- 1Preventative Gynecology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Laser Application in Medical Science Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3School of Public Health, University of Saskatchewan, Saskatoon, SK, Canada
- 4Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran
Purpose: This study was performed to determine the effects of chromium supplementation on the gene expression of insulin, lipid, and inflammatory markers in infertile women with polycystic ovary syndrome (PCOS) who were candidate for in vitro fertilization (IVF).
Methods: Forty women, aged 18–40 years, who had been selected for IVF were recruited in this randomized double-blinded, placebo-controlled trial. They (n = 20/group) were randomly assigned into intervention groups to take either 200 μg/day of chromium or placebo for 8 weeks. Inflammatory markers were measured at baseline and end of the trial. Genes related to insulin, lipid, and inflammation were expressed in peripheral blood mononuclear cells (PBMCs), using RT-PCR method.
Results: Chromium supplementation led to a significant reduction in serum high sensitivity C-reactive protein (hs-CRP) (−1.4 ± 1.5 vs. + 0.2 ± 2.2 mg/L, p = 0.01) compared with the placebo. RT-PCR findings indicated that chromium supplementation upregulated gene expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) (p = 0.01), glucose transporter 1 (GLUT-1) (p = 0.001) and low-density lipoprotein receptor (LDLR) (p = 0.01), as well as downregulated gene expression of interleukin-1 (IL-1) (p = 0.004) in PBMCs of patients with PCOS compared with the placebo. Chromium supplementation had no significant effect on gene expression of IL-8, tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β) and vascular endothelial growth factor (VEGF).
Conclusion: Overall, our findings demonstrated that infertile women with PCOS, who were candidate for IVF benefited from chromium supplementation for 8 weeks in terms of lowering hs-CRP and improving gene expression of PPAR-γ, GLUT-1, LDLR, and IL-1, though chromium had no effect on the gene expression of IL-8, TNF-α, TGF-β, and VEGF.
Clinical Trial Registration Number: http://www.irct.ir:IRCT20170513033941N32.
Introduction
Polycystic ovary syndrome (PCOS), the major cause of anovulation and infertility among women of reproductive age, is the most common endocrine disorder affecting 5–10% of women worldwide (1). Accelerated lipolysis and subsequent dyslipidemia in women with PCOS is associated with metabolic abnormalities including obesity, insulin resistance and type 2 diabetes (T2DM) (2). The reflected changes in the follicular fluid (FF) of dominant follicle (3, 4) can directly affect the quality and metabolism of oocyte. Low-grade chronic inflammation is commonly blamed as an etiopathogenetic mechanism of infertility. Inflammatory cytokines are significantly higher among women with unexplained infertility (5). Pro-inflammatory factors have been shown to be significantly associated with higher failure rates of in vitro fertilization (IVF), especially in the implantation stage, among women with unexplained infertility (6). Studies have found elevated interferon gamma (IFN-γ) and interleukin-2 (IL-2) levels and decreased transforming growth factor beta (TGF-β) levels, an important endogenous anti-inflammatory mediator, (7) in the plasma of women with unexplained infertility compared with fertile controls during the implantation window (luteal phase) (8). Moreover, unexplained infertile patients have demonstrated elevated levels of serum IL-2, IL-4, IL-6, IL-21, tumor necrosis factor alpha (TNF-α), and IFN-γ, compared with fertile women (5).
The beneficial effects of chromium supplements have been already reported on metabolic abnormalities among women suffering from PCOS. In a meta-analysis conducted by Fazelian et al. (9), chromium supplementation decreased serum insulin and free testosterone and improved body weight in patients with PCOS. We have already published advantages of taking chromium supplements for 8 weeks on glycemic control, cardiometabolic risk parameters, and oxidative stress in infertile women with PCOS who were candidate for IVF (10). Further, chromium supplementation in diabetic rats showed anti-diabetic activities, which can be explained through chromium impact on the modulation of peroxisome proliferator-activated receptor gamma (PPAR-γ), insulin receptor substrate 1 (IRS-1) and nuclear factor κB (NF-κB) proteins (11). Similarly, Wang et al. (12) found that compared to obese control rats, those supplemented with chromium had significant improvement in their glucose disposal rates and an elevation in insulin-stimulated p-IRS-1 and phosphatidylinositol-3 kinase activity in skeletal muscles. In another animal experiment, chromium ingestion for 7 weeks, by diabetic rats, significantly decreased inflammatory markers including C-reactive protein (CRP), TNF-α and IL-6 (13).
Considering anti-diabetic and lipid-lowering effects of chromium (11, 14), we hypothesized that chromium supplementation might be beneficial in women with PCOS and candidate for IVF, who suffers from different metabolic abnormalities. According to our best knowledge, nothing has been published about the effects of chromium supplementation on gene expression of insulin, lipid, and inflammatory markers in infertile women with PCOS who were candidate for IVF. This trial was aimed to determine the effects of chromium supplementation on gene expression of insulin, lipid, and inflammatory markers in infertile women who suffered from PCOS and were candidate for IVF.
Materials and Methods
Trial Design and Participants' Characteristics
This randomized, double-blinded, placebo-controlled trial was registered by Iranian website for clinical trials registration (http://www.irct.ir:IRCT20170513033941N32). Forty infertile women with PCOS, aged 18–40 years, who were candidate for IVF without previous history of IVF, were included. This study was conducted in Taleghani Hospital, Tehran, Iran, between February and May 2018. Rotterdam criteria was used to confirm the diagnosis of PCOS (15). Participants with metabolic abnormalities including thyroid dysfunction, diabetes or impaired glucose tolerance were excluded from the study. This investigation was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the research ethics committee of Shahid Beheshti University of Medical Sciences (SUMS), Tehran, Iran. Written informed consent was signed by all individuals.
Intervention
Participants were randomly allocated into intervention groups to take either 200 μg/day chromium picolinate (Nature Made, California, USA) (n = 20) or placebo (Barij Essence, Kashan, Iran) (n = 20) for 8 weeks. Chromium supplements and placebos were matched in terms of shape, appearance, smell and packaging. Randomization was done using computer-generated random numbers. Randomization and allocation process were concealed from both the researchers and participants until the completion of final analyses. Another person, not involved in the trial and unaware of random sequences, assigned the subjects to the numbered bottles of tablets. Adherence to the supplements and placebos, throughout the trial, was monitored asking individuals to bring the medication containers back. To increase the compliance rate, all participants were receiving short daily reminder messages on their cell phones to take the supplements. Study participants completed dietary records at week 0, 4, and 8 of the intervention.
Assessment of Outcomes
Serum high sensitivity C-reactive protein (hs-CRP) levels were considered as the primary outcome and metabolic profiles were measured as the secondary outcomes. Fasting blood samples (15 mL) were collected at the beginning and end of the 8-week intervention at Taleghani Hospital (Tehran, Iran). Hs-CRP levels were measured using commercial ELISA kit (LDN, Nordhorn, Germany) with inter- and intra-assay coefficient variances (CVs) of lower than 7%. Plasma nitric oxide (NO) levels were measured using Griess method (16) with intra-assay CVs of < 5%.
Isolation of Lymphocyte Cells
Blood samples were used to extract lymphocyte cells, using 50% percoll (Sigma-Aldrich, Dorset, UK). Cell count and viability test were conducted using trypan blue, RNA and, DNA extraction (17).
RNA Extraction and Real-Time PCR (RT-PCR)
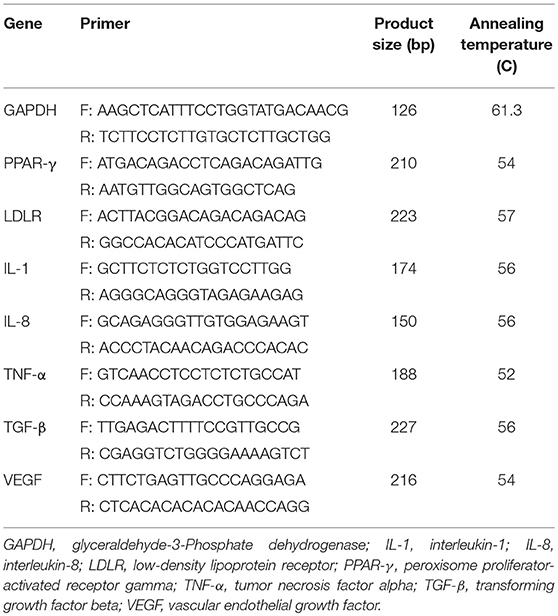
RNA was extracted from blood samples using RNX-plus kit (Cinnacolon, Tehran, Iran). RNA suspension was frozen at −20°C until cDNA was derived. Following the extraction of total RNAs from each sample, RNA was quantified using UV spectrophotometer. Each sample OD 260/280 ratio of 1.7–2.1 represented no contamination with either protein or DNA (17). Using moloney murine leukemia virus reverse transcriptase (RT), isolated RNA was reverse transcribed to cDNA library. Gene expressions of PPAR-γ, glucose transporter 1 (GLUT-1), low-density lipoprotein receptor (LDLR), IL-1, IL-8, TNF-α, TGF-β, and vascular endothelial growth factor (VEGF) were conducted on mononuclear cells (PBMCs) of peripheral blood, using SYBR green detection and Amplicon Kit and applying quantitative RT-PCR and Light Cycler technology (Roche Diagnostics, Rotkreuz, Switzerland) (Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used as a housekeeping gene. Primers were designed using Primer Express Software (Applied Biosystems, Foster City, USA) and Beacon designer software (Takaposizt, Tehran, Iran). Relative transcription levels were calculated using Pffafi or 2−ΔΔCT methods.
Sample Size and Statistical Methods
Sample size was calculated using the established formula for RCTs, with type one (α) and type two errors (β) were considered as 0.05 and 0.20 to provide the power of 80%. Based on a previous published study (18), a mean difference (d) of 1500.0 ng/mL and a standard deviation (SD) of 1496.1 ng/mL for hs-CRP were used to calculate sample size. Considering a probable dropout of 4 subjects in each intervention group, the final sample size was determined to be 20 (16 + 4) subjects per group.
The normality of data was assessed using Kolmogorov-Smirnov test. Analyses were performed using intention-to-treat (ITT) approach. Independent samples t-test was applied to determine the differences in anthropometric measures and gene expression of insulin, lipid and inflammation between intervention groups. The effects of chromium supplementation on inflammatory markers were assessed using repeated measures ANOVA test. Differences in proportions were evaluated by Fisher's exact test. The P < 0.05 were considered statistically significant. Statistical analyses were conducted using the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, Illinois, USA).
Results
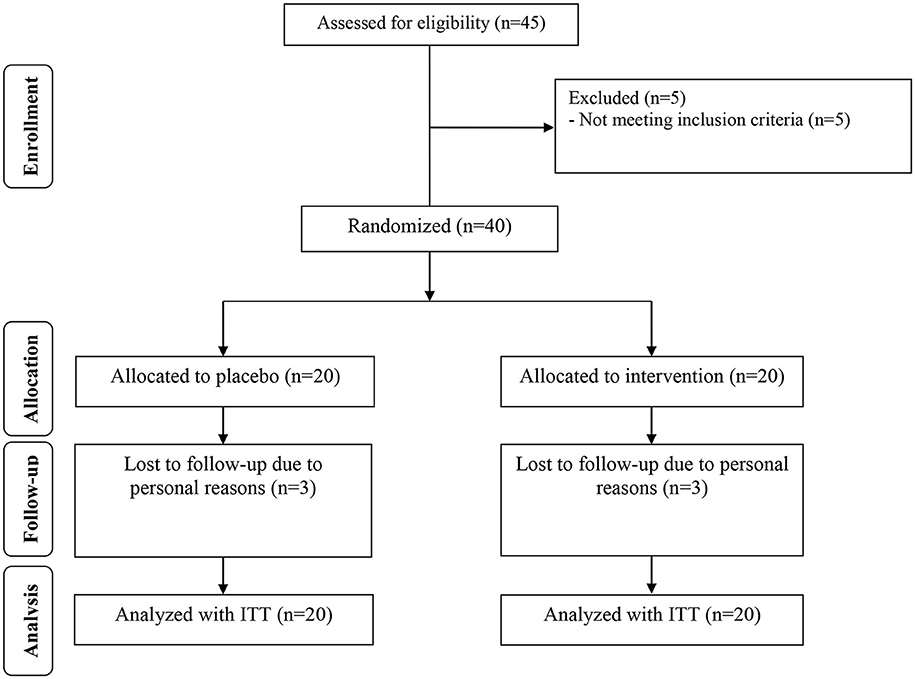
Three dropouts were reported in each intervention group, due to personal reasons. So, overall 34 participants [infertile women with PCOS candidate for IVF receiving chromium (n = 17) and placebo (n = 17)] completed the study (Figure 1). Using ITT approach, all 40 participants (20 in each group) were included in the final data analysis. Compliance rate in this study was high, with more than 90% of tablets were consumed during the intervention in both groups. No side effects were reported following the consumption of chromium in women with PCOS in this study.
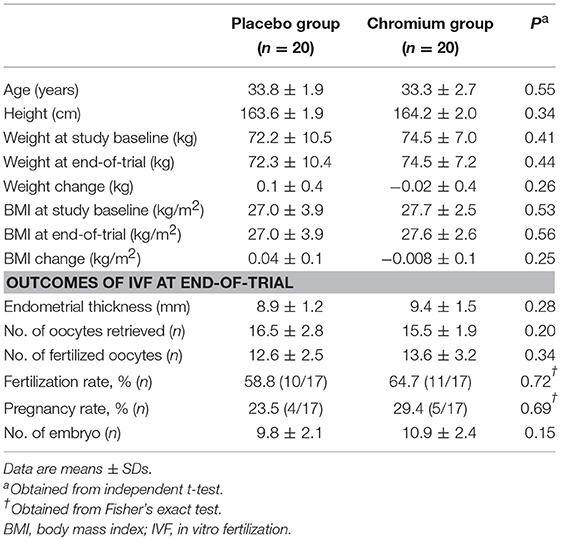
Anthropometric measures were not statistically different between intervention groups (Table 2). Moreover, following chromium supplementation, IVF outcomes including endometrial thickness, number of oocytes retrieved, number of fertilized oocytes, fertilization rate, pregnancy rate and number of embryo were not significantly different between supplemented and placebo groups.
The average of macro- and micro-nutrient intakes was also not significantly different between two intervention groups throughout the treatment (Data not shown).
Chromium supplementation led to a significant reduction in serum hs-CRP (−1.4 ± 1.5 vs. + 0.2 ± 2.2 mg/L, p = 0.01), yet did not affect plasma NO levels (Table 3).
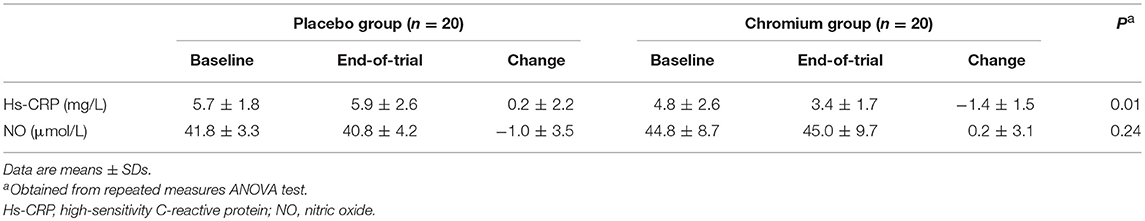
Table 3. Inflammatory markers at baseline and after the 8-week intervention in infertile polycystic ovary syndrome women candidate for in vitro fertilization that received either chromium supplements or placebo.
RT-PCR findings indicated that chromium supplementation significantly upregulated the gene expression of PPAR-γ (p = 0.01), GLUT-1 (p = 0.001) and LDLR (p = 0.01) in PBMCs of patients with PCOS compared with the placebo (Figure 2).
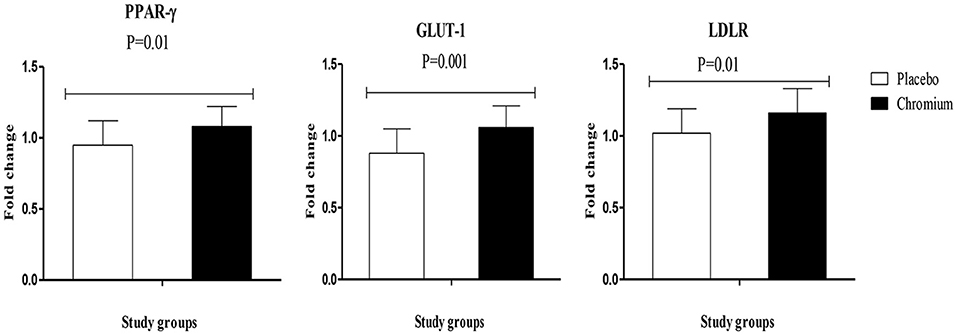
Figure 2. Fold change (means ± SDs) in gene expression levels of PPAR-γ, GLUT-1, and LDLR in women diagnosed with polycystic ovary syndrome who were candidate for in vitro fertilization, receiving chromium supplements and placebo. P-value was obtained from independent t-test. N = 20 in each group. IL-1, interleukin-1; IL-8, interleukin-8; LDLR, oxidized low-density lipoprotein receptor; PPAR-γ, peroxisome proliferator-activated receptor gamma; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.
Chromium supplementation also downregulated the gene expression of IL-1 (P = 0.004) in PBMCs compared with the placebo (Figure 3). There was no significant effect of chromium supplementation on the gene expression of IL-8, TNF-α, TGF-β, and VEGF in PBMCs of patients with PCOS.
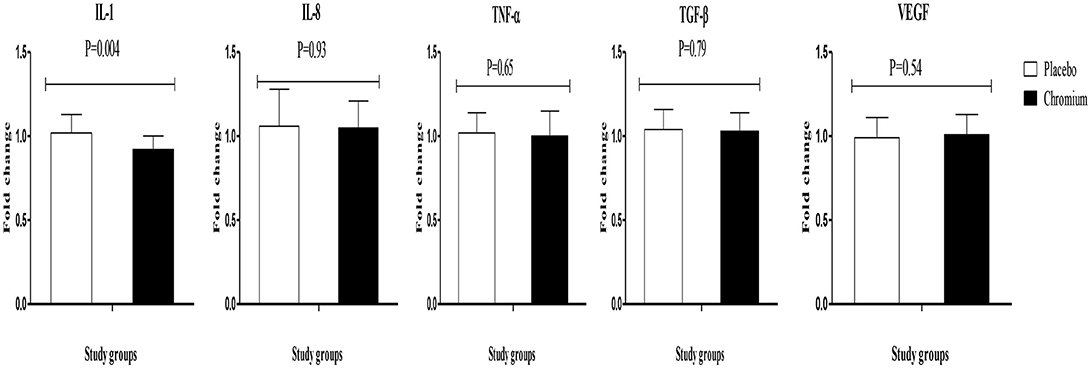
Figure 3. Change (means ± SDs) in gene expression levels of IL-1, IL-8, TNF-α, TGF-β, and VEGF in women diagnosed with polycystic ovary syndrome who were candidate for in vitro fertilization, receiving chromium supplements and placebo. P-value was obtained from independent t-test. N = 20 in each group. IL-1, interleukin-1; IL-8, interleukin-8; LDLR, oxidized low-density lipoprotein receptor; PPAR-γ, peroxisome proliferator-activated receptor gamma; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.
Discussion
We found beneficial effects of chromium administration for 8 weeks in infertile women with PCOS who were candidate for IVF. Chromium supplementation lowered inflammation and improved gene expression of PPAR-γ, GLUT-1, LDLR, and IL-1, though it did not affect the gene expression of IL-8, TNF-α, TGF-β, and VEGF. To our best knowledge, this is the first trial reporting the effects of chromium supplementation on gene expression related to insulin, lipid, and inflammatory markers in infertile women diagnosed with PCOS and candidate for IVF.
Effects of Chromium on Gene Expression of Insulin and Lipid Metabolism
Patients with PCOS are vulnerable to different metabolic abnormalities, such as increased insulin resistance, dyslipidemia and chronic inflammation (19, 20). The current study revealed that chromium supplementation for 8 weeks to infertile women with PCOS who were candidate for IVF significantly increased gene expression of PPAR-γ, GLUT-1, and LDLR. Consistent with our findings, chromium administration to diabetic rats showed anti-diabetic activities, probably related to the impact of chromium on the modulation of PPAR-γ and IRS-1 proteins (11). Wang et al. (12) also found that obese rats supplemented with chromium significantly increased their glucose disposal rates. Moreover, compared with obese controls, these rats showed an elevation in insulin-stimulated p-IRS-1 and phosphatidylinositol-3 kinase activity in skeletal muscles. In another study, chromium, biotin, or their combination significantly upregulated PPAR-γ expression in adipose tissue, which might have an effective insulin-sensitizing impact and reduce insulin resistance in a diabetic rat model (11). Chromium also can promote GLUT-4 trafficking and insulin-stimulated glucose transport (21). Likewise, rats with diabetic retinopathy who were supplemented with chromium for 12 weeks showed improvement in the gene expression of GLUT-1, GLUT-3, and insulin (22). PPAR-γ is the central regulator of insulin, glucose and lipid metabolism which is widely expressed in the adipose tissue. PPAR-γ might improve insulin sensitivity and lipid profiles in patients with type 2 diabetes as well as in diabetic rodent models (23). Current evidence suggests that PPAR-γ agonists may be beneficial in decreasing renal cortical lipids and proteinuria through a mechanism independent of their hypoglycemic impact (24, 25).
Effects of Chromium on Gene Expression of Inflammatory Markers
Our study supported that chromium administration for 8 weeks significantly decreased gene expression of IL-1 and serum hs-CRP levels in infertile women with PCOS who were candidate for IVF, though it did not affect gene expression of IL-8, TNF-α, TGF-β, and VEGF. Current evidence demonstrating the effects of chromium supplementation on the gene expression of inflammatory markers and their circulating levels are scarce and mostly limited to animal studies. Chromium supplementation for 8 weeks to Zucker diabetic fatty (ZDF) rats significantly decreased their serum CRP levels (26). In another study, chromium niacinate ingestion for 7 weeks reduced pro-inflammatory cytokines (TNF-α, IL-6, and CRP) levels, oxidative stress, and lipid concentrations in diabetic rats (13). Further, administration of a nutritional supplement containing chromium picolinate, phosphatidylserine, docosahexaenoic acid, and boron for 12 weeks significantly decreased CRP and TNF-α concentrations in rats fed high-fat diet (27). We found no significant effect of chromium supplementation on IL-8, TNF-α, TGF-β, and VEGF which might be explained through applying different study designs, type and dosages of chromium used, duration of intervention and participants' characteristics. Higher dosages of chromium may significantly improve the metabolic profiles including IL-8, TNF-α, TGF-β, and VEGF. The markers of inflammation and oxidative stress are elevated in many diabetic patients, which positively represents the vascular inflammation (28). Insulin resistance and subsequent vascular inflammation are the major risk factors for developing CVD (29). The suppressing effect of chromium on pro-inflammatory markers may be facilitated by inactivating oxidative stress pathways and reduction of Akt phosphorylation associated with the insulin resistance cascade in the liver (30). Current evidence has shown that increased inflammatory cytokines are involved in the outcomes of IVF. In a study conducted by Haimovici et al. (31), the women who gave live birth to their fetus had lower IL-6 levels. The inhibition of luteal cells producing progesterone in the presence of IL-1β may be one of the factors leading to the failure of the implantation (32). Increased follicular TNF-α and IL-6 might deteriorate the microenvironment in the follicle, thereby negatively affect the quality of oocyte and embryo (33). In another study conducted by Levin et al. (34), higher CRP levels, during IVF stimulation, were significantly associated with the failure of conception. According to the above-mentioned published evidence we assumed that the outcomes of IVF may be influenced by reduced inflammation. A recent study showed that using TNF-α inhibitor and intravenous immunoglobulin significantly improved the outcomes of IVF including; implantation, clinical pregnancy and live birth rates in young infertile women who had high levels of T helper 1/T helper 2 cytokines (35).
There were a few limitations in this study. Due to inadequate funding, we were not able to measure chromium levels and could not assess the effects of chromium on gene expression of oxidative stress.
Overall, our study suggests the advantages of chromium supplementation on lowering inflammatory markers through improving gene expression of PPAR-γ, GLUT-1, LDLR, and IL-1in infertile women with PCOS who were candidate for IVF.
Author Contributions
ZA helped in conception, design and statistical analysis of the manuscript. MA, SZ, NM, EA, and SS contributed in data collection and manuscript drafting. ZA supervised the study.
Funding
This study was supported by a grant from the Vice-chancellor for Research, Shahid Beheshti University of Medical Sciences, and Tehran, Iran.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. (2012) 27:3067–73. doi: 10.1093/humrep/des232
2. Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. (2003) 101:1177–82.
3. Valckx SD, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, et al. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reprod Biol Endocrinol. (2014) 12:13. doi: 10.1186/1477-7827-12-13
4. Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, et al. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod. (2012) 27:3531–9. doi: 10.1093/humrep/des350
5. An LF, Zhang XH, Sun XT, Zhao LH, Li S, Wang WH. Unexplained infertility patients have increased serum IL-2, IL-4, IL-6, IL-8, IL-21, TNFalpha, IFNgamma and increased Tfh/CD4 T cell ratio: increased Tfh and IL-21 strongly correlate with presence of autoantibodies. Immunol Invest. (2015) 44:164–73. doi: 10.3109/08820139.2014.932377
6. Ozkan ZS, Deveci D, Kumbak B, Simsek M, Ilhan F, Sekercioglu S, et al. What is he impact of Th1/Th2 ratio, SOCS3, IL17, and IL35 levels in unexplained infertility? J Reprod Immunol. (2014) 103:53–8. doi: 10.1016/j.jri.2013.11.002
7. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. (1998) 16:137–61
8. Fornari MC, Sarto A, Berardi VE, Martinez MA, Rocha MG, Pasqualini S, et al. Effect of ovaric hyper-stimulation on blood lymphocyte subpopulations, cytokines, leptin and nitrite among patients with unexplained infertility. Am J Reprod Immunol. (2002) 48:394–403.
9. Fazelian S, Rouhani MH, Bank SS, Amani R. Chromium supplementation and polycystic ovary syndrome: a systematic review and meta-analysis. J Trace Elem Med Biol. (2017) 42:92–6. doi: 10.1016/j.jtemb.2017.04.008
10. Jamilian M, Zadeh Modarres S, Amiri Siavashani M, Karimi M, Mafi A, Ostadmohammadi V, et al. The influences of chromium supplementation on glycemic control, markers of cardio-metabolic risk, and oxidative stress in infertile polycystic ovary syndrome women candidate for in vitro fertilization: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. (2018):48–55. doi: 10.1007/s12011-017-1236-3
11. Sahin K, Tuzcu M, Orhan C, Sahin N, Kucuk O, Ozercan IH, et al. Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br J Nutr. (2013) 110:197–205. doi: 10.1017/S0007114512004850
12. Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr. (2006) 136:415–20. doi: 10.1093/jn/136.2.415
13. Jain SK, Rains JL, Croad JL. Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-alpha, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic Biol Med. (2007) 43:1124–31. doi: 10.1016/j.freeradbiomed.2007.05.019
14. Martino F, Puddu PE, Pannarale G, Colantoni C, Martino E, Niglio T, et al. Low dose chromium-polynicotinate or policosanol is effective in hypercholesterolemic children only in combination with glucomannan. Atherosclerosis. (2013) 228:198–202. doi: 10.1016/j.atherosclerosis.2013.02.005
15. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25.
16. Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, de Campos MM, et al. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. (2011) 44:348–50. doi: 10.1016/j.clinbiochem.2010.12.011
17. Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. (2008) 3:1718–28. doi: 10.1038/nprot.2008.171
18. Jamilian M, Bahmani F, Siavashani MA, Mazloomi M, Asemi Z, Esmaillzadeh A. The Effects of chromium supplementation on endocrine profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. (2016) 172:72–8. doi: 10.1007/s12011-015-0570-6
19. Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr. (2015) 34:586–92. doi: 10.1016/j.clnu.2014.09.015
20. Foroozanfard F, Jamilian M, Bahmani F, Talaee R, Talaee N, Hashemi T, et al. Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D-deficient women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Clin Endocrinol. (2015) 83:888–94. doi: 10.1111/cen.12840
21. Chen G, Liu P, Pattar GR, Tackett L, Bhonagiri P, Strawbridge AB, et al. Chromium activates glucose transporter 4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechanism. Mol Endocrinol. (2006) 20:857–70. doi: 10.1210/me.2005-0255
22. Ulas M, Orhan C, Tuzcu M, Ozercan IH, Sahin N, Gencoglu H, et al. Anti-diabetic potential of chromium histidinate in diabetic retinopathy rats. BMC Complement Altern Med. (2015) 15:16. doi: 10.1186/s12906-015-0537-3
23. Arvind K, Pradeepa R, Deepa R, Mohan V. Diabetes & coronary artery disease. Indian J Med Res. (2002)116:163–76
24. Benigni A, Zoja C, Campana M, Corna D, Sangalli F, Rottoli D, et al. Beneficial effect of TGFbeta antagonism in treating diabetic nephropathy depends on when treatment is started. Nephron Exp Nephrol. (2006) 104:e158–68. doi: 10.1159/000094967
25. Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens. (2010) 19:393–402. doi: 10.1097/MNH.0b013e32833aa4ac
26. Jain SK, Croad JL, Velusamy T, Rains JL, Bull R. Chromium dinicocysteinate supplementation can lower blood glucose, CRP, MCP-1, ICAM-1, creatinine, apparently mediated by elevated blood vitamin C and adiponectin and inhibition of NFkappaB, Akt, and Glut-2 in livers of zucker diabetic fatty rats. Mol Nutr Food Res. (2010) 54:1371–80. doi: 10.1002/mnfr.200900177
27. Sahin N, Akdemir F, Orhan C, Aslan A, Agca CA, Gencoglu H, et al. A novel nutritional supplement containing chromium picolinate, phosphatidylserine, docosahexaenoic acid, and boron activates the antioxidant pathway Nrf2/HO-1 and protects the brain against oxidative stress in high-fat-fed rats. Nutr Neurosci. (2012) 15:42–7. doi: 10.1179/1476830512Y.0000000018
28. Donnelly R, Davis KR. Type 2 diabetes and atherosclerosis. Diabetes Obes Metab. (2000) 2 (Suppl. 1):S21–30.
29. Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. (2005) 7:1040–52. doi: 10.1089/ars.2005.7.1040
30. Bartlett HE, Eperjesi F. Nutritional supplementation for type 2 diabetes: a systematic review. Ophthalmic Physiol Opt. (2008) 28:503–23. doi: 10.1111/j.1475-1313.2008.00595.x
31. Haimovici F, Anderson JL, Bates GW, Racowsky C, Ginsburg ES, Simovici D, et al. Stress, anxiety, and depression of both partners in infertile couples are associated with cytokine levels and adverse IVF outcome. Am J Reprod Immunol. (2018) 79:e12832. doi: 10.1111/aji.12832
32. Wang XF, Xing FQ, Chen SL. Interleukin-1beta expression on ovarian granulosa cells and its clinical implication in women undergoing in vitro fertilization. Di Yi Jun Yi Da Xue Xue Bao. (2002) 22:934–6.
33. Wunder DM, Mueller MD, Birkhauser MH, Bersinger NA. Increased ENA-78 in the follicular fluid of patients with endometriosis. Acta Obstet Gynecol Scand. (2006) 85:336–42. doi: 10.1080/00016340500501715
34. Levin I, Gamzu R, Mashiach R, Lessing JB, Amit A, Almog B. Higher C-reactive protein levels during IVF stimulation are associated with ART failure. J Reprod Immunol. (2007) 75:141–44. doi: 10.1016/j.jri.2007.03.004
Keywords: chromium supplementation, gene expression, insulin metabolism, inflammatory markers, polycystic ovary syndrome
Citation: Amiri Siavashani M, Zadeh Modarres S, Mirhosseini N, Aghadavod E, Salehpour S and Asemi Z (2018) The Effects of Chromium Supplementation on Gene Expression of Insulin, Lipid, and Inflammatory Markers in Infertile Women With Polycystic Ovary Syndrome Candidate for in vitro Fertilization: A Randomized, Double-Blinded, Placebo-Controlled Trial. Front. Endocrinol. 9:726. doi: 10.3389/fendo.2018.00726
Received: 06 August 2018; Accepted: 15 November 2018;
Published: 28 November 2018.
Edited by:
Katja Teerds, Human and Animal Physiology, Wageningen University, NetherlandsReviewed by:
Seunghyung Lee, Kangwon National University, South KoreaStephen Franks, Imperial College London, United Kingdom
Copyright © 2018 Amiri Siavashani, Zadeh Modarres, Mirhosseini, Aghadavod, Salehpour and Asemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahrzad Zadeh Modarres, c2hhcnphZC5tb2RhcnJlc0BzYm11LmFjLmly
Zatollah Asemi, YXNlbWlfckB5YWhvby5jb20=
 Mehrnush Amiri Siavashani1
Mehrnush Amiri Siavashani1 Zatollah Asemi
Zatollah Asemi