- Unit of Pediatrics, Department of Human Pathology of Adulthood and Childhood, University of Messina, Messina, Italy
Background: About 85–90% of children born small for gestational age (SGA) experience a catch-up growth that occurs mostly during the first year of life and results in a full stature recovery by the age of 2.
Objective: To investigate the relation between bone maturation (BM) and catch-up growth during the first year of life in SGA infants.
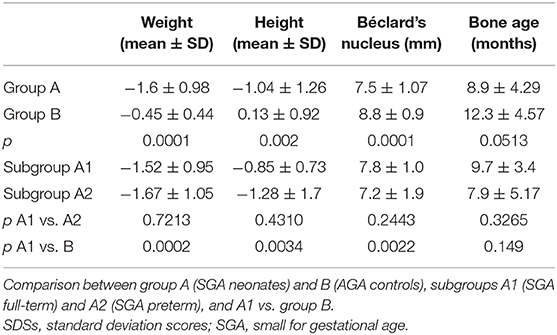
Method: Newborns whose weight and/or length was <-2 SD for gestational age were classified as SGA (group A). The study included a group of 32 SGA, 21 of which are full term [37–41 gestation weeks (GW), subgroup A1] and 11 preterm (30–36 GW, subgroup A2). Control group (B) consisted of 19 full-term and adequate-for-gestational-age (AGA) newborns. All the participants were born in the same hospital and period (January–December 2017). Chromosomal disorders, congenital defects, and maternal chronic diseases were criteria of exclusion. The study population underwent longitudinal evaluation of growth parameters and BM at 0, 3, 6, and 12 months. Assessment of BM was performed by an ultrasonographic (US) study of Béclard's nucleus (NB) (<3 mm at birth, meaning intrauterine delay of BM).
Results: Significantly higher height velocity (HV) was observed in subgroup A2 vs. A1 (32.4 ± 8.0 vs. 25.6 ± 2.9 cm, p = 0.01); nevertheless, more subjects in subgroup A2 had height <-2 SD at year 1 than had subgroup A1 (27.3 vs. 0%, p = 0.01). Intrauterine delay of BM was more common in group A vs. B (59.4 vs. 21.2%, p = 0.0078) and in subgroup A2 vs. A1 (90.9 vs. 42.9%, p = 0.0086). In group A, HV over the first year of life negatively correlates with NB diameter assessed at birth (r = −0.6, p < 0.001) but positively correlates with NB growth (r = 0.52, p < 0.01). Moreover, SGA babies with intrauterine delay of BM showed higher HV and better height gain at 12 months' evaluation than did SGA with adequate BM (29.75 ± 3.1 vs. 23.8 ± 2.7 cm, p = 0.003).
Conclusion: Neonatal BM should be regarded as a predictive factor of SGA height gain during the first year of life. US evaluation of NB is a useful noninvasive technique to identify intrauterine delay of BM, which positively correlates with early postnatal catch-up growth of SGA infants.
Introduction
The term small for gestational age (SGA) describes neonates whose weight and/or length at birth is below the cutoff value of −2 SD for gestational age. In clinical practice, the definition of SGA requires rigorous evaluations: (a) gestational dating, assessed by first-trimester ultrasound exam; (b) accurate anthropometry at birth, including measurements of weight, length, and head circumference; and (c) comparison with data coming from adequate reference population (1–3).
Instead, the concept of intrauterine growth restriction (IUGR) refers to slow fetal growth due to maternal, fetal, and/or placental causes, on the basis of at least two ultrasound exams performed during pregnancy. This process may result in a SGA newborn, even if it is worth noting that a SGA baby is not necessarily IUGR. Indeed, the SGA definition can also include a percentage of neonates (18–22%) who are only constitutionally small (4).
The recognition of SGA condition raises public health issues, as it could imply a variety of short- and long-term consequences including increased perinatal morbidity and mortality, higher risk of short stature, metabolic disorders later in life, and neurocognitive vulnerabilities (2, 5, 6).
With respect to growth, it is well documented that about 85–90% of SGA babies experience a catch-up growth that occurs mostly during the first 12 months of life and results in a full stature recovery by the age of 2 years. The remaining 10–15% of SGA children do not undergo compensatory growth and will remain permanently <-2 SD for height. Indeed, 20% of short-stature adults were born SGA. Finally, the preterm infants born SGA are regarded as a special case, as they may take up to 4 years to achieve a height within the normal range for population (1, 7, 8). The mechanisms of this accelerated linear growth are still not completely clarified (9). Birth weight, birth length, the magnitude of fetal growth retardation and midparental height could be regarded as influencing factors (8, 10, 11).
To the best of our knowledge, bone maturation (BM) assessment in SGA newborns has never been reported to now. As a consequence, there is a lack of specific literature data about the potential influence of BM progression on compensatory growth in this population.
Our prospective study aims to detect the factors that may be involved in early catch-up growth and to investigate the link between BM and postnatal growth during the first year of life in SGA infants.
Materials and Methods
Study Population
The study population consisted of 32 SGA neonates who were born in the same hospital and period (January–December 2017), fulfilling the following inclusion criteria: (a) birth weight and/or length <-2 SD for gestational age; and (b) gestational dating assessed by first-trimester ultrasound exam.
Sick newborns and those with genetic syndromes, chromosomal disorders, congenital defects, neonatal screening positivity, or maternal chronic diseases were excluded from the study.
The entire series of SGA newborns (group A, 18 males) was divided into two subgroups, according to gestational age: 21 full-term SGA [37–41 gestation weeks (GW)] were included in subgroup A1 (11 males) and 11 SGA preterm (30–36 GW) in subgroup A2 (7 males). According to BM assessment at birth, group A was divided into two further subgroups: 13 SGA with adequate BM were part of subgroup A3 and 19 SGA with delayed BM of subgroup A4. The SGA population was compared with controls (group B, 13 males) represented by 19 full-term and adequate-for-gestational-age (AGA) neonates born in the same hospital and period.
Study Design
Newborns who fulfilled the above reported inclusion criteria were enrolled for this prospective one-center study.
The study population underwent longitudinal evaluation of growth parameters (weight, length, and head circumference) and BM at birth and at 3, 6, and 12 months. Bone age was assessed at 12 months of age.
The study design was approved by the local ethical committee of the hospital participating to the study. Informed consent was obtained earlier by the children's parents.
Methods
Gestational age was defined by ultrasound measurement recorded during early pregnancy. Newborns whose weight and/or length was <-2 SD for gestational age were classified as SGA. Anthropometric evaluation was based on standard length, weight, and head circumference measurements. To allow the comparison between different ages and genders, the above-mentioned auxological parameters were expressed as standard deviation scores (SDSs). As recommended by the Italian Society of Pediatric Endocrinology and Diabetology (ISPED), the reference population standards were assessed according to the INeS (Italian Neonatal Study) charts from birth to term (12, 13) and to World Health Organization (WHO) growth charts from term to 24 months (14). Assessment of BM was performed by an ultrasonographic (US) study of the distal femoral epiphyseal ossification center known as Béclard's nucleus (NB) (15, 16). This approach had been previously used for assessment of congenital hypothyroidism, where BM retardation at birth turned out to be a helpful indicator of intrauterine and severe hypothyroidism, with a negative prognostic value on a long-term intellectual outcome (17–21). In all the patients, the presence or absence of the distal femoral epiphyseal nucleus was scored according to the method proposed by Ilicki et al. (17), applied to US evaluation and simplified as follows: bony nucleus diameter was measured using electronic calipers, and BM at birth was considered adequate if NB diameter exceeded 3 mm or delayed if this bony nucleus was absent or its diameter was <3 mm (meaning intrauterine delay of BM). This definition was chosen according to the criteria suggested by Senecal et al. (18) and partially modified by Ilicki et al. (17). The same limit had previously been adopted by Heyerdahl et al. (19). In fact, a knee epiphysis diameter <3 mm implies that the epiphyseal area is <0.07 mm2, which is very similar to the cutoff value (epiphyseal area <0.05 mm2) used by Glorieux et al. (20). This method of BM evaluation had also been used by Wasniewska et al. (21). An echographic study of the distal femoral epiphyseal nucleus was performed with a commercial US machine (Esaote Healthcare Technologies) equipped with a high-frequency linear transducer (7.5–20 MHz). Bone age was estimated by left hand and wrist X-ray at the age of 1 year using Greulich and Pyle's comparative method (22).
Statistical Analysis
Numerical data were expressed as mean, SD, and range; categorical variables were expressed as absolute frequency and percentage. For comparisons of two means, Student's t-test (normally distributed data) and the Mann–Whitney U-test (non-parametric data) were used. Frequency rates were compared by a chi-squared test. Correlations between quantitative variables were assessed using Pearson's correlation analysis. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21 (Armonk, NY, IBM Corp.). A p-value smaller than 0.05 was considered statistically significant.
Results
Main Auxological and Clinical Data at Birth
The clinical features of the entire study population at birth are shown in Table 1. Mean Apgar score at 5 min, body length, and weight were significantly lower in group A than in group B. NB mean diameter at birth was also significantly lower in group A than in control group. Intrauterine delay of BM was documented more frequently in group A than group B (59.4 vs. 21.1%, p = 0.0078) and in subgroup A2 than A1 (90.9 vs. 42.9%, p = 0.0086).
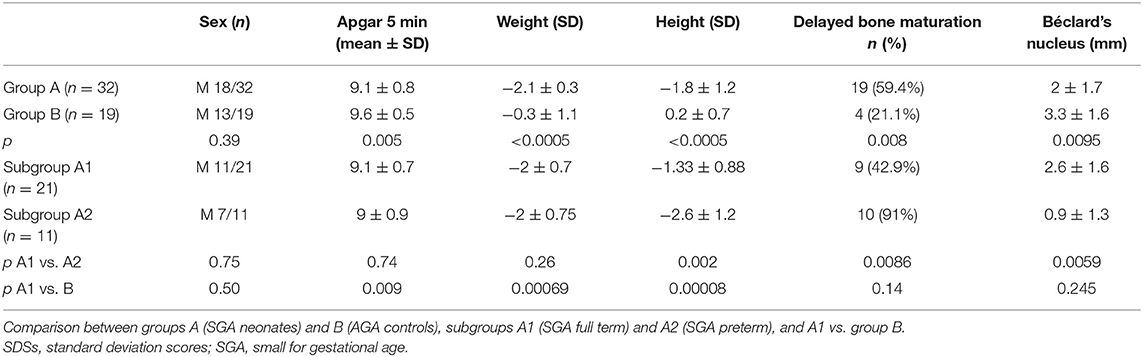
Table 1. Mean ± SDSs and number (n) with percentage (%) data collected at birth in the overall study population.
Growth Monitoring During the First Year of Life
Mean first-year height velocity (HV) of the entire population (group A + B) was 25.5 ± 13.2 cm. Significantly higher HV was observed in subgroup A2 vs. A1 (32.4 ± 8.0 vs. 25.6 ± 2.9 cm, p = 0.01); nevertheless, subjects in subgroup A2 presented more frequently with height <-2 SD than did subgroup A1 (27.3 vs. 0%, p = 0.01). HV was overall higher in group A, if compared with controls, but without reaching statistical significance [group A (28.6 ± 6.5 cm) vs. group B (25.5 ± 2.9 cm), p = 0.10]. The main features of growth pattern for each group and subgroup during follow-up are shown in Figures 1A,B. Subgroup A2 displayed overall a better catch-up growth pattern, which occurred later if compared with the earlier and more gradual stature recovery highlighted in subgroup A1. Indeed, subgroup A2 started catch-up growth only after 3 months of life, with significantly higher HV recorded after this period compared with baseline (p < 0.0005).
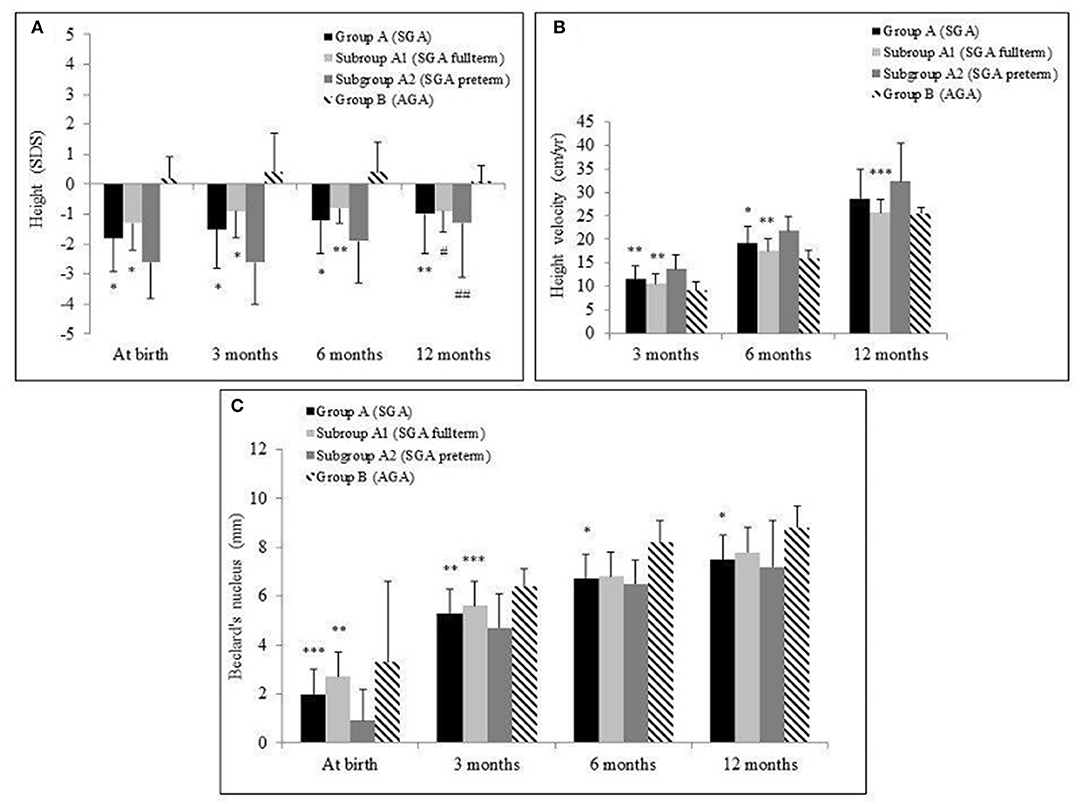
Figure 1. Mean and standard deviation of height standard deviation scores (SDSs) (A), height velocity in cm/year (B), and Béclard's nucleus in mm (C) in the groups A [small for gestational age (SGA)] and B [adequate for gestational age (AGA)] and in the subgroups A1 (SGA full term) and A2 (SGA preterm) during the 12-month follow-up. *p < 0.0005, **p < 0.005, ***p < 0.05. Comparison of group A vs. B and subgroup A1 vs. A2. #p < 0.0005, ##p < 0.005. Comparison of 12-month value vs. at birth in each group.
Group B exhibited a significantly higher body weight (SDSs) than group A during the entire follow-up period, without statistically significant difference between A1 and A2 subgroups.
US evaluation recorded a progressive NB growth during 12 months' follow-up in group A. Instead, group B showed this growth only until the ninth month of follow-up, with no further NB increase (Figure 1C). In group A, HV over the first year of life negatively correlated with NB diameter assessed at birth (r = −0.6, p < 0.001) but positively correlated with NB growth during follow-up (r = 0.52, p < 0.01). This relation was even stronger in subgroup A2 (respectively, r = −0.64, p < 0.01; r = 0.77, p < 0.01).
Growth parameters, BM, and bone age at the end of 12 months' follow-up are presented in Table 2.
Growth Pattern in Small for Gestational Age Infants With Delayed Bone Maturation
SGA infants with documented intrauterine delay of BM (subgroup A4) exhibited a pattern of progressive catch-up growth during the follow-up period. If compared with SGA infants with adequate BM (subgroup A3), subgroup A4 showed higher HV and better height gain (29.75 ± 3.1 cm in A4 subgroup vs. 23.8 ± 2.7 cm in A3 subgroup, p = 0.003) at 12 months' evaluation (Figures 2A,B).
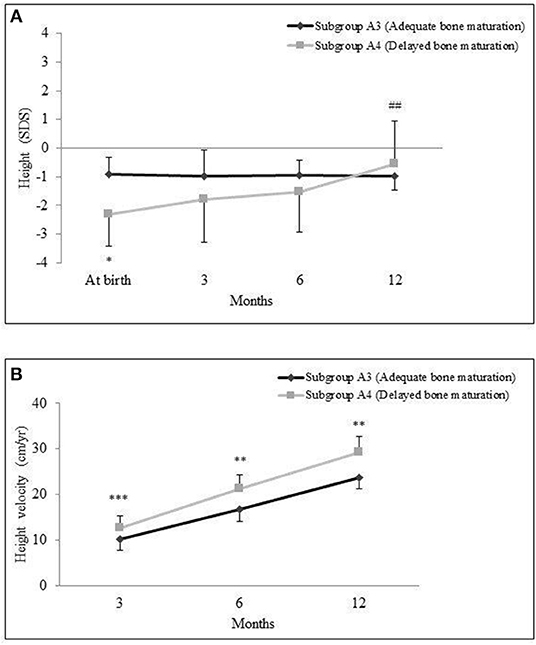
Figure 2. Mean and standard deviation of height standard deviation scores (SDSs) (A) and height velocity in cm/year and (B) in small for gestational age (SGA) babies classified into subgroups A3 (adequate bone maturation) and A4 (delayed bone maturation) during the 12-month follow-up. *p < 0.0005, **p < 0.005, ***p < 0.05. Comparison of subgroup A3 vs. A4. ##p < 0.0005. Comparison of 12-month values vs. at birth.
Moreover, the frequency of a height outcome <-2 SD at the age of 1 year did not significantly differ in these two subgroups (15.8% in A4 subgroup vs. 0% in A3 subgroup; p = 0.13).
At the end of follow-up, bone age evaluation showed similar scores in both subgroups (8.1 ± 5.2 months in A4 subgroup vs. 8.4 ± 4.9 months in A3 subgroup, p = 0.92).
Discussion
It is well known that SGA infants differ in postnatal growth. The typical growth pattern is characterized by a period of accelerated linear growth that occurs mainly in the first 12 months of life and results in a full stature recovery by the age of 2 years. However, 10–15% of SGA children do not undergo compensatory growth, achieving an adult height ~20 cm below their peers' and their target height (7, 8). The pathophysiology of postnatal growth failure is complex and involves a variety of alterations in the growth hormone (GH)/insulin-like growth factor (IGF)-1 axis. Classic GH deficiency is rarely described in SGA infants. In addition, mean IGF-1 and IGF binding protein-3 (IGFBP-3) levels can be reduced, underlying that the mechanisms responsible for growth failure may vary from insufficient IGF-1 production to IGF-1 insensitivity (6, 23). Nevertheless, there is no evidence that circulating levels of GH, IGF-1, and IGFBP-3 could be predictive of subsequent growth, and no biochemical markers have been identified yet (1, 9). During the first 6–12 months of life, spontaneous catch-up growth seems to be influenced predominantly by birth length, birth weight, gestational age, and the magnitude of growth retardation. Only later on (from 2 years of age onward) will the genetic influence of midparental height appear to take over (7, 24). Chromosomopathies or recognized syndromes (such as Silver–Russell or 3M) are associated with an incomplete catch-up growth (8, 10, 11, 25).
To our best knowledge, BM evaluation in SGA newborns has never been reported. It is therefore unknown if a delay of BM could be detected in SGA population at birth. Moreover, the possible influence of BM progression on SGA catch-up growth has never been studied until now.
Our prospective study aims to evaluate the auxological outcome during the first year of life in a cohort of SGA infants and to investigate the frequency of BM retardation at birth and whether BM delay may be involved in early catch-up growth. For this purpose, we chose a noninvasive technique of BM assessment. Indeed, ultrasonographic evaluation of the distal femoral epiphysis has been suggested as a method of determining skeletal maturity in newborn and infants, showing an excellent reliability and correlation with traditional radiological assessment (15, 16, 26).
SGA preterm infants showed overall a better catch-up growth pattern, which occurred later (after the third month of life) and with higher HV than observed in SGA full term, who exhibited instead an earlier and more gradual stature recovery.
In addition, our study showed that there is a positive relationship between BM at birth and HV during the first year of life in SGA infants. Indeed, intrauterine delay of BM positively correlates with catch-up growth, allowing higher HV and better height gain at 12 months' evaluation. These findings are even more evident in SGA preterm. Despite higher HV recorded among the above-mentioned subgroup, SGA preterm exhibited more frequently <-2 SD height outcome at the end of follow-up.
In conclusion, our results suggest for the first time that neonatal BM should be regarded as a predictive factor of SGA height gain during the first year of life. US evaluation of NB turned out to be a useful noninvasive technique to identify intrauterine delay of BM, which seems to positively correlate with early postnatal catch-up growth of SGA infants.
Limitations of the present study were represented by the small sample size; however, this weakness is counterbalanced by the high level of homogeneity exhibited by our population. In addition, our control group included only AGA full term, so it could be considered as only partially representative for the total study population (SGA full term and preterm).
Taking into consideration the lack of specific literature data on neonatal BM and its relationship with gestational age, we could not exclude that the delayed BM of SGA preterm infants in the present study could be related to prematurity. Overall, our data seem to encourage the use of US evaluation of NB, especially among neonates <-2 SD of length at birth. Anyway, to further characterize the influence of BM on SGA catch-up growth, future studies on a wider population are needed.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Messina. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
MW and MC conceived the manuscript. GP, MC, and DC were involved in data collection. GP, MW, and LM were involved in writing of the manuscript and carried out data analysis. MV, GBP, and TA were involved in literature search. All authors approved the submitted version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors declare that the abstract of the present original research article firstly appeared in the proceedings of the European Society for Paediatric Endocrinology 57th annual meeting [Hormone Research in Paediatrics 2018; 90(suppl 1): 355].
References
1. Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. (2007) 92:804–10. doi: 10.1210/jc.2006-2017
2. Saenger P, Czernichow P, Hughes I, Reiter EO. Small for gestational age: short stature and beyond. Endocr Rev. (2007) 28:219–51. doi: 10.1210/er.2006-0039
3. Saggese G, Fanos M, Simi F. SGA children: auxological and metabolic outcomes - the role of GH treatment. J Matern Fetal Neonatal Med. (2013) 26 Suppl 2:64–7. doi: 10.3109/14767058.2013.832870
4. McCowan LM, Figueras F, Anderson NH. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol. (2018) 218:S855–S68. doi: 10.1016/j.ajog.2017.12.004
5. Cho WK, Suh BK. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr. (2016) 59:1–7. doi: 10.3345/kjp.2016.59.1.1
6. Iughetti L, Lucaccioni L, Ferrari F. Challenges in the development and growth of small for gestational age newborns. Expert Rev Endocrinol Metab. (2017) 12:253–60. doi: 10.1080/17446651.2017.1338137
7. Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. (1995) 38:733–9. doi: 10.1203/00006450-199511000-00017
8. Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatr Res. (1995) 38:267–71. doi: 10.1203/00006450-199508000-00022
9. Leger J, Noel M, Limal JM, Czernichow P. Growth factors and intrauterine growth retardation. II. Serum growth hormone, insulin-like growth factor (IGF) I, and IGF-binding protein 3 levels in children with intrauterine growth retardation compared with normal control subjects: prospective study from birth to two years of age. Study Group of IUGR. Pediatr Res. (1996) 40:101–7. doi: 10.1203/00006450-199607000-00018
10. Karlberg J, Albertsson-Wikland K, Kwan CW, Chan FY. Early spontaneous catch-up growth. J Pediatr Endocrinol Metab. (2002) 15(Suppl 5):1243–55.
11. Leger J, Limoni C, Collin D, Czernichow P. Prediction factors in the determination of final height in subjects born small for gestational age. Pediatr Res. (1998) 43:808–12. doi: 10.1203/00006450-199806000-00015
12. Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, et al. Neonatal Anthropometric Charts: The Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. (2010) 51:353–61. doi: 10.1097/MPG.0b013e3181da213e
13. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. (1992) 11:1305–19. doi: 10.1002/sim.4780111005
14. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. (2006) 450:76–85.
15. Leshem E, Bialik V, Hochberg Z. Ultrasonographic assessment of bone maturity in newborns. Horm Res. (2002) 57:180–6. doi: 10.1159/000058379
16. Delle Donne HD Jr, Faundes A, Tristao EG, de Sousa MH, Urbanetz AA. Sonographic identification and measurement of the epiphyseal ossification centers as markers of gestational age. J Clin Ultrasound. (2005) 33:394–400. doi: 10.1002/jcu.20156
17. Ilicki A, Larsson A, Mortensson W. Neonatal skeletal maturation in congenital hypothyroidism and its prognostic value for psychomotor development at 3 years in patients treated early. Horm Res. (1990) 33:260–4. doi: 10.1159/000181532
18. Se'ne'cal J, Grose MC, Vincent A, Simon J, Lefreche JN. Maturation osseuse de foetus et du nouveau-ne'. Archives Francaises de Pe'diatrie. (1977) 29:13–22.
19. Heyerdahl S, Kase BF, Stake G. Skeletal maturation during thyroxine treatment in children with congenital hypothyroidism. Acta Paediatr. (1994) 83:618–22. doi: 10.1111/j.1651-2227.1994.tb13092.x
20. Glorieux J, Desjardins M, Letarte J, Morissette J, Dussault JH. Useful parameters to predict the eventual mental outcome of hypothyroid children. Pediatr Res. (1988) 24:6–8. doi: 10.1203/00006450-198807000-00003
21. Wasniewska M, De Luca F, Cassio A, Oggiaro N, Gianino P, Delvecchio M, et al. In congenital hypothyroidism bone maturation at birth may be a predictive factor of psychomotor development during the first year of life irrespective of other variables related to treatment. Eur J Endocrinol. (2003) 149:1–6. doi: 10.1530/eje.0.1490001
22. Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd edn. Stanford: Stanford University Press (1959).
23. Albertsson-Wikland K, Boguszewski M, Karlberg J. Children born small-for-gestational age: postnatal growth and hormonal status. Horm Res. (1998) 49:7–13. doi: 10.1159/000053080
24. Luo ZC, Albertsson-Wikland K, Karlberg J. Length and body mass index at birth and target height influences on patterns of postnatal growth in children born small for gestational age. Pediatrics. (1998) 102:E72. doi: 10.1542/peds.102.6.e72
25. Beger C, Merker A, Mumm R, Gausche R. Growth prediction of small for gestational age infants within the first weeks after birth. Anthropol Anz. (2018) 74:377–82. doi: 10.1127/anthranz/2018/0820
Keywords: small for gestational age, preterm infant, bone maturation, distal femoral epiphyseal nucleus, catch-up growth
Citation: Pepe G, Calafiore M, Valenzise M, Corica D, Morabito L, Pajno GB, Aversa T and Wasniewska M (2020) Bone Maturation as a Predictive Factor of Catch-Up Growth During the First Year of Life in Born Small for Gestational Age Infants: A Prospective Study. Front. Endocrinol. 11:147. doi: 10.3389/fendo.2020.00147
Received: 30 November 2019; Accepted: 03 March 2020;
Published: 24 March 2020.
Edited by:
M. Loredana Marcovecchio, University of Cambridge, United KingdomReviewed by:
Maria G. Vogiatzi, University of Pennsylvania, United StatesPaul Saenger, New York University Winthrop Hospital, United States
Copyright © 2020 Pepe, Calafiore, Valenzise, Corica, Morabito, Pajno, Aversa and Wasniewska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgia Pepe, Z2lvcmdpYXBlcGUyM0BnbWFpbC5jb20=
 Giorgia Pepe
Giorgia Pepe Mariarosa Calafiore
Mariarosa Calafiore Mariella Valenzise
Mariella Valenzise Domenico Corica
Domenico Corica Letteria Morabito
Letteria Morabito Giovanni Battista Pajno
Giovanni Battista Pajno Tommaso Aversa
Tommaso Aversa Malgorzata Wasniewska
Malgorzata Wasniewska