- 1IRCCS Istituto Nazionale Tumori “Fondazione G. Pascale”, Naples, Italy
- 2Parthenope University of Naples, Naples, Italy
KiSS-1 was first described as a metastasis suppressor gene in malignant melanoma. KiSS-1 encodes a 145 amino-acid residue peptide that is further processed, producing the 54 amino acid metastin and shorter peptides collectively named kisspeptins (KPs). KPs bind and activate KiSS-1R (GPR54). Although the KPs system has been extensively studied for its role in endocrinology of reproductive axis in mammals, its role in cancer is still controversial. Experimental evidences show that KP system exerts an anti-metastatic effect by the regulation of cellular migration and invasion in several cancer types. However, the role of KPs/KiSS-1R is very complex. Genomic studies suggest that KiSS-1/KiSS-1R expression might be different in the various stages of tumor development. Furthermore, overexpression of KiSS-1R has been reported to elicit drug resistance in triple negative breast cancer. In this review, we focused on multiple functions exerted by the KPs/KiSS-1R system in regulating tumor progression.
Introduction
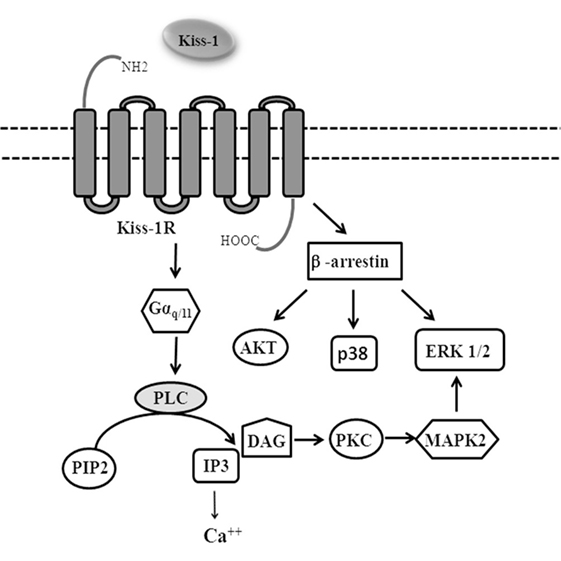
KiSS-1 gene, located on human chromosome 1q32, encodes a precursor peptide of 145 amino acids that subsequently is processed by proteolytic cleavage into shorter peptides collectively defined as kisspeptins (KPs): KP-10, KP-13, KP-14, and KP-54 (metastin). KPs bind to G-protein-coupled receptor 54 (GPR54) also named KiSS-1R that activates the G proteins Gαq/11 (1, 2).
High levels of KiSS-1 have been found in the placenta and in the brain (3) and high expression levels of KiSS-1R were observed to in the placenta, pituitary, pancreas, and spinal cord (2). Low levels of KiSS-1R are present in lymph nodes, peripheral blood lymphocytes, adipose tissue, and spleen (1, 3).
KiSS-1/KiSS-1R coupled to Gαq/11 activates phospholipase C (PLC). PLC activation promotes the hydrolysis of phosphatidylinositol-4,5-bisphosphate which, in turn, leads to the production of two potential “second messengers” inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). The activation of DAG leads to the activation of protein kinase C, ERK1/2, and p38 phosphorylation, while IP3 induces release of intracellular Ca++ (2, 4–6) (Figure 1).
G-protein-coupled receptors are regulated by β-arrestins, which not only desensitizes G-protein signaling, but also acts as molecular scaffolds and activates a series of signaling pathways including ERK 1/2, p38, PI3K/Akt, and cJun N-terminal kinase 3 (7, 8).
G-protein-coupled receptor serin/threonine kinase GRK2 and β-arrestins promote KiSS-1R desensitization by internalization via clathrin-coated pits (9, 10).
The anti-metastatic role of KP has been identified by early studies performed in melanoma and breast cancer cells before its role in regulating the reproductive functions. Although the anti-metastatic role has not been studies extensively as compared to the reproductive function, nevertheless a large body of literature documented the involvement of the KiSS-1/KiSS-1R system in supporting tumor progression. Furthermore, recent studies have demonstrated that plasma levels of KPs are increased in colorectal cancer (CRC) and small renal tumor patients, suggesting that KPs may be considered plasmatic biomarkers in these tumors (11, 12).
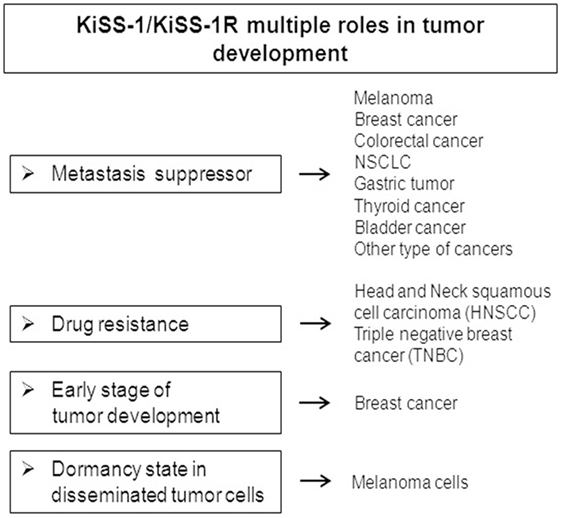
In this review, we focused on multiple functions exerted by the KPs/KiSS-1R system in cancer (Figure 2).
KiSS-1 as a Metastasis Suppressor Gene
First Evidence in Melanoma and Breast Cancer
KiSS-1 was primarily identified as a human melanoma metastasis suppressor gene using subtractive hybridization between the metastatic human melanoma cell line C8161 and non-metastatic variants generated after microcell-mediated transfer of chromosome 6 into C8161 cells to suppress their ability to metastasize (13, 14).
Transfection of KiSS-1 into metastatic human melanoma cell lines suppressed metastasis in athymic nude mice by 50–95% (13). Thus, since KiSS-1 map to the long arm of chromosome 1 and its expression suppresses metastasis of melanoma, it was hypothesized that some regulators of KiSS-1 are encoded by chromosome 6 (14). Indeed, a region of 40cM between 6q16.3 and q23 was identified as an important regulatory region of KiSS-1 (15). Moreover, Shirasaki and co-workers documented the loss of 6q16.3-q23 in more than 50% of melanoma metastases and that loss of heterozygosis of this region associates with the loss of KiSS-1 (16).
Only 1 year after the first paper on melanoma, Lee and colleagues conducted another study on breast cancer demonstrating that human breast carcinoma MDA-MB-435 cells transfected with KiSS-1 expression vector exhibit a lower metastatic, but not tumorigenic potential in comparison with control cells. This study was based on the observations that 1q chromosome is frequently deleted in late stage of human breast carcinomas and that KiSS-1 is located on chromosome 1q32-q41 (17).
The studies that followed to better underline the anti-metastatic role of KiSS-1 in breast cancer, instead, revealed the existence of controversial data for this type of tumors.
In fact a study conducted in 2005 by Martin and colleagues demonstrated that KiSS-1 may not be functioning as a metastasis suppressor in breast cancer cells (18).
They found that the expression of KiSS-1 mRNA had significantly increased in primary tumors in comparison with normal mammary tissues; they also found that levels of KiSS-1 expression were higher in metastatic disease patients compared to healthy individuals, and that this was associated with poor patient prognosis.
This study contrasted the reports which supported the anti-metastatic potential of KiSS-1 in breast cancer. The analysis of KiSS-1 mRNA levels in fresh frozen tissue samples from ductal invasive breast carcinomas revealed a significant reduction of KiSS-1 expression in brain metastases as compared to the primary tumors (19). These results were confirmed in 2012 by Ulasov and co-workers, who found a statistically significant downregulation of KiSS-1 mRNA and protein in brain metastasis compared to primary tumors (20).
So, even though several studies have identified the KiSS-1 gene as a metastasis suppressor in breast cancer, the existence of contradictory data underlines the need to better elucidate its biological role in this particular cancer.
Breast tumors are divided into estrogen receptor α (ERα)-positive and ERα-negative tumors and the role of KiSS-1 and KiSS-1R in the positive group are conflicting.
Estradiol (E2) through its receptor ERα is involved in mammary ductal normal growth and development. It is well established that estrogen is associated with increased breast cancer risk (21).
It has been demonstrated that E2 negatively regulates KiSS-1 and KiSS-1R expression. They showed that in ERα-positive primary tumors, KiSS-1 levels are lower than ERα-negative tumors (22, 23). In contrast, Jarzabek and colleagues demonstrated that ERα-positive breast tumors express higher levels of both KiSS-1 and KiSS-1R than ERα-negative tumors (24).
Recently, KiSS-1R has been documented to induce invasion of triple negative breast cancer (TNBC) cells which lack ERα, progesterone receptor, and human epidermal growth factor receptor 2; also, KiSS-1 mRNA and KiSS-1R mRNA and protein were found to be upregulated in TNBC tissues as compared to normal breast tissue (25).
So far, it is not possible to assign a clear role to KiSS-1/KiSS-1R system in regulating the progression of ERα-positive and ERα-negative breast tumors as the complex cross-talk between KiSS-1 expression and signaling pathways regulated by ERα deserve further investigation.
Studies on Other Tumors
Since the development of metastases is one of the most dangerous complications of solid tumors, many efforts are conducted to discover and characterize new potential anti-metastatic targets.
Colorectal cancer is identified as one of the most frequent and deadly types of cancer; it represents the second most common tumor among women and the third most common among men (26).
A major complication of CRC is disease progression via liver metastases.
Metastases from CRC are strictly associated with matrix metalloproteinase (MMP)-9 expressions (27, 28).
The analysis of KiSS-1 and KiSS-1R expression in colorectal liver metastases showed the existence of a correlation with the patients’ prognosis. CRC tissues with low levels of KiSS-1 express high levels of MMP-9 and metastasize more frequently to distant sites (29).
Accordingly, Chen et al. showed that KiSS-1 gene represses the metastatic potential of CRC cells by inhibiting the expression of MMP-9. Overexpression of KiSS-1 suppressed the proliferation and the invasiveness of HCT-119 CRC cells and enhanced their apoptosis by reducing the expression of MMP-9 through blocking PI3K/Akt/NF-κB pathway (30).
The analysis of KiSS-1 and KiSS-1R expression in normal and malignant tissue samples from 111 patients with colorectal adenocarcinoma showed that KiSS-1 expression levels were much higher in the normal than in the malignant colonic mucosa (31).
Regarding malignant tissues, it has been shown that the expression level of KiSS-1 had a negative correlation with Dukes staging, TNM (tumor, lymph node, and metastasis) staging, tumor size, and lymph node involvement. Reduction of KiSS-1R was also linked to poor prognosis of the patients (32).
Studies of comparison of the miRNA expression profiles in CRC tissues and hepatic metastasis revealed that down-regulation of miR-199b associates with distant metastasis in CRC and a longer median survival. Through human tumor metastasis PCR array, Shen et al. identified KiSS-1 as one of the downstream targets of SIRT1: silencing of SIRT1 upregulates KiSS-1 expression by enhancing the acetylation of the transcription factor CREB which, in turn, may be activated through its binding to the promoter of KiSS-1. Thus, miR-199b regulates SIRT1/CREB/KiSS-1 signaling pathway and might serve as a prognosis marker for patients with CRC (33).
So KiSS-1 and MMP-9 could be considered as prognostic markers in patients with CRC.
An inverse correlation between KiSS-1 and MMP-9 has been demonstrated also in non-small cell lung cancer (NSCLC) patients. It has been reported that KiSS-1 and MMP-9 mRNA and protein correlate with disease stage, metastasis, and survival of the patients. In particular, KiSS-1 expression was found to be lower in the metastatic tissues as compared to the primary tumors, supporting the notion that KiSS-1 may be considered as a metastasis suppressor in NSCLC (34).
The potential use of KiSS-1 and KiSS-1R as favorable prognostic markers in NSCLC has been confirmed by Sun et al. in 2013 (35). They analyzed 56 NSCLC specimens divided into low stage (locally advanced) and metastatic (advanced) disease and they found an inverse correlation between KiSS-1 and KiSS-1R expression and NSCLC progression. In particular, they assessed that the expression levels of KiSS-1 and KiSS-1R were lower in cancer tissues compared to normal tissues; moreover, KiSS-1 and KiSS-1R expression was lower in patients with advanced stage compared to patients with low stage of NSCLC. Additionally, they found that cells collected from low stage disease showed high apoptotic ratio and arrest in G1 phase, suggesting a role of the KiSS-1/KiSS-1R system not only in invasion and migration, but also in apoptosis and cell cycle processes.
Furthermore, studies on cisplatin-resistant NSCLC cells demonstrated that overexpression of exogenous KiSS-1 significantly decreases their invasive capability in vitro and in vivo (36).
KiSS-1 has been shown to inhibit the proliferation and invasion also of gastric carcinoma cells in vitro and in vivo through the downregulation of MMP-9 (37).
A study on 40 gastric cancers divided into two groups according to their high or low KiSS-1 mRNA expression levels and compared with their adjacent normal gastric mucosa, demonstrated that KiSS-1 may represent an independent prognostic factor for gastric cancer patients. Indeed, the low expression of KiSS-1 in tumor tissues was documented to correlate with the propensity of gastric cancers to invade, metastasize, and relapse as well as to worse overall and disease-free survival (38). Immunohistochemical analysis of tissue microarrays from 71 patients with gastric cancer revealed a statistically significant reduction of KiSS-1 in lymph node and liver metastases compared with primary tumors (39).
A potential role of the KiSS-1 gene product, metastin, and its receptor in modulating the biological behavior of thyroid carcinomas has been suggested.
Ringel and co-workers demonstrated that metastin is expressed in normal thyroid and in papillary thyroid carcinomas, while KiSS-1R is overexpressed in papillary thyroid cancer but not in normal thyroid and in follicular adenomas. The expression of KP and its receptor are less common in follicular carcinomas. The authors suggest that the high ability of follicular carcinomas to metastasize is due to their low expression of KiSS-1 product and KiSS-1R. They also show that KiSS-1R activates MAPK, but not Akt in thyroid cancer cells (40). Moreover, KiSS-1 expression is significantly higher in advanced tumors with extra-thyroidal invasion compared to thyroid tumors in the early stages. Decreased expression of KiSS-1R seems to attenuate signaling of the KiSS-1/KiSS-1R system, possibly leading to tumor growth (41).
Another type of cancer in which the expression of KiSS-1 is correlated with stage and tumor grade is bladder cancer. Sanchez-Carbayo and co-workers found lower levels of KiSS-1 in bladder cancer tissues as compared to normal counterpart; also, they documented that the loss of KiSS-1 is associated with bladder cancer progression and clinical outcome (42).
Successively, Cebrian and co-workers identified an epigenetic silencing of KiSS-1 due to a CpG island hypermethylation near to its promoter region, which correlates with lower levels of KiSS-1 transcripts in invasive bladder cancer as compared to superficial tumors. These findings highlight the value of KiSS-1 as a prognostic biomarker (43).
The occurrence of an epigenetic regulation of KiSS-1 expression which favors bladder cancer invasion was confirmed by Zhang and co-workers. They found that ubiquitin-like with PHD and RING finger domains 1 increase the methylation of CpG nucleotides of KiSS-1, thus reducing its expression (44).
Finally, KiSS-1 overexpression has been documented to regulate apoptosis by increasing caspase 3 and Bax and decreasing Bcl-2 and Bax mRNA levels in human osteosarcoma MG-62 and U2OS cells. In these cell lines, KiSS-1 overexpression reduces the extent of both cell proliferation and invasiveness (45).
It has been shown that KiSS-1 exerts an anti-metastatic role in human hepatocellular and renal carcinoma by inhibiting metalloproteinase MMP-9 and MMP-2 activity (46, 47).
In human head and neck squamous cell carcinoma (HNSCC) tumors, loss of KiSS-1 expression has been associated with high metastatic potential compared with non-metastatic tumors (48).
Role of KiSS-1R in the Acquisition of Drug Resistance
Recent papers attribute to KiSS-1/KiSS-1R complex a diverse function from that observed in other tumor types.
Genetic reconstitution of KiSS-1 in cisplatin-resistant HNSCC cells has been shown to induce alterations in cisplatin metabolism thus restoring platinum sensitivity (48).
In TNBC, Blake and co-workers documented that KiSS-1R signaling promotes drug resistance in ERα-negative breast cell lines and in TNBC cells by increasing the expression of the efflux drug transporter breast cancer-resistance protein and also by promoting tyrosine kinase (AXL) expression and activity. The authors demonstrated that KiSS-1R activity is necessary to promote drug resistance in ERα-negative breast cell lines and in TNBC cells, since KiSS-1R antagonist restored cell sensitivity to doxorubicin. These findings suggest the possibility to consider KiSS-1R as a possible novel therapeutic target to restore drug sensitivity in patients affected by TNBC (25).
Role of KiSS-1 in Early Stage of Tumor Development
An additional role of KiSS-1/KiSS-1R was demonstrated by Cho et al. in the early steps of breast cancer development. Using transgenic mice expressing the polyoma middle T antigen under the control of MMTV (mouse mammary tumor virus) long terminal repeat promoter (MMTV-PyMT), the authors found that heterozygous mouse for KiSS-1 or KiSS-1R showed delayed hyperplasia, resulting in a late breast cancer initiation, progression, and lung metastasis. Also, they showed that KiSS-1 and KiSS-1R silencing in pubertal breast epithelium inhibits mammary gland hyperplasia (49). These findings highlight the role of KiSS-1/KiSS-1R complex in the early phase of breast tumor development.
KiSS-1 Induces a Dormancy State in Disseminated Tumor Cells
Nash et al. hypothesized that secreted KiSS-1 was able to keep the disseminated melanoma cells in a state of dormancy inducing a suppression of metastatic colonization to multiple organs. The authors showed that mice injected intravenously with cells expressing KiSS-1 without the secretion sequence developed lung metastasis. Also, the mouse survival fell quickly after the interruption of the dormancy and the reactivation of proliferation. Thus, the possibility to keep tumor cells in a dormant state and obtain high survival through the use of exogenous KiSS-1 therapy has been suggested to represent an important goal for the treatment of tumor patients. In addition, the capability of KiSS-1 to maintain the cells in a dormancy state was documented to occur in the absence of its cognate receptor, raising the possibility that additional KiSS-1 receptors and/or paracrine signals exist with the potential to be selectively targeted (50). Similar hypothesis was formulated by Beck et al., suggesting that many tumor cells do not express KiSS-1 receptor, so a paracrine control may exist between tumor cells expressing KiSS-1 and stromal cells expressing KiSS-1R. KiSS-1 may activate or induce stromal cells to produce secreted factors in the surrounding extracellular milieu that, directly or indirectly, elicit dormancy in metastatic cells (51).
Conclusion
Although initially the KiSS-1/KiSS-1R complex was described to be involved in the onset of puberty, sexual maturity, and pregnancy through direct regulation of the hormone releasing the gonadotropin produced by the hypothalamus, other multiple roles have been proposed for this complex in the tumor development process.
Metastasis is a main cause of death in cancer patients and involves a multi-step process encompassing detachment of cancer cells from a primary tumor, invasion of adjacent tissue, transvasation of blood vessels, and spread through circulation, distant organs colonization. KiSS-1 has been described as a gene suppressor of metastasis in melanoma and more recently in other types of cancer, such as breast cancer, CRC, lung, thyroid, bladder, gastric, and other cancers.
In this mini-review, we highlighted other roles of the KiSS-1/KiSS-1R complex in addition to the role of suppressor gene of metastases. One of the main functions that have been found is the involvement of this complex in drug resistance. The development of drug resistance is still one of the main obstacles in effective cancer treatment. Therefore, there is still an unmet need to identify the molecules that could be targeted in order to overcome resistance in the treatment of tumors and the KiSS-1/KiSS-1R complex may represent a potential target for the treatment of drug-resistant tumors. In fact, it has been described that the KiSS-1/KiSS-1R system is involved in the sensitivity to traditional drugs, since the reconstitution of KiSS-1 in cisplatin-resistant head and neck cancer cells restores platinum sensitivity; in addition, KiSS-1R has been reported to be involved in the process of drug resistance in TNBC.
Furthermore, a potential role of the KiSS-1/KiSS-1R complex has been proposed in the early stage of breast cancer development so this complex is not only involved in the advanced stages of the tumor, but could also represent a good target to hit tumors at an initial and final stage of development.
Finally, a role played by KiSS-1 in the dormancy of disseminated tumor cells and in the suppression of multiple organ metastases was described, representing the possibility of maintaining the tumor in an asymptomatic state. This could be a valid method to intervene on the block of metastatic development through the treatment with KiSS-1 that inducing tumor cell dormancy could block the metastatic process. The perspective that KiSS-1 can be used in clinical treatment is really favorable because KiSS-1 is a natural product that can be administered at high levels to humans without toxicity.
Author Contributions
MM: conception of the work. FF and MM: performed extensive literature search. MM and FF: manuscript drafting. MM and MC: critical revision of the work. MM: final version approval.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers FM, AM and handling Editor declared their shared affiliation.
Funding
MM is funded by University of Naples “Parthenope” (DSMB 187, CUP I62I15000090005).
References
1. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature (2001) 411:613–7. doi:10.1038/35079135
2. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes KPs, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem (2001) 276:34631–6. doi:10.1074/jbc.M104847200
3. Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem (2001) 276:28969–75. doi:10.1074/jbc.M102743200
4. Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology (2008) 149(9):4605–14. doi:10.1210/en.2008-0321
5. Szereszewski JM, Pampillo M, Ahow MR, Offermanns S, Bhattacharya M, Babwah AV. GPR54 regulates ERK1/2 activity and hypothalamic gene expression in a Gα(q/11) and β-arrestin-dependent manner. PLoS One (2010) 5(9):e12964. doi:10.1371/journal.pone.0012964
6. Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides (2009) 30(1):10–5. doi:10.1016/j.peptides.2008.07.025
7. Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell (2009) 17(4):443–58. doi:10.1016/j.devcel.2009.09.011
8. Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci (2011) 36(9):457–69. doi:10.1016/j.tibs.2011.06.003
9. Pampillo M, Camuso N, Taylor JE, Szereszewski JM, Ahow MR, Zajac M, et al. Regulation of GPR54 signaling by GRK2 and {beta}-arrestin. Mol Endocrinol (2009) 23(12):2060–74. doi:10.1210/me.2009-0013
10. Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci (2002) 115(Pt 3):455–65.
11. Canbay E, Ergen A, Bugra D, Yamaner S, Eraltan IY, Buyukuncu Y, et al. Kisspeptin-54 levels are increased in patients with colorectal cancer. World J Surg (2012) 36(9):2218–24. doi:10.1007/s00268-012-1636-7
12. Horstmann M, Krause F, Steinbach D, Twelker L, Grimm MO. Evaluation of plasmatic Kisspetin-10 as a biomarker for malignancy and subtype differentiation in small renal tumours. Urol Int (2017) 98(2):177–83. doi:10.1159/000452108
13. Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst (1996) 88:1731–7. doi:10.1093/jnci/88.23.1731
14. Lee JH, Welch DR. Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. Int J Cancer (1997) 71(6):1035–44. doi:10.1002/(SICI)1097-0215(19970611)71:6<1035::AID-IJC20>3.0.CO;2-B
15. Miele ME, Jewett MD, Goldberg SF, Hyatt DL, Morelli C, Gualandi F, et al. A human melanoma metastasis-suppressor locus maps to 6q16.3-q23. Int J Cancer (2000) 86(4):524–8. doi:10.1002/(SICI)1097-0215(20000515)86:4<524::AID-IJC13>3.0.CO;2-W
16. Shirasaki F, Takata M, Hatta N, Takehara K. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Res (2001) 61(20):7422–5.
17. Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res (1997) 57(12):2384–7.
18. Martin TA, Watkins G, Jiang WG. KiSS-1 expression in human breast cancer. Clin Exp Metastasis (2005) 22(6):503–11. doi:10.1007/s10585-005-4180-0
19. Stark AM, Tongers K, Maass N, Mehdorn HM, Held-Feindt J. Reduced metastasis-suppressor gene mRNA-expression in breast cancer brain metastases. J Cancer Res Clin Oncol (2005) 131(3):191–8. doi:10.1007/s00432-004-0629-9
20. Ulasov IV, Kaverina NV, Pytel P, Thaci B, Liu F, Hurst DR, et al. Clinical significance of KiSS1 protein expression for brain invasion and metastasis. Cancer (2012) 118:2096–105. doi:10.1002/cncr.26525
21. Stingl J. Estrogen and progesterone in normal mammary gland development and cancer. Horm Cancer (2011) 2(2):85–90. doi:10.1007/s12672-010-0055-1
22. Rosen JM. On hormone action in the mammary gland. Cold Spring Harb Perspect Biol (2012) 4(2):a013086. doi:10.1101/cshperspect.a013086
23. Marot D, Bieche I, Aumas C, Esselin S, Bouquet C, Vacher S, et al. High tumoral levels of KiSS1 and G-protein-coupled receptor 54 expression are correlated with poor prognosis of estrogen receptor-positive breast tumors. Endocr Relat Cancer (2007) 14(3):691–702. doi:10.1677/ERC-07-0012
24. Jarzabek K, Kozłowski L, Milewski R, Wołczyński S. KiSS1/GPR54 and estrogen-related gene expression profiles in primary breast cancer. Oncol Lett (2012) 3(4):930–4.
25. Blake A, Dragan M, Tirona RG, Hardy DB, Brackstone M, Tuck AB, et al. G protein-coupled KiSS1 receptor is overexpressed in triple negative breast cancer and promotes drug resistance. Sci Rep (2017) 7:46525. doi:10.1038/srep46525
26. Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet (2010) 375(9719):1030–47. doi:10.1016/S0140-6736(10)60353-4
27. Zucker S, Lysik RM, Zarrabi MH, Moll U. M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res (1993) 53(1):140–6.
28. Onisto M, Garbisa S, Caenazzo C, Freda MP, Di F, Nitti D, et al. Reverse transcription-polymerase chain reaction phenotyping of metalloproteinases and inhibitors involved in tumor matrix invasion. Diagn Mol Pathol (1993) 2(2):74–80. doi:10.1097/00019606-199306000-00002
29. Zhu C, Takasu C, Morine Y, Bando Y, Ikemoto T, Saito Y, et al. KiSS1 associates with better outcome via inhibiting matrix metalloproteinase-9 in colorectal liver metastasis. Ann Surg Oncol (2015) 22(Suppl 3):S1516–23. doi:10.1245/s10434-015-4891-7
30. Chen S, Chen W, Zhang X, Lin S, Chen Z. Overexpression of KiSS-1 reduces colorectal cancer cell invasion by downregulating MMP-9 via blocking PI3K/Akt/NF-κB signal pathway. Int J Oncol (2016) 48(4):1391–8. doi:10.3892/ijo.2016.3368
31. Kostakis ID, Agrogiannis G, Vaiopoulos AG, Mylona E, Patsouris E, Kouraklis G, et al. A clinicopathological analysis of KiSS1 and KiSS1R expression in colorectal cancer. APMIS (2015) 123(7):629–37. doi:10.1111/apm.12397
32. Ji K, Ye L, Ruge F, Hargest R, Mason MD, Jiang WG. Implication of metastasis suppressor gene, KiSS-1 and its receptor KiSS-1R in colorectal cancer. BMC Cancer (2014) 14:723. doi:10.1186/1471-2407-14-723
33. Shen ZL, Wang B, Jiang KW, Ye CX, Cheng C, Yan YC, et al. Downregulation of miR-199b is associated with distant metastasis in colorectal cancer via activation of SIRT1 and inhibition of CREB/KiSS1 signaling. Oncotarget (2016) 7(23):35092–105. doi:10.18632/oncotarget.9042
34. Zheng S, Chang Y, Hodges KB, Sun Y, Ma X, Xue Y, et al. Expression of KiSS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res (2010) 30(3):713–8.
35. Sun YB, Xu S. Expression of KiSS1 and KiSS1R (GPR54) may be used as favorable prognostic markers for patients with non-small cell lung cancer. Int J Oncol (2013) 43(2):521–30. doi:10.3892/ijo.2013.1967
36. Zuco V, Cassinelli G, Cossa G, Gatti L, Favini E, Tortoreto M, et al. Targeting the invasive phenotype of cisplatin-resistant non-small cell lung cancer cells by a novel histone deacetylase inhibitor. Biochem Pharmacol (2015) 94(2):79–90. doi:10.1016/j.bcp.2015.01.002
37. Li N, Wang HX, Zhang J, Ye YP, He GY. KiSS-1 inhibits the proliferation and invasion of gastric carcinoma cells. World J Gastroenterol (2012) 18(15):1827–33. doi:10.3748/wjg.v18.i15.1827
38. Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, et al. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer (2004) 111(6):868–72. doi:10.1002/ijc.20357
39. Guan-Zhen Y, Ying C, Can-Rong N, Guo-Dong W, Jian-Xin Q, Jie-Jun W. Reduced protein expression of metastasis-related genes (nm23, KiSS1, KAI1 and p53) in lymph node and liver metastases of gastric cancer. Int J Exp Pathol (2007) 88(3):175–83. doi:10.1111/j.1365-2613.2006.00510.x
40. Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, et al. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab (2002) 87(5):2399. doi:10.1210/jcem.87.5.8626
41. Savvidis C, Papaoiconomou E, Petraki C, Msaouel P, Koutsilieris M. The role of KiSS1/KiSS1R system in tumor growth and invasion of differentiated thyroid cancer. Anticancer Res (2015) 35(2):819–26.
42. Sanchez-Carbayo M, Capodieci P, Cordon-Cardo C. Tumor suppressor role of KiSS-1 in bladder cancer: loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am J Pathol (2003) 162(2):609–17. doi:10.1016/S0002-9440(10)63854-0
43. Cebrian V, Fierro M, Orenes-Piñero E, Grau L, Moya P, Ecke T, et al. KiSS1 methylation and expression as tumor stratification biomarkers and clinical outcome prognosticators for bladder cancer patients. Am J Pathol (2011) 179(2):540–6. doi:10.1016/j.ajpath.2011.05.009
44. Zhang Y, Huang Z, Zhu Z, Zheng X, Liu J, Han Z, et al. Upregulated UHRF1 promotes bladder cancer cell invasion by epigenetic silencing of KiSS1. PLoS One (2014) 9(10):e104252. doi:10.1371/journal.pone.0104252
45. Yin Y, Tang L, Shi L. The metastasis suppressor gene KiSS-1 regulates osteosarcoma apoptosis and autophagy processes. Mol Med Rep (2017) 15(3):1286–90. doi:10.3892/mmr.2017.6109
46. Shengbing Z, Feng LJ, Bin W, Lingyun G, Aimin H. Expression of KiSS-1 gene and its role in invasion and metastasis of human hepatocellular carcinoma. Anat Rec (Hoboken) (2009) 292(8):1128–34. doi:10.1002/ar.20950
47. Yoshioka K, Ohno Y, Horiguchi Y, Ozu C, Namiki K, Tachibana M. Effects of a KiSS-1 peptide, a metastasis suppressor gene, on the invasive ability of renal cell carcinoma cells through a modulation of a matrix metalloproteinase 2 expression. Life Sci (2008) 83(9–10):332–8. doi:10.1016/j.lfs.2008.06.018
48. Jiffar T, Yilmaz T, Lee J, Hanna E, El-Naggar A, Yu D, et al. KiSS1 mediates platinum sensitivity and metastasis suppression in head and neck squamous cell carcinoma. Oncogene (2011) 30(28):3163–73. doi:10.1038/onc.2011.39
49. Cho SG, Wang Y, Rodriguez M, Tan K, Zhang W, Luo J, et al. Haploinsufficiency in the prometastasis KiSS1 receptor Gpr54 delays breast tumor initiation, progression, and lung metastasis. Cancer Res (2011) 71(20):6535–46. doi:10.1158/0008-5472.CAN-11-0329
50. Nash KT, Phadke PA, Navenot JM, Hurst DR, Accavitti-Loper MA, Sztul E, et al. Requirement of KiSS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J Natl Cancer Inst (2007) 99(4):309–21. doi:10.1093/jnci/djk053
Keywords: kisspeptin, KiSS-1R, tumor development, tumor invasion, metastasis
Citation: Fratangelo F, Carriero MV and Motti ML (2018) Controversial Role of Kisspeptins/KiSS-1R Signaling System in Tumor Development. Front. Endocrinol. 9:192. doi: 10.3389/fendo.2018.00192
Received: 15 January 2018; Accepted: 09 April 2018;
Published: 30 April 2018
Edited by:
Silvia Fasano, Università degli Studi della Campania “Luigi Vanvitelli” Caserta, ItalyReviewed by:
Satoshi Ogawa, Monash University Malaysia, MalaysiaAntimo Migliaccio, Università degli Studi della Campania “Luigi Vanvitelli” Naples, Italy
Floriana Morgillo, Università degli Studi della Campania “Luigi Vanvitelli” Naples, Italy
Copyright: © 2018 Fratangelo, Carriero and Motti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Letizia Motti, bW90dGlAdW5pcGFydGhlbm9wZS5pdA==
 Federica Fratangelo
Federica Fratangelo Maria Vincenza Carriero1
Maria Vincenza Carriero1 Maria Letizia Motti
Maria Letizia Motti