doi: 10.1016/j.jas.2006.09.007
Vogel, J. C. (1993). “4 - variability of carbon isotope fractionation during photosynthesis,” in Stable Isotopes and Plant Carbon-water Relations, eds. J. R. Ehleringer, A. E. Hall, and G. D. Farquhar (San Diego: Academic Press), 29–46. doi: 10.1016/B978-0-08-091801-3.50010-6
Wallace, M., Jones, G., Charles, M., Fraser, R., Halstead, P., Heaton, T. H. E., et al. (2013). Stable carbon isotope analysis as a direct means of inferring crop water status and water management practices. World Archaeol. 45, 388–409. doi: 10.1080/00438243.2013.821671
Wallace, M. P., Jones, G., Charles, M., Fraser, R., Heaton, T. H. E., and Bogaard, A. (2015). Stable carbon isotope evidence for neolithic and bronze age crop water management in the Eastern Mediterranean and Southwest Asia. PLoS ONE 10:e0127085. doi: 10.1371/journal.pone.0127085
Weber, S. (2003). “Archaeobotany at Harappa,” in Indus Ethnobiology: New Perspectives from the Field, eds. S. Weber and B. Belcher (New York: Lexington Books), 175–198.
Weber, S., Kashyap, A., and Harriman, D. (2010b). Does size matter: the role and significance of cereal grains in the Indus civilization. Archaeol. Anthropol. Sci. 2, 35–43. doi: 10.1007/s12520-010-0025-0
Weber, S. A., Barela, T., and Lehman, H. (2010a). Ecological continuity: an explanation for agricultural diversity in the indus civilization and beyond. Man and Environment XXXV, 62–75.
Yang, J., Zhang, D., Yang, X., Wang, W., Perry, L., Fuller, D. Q., et al. (2022). Sustainable intensification of millet–pig agriculture in Neolithic North China. Nat. Sustain. 5, 780–786. doi: 10.1038/s41893-022-00905-9
Keywords: charred grain, carbon isotope, nitrogen isotope, Indus valley civilization, stable isotope analysis, cultivation practices
Citation: James N, Winter-Schuh C, Kenoyer JM, D'Alpoim Guedes J and Makarewicz CA (2025) Differences in the isotopic composition of individual grains and aggregated seed samples affect interpretation of ancient plant cultivation practices. Front. Environ. Archaeol. 4:1510394. doi: 10.3389/fearc.2025.1510394
Received: 12 October 2024; Accepted: 21 April 2025;
Published: 26 June 2025.
Edited by:
Xinyi Liu, Washington University in St. Louis, United StatesReviewed by:
Ferran Antolín, Deutsches Archäologisches Institut, GermanyMike J. Church, Durham University, United Kingdom
Copyright © 2025 James, Winter-Schuh, Kenoyer, D'Alpoim Guedes and Makarewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheryl A. Makarewicz, Yy5tYWthcmV3aWN6QHVmZy51bmkta2llbC5kZQ==
 Nathaniel James
Nathaniel James Christine Winter-Schuh
Christine Winter-Schuh J. Mark Kenoyer
J. Mark Kenoyer Jade D'Alpoim Guedes
Jade D'Alpoim Guedes Cheryl A. Makarewicz
Cheryl A. Makarewicz