- College of Nursing, University of Cincinnati, Cincinnati, OH, United States
The Metabolic Syndrome (MetS) is recognized as a predictor of cardiovascular outcomes and type 2 diabetes (T2DM). The MetS is a constellation of clinical and metabolic risk factors that include abdominal obesity, dyslipidemia, glucose intolerance, and hypertension. There are ethnic and racial differences in the prevalence of MetS and its components. In general, African-Americans have lower prevalence of MetS when compared to whites, but suffer disproportionately from higher cardiovascular mortality and T2DM. Specifically, African-American women (AAW) have higher rates of T2DM and cardiovascular mortality despite a more favorable lipid and lipoprotein profile. This is paradoxical. However, there is a general upward trend in the prevalence of MetS in the US. The reasons are debatable, but could be multifactorial, including genetics and environmental factors. Thus, there is a need to understand the increasing trend in the MetS, its components, and the associated outcomes for AAW. Therefore, the purpose of this mini review is to (1) understand the increasing prevalence of MetS and its components in AAW and (2) provide suggestions for future prevention of cardiovascular disease and T2DM in AAW.
Introduction
The metabolic syndrome (MetS) is a constellation of interrelated clinical and metabolic risk factors; including abdominal obesity, dyslipidemia, glucose intolerance, and hypertension that are associated with increased risk for cardiovascular disease (CVD) and type 2 diabetes (T2DM) (1–5). The mechanisms of MetS are not fully understood; however, it appears that insulin resistance is the major underpinning (6, 7). In fact, insulin resistance is related to obesity, T2DM, and hypertension all of which have dramatically increased over the last four decades. Ford et al. estimated the age-adjusted prevalence of the MetS to be 23.7% in the Third National Health and Nutrition Examination Survey (NHANES III) data (1988–1994), with similar prevalence for men (24%) and women (23.4%) (2). In the NHANES III, there were ethnic/racial differences in the prevalence of MetS, with African-American (AA) men having the lowest prevalence (16.4%) compared to white men (24.8%) and similar among AA women (AAW) (25.7%) and white women (22.8%) (2). In a recent report, Mozumdar and Liguori comparing the prevalence of the MetS in the NHANES III and NHANES 1999–2006 data found an increase in the age-adjusted prevalence of MetS from 29.2 ± 1.0 to 34.2 ± 0.7% (8). For AAW, the prevalence of MetS increased from 30.6 to 36.5%, respectively (8). This represented an absolute change of 5.9% and a relative change of 19.3% among AAW. The major increase in MetS for women was attributed to obesity [waist circumference (WC)] increased from 46.0 ± 1.4 to 58.0 ± 1.1%; hypertension 27.8 ± 0.9 to 36.6 ± 0.8%; hypertriglyceridemia 24.7 ± 1.2 to 27.6 ± 0.8%; hyperglycemia 24.2 ± 1.2 to 29.2 ± 1.0% (8). The authors found the greatest increase in MetS among young AAW, 20–39 years, which was attributed to increases in WC and hypertension (8). Moore et al. examined the increasing trends in MetS in the NHANES for three time-periods, 1988–1994, 1999–2006, and 2007–2012 and reported that MetS increased from 25.29, 24.99, and 34.17%, respectively (9). In this study, the prevalence of MetS for AAW increased from 14.48, 14.02 to 20.89%, compared to 15.28, 17.37 to 25.08% for white women, for the corresponding time-periods. There are several reasons for the increases in MetS among women; these include aging, increases in obesity, T2DM, hypertension, and physical inactivity. In addition, there were racial/ethnic differences in the components of MetS observed in the NHANES data. Because of the dipartites in T2DM and cardiovascular outcomes in AAW when compared to white women, it is of utmost importance to understand the trends of MetS and its components in AAW. Thus, the purpose of this Mini Review is to (1) understand the increasing prevalence of MetS and its components in AAW and (2) provide suggestions for future prevention of CVD and T2DM in AAW.
Definition of the MetS
In the US, the National Cholesterol Education Program (NCEP), Adult Treatment Panel ATP III (NCEP-ATP) is used to define MetS (Table 1) (1). The American Diabetes Association and the American Association of Clinical Endocrinologist has modified this definition to include a lower reference for glucose intolerance (10, 11).

Table 1. Metabolic syndrome criteria in women according to the National Cholesterol Education Program (NCEP), Adult Treatment Panel ATP III (NCEP-ATP).
According to NCEP-ATP, three or more of the following criteria constitutes MetS in women; abdominal obesity WC ≥88 cm; Hypertriglyceridemia ≥150 mg/dL; Low HDL-C <50 mg/dL; elevated blood pressure (BP) ≥130/85 mmHg; elevated fasting glucose ≥ 100 mg/dL (Table 1). Recent studies have shown ethnic/racial differences in not only the MetS but also its components (2, 4, 8, 9).
Components of the MetS in AAW
Obesity
The prevalence of obesity has increased steadily over the past decades with over two-thirds of US adults being either overweight or obese (12–14). Obesity is clinically defined as body mass index (BMI) >30 kg/m2. However, data suggest that a more accurate reflection of true metabolic risk is central adiposity, assessed clinically by WC (15). Conversely, WC plays a critical role in the development of MetS and appears to antecede the development of other MetS components (4, 12, 14). Thus, the debate as to whether WC is superior to other anthropometric indices is ongoing and varies among gender and racial/ethnic groups (16).
The NCEP-ATP defines abdominal obesity as WC >88 cm in women and varies among racial/ethnic populations (1). Ford et al., using the NHANES III data, reported 46.3% of women had abdominal obesity (37.2% white and 44.6% black) (2). There has been an increasing trend in WC for AAW, according to NHANES, 1988–1994 (38.25%); 1999–2006 (56.7%); and 2007–2012 (68.8%) (9). Therefore two-thirds of AAW manifest abdominal obesity. In a study by our group, 35.5% of AAW had MetS (17). Most importantly, WC was the most common parameter to likely meet the MetS in our studies (17, 18). In the Jackson Heart Study (JHS), 76.5% of AAW had WC ≥88 cm (5). In another study of AAs and whites, 30–64 years, WC was the most powerful tool to predict MetS (19). Furthermore, Shen and colleagues reported that WC had the strongest association with health-risk indicators, followed by BMI and MetS components (15).
Another factor that contributes to MetS is intraabdominal visceral adiposity. There are ethnic/racial differences in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) (20–22). In general, VAT is more pathogenic and has a stronger association with insulin resistance and MetS (20–22). For the same BMI, AAW have lower VAT when compared to their white counterparts (20–23). However, AAW are more insulin resistant despite the lower VAT. This is paradoxical. In a study by Liu et al. examining SAT and VAT with cardiometabolic risk factors between AAW in the JHS and white women in the Framingham Heart Study (FHS) found that the associations between VAT were stronger in the FHS than in the JHS (20). These results may partly contribute to the apparent paradox of lower VAT in the setting of cardiometabolic risk factors in AAW when compared to their white counterparts. However, there were limitations in conduct of this study. First, there were different protocols for the CT-scan measurement of adipose tissue volume. Second, both studies were single center studies of AA and white women, thus the results cannot be generalizable. Third, these studies were cross-sectional and, therefore, the authors could not determine the causal relationship between abdominal VAT, SAT, and MetS. Of note, similar paradoxical relationships have been found in AA children (24), adolescence (25), young adults (26), and older adults in the Insulin Resistance Atherosclerosis Study (IRAS) (27) and Atherosclerosis Risk in the Community (ARIC) (28) studies. The reasons for the paradoxical relationship between VAT and obesity remain unknown, but may be related to other environmental and genetic factors. Thus, the consequences of VAT and cardiometabolic risk factors warrant further investigation in AAW.
High Blood Pressure
The prevalence of hypertension among AAs is one of the highest is the world. Hypertension is more common in AAs than whites and occurs in approximately 41.2 vs 28%, respectively (29, 30). Data from NHANES demonstrate that the prevalence of hypertension in young adults, 20–40 years is twofold higher in AAs than in their white counterparts (9). The higher rates of hypertension in AAs contribute to the excess CVD morbidity and mortality in AAs with and without diabetes, lipid/lipoprotein disorders, or obesity (2–6, 8, 9, 18, 19, 24, 25, 30, 31). The American Heart Association reports that AAW suffer disproportionally from higher rates hypertension and CVD mortality when compared to white women (32). The exact causes of hypertension in AAW are unknown. However, it has been speculated that genetic and environmental factors, increased vascular reactivity, and increase renal sodium reabsorption are contributing factors (32, 33). Insulin resistance has been associated with BP in several populations; however, there is a very weak relationship in AAs when compared to whites (6, 7, 20, 21, 26–30). This is paradoxical in AAs.
There is evidence for increasing trends in BP in the NHANES cohort from 1988 to 1999 (33.92%), 1999 to 2006 (40.62%), and 2007 to 2012 (42.72%) (9). In addition, the prevalence of hypertension also increased among AAW for the same time-periods (38.5, 45.1, and 50.1%, respectively) (Table 2). Hence, Gaillard et al. found that 35.7% of AAW with family history of T2DM were hypertensive, and that increasing BP was associated with increasing prevalence of MetS and its components (31). Similarly, the JHS reported 73.1% of AAW were hypertensive (5). Given the havoc and the cardiovascular morbidity and mortality associated with hypertension, it is important to initiate early intervention strategies in AAW to control BP.
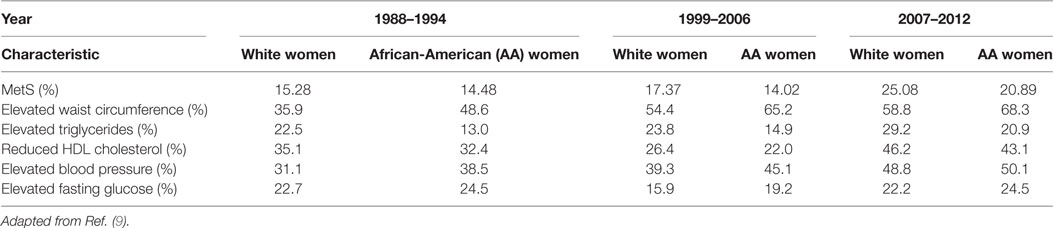
Table 2. Prevalence of metabolic syndrome (MetS) in US white and African-American female adults, National Health and Nutrition Examination Survey, 1988–2012.
Glucose Intolerance and T2DM
Diabetes and prediabetes has become epidemic in the US affecting 29.1 million and 89 million adults, in the US, respectively (34). Patients with prediabetes and T2DM have greater cardiovascular risks and events (i.e., congestive heart failure, stroke) as well as the associated morbidity and mortality than in non-diabetic subjects (34, 35).
The Center for Disease Control and Prevention report that risk of diabetes is 77% higher among AAs than among whites.1 In fact, AAW are twice as likely to be diagnosed with T2DM when compared to whites (36, 37). The reasons for the higher prevalence of diabetes in AAs are associated with genetics and environmental factors (i.e., low physical activity, poor dietary/nutrition practices, stress). Most importantly, the fundament lesion for T2DM is beta-cell dysfunction and insulin resistance, which are higher in AAW when compared to white women (38–41). Similar findings have been demonstrated in AA children (29, 41), young adults (26), and middle aged adults in the IRAS (27) and ARIC (28) studies.
Type 2 diabetes is an outcome of MetS. Therefore, individuals with T2DM have higher prevalence of MetS that those without MetS. AAs have a disproportionate higher burden of T2DM, independent of BMI when compared to whites (20, 28, 36, 37, 39). The higher prevalence of glucose intolerance, prediabetes, and T2DM has been attributed in part to the greater insulin resistance in AAs compared to whites (37–42). In particular, AAW, with and without glucose intolerance, manifest greater insulin resistance than their white counterparts (36–39). In this regard, interventions such as the Diabetes Prevention Program (DPP) are effective strategies to improve insulin resistance and prevent progression to diabetes (43). In this regard, 53% of subjects in the DPP had MetS (44).
In the NHANES, the prevalence of fasting glucose meeting the NCEP-ATP criteria did not significantly change over the three time-periods, including AAW (Table 2). The lack of remarkable changes in fasting glucose over time is surprising in view of the increases in obesity among AAW. Thus, obesity is considered a major contributor to glucose dysregulation and insulin resistance. Nevertheless, it may be possible that AAW may be more sensitive to adverse changes in obesity given their higher prevalence of diabetes compared to whites (45). In addition, changes in the categories and classification of glucose intolerance, prediabetes, and T2DM may also contribute to the lack of significant increases in T2DM (10, 42, 46). In this regard, Bullard et al. examined the estimated prevalence of prediabetes in the NHANES (1999–2010) and reported a 21% increase (46). The authors reported the greatest increase was found among adolescent girls and women.
Prediabetes is a precursor to T2DM and is associated with higher cardiovascular risk and events in several populations (36–42, 46). In this regard, Osei et al. reported that first-degree relatives of AAs with normal glucose tolerance who progressed to impaired glucose tolerance and T2DM after 6 years manifest insulin resistance, beta cell dysfunction, and weight gain before diagnosis of T2DM compared to those who did not progress to T2DM (47). In the IRAS study, D’Agostino et al. examined whether CVD risk factors predicted future development of T2DM in 5 years (48). The authors concluded that for each CVD risk factor (hypertension, hypertriglyceridemia, low HDL-C, and impaired glucose tolerance) there was a doubling of risk for conversion to diabetes when compared to those without risk factors (48). Most importantly, impaired glucose tolerance was the strongest risk factor associated with conversion to diabetes in this multiethnic population, which included AA (39).
Dyslipidemia
Dyslipidemia is defined as abnormalities in cholesterol, low density lipoprotein cholesterol (LDL-C), hypertriglyceridemia, and HDL-C (1, 3, 4, 6, 7, 20, 32, 49). These alterations in lipids/lipoproteins have been associated with increased cardiovascular morbidity and mortality (i.e., myocardial infarction, stroke, and heart failure). Thus, dyslipidemia constitute a leading cause of deaths in the US. With respect to the MetS, high triglycerides and low HDL-C are major components. The traditional lipid profile found in MetS has been attributed to insulin resistance and obesity. Major epidemiological studies have confirmed this atherogenic profile in several ethic/racial populations (49–51). However, AAs have lower triglycerides and higher HDL-C levels than whites (48–50). Thus, theoretically, this favorable lipid profile should protect AAs from coronary heart disease. Surprisingly, AAs with insulin resistance have lower triglycerides and higher HDL-C when compared to whites (15–18, 26–30). Thus, there is a paradoxical relationship between lipids/lipoproteins and CVD in AAs (6). This paradox has been found in AA children in the Bogalusa Heart Study (25, 41), in young adults in the CARDIA study (26), and for adults in the ARIC (28), IRAS (27, 37), and JHS (5).
The increasing prevalence of obesity in the US predisposes the population to alterations in lipids/lipoproteins. In the NHANES—1988–1994, 1999–2006, 2007–2012, there has been a modest increase in the prevalence of elevated triglycerides and low HDL-C levels, including AAW (Table 2). The reasons are uncertain, but could reflect the increasing rate of obesity, lack of physical activity, and higher caloric consumption among AAW (52). In addition, several studies have examined the subclasses of lipids/lipoproteins using nuclear magnetic resonance (NMR) technique to study the atherogenic properties of the lipoprotein concentration and particle size. Traditionally, measured LDL-C levels are positively and HDL-C levels are negatively associated with coronary heart disease in whites, but it remains controversial in blacks (53–57). In contrast, NMR-derived HDL particles inversely correlated with incident coronary heart disease in all populations, including Blacks (56, 57). Thus, we have previously demonstrated that AAW have more favorable NMR-derived lipoprotein profiles (53). But these findings cannot explain the paradoxically higher CVD mortality in AAW, thus requires further elucidation (6, 53–57).
Genetics of the MetS
The MetS consists of five components that individually may be genetically determined. Thus, several authorities have argued and debated if there is a single genetic entity associated with MetS (58–62). Moreover, other authorities have argued that MetS is a sum of its components and a single genetically determined Mets does not exist (60–63). Also, there is no known genetic mutation that has been identified as a genetic predictor or marker for MetS. Furthermore, there has not been a single nucleotide polymorphism identified for MetS (58–63). Given the current scanty literature on this issue, there is urgent need to conduct more genetic studies in AAs and ethnic/racial populations with MetS.
Prevention of Met Syndrome
The major determinant of the components of MetS is obesity, which is associated with insulin resistance. Thus, weight loss has been recommended for managing and preventing MetS. In this context, the DPP, the largest diabetes primary intervention program of multiethnic/multiracial US adults with prediabetes has been very effective in preventing progression to T2DM (43). In the DPP, 53% of patients had MetS (44). Most remarkable, the DPP recommended modest lifestyle changes, specifically 7% weight loss, combining caloric restriction (low fat) and increases in physical activity (150 min/week). These modest lifestyle recommendations resulted in reduction in the development of T2DM by 58% in all the populations, including AAs compared to 31% in those receiving metformin (43). The success of this program has been adapted by the Centers for Disease Control and Prevention and other health-care organizations (64, 65). Thus, the benefits of DPP extend beyond weight loss and included lower glucose, lipids/lipoproteins levels and improved BP all of which are components of MetS. It is worthy to note that the improvement in MetS components had been sustained for at least 20 years in individuals who participated in the DPP program (44).
Strengths and Limitations
In this mini review, we examined the literature and data for trends in the MetS and its components from NHANES 1988 to 2012 (2–9). The strength in this data is the large sample size of US represented adults and the ability to compare sequential data from multiple studies. A major limitation is that we did not perform analysis of the raw data nor was this a meta-analysis. Therefore, the data consist of only literature review with emphasis on AAW. Therefore, any conclusion is tentative and would require validation studies. In addition, we did not measure other factors such as, inflammation, oxidation, socioeconomic status, and environmental factors that are also known to be associated with the MetS in other large epidemiological studies (66–69). Moreover, there is only limited information on genetics and MetS in AAW. Other studies examining the contributions of these factors in AAW are warranted.
In summary, this mini review, discussed the increasing prevalence of MetS and its components in the NHANES data from 1988 to 2012. The data demonstrate and increase in MetS among US and AAW. We conclude that the increasing prevalence of obesity and its associated risk factors contribute to the increases in MetS in the US and in AAW. Given these observations, we believe intervention strategies (i.e., DPP) aimed at reducing obesity should be a national priority in combating MetS and its associated cardiovascular outcomes and T2DM, especially among AAW.
Author Contributions
The author has developed the ideas and content of the manuscript.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnote
References
1. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive Summary. Circulation (2005) 112:e285–9. doi:10.1161/CIRCULATIONAHA.105.169405
2. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA (2002) 287(3):356–9. doi:10.1001/jama.287.3.356
3. Clark LT, El-Atat F. Metabolic syndrome in African Americans: implications for preventing coronary heart disease. Clin Cardiol 2007; 30:161–164. doi:10.1002/clc.20003
4. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988e1994. Arch Intern Med (2003) 163:427e36. doi:10.1001/archinte.163.4.427
5. Taylor H, Liu J, Wilson G, Golden SH, Brunson CD, Steefes M, et al. Distinct component profiles and high risk among African Americans with metabolic syndrome. The Jackson Heart Study. Diabetes Care (2008) 31:1248–53. doi:10.2337/dc07-1810
6. Gaillard T, Schuster D, Osei K. Metabolic syndrome in Black people of African diaspora: the paradox of current classification, definition and criteria. Ethn Dis (2009) 19(2 Suppl 2):S2–1-7.
7. Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J (2005) 149:33–45. doi:10.1016/j.ahj.2004.07.013
8. Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. Adults: NHANES III to NHANES 1999–2006. Diabetes Care (2011) 34:216–9. doi:10.2337/dc10-0879
9. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis (2017) 14:160287. doi:10.5888/pcd14.160287
10. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care (2002) 25(Suppl 1):S33–49. doi:10.2337/diacare.25.2007.S33
11. The American Association of Clinical Endocrinologists (AACE). Medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management – 2002 update. Endocr Pract (2002) 8(Suppl 1):40–64. doi:10.4158/EP.8.S1.40
12. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA (2002) 288(14):1723–7. doi:10.1001/jama.288.14.1723
13. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA (2014) 311(8):806–14. doi:10.1001/jama.2014.732
14. Ogden CL. Disparities in obesity prevalence in the United States: black women at risk. Am J Clin Nutr (2009) 89(4):1001–2. doi:10.3945/ajcn.2009.27592
15. Shen W, Punyanitya M, Chen J, Gallager D, Albu A, Pi-Sunyer X, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity (2006) 14(4):727–36. doi:10.1038/oby.2006.83
16. Sumner AE, Sen S, Ricks M, Frempong BA, Sebring NG, Kushner H. Determining the waist circumference in African Americans which best predicts insulin resistance. Obesity (Silver Spring) (2008) 16:841–6. doi:10.1038/oby.2008.11
17. Gaillard T, Schuster D, Osei K. Differential impact of serum glucose, triglycerides and HDL-C on cardiovascular risk factor burden in nondiabetic, obese African American Women: implications for the prevalence of metabolic syndrome. Metabolism (2010) 59(8):1115–23. doi:10.1016/j.metabol.2009.09.035
18. Meis SB, Schuster D, Gaillard T, Osei K. Metabolic syndrome in non-diabetic, obese first degree relatives of African American patients with type 2 diabetes. African American triglycerides-HDL and insulin resistance paradox. Ethn Dis (2006) 16(4):830–6.
19. Beydoun MS, Kuczmarski MT, Wang Y, Mason MA, Evans MK, Zonderman AB. Receiver-operating characteristics of adiposity for metabolic syndrome: the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Public Health Nutr (2011) 14(1):77–92. doi:10.1017/S1368980010002648
20. Liu J, Coady S, Carr JJ, Hoffmann U, Taylo HA, Fox CS. Differential associations of abdominal visceral, subcutaneous adipose tissue with cardiometabolic risk factors between African and European Americans. Obesity (2014) 22:811–8. doi:10.1002/oby.20307
21. Wildman RP, Janssen I, Khan UI, Thurston R, Barinas-Mitchell E, El Khoudary SR, et al. Subcutaneous adipose tissue in relation to subclinical atherosclerosis and cardiometabolic risk factors in midlife women. Am J Clin Nutr (2011) 93:719–26. doi:10.3945/ajcn.110.007153
22. Carroll JF, Fulda KG, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, et al. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) (2009) 17(7):1420–7. doi:10.1038/oby.2008.657
23. Despres JP. Visceral obesity, insulin resistance, and dyslipidemia: contribution of endurance exercise training to the treatment of the plurimetabolic syndrome. Exerc Sport Sci Rev (1997) 25:271–300.
24. Weiss R, Dziura J, Burget TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med (2004) 350(23):2362–74. doi:10.1056/NEJMoa031049
25. Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis (2012) 22:141–8. doi:10.1016/j.numecd.2010.05.006
26. Carnethon MR, Loria CM, Hill JO, Sidney S, Savage PJ, Liu K. Risk factors for the metabolic syndrome the coronary artery risk development in young adults (CARDIA) study, 1985–2001. Diabetes Care (2004) 27:2707–15. doi:10.2337/diacare.27.11.2707
27. Palaniappan L, Carnethon MR, Wang Y, Hanley AJ, Fortmann SP, Haffner SM, et al. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care (2004) 27(3):788–93. doi:10.2337/diacare.27.3.788
28. Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity and the incidence of type 2 diabetes in black and white adults. Atherosclerosis Risk in the Community (ARIC) 1987–1998. Diabetes Care (2002) 25(8):1358–64. doi:10.2337/diacare.25.8.1358
29. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief (2013) 133:1–8.
30. Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension United States, 2003 to 2012. Hypertension (2015) 65:54–61. doi:10.1161/HYPERTENSIONAHA.114.04012
31. Gaillard T, Schuster D, Osei K. Independent role of blood pressure on cardiovascular risk factors in nondiabetic, obese African-American women with family history of type 2 diabetes: implications for metabolic syndrome components. J Am Soc Hypertens (2009) 3(1):25–34. doi:10.1016/j.jash.2008.07.003
32. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation (2015) 131:e29–322. doi:10.1161/CIR.0000000000000157
33. Adbul-Ghani MA, DeFronzo RA. Lowering plasma glucose concentration by inhibiting renal sodium-glucose cotransport. J Intern Med (2014) 276(4):352–63. doi:10.1111/joim.12244
34. American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care (2017) 40(Suppl 1):S1–135.
35. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht M, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med (2010) 362:800–11. doi:10.1056/NEJMoa0908359
36. Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care (2006) 29:1263–8. doi:10.2337/dc06-0062
37. Lee CC, Haffner SM, Wagenknecht LE, Lorenzo C, Norris JM, Bergman RN, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care (2013) 36:901–7. doi:10.2337/dc12-1316
38. Osei K, Gaillard T, Schuster DP. Pathogenetic mechanisms of impaired glucose tolerance and type II diabetes in African-Americans. The significance of insulin secretion, insulin sensitivity, and glucose effectiveness. Diabetes Care (1997) 20(3):396–404. doi:10.2337/diacare.20.3.396
39. Lorenzo C, Wagenknecht LE, D’Agostino RB Jr, Rewers MJ, Karter AJ, Haffner SM. Insulin resistance, β-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes Care (2010) 33:67–72. doi:10.2337/dc09-1115
40. Osei K, Gaillard T. Ethnic difference in glucose effectiveness and disposition index in overweight/obese African American and White women with prediabetes: a study of compensatory mechanisms. Diabetes Res Clin Pract (2017) 130:278–85. doi:10.1016/j.diabres.2017.02.020
41. Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood. The Bogalusa Heart Study. Arch Pediatr Adolesc Med (2010) 164(2):124–8. doi:10.1001/archpediatrics.2009.268
42. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA (2015) 314(10):1021–9. doi:10.1001/jama.2015.10029
43. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med (2002) 346:393–403. doi:10.1056/NEJMoa012512
44. Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med (2005) 142:611–9. doi:10.7326/0003-4819-142-8-200504190-00009
45. Albrecht SS, Mayer-Davis E, Popkin BM. Secular and race/ethnic trends in glycemic outcomes by BMI in US adults: the role of waist circumference. Diabetes Metab Res Rev (2017) 33(5):e2889. doi:10.1002/dmrr.2889
46. Bullard KM, Saydah SH, Imperatore G, Cowie CC, Gregg EW, Geiss LS, et al. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care (2013) 36:2286–93. doi:10.2337/dc12-2563
47. Osei K, Rhinesmith S, Gaillard T, Schuster D. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care (2004) 27(6):1439–46. doi:10.2337/diacare.27.6.1439
48. D’Agostino RB Jr, Hamman RF, Karter AJ, Mykkanen L, Wagenknecht LE, Haffner SM, et al. Cardiovascular disease risk factors predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care (2004) 27(9):2234–40. doi:10.2337/diacare.27.9.2234
49. Haffner SM, D’Agostino R, Goff D, Howard B, Festa A, Saad MF, et al. LDL size in African Americans, Hispanics, and non-Hispanic whites: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol (1999) 19(9):2234–40. doi:10.1161/01.ATV.19.9.2234
50. Sumner AE, Finley KB, Genovese DJ, Criqui M, Boston R. Fasting triglycerides and the triglyceride-HDL-cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med (2005) 165:1395–400. doi:10.1001/archinte.165.12.1395
51. Li C, Ford ES, Meng YX, Mokdad AH, Reaven GM. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol (2008) 7:4. doi:10.1186/1475-2840-7-4
52. Carson JAS, Michalsky L, Latson B, Banks K, Tong L, Gimpel N, et al. The cardiovascular health of Urban African Americans: diet-related results from the genes, nutrition, exercise, wellness, and spiritual growth (GoodNEWS) trial. J Acad Nutr Diet (2012) 112:1852–8. doi:10.1016/j.jand.2012.06.357
53. Gaillard T, Osei K. Ethnic differences in serum lipids and lipoproteins in overweight/obese African-American and white American women with pre-diabetes: significance of NMR-derived lipoprotein particle concentrations and sizes. BMJ Open Diabetes Res Care (2016) 4:e000246. doi:10.1136/bmjdrc-2016-000246
54. Srinivasan SR, Li S, Chen W, Tang R, Bond MD, Boerwinkle E, et al. Q192R polymorphism of the paraoxanase 1 gene and its association with serum lipoprotein variables and carotid artery intima-media thickness in young adults from a biracial community. The Bogalusa Heart Study. Atherosclerosis (2004) 117(1):167–74. doi:10.1016/S0021-9150(04)00372-7
55. Davis KA, Crow JA, Shambers HW, Meek EC, Chambers JE. Racial differences in paraoxonase-1 (PON1): a factor in the health of southerners? Environ Health Prespect (2009) 117(8):1126–31. doi:10.1289/ehp.0900569
56. Vora AN, Ouyang P, Bittner V, Tardif JC, Walters DD, Vaidya D. Racial differences of lipoprotein subclass distributions in postmenopausal women. Ethn Dis (2008) 18(2):176–80.
57. Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation (2013) 128:1189–97. doi:10.1161/CIRCULATIONAHA.113.002671
58. Loos RJF, Katxmarzyk PT, Rao DC, Rice T, Leon AS, Skinner JS, et al. Genorme-wide linkage scan for the metabolic syndrome in the HERITAGE Family Study. J Clin Endo Meta (2003) 88(12):5935–43. doi:10.1210/jc.2003-030553
59. Liang J, Le TH, Edwards ERV, Tayo B, Gaulton KJ, Smith JA, et al. Single-trait and multi-trait genome-wide association analyses identify novel loci for blood pressure in African-ancestry populations. PLoS Genet (2017) 13(5):e1006728. doi:10.1371/journal.pgen.1006728
60. Edwards KL, Huttle CM, Wan JY, Kim H, Monks SA. Genome-wide linkage scan for the metabolic syndrome: the GENNID study. Obesity (2008) 16:1596–601. doi:10.1038/oby.2008.236
61. Klimentidis YC, Arora A, Zhou J, Kittles R, Allison DB. The genetic contribution of West-African ancestry to protection against central obesity in African-American men but not women: results form the ARIC and MESA studies. Front Genet (2016) 7:89. doi:10.3389/fgene.2016.00089
62. Taylor JY, Sun YV, Hunt SC, Kardia SLR. Hypertension among African American women across generations. Biol Res Nurs (2010) 12(2):149–55. doi:10.1177/1099800410371225
63. Smith LM, Yao-Borengasser A, Starks T, Tripputi M, Kern PA, Rasouli N. Insulin resistance in African-American and Caucasian women: differences in lipotoxicity, adipokines, and gene expression in adipose tissue and muscle. Clin Endocrinol Metab (2010) 95(9):4441–8. doi:10.1210/jc.2010-0017
64. Ely EK, Gruss SM, Luman ET, Ali MI, Nhim K, Rolka DB, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care (2017) 40(10):1331–41. doi:10.2337/dc16-2099
65. Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the US. The National Diabetes Prevention Program. Am J Prev Med (2013) 44:S346–51. doi:10.1016/j.amepre.2012.12.009
66. Hyatt TC, Phadke RP, Hunter GR, Bush NC, Munoz AJ, Gower BA. Insulin sensitivity in African-American and white women: association with inflammation. Obesity (Silver Spring) (2009) 17(2):276–82. doi:10.1038/oby.2008.549
67. Hill JO, Galloway JM, Goley A, Marrero DG, Minners R, Montgomery B, et al. Scientific statement: socioecological determinants of prediabetes and type 2 diabetes. Diabetes Care (2013) 36(8):2430–9. doi:10.2337/dc13-1161
68. Gaillard TR, Schuster DP, Bossetti BM, Green PA, Osei K. The impact of socioeconomic status on cardiovascular risk factors in African Americans at high risk for type II diabetes: implications for syndrome X. Diabetes Care (1997) 20(5):745–52. doi:10.2337/diacare.20.5.745
Keywords: metabolic syndrome, African-American women, obesity, hypertension, type 2 diabetes
Citation: Gaillard TR (2018) The Metabolic Syndrome and Its Components in African-American Women: Emerging Trends and Implications. Front. Endocrinol. 8:383. doi: 10.3389/fendo.2017.00383
Received: 14 July 2017; Accepted: 26 December 2017;
Published: 22 January 2018
Edited by:
Elias S. Siraj, Eastern Virginia Medical School, United StatesCopyright: © 2018 Gaillard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trudy R. Gaillard, dHJ1ZHkuZ2FpbGxhcmRAdWMuZWR1, dGdhaWxsYXJAZml1LmVkdQ==
†Present address: Trudy R. Gaillard, Nicole Wertheim College of Nursing and Health Sciences, Florida International University, Miami, FL, Unites States
 Trudy R. Gaillard
Trudy R. Gaillard