- 1Discipline of Clinical Psychology, Graduate School of Health, University of Technology Sydney, Sydney, NSW, Australia
- 2Library Section for Medicine and Health Sciences, NTNU University Library, NTNU–Norwegian University of Science and Technology, Trondheim, Norway
- 3Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Sciences, NTNU–Norwegian University of Science and Technology, Trondheim, Norway
Ranked highly in its association with serious medical comorbidities, obesity, a rapidly growing epidemic worldwide, poses a significant socio-economic burden. While bariatric procedures offer the most efficacious treatment for weight loss, a subset of patients risk weight recidivism. Due to the heterogeneity of obesity, it is likely that there are phenotypes or sub-groups of patients that require evidence-based psychological support to produce more sustainable outcomes. So far, however, characteristics of patients have not led to a personalized treatment algorithm for bariatric surgery. Maintenance of weight loss following bariatric surgery requires long-term modification of eating behaviors and physical activity. A recent Clinical Obesity Maintenance Model (COMM) proposed a conceptual framework of salient constructs, including the role of habit, behavioral clusters, emotion dysregulation, mood, health literacy, and executive function as interconnected drivers of obesity maintaining behaviors relevant to the field of bariatric psychology. The primary aim of this concise review is to bring together emerging findings from experimental and epidemiological studies relating to the COMM constructs that may inform the assessment and follow up of bariatric surgery. We also aim to explain the phenotypes that need to be understood and screened prior to bariatric surgery to enable better pre-surgery intervention and optimum post-surgery response.
Introduction
Associated with serious medical comorbidities, obesity is a major risk factor for preventable mortality and morbidity worldwide (1). Bariatric surgery has been shown to be an effective treatment for obesity resulting in greater weight loss than non-surgical treatments (2–4). Although there is a lack of consensus about what constitutes significant weight regain in bariatric surgery studies (5), there is consensus that a subset of patients risk weight recidivism (6–8), and up to 50% of patients experience weight regain within 2 years after surgery (5).
Researchers have suggested that the mechanisms that aid initial weight loss are theoretically distinct from those associated with weight loss maintenance [e.g., (9)]. A growing number of recent studies have indicated that in addition to metabolic and surgical explanations, post-surgical weight regain may be influenced by maladaptive eating, lifestyle behaviors, and psychological co-morbidities (10). Thus, the development of a sound theoretical framework will influence the design of future studies and contribute to greater pre-surgical readiness and improved treatment of post-surgical challenges that impact weight recidivism (11).
Incorporating evidence from the fields of eating disorders (ED), neuropsychology, and obesity, the Clinical Obesity Maintenance Model (COMM; 12) has highlighted the need to examine the behavioral and psychological mechanisms that underpin longer-term weight loss maintenance. The specific executive function (EF) deficits at the center of this model [Figure 1; (12)], can be considered to address readiness for bariatric surgery or weight recidivism following surgery. In addition, the COMM suggests that emotion dysregulation, maladaptive habits, behavioral clusters, health literacy (HL), and mood interact with executive functioning and impact eating and physical inactivity leading to maintain obesity (12). In this mini review, we aim to summarize research on the COMM constructs from the past 7 years and provide a theoretical framework of the mechanisms that may be implicated in unsuccessful outcomes following bariatric surgery. Psychological underpinnings associated with post-surgery weight regain will be elucidated and research gaps identified.
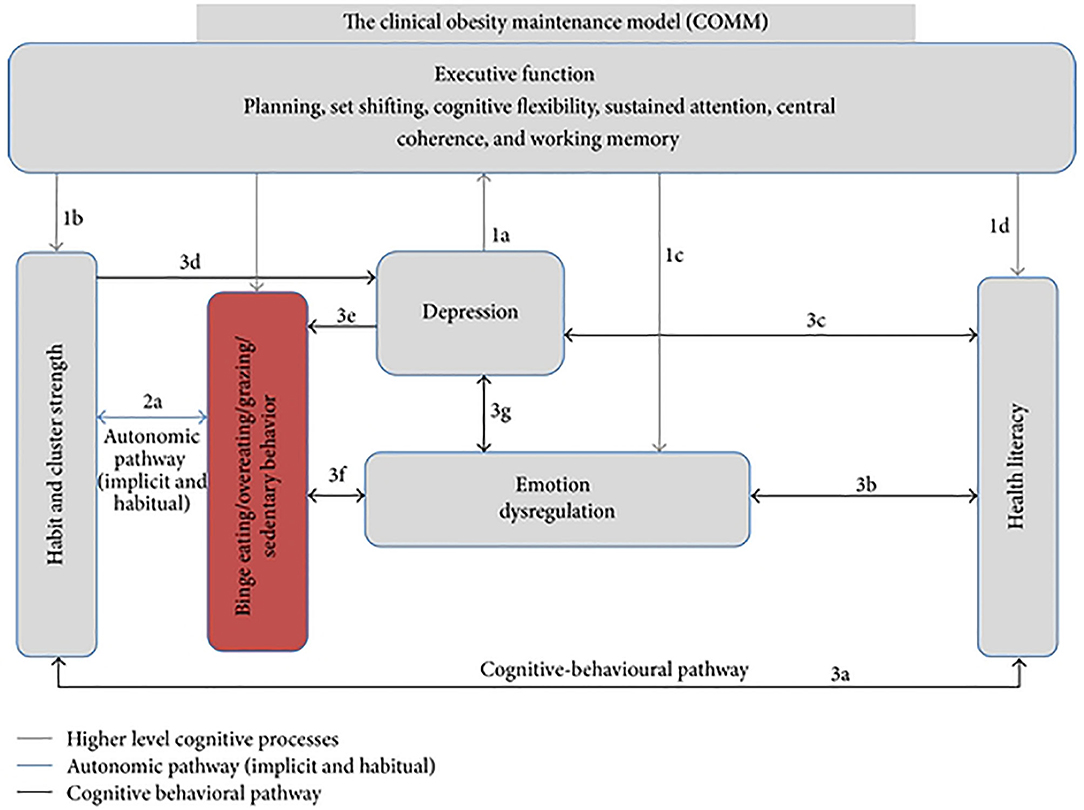
Figure 1. Hypothesized model of the Clinical Obesity Maintenance Model (COMM). The letters indicate direct pathways between variables. From Raman et al. (12). The figure is licensed under Creative Commons Attribution 4.0. License; https://creativecommons.org/licenses/by/4.0.
Executive Function
The challenge of weight recidivism has prompted researchers to explore beyond the physiological and psychological aspects of obesity (9, 13). As outlined in the COMM, emerging evidence has suggested that obesity-related disordered eating behaviors (DEB) are linked to deficits in executive functioning (EF) independent of differences in intelligence, level of education, and after controlling for gender, obesity severity, and age (14–18). Although not a unitary construct, EF refers to a set of cognitive processes and behavioral competencies that are involved in initiating and executing strategies, sustaining or flexibly redirecting attention, inhibiting inappropriate behavioral responses, planning, sequencing, and achievement of complex goal-oriented behavior, and cognitive flexibility (19).
A recent meta-analysis found a significant inverse relationship between obesity and EF, including cognitive flexibility, inhibition, decision-making, and planning (17). Nevertheless, the nature by which executive deficits are associated with obesity is unclear. Several plausible explanations have been proposed, including inflammation driven factors, changes in brain-derived neurotrophic factor, dopamine dysregulation implicated in hyperphagia, vascular diseases, neuroendocrine changes, and leptin [e.g., (20–22)]. Each of these factors may independently or collectively contribute to executive deficits in obesity and may influence bariatric surgery outcomes. Furthermore, the direction of this relationship remains unclear. Most studies contend that causality may occur in either direction, with impaired EF increasing the risk of obesity or obesity impacting on EF. For example, a recent systematic review found evidence of a reciprocal influence between obesity and EF (23). Supporting this interpretation, bariatric patients have demonstrated improvements in EF following surgical weight loss (24).
Executive deficits manifested through challenges in self-regulation of eating behaviors may lead to poorer weight loss maintenance after bariatric surgery (25). For example, a study of 37 bariatric surgery patients found that EF was strongly associated with adherence to post-surgical guidelines shortly after surgery (25). Supporting this notion, a recent study has shown that EF predicts a higher body mass index (BMI) 12 months after bariatric surgery (26). Similarly, poorer executive performance 12 weeks post-surgery has been found to be indicative of reduced weight loss at the 3 years follow up (27). Given this body of recent evidence, future research should further explore how best to consider executive deficits in the pre-surgery screening and follow-up of bariatric candidates.
Disordered Eating Behaviors and Eating Disorders
A substantial evidence-base supports the prevalence of disordered eating behaviors (DEB) and eating disorders (ED) in bariatric surgery outcomes [e.g., (28–31)] with two recent studies finding a higher prevalence of ED in post-surgical bariatric patients with weight regain (32, 33). In addition, Binge Eating Disorder (BED) is highly prevalent among bariatric patients (34). BED refers to eating an excessive amount of food in a discrete period of time, accompanied by a sense of LOC over eating (35). Although gastrointestinal modifications may help to restrict portion sizes post-surgery, new DEB may develop as a compensatory mechanism, with a higher frequency of energy intake (36). In support of this view, recent cross-sectional and longitudinal studies have found evidence on the association between grazing (i.e., intake of smaller portions of food over extended periods) and post-surgical weight regain [e.g., (37)]. Furthermore, researchers have emphasized the role of other DEB, such as emotional eating, night eating syndrome, and picking and nibling in bariatric surgery outcomes (37–39). One longitudinal study found that about 65% of patients with weight regain reported pre-surgery DEB (32). Thus, comprehensive pre and post-surgery assessments and intervention for ED and DEB may hold promise for optimizing bariatric outcomes.
Depression
An association between depression and obesity has been long supported by clinical and epidemiological studies (40, 41) with a recent meta-analyses providing further evidence of this relationship in bariatric surgery patients (42). A review on the psychological outcomes after bariatric surgery found that pre-surgery depression symptoms reduced at 6, 12, and 24 months after bariatric surgery; however, from 36 months onwards, depression symptoms increased and returned to pre-surgery levels (43). Similarly, a population-based study of 4,793 participants found that bariatric surgery patients had higher levels of depression than others with similar BMI and that initial reductions in depression were not maintained at the follow-up (44). Other studies have also shown that improvements in depressive symptoms following bariatric surgery may not be maintained after the initial post-surgery years (i.e., 1–3 years) and that depressive symptoms may return to baseline or worsen in some patients (45–47).
Post-surgery weight regain and depression may act as a risk factor for one another. Weight regain after surgery has been indicated as a significant risk factor for recurring or elevated depression post-surgery (48, 49). These findings are consistent with the notion that rapid post-surgery weight loss only temporarily aids the remission of depression, which later re-occurs once the surgical benefits decline (43, 50, 51). Similarly, studies have shown that depressed mood is associated with unhealthy lifestyle habits (52), emotional eating, and loss of control (LOC) eating [e.g., (53, 54)]. In particular, post-surgery depressive symptoms have been associated with ED and weight recidivism (42, 55–57). Therefore, the role of depression has important implications for post-surgery functioning and should be monitored and addressed through focused evidence-based interventions.
Emotion Dysregulation
Individuals with obesity often demonstrate a dysregulated physiological response to intense emotion, known as emotional eating. Specific aspects of emotion dysregulation, such as a lowered tendency to act with emotional awareness (58), difficulty identifying emotions (59), and limited access to emotion regulation strategies (60) have been implicated. A high prevalence of emotion dysregulation in pre-surgery bariatric patients has been shown (61), and pre-surgery maladaptive eating was in one study initiated by both avoidance (of negative affect) and approach (reward sensitivity) behaviors (62). More, emotion dysregulation fully mediated the associations between emotional eating as well as eating in the absence of hunger in another study of bariatric surgery candidates (63). Distress tolerance, an aspect of emotion regulation, has been found in one study to be unrelated to 2-years post-surgical weight loss outcomes but delineated individuals opting for bariatric surgery (64). In contrast, bariatric surgery patients with greater weight loss more frequently applied emotion regulation strategies post-surgery than pre-surgery, compared to patients with lower weight loss (65). Furthermore, there is ample empirical support for a direct link between emotion dysregulation and BED (63, 65, 66), highly prevalent in bariatric patients (30, 34, 43). Accumulating evidence thus indicates that emotional regulatory factors may act as drivers to initiate and/or maintain DEB and ED in bariatric surgery patients. Consequently, future research should further explore the emotional determinants of longer-term weight loss maintenance following bariatric surgery.
Habit
Habit has been defined as “a process by which a stimulus generates an impulse to act as a result of a learned stimulus-response association” (67). Behaviors driven by habitual automaticity require powerful intentions to override, and developing new habits involves a gradual transfer in cognitive control from purposeful actions to automatic processes (68). Poor dietary choices are likely to be perpetuated by habit (12). For example, habit strength and energy intake were significantly associated in a recent ecological momentary assessment study (69). Furthermore, researchers have asserted that grazing, a DEB described as “mindless,” “distracted,” and “non-anticipated,” should be studied as a habitual and automatic behavior (70). This was supported in a review where a link between grazing and weight recidivism post-bariatric surgery, independent of surgery type and contextual concept of grazing was found (37). In addition, habit has been shown to partially regulate physical activity (71). More, irrespective of the amount of weight loss 6 and 12 months following bariatric surgery, patients may persist with lifestyle habits, such as physical inactivity, lower consumption of protein, fruit, and vegetables, and higher consumption of carbohydrates, sugars, and fats that place them at a high risk for weight regain (72, 73). Maladaptive pre-surgery habits and DEB may thus pose significant challenges to optimal longer-term surgical outcomes (74). Future assessments of bariatric surgery candidates should consider pre-surgery habits as a major driver of weight loss practices (75, 76).
Health Literacy
Health Literacy (HL) is another construct in the COMM that has been included as a modifiable determinant of obesity maintaining behaviors. HL has been defined as the “cognitive and social skills that determine the motivation and ability of individuals to gain access to, understand, and use information in ways which promote and maintain good health” (77). The COMM considers HL a logical prerequisite to healthy eating behaviors and an active lifestyle. Lower levels of HL are associated with excessive body weight and difficulties overcoming obesity (78) as well as increased use of unhealthy weight loss methods (79), poorer weight loss following bariatric surgery (80), and lower levels of physical activity (81). A patient's HL also contributes to decision-making regarding bariatric surgery. For example, research has found that patients with higher HL and education are more likely to elect bariatric surgery, whereas patients with lower HL and less education are less likely to opt for bariatric surgery (82). Similarly, higher pre-surgical HL is associated with successful weight loss outcomes 12 months after bariatric surgery (83). Evidence also suggests that targeting HL through primary health care interventions results in a significant reduction in weight (84). Therefore, improving one's HL may guide patients to make informed decisions about their treatment and better understand the potential implications of surgery and the barriers to longer-term weight management.
Behavioral Clusters
Clustering is the co-occurrence of several risk behaviors all of which share an underlying association (85). In obesity, behavioral clusters may interact in multiple ways to maintain the condition, with potentially synergistic effects (86). In support of this view, a study found that bariatric candidates who reported pre-surgical grazing behaviors also reported more alcohol use, less physical activity, and more difficulties in post-surgery lifestyle modification (36). Similarly, longitudinal studies have indicated that pre-surgery problematic alcohol, substance, and tobacco use to be reliable correlates of post-surgery problematic alcohol and substance use in bariatric patients (87–89). Whether these maladaptive clusters are activated as coping and/or compensatory reward mechanisms in a caloric-restricted new lifestyle following bariatric surgery is yet to be explored (90). Similar to previous research that showed smoking, excessive alcohol use, unhealthy eating, and sedentary lifestyle as the salient “big four” modifiable causes of obesity (91), the above findings highlight how pattern recognition of behavioral clusters can inform future research and development of targeted interventions for bariatric surgery candidates. In addition, there is a need for bariatric research to delineate alcohol use disorder from other drug use to help identify behavioral clusters that may further inform post-surgery complications (87).
Conclusions, Limitations, and Future Research
This mini review aimed to provide a theoretical framework for bariatric psychology by summarizing emerging evidence on the psychological and behavioral constructs incorporated in the COMM. The findings of this review should be considered in light of several limitations. Firstly, a comprehensive delineation on outcome research findings from extant bariatric literature was beyond the scope of this review. For example, the magnitude and rate of weight loss outcomes and other psychological co-morbidities observed post-surgically have previously been shown to differ across types of bariatric surgeries and obesity class (92, 93). Secondly, EF, a central COMM component, has been presented as an overarching construct on account of brevity. Specific aspects of EF, in particular, cognitive flexibility may have important implications for ED and DEB and researchers have called for improved methods in pre-surgery cognitive assessment to help clarify longer term post-surgery outcomes [e.g., (94)]. Similarly, the role of depression in bariatric psychology was discussed in a broader context. Lifetime prevalence rate, type, and severity of depression and associated secondary comorbidities may play an important role in longer-term post-surgery outcomes and need to be carefully assessed at pre-surgery screening (95).
This concise review has outlined key modifiable factors and their putative pathways as interactive drivers of weight recidivism after bariatric surgery. For example, given the established links between EF and DEB, extant studies have begun to incorporate EF, binge eating, and grazing behaviors as outcome measures in the fields of eating behaviors and bariatric psychology [e.g., (36, 37, 96–98)]. Similarly, in a study comparing healthy controls and bariatric surgery patients with and without depression, obesity and depression were shown to have an additive effect on executive performance (99). This highlights the importance of addressing depression and executive deficits before and after bariatric surgery to enhance longer-term surgical outcomes. In addition, the COMM's top-down approach proposes that modification of habits rely on executive processes, which contribute to difficulties in adherence to sustainable eating following bariatric surgery. While executive processes are not typically required to perform habitual behaviors, current research has shown that EF is heavily involved when individuals aim to modify and develop new habits (100).
Under-recognized psychological difficulties and under-treated mental health may negatively impact bariatric surgery outcomes (101). To this effect, weight recidivism-specific HL, specially formulated therapies targeting ED, maladaptive habits and behavioral clusters, specific emotion regulation strategies, and evidence-based psychological therapies for depression may be offered prior to and/or following bariatric surgery. Future research could further explore these associations to gain insight into the determinants of the longer-term efficacy of bariatric surgery. The theoretical framework of COMM should also be further evaluated in bariatric studies, including the use of structural equation modeling and randomized controlled trials.
To conclude, post-surgical weight recidivism is an important public and socio-economic health issue. In addition, regaining weight after bariatric surgery has a significant impact on the patient's mental health and may lead to a recurrence of serious psychological comorbidities. A comprehensive pre-surgical psychological and behavioral assessment partnered with post-surgical management is vital in this regard. As set forth in this review, the underlying mechanisms outlined in the COMM call for a more integrative, multipronged approach in bariatric surgery assessment and care.
Author Contributions
TE-N and JR conceived the study and were in charge of the overall direction and planning. LJ and DS contributed to the acquisition and screening of data. JR took the lead in writing the manuscript. All authors provided critical feedback and helped shape the review, analysis, and manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. 10 Facts on Obesity. (2017). Retrieved from: https://www.who.int/features/factfiles/obesity/en (accessed August 04, 2020).
2. Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. (2014) 2014:CD003641. doi: 10.1002/14651858.CD003641.pub4
3. Courcoulas AP, Yanovski SZ, Bonds D, Eggerman TL, Horlick M, Staten MA, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. (2014) 149:1323–9. doi: 10.1001/jamasurg.2014.2440
4. Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. (2017) 377:1143–55. doi: 10.1056/NEJMoa1700459
5. Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. (2013) 23:1922–33. doi: 10.1007/s11695-013-1070-4
6. Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. (2013) 310:2416–25. doi: 10.1001/jama.2013.280928
7. King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. (2018) 320:1560–9. doi: 10.1001/jama.2018.14433
8. Panagiotou OA, Markozannes G, Kowalski R, Gazula A, Di M, Bond DS, et al. Short- and Long-Term Outcomes after Bariatric Surgery in the Medicare Population. Rockville, MD: Agency for Healthcare Research and Quality (2018).
9. Gettens KM, Gorin AA. Executive function in weight loss and weight loss maintenance: a conceptual review and novel neuropsychological model of weight control. J Behav Med. (2017) 40:687–701. doi: 10.1007/s10865-017-9831-5
10. Kushner RF, Sorensen KW. Prevention of weight regain following bariatric surgery. Curr Obes Rep. (2015) 4:198–206. doi: 10.1007/s13679-015-0146-y
11. Jumbe SE. Post-surgical cliff after weight loss surgery: accounts of patients and their health care professionals (Doctoral dissertation). University of the West of England, Bristol, United Kingdom (2017). Retrieved from: https://uwe-repository.worktribe.com/OutputFile/884532 (accessed August 04, 2020).
12. Raman J, Smith E, Hay P. The clinical obesity maintenance model: an integration of psychological constructs including mood, emotional regulation, disordered overeating, habitual cluster behaviours, health literacy and cognitive function. J Obes. (2013) 2013:240128. doi: 10.1155/2013/240128
13. Gilbert M, Raman J, Sui Z. Cognitive remediation-enabled cognitive behaviour therapy for obesity: a case series. Eat Weight Disord. (2019). doi: 10.1007/s40519-019-00823-4. [Epub ahead of print].
14. Jansen A, Houben K, Roefs A. A cognitive profile of obesity and its translation into new interventions. Front Psychol. (2015) 6:1807. doi: 10.3389/fpsyg.2015.01807
15. Lavagnino L, Arnone D, Cao B, Soares JC, Selvaraj S, Lavagnino L. Inhibitory control in obesity and binge eating disorder: a systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci Biobehav Rev. (2016) 68:714–26. doi: 10.1016/j.neubiorev.2016.06.041
16. Hayes JF, Eichen DM, Barch DM, Wilfley DE. Executive function in childhood obesity: promising intervention strategies to optimize treatment outcomes. Appetite. (2018) 124:10–23. doi: 10.1016/j.appet.2017.05.040
17. Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev. (2018) 84:225–44. doi: 10.1016/j.neubiorev.2017.11.020
18. Segura-Serralta M, Ciscar S, Blasco L, Oltra-Cucarella J, Roncero M, Espert R, et al. Contribution of executive functions to eating behaviours in obesity and eating disorders. Behav Cogn Psychother. (2020). doi: 10.1017/S1352465820000260. [Epub ahead of print].
19. Robbins TW. Dissociating executive functions of the prefrontal cortex. In: Roberts AC, Robbins TW, Weiskrantz L, editors. The Prefrontal Cortex. Oxford: Oxford University Press (1998). p. 117–30.
20. Yao L, Bhatta A, Xu Z, Lucas R, Huo Y, Bagi Z, et al. Obesity-induced inflammation of the visceral adipose tissue is mediated by upregulation of arginase 1 in vascular endothelial cells. Circulation. (2016) 134:A14793. doi: 10.1161/circ.134.suppl_1.14793
21. Small DM. Dopamine adaptations as a common pathway for neurocognitive impairment in diabetes and obesity: a neuropsychological perspective. Front Neurosci. (2017) 11:134. doi: 10.3389/fnins.2017.00134
22. Rashidian H, Rosenblat JD, McIntyre RS, Mansur RB. Leptin, obesity, and response to ketamine. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 98:109773. doi: 10.1016/j.pnpbp.2019.109773
23. Favieri F, Forte G, Casagrande M. The executive functions in overweight and obesity: a systematic review of neuropsychological cross-sectional and longitudinal studies. Front Psychol. (2019) 10:2126. doi: 10.3389/fpsyg.2019.02126
24. Handley JD, Williams DM, Caplin S, Stephens JW, Barry J. Changes in cognitive function following bariatric surgery: a systematic review. Obes Surg. (2016) 26:2530–7. doi: 10.1007/s11695-016-2312-z
25. Galioto R, Bond D, Gunstad J, Pera V, Rathier L, Tremont G. Executive functions predict weight loss in a medically supervised weight loss programme. Obes Sci Pract. (2016) 2:334–40. doi: 10.1002/osp4.70
26. Spitznagel MB, Garcia S, Miller LA, Strain G, Devlin M, Wing R, et al. Cognitive function predicts weight loss after bariatric surgery. Surg Obes Relat Dis. (2013) 9:453–9. doi: 10.1016/j.soard.2011.10.008
27. Spitznagel MB, Alosco M, Galioto R, Strain G, Devlin M, Sysko R, et al. The role of cognitive function in postoperative weight loss outcomes: 36-month follow-up. Obes Surg. (2014) 24:1078–84. doi: 10.1007/s11695-014-1205-2
28. Meule A, Heckel D, Jurowich CF, Vögele C, Kübler A. Correlates of food addiction in obese individuals seeking bariatric surgery. Clin Obes. (2014) 4:228–36. doi: 10.1111/cob.12065
29. Conceição EM, Utzinger LM, Pisetsky EM. Eating disorders and problematic eating behaviours before and after bariatric surgery: characterization, assessment and association with treatment outcomes. Eur Eat Disord Rev. (2015) 23:417–25. doi: 10.1002/erv.2397
30. Mitchell JE, King WC, Courcoulas A, Dakin G, Elder K, Engel S, et al. Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Disord. (2015) 48:215–22. doi: 10.1002/eat.22275
31. Parker K, Brennan L. Measurement of disordered eating in bariatric surgery candidates: a systematic review of the literature. Obes Res Clin Pract. (2015) 9:12–25. doi: 10.1016/j.orcp.2014.01.005
32. Conceição EM, Mitchell JE, Pinto-Bastos A, Arrojado F, Brandão I, Machado PPP. Stability of problematic eating behaviors and weight loss trajectories after bariatric surgery: a longitudinal observational study. Surg Obes Relat Dis. (2017) 13:1063–70. doi: 10.1016/j.soard.2016.12.006
33. Mauro MFFP, Papelbaum M, Brasil MAA, Carneiro JRI, Coutinho ESF, Coutinho W, et al. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta-analysis. Obes Rev. (2019) 20:1413–25. doi: 10.1111/obr.12907
34. Smith KE, Orcutt M, Steffen KJ, Crosby RD, Cao L, Garcia L, et al. Loss of control eating and binge eating in the 7 years following bariatric surgery. Obes Surg. (2019) 29:1773–80. doi: 10.1007/s11695-019-03791-x
35. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association (2013).
36. Nicolau J, Ayala L, Rivera R, Speranskaya A, Sanchís P, Julian X, et al. Postoperative grazing as a risk factor for negative outcomes after bariatric surgery. Eat Behav. (2015) 18:147–50. doi: 10.1016/j.eatbeh.2015.05.008
37. Pizato N, Botelho PB, Gonçalves V, Dutra ES, de Carvalho K. Effect of grazing behavior on weight regain post-bariatric surgery: a systematic review. Nutrients. (2017) 9:1322. doi: 10.3390/nu9121322
38. de Zwaan M, Marschollek M, Allison KC. The night eating syndrome (NES) in bariatric surgery patients. Eur Eat Disord Rev. (2015) 23:426–34. doi: 10.1002/erv.2405
39. Brode CS, Mitchell JE. Problematic eating behaviors and eating disorders associated with bariatric surgery. Psychiatr Clin North Am. (2019) 42:287–97. doi: 10.1016/j.psc.2019.01.014
40. Rivera M, Locke AE, Corre T, Czamara D, Wolf C, Ching-Lopez A, et al. Interaction between the FTO gene, body mass index and depression: meta-analysis of 13701 individuals. Br J Psychiatry. (2017) 211:70–6. doi: 10.1192/bjp.bp.116.183475
41. Porras-Segovia A, Rivera M, Molina E, Lopez-Chaves D, Gutierrez B, Cervilla J. Physical exercise and body mass index as correlates of major depressive disorder in community-dwelling adults: results from the PISMA-ep study. J Affect Disord. (2019) 251:263–9. doi: 10.1016/j.jad.2019.01.050
42. Müller A, Hase C, Pommnitz M, de Zwaan M. Depression and suicide after bariatric surgery. Curr Psychiatry Rep. (2019) 21:84. doi: 10.1007/s11920-019-1069-1
43. Spirou D, Raman J, Smith E. Psychological outcomes following surgical and endoscopic bariatric procedures: a systematic review. Obes Rev. (2020) 21:e12998. doi: 10.1111/obr.12998
44. Gulliford MC, Charlton J, Booth HP, Fildes A, Ashworth M, Rudisill C. Costs and outcomes of increasing access to bariatric surgery for obesity: cohort study and cost-effectiveness analysis using electronic health records. Health Serv Deliv Res. (2016) 4:65–71. doi: 10.3310/hsdr04170
45. Booth H, Khan O, Prevost AT, Reddy M, Charlton J, Gulliford M. Impact of bariatric surgery on clinical depression. Interrupted time series study with matched controls. J Affect Disord. (2015) 174:644–9. doi: 10.1016/j.jad.2014.12.050
46. Ivezaj V, Grilo CM. When mood worsens after gastric bypass surgery: characterization of bariatric patients with increases in depressive symptoms following surgery. Obes Surg. (2015) 25:423–9. doi: 10.1007/s11695-014-1402-z
47. Ribeiro G, Giapietro H, Belarmino L, Salgado-Junior W. Depression, anxiety, and binge eating before and after bariatric surgery: problems that remain. Arq Brasil Cirurg Digest. (2018) 31:E1356. doi: 10.1590/0102-672020180001e1356
48. Mitchell JE, King WC, Chen JY, Devlin MJ, Flum D, Garcia L, et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity. (2014) 22:1799–806. doi: 10.1002/oby.20738
49. White MA, Kalarchian MA, Levine MD, Masheb RM, Marcus MD, Grilo CM. Prognostic significance of depressive symptoms on weight loss and psychosocial outcomes following gastric bypass surgery: a prospective 24-month follow-up study. Obes Surg. (2015) 25:1909–16. doi: 10.1007/s11695-015-1631-9
50. Andersen JR. Quality of life following laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. (2015) 11:76–8. doi: 10.1016/j.soard.2014.06.003
51. Lynch A. “When the honeymoon is over, the real work begins:” gastric bypass patients' weight loss trajectories and dietary change experiences. Soc Sci Med. (2016) 151:241–9. doi: 10.1016/j.socscimed.2015.12.024
52. Romain AJ, Marleau J, Baillot A. Impact of obesity and mood disorders on physical comorbidities, psychological well-being, health behaviours and use of health services. J Affect Disord. (2018) 225:381–8. doi: 10.1016/j.jad.2017.08.065
53. van Strien T, Konttinen H, Homberg JR, Engels RC, Winkens LH. Emotional eating as a mediator between depression and weight gain. Appetite. (2016) 100:216–24. doi: 10.1016/j.appet.2016.02.034
54. Mills JG, Larkin TA, Deng C, Thomas SJ. Weight gain in major depressive disorder: linking appetite and disordered eating to leptin and ghrelin. Psychiatry Res. (2019) 279:244–51. doi: 10.1016/j.psychres.2019.03.017
55. Wiedemann AA, Ivezaj V, Grilo CM. An examination of emotional and loss-of-control eating after sleeve gastrectomy surgery. Eat Behav. (2018) 31:48–52. doi: 10.1016/j.eatbeh.2018.07.008
56. King WC, Belle SH, Hinerman AS, Mitchell JE, Steffen KJ, Courcoulas AP. Patient behaviors and characteristics related to weight regain after Roux-en-Y gastric bypass: a multicenter prospective cohort study. Ann Surg. (2019). doi: 10.1097/SLA.0000000000003281. [Epub ahead of print].
57. Freire CC, Zanella MT, Segal A, Arasaki CH, Matos MIR, Carneiro G. Associations between binge eating, depressive symptoms and anxiety and weight regain after Roux-en-Y gastric bypass surgery. Eat Weight Disord. (2020). doi: 10.1007/s40519-019-00839-w. [Epub ahead of print].
58. Höppener MM, Larsen JK, van Strien T, Ouwens MA, Winkens LHH, Eisinga R. Depressive symptoms and emotional eating: mediated by mindfulness? Mindfulness. (2019) 10:670–8. doi: 10.1007/s12671-018-1002-4
59. Kass AE, Wildes JE, Coccaro EF. Identification and regulation of emotions in adults of varying weight statuses. J Health Psychol. (2019) 24:941–52. doi: 10.1177/1359105316689604
60. Braden A, Musher-Eizenman D, Watford T, Emley E. Eating when depressed, anxious, bored, or happy: are emotional eating types associated with unique psychological and physical health correlates? Appetite. (2018) 125:410–7. doi: 10.1016/j.appet.2018.02.022
61. Schäfer L, Hübner C, Carus T, Herbig B, Seyfried F, Kaiser S, et al. Identifying prebariatric subtypes based on temperament traits, emotion dysregulation, and disinhibited eating: a latent profile analysis. Int J Eat Disord. (2017) 50:1172–82. doi: 10.1002/eat.22760
62. Ouellette AS, Rodrigue C, Lemieux S, Tchernof A, Biertho L, Bégin C. An examination of the mechanisms and personality traits underlying food addiction among individuals with severe obesity awaiting bariatric surgery. Eat Weight Disord. (2017) 22:633–40. doi: 10.1007/s40519-017-0440-7
63. Baldofski S, Rudolph A, Tigges W, Herbig B, Jurowich C, Kaiser S, et al. Weight bias internalization, emotion dysregulation, and non-normative eating behaviors in prebariatric patients. Int J Eat Disord. (2016) 49:180–5. doi: 10.1002/eat.22484
64. Koball AM, Himes SM, Sim L, Clark MM, Collazo-Clavell ML, Mundi M, et al. Distress tolerance and psychological comorbidity in patients seeking bariatric surgery. Obes Surg. (2016) 26:1559–64. doi: 10.1007/s11695-015-1926-x
65. Efferdinger C, König D, Klaus A, Jagsch R. Emotion regulation and mental well-being before and six months after bariatric surgery. Eat Weight Disord. (2017) 22:353–60. doi: 10.1007/s40519-017-0379-8
66. Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, Giel KE. Emotion regulation model in binge eating disorder and obesity: a systematic review. Neurosci Biobehav Rev. (2015) 49:125–34. doi: 10.1016/j.neubiorev.2014.12.008
67. Gardner B. A review and analysis of the use of ‘habit’ in understanding, predicting and influencing health-related behaviour. Health Psychol Rev. (2015) 9:277–95. doi: 10.1080/17437199.2013.876238
68. Orbell S, Verplanken B. The strength of habit. Health Psychol Rev. (2015) 9:311–7. doi: 10.1080/17437199.2014.992031
69. Wouters S, Jacobs N, Duif M, Lechner L, Thewissen V. Negative affective stress reactivity: the dampening effect of snacking. Stress Health. (2018) 34:286–95. doi: 10.1002/smi.2788
70. Heriseanu AI, Hay P, Corbit L, Touyz S. Grazing in adults with obesity and eating disorders: a systematic review of associated clinical features and meta-analysis of prevalence. Clin Psychol Rev. (2017) 58:16–32. doi: 10.1016/j.cpr.2017.09.004
71. Gardner B, Phillips LA, Judah G. Habitual instigation and habitual execution: definition, measurement, and effects on behaviour frequency. Br J Health Psychol. (2016) 21:613–30. doi: 10.1111/bjhp.12189
72. King WC, Bond DS. The importance of preoperative and postoperative physical activity counseling in bariatric surgery. Exerc Sport Sci Rev. (2013) 41:26–35. doi: 10.1097/JES.0b013e31826444e0
73. Soares FL, Bissoni de Sousa L, Corradi-Perini C, Ramos da Cruz MR, Nunes MG, Branco-Filho AJ. Food quality in the late postoperative period of bariatric surgery: an evaluation using the bariatric food pyramid. Obes Surg. (2014) 24:1481–6. doi: 10.1007/s11695-014-1198-x
74. Correia Horvath JD, Dias de Castro ML, Kops N, Kruger Malinoski N, Friedman R. Obesity coexists with malnutrition? Adequacy of food consumption by severely obese patients to dietary reference intake recommendations. Nutr Hospit. (2014) 29:292–9. doi: 10.3305/nh.2014.29.2.7053
75. Matei R, Thuné-Boyle I, Hamer M, Iliffe S, Fox KR, Jefferis BJ, et al. Acceptability of a theory-based sedentary behaviour reduction intervention for older adults (‘On Your Feet to Earn Your Seat’). BMC Public Health. (2015) 15:606. doi: 10.1186/s12889-015-1921-0
76. Cleo G, Isenring E, Thomas R, Glasziou P. Could habits hold the key to weight loss maintenance? A narrative review. J Hum Nutr Dietet. (2017) 30:655–64. doi: 10.1111/jhn.12456
77. Berkman ND, Davis TC, McCormack L. Health literacy: what is it? J Health Commun. (2010) 15:9–19. doi: 10.1080/10810730.2010.499985
78. Michou M, Panagiotakos DB, Costarelli V. Low health literacy and excess body weight: a systematic review. Central Eur J Public Health. (2018) 26:234–41. doi: 10.21101/cejph.a5172
79. Raffoul A, Hammond D. Correlates of weight-loss methods among young adults in Canada. Obesity. (2018) 26:1357–64. doi: 10.1002/oby.22218
80. Mahoney ST, Strassle PD, Farrell TM, Duke MC. Does lower level of education and health literacy affect successful outcomes in bariatric surgery? J Laparoendosc Adv Surg Tech A. (2019) 29:1011–5. doi: 10.1089/lap.2018.0806
81. Laz TH, Rahman M, Pohlmeier AM, Berenson AB. Level of nutrition knowledge and its association with weight loss behaviors among low-income reproductive-age women. J Commun Health. (2015) 40:542–8. doi: 10.1007/s10900-014-9969-9
82. Cayci HM, Erdogdu UE, Demirci H, Ardic A, Topak NY, Taymur I. Effect of health literacy on help-seeking behavior in morbidly obese patients agreeing to bariatric surgery. Obes Surg. (2018) 28:791–7. doi: 10.1007/s11695-017-2882-4
83. Erdogdu UE, Cayci HM, Tardu A, Demirci H, Kisakol G, Guclu M. Health literacy and weight loss after bariatric surgery. Obes Surg. (2019) 29:3948–53. doi: 10.1007/s11695-019-04060-7
84. Faruqi N, Spooner C, Joshi C, Lloyd J, Dennis S, Stocks N, et al. Primary health care-level interventions targeting health literacy and their effect on weight loss: a systematic review. BMC Obes. (2015) 2:6. doi: 10.1186/s40608-015-0035-7
85. Teh CH, Teh MW, Lim KH, Kee CC, Sumarni MG, Heng PP, et al. Clustering of lifestyle risk behaviours and its determinants among school-going adolescents in a middle-income country: a cross-sectional study. BMC Public Health. (2019) 19:1177. doi: 10.1186/s12889-019-7516-4
86. Biddle SJH, Pearson N, Salmon J. Sedentary behaviors and adiposity in young people: causality and conceptual model. Exerc Sport Sci Rev. (2018) 46:18–25. doi: 10.1249/jes.0000000000000135
87. Spadola CE, Wagner EF, Dillon FR, Trepka MJ, De La Cruz-Munoz N, Messiah SE. Alcohol and drug use among postoperative bariatric patients: a systematic review of the emerging research and its implications. Alcohol Clin Exp Res. (2015) 39:1582–601. doi: 10.1111/acer.12805
88. Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res. (2016) 76:16–29. doi: 10.1016/j.jpsychires.2016.01.009
89. Kanji S, Wong E, Akioyamen L, Melamed O, Taylor VH. Exploring pre-surgery and post-surgery substance use disorder and alcohol use disorder in bariatric surgery: a qualitative scoping review. Int J Obes. (2019) 43:1659–74. doi: 10.1038/s41366-019-0397-x
90. Blackburn AN, Hajnal A, Leggio L. The gut in the brain: the effects of bariatric surgery on alcohol consumption. Addict Biol. (2017) 22:1540–53. doi: 10.1111/adb.12436
91. Poortinga W. The prevalence and clustering of four major lifestyle risk factors in an English adult population. Prev Med. (2007) 44:124–8. doi: 10.1016/j.ypmed.2006.10.006
92. De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. (2016) 22:681–703. doi: 10.3748/wjg.v22.i2.681
93. Arterburn D, Wellman R, Emiliano A, Smith S, Odegaard A, Murali S, et al. Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med. (2018) 169:741–55. doi: 10.7326/M17-2786
94. Spitznagel MB, Hawkins M, Alosco M, Galioto R, Garcia S, Miller L, et al. Neurocognitive effects of obesity and bariatric surgery. Eur Eat Disord Rev. (2015) 23:488–95. doi: 10.1002/erv.2393
95. Da Silva VB, Da Silva RB, Azorin JM, Belzeaux R. Mood disorders are highly prevalent but underdiagnosed among patients seeking bariatric surgery. Obes Surg. (2015) 25:543–4. doi: 10.1007/s11695-014-1557-7
96. Dohle S, Diel K, Hofmann W. Executive functions and the self-regulation of eating behavior: a review. Appetite. (2018) 124:4–9. doi: 10.1016/j.appet.2017.05.041
97. Turton R, Nazar BP, Burgess EE, Lawrence NS, Cardi V, Treasure J, et al. To go or not to go: a proof of concept study testing food-specific inhibition training for women with eating and weight disorders. Eur Eat Disord Rev. (2018) 26:11–21. doi: 10.1002/erv.2566
98. Preuss H, Leister L, Pinnow M, Legenbauer T. Inhibitory control pathway to disinhibited eating: a matter of perspective? Appetite. (2019) 141:104297. doi: 10.1016/j.appet.2019.05.028
99. Restivo MR, McKinnon MC, Frey BN, Hall GB, Syed W, Taylor VH. The impact of obesity on neuropsychological functioningin adults with and without major depressive disorder. PLoS ONE. (2017) 12:e0176898. doi: 10.1371/journal.pone.0176898
100. Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev. (2016) 10:277–96. doi: 10.1080/17437199.2016.1151372
Keywords: obesity, bariatric surgery, disordered eating, executive function, depression, health literacy, emotion dysregulation, habitual cluster behaviors
Citation: Raman J, Spirou D, Jahren L and Eik-Nes TT (2020) The Clinical Obesity Maintenance Model: A Theoretical Framework for Bariatric Psychology. Front. Endocrinol. 11:563. doi: 10.3389/fendo.2020.00563
Received: 08 April 2020; Accepted: 10 July 2020;
Published: 14 August 2020.
Edited by:
Luca Busetto, University of Padua, ItalyReviewed by:
Martina De Zwaan, Hannover Medical School, GermanyEva M. Conceição, University of Minho, Portugal
Copyright © 2020 Raman, Spirou, Jahren and Eik-Nes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trine Tetlie Eik-Nes, dHJpbmUudC5laWstbmVzQG50bnUubm8=
 Jayanthi Raman
Jayanthi Raman Dean Spirou
Dean Spirou Lisbeth Jahren
Lisbeth Jahren Trine Tetlie Eik-Nes
Trine Tetlie Eik-Nes