- 1Department of Epidemiology and Health Statistics, School of Public Health, Kunming Medical University, Kunming, China
- 2School of Public Health, Sun Yat-sen University, Guangzhou, China
- 3Shandong Cancer Hospital Affiliated to Shandong University, Shandong Academy of Medical Sciences, Jinan, China
- 4Wuqing Center for Disease Control and Prevention, Tianjin, China
- 5Department of Health Education and Health Behavior, School of Public Health, Fudan University, Shanghai, China
- 6Karolinska Institutet, Stockholm, Sweden
Background: The association of pulse pressure and peripheral arterial disease (PAD) has seldom been examined using a prospective design. This study aimed to investigate the association of pulse pressure with PAD incidence in an elderly general population.
Methods: We utilized data from a cohort conducted in Beijing with additionally 2-year follow-up time. PAD was defined as an ankle brachial index value <0.9 in either leg. Cox proportional hazard regression model was used to quantify the magnitude of pulse pressure on PAD incidence.
Results: During a 2-year follow-up time, 357 of 4,201 (8.5%) participants developed PAD with 105 (6.9%) men and 252 (9.4%) women, respectively. After adjusting for baseline age, sex, body mass index, hypertension, diabetes, total cholesterol, and high-density lipoprotein cholesterol, and smoking, the hazard ratio and 95% confidence interval for people with pulse pressure greater than 60 mmHg was 2.20 (1.53, 3.15) compared with those whose pulse pressure was less than 40 mmHg. A linear trend was observed for the association of pulse pressure with PAD.
Conclusion: Higher pulse pressure was associated with higher PAD incidence.
Introduction
Peripheral arterial disease (PAD) is a common circulatory problem in which narrowed arteries reduce blood flow to limbs (1, 2). It is also a strong predictor for cardiovascular diseases, mortality, and stroke, independent of traditional cardiovascular risk factors (3, 4). Identification of those with PAD has profound public health and clinical implications for earlier disease prevention and treatment (5).
A number of risk factors have been identified for PAD risk assessment, such as smoking, diabetes, height, and dyslipidemia (6–9). Previous investigations suggested higher pulse pressure may be an independent predictor for PAD and this relationship was observed in several studies (10–12). However, most of these studies were based on cross-sectional design or case–control setting in which the temporal order was difficult to establish due to the weakness of study design. A prospective investigation of the association between pulse pressure and PAD remains scarce.
The present study aimed to examine the incidence of PAD, and to assess its association with pulse pressure in a general senior population.
Patients and Methods
Study Design and Participants
This study was a prospective cohort with participants originally recruited for chronic diseases and risk factors assessment in Beijing in 2007 with additional 2-year follow-up until 2009. A more detailed study design was described previously (13, 14). The present study included participants who were 50 years and over. In brief, 5,885 participants from 38 communities were recruited. During the 2-year follow-up duration, 1,095 participants emigrated or relocated, 402 refused to attend the follow-up examination, and 19 died. Of the remaining participants, 164 had baseline PAD and 4 had missing data on core covariates; the final analysis included 4,201 people who were free of PAD at baseline. All participants provided written informed consent to attend this study and subsequent follow-up examination, and the study protocol and ethical approval was obtained from the Ethic Committee of Beijing Municipal Science and Technology Commission.
Measurements
The health interview was performed by trained medical staff at community clinics using a well-established questionnaire to determine demographic and behavioral characteristics of the study population. Information regarding birthday, gender, and smoking status were collected. Physical examination included anthropometric measurements, blood pressure, and medical history. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, with the subject standing barefoot in light clothes. Waist circumference was measured to the nearest 0.1 cm at the mid-point between the 12th rib and right anterior superior iliac spine. Body mass index (BMI) was calculated as weight (kilogram) to be divided by square of height (meter). Blood pressure was measured using standard mercury sphygmomanometer on the right arm in sitting position after the participants rested for 5 min. Phase 1 and phase 5 Korotkoff sound was used as systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively. Blood pressure was measured twice with the average results for the data analysis. Medical history was obtained from medical record and confirmed by local general practitioners.
Blood samples were collected from all the participants after an overnight fasting. All the biochemical measurements were conducted in the central laboratory of Peking University People’s Hospital. Concentrations of fasting glucose, total cholesterol (TC), high-density lipoprotein cholesterol were measured using an auto analyzer (Hitachi 717, Hitachi Instruments, Inc., Tokyo, Japan). Hypertension was defined as SBP ≥140 mmHg, DBP ≥90 mmHg, or current medication for hypertension, and diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L or current medication for diabetes.
Determination of Pulse Pressure and PAD
Pulse pressure was calculated as the SBP minus the DBP. The ankle brachial index (ABI) was determined complying with a standard protocol. After 5 min of rest, a standard mercury sphygmomanometer and a Doppler stethoscope with 5 mHz probe (Nicolet, Elite 100 R, 5 mHz probe, USA) were used to determine the bilateral brachial, tibial, and dorsal arteries with participants in supine position. Measurements were carried out twice and averaged for analysis. ABI was calculated as the ratio of the highest SBP in the leg to the highest SBP in the arm. The lower value of ABIs was used as the patient-specific ABI for analysis. ABI <0.9 was considered as PAD (15).
Statistical Analysis
Continuous variables were presented as mean ± SD and categorical variables were presented as frequencies and proportions. In the descriptive analysis, we present the basic characteristics of study subjects in men and women separately. Then in the exploratory analysis, we examined the association between pulse pressure and PAD using Cox proportional hazard regression models. Before treating pulse pressure as a continuous variable, pulse pressure was categorized into quartiles with the lowest quartiles as the reference. Three models were used for the analysis. The first model only included pulse pressure followed by the second model adjusted for age and gender as confounders. The third model was further adjusted for BMI, high-density lipoprotein cholesterol, TC, hypertension, diabetes, and smoking status. Hazard ratios (HRs) with 95% confidence interval (CI) were also presented. Kaplan–Meier method was used to estimate the PAD-free probability for different pulse pressure groups. All the analyses were two-tailed and P < 0.05 was considered to be statistically significant. All the statistics were obtained using R 3.1.
Results
Basic Characteristics
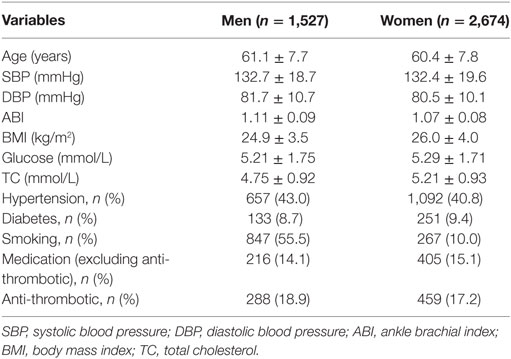
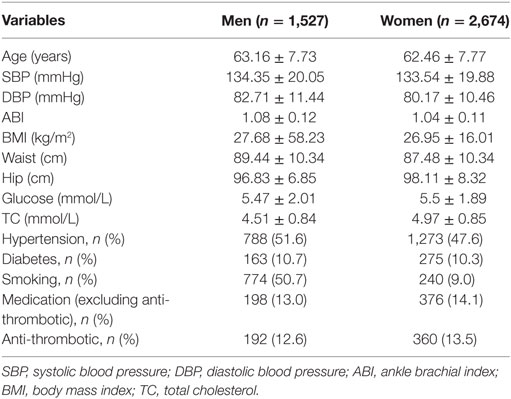
Among the 4,201 study participants, 1,527 (36.3%) were men and 2,674 (63.7%) were women. As shown in Table 1, the mean age was 61.1 (SD, 7.7) for men and 60.4 (SD, 7.8) for women. The mean ABI was 1.09 (SD, 0.08) with 1.11 (SD, 0.09) for men and 1.07 (SD, 0.08) for women. Tables 2 and 3 described the characteristics by PAD status. During a median 2-year (range: 1.8–2.3 years) follow-up time, 357 (8.5%) participants developed PAD with 105 (6.9%) men and 252 (9.4%) women corresponding to 33.9 per 1,000 person-year in men and 45.5 per 1,000 person-year in women, respectively.
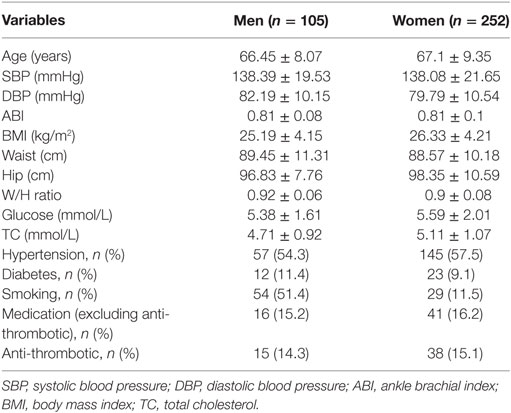
Table 3. Characteristics of study participants who developed peripheral arterial disease at follow-up.
Association between Pulse Pressure and PAD Incidence
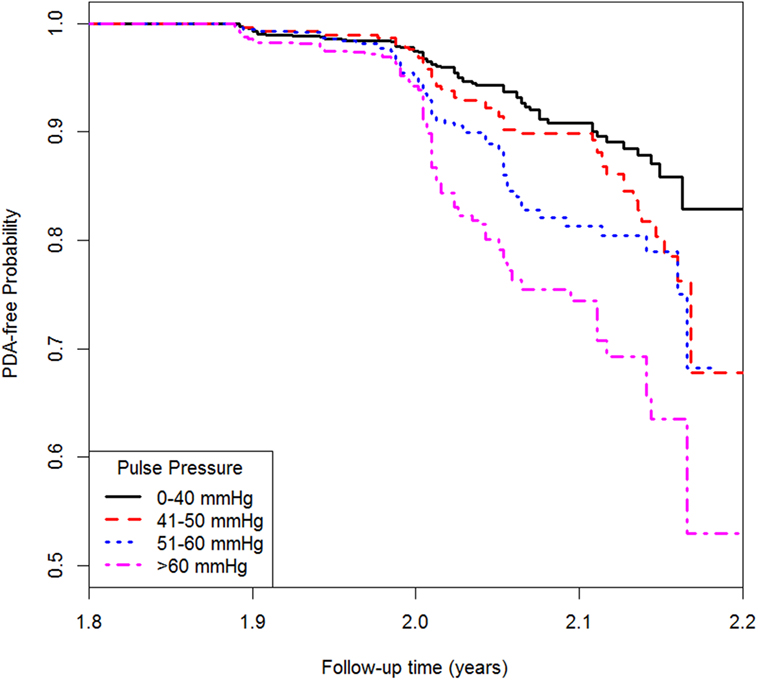
Table 4 shows the association between pulse pressure and PAD. Model 1 only included pulse pressure, while Model 2 was adjusted for age and gender followed by Model 3 adjusted for age, gender, BMI, hypertension, diabetes, TC, high-density lipoprotein cholesterol, and smoking. In Model 3, each 10 mmHg increase in pulse pressure was associated with 19% higher risk of PAD when treating pulse pressure as a continuous variable. The HR (95% CI) for people with pulse pressure greater than 60 mmHg was 2.20 (1.53, 3.15) compared with those whose pulse pressure was less than 40 mmHg. Similar result was shown from the Kaplan–Meier plot in Figure 1.
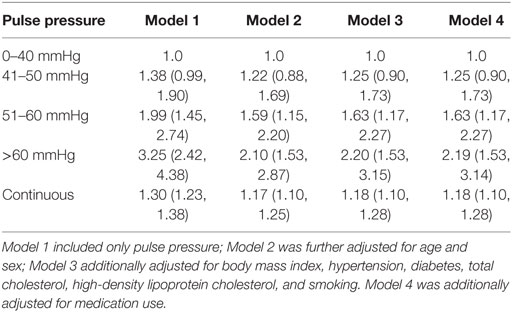
Table 4. Association between pulse pressure and peripheral arterial disease incidence, hazard ratio (95% confidence interval).
Discussion
In the present study, we examined the incidence of PAD and its association with pulse pressure in prospective cohort. We found that higher pulse pressure was associated with high risk of PAD and this association was independent of several risk factors including age, gender, high-density lipoprotein cholesterol, TC, hypertension, diabetes, and smoking status.
There are few studies of PAD incidence based on ABI. The Limburg study reported PAD incidence rate was 17.8 in men and 22.9 in women per 1,000 person-year at the age of 65 and over (16) which is lower than our study. The difference may be due to the different study population, follow-up time, or socioeconomic status. Several observational studies have been conducted to examine whether pulse pressure is associated with PAD (17, 18). One study from Finland reported one 1 mmHg increase of pulse pressure to be associated with 1.03 odds ratio of PAD which was approximately 1.34 odds ratio for 10 mmHg increase of pulse pressure (11). Our previous study also found 5-mmHg increase in pulse pressure was associated with a 17 and 15% increase of PAD risk in men and women (10). A more recent study from Japan observed an odds ratio for PAD was 1.30 for increasing pulse pressure (12). However, all of these studies are based on cross-sectional design or in a case–control setting. It might be difficult to determine if it is pulse pressure affect PAD or the opposite. Our current study, by using the prospectively collected data, found 10 mmHg to be associated with 19% higher risk for 2-year PAD incidence risk. This finding is consistent with published studies while has more strength in terms of its longitudinal cohort design and avoid reverse causation.
In this study, we observed women had higher incidence of PAD compared with men, which was also observed in The Limburg Study (15). Several factors might contribute to the sex difference in PAD incidence. Firstly, women were less physically active on than men nowadays in China, especially for the elderly (19). Physical activity was a protective factor for PAD; less physical activity could contribute to higher PAD incidence. Secondly, previous studies also revealed sex hormone and menopausal status were associated with lower risk of cardiovascular diseases for women. However, this phenomenon was not observed for PAD. In the Framingham Heart Study, sex hormone concentrations were not associated PAD risk in neither pre-menopausal nor post-menopausal women (20). Thirdly, heart rate was reversely correlated with ABI (21) and women tended to have higher heart rate and heart rate variability than men (22). However, more research is needed to clarify this sex inequality and more attention should be paid to women with higher risks of PAD.
Several potential molecular mechanisms might explain the observed association between pulse pressure and PAD. Pulse pressure was found to impair vascular endothelial function (23), and persistence of endothelial dysfunction could lead to arterial stiffness, atherosclerosis, and PAD (24, 25). Additionally, the ENPP1 Q121 variant could increase pulse pressure and reduced insulin signaling (26). Insulin resistance was strongly and independently associated with PAD (27). Moreover, pulse pressure promotes the apoptosis of vascular smooth muscle cell and this effect is independent of endothelial and mitogen-activated protein kinase (28). The synergistic effects of all these processes and lipid accumulation (29) accelerate the narrowing of the arteries in the lower extremities and aggravate intermittent claudication.
In order to examine if the observed association was independent of traditional risk factors of PAD, we additionally adjusted for several variables in our analyses. In the multivariable adjusted models, the magnitude of pulse press on PAD incidence was attenuated compared with the unadjusted models. Two reasons might explain this effect size decrease. These adjusted factors, such as age and gender, might be the confounders for both PAD and pulse pressure; thus, the HR will decrease after removing the confounding effects by model adjustment. Other risk factors, such as hypertension, diabetes, or lipid fractions, might mediate the effect from pulse pressure to PAD. However, these explanations need to be further tested in future studies. A few studies examined the relationship between ABI and flow-mediated vasodilation and found they were strongly correlated (30). However, we did not measure flow-mediated vasodilation in our current study.
Limitations
Although the data used in our study was collected prospectively, the median follow-up time was only 2 years. In this study, we only considered several important confounders in the analyses; a residual confounding could still bias the observed association. Additional analysis by collecting more variables, such as genetic variants (31) and other biomarkers (32–34), should be useful. The present study was conducted in an Asia population; the generalization of the results to other ethnic groups may be limited.
Conclusion
Higher pulse pressure was associated with PAD incidence, and this association might be mediated by several mechanisms.
Ethics Statement
This study was carried out in accordance with the recommendations of Ethic Committee of Beijing Municipal Science and Technology Commission with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Ethic Committee of Beijing Municipal Science and Technology Commission’.
Author Contributions
YM, YZ, HY, and JY designed this study. YZ and GY analyzed these data. All authors drafted this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res (2015) 116(9):1509–26. doi:10.1161/CIRCRESAHA.116.303849
2. Kullo IJ, Rooke TW. Clinical practice. Peripheral artery disease. N Engl J Med (2016) 374(9):861–71. doi:10.1056/NEJMcp1507631
3. Freisinger E, Malyar NM, Reinecke H. Peripheral artery disease is associated with high in-hospital mortality particularly in males with acute myocardial infarction in a nationwide real-world setting. Vasa (2016) 45(2):169–74. doi:10.1024/0301-1526/a000512
4. Tziomalos K, Giampatzis V, Bouziana S, Pavlidis A, Spanou M, Papadopoulou M, et al. Predictive value of the ankle brachial index in patients with acute ischemic stroke. Vasa (2014) 43(1):55–61. doi:10.1024/0301-1526/a000328
5. Zhan Y, Zhuang J, Dong Y, Xu H, Hu D, Yu J. Predicting the prevalence of peripheral arterial diseases: modelling and validation in different cohorts. Vasa (2016) 45(1):31–6. doi:10.1024/0301-1526/a000492
6. Zhan Y, Yu J, Ding R, Sun Y, Hu D. Triglyceride to high density lipoprotein cholesterol ratio, total cholesterol to high density lipoprotein cholesterol ratio and low ankle brachial index in an elderly population. Vasa (2014) 43(3):189–97. doi:10.1024/0301-1526/a000348
7. Husmann M, Jacomella V, Thalhammer C, Amann-Vesti BR. Markers of arterial stiffness in peripheral arterial disease. Vasa (2015) 44(5):341–8. doi:10.1024/0301-1526/a000452
8. Gavier B, Vazquez F, Gandara E. Antiphospholipid antibodies and lower extremity peripheral arterial disease – a systematic review and meta-analysis. Vasa (2016) 45(4):325–30. doi:10.1024/0301-1526/a000545
9. Wang Y, Li J, Zhao D, Wei Y, Hou L, Hu D, et al. Prevalence and characteristics of atherosclerosis and peripheral arterial disease in a Chinese population of Inner Mongolia. Vasa (2011) 40(1):49–56. doi:10.1024/0301-1526/a000069
10. Zhan Y, Yu J, Chen R, Sun Y, Fu Y, Zhang L, et al. Prevalence of low ankle brachial index and its association with pulse pressure in an elderly Chinese population: a cross-sectional study. J Epidemiol (2012) 22(5):454–61. doi:10.2188/jea.JE20110140
11. Korhonen P, Kautiainen H, Aarnio P. Pulse pressure and subclinical peripheral artery disease. J Hum Hypertens (2014) 28(4):242–5. doi:10.1038/jhh.2013.99
12. Kiuchi S, Hisatake S, Watanabe I, Toda M, Kabuki T, Oka T, et al. Pulse pressure and upstroke time are useful parameters for the diagnosis of peripheral artery disease in patients with normal ankle brachial index. Cardiol Res (2016) 7(5):161–6. doi:10.14740/cr508e
13. Zhan Y, Yu J, Chen R, Gao J, Ding R, Fu Y, et al. Socioeconomic status and metabolic syndrome in the general population of China: a cross-sectional study. BMC Public Health (2012) 12:921. doi:10.1186/1471-2458-12-921
14. Zhan Y, Zhang F, Lu L, Wang J, Sun Y, Ding R, et al. Prevalence of dyslipidemia and its association with insomnia in a community based population in China. BMC Public Health (2014) 14:1050. doi:10.1186/1471-2458-14-1050
15. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation (2012) 126(24):2890–909. doi:10.1161/CIR.0b013e318276fbcb
16. Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol (2001) 153(7):666–72. doi:10.1093/aje/153.7.666
17. Tseng CH. Pulse pressure as a risk factor for peripheral vascular disease in type 2 diabetic patients. Clin Exp Hypertens (2003) 25(8):475–85. doi:10.1081/CEH-120025331
18. Subramaniam T, Khaing Nang EE, Lim SC, Wu Y, Khoo CM, Lee J, et al. Distribution of ankle—brachial index and the risk factors of peripheral artery disease in a multi-ethnic Asian population. Vasc Med (2011) 16(2):87–95. doi:10.1177/1358863X11400781
19. Zang J, Ng SW. Age, period and cohort effects on adult physical activity levels from 1991 to 2011 in China. Int J Behav Nutr Phys Act (2016) 13:40. doi:10.1186/s12966-016-0364-z
20. Haring R, Travison TG, Bhasin S, Vasan RS, Wallaschofski H, Davda MN, et al. Relation between sex hormone concentrations, peripheral arterial disease, and change in ankle-brachial index: findings from the Framingham Heart Study. J Clin Endocrinol Metab (2011) 96(12):3724–32. doi:10.1210/jc.2011-1068
21. Su HM, Lee KT, Chu CS, Lee MY, Lin TH, Voon WC, et al. Effects of heart rate on brachial-ankle pulse wave velocity and ankle-brachial pressure index in patients without significant organic heart disease. Angiology (2007) 58(1):67–74. doi:10.1177/0003319706295481
22. Jandackova VK, Scholes S, Britton A, Steptoe A. Are changes in heart rate variability in middle-aged and older people normative or caused by pathological conditions? Findings from a large population-based longitudinal cohort study. J Am Heart Assoc (2016) 5(2): e002365. doi:10.1161/JAHA.115.002365
23. Beigel R, Dvir D, Arbel Y, Shechter A, Feinberg MS, Shechter M. Pulse pressure is a predictor of vascular endothelial function in middle-aged subjects with no apparent heart disease. Vasc Med (2010) 15(4):299–305. doi:10.1177/1358863X10373300
24. Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev (2010) 9(12):830–4. doi:10.1016/j.autrev.2010.07.016
25. Brevetti G, Silvestro A, Di Giacomo S, Bucur R, Di Donato A, Schiano V, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg (2003) 38(2):374–9. doi:10.1016/S0741-5214(03)00124-1
26. Bacci S, Di Paola R, Menzaghi C, Di Fulvio P, Di Silvestre S, Pellegrini F, et al. ENPP1 Q121 variant, increased pulse pressure and reduced insulin signaling, and nitric oxide synthase activity in endothelial cells. Arterioscler Thromb Vasc Biol (2009) 29(10):1678–83. doi:10.1161/ATVBAHA.109.189191
27. Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999 to 2004. Circulation (2008) 118(1):33–41. doi:10.1161/CIRCULATIONAHA.107.721878
28. Birney YA, Sweeney CH, Cappadona CR, Sitzmann JV, Cummins PM, Redmond EM, et al. Pulse pressure-induced transmural fluid flux increases bovine aortic smooth muscle cell apoptosis in a mitogen activated protein kinase dependent manner. J Vasc Res (2004) 41(4):364–74. doi:10.1159/000080700
29. Kiefer CR, McKenney JB, Trainor JF, Snyder LM. Pulse-pressure-driven neutral lipid accumulation and correlative proinflammatory markers of accelerated atherogenesis. Atherosclerosis (2005) 183(1):17–24. doi:10.1016/j.atherosclerosis.2005.02.023
30. Kajikawa M, Maruhashi T, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, et al. Borderline ankle-brachial index value of 0.91-0.99 is associated with endothelial dysfunction. Circ J (2014) 78(7):1740–5. doi:10.1253/circj.CJ-14-0165
31. Kullo IJ, Leeper NJ. The genetic basis of peripheral arterial disease: current knowledge, challenges, and future directions. Circ Res (2015) 116(9):1551–60. doi:10.1161/CIRCRESAHA.116.303518
32. Zhan Y, Dong Y, Tang Z, Zhang F, Hu D, Yu J. Serum uric acid, gender, and low ankle brachial index in adults with high cardiovascular risk. Angiology (2015) 66(7):687–91. doi:10.1177/0003319714566228
33. Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Esponda OL, et al. Impaired vascular endothelial growth factor A and inflammation in patients with peripheral artery disease. Angiology (2014) 65(8):683–90. doi:10.1177/0003319713501376
Keywords: pulse pressure, peripheral arterial disease, ankle brachial index, blood pressure, cohort study
Citation: Mao Y, Huang Y, Yu H, Xu P, Yu G, Yu J and Zhan Y (2017) Incidence of Peripheral Arterial Disease and Its Association with Pulse Pressure: A Prospective Cohort Study. Front. Endocrinol. 8:333. doi: 10.3389/fendo.2017.00333
Received: 02 August 2017; Accepted: 10 November 2017;
Published: 24 November 2017
Edited by:
Mingyi Wang, National Institutes of Health (NIH), United StatesReviewed by:
Francesca Seta, Boston University School of Medicine, United StatesJavier Ena, Hospital Marina Baixa, Spain
Copyright: © 2017 Mao, Huang, Yu, Xu, Yu, Yu and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haining Yu, eXUuaGFpbmluZ0BvdXRsb29rLmNvbQ==
 Yong Mao
Yong Mao Yixiang Huang2
Yixiang Huang2 Haining Yu
Haining Yu