- 1 Department of Endocrinology, Pathophysiology and Applied Biology – Center of Excellence on Neurodegenerative Diseases, Università degli Studi di Milano, Milano, Italy
- 2 Giovanni Armenise-Harvard Foundation Laboratory, Department of Pharmacological Sciences, Università degli Studi di Milano, Milano, Italy
- 3 Laboratory of Biochemistry, Molecular Biology of Lipids and Mass Spectrometry “Giovanni Galli”, Department of Pharmacological Sciences, Università degli Studi di Milano, Milano, Italy
- 4 Instituto Cajal, Consejo Superior de Investigaciones Científicas, Madrid, Spain
Several reviews have so far pointed out on the relevant physiological and pharmacological role exerted by neuroactive steroids in the central nervous system. In the present review we summarize observations indicating that synthesis and metabolism of neuroactive steroids also occur in the peripheral nerves. Interestingly, peripheral nervous system is also a target of their action. Indeed, as here reported neuroactive steroids are physiological regulators of peripheral nerve functions and they may also represent interesting therapeutic tools for different types of peripheral neuropathy.
Introduction
Recent studies have shown that peripheral nerves synthesize and metabolize neuroactive steroids. The term neuroactive steroid refers to those steroids acting in the nervous system and includes steroids produced by the nervous system (i.e., neurosteroids) and hormonal steroids coming from classical steroidogenic tissues (i.e., gonads and adrenal glands; Melcangi et al., 2008). Moreover, peripheral nerves express receptors for neuroactive steroids and consequently represent a target for them. Indeed, neuroactive steroids are involved in the regulation of different functions of peripheral nerves, including Schwann cell proliferation and myelination (Chan et al., 1998, 2000; Desarnaud et al., 1998, 2000; Fex Svenningsen and Kanje, 1999; Lubischer and Bebinger, 1999; Guennoun et al., 2001; Mercier et al., 2001; Azcoitia et al., 2003; Rodriguez-Waitkus et al., 2003; Tanzer and Jones, 2004; Melcangi et al., 2005; Magnaghi et al., 2006, 2007).
Peripheral neuropathy is one of the most common disorders with a prevalence of about 2.4% that rises with aging to 8% in the general population (England and Asbury, 2004). Peripheral neuropathy may be inherited, such as those referred to Charcot–Marie–Tooth (CMT) disease including demyelinating and axonal variants, or acquired, like for instance those occurring during aging process, after physical injury, in systemic or metabolic disorders (e.g., diabetes mellitus, vitamin deficiencies, alcoholism, kidney failure, cancer, etc.), in infections and autoimmune disorders (e.g., AIDS, hepatitis, Guillain–Barré syndrome, Lyme disease, rheumatoid arthritis, leprosy, sarcoidosis, syphilis, systemic lupus erythematosus, etc.), after exposure to toxic compounds and during drug treatment (e.g., chemotherapeutic, antiretroviral, anti-tuberculosis medications, antimicrobial drugs, lithium, etc.). Currently, therapeutic arsenal for these peripheral neuropathies is very limited. Therefore, the finding that neuroactive steroids act as protective agents in different experimental models of peripheral neuropathy is highly promising (Leonelli et al., 2006; Melcangi and Garcia-Segura, 2006, 2010; Roglio et al., 2008b; Schumacher et al., 2008). At present, corticosteroids are the only steroids used for clinical management of peripheral neuropathy, due to their anti-inflammatory actions. However, the design of a randomized controlled trial for the treatment of mild idiopathic carpal tunnel syndrome (CTS) with local injection of 17 alpha-hydroxyprogesterone caproate, a synthetic derivative of progesterone (PROG), has been recently reported (Milani et al., 2010).
We will here review the state of the art on the synthesis, actions, and therapeutic implications of neuroactive steroids in the peripheral nervous system (PNS).
Synthesis of Neuroactive Steroids Occurs in the Peripheral Nerves
Peripheral nerves are a source of neuroactive steroids (Mellon and Deschepper, 1993; Schumacher et al., 2003; Melcangi et al., 2008; Pelletier, 2010). The first step of steroidogenesis is the transport of cholesterol from intracellular stores to the inner mitochondrial membrane, where cytochrome P450 side chain cleavage (P450scc), the enzyme that converts cholesterol to pregnenolone, is located. This transport is facilitated by translocator protein-18kDa (TSPO) and steroidogenic acute regulatory protein (StAR), two molecules that, together with P450scc, are expressed by Schwann cells (Benmessahel et al., 2004; Papadopoulos et al., 2006).
Moreover, Schwann cells, as well as dorsal root ganglia (DRG), also express other steroidogenic enzymes such as 3α-hydroxysteroid dehydrogenase (i.e., the enzyme converting PREG into PROG; Koenig et al., 1995; Guennoun et al., 1997; Chan et al., 2000; Schumacher et al., 2001; Coirini et al., 2003; Rodriguez-Waitkus et al., 2003; Schaeffer et al., 2010), 5α-reductase (5α-R), converting PROG and testosterone (T) into dihydroprogesterone (DHP) and dihydrotestosterone (DHT) respectively, and 3α-hydroxysteroid dehydrogenase, converting DHP and DHT into tetrahydroprogesterone (THP) and 5α-androstane-3α, 17β-diol (3α-diol) respectively (Melcangi et al., 1990, 2001a; Yokoi et al., 1998; Schaeffer et al., 2010). Synthesis of neuroactive steroids in peripheral nerves has been also confirmed by the assessment of their levels. For instance, using liquid chromatography tandem mass spectrometry analysis, levels of PREG, PROG, and its derivatives (i.e., DHP, THP and isopregnanolone), dehydroepiandrosterone (DHEA), T, and its derivatives (i.e., DHT and 3α-diol) have been determined in the sciatic nerve of male and female rats (Caruso et al., 2008a,b, 2010; Pesaresi et al., 2010b). These studies have shown that local levels of neuroactive steroids in the peripheral nerve do not directly reflect plasma levels. In addition, these studies have revealed that the PNS adapts its local levels of neuroactive steroids in response to gonadectomy with sex specificity and depending on the duration of the peripheral modifications (Caruso et al., 2010).
Physiological Control by Neuroactive Steroids on Peripheral Nerves
Peripheral Nerves as Targets of Neuroactive Steroids
Peripheral nerves and Schwann cells not only synthesize and metabolize neuroactive steroids, but are also targets for these molecules. Classical intracellular steroid receptors, such as PROG (PR), estrogen, glucocorticoid, and mineralocorticoid receptors, have been detected in rat sciatic nerve and in Schwann cells (Jung-Testas et al., 1996; Melcangi et al., 2001b; Groyer et al., 2006). Also, androgen receptor (AR) expression has been demonstrated in the endoneurial compartment of rat sciatic nerve (Magnaghi et al., 1999; Jordan et al., 2002). Through the activation of these classical steroid receptors, neuroactive steroids such as PROG, DHP, T, DHT, DHEA, estrogens, and corticosteroids, affect the development and function of the PNS.
In addition, in the central nervous system, neuroactive steroids such as 3alpha-hydroxy-derivatives of dihydroprogesterone, dihydrotestosterone, and deoxycorticosterone are known to bind to neurotransmitter receptor channels and to modulate their activity (Lambert et al., 2003; Belelli and Lambert, 2005; Zheng, 2009). This mechanism of action also operates in the PNS, since neurotransmitter receptors modulated by neuroactive steroids, such as GABA-A (i.e., α2, α3, β1, β2, and β3 subunits) and GABA-B (i.e., GABA-B1 and GABA-B2) receptors, have been identified in peripheral nerves and Schwann cells (Melcangi et al., 1999; Magnaghi et al., 2004a). Moreover, rat sural nerve expresses NMDA receptor 1 subunit, glutamate receptor 1 (GluR1), AMPA subunit, and GluR 5, 6, 7 kainate subunits (Coggeshall and Carlton, 1998; Verkhratsky and Steinhauser, 2000). Schwann cells of mammalian peripheral vestibular system express GluR 2, 3, 4 (Dememes et al., 1995; Verkhratsky and Steinhauser, 2000). Finally, the presence of sigma 1 receptor (i.e., an atypical neuro-modulatory receptor) in Schwann cells of rat sciatic nerve has been confirmed (Palacios et al., 2004). Therefore, neuroactive steroids may also regulate PNS physiology through the modulation of the activity of neurotransmitter receptors and by non-classical steroid receptors, such as the sigma 1 receptor.
Effects, Mechanisms of Action, and Influence of Sex
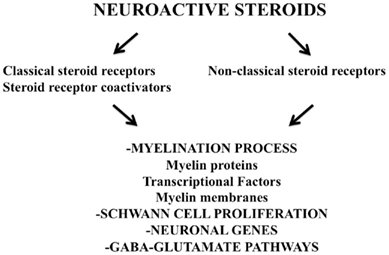
One of best characterized actions of neuroactive steroids in peripheral nerves is the regulation of the myelination program. Classical and non-classical steroid receptors mediate physiological actions of neuroactive steroids on the proliferation of Schwann cells and on their expression of myelin proteins and of transcription factors involved in the regulation of myelination (Figure 1). Indeed, PROG and its derivatives are able to modulate the expression of myelin proteins of the PNS, such as glycoprotein zero (P0) and the peripheral myelin protein 22 (PMP22). Namely, the expression of P0 in sciatic nerve of adult male rats, as well as that in rat Schwann cell culture, was increased by the treatment with PROG, DHP, or THP, while in case of PMP22, only THP was effective (Melcangi et al., 1999, 2005). Similar effects are also exerted by derivatives of T. Thus, orchidectomy in adult male rat decreased the expression of P0 and PMP22 in the sciatic nerve (Magnaghi et al., 1999, 2004b). The subsequent treatment with DHT or 3α-diol (i.e., two derivatives of T) restored the levels of P0, while in case of PMP22 only 3α-diol was effective (Magnaghi et al., 1999, 2004b). A very similar pattern of effects was also evident in cultures of rat Schwann cells (Melcangi et al., 2005). The mechanism involved in these effects was different depending on the myelin protein considered. Thus, observations performed with receptor agonists or antagonists suggest that the expression of P0 is under the control of classical intracellular receptors, such as PR and AR, while a role for non-classical mechanisms, like the modulation of GABA-A receptor, may be hypothesized in case of PMP22 (Melcangi et al., 2005). Activation of a classical steroid receptor, such as PR, clearly suggests that the effect of PROG derivatives on P0 expression is due to a classical steroid genomic effect. This hypothesis is supported by the finding that putative progesterone responsive elements are present on P0 gene (Magnaghi et al., 1999) and that a coactivator, such as steroid receptor coactivator-1 (SRC-1), participates in the regulation of P0 gene expression by DHP. Indeed, the effect of this neuroactive steroid on P0 expression in an immortalized cell line of Schwann cell (i.e., MSC80 cells) stably transfected to over- or down-express SRC-1 was increased or completely lost, respectively (Cavarretta et al., 2004). A role for AR in controlling expression of P0 may be also hypothesized. Indeed, in vivo treatment with an AR antagonist, such as flutamide, decreased the synthesis of P0 in rat sciatic nerve (Magnaghi et al., 2004b). Interestingly, inhibition of AR influenced P0 synthesis in adult age only. This age-linked effect is different from what we have observed after the in vivo treatment with mifepristone, where PR antagonist was only able to decrease the synthesis of P0 at postnatal day 20 (Melcangi et al., 2003b). A possible hypothesis could be that PROG derivatives may be necessary for inducing P0 synthesis during the first steps of the myelination process, while the subsequent intervention of T derivatives will participate in the maintenance of this process.
At variance to what observed on P0, expression of PMP22 seems to be under the control of GABA-A receptor. As we observed in Schwann cell cultures, treatment with antagonist (i.e., bicuculline) or agonist (i.e., muscimol) of GABA-A receptor abolished or mimicked respectively the stimulatory effect exerted by THP on PMP22 (Melcangi et al., 2005).
Expression of P0 and PMP22 is also affected in a sex-dependent manner. Thus, observations obtained in culture of rat Schwann cells have indicated that PROG or DHP treatment induced a stimulatory effect on P0 mRNA levels only in male, while THP was only effective in female cells. Similarly, the expression of PMP22 was stimulated by PROG in cultures from males and by THP in cultures from females (Magnaghi et al., 2006).
Neuroactive steroids regulate myelination program by affecting also the expression of transcription factors (Figure 1). Data obtained in culture of rat Schwann cells (Guennoun et al., 2001; Mercier et al., 2001) have indicated that PROG stimulates the gene expression of Krox-20, Krox-24, Egr-3, and FosB. Moreover, the expression of Krox-20 was also stimulated by DHP and THP, while that of another transcription factor, such as Sox-10, was only stimulated by DHP (Magnaghi et al., 2007).
P0 and PMP22 play an important role for the maintenance of the multilamellar structure of PNS myelin (D’Urso et al., 1990). Therefore, in agreement with the effect exerted on the proteins of peripheral myelin, PROG is also able to stimulate the synthesis of myelin membranes (Figure 1). For instance, PROG accelerates the time of initiation and enhances the rate of myelin synthesis in Schwann cells co-cultured with DRG neurons (Chan et al., 1998, 2000).
Moreover, also the axonal compartment of PNS neurons is a target for the action of neuroactive steroids. Thus, PROG affects the expression of neuronal genes involved in the myelination process by Schwann cells. For instance, in co-culture of Schwann cells and DRG neurons two genes, like a small Ras-like GTP-binding protein (Rap 1b) and phosphoribosyl diphosphate synthase-associated protein, which are induced in co-cultures during myelin synthesis, were also induced by PROG treatment (Chan et al., 2000; Rodriguez-Waitkus et al., 2003). Finally, the blockade of PR by mifepristone during development resulted in axonal impairment in the sciatic nerve of male rats (Melcangi et al., 2003b).
Neuroactive steroids are also able to affect Schwann cell proliferation (Figure 1). For instance, a stimulatory effect of PROG has been detected in vitro (Fex Svenningsen and Kanje, 1999; Bartolami et al., 2003). Interestingly, also this effect of PROG seems to be dependent on the sex of the animals. Indeed, PROG increased Schwann cell proliferation in cultures of segments of rat sciatic nerve from females, but was ineffective in cultures from males (Fex Svenningsen and Kanje, 1999). Androgens also affect Schwann cell proliferation. For instance, the number of terminal Schwann cells unsheathing the synaptic junction between motor nerve endings and muscles decreased after castration and this effect was counteracted by T replacement (Lubischer and Bebinger, 1999).
Steroid coactivators, which as previously mentioned participate in the effects exerted by neuroactive steroids on myelin proteins, are also able to affect cell proliferation. For instance, cell proliferation in an immortalized line of Schwann cells (i.e., MSC80 cells) overexpressing SRC-1 was slower than in cells in which the coactivator expression was down regulated (Melcangi et al., 2005). In contrast, overexpression of another coactivator, such as steroid receptor RNA activator (SRA), induced an increase in the proliferation of MSC80 cells (Melcangi et al., 2005).
Finally, metabolites of PROG, such as THP, acting on GABA-A receptors are also able to influence GABA and glutamate pathways in Schwann cells (Figure 1). Thus, this neuroactive steroid stimulates GABA synthesis by increasing the levels of glutamic acid decarboxylase of 67 kDa (Magnaghi et al., 2010) and the activity of excitatory amino acid carrier 1 and therefore, glutamate uptake (Perego et al., 2011).
The Levels of Neuroactive Steroids in Peripheral Nerves are Affected by Pathological Events
As demonstrated in several experimental models, the levels of neuroactive steroids present in peripheral nerves are affected by traumatic injury and pathologies. Namely, in the experimental model of crush injury the levels of PREG, DHP, and THP present in the distal portion of injured sciatic nerve were lowered (Roglio et al., 2008a). The drop of DHP was not explained by a decrease in the levels of its first substrate (i.e., PROG) that were unchanged, but by a decrease in the expression of the enzyme 5α-R (i.e., enzyme converting PROG into DHP; Roglio et al., 2008a).
Changes in the levels of neuroactive steroids have been also reported in an experimental model of Charcot–Marie–Tooth type 1 (CMT1A; Caruso et al., 2008b) or of diabetic neuropathy (Caruso et al., 2008a; Pesaresi et al., 2010b). Interestingly, in these experimental models the levels of neuroactive steroids were affected in a sex-dimorphic way by the pathology. For instance, as demonstrated in the sciatic nerve of male and female PMP22 transgenic rats (i.e., an experimental model of CMT1A) the levels of isopregnanolone and of 3α-diol, which are exclusively detectable in sciatic nerve of female and male rats respectively, were strongly decreased (Caruso et al., 2008b). In sciatic nerve of streptozotocin (STZ)- treated animals (i.e., an experimental model of diabetes inducing peripheral neuropathy), the levels of PREG, T, DHT, and 3α-diol were significantly decreased in males but not in females, while PROG, THP, and isopregnanolone were decreased only in females (Pesaresi et al., 2010b).
Neuroactive Steroids as Protective Agents in Peripheral Nervous System
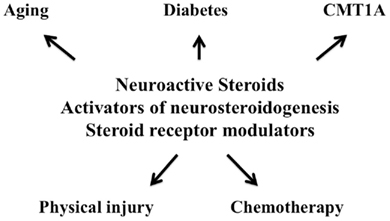
Neuroactive steroids are not only protective agents in the central nervous system as extensively demonstrated in several experimental models (Melcangi et al., 2000a; Lapchak and Araujo, 2001; McCullough and Hurn, 2003; Ciriza et al., 2004, 2006; Griffin et al., 2004; Melcangi and Mensah-Nyagan, 2006; Aguado-Llera et al., 2007; Schumacher et al., 2007; Pesaresi et al., 2010a), but they are also effective on peripheral neuropathies (Figure 2).
Aging
Aging induces important biochemical (e.g., a decrease in the synthesis of P0 and PMP22) and morphological changes in peripheral nerves, such as atrophy of the large myelinated fibers, myelin sheaths increase in thickness and show various irregularities (i.e., myelin ballooning, splitting, infolding, reduplication, and remyelination; Azcoitia et al., 2003; Melcangi et al., 2003a). Neuroactive steroids, such as PROG or its derivatives, are able to stimulate the low expression of P0 and PMP22 in the sciatic nerve of aged rats (Melcangi et al., 1998, 2000c, 2003a). Moreover, they have also clear beneficial effects on the number and shape of myelinated fibers as well as on the frequency of myelin abnormalities (Azcoitia et al., 2003; Melcangi et al., 2003a).
All these effects seem to be a peculiarity of PROG and its derivatives, because neither T nor DHT or 3α-diol were able to influence the morphological parameters analyzed in these experiments (Azcoitia et al., 2003; Melcangi et al., 2003a).
Physical Injury
Protective and regenerative effects of neuroactive steroids have been well characterized in experimental models of degeneration occurring after physical injury of peripheral nerves. For instance, (1) PROG or DHP, increase gene expression of P0 after nerve transection (Melcangi et al., 2000b); (2) PREG and PROG counteract the decrease of the amounts of myelin membranes induced by a cryolesion in the sciatic nerve of the mouse (Koenig et al., 1995); (3) In guided regeneration of facial nerve of rabbit, PROG induces an increase in the number of Schwann cell nuclei, of non-myelinated and myelinated nerve fibers (with increase also in their diameters), as well as in the g-ratio of myelinated nerve fibers (Chavez-Delgado et al., 2005); and (4) In crush injury model, DHP and/or P counteract biochemical alterations (i.e., myelin proteins and Na+,K+-ATPase pump) and stimulate reelin gene expression. These two neuroactive steroids also counteract nociception impairment, and DHP treatment significantly decreases the up-regulation of myelinated fibers’ density occurring in crushed nerves (Roglio et al., 2008a).
Moreover, promising results have been also obtained with other neuroactive steroids. For instance, T and its derivative, DHT, accelerate regeneration, and functional recovery of injured nerves (Yu, 1982; Vita et al., 1983; Jones et al., 2001; Tanzer and Jones, 2004; Huppenbauer et al., 2005). DHEA is protective after rat sciatic nerve transection, reducing the extent of denervation atrophy and inducing an earlier onset of axonal regeneration (Ayhan et al., 2003). DHEA also promotes a faster return to normal values of sciatic function index and increases the number of myelinated fibers and of fiber diameters after nerve crush injury in rats (Gudemez et al., 2002). Similar results have been obtained after crush injury in mice, using 17β-estradiol (Islamov et al., 2002).
Chemotherapy-Induced Peripheral Neurotoxicity
Effects of neuroactive steroids have been also evaluated in an experimental model of chemotherapy-induced peripheral neurotoxicity, such as those due to docetaxel (i.e., a semisynthetic taxane widely employed as antineoplastic agent for the treatment of breast, ovarian, and non-small cell lung cancer; Roglio et al., 2009). We demonstrated that treatment with DHP or P counteracted docetaxel-induced neuropathy, preventing nerve conduction velocity (NCV) and thermal threshold changes, and degeneration of skin nerves in the footpad. Neuroactive steroids also counteracted the changes in gene expression of several myelin proteins, such as P0, PMP22, myelin, and lymphocyte-associated protein and myelin basic protein (MBP) induced by docetaxel in sciatic nerve. Most nerve abnormalities observed during the treatment with docetaxel spontaneously recovered after drug withdrawal, similarly to what occurs in patients. However, results of midterm follow-up experiments indicate that animals treated with DHP or P show a faster recovery (Roglio et al., 2009).
Neuropathic pain is another important consequence of peripheral nerve damage. Indeed, neuroactive steroids exert a beneficial effect also on this component. For instance, DHP or THP treatment suppresses neuropathic symptoms (allodynia/hyperalgesia) evoked by antineoplastic drugs such as vincristine (Meyer et al., 2010) or oxaliplatin (Meyer et al., 2011).
Diabetic Neuropathy
Another experimental model in which protective effects of neuroactive steroids have been ascertained is diabetic neuropathy. Thus, in STZ-treated rats, PROG or its derivatives improve sciatic NCV, myelin protein mRNA levels (i.e., P0 and PMP22), Na+, K+-ATPase activity, thermal threshold, skin innervation density (Leonelli et al., 2007) and counteract the increase in the number of fibers with myelin infoldings (Veiga et al., 2006). Androgens, such as T or its derivatives exert similar effects (Roglio et al., 2007), while DHEA prevents not only neuronal but also vascular dysfunction in the sciatic nerve of STZ-rats (Yorek et al., 2002).
Influence of Hormonal Environment
As recently demonstrated, endogenous glucocorticoids exert a role in myelination after sciatic nerve injury. For instance, crush injury performed in adrenalectomized rats affects myelination processes in term of MBP expression and morphological parameters of myelin sheaths (Morisaki et al., 2010). Thus, adrenalectomized animals showed reduced MBP mRNA and protein levels and decreased myelin thickness in comparison to sham-operated animals. These alterations were prevented by low-dose corticosterone replacement.
Recent results have shown that gonadal steroid hormonal environment also influences diabetic neuropathy. Interestingly, this effect is sex-dimorphic. Thus, ovariectomy, but not orchidectomy, significantly counteract STZ-induced alterations on NCV, Na+, K+-ATPase activity, and expression of myelin proteins, such as P0 and PMP22 (Pesaresi et al., 2011). The effect of ovariectomy is associated with an increase in the levels of neuroactive steroids (e.g., DHEA, T, and DHT) in the sciatic nerve of diabetic rats (Pesaresi et al., 2011).
These observations, together with the finding that peripheral neuropathies show sex differences in their incidence, symptomatology, and nerodegenerative outcome (Melcangi and Garcia-Segura, 2010) may have strong implications for the development of new sex-oriented therapies based on the use of neuroactive steroids.
Neuroprotective Effects by Steroid Receptor Modulators
An alternative to the use of P, DHP, and THP may be the use of molecules that target PR and/or GABA-A receptor (Melcangi et al., 2005). Indeed, it has been demonstrated that the treatment with a PR antagonist (i.e., onapristone) was able to reduce the overexpression of PMP22 and to improve CMT phenotype in an experimental model of CMT1A (i.e., PMP22-transgenic rats; Sereda et al., 2003; Meyer zu Horste et al., 2007). This opens the possibility of using selective PR modulators, perhaps in combination with GABA-A ligands, for the treatment of peripheral neuropathy. Selective estrogen receptor modulators may also offer a potential therapeutic interest since the selective estrogen receptor modulator LY117018 has been shown to enhance functional recovery after injury of the sciatic nerve (McMurray et al., 2003).
In situ Induction of Neuroactive Steroids as Therapeutic Tools
Another alternative for a therapeutic strategy with neuroactive steroids themselves, or with specific synthetic ligands of their receptors, might be the use of pharmacological agents that increase the synthesis of endogenous neuroactive steroids within the nervous system. For instance, this is possible with ligands of TSPO, which promoting the translocation of cholesterol to the inner mitochondrial membrane increase the synthesis of neuroactive steroids. As reported, a TSPO ligand like SSR180575 was able to increase the survival of facial nerve motoneurons after axotomy and the regeneration of peripheral nerves (Ferzaz et al., 2002). Another TSPO ligand, such as Ro5-4864, exerted a beneficial effect on morphological parameters of the sciatic nerve of aged male rats, significantly increasing the total number of myelinated fibers and decreasing the percentage of fibers with myelin decompaction (Leonelli et al., 2005). Moreover, Ro5-4864 is effective on STZ-induced diabetic neuropathy and significantly stimulates the low levels of PREG, PROG, and DHT observed in the sciatic nerves of diabetic rats (Giatti et al., 2009). In agreement with the protective actions of these neuroactive steroids, activation of TSPO counteracted the impairment of NCV and thermal threshold, restored skin innervation density and P0 gene expression, and improved Na+, K+-ATPase activity (Giatti et al., 2009).
Etifoxine (i.e., a TSPO ligand used for the treatment of anxiety disorders) has been demonstrated to activate neuroactive steroid synthesis (Verleye et al., 2005). In experimental models of peripheral nerve lesion, etifoxine treatment improved peripheral nerve regeneration and functional recovery, increasing axonal growth, causing a marked reduction in the number of macrophages and improving recovery of locomotion, motor coordination and sensory functions (Girard et al., 2008).
Finally, also liver X receptors (LXRs) might be an interesting therapeutic target (Cermenati et al., 2010). LXRs are ligand activated transcription factors that belong to the nuclear receptor superfamily that serving as cholesterol sensors prevent excessive intracellular accumulation of cholesterol.
As recently demonstrated, sciatic nerve expresses functional LRXα and β isoforms. Treatment with a synthetic ligand of this receptors (i.e., GW3965) resulted in an increase of neurosteroidogenesis in the sciatic levels of diabetic animals. Thus, an increase of the levels of PREG, PROG, DHP, and 3α-diol and of molecules and enzymes involved in their synthesis, such as StAR, P450scc, and 5α-R, as well as of classical LXR targets involved in cholesterol efflux, such as ABCA1 and ABCG1 was observed. These changes in neurosteroidogenesis machinery were associated with neuroprotective effects on thermal nociceptive activity, NCV and Na+, K+-ATPase activity (Cermenati et al., 2010). Interestingly, recent results have indicated that LXRs may also affect peripheral myelination opening new perspectives in the treatment of peripheral neuropathy. Indeed, knock-out of LXR in mice results in an alteration of the phenotype of myelin sheaths surrounding axons (i.e., thinner myelin sheaths), without affecting the diameters and number of axons (Makoukji et al., 2011).
Concluding Remarks
Observations here reviewed indicate that the PNS is a target for the effects of neuroactive steroids. In addition, neuroactive steroids themselves or pharmacological approaches acting on their receptors or their synthesis might represent potential therapeutic tools for different forms of peripheral neuropathies. This is extremely interesting because in many situations there are no effective treatments that can stop or reverse peripheral nerve damage. Thus, a sustained research and development effort on this experimental field might permit in a close future a promising translation to clinical research.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The financial support of Fondazione San Paolo (Progetto Neuroscienze, PF-2009.1180) and Fondazione Italiana Sclerosi Multipla (2010/23) to Roberto Cosimo Melcangi and Ministerio de Ciencia e Innovación, Spain (BFU2008-02950-C03-01) to Luis Miguel Garcia-Segura is gratefully acknowledged.
References
Aguado-Llera, D., Arilla-Ferreiro, E., Chowen, J. A., Argente, J., Puebla-Jimenez, L., Frago, L. M., and Barrios, V. (2007). 17Beta-estradiol protects depletion of rat temporal cortex somatostatinergic system by beta-amyloid. Neurobiol. Aging 28, 1396–1409.
Ayhan, S., Markal, N., Siemionow, K., Araneo, B., and Siemionow, M. (2003). Effect of subepineurial dehydroepiandrosterone treatment on healing of transected nerves repaired with the epineurial sleeve technique. Microsurgery 23, 49–55.
Azcoitia, I., Leonelli, E., Magnaghi, V., Veiga, S., Garcia-Segura, L. M., and Melcangi, R. C. (2003). Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol. Aging 24, 853–860.
Bartolami, S., Auge, C., Travo, C., Venteo, S., Knipper, M., and Sans, A. (2003). Vestibular Schwann cells are a distinct subpopulation of peripheral glia with specific sensitivity to growth factors and extracellular matrix components. J. Neurobiol. 57, 270–290.
Belelli, D., and Lambert, J. J. (2005). Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 6, 565–575.
Benmessahel, Y., Troadec, J. D., Cadepond, F., Guennoun, R., Hales, D. B., Schumacher, M., and Groyer, G. (2004). Downregulation of steroidogenic acute regulatory protein (StAR) gene expression by cyclic AMP in cultured Schwann cells. Glia 45, 213–228.
Caruso, D., Pesaresi, M., Maschi, O., Giatti, S., Garcia-Segura, L. M., and Melcangi, R. C. (2010). Effects of short- and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J. Neuroendocrinol. 22, 1137–1147.
Caruso, D., Scurati, S., Maschi, O., De Angelis, L., Roglio, I., Giatti, S., Garcia-Segura, L. M., and Melcangi, R. C. (2008a). Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem. Int. 52, 560–568.
Caruso, D., Scurati, S., Roglio, I., Nobbio, L., Schenone, A., and Melcangi, R. C. (2008b). Neuroactive steroid levels in a transgenic rat model of CMT1A neuropathy. J. Mol. Neurosci. 34, 249–253.
Cavarretta, I. T., Martini, L., Motta, M., Smith, C. L., and Melcangi, R. C. (2004). SRC-1 is involved in the control of the gene expression of myelin protein P0. J. Mol. Neurosci. 24, 217–226.
Cermenati, G., Giatti, S., Cavaletti, G., Bianchi, R., Maschi, O., Pesaresi, M., Abbiati, F., Volonterio, A., Saez, E., Caruso, D., Melcangi, R. C., and Mitro, N. (2010). Activation of the liver X receptor increases neuroactive steroid levels and protects from diabetes-induced peripheral neuropathy. J. Neurosci. 30, 11896–11901.
Chan, J. R., Phillips, L. J. II, and Glaser, M. (1998). Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc. Natl. Acad. Sci. U.S.A. 95, 10459–10464.
Chan, J. R., Rodriguez-Waitkus, P. M., Ng, B. K., Liang, P., and Glaser, M. (2000). Progesterone synthesized by Schwann cells during myelin formation regulates neuronal gene expression. Mol. Biol. Cell 11, 2283–2295.
Chavez-Delgado, M. E., Gomez-Pinedo, U., Feria-Velasco, A., Huerta-Viera, M., Castaneda, S. C., Toral, F. A., Parducz, A., Anda, S. L., Mora-Galindo, J., and Garcia-Estrada, J. (2005). Ultrastructural analysis of guided nerve regeneration using progesterone- and pregnenolone-loaded chitosan prostheses. J. Biomed. Mater. Res. B Appl. Biomater. 74, 589–600.
Ciriza, I., Azcoitia, I., and Garcia-Segura, L. M. (2004). Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J. Neuroendocrinol. 16, 58–63.
Ciriza, I., Carrero, P., Frye, C. A., and Garcia-Segura, L. M. (2006). Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J. Neurobiol. 66, 916–928.
Coggeshall, R. E., and Carlton, S. M. (1998). Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J. Comp. Neurol. 391, 78–86.
Coirini, H., Gouezou, M., Delespierre, B., Liere, P., Pianos, A., Eychenne, B., Schumacher, M., and Guennoun, R. (2003). Characterization and regulation of the 3beta-hydroxysteroid dehydrogenase isomerase enzyme in the rat sciatic nerve. J. Neurochem. 84, 119–126.
Dememes, D., Lleixa, A., and Dechesne, C. J. (1995). Cellular and subcellular localization of AMPA-selective glutamate receptors in the mammalian peripheral vestibular system. Brain Res. 671, 83–94.
Desarnaud, F., Bidichandani, S., Patel, P. I., Baulieu, E. E., and Schumacher, M. (2000). Glucocorticosteroids stimulate the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. Brain Res. 865, 12–16.
Desarnaud, F., Do Thi, A. N., Brown, A. M., Lemke, G., Suter, U., Baulieu, E. E., and Schumacher, M. (1998). Progesterone stimulates the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. J. Neurochem. 71, 1765–1768.
D’Urso, D., Brophy, P. J., Staugaitis, S. M., Gillespie, C. S., Frey, A. B., Stempak, J. G., and Colman, D. R. (1990). Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron 4, 449–460.
Ferzaz, B., Brault, E., Bourliaud, G., Robert, J. P., Poughon, G., Claustre, Y., Marguet, F., Liere, P., Schumacher, M., Nowicki, J. P., Fournier, J., Marabout, B., Sevrin, M., George, P., Soubrie, P., Benavides, J., and Scatton, B. (2002). SSR180575 (7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide), a peripheral benzodiazepine receptor ligand, promotes neuronal survival and repair. J. Pharmacol. Exp. Ther. 301, 1067–1078.
Fex Svenningsen, A., and Kanje, M. (1999). Estrogen and progesterone stimulate Schwann cell proliferation in a sex- and age-dependent manner. J. Neurosci. Res. 57, 124–130.
Giatti, S., Pesaresi, M., Cavaletti, G., Bianchi, R., Carozzi, V., Lombardi, R., Maschi, O., Lauria, G., Garcia-Segura, L. M., Caruso, D., and Melcangi, R. C. (2009). Neuroprotective effects of a ligand of translocator protein-18kDa (Ro5-4864) in experimental diabetic neuropathy. Neuroscience 164, 520–529.
Girard, C., Liu, S., Cadepond, F., Adams, D., Lacroix, C., Verleye, M., Gillardin, J. M., Baulieu, E. E., Schumacher, M., and Schweizer-Groyer, G. (2008). Etifoxine improves peripheral nerve regeneration and functional recovery. Proc. Natl. Acad. Sci. U.S.A. 105, 20505–20510.
Griffin, L. D., Gong, W., Verot, L., and Mellon, S. H. (2004). Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 10, 704–711.
Groyer, G., Eychenne, B., Girard, C., Rajkowski, K., Schumacher, M., and Cadepond, F. (2006). Expression and functional state of the corticosteroid receptors and 11 beta-hydroxysteroid dehydrogenase type 2 in Schwann cells. Endocrinology 147, 4339–4350.
Gudemez, E., Ozer, K., Cunningham, B., Siemionow, K., Browne, E., and Siemionow, M. (2002). Dehydroepiandrosterone as an enhancer of functional recovery following crush injury to rat sciatic nerve. Microsurgery 22, 234–241.
Guennoun, R., Benmessahel, Y., Delespierre, B., Gouezou, M., Rajkowski, K. M., Baulieu, E. E., and Schumacher, M. (2001). Progesterone stimulates Krox-20 gene expression in Schwann cells. Brain Res. Mol. Brain Res. 90, 75–82.
Guennoun, R., Schumacher, M., Robert, F., Delespierre, B., Gouezou, M., Eychenne, B., Akwa, Y., Robel, P., and Baulieu, E. E. (1997). Neurosteroids: expression of functional 3beta-hydroxysteroid dehydrogenase by rat sensory neurons and Schwann cells. Eur. J. Neurosci. 9, 2236–2247.
Huppenbauer, C. B., Tanzer, L., DonCarlos, L. L., and Jones, K. J. (2005). Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J. Neurosci. 25, 4004–4013.
Islamov, R. R., Hendricks, W. A., Jones, R. J., Lyall, G. J., Spanier, N. S., and Murashov, A. K. (2002). 17Beta-estradiol stimulates regeneration of sciatic nerve in female mice. Brain Res. 943, 283–286.
Jones, K. J., Brown, T. J., and Damaser, M. (2001). Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res. Brain Res. Rev. 37, 372–382.
Jordan, C. L., Price, R. H. Jr., and Handa, R. J. (2002). Androgen receptor messenger RNA and protein in adult rat sciatic nerve: implications for site of androgen action. J. Neurosci. Res. 69, 509–518.
Jung-Testas, I., Schumacher, M., Robel, P., and Baulieu, E. E. (1996). Demonstration of progesterone receptors in rat Schwann cells. J. Steroid Biochem. Mol. Biol. 58, 77–82.
Koenig, H. L., Schumacher, M., Ferzaz, B., Thi, A. N., Ressouches, A., Guennoun, R., Jung-Testas, I., Robel, P., Akwa, Y., and Baulieu, E. E. (1995). Progesterone synthesis and myelin formation by Schwann cells. Science 268, 1500–1503.
Lambert, J. J., Belelli, D., Peden, D. R., Vardy, A. W., and Peters, J. A. (2003). Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 71, 67–80.
Lapchak, P. A., and Araujo, D. M. (2001). Preclinical development of neurosteroids as neuroprotective agents for the treatment of neurodegenerative diseases. Int. Rev. Neurobiol. 46, 379–397.
Leonelli, E., Ballabio, M., Consoli, A., Roglio, I., Magnaghi, V., and Melcangi, R. C. (2006). Neuroactive steroids: a therapeutic approach to maintain peripheral nerve integrity during neurodegenerative events. J. Mol. Neurosci. 28, 65–76.
Leonelli, E., Bianchi, R., Cavaletti, G., Caruso, D., Crippa, D., Garcia-Segura, L. M., Lauria, G., Magnaghi, V., Roglio, I., and Melcangi, R. C. (2007). Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: a multimodal analysis. Neuroscience 144, 1293–1304.
Leonelli, E., Yague, J. G., Ballabio, M., Azcoitia, I., Magnaghi, V., Schumacher, M., Garcia-Segura, L. M., and Melcangi, R. C. (2005). Ro5-4864, a synthetic ligand of peripheral benzodiazepine receptor, reduces aging-associated myelin degeneration in the sciatic nerve of male rats. Mech. Ageing Dev. 126, 1159–1163.
Lubischer, J. L., and Bebinger, D. M. (1999). Regulation of terminal Schwann cell number at the adult neuromuscular junction. J. Neurosci. 19, RC46.
Magnaghi, V., Ballabio, M., Cavarretta, I. T., Froestl, W., Lambert, J. J., Zucchi, I., and Melcangi, R. C. (2004a). GABAB receptors in Schwann cells influence proliferation and myelin protein expression. Eur. J. Neurosci. 19, 2641–2649.
Magnaghi, V., Ballabio, M., Gonzalez, L. C., Leonelli, E., Motta, M., and Melcangi, R. C. (2004b). The synthesis of glycoprotein P0 and peripheral myelin protein 22 in sciatic nerve of male rats is modulated by testosterone metabolites. Brain Res. Mol. Brain Res. 126, 67–73.
Magnaghi, V., Ballabio, M., Roglio, I., and Melcangi, R. C. (2007). Progesterone derivatives increase expression of Krox-20 and Sox-10 in rat Schwann cells. J. Mol. Neurosci. 31, 149–157.
Magnaghi, V., Cavarretta, I., Zucchi, I., Susani, L., Rupprecht, R., Hermann, B., Martini, L., and Melcangi, R. C. (1999). P0 gene expression is modulated by androgens in the sciatic nerve of adult male rats. Brain Res. Mol. Brain Res. 70, 36–44.
Magnaghi, V., Parducz, A., Frasca, A., Ballabio, M., Procacci, P., Racagni, G., Bonanno, G., and Fumagalli, F. (2010). GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J. Neurochem. 112, 980–990.
Magnaghi, V., Veiga, S., Ballabio, M., Gonzalez, L. C., Garcia-Segura, L. M., and Melcangi, R. C. (2006). Sex-dimorphic effects of progesterone and its reduced metabolites on gene expression of myelin proteins by rat Schwann cells. J. Peripher. Nerv. Syst. 11, 111–118.
Makoukji, J., Shackleford, G., Meffre, D., Grenier, J., Liere, P., Lobaccaro, J. M., Schumacher, M., and Massaad, C. (2011). Interplay between LXR and Wnt/beta-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J. Neurosci. 31, 9620–9629.
McCullough, L. D., and Hurn, P. D. (2003). Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol. Metab. 14, 228–235.
McMurray, R., Islamov, R., and Murashov, A. K. (2003). Raloxifene analog LY117018 enhances the regeneration of sciatic nerve in ovariectomized female mice. Brain Res. 980, 140–145.
Melcangi, R. C., Azcoitia, I., Ballabio, M., Cavarretta, I., Gonzalez, L. C., Leonelli, E., Magnaghi, V., Veiga, S., and Garcia-Segura, L. M. (2003a). Neuroactive steroids influence peripheral myelination: a promising opportunity for preventing or treating age-dependent dysfunctions of peripheral nerves. Prog. Neurobiol. 71, 57–66.
Melcangi, R. C., Leonelli, E., Magnaghi, V., Gherardi, G., Nobbio, L., and Schenone, A. (2003b). Mifepristone (RU 38486) influences expression of glycoprotein Po and morphological parameters at the level of rat sciatic nerve: in vivo observations. Exp. Neurol. 184, 930–938.
Melcangi, R. C., Cavarretta, I., Magnaghi, V., Ciusani, E., and Salmaggi, A. (2000a). Corticosteroids protect oligodendrocytes from cytokine-induced cell death. Neuroreport 11, 3969–3972.
Melcangi, R. C., Magnaghi, V., Galbiati, M., Ghelarducci, B., Sebastiani, L., and Martini, L. (2000b). The action of steroid hormones on peripheral myelin proteins: a possible new tool for the rebuilding of myelin? J. Neurocytol. 29, 327–339.
Melcangi, R. C., Magnaghi, V., and Martini, L. (2000c). Aging in peripheral nerves: regulation of myelin protein genes by steroid hormones. Prog. Neurobiol. 60, 291–308.
Melcangi, R. C., Cavarretta, I. T., Ballabio, M., Leonelli, E., Schenone, A., Azcoitia, I., Garcia-Segura, L. M., and Magnaghi, V. (2005). Peripheral nerves: a target for the action of neuroactive steroids. Brain Res. Brain Res. Rev. 48, 328–338.
Melcangi, R. C., Celotti, F., Ballabio, M., Poletti, A., and Martini, L. (1990). Testosterone metabolism in peripheral nerves: presence of the 5 alpha-reductase-3 alpha-hydroxysteroid-dehydrogenase enzymatic system in the sciatic nerve of adult and aged rats. J. Steroid Biochem. 35, 145–148.
Melcangi, R. C., and Garcia-Segura, L. M. (2006). Therapeutic approaches to peripheral neuropathy based on neuroactive steroids. Expert Rev. Neurother. 6, 1121–1125.
Melcangi, R. C., and Garcia-Segura, L. M. (2010). Sex-specific therapeutic strategies based on neuroactive steroids: in search for innovative tools for neuroprotection. Horm. Behav. 57, 2–11.
Melcangi, R. C., Garcia-Segura, L. M., and Mensah-Nyagan, A. G. (2008). Neuroactive steroids: state of the art and new perspectives. Cell. Mol. Life Sci. 65, 777–797.
Melcangi, R. C., Magnaghi, V., Cavarretta, I., Martini, L., and Piva, F. (1998). Age-induced decrease of glycoprotein Po and myelin basic protein gene expression in the rat sciatic nerve. Repair by steroid derivatives. Neuroscience 85, 569–578.
Melcangi, R. C., Magnaghi, V., Cavarretta, I., Zucchi, I., Bovolin, P., D’Urso, D., and Martini, L. (1999). Progesterone derivatives are able to influence peripheral myelin protein 22 and P0 gene expression: possible mechanisms of action. J. Neurosci. Res. 56, 349–357.
Melcangi, R. C., Magnaghi, V., Galbiati, M., and Martini, L. (2001a). Formation and effects of neuroactive steroids in the central and peripheral nervous system. Int. Rev. Neurobiol. 46, 145–176.
Melcangi, R. C., Magnaghi, V., Galbiati, M., and Martini, L. (2001b). Glial cells: a target for steroid hormones. Prog. Brain Res. 132, 31–40.
Melcangi, R. C., and Mensah-Nyagan, A. G. (2006). Neuroprotective effects of neuroactive steroids in the spinal cord and peripheral nerves. J. Mol. Neurosci. 28, 1–2.
Mellon, S. H., and Deschepper, C. F. (1993). Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 629, 283–292.
Mercier, G., Turque, N., and Schumacher, M. (2001). Early activation of transcription factor expression in Schwann cells by progesterone. Brain Res. Mol. Brain Res. 97, 137–148.
Meyer, L., Patte-Mensah, C., Taleb, O., and Mensah-Nyagan, A. G. (2010). Cellular and functional evidence for a protective action of neurosteroids against vincristine chemotherapy-induced painful neuropathy. Cell. Mol. Life Sci. 67, 3017–3034.
Meyer, L., Patte-Mensah, C., Taleb, O., and Mensah-Nyagan, A. G. (2011). Allopregnanolone prevents and suppresses oxaliplatin-evoked painful neuropathy: multi-parametric assessment and direct evidence. Pain 152, 170–181.
Meyer zu Horste, G., Prukop, T., Liebetanz, D., Mobius, W., Nave, K. A., and Sereda, M. W. (2007). Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann. Neurol. 61, 61–72.
Milani, P., Mondelli, M., Ginanneschi, F., Mazzocchio, R., and Rossi, A. (2010). Progesterone – new therapy in mild carpal tunnel syndrome? Study design of a randomized clinical trial for local therapy. J. Brachial Plex. Peripher. Nerve Inj. 5, 11.
Morisaki, S., Nishi, M., Fujiwara, H., Oda, R., Kawata, M., and Kubo, T. (2010). Endogenous glucocorticoids improve myelination via Schwann cells after peripheral nerve injury: an in vivo study using a crush injury model. Glia 58, 954–963.
Palacios, G., Muro, A., Verdu, E., Pumarola, M., and Vela, J. M. (2004). Immunohistochemical localization of the sigma1 receptor in Schwann cells of rat sciatic nerve. Brain Res. 1007, 65–70.
Papadopoulos, V., Lecanu, L., Brown, R. C., Han, Z., and Yao, Z. X. (2006). Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience 138, 749–756.
Pelletier, G. (2010). Steroidogenic enzymes in the brain: morphological aspects. Prog. Brain Res. 181, 193–207.
Perego, C., Cairano, E. S., Ballabio, M., and Magnaghi, V. (2011). Neurosteroid allopregnanolone regulates EAAC1-mediated glutamate uptake and triggers actin changes in Schwann cells. J. Cell. Physiol. doi: 10.1002/jcp.22898. [Epub ahead of print].
Pesaresi, M., Giatti, S., Calabrese, D., Maschi, O., Caruso, D., and Melcangi, R. C. (2010a). Dihydroprogesterone increases the gene expression of myelin basic protein in spinal cord of diabetic rats. J. Mol. Neurosci. 42, 135–139.
Pesaresi, M., Maschi, O., Giatti, S., Garcia-Segura, L. M., Caruso, D., and Melcangi, R. C. (2010b). Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm. Behav. 57, 46–55.
Pesaresi, M., Giatti, S., Cavaletti, G., Abbiati, F., Calabrese, D., Bianchi, R., Caruso, D., Garcia-Segura, L. M., and Melcangi, R. C. (2011). Sex differences in the manifestation of peripheral diabetic neuropathy in gonadectomized rats: a correlation with the levels of neuroactive steroids in the sciatic nerve. Exp. Neurol. 228, 215–221.
Rodriguez-Waitkus, P. M., Lafollette, A. J., Ng, B. K., Zhu, T. S., Conrad, H. E., and Glaser, M. (2003). Steroid hormone signaling between Schwann cells and neurons regulates the rate of myelin synthesis. Ann. N. Y. Acad. Sci. 1007, 340–348.
Roglio, I., Bianchi, R., Camozzi, F., Carozzi, V., Cervellini, I., Crippa, D., Lauria, G., Cavaletti, G., and Melcangi, R. C. (2009). Docetaxel-induced peripheral neuropathy: protective effects of dihydroprogesterone and progesterone in an experimental model. J. Peripher. Nerv. Syst. 14, 36–44.
Roglio, I., Bianchi, R., Giatti, S., Cavaletti, G., Caruso, D., Scurati, S., Crippa, D., Garcia-Segura, L. M., Camozzi, F., Lauria, G., and Melcangi, R. C. (2007). Testosterone derivatives are neuroprotective agents in experimental diabetic neuropathy. Cell. Mol. Life Sci. 64, 1158–1168.
Roglio, I., Bianchi, R., Gotti, S., Scurati, S., Giatti, S., Pesaresi, M., Caruso, D., Panzica, G. C., and Melcangi, R. C. (2008a). Neuroprotective effects of dihydroprogesterone and progesterone in an experimental model of nerve crush injury. Neuroscience 155, 673–685.
Roglio, I., Giatti, S., Pesaresi, M., Bianchi, R., Cavaletti, G., Lauria, G., Garcia-Segura, L. M., and Melcangi, R. C. (2008b). Neuroactive steroids and peripheral neuropathy. Brain Res. Rev. 57, 460–469.
Schaeffer, V., Meyer, L., Patte-Mensah, C., and Mensah-Nyagan, A. G. (2010). Progress in dorsal root ganglion neurosteroidogenic activity: basic evidence and pathophysiological correlation. Prog. Neurobiol. 92, 33–41.
Schumacher, M., Guennoun, R., Mercier, G., Desarnaud, F., Lacor, P., Benavides, J., Ferzaz, B., Robert, F., and Baulieu, E. E. (2001). Progesterone synthesis and myelin formation in peripheral nerves. Brain Res. Brain Res. Rev. 37, 343–359.
Schumacher, M., Guennoun, R., Stein, D. G., and De Nicola, A. F. (2007). Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol. Ther. 116, 77–106.
Schumacher, M., Sitruk-Ware, R., and De Nicola, A. F. (2008). Progesterone and progestins: neuroprotection and myelin repair. Curr. Opin. Pharmacol. 8, 740–746.
Schumacher, M., Weill-Engerer, S., Liere, P., Robert, F., Franklin, R. J., Garcia-Segura, L. M., Lambert, J. J., Mayo, W., Melcangi, R. C., Parducz, A., Suter, U., Carelli, C., Baulieu, E. E., and Akwa, Y. (2003). Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol. 71, 3–29.
Sereda, M. W., Meyer zu Horste, G., Suter, U., Uzma, N., and Nave, K. A. (2003). Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A). Nat. Med. 9, 1533–1537.
Tanzer, L., and Jones, K. J. (2004). Neurotherapeutic action of testosterone on hamster facial nerve regeneration: temporal window of effects. Horm. Behav. 45, 339–344.
Veiga, S., Leonelli, E., Beelke, M., Garcia-Segura, L. M., and Melcangi, R. C. (2006). Neuroactive steroids prevent peripheral myelin alterations induced by diabetes. Neurosci. Lett. 402, 150–153.
Verkhratsky, A., and Steinhauser, C. (2000). Ion channels in glial cells. Brain Res. Brain Res. Rev. 32, 380–412.
Verleye, M., Akwa, Y., Liere, P., Ladurelle, N., Pianos, A., Eychenne, B., Schumacher, M., and Gillardin, J. M. (2005). The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol. Biochem. Behav. 82, 712–720.
Vita, G., Dattola, R., Girlanda, P., Oteri, G., Lo Presti, F., and Messina, C. (1983). Effects of steroid hormones on muscle reinnervation after nerve crush in rabbit. Exp. Neurol. 80, 279–287.
Yokoi, H., Tsuruo, Y., and Ishimura, K. (1998). Steroid 5alpha-reductase type 1 immunolocalized in the rat peripheral nervous system and paraganglia. Histochem. J. 30, 731–739.
Yorek, M. A., Coppey, L. J., Gellett, J. S., Davidson, E. P., Bing, X., Lund, D. D., and Dillon, J. S. (2002). Effect of treatment of diabetic rats with dehydroepiandrosterone on vascular and neural function. Am. J. Physiol. Endocrinol. Metab. 283, E1067–E1075.
Yu, W. H. (1982). Effect of testosterone on the regeneration of the hypoglossal nerve in rats. Exp. Neurol. 77, 129–141.
Keywords: progesterone, testosterone, metabolism, peripheral neuropathy, steroidogenesis, neuroprotection
Citation: Melcangi RC, Giatti S, Pesaresi M, Calabrese D, Mitro N, Caruso D and Garcia-Segura LM (2011) Role of neuroactive steroids in the peripheral nervous system. Front. Endocrin. 2:104. doi: 10.3389/fendo.2011.00104
Received: 04 July 2011;
Paper pending published: 12 August 2011;
Accepted: 05 December 2011;
Published online: 27 December 2011.
Edited by:
Hubert Vaudry, University of Rouen, FranceReviewed by:
Mihail Gr. Coculescu, University of Medicine and Pharmacy Carol Davila, RomaniaMichael Schumacher, INSERM, France
Copyright: © 2011 Melcangi, Giatti, Pesaresi, Calabrese, Mitro, Caruso and Garcia-Segura. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Roberto Cosimo Melcangi, Department of Endocrinology, Pathophysiology and Applied Biology – Center of Excellence on Neurodegenerative Diseases, Università degli Studi di Milano, Via Balzaretti 9, 20133 Milano, Italy. e-mail:cm9iZXJ0by5tZWxjYW5naUB1bmltaS5pdA==

 Silvia Giatti1
Silvia Giatti1