- Departments of Biology and Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada
In all vertebrates, the thyroid axis is an endocrine feedback system that affects growth, differentiation, and reproduction, by sensing and translating central and peripheral signals to maintain homeostasis and a proper thyroidal set-point. Fish, the most diverse group of vertebrates, rely on this system for somatic growth, metamorphosis, reproductive events, and the ability to tolerate changing environments. The vast majority of the research on the thyroid axis pertains to mammals, in particular rodents, and although some progress has been made to understand the role of this endocrine axis in non-mammalian vertebrates, including amphibians and teleost fish, major gaps in our knowledge remain regarding other groups, such as elasmobranchs and cyclostomes. In this review, we discuss the roles of the thyroid axis in fish and its contributions to growth and development, metamorphosis, reproduction, osmoregulation, as well as feeding and nutrient metabolism. We also discuss how thyroid hormones have been/can be used in aquaculture, and potential threats to the thyroid system in this regard.
Introduction
The thyroid gland is a key metabolic regulator in the body of animals. An intact axis between the brain, thyroid, and peripheral tissues is essential to modulate energy expenditure and homeostasis (1). An imbalance in energy homeostasis results in the release of brain or peripheral signals, which communicate to the thyroid to increase or decrease energy expenditure, by modulating the release of thyroid hormones (THs). In mammals, there is clear evidence that increased TH production/release induces increases in metabolic rate (2), weight loss (3), and cardiac output (4), while decreased TH production/release leads to opposite effects. In all vertebrates, THs are key hormones that influence a number of physiological processes including growth, development/morphogenesis, and metabolism (5). However, in fish, the role of the thyroid is incompletely understood. Although homology in genetic mechanisms exists between mammals and fish (6) and THs are generally conserved in structure and function (7), the thyroid system is not always analogous between groups.
Fish [Chondrichthyes (i.e., cartilaginous fish: sharks, skates, rays), Osteichthyes (i.e., bony fish: ray-finned and lobe-finned fish) and Agnatha (i.e., jawless fish: hagfish and lamprey)] (8) make up approximately 48% of all vertebrates (9), contributing to the 73,327 of total vertebrate species described (10). This diversity has led to wide variations within ecological niches, physiological mechanisms and local adaptations. In the context of the thyroid, major differences in terms of morphology, physiology, and regulation are seen within and between species.
The thyroid was first described in fish in the 19th century (11). Later studies compared the structure/location of the gland in different fish species [e.g., gill tissue in rainbow trout (Oncorhynchus mykiss) (12)], and uncovered the role of the thyroid as a regulator of metabolic activity (13), and the role of the pituitary [sailfin molly (Poecilia latipinna) (14)] and hypothalamus [African lungfish (Protopterus annectens) (15)] in the regulation of thyroid function. Despite over a century of research, our knowledge of the physiology of the fish thyroid is still incomplete, and previously published reviews focus on teleosts and on specific functions of the thyroid [e.g., metamorphosis (16); reproduction (17)].
This review provides a general overview of our current knowledge on the actions of thyroid hormones in fish (not only teleosts but also other groups), including those on growth and development, reproduction, osmoregulation, and feeding/metabolism, how thyroid function may be affected by intrinsic and extrinsic factors, and how this knowledge could be used by the aquaculture industry.
Thyroid Hormones and the Thyroid Axis
Regulation of Secretion
THs consist of two forms, thyroxine (or tetraiodothyronine, T4) and the biologically active triiodothyronine (T3) (18). Although T4 is the predominant circulating form, T3 is more biologically active (19). Conversion of T4 to T3 occurs in central and peripheral tissues (e.g., brain, gut, liver) by enzymatic removal (5’-monodeiodination, 5’-MDA) of an iodide unit on the outer ring of T4 (20).
In vertebrates, the secretion of THs is regulated by the hypothalamus-pituitary-thyroid (HPT) axis (hereafter also referred to as the thyroid axis). The prime stimulatory hormone for the thyroid gland/follicle is thyrotropin (TSH), from thyrotropes of the anterior pituitary. In higher vertebrates, thyrotropin-releasing hormone (TRH) is the main stimulator of TSH release, whereas some neurotransmitters, dopamine (DA), and somatostatin (SS), act as inhibitors (21, 22). Serum TH levels have direct inhibitory effects on the synthesis and release of both hypothalamic TRH and pituitary TSH (23). While it is clear in mammals that TRH stimulates release of TSH from the anterior pituitary, the role of TRH in activating the fish thyroid axis is not clear (17).
In teleosts, there seems to be species-specific differences in TRH action on thyrotropes. In bighead carp (Aristichthys nobilis), TRH treatment of pituitary cells increases TSHβ messenger RNA (mRNA) expression levels (24). However, in common carp (Cyprinus carpio) (25, 26) and coho salmon (Oncorhynchus kisutch) (27), TRH does not directly affect TSH expression or release from the pituitary. It has been suggested that, in some teleosts, corticotropin-releasing hormone (CRH) may play a greater role as a TSH stimulator than TRH (27, 28).
There is evidence that TRH stimulates the secretion of growth hormone (GH), prolactin (PRL), adrenocorticotropic hormone (ACTH), and melanocyte stimulating hormone alpha (α-MSH) in fish (29). TRH evokes release of proopiomelanocortin (POMC)-derived peptides (α-MSH and ACTH) (30) and GH (31) from goldfish (Carassius auratus) anterior pituitaries, and PRL synthesis and release in common carp (25). It is possible that TRH-induced increases in T4 plasma levels, as seen in rainbow trout and Arctic charr (Salvelinus alpinus) (32), might occur through stimulation of TSH release or other pituitary hormones such as GH and PRL.
Similar to mammalian TSH, fish TSH is a glycoprotein that comprises a hormone-specific β subunit (TSHβ) coupled to a glycoprotein α subunit (GSUα) [e.g., teleosts (33), elasmobranchs (34)]. The α subunit is common to TSH and gonadotropins [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] whereas the β subunit confers hormonal specificity (34). TSH mRNA is mainly expressed in teleost pituitary tissue, although ectopic expression occurs, particularly in gonads (33).
TSH exerts its actions by binding to TSH receptors (G protein-coupled receptors) on the basal membrane of thyroid follicles (33). Two TSH receptor sequences have been identified in most teleost groups but only one receptor gene has been identified in the coelacanth and elephant shark genomes (34). Evidence suggests that, in fish, TSH has a stimulatory effect on the synthesis/release of THs and iodide uptake. For example, incubating thyroid glands from the sea catfish (Galeichthys felis) in vitro for 3 days with mammalian TSH increases T4 release and thyrocyte height (35); in vivo injections with mammalian TSH increase thyrocyte height and follicle proliferation in coho salmon (36), and circulating T4 levels in mummichog (Fundulus heteroclitus) (37) and brook trout (Salvelinus fontinalis) (38).
The release of pituitary TSH is inhibited by DA (39) and SS (40), neuropeptides, and by negative feedback actions by T4 and T3. In goldfish, treatment with SS suppresses radioiodide uptake by thyroid follicles but does not lower plasma T4 in TSH-injected goldfish, supporting the role of SS as a TSH inhibiting factor in this species (41). Appetite regulating peptides also affect TSH expression/release at the pituitary, as leptin and β-endorphin stimulate, whereas galanin and neuropeptide Y (NPY) inhibit TSH pituitary mRNA expression in bighead carp (42).
In mammals, THs exert an inhibitory feedback action on TRH and TSH expression by binding to TRβ located on the TRH promoter in the hypothalamus (43, 44), and inhibiting the transcription of both TSHα and TSHβ in the pituitary (45). In fish, there is no clear evidence of TH inhibition on TRH. Injections of T4 in common carp have no effect on hypothalamic TRH expression, but increase hypothalamic CRH binding protein expression (46), which might result in CRH inactivation and in the modulation of TSH synthesis in the pituitary, as seen in mammals (47). There is however evidence in fish for feedback control of THs at the pituitary level, as THs decrease pituitary TSHβ expression both in vivo [e.g., goldfish (48); turbot (Scophthalmus maximus) (49); European eel (Anguilla Anguilla) (50)] and in vitro [goldfish (51)].
Thyroid Hormone Synthesis Sites and Peripheral Regulation
Synthesis of THs occur in thyroid follicles—a single layer of epithelial cells (thyrocytes) enclosing a colloid-filled space (52). In mammals, and most vertebrates, the thyroid gland is an encapsulated gland in the neck region. In fish, the thyroid gland can be either compact/encapsulated [e.g., Chondrichthyes or cartilaginous fish, such as sharks and rays, and Chondrostei, such as sturgeons] or more commonly diffusely arranged in the pharyngeal, heart, and kidney regions [e.g., most teleosts with a few exceptions such as Tetraodontiformes and Lophiiformes] (53–55). In larval lampreys, the site of TH synthesis is the subpharyngeal endostyle, a filter-feeding apparatus, which transforms into typical follicular thyroid tissue during metamorphosis (56).
Synthesis of THs requires iodine, that, in most fish, is assimilated by diet or from water via the gills (57), and thyroid uptake of iodine requires TSH binding to follicles. Evidence on TSH stimulation of iodide uptake in teleost fish is scarce as the spatial distribution of thyroid follicles makes it difficult to measure radioiodide uptake (38), but it has been shown in elasmobranchs, who have an encapsulated thyroid [e.g., lesser spotted dogfish (Scyliorhinus canicula) (58)].
Once secreted from follicles, THs require peripheral regulation to exert their effects. Iodothyronine deiodinases are selenoenzymes that regulate TH availability and disposal. Several isoforms of deiodinases (DIOs) with different catalytic properties (type 1, 2, and 3, or DIO1, DIO2, DIO3) and tissue- and developmental stage-specific expressions exist (59). In mammals, DIO2 is part of the activating pathway [or outer ring-deiodination (ORD)] as it converts T4 to T3, whereas DIO3 is part of inactivation [inner ring-deiodination (IRD)] as it converts T4 and T3 to inactive metabolites [reverse triiodothyronine (rT3) and 3,3′-diiodothyronine (T2)] (59, 60). DIO1 is capable of both activation (ORD) and inactivation (IRD), processing T4 to T3 and rT3 to T2, respectively (61, 62). Similar DIOs have been shown in fish (57, 63–66). However, fish DIOs differ in some respects from their mammalian counterparts (20). For example, teleostean DIO1 is resistant to propylthiouracil (PTU, inhibitor of thyroperoxidase, TPO—responsible for iodide to iodine oxidation in thyroid follicles) inhibition, and teleosts have relatively higher levels of hepatic DIO2 activity and expression compared to other vertebrates (67).
Regulation by Circadian and Seasonal Rhythms
Several studies have shown circadian and seasonal cycles of THs and thyroid axis components. In mammals, circadian cycles of TRH and TSH are controlled by “pacemakers” within the superchiasmatic nucleus (SCN) of the hypothalamus. These in turn regulate circulating TH levels (68). The pineal gland—which produces melatonin, and controls sleep patterns in a circadian and seasonal manner—also has an inhibitory influence on circulating THs (69). Studies in hamsters show that melatonin inhibits the release of TSH and increases DIO3 expression during winter months (short photoperiod), and stimulates TSH release in summer (long photoperiods), increases DIO2 expression and decreases DIO3 expression, thus controlling the availability and metabolism of THs (70, 71).
Several studies in fish have shown that thyroid axis components respond to environmental cues (72) and undergo circadian and seasonal cycles (73). Pituitary transcript expression levels of TSH and DIO exhibit distinct rhythms. In red drum (Sciaenops ocellatus), seasonal rhythms of T4 correlate with pituitary TSH subunits (TSHα, TSHβ) and DIO3 gene expression cycles (74), and in Arctic charr, hypothalamic DIO2 expression is decreased during late summer (75). In fish, there is evidence that the saccus vasculosus (SV, an organ only observed in fish, situated on the ventral side of the diencephalon, posterior to the pituitary gland) is the seasonal sensor in the brain. The SV expresses TSH and DIO2, suggesting that this organ might play a central role in seasonal changes in THs, albeit probably linked to reproduction (76). In precocious male masu salmon (Oncorhynchus masou), the SV responds to changes in light, with salmon kept under long periods of light displaying high TSHβ and DIO2 protein levels, the opposite occurring with exposure to short periods of light (77).
TH circadian cycles have been shown in several fish species [see (73)], including Atlantic salmon (Salmo salar) (78), winter flounder (Pseudopleuronectes americanus) (79), goldfish (80), and red drum (81), although the time of the peak of TH appears to be species-specific. There also appears to be sex-specific TH rhythms, as in rainbow trout, TH levels increase during the day and decrease at night in males, and increase at night and decrease in the morning in females (82). Seasonal variations in THs also exist, often related to migration and reproduction [e.g., channel catfish (Ictalurus punctatus) (83); Atlantic cod (Gadus morhua) (84); rainbow trout (85)].
Mechanism of Action and General Actions of Thyroid Hormones
The ability of THs to exert their many pleiotropic effects relies on efficient transport, bioactivation, and genomic/nongenomic actions at target tissues.
Thyroid Hormone Transport
In higher vertebrates, THs are transported by plasma TH-binding proteins: thyroxine-binding globulin (TBG), transthyretin (TTR), and albumin. The primary plasma TH-binding molecules in fish consist of albumin and prealbumin, the latter now identified as TTR (86). A TBG-like protein has not yet been identified in fish. In contrast to mammals, fish TTR binds T3 more avidly than T4 (57), possibly making albumin the main T4 binding protein (86).
Due to the lipophilic nature of THs, it was previously assumed that passive diffusion across lipid bilayers of plasma membranes occurred. It is now believed that THs enter target cells via facilitated transport by several ATP-dependent transporters including the monocarboxylate transporters (MCTs) such as MCT8, organic anion transporter polypeptides (OATPs, predominately present in brain capillaries), large neutral amino acid transporters (LATs), and the sodium/taurocholate co-transporting polypeptide (SLC10A1, also known as NTCP) (87, 88).
With the exception of some studies on the role of MCT8 in zebrafish (Danio rerio) development, little is known about TH transporters in fish. The tissue distribution of TH transporters appears to vary between fish models. MCT8 mRNA is expressed in brain, spinal cord and vascular system in zebrafish (89) and mostly in the liver of fathead minnow (Pimephales promelas) (90). OATP1C1 is expressed primarily in the liver and brain in zebrafish (91, 92), and in the gonad, liver, and brain in fathead minnow (90).
The expression of TH transporter transcripts shows an inverse relationship to circulating TH levels. In fathead minnow, exogenous T3 administration leads to a reduction in liver OATP1C1 transcript abundance (90), while treatment with oral PTU increases brain MCT8 expression (93). In zebrafish, MCT8 seems to mediate T3 transport across the blood brain barrier (BBB) (89) and MCT8-deficient zebrafish have altered nervous system development (94). The role of OATPs in fish remains unclear but in zebrafish, OATP1C1 deficiency leads to hyperactivity of the thyroid and the development of goiter (thyroid follicle enlargement), possibly as a consequence of low TH levels as a result of reduced transport into target cells (91).
Thyroid Hormone Nuclear Receptors
THs affect physiological processes by regulating expression of genes in target tissues (genomic actions) (95). Within target cells, T3 binds to thyroid hormone receptors (TRs). TRs are located on thyroid response elements (TRE) of the DNA, located at T3 target gene promoter sites (96). Nuclear TRs act as ligand-modulated transcription factors, In the absence of T3, TR represses transcription by recruiting corepressors [e.g., nuclear-receptor co-repressor (NCoR)/silencing-mediator for retinoid/thyroid hormone receptors (SMRT)], whereas in the presence of T3, TRs recruit coactivators [e.g., steroid receptor coactivator (SRC), p300/CREB-binding protein (CBP)] to facilitate transcription (96). Therefore, the transcription rate of target genes depends on the binding of T3 to TRs.
TRs are products of two different genes, c-erbAα and c-erbAβ (or TRα and TRβ) (97, 98). The TR binds to a TRE as a monomer, a homodimer (α/α, α/β, β/β) or a heterodimer, in which a TR isoform dimerizes with the retinoid X receptor (RXR) (99). TRα and TRβ each have different isoforms that have different tissue distributions (e.g., in mice, TRα1 and TRβ1 are expressed in all tissues, but TRα1 is predominantly expressed in the heart and brain, whereas TRβ1 is predominant in skeletal muscle, kidney, and liver) and binding capacities (TRα2 and TRα3 isoforms are truncated and are unable to bind T3) (98).
In fish, several species-dependent TR isoforms have been identified. For example, Japanese flounder (Paralichthys olivaceus), Atlantic salmon, and Atlantic halibut (Hippoglossus hippoglossus) have two distinct TRα genes, while conger eels (Conger myriaster) have two subtypes of each TRα and TRβ genes (100–102). Goldfish have three unique TRα isoforms (TRα-1, TRα-2, and TRα-truncated) all similarly expressed in pituitary, brain, liver, gonads, and gut (103). The goldfish truncated form may inhibit transcription of functional TRs by competition for TREs (103, 104). In tilapia, two isoforms of TRβ exist—a short (S-TRβ1) and long (L-TRβ1) isoform—differing by nine amino acids. T3 and T2 bind to activate L-TRβ1, but not S-TRβ1, and regulate TRβ expression in vivo (105).
Differences in the number/type/specificity of isoforms, and tissues distributions might indicate species-specific differential splicing, target cells, and functions, although it must be noted that transcript expression levels might not reflect protein levels, for which information is lacking (95).
Non-Nuclear Thyroid Hormone Receptors
THs have the ability to act both non-genomically and extracellularly—within the cytoplasm or plasma membrane—in a very rapid manner. THs activate intracellular pathways and other transcription factors such as the mitogen-activated protein kinase (MAPK) (106, 107) or phosphatidylinositol 3-kinase (PI3K) pathways (108, 109) by binding to the integrin αVβ3 TH specific plasma membrane receptor (110). Non-genomic actions may have downstream long-term specific nuclear effects (cell proliferation, gene transcription) leading to cross-talk between non-genomic and genomic action of THs (111).
There is very limited evidence showing direct non-genomic actions of THs in fish, as non-genomic and genomic effects can overlap in the nucleus. In embryonic zebrafish, T4, but not T3, regulates sodium currents through the MAPK pathway requiring the integrin αVβ3 receptor (112). It has also been suggested that, in fish, THs regulate mitochondrial respiration (113), similar to what is seen in rodents, for which TH binding sites have been shown in mitochondrial membranes (114).
Actions of T2
Although most studies focus on the actions of T4 and T3, recent evidence shows that T2, a product of T3 ORD, is also biologically active and binds to TRβ in teleosts (105). In rodents, administration of T2 increases metabolic rate and has hypolipidemic effects (115). In fish, T2 regulates the transcription of genes associated with cell signalling and transcriptional pathways in the liver of Nile tilapia (Oreochromis niloticus) (116) and stimulates mitochondrial respiration of liver and muscle in goldfish (117). T2 (like T4 and T3) also decreases DIO1 and DIO2 activities in the liver of killifish (Fundulus heteroclitus) (66), and regulates thermal acclimation in zebrafish (118) and growth in tilapia (119). Therefore, while previously viewed as an inactive TH, T2 may have a larger role than originally thought.
Role of the Thyroid Axis on Somatic Development and Growth
In fish, as in all vertebrates, THs are crucial for the proper development of both embryos and adults, and are involved in major life transitions and metamorphosis in some species (52, 120, 121).
Maternal Origin of Thyroid Hormones and Importance in Egg and Larval Development
In early mammalian development, an embryo relies solely on maternal THs as its thyroid gland is not yet fully functional (121). THs are actively transported from the mother to the embryo across tissue barriers—including the placenta and BBB—and act on embryonic target cells (121).
The diverse modes of reproduction in fish (122) result in species-specific thyroid-mediated development, due to the variety of mechanisms by which maternal transfer of THs into the egg/embryo occurs (123).
Most fish have external fertilization and are oviparous [i.e., produce eggs that develop and hatch in the external environment (124)]. Others have internal fertilization and the egg/embryo develops within the mother. In viviparity, eggs develop and hatch within the mother before being released as live young to the external environment (124). In yolk sac, or lecithotrophic viviparity, eggs are retained inside the female until fully developed, with no maternal chemical contribution beyond yolk. In matrotrophic viviparity, the embryos receive additional nutrition from the mother (e.g., maternal proteins and lipid-rich histotroph secreted from the uterus in histotrophy; unfertilized eggs/other embryos in oophagy/adelphophagy; or through placenta-like structures) (125, 126).
In oviparous fish, there is evidence that THs are transferred from female fish to eggs (127). Fathead minnow and zebrafish eggs display high TH levels and high transcript levels of thyroid-related transcripts (TRα, TRβ, DIO1, DIO2, DIO3, TPO, sodium-iodide symporter, TRH-receptor, TSH-receptor, TG, and TTR) before 2–3 days post-fertilization (dpf)—time at which endogenous TH production begins—suggesting a maternal transfer of THs (123). In alligator gar (Atractosteus spatula) and spotted gar (Lepisosteus oculatus), injecting females with THs or TSH results in increases in the concentrations of T4 and T3 in early embryos (128). As well, maternal injections and egg immersion have been shown to increase pigment concentrations in larval tissues, hatching and larval growth rate, swim bladder inflation, muscle development, larval metabolic capacity, and metamorphosis [e.g., Sterlet sturgeon (Acipenser ruthenus) (129, 130); piracanjuba (Brycon orbignyanus) (131); matrinxã (Brycon amazonicus) (132); zebrafish (133); goldfish (134)]. Interestingly, it appears that T4 concentrations are greater than T3 concentrations in eggs of most freshwater (FW) fish, whereas T3 concentrations are greater in seawater (SW) fish (135), suggesting differential TH utilization during egg development.
Less is known about maternal transfer of THs in viviparous species. In the lecithotrophic viviparous dogfish (Squalus acanthias), 5′-MDA activity (an indicator of the production rate of the active thyroid hormone T3) is present in yolk sac embryos and may be of maternal origin (136), and in Korean rockfish (Sebastes schlegelii), maternal T3 injections improve growth and survival of young in utero (137). In matrotrophic viviparity, there is an association between embryos and maternal structures, suggesting that maternal THs could be exchanged (125). In surfperch (Neoditrema ransonnetii)—a matrotrophic teleost in which embryos are sustained by ovarian cavity fluid (OCF) ingestion and by nutrient absorption via enlarged hindgut—OCF and fetal plasma contain high TTR levels. TTR plasma levels are higher in pregnant fish than in non-pregnant fish, and large amounts of maternal TTR are taken up by fetal intestinal epithelial cells (enterocytes), indicating that maternal TTR is secreted into OCF and taken up by fetal enterocytes, presumably to deliver THs to developing embryos (138). In the viviparous bonnethead shark (Sphyrna tiburo), yolk-dependent embryos undergo yolk-sac modification in which the fetal portion of a placenta attaches to the maternal uterine wall near mid-gestation, which facilitates direct exchanges of blood and nutrients between the mother and embryo (139). In this species, T3 in yolk increases from pre- to post-ovulation and peaks during the pregnancy stage, and maternal serum T3 concentrations increase as development progresses, suggesting that maternal THs are needed for development of the egg/embryo (140).
The Thyroid and Growth Axes
In fish, as in mammals, somatic growth is regulated by hormones of the growth (or hypothalamic–pituitary–somatotropic, HPS) axis, i.e., growth-hormone releasing hormone (GHRH) from the hypothalamus, and growth hormone (GH) produced by somatotrophs in the anterior pituitary. GH release is stimulated by GHRH and other secretagogues (e.g., ghrelin) and inhibited by SS (141). GH has direct and indirect actions on tissues via the stimulation and release of insulin-like growth factors I and II (IGF-I, IGF-II) by the liver. These act on tissues to promote cellular proliferation and differentiation (142, 143).
Embryonic differentiation/organogenesis and growth in teleosts is regulated by THs, likely by triggering both GH [e.g., THs increase GH mRNA transcription in rainbow trout (144) and carp (145), and increase synthesis and release in hybrid tilapia (146)] and IGF-I [e.g., THs induce in vivo and in vitro synthesis/release in Mozambique tilapia (Oreochromis mossambicus) (147)]. Since THs are crucial regulators of growth (148, 149), inhibition of thyroid function results in impairment in the development of brain, skeleton, and other organs, as well as in pigmentation. For example, in zebrafish, treatment with T3 increases IGF-1 expression and enhances swim bladder and eye development but IGF-1 receptor blockade suppresses these effects of T3 on swim bladder and eye (150).
Interactions Between Thyroid and Growth Axes
Components of the thyroid axis have been shown to affect the GH/IGF-I axis in vertebrates. TRH stimulates the secretion of GH by acting directly upon GH cells in amphibians (151, 152) and reptiles (152, 153). In rodents, THs have been shown to stimulate GH synthesis and secretion (154, 155), upregulate SS receptors (156) and increase SS immunoreactivity and release (157).
In fish, the effects of the thyroid axis on growth are not clear, as components have been shown to have both inhibitory and stimulatory effects. TRH increases GH secretion in vivo in goldfish (158) and tilapia hybrid (Oreochromis niloticus x Oreochromis aureus) (146), and in vitro in common carp pituitary fragments (159), but not in tilapia hybrid (146) or sailfin molly (160). TSH injections increase GH plasma levels in several species including Nile tilapia (146), killifish (161, 162), coho salmon (163), rainbow trout (164), and Indian carp (Cirrhinus mrigala) (165).
THs also affect the growth axis in fish, although results are inconsistent. In vivo treatment with T4 or T3 decreases both pituitary and serum GH levels in female European eel (166) but has no effect on GH levels in goldfish (51). T4 administration to aquarium water increases somatotroph activity in red belly tilapia (Coptodon zillii) (167), and in vivo T3 injections increase pituitary GH mRNA expression in rainbow trout (144) and GH plasma levels in hybrid tilapia (146). THs also act on liver to stimulate IGF-I synthesis/secretion: T3 increases hepatic IGF-I mRNA levels both in vitro and in vivo in Mozambique tilapia (147) and zebrafish (168), but not in coho salmon (169) or silver sea bream (Sparus sarba) (170). T3 may regulate IGF-I expression by binding to liver GH receptors [e.g., coho salmon (169)] or TRs [e.g., rainbow trout (171)], although this action seems species-specific.
Whereas the thyroid axis can affect growth, components of the growth axis affect the thyroid. In mammals, the thyroid axis is stimulated by GH, as seen by increases in TH levels following GH treatment (172), and inhibited by SS (173). In humans, ghrelin decreases TSH-induced production of thyroglobulin and mRNA expression of TPO in thyroid cells (174), while SS treatment decreases the volume of TSH-cells and serum concentrations of TSH in rats (175) but has no effect on serum TSH and TH levels in humans (176).
In fish, there is also evidence for a role of the GH axis in regulating thyroid function. TSH receptor expression is up-regulated in transgenic grass carp overexpressing GH (177), and in European eel, GH stimulates thyroid follicles to release T4 and enhances peripheral 5’-MDA activity (178). In mummichog, hypophysectomy prevents TSH-induced secretion of T4 and treatment with ovine GH restores this response (162). Information on the role of ghrelin and SS on the thyroid axis is scarce. Plasma TH levels are inversely correlated with SS plasma levels in rainbow trout (179), and burbot (Lota lota) have decreased plasma ghrelin and TH levels pre-spawning (180), suggesting an interaction between SS, ghrelin and THs.
Ecological Importance of Thyroid-Mediated Development
THs are particularly important for the development of the central nervous system (CNS) and for ecological/ecosystem shifts within fish. The plasticity of the fish nervous system allows it to regenerate after injury and be remodeled during life history shifts, processes in which THs are most likely implicated. This has been demonstrated in zebrafish submitted to optic nerve injury, in which the re-innervation of the optic tectum is accelerated when T3 plasma levels are lowered with a TRβ antagonist and iopanoic acid (IOP, inhibits TH release and reduces peripheral T4 to T3 conversion) (181).
In the case of migrating anadromous species, T3 induces the proliferation of olfactory receptor neurons (which are crucial for natal stream imprinting) in olfactory epithelium (182) and T4 induces a switch from UV to blue opsin photoreceptors in the retinas of young coho salmon and rainbow trout (183)—which allows better visual contrast for feeding before a SW migration (184). In masu salmon, T3 binding in the brain is tissue-specific during the parr-smolt transformation: At both life stages, T3 binding is highest in the olfactory epithelium, and smolts show higher binding compared to parr in this region (185). This suggests that THs play an important role in functional changes of the brain and olfactory epithelium, playing a preparatory role for shifting between aquatic habitats.
Metamorphosis
Fish metamorphosis refers to the dramatic changes seen in flatfish, lampreys, and eels, but also be applied to any irreversible post-embryonic developmental event that affects multiple physiological or morphological traits (excluding those related to sexual maturation, reproduction, or senescence) seen in several FW and marine species (56, 186). THs are key regulators of teleost metamorphosis, which involves cellular and molecular remodeling that lead to developmental changes (16). Typically, thyroid activity is low during pre-metamorphosis (i.e., low TH levels, with reduced DIO and TR expression), increases during the metamorphic event, peaks during developmental changes (metamorphic climax), and decreases to pre-metamorphic levels (16, 186).
In flatfish, pelagic larvae develop symmetrically with eyes on each side of the head, and morph into asymmetric benthic juveniles following the migration of one eye to the opposite side of the head to become right- or left-eyed, a species-specific distinction [e.g., right-eyed Atlantic halibut (187), left-eyed Japanese flounder (100) and left- or right-eyed Starry flounder (188)]. In Senegalese sole (Solea senegalensis), increases in TH circulating levels, pituitary TSHβ, and whole body thyroglobulin and TR transcript levels (189) coincide with metamorphic climax and activity in thyroid follicles (190). Similarly, during Atlantic halibut metamorphosis, the vast majority of transcripts expressed in the head transcriptome are related to the thyroid axis (187).
In sea lamprey (Petromyzon marinus), the blind, sedentary, filter-feeding larvae metamorphose into free-swimming juveniles. This involves major changes including the development/transformation of adult kidneys, GIT, gills, and the development of the eyes (56). Interestingly, as opposed to other fish, lamprey metamorphosis coincides with a drop in serum endostyle cells-derived TH levels, is blocked by TH treatment and is stimulated by goitrogens (which suppress TH levels), but the mechanisms by which this occurs are still unclear (56, 191).
In diadromous species, which migrate between SW and FW, metamorphosis induces morphological and physiological changes (e.g., changes in body shape, pigmentation, kidneys, gut, eyes, osmoregulation, metabolism) that prepare the fish to survive in a new habitat (186). In anadromous salmonids (e.g., Oncorhynchus, Salmo and Salvelinus), fish hatch and grow in FW before migrating to SW where most of the somatic growth takes place. Smoltification [or parr (FW fish)–smolt (SW fish) transformation] refers to the changes in physiology, behavior, and morphology that occur in juvenile salmonids prior to this migration. These include pigmentation changes (i.e., body and darkening of fins) and changes in olfactory receptors and osmoregulatory adaptation (192–195), all associated with a surge in TH levels. For example, TH treatment induces downstream migration in Atlantic (196), coho, chum (Oncorhynchus keta) and sockeye (Oncorhynchus nerka) salmon (197), and TSH injections or TH treatment increase purine synthesis, which is responsible for skin silvering in rainbow trout (198) and brook trout (199).
In contrast to salmonids, eels hatch and develop as marine larvae [flat and transparent marine larvae (leptocephali)] and undergo a SW to FW (catadromous) migration. Larvae transform into transparent “glass eels,” which move to FW and complete metamorphosis to become juvenile “elvers.” These then undergo a secondary metamorphic event (silvering) and return to the ocean for spawning. In Japanese eel, the change from leptocephalus larvae to glass eel is characterized by an increase in TH levels and TSHβ expression, with TSHβ levels peaking at the glass eel stage and THs increasing into the juvenile stages (200).
Many teleosts undergo subtle irreversible post-embryonic morphological and physiological changes that have been defined as a metamorphosis and are regulated in part by THs (186). These include the development of the fins and the appearance of adult stripes in zebrafish (133), and changes in coloration and swimming behavior marine fish such as red sea bream (Pagrus major) (201), grouper (Epinephelus coioides) (202), surgeonfish (Acanthurus triostegus), and clown fish (Amphiprion ocellaris) (203).
Reproduction
THs regulate many aspects of the reproductive system, including formation of gametes and steroids, and sexual behavior in both males and females. In vertebrates, the hypothalamus-pituitary-gonadal (HPG) axis regulates reproduction: gonadotropin releasing hormone (GnRH) from the hypothalamus stimulates the pituitary to release gonadotropins (GTH) [luteinizing hormone (LH) and follicle stimulating hormone (FSH)] which act on gonads to regulate gametogenesis and steroidogenesis [e.g., in mammals (204) and fish (205)]. There is growing evidence of a crosstalk between the thyroid and HPG axes in several vertebrates (e.g., mammals, amphibians, fish) (206).
In mammals, the link between thyroid and reproductive function is well established. THs and TSH can affect gonadal development and sex steroid hormone synthesis and actions, and thyroid dysfunction is associated with decreased fertility, impaired gonadal function and disruption of seasonal cycles in both in males and females (207–210). In fish, the link between THs and reproduction is not clear, as inconsistent results have been reported, likely due to the diversity in reproductive strategies, and methods used to investigate TH actions (211).
Thyroid Hormone and Reproductive Cycles
Several studies have shown correlations between circulating THs and reproductive cycles (e.g., gamete formation and maturation, and spawning/hatching events) in fish, but between species, the nature of these relationships vary. Among teleosts, some species display peaks in plasma THs during gametogenesis [e.g., rainbow trout (212); brook trout (213) and/or during spawning [e.g., climbing perch (Anabas testudineus) (214); sea lamprey (215)], whereas others display decreases in TH levels during gonad maturation [e.g., Mozambique tilapia (216)], before [e.g., sockeye salmon (217)] or during spawning [e.g., winter flounder (79)]. In the jawless Pacific sea lamprey, both males and females show peaks in plasma THs during gametogenesis and spawning (215, 218).
In the Chondrostei stellate sturgeon (Acipenser stellatus) and lake sturgeon (Acipenser fulvescens), THs are correlated with increased gonad maturation during the spawning season (219, 220), while in immature and previtellogenic individuals, changes in THs during the reproductive season are more closely correlated with temperature, feeding, and growth [e.g., great sturgeon (Huso huso) (221) and lake sturgeon (220)].
Very little is known about the role of THs in elasmobranch reproduction. In oviparous elasmobranchs, thyroid activity and TH levels are usually lowest in immature females in the non-breeding season, and greatest during egg development and vitellogenesis during the reproductive season [e.g., lesser spotted dogfish (222); brownbanded bamboo shark (Chiloscyllium punctatum) (223)]. Complete thyroid removal inhibits seasonal gonad development [e.g., spotted dogfish (224)]. A similar correlation between thyroidal function and female reproduction has been shown in viviparous elasmobranchs. In the Atlantic stingray (Dasyatis sabina), circulating T3 levels and thyroid activity are low in immature individuals and high in females undergoing oogenesis, and, from ovulation throughout gestation (225, 226). Similarly, in the torpedo (Torpedo ocellata), thyroid activity is high in gestating females (227). However, in female dogfish, thyroid activity does not seem to be associated with reproductive events, but rather with migration (228).
Evidence of Expression of Deiodinases, Thyroid Hormone Receptors, and Thyrotropin Receptors in Gonads
Deiodinases
DIOs have been shown to be present in gonads [e.g., mammals (229, 230); amphibians (231); reptiles (232)] and to be involved in reproductive cyclicity. In mammals, 5’-MDA activity is elevated during gonad development and differentiation [e.g., horse ovary (233); pig testis (230)]. In western clawed frog (Silurana tropicalis) gonads, DIO2 and DIO3 expressions increase and DIO1 expression decreases throughout the development into adult (231). Moreover, gender-specific roles of DOIs have been suggested in lower vertebrates. Adult western clawed frog testis show higher expression of DIO1, DIO2, and DIO3 than ovary (231), and in breeding green anole lizards (Anolis carolinensis), DIO2 and DIO3 expression levels are high in testes and ovaries, respectively (232).
Although DIO1, DIO2, and DIO3 activity/expression has been shown in the gonads of several fish [including striped parrotfish (Scarus iseri) (234), European sea bass (Dicentrarchus labrax) (235), goldfish (236), Nile tilapia (237), sapphire devil (Chrysiptera cyanea) (238), and rainbow trout (239)] their role in gonadal thyroid metabolism is not clear.
A gender-specific expression of DIO1 and DOI2 has been shown in parrotfish, with higher expression levels in ovaries than testes, suggesting that ovaries may require more bioactive THs than testes (234). Whereas there is no evidence for a role of DIO1 in the gonads, DIO2 has been implicated in the regulation of gonad maturation and gametogenesis. In zebrafish, DIO2 deficiency results in delayed sexual maturity and reduced gametogenesis and spawning in both males and females (240). Conversely, high DIO2 activity/expression in gonads [e.g., female tilapia (216); male rainbow trout, (239)], may ensure appropriate levels of T3 needed for gametogenesis. In the sapphire devil, transcript levels of ovary DIO3 increase as vitellogenesis progresses, suggesting that high DIO3 expression might prevent excess TH buildup (238).
Thyroid Hormone Receptors
TRs are expressed in gonads of teleosts such as goldfish (103, 236), striped parrotfish (234), Korean rockfish (241), black porgy (Acanthopagrus schlegelii) (242), and fathead minnow (243), and their expressions appear to be gender-dependent and species-specific. The expressions of TRα and TRβ are higher in ovary than in testis in mature Korean rockfish (241), mature goldfish (103), and developing fathead minnow (243), but higher in testis than the ovary in striped parrotfish (234).
In fish that change sex as part of their life-history strategy, TR subtypes display expression changes in regard to gender. In protandrous (sex change from male to female) black porgy, TRα mRNA expression is low in immature testis and increases at maturation. During sex change, TRα expression decreases then subsequently increases during ovary development and maturation and TRβ expression is highest in mature ovary after sex change than in any other gonadal or sex stage (242). These results suggest that TRα is critical for both testis and ovary development, and TRβ might only be required in the ovary of this species, similar to fathead minnow (243). The significance of this differential expression is yet to be uncovered, but most likely important in cell-specific proliferation and differentiation in gonads, albeit, dependent on sex.
Thyrotropin Receptors
Thyrotropin receptor (TSHR) expression has been detected in gonads of several species, including European sea bass (244), walking catfish (Clarias batrachus) (245), channel catfish (246), striped bass (Morone saxatilis) (247), Biwa trout (Oncorhynchus rhodurus) (248), and sunrise sculpin (Pseudobennius cottoides) (248).
TSHR expression levels increase during ovarian and testicular maturation in European sea bass (244), channel catfish (246) and striped bass (247), and peak during spermatogenesis in sunrise sculpin (248), suggesting a direct role of TSH and TSHR in gametogenesis. In walking catfish, GnRH treatment increases TSHR mRNA expression in gonads, suggesting a positive correlation between TH levels and reproduction (245).
Thyroid and Hypothalamus-Pituitary-Gonadal Axes
In fish, as in mammals, the thyroid influences the HPG axis in a gender-, development-, and species-specific manner. The effects of the thyroid axis on reproductive processes of fish occur via actions at all levels of the HPG axis, i.e., the hypothalamus, pituitary, and gonads.
In the hypothalamus, the effects of THs on GnRH appear to depend on the species and the reproductive-stage considered, as well as the specific population of GnRH neurons. In male mature recrudescent (active gametogenesis) air-breathing catfish (Clarias gariepinus), thiourea-induced TH depletion reduces the number of hypothalamic GnRH immunoreactive neuronal cells and fibers (249). In immature male Nile tilapia, T3 treatment suppresses terminal nerve GnRH mRNA, but does not significantly affect preoptic or midbrain GnRH mRNA levels or the number of hypothalamic GnRH neurons (250), suggesting central-specific TH action dependent on reproductive stage.
Studies have shown that THs may act at the pituitary level to inhibit gonadotropin secretion. Hypothyroid conditions decrease pituitary LH immunoreactivity and LH circulating levels in male recrudescent air-breathing catfish (249), and, in recrudescent goldfish, administration of T3 decreases pituitary LH mRNA expression in males (251) and attenuates GnRH-induced LH secretion in females (252).
Gonadal steroidogenesis occurs in Leydig cells of testes and thecal and granulosa cells of ovaries, and starts with the transport of cholesterol into the mitochondria mediated by steroidogenic acute regulatory protein (StAR), where it is converted into pregnenolone, which is sequentially converted into active steroids such as progesterone (P), 17α-hydroxy-20β-dihydroprogesterone (DHP), the androgens testosterone (T) and 11-ketotestosterone (11-KT, the predominant androgen in fish), and estradiol-17β (E2) by several steroidogenic enzymes (253). In male vertebrates, Sertoli and Leydig cells are responsible for spermatogenesis and androgen biosynthesis, respectively, whereas oogenesis is stimulated by ovarian estrogen and progestins in females (254).
There is evidence in fish that THs increase spermatogenesis and androgen secretion in males and estrogen and progestin secretion in females. In zebrafish testis, T3 stimulates spermatogenesis by increasing the division of spermatogonia and Sertoli cells (255, 256), increasing the production of IGF-III (insulin-like growth factor-III, a stimulatory growth factor of spermatogenesis) by Sertoli cells, and enhancing the gonadotropin-induced synthesis and release of androgens by Leydig cells (257). In male goldfish, treatment with T3 decreases expression of CYP19 (aromatase, which converts androgens into estrogens) thus increasing the androgen to estrogen (A:E) ratio (251), and inhibiting T3 synthesis with monocrotophos (organophosphate pesticide) increases CYP19 expression and reduces the A:E ratio (258). In contrast, in cultured adult zebrafish testis, T3 does not affect the release of 11-KT, or AR and CYP19 mRNA expressions (255), and in juvenile common carp, treatment with T4 has no effect on testis diameter or number of spermatogonia (259). In mid to late recrudescent male goldfish, T3 decreases circulating E2 levels and expression levels of testis estrogen receptor subtypes (ERα, ERβ1, and ERβ2) during mid-recrudescence (251), but has no effect in late or regressed gonads (51). This suggests that THs are essential for spermatogenesis in males but are reproductive stage-specific and seem to have the greatest effect in periods of active spermatogenesis.
In mid-recrudescent female goldfish, in vivo T3 treatment decreases the expressions of estrogen receptors (ERα and ERβ1) and CYP19 in ovary (251), and in recrudescent female air-breathing catfish, T4 treatment decreases CYP19 immunoreactivity and E2 levels in ovary (260), while thiourea-induced TH depletion increase ovarian expression of CYP19 (261). In oocytes of pre-spawning climbing perch, in vitro T3 treatment increases progesterone release (262) and 3β-hydroxysteroid dehydrogenase (3β-HSD, which converts pregnenolone to progesterone) activity (263), and enhances gonadotropin-induced E2 secretion in ovarian follicles from spawning rainbow trout (264).Therefore, similar to male testes, the actions of TH in ovaries appear more pronounced during active periods of gametogenesis. It has been suggested that in seasonal species such as goldfish, THs might inhibit oogenesis/vitellogenesis during non-spawning season, allowing fish to allocate their energy to somatic growth (251, 265).
Very few studies have been performed in elasmobranchs. In the oviparous female dogfish, thyroidectomy impairs ovarian follicular development (224). Both male and female spiny dogfish show correlations between gonad follicle and thyroid growth, with female follicular cell height showing a positive relationship to thyroid weight (228).
While THs affect reproductive tissues, the thyroid axis is also regulated by reproductive hormones. In fish, treatment with E2 appears to have inhibitory effects on TH levels, as seen by E2 induced decrease in thyroid epithelial cell height and thyroid activity [e.g., European eel (266) and rainbow trout (267)], decreases in plasma TH levels (usually T3) [e.g., European eel (266), Atlantic salmon (268) and southern hemisphere lamprey (Geotria austrails) (269)], decreases in hepatic T3 production [e.g., trout (264, 270) and masu salmon (271)], increases in TSH [e.g., rainbow trout (270) and masu salmon (271)], and decrease in gonad TRα expression in male and female fathead minnow (272). Like estrogens, androgens might also affect the thyroid axis in fish (273). Androgens have been shown to enhance thyroidal function in most teleosts examined [e.g., striped catfish (Mystus vittatus) (274); rainbow trout (275); masu salmon (276); coho salmon (277), striped catfish (274)]. In Japanese medaka (Oryzias latipes) (278) and coho salmon smolt (277), 11-KT (medaka), and 17α-methyltestosterone (MT, coho) administration in larval males causes thyroid follicle hypertrophy and enhances 5’-MDA activity (279). However, MT treatment induces a dose-dependent decrease in plasma T4 and inhibits the smoltifying effects of T4 in masu salmon (276).
Role of Thyroid Hormones in Osmoregulation
In mammals, the kidney is the major osmoregulatory organ, and THs influence renal development, kidney hemodynamics, glomerular filtration rate and ion and water homeostasis (280) and thyroid dysfunction affects renal function (280).
In fish, osmoregulation is accomplished by the kidneys and GIT, but mainly by gills (via chloride cells) in teleosts and rectal gland in elasmobranchs (281). Compared to the outside water, the internal environment of marine fish is hypoosmotic, while that of a FW fish is hyperosmotic. Most species live in relatively constant habitats and can only survive within a narrow range of salinities (stenohaline). However, other species are able to adapt to a wide range of salinities (euryhaline) and some undergo drastic osmotic changes as they migrate [from SW to FW (anadromy) or from FW to SW (catadromy)] (282).
Several hormones control osmoregulation in fish. In euryhaline fish, cortisol (a glucocorticoid secreted by kidney) is considered the main SW adapting hormone whereas prolactin (PRL, which promotes ion uptake and inhibits ion secretion) is viewed as a FW adapting hormone; GH and IGF-I have also been implicated in the control of SW adaptation (283, 284). The thyroid axis has been shown to regulate osmoregulatory changes in fish, most likely through interactions with cortisol/GH and PRL (283, 284).
Salinity Tolerance in Salmonids
Several studies have examined the role of the thyroid axis in determining tolerance to changing salinities in salmonids. Salinity tolerance (capacity to withstand SW) increases after TH treatment in FW coho salmon (285, 286), Atlantic salmon (287, 288), pink (Oncorhynchus gorbuscha) and sockeye salmon (289), and sockeye salmon transferred from FW to SW have increased gill TRα, TRβ1, and TRβ2 mRNA and increased TH levels (290). In Atlantic salmon, T3 increases the binding affinity of cortisol to gill cortisol receptors, an effect synergistic when co-injected with GH (291)—indicative of increased SW tolerance. In amago salmon (Oncorhynchus rhodurus), T4 treatment potentiates the action of GH on gill Na+/K+-ATPase (NKA, major ion pump) (292), while there is a synergistic effect in gill NKA activity in Atlantic salmon (291) and rainbow trout (293) when co-injected with T3 and GH.
Atlantic salmon injected with PRL limits cortisol receptor binding affinity and decreases NKA activity, reducing SW tolerance. In coho salmon, PRL alone has no effect on plasma T3 levels and decreases plasma T4 levels, and when PRL is co-injected with TSH it prolongs the TSH-induced elevation of TH levels (294). In brook trout (Salvelinus fontinalis) co-injections of TSH and PRL increase plasma T3 levels, hepatic T3 content and 5’-MDA rates compared with TSH-treated animals (295), suggesting an interaction between TSH and PRL.
Evidence in Other Euryhaline Fish
THs have also been shown to affect the osmoregulatory capabilities of other euryhaline species. In Mozambique tilapia, TH injections increase gill NKA activity (296), potentiate the action of cortisol on gill NKA activity (297) and increases chloride cell size (a function of ionoregulatory ability) (296).
In summer flounder (Paralichthys dentatus), which move from high to low salinity ocean water during metamorphosis, SW tolerance increases after TH treatment in individuals undergoing metamorphosis, suggesting that, similar to anadromous salmon, THs regulate the development of osmoregulatory mechanisms necessary for the transition to FW to SW (298). In gilthead sea bream (Sparus aurata), exposure to low salinity increases T4 levels and decreases gill DIO1 activity (299), while high salinity decreases T4 levels and increases pituitary TSHβ and gill NKA activity (300). However, in grass carp (Ctenophayngodon idella), an increased salinity decreases T3 and TSH levels, and increases T4 serum levels (301).
Marine and euryhaline elasmobranchs in SW regulate urea and other body fluid solutes [trimethylamine oxide (TMAO), Na+, Cl−] such that they remain iso- or slightly hyperosmotic to their environment (302). While little information is available, it seems that the thyroid axis may contribute to elasmobranch osmoregulation. In Atlantic stingray, plasma urea levels and osmotic concentration increase following thyroidectomy and decrease after T4 replacement therapy, possibly due to the regulation of urea efflux or metabolism (303). In dogfish, 5’-MDA liver activity increases in the presence of TMAO (protein stabilizer that counteracts urea buildup) and TMAO + urea (136), suggesting a role of THs in urea metabolism, as seen in goldfish, for which T4 increases ammonia production and excretion (304, 305).
Feeding and Nutrient Homeostasis
The nutritional energy provided by food intake is essential for activity, growth, and maintenance of bodily functions. In fish (306) as in mammals (307), food intake is mainly regulated by brain feeding centers controlled by central and peripheral endocrine signals, which either stimulate [orexigenic peptides, such as orexin, agouti-related protein (AgRP), and neuropeptide Y (NPY)] or inhibit [anorexigenic signals, such as cocaine- and amphetamine-regulated transcript (CART) and α-melanocyte-stimulating hormone (α-MSH) derived from POMC] feeding behavior. Feeding centers receive information about nutritional status from the periphery [e.g., gastrointestinal tract (GIT)] either via the general circulation or the brainstem/vagal complex. These peripheral signals include ghrelin, cholecystokinin (CCK), peptide YY (PYY), and leptin. Usually, when food intake is restricted, the expression of orexigenic hormones increases while that of anorexigenic hormones decreases (306, 308).
Role of the Thyroid Axis in Feeding/Food Intake
In mammals, the thyroid axis regulates food intake, body weight (309) and metabolic/nutrient homeostasis (310). The thyroid axis can influence feeding via the actions of TRH and THs in the brain, THs in the periphery, and also be influenced by endocrine appetite-regulating signals (e.g., NPY, leptin).
In rodents, central administration of TRH or TSH decreases food intake (311, 312) whereas TH injections increase feeding (313, 314). Conversely, food deprivation decreases hypothalamic TRH and pituitary TSHβ mRNA expression, and peripheral T3 serum levels (315), while refeeding increases hypothalamic TRH mRNA expression, increases plasma TSH, and normalizes circulating T3 levels (316).
Interactions between the thyroid axis and appetite-regulating signals have been shown in mammals. In rats, although TRH neurons contain NPY receptors (317), TRH does not stimulate NPY neurons (318), but goats injected with NPY show a dose-dependent increase in TH levels (319). TRH neurons excite orexin neurons (318) and orexin has been reported to either increase (320) or decrease (321) hypothalamic TRH levels. Interestingly, some hypothalamic TRH neurons co-secrete CART but the nature of this interaction is unclear (322). It has been suggested that the anorexigenic actions of TRH are mediated in part by the inhibition of melanin-concentrating hormone (MCH, an orexigenic neuropeptide) (318), while the orexigenic effect of THs might occur via decreases in the expression of anorexigenic factors such as POMC, CART, and MC4R (melanocortin 4 receptor, activated by α-MSH and AgRP to reduce food intake) (310, 323, 324), and increases in the expression of appetite stimulators such as NPY (325). Leptin (a adipose satiety signal) increases TRH expression directly by binding to its receptors at TRH neurons (326), or indirectly via decreases in AgRP and NPY and increases in α-MSH (which innervate TRH neurons) (309, 327). There is no clear evidence of a correlation between THs and leptin expression and circulating levels (328, 329).
In fish, interactions between feeding and thyroid status have been shown in several species. In green sunfish (Lepomis cyanellus), high thyroid activity correlates with increased food intake (330), whereas in Amur sturgeon (Acipenser schrenckii), low serum TH levels correlate to low feeding rates (331). In climbing perch, exposure to thiourea (TPO inhibitor) decreases food consumption (332). Reduced food ration in green sunfish (330) and long-term starvation in rainbow trout (333) decreases the sensitivity of thyroid tissues to TSH, resulting in a decrease in TH levels. In winter flounder, hypothalamic TRH expression increases during fasting (334) but decreases in common carp (335), and in goldfish, TRH injections increase food intake (336).
Little is known about interactions between the thyroid axis and appetite regulators in fish. In goldfish, TRH injections increase the brain expression of orexin, orexin receptor, and CART (336). In bighead carp pituitaries, leptin increases TSHα and TSHβ expression (42), and in grass carp, leptin and ObRb expression levels increase in hepatocytes incubated with low doses of T3 (although high doses inhibit expression) (337). In fasted burbot, plasma T4 and TSH correlate with increased plasma leptin levels (180).
All together, these results suggest that in fish, the thyroid axis plays a role in regulating appetite, and responds to changes in feeding status.
Thyroid Hormones, Nutrient Synthesis, and Metabolism
Nutrients and how efficiently they are metabolized have been shown to influence and be influenced by the thyroid axis. In mammals, hyperthyroidism is associated with high metabolism—increased fat breakdown, weight loss, increased liver cholesterol synthesis and clearance, and low serum cholesterol—while the opposite occurs in hypothyroidism (338). For example, in rats, T3 increases caloric intake and leads to increased lipolysis (by fatty acid β-oxidation) (339), while hypothyroid female rats have reduced hepatic mRNA expressions associated with cholesterol uptake and lipid oxidation (340). Conversely, the quality of nutrients influences the thyroid axis and TH production. Rats fed fish oil diets have higher liver TR expression and increased thyroid signaling associated with lipid metabolism than rats fed soybean oil diets (341), and rats fed diets supplemented with Yucca schidigera (which contains saponins that decrease GIT nutrient absorption), have lower THs levels than control animals (342).
In fish, THs influence nutrient metabolism of lipids, proteins and carbohydrates (343) in a species-specific manner. T4 treatment promotes lipolysis, stimulates lipid mobilization, and decreases lipid stores (e.g., as seen by decreased total lipids and increased lipolytic enzyme activity) in coho salmon (344), and increases lipid efficiency, plasma cholesterol and triglyceride levels in Sterlet sturgeon (345). Body protein content decreases in European eel (glass stage) treated with THs (346), and walking catfish exposed to thiourea (347). THs also affect glucose and related carbohydrate metabolism pathways. Following TH treatment, plasma glucose levels increase in red sea bream (348), gilthead sea bream (349), and European eel (346), but decrease in rainbow trout (350). TH treatment increases liver gluconeogenic pathways in gilthead sea bream in vivo (349), and expression of transcripts associated with glycolytic pathways [i.e., glucokinase (GK), glucose-6-phosphatase (G6Pase), glycogen synthase (GS), and glycogen phosphorylase (GP)] in silver sea bream hepatocytes in vitro (351). However, RNA-seq analysis conducted in liver of tilapia treated with T3 shows a down-regulation of several pathways related to carbohydrate metabolism (i.e., amino sugars synthesis, galactose and mannose metabolism, tricarboxylic acid cycle) (116).
The quality of the food (i.e., protein, carbohydrate, or lipid content) also influences the thyroid axis in fish. For example, low protein diets reduce plasma T4 levels and/or 5’-MDA activity in rainbow trout (352) and brook trout (353). Similarly, in Japanese flounder, fish meal-fed fish have higher levels of T3 than fish fed with fish protein concentrate (FPC) or soy protein concentrate (SPC) (353). Rainbow trout fed a diet with low carbohydrates have low 5’-MDA activity compared to fish fed a carbohydrate-rich diet (354). Under a diet with low salmon oil content, rainbow trout have reduced plasma T4 and increased plasma T3 levels, while a high salmon oil diet leads to high plasma T4 and low T3 (354).
Relevance of the Thyroid Axis in Aquaculture
The basic premise to aquaculture systems is to maximize growth at a minimum cost, producing an aesthetic product with high nutritional value (355). The bottlenecks in aquaculture are often the survival of larval and juvenile stages, and successful spawning. Manipulations or disruptions of the thyroid axis could potentially have positive (e.g., increased developmental and reproductive success, hatching, and growth rates) or negative (e.g., skeletal deformations, depressed food intake) effects in the aquaculture industry.
Thyroid Hormones Could Be Used to Enhance Early Survival and Development in Fish
THs are important in the development and growth of fish, particularly during early life stages. In aquaculture settings, high mortality rates are seen in early life stages and several species develop skeletal deformities or abnormal pigmentations which might compromise the aspect of the fish and render it improper for sale [e.g., Atlantic salmon (356); Atlantic cod (357); flatfish (358)].
Many studies have reported positive effects of TH treatment in newly fertilized eggs and larvae to enhance hatching, post-embryonic growth and larval survival. For example, immersion in T4 reduces the hatching period, the number of physical deformities, and mortality rate in Asian stinging catfish (Heteropneustes fossilis) eggs, (359), and induces faster development (i.e., gut formation, swim bladder development, yolk absorption) in freshwater carp (Catla catla) larvae (360). Similar positive effects have been shown in Pacific threadfin (Polydactylus sexfilis) (361), spotted gar (128), rainbow trout (362), milkfish (Chanos chanos) (363), grouper (202), and chum salmon (364), as well as a number of South American fish [e.g., piracanjuba (131); matrinxã (132); dourado (Salminus maxillosus) (365)].
However, negative effects of THs have also been reported. T4 immersion results in reduced hatching, growth rate, and yolk content in alligator gar (128), decreased pigmentation in Atlantic salmon (366), major abnormalities in Nile tilapia [i.e., abnormal shaped pectoral fins, lordosis, and scoliosis (spinal curvature)] (367) and albinism in Japanese flounder—possibly via inhibition of pigment production or impairment of melanophore development due to precocious metamorphosis (368).
Overall, these studies suggest that the effects of TH on eggs and larvae might be dose- and species-dependent.
Thyroid Hormones Can Control and Optimize the Time of Salmonid Smoltification
As there are individual variations in growth rates in fish, THs (which are involved in stimulating both growth and smoltification) have been used to accelerate growth and promote the achievement of SW tolerance in several salmonids (369). TH treatments could also be useful in inducing promote out-of-season growth and smoltification.
Smoltification is controlled by environmental cues (mainly photoperiod and temperature), which induce changes in the thyroid axis (370–372) and only occurs when a threshold weight has been reached (373). In aquaculture, the period following the transfer of fish from FW to SW is critical, as the performance (including optimal growth rates) of the fish after transfer depends upon a successful Parr-Smolt transformation (374).
A well-timed TH induction of smoltification may be advantageous in species which are released and recaptured [e.g., kokanee salmon (Oncorhynchus nerka) (375)] to ensure the return of adult fish to release sites, as fish with the highest whole body T4 content display increased odor attractions and more accurate homing behavior compared to fish with low T4 levels (376). In Atlantic salmon smolts following transfer to SW, there is a transient suppression of appetite and growth (for up to 30 days) (374, 377), and THs treatment at the right time and the right dose during the parr phase might lessen this inhibition. However, T4 administration in late Atlantic salmon parr depressed olfactory bulb response to L-alanine (nasal stimulant in salmon) and inhibited 5’-MDA, so timing of induction is critical (378).
Thyroid Hormones Could Enhance Reproduction
THs may potentially be used to enhance reproduction in some aquaculture species by enhancing offspring survival and market value [e.g., increase quality of eggs for sturgeon caviar production (379)]. Higher embryonic/larval survival rates and hatching rates have been shown in fertilized eggs treated with THs [e.g., Pacific threadfin (361); Sterlet sturgeon (130)] or following maternal TH injections [e.g., greater amberjack (Seriola dumerili), Japanese whitling (Sillago japonica), red spotted grouper (Epinephelus akaara), red sea bream, and Japanese parrotfish (Oplegnathus fasciatus) (380, 381); striped bass (382)]. In Medaka, administration of T3 prior to spawning increases E2 production and oocyte growth, showing that T3 administration can enhance final oocyte maturation (383).
The use of THs to enhance reproduction has been successfully used in large scale aquaculture production of some species [e.g., goldstriped amberjack (Seriola lalandi) (380); Korean rockfish (137)]. In goldstriped amberjack, maternal injections of T3 reduce mortality during early development and growth, and larval survival increased from less than 1.0% when seed production began in 1985, to 7.3% by 1994 following implementation of T3 injections (380).
Thyroid Disruption by Anthropogenic Actions as a Threat to Aquaculture
Pollutants
Thyroid disruption by exposure to environmental toxicants such as metals [e.g., cadmium (384)], pesticides [e.g., organophosphorous pesticides (385)], and pollutants [e.g., polychlorinated biphenyls, PCBs (386)] could result in increased larval mortality and developmental deficiencies (387) depending on the aquaculture system and species.
With increasing anthropogenic and industrial activities, heavy metals can become soluble and accumulate to toxic levels, and potentially affect the thyroid axis (388). Cadmium decreases TH levels in rainbow trout (389), while chromium exposure reduces TH levels in European eel (390), and induces thyroid follicle hypertrophy and increases in serum TH levels in spotted snakehead (Channa punctatus) (391). Exposure to mercury decreases circulating TH levels in spotted snakehead (392) and increases the T4:T3 ratio—suggesting an inhibition of 5’-MDA activity—in yellowfin sea bream (Acanthopagrus latus) (393).
Organophosphorus pesticides (OPs) can inhibit growth and development of fish. Dimethoate decreases serum TH levels and increases TSH levels in roho labeo (Labeo rohita) (394), chlorpyrifos decrease serum TH and TSH levels in Asian stinging catfish (395), and decreases in TH levels inhibits development of sensory organs (eyes, olfactory organ, and lateral line) and decreases survival rates in surgeonfish (396). In goldfish, monocrotophos decrease TH levels, and up-regulate pituitary TSHβ and hepatic DIO1 and DIO3 expressions (397). In Senegalese sole, exposure to malathion affects growth patterns (eye migration, skeletal disorders), reduces thyroid follicle size, and induces decreased thyroid signaling (as seen by low TRβ mRNA levels) (398).
PCB exposure induces higher rates of thyroid metabolism (i.e., deiodination, glucuronidation, and sulfatation) and lower TH levels in European sea bass (399), coho salmon (400), and rainbow trout (401), but not in European flounder (Platichthys flesus) (402).
Therefore, while some mechanisms of interaction between environmental toxicants and the thyroid axis are unknown, toxicants can have negative effects on thyroid economy of fish, and could potentially affect growth and production of aquaculture species.
Climate Changes
Climate change brings about changes in the aquatic environment, such as increases in temperature and acidification, which deeply affect fish physiology (403) and aquaculture practices (404), and might have potential effects on the thyroid axis.
Warmer temperatures have been shown to decrease the sensitivity of fish to THs in zebrafish (118, 405) and mosquito fish (Gambusia holbrooki) (406), and in surgeonfish, a 3°C increase in temperature induces lower TH levels and a disrupted development of sensory organ, an effect that can be reversed by treating the fish with THs (396). In addition, thermally challenged fish may produce less viable gametes, with fitness implications that could affect species at the population level (407). In Japanese Medaka, high temperatures decrease the number of spawned eggs, an effect amplified by a reduction in TH levels (by sodium perchlorate exposure) (408). Similarly, seasonal spawners such as goldfish exhibit high TH levels post-spawning in the summer (when water temperatures are the highest) as a way to inhibit pituitary LH and gonadal aromatase (265). While these temperature-mediated effects have not held true for all fish species [e.g., Atlantic cod (84, 409)], an earlier than normal increase in water temperatures as a result of climate change, might disrupt thyroid cycles and inhibit reproductive capabilities in some fish.
The thyroid axis is also sensitive to ambient acidity. For example, exposure to acid water increases T4 plasma levels in the climbing perch (Anabas testudineus) (410) and brown trout (Salmo trutta) (411), and a decrease in T3 levels in Atlantic Salmon (412).
Changes associated with climate may differentially affect specific life-history stages of fish (e.g., species that undergo substantial metamorphic events), which may result in plastic responses that lead to deficiencies later in life. These abiotic changes are poorly understood in the context of the thyroid axis and fish, but require attention for future climate scenarios and aquaculture practices.
Summary and Conclusion
Thyroid hormones have diverse effects and play an important role in the maintenance of a normal physiological state in vertebrates. While similarities exist between fish and other vertebrates exist, fish thyroidal systems present unique features (see Table 1, Figure 1) and functions owing to the diversity in fish anatomies, habitats, and life cycles.
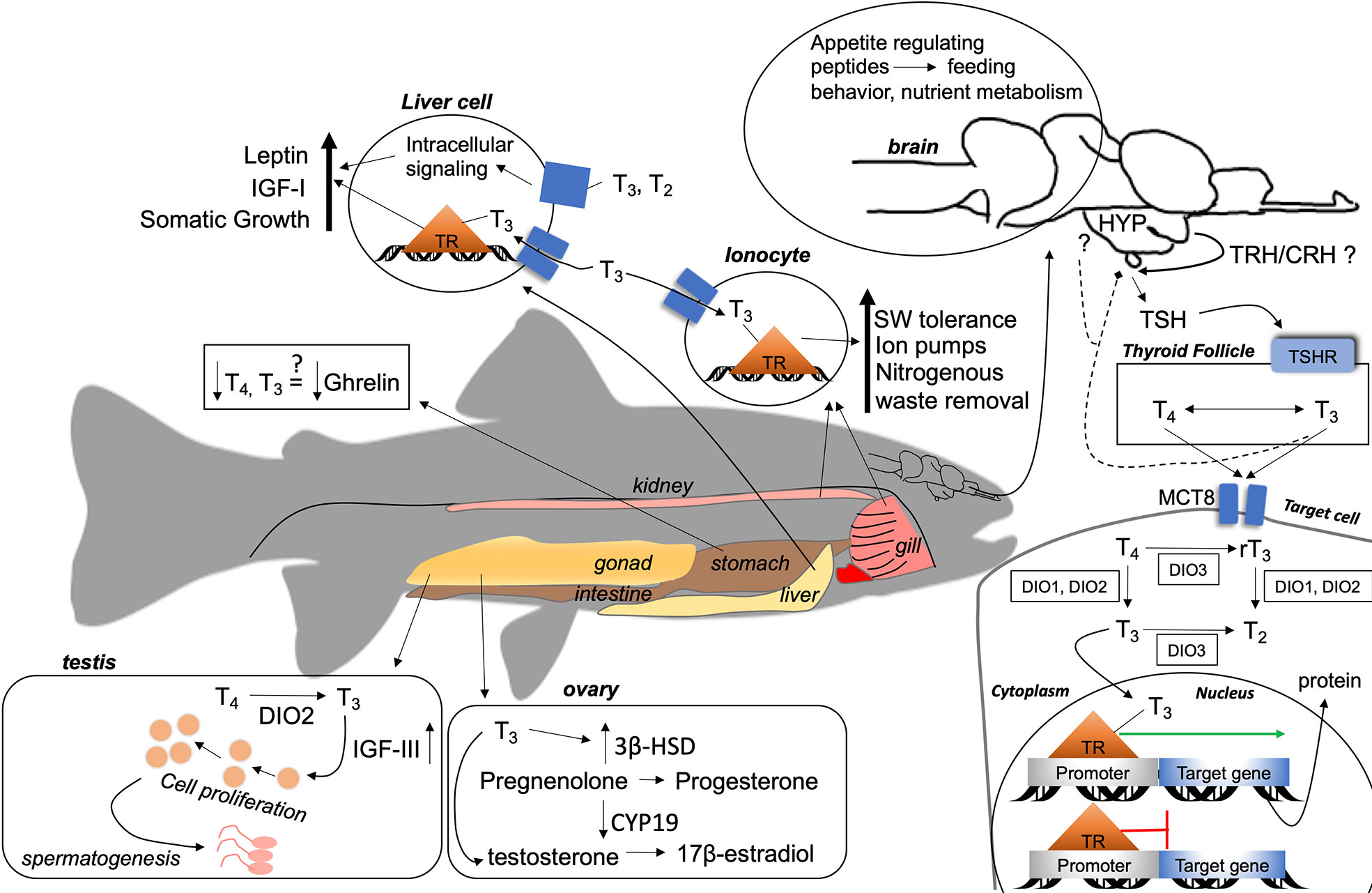
Figure 1 A summary of the general actions of thyroid hormones (THs) in fish. A thyrotropin (TSH)-releasing factor [thyrotropin-releasing hormone (TRH)/corticotropin-releasing hormone (CRH)] stimulates the anterior pituitary to release TSH, which binds to TSHR on the membrane of thyroid follicles. Intracellular processes produce T4 and T3 that enter the circulation to target cells (solid line) or feedback (dashed line) to the hypothalamus–pituitary axis. THs enter target cells through membrane transporters (e.g., MCT8), where bioactivation of T4 to T3 occurs through DIO1 and DIO2, or further metabolization to rT3 or T2 through DIO1, DIO2, or DIO3. THs enter the target cells nucleus from the cytoplasm and bind to TRs located on promoter regions of a thyroid hormone response element (TRE). When T3 is bound, gene transcription occurs (green arrow), otherwise transcription is repressed (red line). THs may act on various tissues in fish, as shown by general mechanisms in central and peripheral tissues. Question marks indicate evidence of effects of THs, but no known mechanism of action by THs in fish. Arrows that point up indicate that THs increase activity, production, or synthesis. Down arrows indicate repression or reduction of synthesis/production. HYP, hypothalamus; TRH, thyrotropin-releasing hormone; CRH, corticotropin-releasing hormone; TSH, thyrotropin, TSHR, thyrotropin receptor; MCT8, monocarboxylase transporter 8; T4 thyroxine, T3 triiodothyronine; rT3, reverse triiodothyronine; T2, diiodothyronine; DIO1, deiodinase I; DIO2, deiodinase II; DIO3, deiodinase III; TR, thyroid receptor; IGF-I, insulin-like growth factor I; IGF-III, insulin-like growth factor III; 3β-HSD, 3β-hydroxysteroid dehydrogenase; CYP19, aromatase.
The follicular structure of the thyroid is conserved in vertebrates, but most fish have diffuse glands making it more difficult to study. The mechanisms by which fish synthesize and metabolize THs is similar to those in mammals (i.e., THs requires thyroglobulin, iodine, and TPO, and DIOs are needed to activate/inactivate THs), but fish might have different isoforms of enzymes which have different properties/actions/locations (e.g., DIO1 is insensitive to PTU and DIOs are located in various tissues), suggesting diverse TH metabolisms.
Evidence suggests that TRH may not be the major TSH-releasing factor at the pituitary in fish, but rather be responsible for the secretion of GH, PRL, and ACTH, which in turn might affect TSH. THs appear to exert an inhibitory feedback action on TSH, but there is no clear evidence for TRH. More advanced molecular techniques (e.g., RNA-sequencing) and in vivo studies may help to shed light on the true nature and interactions of TRH in fish.
Existing literature has highlighted the actions of TH in fish via genomic (binding to species specific isoforms of TRs) mechanisms. However, the non-genomic mechanisms by which THs act are poorly understood, as these processes can overlap with genomic actions. As in all vertebrates, T3 is the main biologically active form of TH, but metabolized THs (e.g., T2 and Tetrac) previously deemed inactive, are proving to have a role in regulating metabolism (413).
In fish, THs regulate many aspects of reproduction, including gonad maturation, steroidogenesis, and sexual behavior, and can affect the time of spawning, quality of eggs, and fertilization rates and development of eggs/larvae. There are also deep complex interactions between the thyroid axis and growth (e.g., GH, IGF-1) and feeding/appetite (e.g., NPY, POMC) regulators, however, a good knowledge of these interactions is still lacking. A better understanding of the control of THs on reproduction, growth, and development, and feeding might provide invaluable insights in aquaculture species/practices and may especially be important to maximize growth while reducing production costs in the ever-growing aquaculture industry.
Any alteration of the thyroid axis by environmental anthropogenic pollutants (effluents containing thyroid disrupting compounds) could have serious physiological and ecological consequences. Understanding specific mechanisms of action of these pollutants might help to substantiate their potential long term affects, and help fisheries managers regulate wild populations under threat from these compounds.
Finally, climate change is an additional stress to aquatic ecosystems, affecting both water temperature and shifting carbon dioxide concentrations through direct and indirect effects. Owing to the aquatic habitat of fish, the thyroid axis shows trends in seasonality (414), and is affected by external factors such as temperature, salinity, and pH (118), begging the question on how climate change might alter thyroid signaling.
Author Contributions
CKD and HV both designed, wrote, and approved the final version of the manuscript.
Funding
The authors acknowledge funding from the Natural Sciences and Engineering Research Council (NSERC) Discovery Grant, 261414-03 (HV).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge former and current researchers adding to the field of fish thyroid biology.
References
1. McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann New York Acad Sci (2014) 1311:77–87. doi: 10.1111/nyas.12374
2. Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid (2008) 18(2):141–4. doi: 10.1089/thy.2007.0266
3. Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol (2010) 316(2):165–71. doi: 10.1016/j.mce.2009.06.005
4. Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, et al. Thyroid hormone treatment after coronary–artery bypass surgery. New Engl J Med (1995) 333(23):1522–7. doi: 10.1056/NEJM199512073332302
5. Rabah SA, Gowan IL, Pagnin M, Osman N, Richardson SJ. Thyroid hormone distributor proteins during development in vertebrates. Front Endocrinol (2019) 10:506. doi: 10.3389/fendo.2019.00506
6. van de Pol I, Flik G, Gorissen M. Comparative physiology of energy metabolism: Fishing for endocrine signals in the early vertebrate pool. Front Endocrinol (2017) 8(36):1–18. doi: 10.3389/fendo.2017.00036
7. Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic–pituitary–thyroid (HPT) axis. Crit Rev Toxicol (2007) 37:11–53. doi: 10.1080/10408440601123446
9. Fricke R, Eschmeyer WN, van der Laan R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (Accessed June 01, 2020).
10. IUCN. The IUCN Red List of Threatened Species. Available at: https://www.iucnredlist.org/, (Accessed July15, 2020).
11. Simon J, Green JH. On the comparative anatomy of the thyroid gland. Philos Trans R Soc London (1844) 134:295–303. doi: 10.1098/rstl.1844.0010
12. Gudernatsch JF. The thyreoid gland of the teleosts. J Morphol (1911) 21(S1):709–82. doi: 10.1002/jmor.1050210502
13. Lynn WG, Wachowski HE. The thyroid gland and its functions in cold–blooded vertebrates. Q Rev Biol (1951) 26(2):123–68. doi: 10.1086/398076
14. Ball JN. Brood–production after hypophysectomy in the viviparous teleost Mollienesia latipinna Le Sueur. Nature (1962) 194(4830):787–. doi: 10.1038/194787a0
15. Kreider MS, Winokur A, Manaker S, Pack AI, Fishman AP. Characterization of thyrotropin–releasing hormone in the central nervous system of African lungfish. Gen Comp Endocrinol (1988) 72(1):115–22. doi: 10.1016/0016-6480(88)90186-4
16. Campinho MA. Teleost metamorphosis: The role of thyroid hormone. Front Endocrinol (2019) 10:383. doi: 10.3389/fendo.2019.00383
17. Blanton ML, Specker JL. The hypothalamic–pituitary–thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit Rev Toxicol (2007) 37:97–115. doi: 10.1080/10408440601123529
18. Gavrila A, Hollenberg A. The hypothalamic–pituitary–thyroid axis: physiological regulation and clinical implications. In: Luster M, Duntas L, Wartofsky L, editors. The Thyroid and Its Diseases. Chamonix, CH: Springer (2019). p. 13–23.
19. Stathatos N. Thyroid Physiology. Med Clinics North America (2012) 96(2):165–73. doi: 10.1016/j.mcna.2012.01.007
20. Eales JG, MacLatchy DL, Sweeting RM. Thyroid hormone deiodinase systems in salmonids, and their involvement in the regulation of thyroidal status. Fish Physiol Biochem (1993) 11(1):313–21. doi: 10.1007/BF00004580
21. Fliers E, Kalsbeek A, Boelen A. Beyond the fixed setpoint of the hypothalamus–pituitary–thyroid axis. Eur J Endocrinol (2014) 171(5):R197–208. doi: 10.1530/EJE-14-0285
22. Roelfsema F, Boelen A, Kalsbeek A, Fliers E. Regulatory aspects of the human hypothalamus–pituitary–thyroid axis. Best Pract Res Clin Endocrinol Metab (2017) 31(5):487–503. doi: 10.1016/j.beem.2017.09.004
23. Costa–e–Sousa RH, Hollenberg AN. Minireview: The neural regulation of the hypothalamic–pituitary–thyroid axis. Endocrinology (2012) 153:4128–35. doi: 10.1210/en.2012-1467
24. Chatterjee A, Hsieh YL, Yu JYL. Molecular cloning of cDNA encoding thyroid stimulating hormone β subunit of bighead carp Aristichthys nobilis and regulation of its gene expression. Mol Cell Endocrinol (2001) 174(1):1–9. doi: 10.1016/S0303-7207(01)00392-6
25. Kagabu Y, Mishiba T, Okino T, Yanagisawa T. Effects of thyrotropin–releasing hormone and its metabolites, cyclo(his–pro) and trh–oh, on growth hormone and prolactin synthesis in primary cultured pituitary cells of the common carp, Cyprinus carpio. Gen Comp Endocrinol (1998) 111(3):395–403. doi: 10.1006/gcen.1998.7124
26. Geven EJW, Flik G, Klaren PHM. Central and peripheral integration of interrenal and thyroid axes signals in common carp (Cyprinus carpio L.). J Endocrinol (2009) 200(1):117–23. doi: 10.1677/JOE-08-0410
27. Larsen DA, Swanson P, Dickey JT, Rivier J, Dickhoff WW. In vitro thyrotropin–releasing activity of corticotropin–releasing hormone–family peptides in coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol (1998) 109(2):276–85. doi: 10.1006/gcen.1997.7031
28. De Groef B, Van Der Geyten S, Darras VM, Kühn ER. Role of corticotropin–releasing hormone as a thyrotropin–releasing factor in non–mammalian vertebrates. Gen Comp Endocrinol (2006) 146(1):62–8. doi: 10.1016/j.ygcen.2005.10.014
29. Galas L, Raoult E, Tonon M-C, Okada R, Jenks BG, Castaño JP, et al. TRH acts as a multifunctional hypophysiotropic factor in vertebrates. Gen Comp Endocrinol (2009) 164(1):40–50. doi: 10.1016/j.ygcen.2009.05.003
30. Tran TN, Fryer JN, Bennett HPJ, Tonon MC, Vaudry H. TRH stimulates the release of POMC–derived peptides from goldfish melanotropes. Peptides (1989) 10(4):835–41. doi: 10.1016/0196-9781(89)90122-8
31. Trudeau VL, Somoza GM, Nahorniak CS, Peter RE. Interactions of estradiol with gonadotropin–releasing hormone and thyrotropin–releasing hormone in the control of growth hormone secretion in the goldfish. Neuroendocrinology (1992) 56(4):483–90. doi: 10.1159/000126265
32. Eales JG, Himick BA. The effects of TRH on plasma thyroid hormone levels of rainbow trout (Salmo gairdneri) and arctic charr (Salvelinus alpinus). Gen Comp Endocrinol (1988) 72(3):333–9. doi: 10.1016/0016-6480(88)90155-4
33. MacKenzie DS, Jones RA, Miller TC. Thyrotropin in teleost fish. Gen Comp Endocrinol (2009) 161(1):83–9. doi: 10.1016/j.ygcen.2008.12.010
34. Maugars G, Dufour S, Cohen–Tannoudji J, Quérat B. Multiple thyrotropin β–subunit and thyrotropin receptor–related genes arose during vertebrate evolution. PloS One (2014) 9(11):e111361–e. doi: 10.1371/journal.pone.0111361
35. Jackson RG, Sage M. A comparison of the effects of mammalian TSH on the thyroid glands of the teleost Galeichthys felis and the elasmobranch Dasyatis sabina. Comp Biochem Physiol Part A: Physiol (1973) 44(3):867–70. doi: 10.1016/0300-9629(73)90149-7
36. Nishioka RS, Grau EG, Lai KV, Bern HA. Effect of thyroid–stimulating hormone on the physiology and morphology of the thyroid gland in coho salmon, Oncorhynchus kisutch. Fish Physiol Biochem (1987) 3(2):63–71. doi: 10.1007/BF02183000
37. Grau GE, Stetson MH. The effects of prolactin and TSH on thyroid function in Fundulus heteroclitus. Gen Comp Endocrinol (1977) 33(3):329–35. doi: 10.1016/0016-6480(77)90047-8
38. Chan HH, Eales JG. Influence of bovine TSH on plasma thyroxine levels and thyroid function in brook trout, Salvelinus fontinalis (Mitchill). Gen Comp Endocrinol (1976) 124:343–58. doi: 10.1016/0016-6480(76)90155-6
39. Scanlon MF, Weightman DR, Shale DJ, Mora B, Heath M, Snow MH, et al. Dopamine is a physiological regulator of thyrotropin (TSH) secretion in normal man. Clin Endocrinol (1979) 10(1):7–15. doi: 10.1111/j.1365-2265.1979.tb03028.x
40. Tanjasiri P, Kozbur X, Florsheim WH. Somatostatin in the physiologic feedback control of thyrotropin secretion. Life Sci (1976) 19(5):657–60. doi: 10.1016/0024-3205(76)90162-4
41. Peter RE, McKeown BA. Hypothalamic control of prolactin and thyrotropin secretion in teleosts, with special reference to recent studies on the goldfish. Gen Comp Endocrinol (1975) 25(2):153–65. doi: 10.1016/0016-6480(75)90186-0
42. Chowdhury I, Chien JT, Chatterjee A, Yu JYL. In vitro effects of mammalian leptin, neuropeptide–Y, β–endorphin and galanin on transcript levels of thyrotropin β and common α subunit mRNAs in the pituitary of bighead carp (Aristichthys nobilis). Comp Biochem Physiol Part B: Biochem Mol Biol (2004) 139(1):87–98. doi: 10.1016/j.cbpc.2004.06.007
43. Dupré SM, Guissouma H, Flamant F, Seugnet I, Scanlan TS, Baxter JD, et al. Both thyroid hormone receptor (TR)β1 and TRβ2 isoforms contribute to the regulation of hypothalamic thyrotropin–releasing hormone. Endocrinology (2004) 145(5):2337–45. doi: 10.1210/en.2003-1209
44. Hollenberg AN, Monden T, Flynn TR, Boers ME, Cohen O, Wondisford FE. The human thyrotropin–releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements. Mol Endocrinol (1995) 9(5):540–50. doi: 10.1210/mend.9.5.7565802
45. Shupnik MA, Chin WW, Habener JF, Ridgway EC. Transcriptional regulation of the thyrotropin subunit genes by thyroid hormone. J Biol Chem (1985) 260(5):2900–3.
46. Geven EJW, Verkaar F, Flik G, Klaren PHM. Experimental hyperthyroidism and central mediators of stress axis and thyroid axis activity in common carp (Cyprinus carpio L.). J Mol Endocrinol (2006) 37(3):443–52. doi: 10.1677/jme.1.02144
47. Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor–binding proteins. Nature (1991) 349(6308):423–6. doi: 10.1038/349423a0
48. Yoshiura Y, Sohn YC, Munakata A, Kobayashi M, Aida K. Molecular cloning of the cDNA encoding the β subunit of thyrotropin and regulation of its gene expression by thyroid hormones in the goldfish, Carassius auratus. Fish Physiol Biochem (1999) 21:201–10. doi: 10.1023/A:1007884527397
49. Pradet–Balade B, Burel C, Dufour S, Boujard T, Kaushik SJ, Quérat B, et al. Thyroid hormones down–regulate thyrotropin β mRNA level in vivo in the turbot (Psetta maxima). Fish Physiol Biochem (1999) 20(3):193–9. doi: 10.1023/A:1007791415780
50. Pradet–Balade B, Schmitz M, Salmon C, Dufour S, Quérat B. Down–regulation of TSH subunit mRNA levels by thyroid hormones in the European eel. Gen Comp Endocrinol (1997) 108(2):191–8. doi: 10.1006/gcen.1997.6960
51. Allan ERO, Habibi HR. Direct effects of triiodothyronine on production of anterior pituitary hormones and gonadal steroids in goldfish. Mol Reprod Dev (2012) 79:592–602. doi: 10.1002/mrd.22066
52. Power DM, Llewellyn L, Faustino M, Nowell MA, Björnsson BT, Einarsdottir IE, et al. Thyroid hormones in growth and development of fish. Comp Biochem Physiol – C Toxicol Pharmacol (2001) 130(4):447–59. doi: 10.1016/S1532-0456(01)00271-X
53. Chanet B, Meunier FJ. The anatomy of the thyroid gland among “fishes”:Phylogenetic implications for the Vertebrata. Cybium (2014) 38(2):90–116. doi: 10.26028/cybium/2014-382-002
54. Geven EJW, Nguyen NK, Van Den Boogaart M, Spanings FAT, Flik G, Klaren PHM. Comparative thyroidology: Thyroid gland location and iodothyronine dynamics in Mozambique tilapia (Oreochromis mossambicus Peters) and common carp (Cyprinus carpio L.). J Exp Biol (2007) 210:4005–15. doi: 10.1242/jeb.010462
55. Gorbman A. 4 – Thyroid Function and Its Control in Fishes. In: Hoar WS, Randall DJ, editors. Fish Physiology. 2. New York City, NY: Academic Press (1969). p. 241–74.
56. Manzon RG, Manzon LA. Lamprey metamorphosis: Thyroid hormone signaling in a basal vertebrate. Mol Cell Endocrinol (2017) 459:28–42. doi: 10.1016/j.mce.2017.06.015
57. Eales JG. The relationship between ingested thyroid hormones, thyroid homeostasis and iodine metabolism in humans and teleost fish. Gen Comp Endocrinol (2019) 280:62–72. doi: 10.1016/j.ygcen.2019.04.012
58. Dent JN, Dodd JM. Some effects of mammalian thyroid stimulating hormone, elasmobranch pituitary gland extracts and temperature on thyroidal activity in newly hatched dogfish (Scyliorhinus caniculus). J Endocrinol (1961) 22(4):395–NP. doi: 10.1677/joe.0.0220395
59. St Germain DL, Galton VA, Hernandez A. Minireview: Defining the roles of the iodothyronine deiodinases: Current concepts and challenges. Endocrinology (2009) 150(3):1097–107. doi: 10.1210/en.2008-1588
60. Luongo C, Dentice M, Salvatore D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol (2019) 15(8):479–88. doi: 10.1038/s41574-019-0218-2
61. Kelly G. Peripheral metabolism of thyroid hormones: A review. Altern Med Rev: A J Clin Ther (2000) 5(4):306–33.
62. Gereben B, Anikó Z, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: Local action with general consequences. Cell Mol Life Sci CMLS (2008) 65:570–90. doi: 10.1007/s00018-007-7396-0
63. Eales JG, Brown SB. Measurement and regulation of thyroidal status in teleost fish. Rev Fish Biol Fish (1993) 3(4):299–347. doi: 10.1007/BF00043383
64. Bianco AC, Salvatore D, Gereben BZ, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev (2002) 23(1):38–89. doi: 10.1210/edrv.23.1.0455
65. Jarque S, Piña B. Deiodinases and thyroid metabolism disruption in teleost fish. Environ Res (2014) 135:361–75. doi: 10.1016/j.envres.2014.09.022
66. Garcı́a –G C, Jeziorski MC, Valverde –R C, Orozco A. Effects of iodothyronines on the hepatic outer–ring deiodinating pathway in killifish. Gen Comp Endocrinol (2004) 135(2):201–9. doi: 10.1016/j.ygcen.2003.09.010
67. Orozco A, Valverde –RC. Thyroid hormone deiodination in fish. Thyroid (2005) 15(8):799–813. doi: 10.1089/thy.2005.15.799
68. Philippe J, Dibner C. Thyroid circadian timing: Roles in physiology and thyroid malignancies. J Biol Rhythms (2014) 30(2):76–83. doi: 10.1177/0748730414557634
69. Vriend J. Influence of the pineal gland and circadian rhythms in circulating levels of thyroid hormones of male hamsters. J Pineal Res (1984) 1(1):15–22. doi: 10.1111/j.1600-079X.1984.tb00191.x
70. Milesi S, Simonneaux V, Klosen P. Downregulation of deiodinase 3 is the earliest event in photoperiodic and photorefractory activation of the gonadotropic axis in seasonal hamsters. Sci Rep (2017) 7(1):17739–. doi: 10.1038/s41598-017-17920-y
71. Sáenz de Miera C, Sage–Ciocca D, Simonneaux V, Pévet P, Monecke S. Melatonin–independent photoperiodic entrainment of the circannual TSH Rhythm in the pars tuberalis of the European hamster. J Biol Rhythms (2018) 33(3):302–17. doi: 10.1177/0748730418766601
72. Grau GE. Environmental influences on thyroid function in teleost fish. Am Zoologist (1988) 28(2):329–35. doi: 10.1093/icb/28.2.329
73. Cowan M, Azpeleta C, López–Olmeda JF. Rhythms in the endocrine system of fish: a review. J Comp Physiol B (2017) 187(8):1057–89. doi: 10.1007/s00360-017-1094-5
74. Jones RA, Cohn WB, Miller TC, Jaques JT, MacKenzie DS. Cyclic mRNA expression of thyrotropin subunits and deiodinases in red drum, Sciaenops ocellatus. Gen Comp Endocrinol (2013) 194:248–56. doi: 10.1016/j.ygcen.2013.09.017
75. Striberny A, Jørgensen EH, Klopp C, Magnanou E. Arctic charr brain transcriptome strongly affected by summer seasonal growth but only subtly by feed deprivation. BMC Genomics (2019) 20(1):529–. doi: 10.1186/s12864-019-5874-z
76. Ikegami K, Yoshimura T. Comparative analysis reveals the underlying mechanism of vertebrate seasonal reproduction. Gen Comp Endocrinol (2016) 227:64–8. doi: 10.1016/j.ygcen.2015.05.009
77. Nakane Y, Ikegami K, Iigo M, Ono H, Takeda K, Takahashi D, et al. The saccus vasculosus of fish is a sensor of seasonal changes in day length. Nat Commun (2013) 4(1):2108. doi: 10.1038/ncomms3108
78. Ebbesson LOE, Björnsson BT, Ekström P, Stefansson SO. Daily endocrine profiles in parr and smolt Atlantic salmon. Comp Biochem Physiol Part A: Mol Integr Physiol (2008) 151(4):698–704. doi: 10.1016/j.cbpa.2008.08.017
79. Eales JG, Fletcher GL. Circannual cycles of thyroid hormones in plasma of winter flounder (Pseudopleuronectes americanus Walbaum). Can J Zool (1982) 60(3):304–9. doi: 10.1139/z82-040
80. Spieler RE, Noeske TA. Diel variations in circulating levels of triiodothyronine and thyroxine in goldfish, Carassius auratus. Can J Zool (1979) 57(3):665–9. doi: 10.1139/z79-079
81. Leiner KA, Han GS, MacKenzie DS. The effects of photoperiod and feeding on the diurnal rhythm of circulating thyroid hormones in the red drum, Sciaenops ocellatus. Gen Comp Endocrinol (2000) 120(1):88–98. doi: 10.1006/gcen.2000.7539
82. Ganzha EV, Pavlov ED. Diurnal dynamics of thyroid and sex steroid hormones in the blood of rainbow trout juveniles. Inland Water Biol (2019) 12(3):333–6. doi: 10.1134/S1995082919030064
83. Loter TC, MacKenzie DS, McLeese J, Eales JG. Seasonal changes in channel catfish thyroid hormones reflect increased magnitude of daily thyroid hormone cycles. Aquaculture (2007) 262(2):451–60. doi: 10.1016/j.aquaculture.2006.09.017
84. Comeau LA, Campana SE, Hanson JM, Chouinard GA. Seasonal changes of thyroid hormones in field–collected Atlantic cod in relation to condition indices, water temperature and photoperiod. J Fish Biol (2000) 57(3):571–88. doi: 10.1111/j.1095-8649.2000.tb00261.x
85. Cyr DG, Eales JG. Influence of thyroidal status on ovarian function in rainbow trout, Salmo gairdneri. J Exp Zool (1988) 248(1):81–7. doi: 10.1002/jez.1402480110
86. Power DM, Elias NP, Richardson SJ, Mendes J, Soares CM, Santos CRA. Evolution of the thyroid hormone–binding protein, transthyretin. Gen Comp Endocrinol (2000) 119(3):241–55. doi: 10.1006/gcen.2000.7520
87. Heijlen M, Houbrechts AM, Darras VM. Zebrafish as a model to study peripheral thyroid hormone metabolism in vertebrate development. Gen Comp Endocrinol (2013) 188:289–96. doi: 10.1016/j.ygcen.2013.04.004
88. Bernal J, Guadaño–Ferraz A, Morte B. Thyroid hormone transporters—functions and clinical implications. Nat Rev Endocrinol (2015) 11(7):406–17. doi: 10.1038/nrendo.2015.66
89. Groeneweg S, van Geest FS, Peeters RP, Heuer H, Visser WE. Thyroid hormone transporters. EndocrRev (2019) 41(2):146–201. doi: 10.1210/endrev/bnz008
90. Muzzio AM, Noyes PD, Stapleton HM, Lema SC. Tissue distribution and thyroid hormone effects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion–transporting polypeptides (Oatps) in a teleost fish. Comp Biochem Physiol Part A: Mol Integr Physiol (2014) 167:77–89. doi: 10.1016/j.cbpa.2013.09.019
91. Admati I, Wasserman–Bartov T, Tovin A, Rozenblat R, Blitz E, Zada D, et al. Neural alterations and hyperactivity of the hypothalamic–pituitary–thyroid axis in oatp1c1 deficiency. Thyroid (2019) 30(1):161–74. doi: 10.1089/thy.2019.0320
92. Zada D, Blitz E, Appelbaum L. Zebrafish – An emerging model to explore thyroid hormone transporters and psychomotor retardation. Mol Cell Endocrinol (2017) 459:53–8. doi: 10.1016/j.mce.2017.03.004
93. Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. Low level exposure to the flame retardant BDE–209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ Sci Technol (2013) 47(17):10012–21. doi: 10.1021/es402650x
94. Vatine GD, Zada D, Lerer–Goldshtein T, Tovin A, Malkinson G, Yaniv K, et al. Zebrafish as a model for monocarboxyl transporter 8–deficiency. J Biol Chem (2013) 288(1):169–80. doi: 10.1074/jbc.M112.413831
95. Cheng S–Y, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev (2010) 31(2):139–70. doi: 10.1210/er.2009-0007
96. Paul BD, Buchholz DR, Fu L, Shi YB. SRC–p300 coactivator complex is required for thyroid hormone–induced amphibian metamorphosis. J Biol Chem (2007) 282:7472–81. doi: 10.1074/jbc.M607589200
97. Forrest D, Vennström B. Functions of thyroid hormone receptors in mice. Thyroid (2000) 10(1):41–52. doi: 10.1089/thy.2000.10.41
98. Ortiga–Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol (2014) 10(10):582–91. doi: 10.1038/nrendo.2014.143
99. Bhagavan NV. CHAPTER 30 – Endocrine Metabolism I: Introduction. In: Bhagavan NV, editor. Medical Biochemistry, 4th ed. San Diego: Academic Press (2002). p. 699–727.
100. Yu J, Fu Y, Shi Z. Coordinated expression and regulation of deiodinases and thyroid hormone receptors during metamorphosis in the Japanese flounder (Paralichthys olivaceus). Fish Physiol Biochem (2017) 43(2):321–36. doi: 10.1007/s10695-016-0289-0
101. Marchand O, Safi R, Escriva H, Rompaey EV, Prunet P, Laudet V. Molecular cloning and characterization of thyroid hormone receptors in teleost fish. J Mol Endocrinol (2001) 26(1):51–65. doi: 10.1677/jme.0.0260051
102. Kawakami Y, Tanda M, Adachi S, Yamauchi K. Characterization of thyroid hormone receptor α and β in the metamorphosing Japanese conger eel, Conger myriaster. Gen Comp Endocrinol (2003) 132(2):321–32. doi: 10.1016/S0016-6480(03)00087-X
103. Nelson ER, Habibi HR. Molecular characterization and sex–related seasonal expression of thyroid receptor subtypes in goldfish. Mol Cell Endocrinol (2006) 253(1–2):83–95. doi: 10.1016/j.mce.2006.05.003
104. Nelson ER, Habibi HR. Thyroid receptor subtypes: Structure and function in fish. Gen Comp Endocrinol (2009) 161(1):90–6. doi: 10.1016/j.ygcen.2008.09.006
105. Mendoza A, Navarrete–Ramírez P, Hernández–Puga G, Villalobos P, Holzer G, Renaud JP, et al. 3,5–T2 Is an alternative ligand for the thyroid hormone receptor β1. Endocrinology (2013) 154(8):2948–58. doi: 10.1210/en.2013-1030
106. Cayrol F, Sterle HA, Díaz Flaqué MC, Barreiro Arcos ML, Cremaschi GA. Non–genomic actions of thyroid hormones regulate the growth and angiogenesis of t cell lymphomas. Front Endocrinol (2019) 10:63–. doi: 10.3389/fendo.2019.00063
107. Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol (2016) 12:111–21. doi: 10.1038/nrendo.2015.205
108. Hiroi Y, Kim H–H, Ying H, Furuya F, Huang Z, Simoncini T, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci (2006) 103(38):14104 LP–9. doi: 10.1073/pnas.0601600103
109. Moeller LC, Cao X, Dumitrescu AM, Seo H, Refetoff S. Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor beta through the phosphatidylinositol 3–kinase pathway. Nuclear Receptor Signaling (2006) 4:e020–e. doi: 10.1621/nrs.04020
110. Bergh JJ, Lin H–Y, Lansing L, Mohamed SN, Davis FB, Mousa S, et al. Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen–activated protein kinase and induction of angiogenesis. Endocrinology (2005) 146(7):2864–71. doi: 10.1210/en.2005-0102
111. De Vito P, Balducci V, Leone S, Percario Z, Mangino G, Davis PJ, et al. Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players. Steroids (2012) 77:988–95. doi: 10.1016/j.steroids.2012.02.018
112. Yonkers MA, Ribera AB. Molecular components underlying nongenomic thyroid hormone signaling in embryonic zebrafish neurons. Neural Dev (2009) 4:20. doi: 10.1186/1749-8104-4-20
113. Oommen OV, Sreejith P, Beyo RS, Divya L, Vijayasree AS, Manju M. Thyroid hormone regulates mitochondrial respiration as well as antioxidant defense in teleosts too. J Endocrinol Reprod (2006) 10:96–105.
114. Hashizume K, Ichikawa K. Localization of 3,5,3′–L–triiodothyronine receptor in rat kidney mitochondrial membranes. Biochem Biophys Res Commun (1982) 106(3):920–6. doi: 10.1016/0006-291X(82)91798-3
115. Senese R, de Lange P, Petito G, Moreno M, Goglia F, Lanni A. 3,5–Diiodothyronine: A novel thyroid hormone metabolite and potent modulator of energy metabolism. Front Endocrinol (2018) 9:427. doi: 10.3389/fendo.2018.00427
116. Olvera A, Martyniuk CJ, Buisine N, Jiménez–Jacinto V, Sanchez–Flores A, Sachs LM, et al. Differential transcriptome regulation by 3,5–T2 and 3’,3,5–T3 in brain and liver uncovers novel roles for thyroid hormones in tilapia. Sci Rep (2017) 7(1):15043. doi: 10.1038/s41598-017-14913-9
117. Leary SC, Barton KN, Ballantyne JS. Direct effects of 3,5,3′–triiodothyronine and 3,5–diiodothyronine on mitochondrial metabolism in the goldfish Carassius auratus. Gen Comp Endocrinol (1996) 104(1):61–6. doi: 10.1006/gcen.1996.0141
118. Little AG, Kunisue T, Kannan K, Seebacher F. Thyroid hormone actions are temperature–specific and regulate thermal acclimation in zebrafish (Danio rerio). BMC Biol (2013) 11(1):26. doi: 10.1186/1741-7007-11-26
119. Pamela N–R, Maricela L, Carlos Valverde R, Aurea O. 3,5–di–iodothyronine stimulates tilapia growth through an alternate isoform of thyroid hormone receptor β1. J Mol Endocrinol (2014) 52(1):1–9. doi: 10.1530/JME-13-0145
120. Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol (2014) 221(3):R87–R103. doi: 10.1530/JOE-14-0025
121. Stepien BK, Huttner WB. Transport, metabolism, and function of thyroid hormones in the developing mammalian brain. Front Endocrinol (2019) 10:1–16. doi: 10.3389/fendo.2019.00209
122. Godwin J, Phillips M. Modes of Reproduction in Fishes. In: Skinner MK, editor. Encyclopedia of Reproduction, 2nd ed. Oxford: Academic Press (2018). p. 23–31.
123. Vergauwen L, Cavallin JE, Ankley GT, Bars C, Gabriëls IJ, Michiels EDG, et al. Gene transcription ontogeny of hypothalamic–pituitary–thyroid axis development in early–life stage fathead minnow and zebrafish. Gen Comp Endocrinol (2018) 266:87–100. doi: 10.1016/j.ygcen.2018.05.001
124. Sloman KA. The Diversity of Fish Reproduction: An Introduction. In: Farrell AP, editor. Encyclopedia of Fish Physiology. San Diego: Academic Press (2011). p. 613–5.
125. Wourms JP, Demski LS. The Reproduction and Development of Sharks, Skates, Rays and Ratfishes: Introduction, History, Overview, and Future Prospects. In: Demski LS, Wourms JP, editors. The Reproduction and Development of Sharks, Skates, Rays and Ratfishes. Dordrecht: Springer Netherlands (1993). p. 7–21.
126. Hamlett WC. Ontogeny of the umbilical cord and placenta in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Environ Biol Fish (1993) 38(1):253–67. doi: 10.1007/BF00842921
127. Lam TJ. Hormones and Egg/Larval Quality in Fish. J World Aquaculture Soc (1994) 25(1):2–12. doi: 10.1111/j.1749-7345.1994.tb00798.x
128. Castillo S, Bollfrass K, Mendoza R, Fontenot Q, Lazo JP, Aguilera C, et al. Stimulatory effect of thyroid hormones improves larval development and reproductive performance in alligator gar (Atractosteus spatula) and spotted gar (Lepisosteus oculatus). Aquaculture Res (2015) 46(9):2079–91. doi: 10.1111/are.12363
129. Abdollahpour H, Falahatkar B, Efatpanah I, Meknatkhah B, Van Der Kraak G. Influence of thyroxine on spawning performance and larval development of Sterlet sturgeon Acipenser ruthenus. Aquaculture (2018) 497:134–9. doi: 10.1016/j.aquaculture.2018.07.033
130. Alinezhad S, Abdollahpour H, Jafari N, Falahatkar B. Effects of thyroxine immersion on sterlet sturgeon (Acipenser ruthenus) embryos and larvae: Variations in thyroid hormone levels during development. Aquaculture (2020) 519:734745–. doi: 10.1016/j.aquaculture.2019.734745
131. Landines MA, Sanabria AI, Senhorini JA, Urbinati EC. The influence of triiodothyronine (T3) on the early development of piracanjuba (Brycon orbignyanus). Fish Physiol Biochem (2010) 36(4):1291–6. doi: 10.1007/s10695-010-9410-y
132. Urbinati EC, Vasques LH, Senhorini JA, Souza VL, Gonçalves FD. Larval performance of matrinxã, Brycon amazonicus (Spix & Agassiz 1829), after maternal triiodothyronine injection or egg immersion. Aquaculture Res (2008) 39(13):1355–9. doi: 10.1111/j.1365-2109.2008.02002.x
133. Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci (1997) 94(24):13011. doi: 10.1073/pnas.94.24.13011
134. Reddy PK, Lam TJ. Effect of thyroid hormones on morphogenesis and growth of larvae and fry of telescopic–eye black goldfish, Carassius auratus. Aquaculture (1992) 107(4):383–94. doi: 10.1016/0044-8486(92)90085-Y
135. Tagawa M, Tanaka M, Matsumoto S, Hirano T. Thyroid hormones in eggs of various freshwater, marine and diadromous teleosts and their changes during egg development. Fish Physiol Biochem (1990) 8(6):515–20. doi: 10.1007/BF00003409
136. Leary SC, Ballantyne JS, Leatherland JF. Evaluation of thyroid hormone economy in elasmobranch fishes, with measurements of hepatic 5′–monodeiodinase activity in wild dogfish. J Exp Zool (1999) 284(5):492–9. doi: 10.1002/(SICI)1097-010X(19991001)284:5<492::AID-JEZ4>3.0.CO;2-A
137. Kang D–Y, Chang YJ. Effects of maternal injection of 3,5,3′–triiodo–l–thyronine (T3) on growth of newborn offspring of rockfish, Sebastes schlegeli. Aquaculture (2004) 234(1):641–55. doi: 10.1016/j.aquaculture.2004.01.011
138. Nakamura O, Suzuki R, Asai K, Kaji H, Kaneko T, Takahashi Y, et al. Transport of maternal transthyretin to the fetus in the viviparous teleost Neoditrema ransonnetii (Perciformes, Embiotocidae). J Comp Physiol B (2020) 190(2):231–41. doi: 10.1007/s00360-020-01261-w
139. Wourms JP. Viviparity: The Maternal–Fetal Relationship in Fishes. Am Zoologist (2015) 21(2):473–515. doi: 10.1093/icb/21.2.473
140. McComb DM, Gelsleichter J, Manire CA, Brinn R, Brown CL. Comparative thyroid hormone concentration in maternal serum and yolk of the bonnethead shark (Sphyrna tiburo) from two sites along the coast of Florida. Gen Comp Endocrinol (2005) 144(2):167–73. doi: 10.1016/j.ygcen.2005.05.005
141. Rodriguez–Arnao J, Miell JP, Ross RJM. Influence of thyroid hormones on the GH–IGF–I axis. Trends Endocrinol Metab (1993) 4(5):169–73. doi: 10.1016/1043-2760(93)90107-P
142. Blanco AM. Hypothalamic– and pituitary–derived growth and reproductive hormones and the control of energy balance in fish. Gen Comp Endocrinol (2020) 287:113322. doi: 10.1016/j.ygcen.2019.113322
143. Triantaphyllopoulos KA, Cartas D, Miliou H. Factors influencing GH and IGF–I gene expression on growth inteleost fish: How can aquaculture industry benefit? Rev Aquaculture (2019) 12(3):1637–62. doi: 10.1111/raq.12402
144. Moav B, McKeown BA. Thyroid hormone increases transcription of growth hormone mRNA in rainbow trout pituitary. Hormone Metab Res (1992) 24(1):10–4. doi: 10.1055/s-2007-1003242
145. Farchi–Pisanty O, Hackett PB Jr., Moav B. Regulation of fish growth hormone transcription. Mol Marine Biol Biotechnol (1995) 4(3):215–23.
146. Melamed P, Eliahu N, Levavi–Sivan B, Ofir M, Farchi–Pisanty O, Rentier–Delrue F, et al. Hypothalamic and thyroidal regulation of growth hormone in tilapia. Gen Comp Endocrinol (1995) 97(1):13–30. doi: 10.1006/gcen.1995.1002
147. Schmid AC, Lutz I, Kloas W, Reinecke M. Thyroid hormone stimulates hepatic IGF–I mRNA expression in a bony fish, the tilapia Oreochromis mossambicus, in vitro and in vivo. Gen Comp Endocrinol (2003) 130(2):129–34. doi: 10.1016/S0016-6480(02)00577-4
148. Bolotovskiy AA, Levin BA. Effects of thyroid hormones on vertebral numbers in two cyprinid fish species: Rutilus rutilus (Linnaeus, 1758) and Abramis brama (Linnaeus, 1758). J Appl Ichthyol (2018) 34(2):449–54. doi: 10.1111/jai.13659
149. Keer S, Cohen K, May C, Hu Y, McMenamin S, Hernandez LP. Anatomical assessment of the adult skeleton of zebrafish reared under different thyroid hormone profiles. Anatomical Record (2019) 302(10):1754–69. doi: 10.1002/ar.24139
150. Molla MHR, Hasan MT, Jang WJ, Soria Diaz CD, Appenteng P, Marufchoni H, et al. Thyroid hormone–induced swim bladder and eye maturation are transduced by IGF–1 in zebrafish embryos. Aquaculture Res (2019) 50(11):3462–70. doi: 10.1111/are.14305
151. Gracia–Navarro F, Castaño JP, Malagón MM, Torronteras R. Subcellular responsiveness of amphibian growth hormone cells after TSH–releasing hormone stimulation. Gen Comp Endocrinol (1991) 84(1):94–103. doi: 10.1016/0016-6480(91)90068-H
152. Hall TR, Chadwick A. Effects of synthetic mammalian thyrotrophin releasing hormone, somatostatin and dopamine on the secretion of prolactin and growth hormone from amphibian and reptilian pituitary glands incubated in vitro. J Endocrinol (1984) 102(2):175–80. doi: 10.1677/joe.0.1020175
153. Denver RJ, Licht P. Thyroid status influences in vitro thyrotropin and growth hormone responses to thyrotropin–releasing hormone by pituitary glands of hatchling slider turtles (Pseudemys scripta elegans). J Exp Zool (1988) 246(3):293–304. doi: 10.1002/jez.1402460309
154. Hervas F, de Escobar GM, del Rey FE. Rapid effects of single small doses of l–thyroxine and triiodo–l–thyronine on growth hormone, as studied in the rat by radioimmunoassay. Endocrinology (1975) 97(1):91–101. doi: 10.1210/endo-97-1-91
155. Dobner PR, Kawasaki ES, Yu LY, Bancroft FC. Thyroid or glucocorticoid hormone induces pre–growth–hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci (1981) 78(4):2230–4. doi: 10.1073/pnas.78.4.2230
156. James RA, Sarapura VD, Bruns C, Raulf F, Dowding JM, Gordon DF, et al. Thyroid hormone–induced expression of specific somatostatin receptor subtypes correlates with involution of the TtT–97 murine thyrotrope tumor. Endocrinology (1997) 138(2):719–24. doi: 10.1210/endo.138.2.4951
157. Berelowitz M, Maeda K, Harris S, Frohman L. The effect of alterations in the pituitary–thyroid axis on hypothalamic content and in vitro release of somatostatin–like immunoreactivity. Endocrinology (1980) 107(1):24–9. doi: 10.1210/endo-107-1-24
158. Cook AF, Peter RE. The effects of somatostatin on serum growth hormone levels in the goldfish, Carassius auratus. Gen Comp Endocrinol (1984) 54(1):109–13. doi: 10.1016/0016-6480(84)90205-3
159. Lin XW, Lin HR, Peter RE. The regulatory effects of thyrotropin–releasing hormone on growth hormone secretion from the pituitary of common carp in vitro. Fish Physiol Biochem (11(1–6) 1993:71–6. doi: 10.1007/BF00004552
160. Batten TFC, Wigham T. Effects of TRH and somatostatin on releases of prolactin and growth hormone in vitro by the pituitary of Poecilia latipinna. Cell Tissue Res (1984) 237(3):595–603. doi: 10.1007/BF00228444
161. Pickford G. The response of hypophysectomized male killifish to prolonged treatment with small doses of thyrotropin. Endocrinology (1954) 55:589–92. doi: 10.1210/endo-55-5-589
162. Grau GE, Stetson MH. Growth hormone is thyrotropic in Fundulus heteroclitus. Gen Comp Endocrinol (1979) 39(1):1–8. doi: 10.1016/0016-6480(79)90186-2
163. Higgs DA, Donaldson EM, Dye HM, McBride JR. Influence of bovine growth hormone and l–thyroxine on growth, muscle composition, and histological structure of the gonads, thyroid, pancreas, and pituitary of coho salmon (Oncorhynchus kisutch). J Fish Res Board Canada (1976) 33(7):1585–603. doi: 10.1139/f76-199
164. Leatherland JF, Farbridge KJ. Chronic fasting reduces the response of the thyroid to growth hormone and TSH, and alters the growth hormone–related changes in hepatic 5′–monodeiodinase activity in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol (1992) 87(3):342–53. doi: 10.1016/0016-6480(92)90040-Q
165. Bandyopadhyay S, Bhattacharya S. Purification and properties of an indian major carp (Cirrhinus mrigala, Ham.) pituitary thyrotropin. Gen Comp Endocrinol (1993) 90(2):192–204. doi: 10.1006/gcen.1993.1074
166. Rousseau K, Belle NL, Sbaihi M, Marchelidon J, Schmitz M, Dufour S. Evidence for a negative feedback in the control of eel growth hormone by thyroid hormones. J Endocrinol (2002) 175(3):605–13. doi: 10.1677/joe.0.1750605
167. Leatherland JF, Hyder M. Effect of thyroxine on the ultrastructure of the hypophyseal proximal pars distalis in Tilapia zillii. Can J Zool (1975) 53(6):686–90. doi: 10.1139/z75-083
168. Wang Y, Zhang S. Expression and regulation by thyroid hormone (TH) of zebrafish IGF–I gene and amphioxus IGFl gene with implication of the origin of TH/IGF signaling pathway. Comp Biochem Physiol Part A: Mol Integr Physiol (2011) 160(4):474–9. doi: 10.1016/j.cbpa.2011.08.005
169. Pierce AL, Fukada H, Dickhoff WW. Metabolic hormones modulate the effect of growth hormone (GH) on insulin–like growth factor–I (IGF–I) mRNA level in primary culture of salmon hepatocytes. J Endocrinol (2005) 184(2):341–9. doi: 10.1677/joe.1.05892
170. Leung LY, Kwong AKY, Man AKY, Woo NYS. Direct actions of cortisol, thyroxine and growth hormone on IGF–I mRNA expression in sea bream hepatocytes. Comp Biochem Physiol Part A: Mol Integr Physiol (2008) 151(4):705–10. doi: 10.1016/j.cbpa.2008.08.023
171. MacLatchy DL, Eales JG. Intra– and extra–cellular sources of T3 binding to putative thyroid hormone receptors in liver, kidney, and gill nuclei of immature rainbow trout, Oncorhynchus mykiss. J Exp Zool (1992) 262(1):22–9. doi: 10.1002/jez.1402620105
172. Yamauchi I, Sakane Y, Yamashita T, Hirota K, Ueda Y, Kanai Y, et al. Effects of growth hormone on thyroid function are mediated by type 2 iodothyronine deiodinase in humans. Endocrine (2018) 59(2):353–63. doi: 10.1007/s12020-017-1495-y
173. Lamberts SWJ, Reubi JC, Krenning EP. Chapter 17 Somatostatin. 10. Stamford, CT: Elsevier (1997). p. 403–19.
174. Barington M, Brorson MM, Hofman–Bang J, Rasmussen ÅK, Holst B, Feldt–Rasmussen U. Ghrelin–mediated inhibition of the TSH–stimulated function of differentiated human thyrocytes ex vivo. PloS One (2017) 12(9):e0184992. doi: 10.1371/journal.pone.0184992
175. Milosević V, Sekulić M, Brkić B, Lovren M, Starcević V. Effect of centrally administered somatostatin on pituitarythyrotropes in male rats. Histochem J (2000) 32(9):565–9. doi: 10.1023/a:1004158412915
176. De Rosa G, Corsello SM, Della Casa S, De Rosa E, Raimondo S. Effect of somatostatin on the pituitary–thyroid axis. Annales d’Endocrinolo (Paris) (1983) 44(6):355–60.
177. Chen J, Cao M, Zhang A, Shi M, Tao B, Li Y, et al. Growth hormone overexpression disrupts reproductive status through actions on leptin. Front Endocrinol (2018) 9:131. doi: 10.3389/fendo.2018.00131
178. de Luze A, Leloup J. Fish growth hormone enhances peripheral conversion of thyroxine to triiodothyronine in the eel (Anguilla anguilla L.). Gen Comp Endocrinol (1984) 56(2):308–12. doi: 10.1016/0016-6480(84)90045-5
179. Holloway AC, Sheridan MA, Van Der Kraak G, Leatherland JF. Correlations of plasma growth hormone with somatostatin, gonadal steroid hormones and thyroid hormones in rainbow trout during sexual recrudescence. Comp Biochem Physiol – B Biochem Mol Biol (1999) 123(3):251–60. doi: 10.1016/S0305-0491(99)00059-0
180. Nieminen P, Mustonen A–M, Hyvärinen H. Fasting reduces plasma leptin– and ghrelin–immunoreactive peptide concentrations of the burbot (Lota lota) at 2°C but not at 10°C. Zool Sci (2003) 20(9):1109–15. doi: 10.2108/zsj.20.1109
181. Bhumika S, Lemmens K, Vancamp P, Moons L, Darras VM. Decreased thyroid hormone signaling accelerates the reinnervation of the optic tectum following optic nerve crush in adult zebrafish. Mol Cell Neurosci (2015) 68:92–102. doi: 10.1016/j.mcn.2015.04.002
182. Lema SC, Nevitt GA. Evidence that thyroid hormone induces olfactory cellular proliferation in salmon during a sensitive period for imprinting. J Exp Biol (2004) 207(19):3317 LP–27. doi: 10.1242/jeb.01143
183. Cheng CL, Gan KJ, Flamarique I. Thyroid hormone induces a time–dependent opsin switch in the retina of salmonid fishes. Invest Ophthalmol Visual Sci (2009) 50(6):3024–32. doi: 10.1167/iovs.08-2713
184. Flamarique IN, Browman HI. Foraging and prey–search behaviour of small juvenile rainbow trout (Oncorhynchus mykiss) under polarized light. J Exp Biol (2001) 204(14):2415–22.
185. Kudo H, Tsuneyoshi Y, Nagae M, Adachi S, Yamauchi K, Ueda H, et al. Detection of thyroid hormone receptors in the olfactory system and brain of wild masu salmon, Oncorhynchus masou (Brevoort), during smolting by in vitro autoradiography. Aquaculture Res (1994) 25(S2):171–81. doi: 10.1111/are.1994.25.s2.171
186. McMenamin SK, Parichy DM. Metamorphosis in teleosts. Curr Topics Dev Biol (2013) 103:127–65. doi: 10.1016/B978-0-12-385979-2.00005-8
187. Alves RN, Gomes AS, Stueber K, Tine M, Thorne MAS, Smáradóttir H, et al. The transcriptome of metamorphosing flatfish. BMC Genomics (2016) 17(1):413. doi: 10.1186/s12864-016-2699-x
188. Bergstrom CA. Morphological evidence of correlational selection and ecological segregation between dextral and sinistral forms in a polymorphic flatfish, Platichthys stellatus. J Evol Biol (2007) 20(3):1104–14. doi: 10.1111/j.1420-9101.2006.01290.x
189. Campinho MA, Silva N, Roman–Padilla J, Ponce M, Manchado M, Power DM. Flatfish metamorphosis: A hypothalamic independent process? Mol Cell Endocrinol (2015) 404:16–25. doi: 10.1016/j.mce.2014.12.025
190. Campinho MA, Silva N, Martins GG, Anjos L, Florindo C, Roman–Padilla J, et al. A thyroid hormone regulated asymmetric responsive centre is correlated with eye migration during flatfish metamorphosis. Sci Rep (2018) 8(1):12267. doi: 10.1038/s41598-018-29957-8
191. Youson JH. Is lamprey metamorphosis regulated by thyroid hormones? Am Zoologist (2015) 37(6):441–60. doi: 10.1093/icb/37.6.441
192. Stefansson SO, Björnsson B, Ebbesson LOE, McCormick SD. Smoltification. In: Finn RN, Kapoor BG, editors. Fish Larval Physiology. Enfield, NH: Science Publishers (2008). p. 639–81.
193. Björnsson BT, Stefansson SO, McCormick SD. Environmental endocrinology of salmon smoltification. Gen Comp Endocrinol (2011) 170(2):290–8. doi: 10.1016/j.ygcen.2010.07.003
194. McCormick SD. Smolt Physi28ology and Endocrinology. In: FishPhysiology. Cambridge, MA: Academic Press (2012). p. 199–251.
195. Hoar WS. The physiology of smolting salmonids. In: Hoar WS, Randall D, editors. Fish physiology. XIB. New York: Academic Press (1988).
196. Godin J–G, Dill P, Drury D. Effects of thyroid hormones on behavior of yearling Atlantic salmon (Salmo salar). J Fish Res Board Canada (2011) 31:1787–90. doi: 10.1139/f74-227
197. Iwata M. Downstream migratory behavior of salmonids and its relationship with cortisol and thyroid hormones: A review. Aquaculture (1995) 135(1):131–9. doi: 10.1016/0044-8486(95)01000-9
198. Premdas F, Eales J. The influence of TSH and ACTH on purine and pteridine deposition in the skin of rainbow trout (Salmo gairdneri). Can J Zool (1976) 54:576–81. doi: 10.1139/z76-067
199. Chua D, Eales JG. Thyroid function and dermal purines in the brook trout, Salvelinus fontinalis (Mitchill). Can J Zool (1971) 49(12):1557–61. doi: 10.1139/z71-226
200. Sudo R, Okamura A, Kuroki M, Tsukamoto K. Changes in the role of the thyroid axis during metamorphosis of the Japanese eel, Anguilla japonica. J Exp Zool Part A: Ecol Genet Physiol (2014) 321(7):357–64. doi: 10.1002/jez.1861
201. Hirata Y, Kurokura H, Kasahara S. Effects of thyroxine and thiourea on the development of larval red sea bream Pagrus major. Nippon Suisan Gakkasishi (1989) 55(7):1189–95. doi: 10.2331/suisan.55.1189
202. de Jesus EG, Toledo JD, Simpas MS. Thyroid hormones promote early metamorphosis in grouper (Epinephelus coioides) larvae. Gen Comp Endocrinol (1998) 112(1):10–6. doi: 10.1006/gcen.1998.7103
203. Roux N, Salis P, Laudet V. Metamorphosis of marine fish larvae and thyroid hormones. Biol Aujourd’hui (2019) 213(1–2):27–33. doi: 10.1051/jbio/2019010
204. Acevedo–Rodriguez A, Kauffman AS, Cherrington BD, Borges CS, Roepke TA, Laconi M. Emerging insights into hypothalamic–pituitary–gonadal axis regulation and interaction with stress signalling. J Neuroendocrinol (2018) 30(10):e12590–e. doi: 10.1111/jne.12590
205. Biran J, Levavi–Sivan B. Endocrine Control of Reproduction, Fish. In: Skinner MK, editor. Encyclopedia of Reproduction, 2nd ed. Oxford: Academic Press (2018). p. 362–8.
206. Duarte–Guterman P, Navarro–Martín L, Trudeau VL. Mechanisms of crosstalk between endocrine systems: Regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen Comp Endocrinol (2014) 203:69–85. doi: 10.1016/j.ygcen.2014.03.015
207. De Vincentis S, Monzani ML, Brigante G. Crosstalk between gonadotropins and thyroid axis. Minerva Ginecol (2018) 70(5):609–20. doi: 10.23736/S0026-4784.18.04271-5
208. Holsberger DR, Buchold GM, Leal MC, Kiesewetter SE, O’Brien DA, Hess RA, et al. Cell–cycle inhibitors p27Kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod (2005) 72(6):1429–36. doi: 10.1095/biolreprod.105.040386
209. Moenter SM, Woodfill C, Karsch FJ. Role of the thyroid gland in seasonal reproduction: thyroidectomy blocks seasonal suppression of reproductive neuroendocrine activity in ewes. Endocrinology (1991) 128(3):1337–44. doi: 10.1210/endo-128-3-1337
210. Anderson GM, Barrell GK. Effects of thyroidectomy and thyroxine replacement on seasonal reproduction in the red deer hind. Reproduction (1998) 113(2):239–50. doi: 10.1530/jrf.0.1130239
211. Raine JC. Chapter 5 – Thyroid Hormones and Reproduction in Fishes. In: Norris DO, Lopez KH, editors. Hormones and Reproduction of Vertebrates. London: Academic Press (2011). p. 83–102.
212. Osborn RH, Simpson TH, Youngson AF. Seasonal and diurnal rhythms of thyroidal status in the rainbow trout, Salmo gairdneri Richardson. J Fish Biol (1978) 12(6):531–40. doi: 10.1111/j.1095-8649.1978.tb04199.x
213. White BA, Henderson NE. Annual variations in the circulating levels of thyroid hormones in the brook trout, Salvelinus fontinalis, as measured by radioimmunoassay. Can J Zool (1977) 55(3):475–81. doi: 10.1139/z77-064
214. Chakraborti P, Bhattacharya S. Plasma thyroxine levels in freshwater perch: Influence of season, gonadotropins, and gonadal hormones. Gen Comp Endocrinol (1984) 53(2):179–86. doi: 10.1016/0016-6480(84)90240-5
215. Sower SA, Plisetskaya E, Gorbman A. Changes in plasma steroid and thyroid hormones and insulin during final maturation and spawning of the sea lamprey, Petromyzon marinus. Gen Comp Endocrinol (1985) 58(2):259–69. doi: 10.1016/0016-6480(85)90342-9
216. Weber GM, Okimoto DK, Richman NH, Grau EG. Patterns of thyroxine and triiodothyronine in serum and follicle–bound oocytes of the tilapia, Oreochromis mossambicus, during oogenesis. Gen Comp Endocrinol (1992) 85(3):392–404. doi: 10.1016/0016-6480(92)90084-W
217. Biddiscombe S, Idler DR. Plasma levels of thyroid hormones in sockeye salmon (Oncorhynchus nerka) decrease before spawning. Gen Comp Endocrinol (1983) 52(3):467–70. doi: 10.1016/0016-6480(83)90187-9
218. Mesa MG, Bayer JM, Bryan MB, Sower SA. Annual sex steroid and other physiological profiles of Pacific lampreys (Entosphenus tridentatus). Comp Biochem Physiol Part A: Mol Integr Physiol (2010) 155(1):56–63. doi: 10.1016/j.cbpa.2009.09.019
219. Dettlaff TA, Davydova SI. Differential sensitivity of cells of follicular epithelium and oocytes in the stellate sturgeon to unfavorable conditions, and correlating influence of triiodothyronine. Gen Comp Endocrinol (1979) 39(2):236–43. doi: 10.1016/0016-6480(79)90228-4
220. Plohman JC, Dick TA, Eales JG. Thyroid of lake sturgeon, Acipenser fulvescens: Hormone levels in blood and tissues. Gen Comp Endocrinol (2002) 125(1):47–55. doi: 10.1006/gcen.2001.7733
221. Falahatkar B. Endocrine changes during the previtellogenic stage of the great sturgeon, Huso huso (Linnaeus, 1758). J Appl Ichthyol (2015) 31(5):830–8. doi: 10.1111/jai.12813
222. Clements M. Proteolysis by the thyroid and other tissues of the male and female dogfish, Scyliorhinus (Scyllium) canicula. J Exp Zool (1957) 136(2):249–58. doi: 10.1002/jez.1401360204
223. Alimi R, Savari A, Movahedinia A, Zakeri M, Salamat N. Thyroid hormones changes in reproduction season of brownbanded bamboo shark (Chiloscyllium punctatum) from the Persian Gulf. J Vet Res (2015) 70(2):189–94.
224. Lewis M, Dodd JM. Thyroid function and the ovary in the spotted dogfish, Scyliorhinus canicula. J Endocrinol (1974) 63(2):63p.
225. Volkoff H, Wourms JP, Amesbury E, Snelson FF. Structure of the thyroid gland, serum thyroid hormones, and the reproductive cycle of the Atlantic stingray, Dasyatis sabina. J Exp Zool (1999) 284(5):505–16. doi: 10.1002/(SICI)1097-010X(19991001)284:5<505::AID-JEZ6>3.0.CO;2-1
226. Sage M. The Evolution of Thyroidal Function in Fishes. Am Zoologist (1973) 13(3):899–905. doi: 10.1093/icb/13.3.899
227. Zezza P. Tiroide, maturita sessuale e gestazione in Torpedo ocellata. Bollettino della Societa Italiano di Biol Sperimentale (1937) 12:74–6.
228. Woodhead AD. Thyroid activity in the ovo–viviparous elasmobranch Squalus acanthias. J Zool (1966) 148(2):238–75. doi: 10.1111/j.1469-7998.1966.tb02950.x
229. Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril (1993) 59(6):1187–90. doi: 10.1016/S0015-0282(16)55974-3
230. Ślebodzińska E, Ślebodziński AB, Kowalska K. Evidence for the presence of 5′–deiodinase in mammalian seminal plasma and for the increase in enzyme activity in the prepubertal testis. Int J Androl (2000) 23(4):218–24. doi: 10.1046/j.1365-2605.2000.00233.x
231. Duarte–Guterman P, Trudeau VL. Transcript profiles and triiodothyronine regulation of sex steroid– and thyroid hormone–related genes in the gonad–mesonephros complex of Silurana tropicalis. Mol Cell Endocrinol (2011) 331(1):143–9. doi: 10.1016/j.mce.2010.09.004
232. Kang H, Kenealy TM, Cohen RE. The hypothalamic–pituitary–gonadal axis and thyroid hormone regulation interact to influence seasonal breeding in green anole lizards (Anolis carolinensis). Gen Comp Endocrinol (2020) 292:113446. doi: 10.1016/j.ygcen.2020.113446
233. Ślebodziński AB. Ovarian iodide uptake and triiodothyronine generation in follicular fluid: The enigma of the thyroid ovary interaction. Domest Anim Endocrinol (2005) 29(1):97–103. doi: 10.1016/j.domaniend.2005.02.029
234. Johnson KM, Lema SC. Tissue–specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri). Gen Comp Endocrinol (2011) 172(3):505–17. doi: 10.1016/j.ygcen.2011.04.022
235. Isorna E, Vallés R, Servili A, Falcón J, Muñoz–Cueto JA. Cloning and gene expression of deiodinase enzymes and thyroid hormone receptors in the European sea bass, Dicentrarchus labrax. Cybium (2008) 32(2SUPPL.):64. doi: 10.26028/cybium/2008-322SP-025
236. Marlatt VL, Gerrie E, Wiens S, Jackson F, Moon TW, Trudeau VL. Estradiol and triiodothyronine differentially modulate reproductive and thyroidal genes in male goldfish. Fish Physiol Biochem (2012) 38(2):283–96. doi: 10.1007/s10695-011-9506-z
237. Coimbra AM, Reis–Henriques MA, Darras VM. Circulating thyroid hormone levels and iodothyronine deiodinase activities in Nile tilapia (Oreochromis niloticus) following dietary exposure to Endosulfan and Aroclor 1254. Comp Biochem Physiol Part C: Toxicol Pharmacol (2005) 141(1):8–14. doi: 10.1016/j.cca.2005.04.006
238. Hur S–P, Mahardini A, Takeuchi Y, Imamura S, Wambiji N, Rizky D, et al. Expression profiles of types 2 and 3 iodothyronine deiodinase genes in relation to vitellogenesis in a tropical damselfish, Chrysiptera cyanea. Gen Comp Endocrinol (2020) 285:113264. doi: 10.1016/j.ygcen.2019.113264
239. Sambroni E, Gutieres S, Cauty C, Guiguen Y, Breton B, Lareyre J–J. Type II iodothyronine deiodinase is preferentially expressed in rainbow trout (Oncorhynchus mykiss) liver and gonads. Mol Reprod Dev (2001) 60(3):338–50. doi: 10.1002/mrd.1096
240. Houbrechts A, Van Houcke J, Darras V. Disruption of deiodinase type 2 in zebrafish disturbs male andfemale reproduction. J Endocrinol (2019)241:111–23. doi: 10.1530/JOE-18-0549
241. Muhammad DS, Wang Y, Wang W, Jakhrani F, Qi J. Isolation and characterization of thyroid hormone receptors (TR alpha and TR beta) in black rock fish, Sebastes schlegelii. Pakistan J Zool (2012) 44:1215–23.
242. An KW, An MI, Nelson ER, Habibi HR, Choi CY. Gender–related expression of TRα and TRβ in the protandrous black porgyagrus schlegeli, during sex change processes. Acanthop Gen Comp Endocrinol (2010) 165(1):11–8. doi: 10.1016/j.ygcen.2009.05.016
243. Filby AL, Tyler CR. Cloning and characterization of cDNAs for hormones and/or receptors of growth hormone, insulin–like growth factor–I, thyroid hormone, and corticosteroid and the gender–, tissue–, and developmental–specific expression of their mRNA transcripts in fathead minnow (Pimephales promelas). Gen Comp Endocrinol (2007) 150(1):151–63. doi: 10.1016/j.ygcen.2006.07.014
244. Rocha A, Gómez A, Galay–Burgos M, Zanuy S, Sweeney GE, Carrillo M. Molecular characterization and seasonal changes in gonadal expression of a thyrotropin receptor in the European sea bass. Gen Comp Endocrinol (2007) 152(1):89–101. doi: 10.1016/j.ygcen.2007.03.001
245. Bhat IA, Rather MA, Saha R, Ganie PA, Sharma R. Identification and expression analysis of thyroid stimulating hormone receptor (TSHR) in fish gonads following LHRH treatment. Proc Natl Acad Sci India Section B: Biol Sci (2017) 87(3):719–26. doi: 10.1007/s40011-015-0640-8
246. Goto–Kazeto R, Kazeto Y, Trant JM. Molecular cloning, characterization and expression of thyroid–stimulating hormone receptor in channel catfish. Gen Comp Endocrinol (2009) 161(3):313–9. doi: 10.1016/j.ygcen.2009.01.009
247. Kumar RS, Ijiri S, Kight K, Swanson P, Dittman A, Alok D, et al. Cloning and functional expression of a thyrotropin receptor from the gonads of a vertebrate (bony fish): Potential thyroid–independent role for thyrotropin in reproduction. Mol Cell Endocrinol (2000) 167(1–2):1–9. doi: 10.1016/S0303-7207(00)00304-X
248. Hirai T, Oba Y, Nagahama Y. Fish gonadotropin receptors: Molecular characterization and expression during gametogenesis. Fish Sci (2002) 68(sup1):675–8. doi: 10.2331/fishsci.68.sup1_675
249. Swapna I, Rajasekhar M, Supriya A, Raghuveer K, Sreenivasulu G, Rasheeda MK, et al. Thiourea–induced thyroid hormone depletion impairs testicular recrudescence in the air–breathing catfish, Clarias gariepinus. Comp Biochem Physiol Part A: Mol Integr Physiol (2006) 144(1):1–10. doi: 10.1016/j.cbpa.2006.01.017
250. Parhar IS, Soga T, Sakuma Y. Thyroid hormone and estrogen regulate brain region–specific messenger ribonucleic acids encoding three gonadotropin–releasing hormone genes in sexually immature male fish, Oreochromis niloticus. Endocrinology (2000) 141(5):1618–26. doi: 10.1210/endo.141.5.7460
251. Nelson ER, Allan ERO, Pang FY, Habibi HR. Thyroid hormone and reproduction: Regulation of estrogen receptors in goldfish gonads. Mol Reprod Dev (2010) 77(9):784–94. doi: 10.1002/mrd.21219
252. Ma Y, Ladisa C, Chang JP, Habibi HR. Seasonal related multifactorial control of pituitary gonadotropin and growth hormone in female goldfish: Influences of neuropeptides and thyroid hormone. Front Endocrinol (2020) 11:175. doi: 10.3389/fendo.2020.00175
253. Rajakumar A, Senthilkumaran B. Steroidogenesis and its regulation in teleost–a review. Fish Physiol Biochem (2020) 46(3):803–18. doi: 10.1007/s10695-019-00752-0
254. Yaron Z, Levavi–Sivan B. Endocrine Regulation of Fish Reproduction. In: Farrell AP, editor. Encyclopedia of Fish Physiology. San Diego: Academic Press (2011). p. 1500–8.
255. Morais R, Nóbrega R, Gómez–González N, Schmidt R, Bogerd J, Franca L, et al. Thyroid hormone stimulates the proliferation of Sertoli cells and single type A spermatogonia in adult zebrafish (Danio rerio) testis. Endocrinology (2013) 154(11):4365–76. doi: 10.1210/en.2013-1308
256. Safian D, Morais RDVS, Bogerd J, Schulz RW. Igf binding proteins protect undifferentiated spermatogonia in the zebrafish testis against excessive differentiation. Endocrinology (2016) 157(11):4423–33. doi: 10.1210/en.2016-1315
257. Tovo–Neto A, da Silva Rodrigues M, Habibi HR, Nóbrega RH. Thyroid hormone actions on male reproductive system of teleost fish. Gen Comp Endocrinol (2018) 265:230–6. doi: 10.1016/j.ygcen.2018.04.023
258. Zhang X, Liu W, Wang J, Tian H, Wang W, Ru S. Quantitative analysis of in–vivo responses of reproductive and thyroid endpoints in male goldfish exposed to monocrotophos pesticide. Comp Biochem Physiol Part – C: Toxicol Pharmacol (2018) 211:41–7. doi: 10.1016/j.cbpc.2018.05.010
259. Timmermans LP, Chmilevsky DA, Komen H, Schipper H. Precocious onset of spermatogenesis in juvenile carp (Cyprinus carpio L., Teleostei) following treatment with low doses of L–thyroxine. Eur J Morphol (1997) 35(5):344–53. doi: 10.1076/ejom.35.5.344.13085
260. Supriya A, Raghuveer K, Swapna I, Rasheeda MK, Kobayashi T, Nagahama Y, et al. Thyroid hormone modulation of ovarian recrudescenceof air–breathing catfish Clarias gariepinus. Fish Physiol Biochem (2005) 31(2):267–. doi: 10.1007/s10695-006-0034-1
261. Rasheeda MK, Sreenivasulu G, Swapna I, Raghuveer K, Wang DS, Thangaraj K, et al. Thiourea–induced alteration in the expression patternsof some steroidogenic enzymes in the air–breathing catfish Clarias gariepinus. Fish Physiol Biochem (2005) 31(2):275. doi: 10.1007/s10695-006-0036-z
262. Guin S, Bandyopadhyay A, Jana NR, Bhattacharya S. Thyroid hormone stimulates progesterone release from the ovary of a fish, Anabas testudineus. Curr Sci (1993)64(5):327–9. doi: 10.1677/joe.0.1580319
263. Datta M, Nagendra Prasad RJ, Bhattacharya S. Thyroid hormone regulation of perch ovarian 3β–hydroxysteroid dehydrogenase/Δ5–Δ4–isomerase activity: Involvement of a 52–kDa protein. Gen Comp Endocrinol (1999) 113(2):212–20. doi: 10.1006/gcen.1998.7175
264. Cyr DG, Eales JG. In vitro effects of thyroid hormones on gonadotropin–induced estradiol–17β secretion by ovarian follicles of rainbow trout, Salmo gairdneri. Gen Comp Endocrinol (1988) 69(1):80–7. doi: 10.1016/0016-6480(88)90055-X
265. Habibi HR, Nelson ER, Allan ERO. New insights into thyroid hormone function and modulation of reproduction in goldfish. Gen Comp Endocrinol (2012) 175(1):19–26. doi: 10.1016/j.ygcen.2011.11.003
266. Olivereau M, Leloup J, De Luze A, Olivereau J. Effet de l’oestradiol sur l’axe hypophyso–thyroïdien de l’anguille. Gen Comp Endocrinol (1981) 43(3):352–63. doi: 10.1016/0016-6480(81)90295-1
267. Leatherland JF. Effects of 17β–estradiol and methyl testosterone on the activity of the thyroid gland in rainbow trout, Salmo gairdneri Richardson. Gen Comp Endocrinol (1985) 60(3):343–52. doi: 10.1016/0016-6480(85)90067-X
268. McCormick SD, O’Dea MF, Moeckel AM, Lerner DT, Björnsson BT. Endocrine disruption of parr–smolt transformation and seawater tolerance of Atlantic salmon by 4–nonylphenol and 17β–estradiol. Gen Comp Endocrinol (2005) 142(3):280–8. doi: 10.1016/j.ygcen.2005.01.015
269. Leatherland JF, Macey DJ, Hilliard RW, Leatherland A, Potter IC. Seasonal and estradiol–17β–stimulated changes in thyroid function of adult Geotria australis, a southern hemisphere lamprey. Fish Physiol Biochem (1990) 8(5):409–. doi: 10.1007/BF00003372
270. Flett PA, Leatherland JF. Dose–related effects of 17βoestradiol (E2) on liver weight, plasma E2, protein, calcium and thyroid hormone levels, and measurement of the binding of thyroid hormones to vitellogenin in rainbow trout, Salmo gairdneri Richardson. J Fish Biol (1989) 34(4):515–27. doi: 10.1111/j.1095-8649.1989.tb03332.x
271. Yamada H, Horiuchi R, Gen K, Yamauchi K. Involvement of four hormones in thyroxine deiodination in several tissues of immature yearling masu salmon, Oncorhynchus masou. Zool Sci (1993) 10(4):587–96.
272. Filby AL, Thorpe KL, Maack G, Tyler CR. Gene expression profiles revealing the mechanisms of anti–androgen– and estrogen–induced feminization in fish. Aquat Toxicol (2007) 81(2):219–31. doi: 10.1016/j.aquatox.2006.12.003
273. Leet JK, Gall HE, Sepúlveda MS. A review of studies on androgen and estrogen exposure in fish early life stages: effects on gene and hormonal control of sexual differentiation. J Appl Toxicol (2011) 31(5):379–98. doi: 10.1002/jat.1682
274. Singh TP. Maintenance of thyroid activity with some steroids in hypophysectomized and gonadectomized catfish, Mystus vittatus (Bloch). Experientia (1969) 25(4):431–. doi: 10.1007/BF01899967
275. Hunt DWC, Eales JG. The influence of testosterone propionate on thyroid function of immature rainbow trout, Salmo gairdneri Richardson. Gen Comp Endocrinol (1979) 37(1):115–21. doi: 10.1016/0016-6480(79)90053-4
276. Ikuta K, Aida K, Okumoto N, Hanyu I. Effects of thyroxine and methyltestosterone on smoltification of masu salmon (Oncorhynchus masu). Aquaculture (1985) 45:289–303. doi: 10.1016/0044-8486(85)90276-5
277. Shelbourn J, Clarke W, McBride J, Fagerlund U, Donaldson E. The use of 17α–methyltestosterone and 3,5,3′–triiodo–L–thyronine for sterilizing and accelerating the growth of zero–age coho salmon smolts (Oncorhynchus kisutch). Aquaculture (1992) 103:85–99. doi: 10.1016/0044-8486(92)90281-O
278. León A, Teh SJ, Hall LC, Teh FC. Androgen disruption of early development in Qurt strain medaka (Oryzias latipes). Aquat Toxicol (2007) 82(3):195–203. doi: 10.1016/j.aquatox.2007.02.012
279. Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev Fish Biol Fish (1996) 6(2):165–200. doi: 10.1007/BF00182342
280. Iglesias P, Bajo MA, Selgas R, Díez JJ. Thyroid dysfunction and kidney disease: An update. Rev Endocr Metab Disord (2017) 18(1):131–44. doi: 10.1007/s11154-016-9395-7
281. Evans D. A brief history of fish osmoregulation: the central role of the Mt. Desert Island Biological Laboratory. Front Physiol (2010) 1:13. doi: 10.3389/fphys.2010.00013
282. Evans DH. Osmoregulation in Fishes: An Introduction. In: Farrell AP, editor. Encyclopedia of Fish Physiology. San Diego: Academic Press (2011). p. 1348–53.
283. McCormick SD. The Hormonal Control of Osmoregulation in Teleost Fish. In: Farrell AP, editor. Encyclopedia of Fish Physiology. San Diego: Academic Press (2011). p. 1466–73.
284. McCormick SD. Endocrine control of osmoregulation in teleost fish. Vol. 41. Oxford, UK: American Zoologist (2001). p. 781–94.
285. Young G, Björnsson BT, Prunet P, Lin RJ, Bern HA. Smoltification and seawater adaptation in coho salmon (Oncorhynchus kisutch): Plasma prolactin, growth hormone, thyroid hormones, and cortisol. Gen Comp Endocrinol (1989) 74(3):335–45. doi: 10.1016/S0016-6480(89)80029-2
286. Refstie T. The effect of feeding thyroid hormones on saltwater tolerance and growth rate of Atlantic salmon. Can J Zool (1982) 60(11):2706–12. doi: 10.1139/z82-346
287. Saunders RL, McCormick SD, Henderson EB, Eales JG, Johnston CE. The effect of orally administered 3,5,3′–triiodo–L–thyronine on growth and salinity tolerance of Atlantic salmon (Salmo salar L.). Aquaculture (1985) 45(1):143–56. doi: 10.1016/0044-8486(85)90265-0
288. McCormick SD, Saunders RL. Influence of ration level and salinity on circulating thyroid hormones in juvenile Atlantic salmon (Salmo salar). Gen Comp Endocrinol (1990) 78(2):224–30. doi: 10.1016/0016-6480(90)90009-B
289. Baggerman B. Salinity Preference, Thyroid activity and the seaward migration of four species of Pacific salmon (Oncorhynchus). J Fish Res Board Canada (1960) 17(3):295–322. doi: 10.1139/f60-023
290. Shin HS, Choi YJ, Kim NN, Lee J, Ueda H, Choi CY. Effects of exogenous cortisol and seawater adaptation on thyroid hormone receptors in the smolt stage of the sockeye salmon, Oncorhynchus nerka. Ichthyol Res (2014) 61(1):9–16. doi: 10.1007/s10228-013-0365-8
291. Shrimpton JM, McCormick SD. Regulation of gill cytosolic corticosteroid receptors in juvenile Atlantic salmon: Interaction effects of growth hormone with prolactin and triiodothyronine. Gen Comp Endocrinol (1998) 112(2):262–74. doi: 10.1006/gcen.1998.7172
292. Miwa S, Inui Y. Effects of l–thyroxine and ovine growth hormone on smoltification of amago salmon (Oncorhynchus rhodurus). Gen Comp Endocrinol (1985) 58(3):436–42. doi: 10.1016/0016-6480(85)90116-9
293. Shrimpton JM, McCormick SD. Responsiveness of gill Na+/K+–ATPase to cortisol is related to gill corticosteroid receptor concentration in juvenile rainbow trout. J Exp Biol (1999) 202(8):987.
294. Leatherland JF. Effect of Ambient Salinity, Food–deprivation and prolactin on the thyroidal response to tsh, and in vitro hepatic t4 to t3 conversion in yearling coho salmon, Oncorhynchus kisutch. Acta Zool (1982) 63(1):55–64. doi: 10.1111/j.1463-6395.1982.tb00759.x
295. Leatherland JF, Flett PA. Effect of propranolol in combination with TSH and ovine prolactin on plasma thyroid hormone levels and in vitro hepatic monodeiodination of thyroxine in brook charr, Salvelinus fontinalis (Mitchill). Comp Biochem Physiol A Comp Physiol (1988) 91(2):371–6. doi: 10.1016/0300-9629(88)90433-1
296. Subash Peter MC, Lock RAC, Wendelaar Bonga SE. Evidence for an osmoregulatory role of thyroid hormones in the freshwater Mozambique tilapia Oreochromis mossambicus. Gen Comp Endocrinol (2000) 120(2):157–67. doi: 10.1006/gcen.2000.7542
297. Dangé AD. Branchial Na+–K+–ATPase activity in freshwater or saltwater acclimated tilapia, Oreochromis (Sarotherodon) mossambicus: Effects of cortisol and thyroxine. Gen Comp Endocrinol (1986) 62(2):341–3. doi: 10.1016/0016-6480(86)90125-5
298. Schreiber AM, Specker JL. Metamorphosis in the summer flounder, Paralichthys dentatus: Thyroidal status influences salinity tolerance. J Exp Zool (1999) 284:414–24. doi: 10.1002/(SICI)1097-010X(19990901)284:4<414::AID-JEZ8>3.0.CO;2-E
299. Klaren PHM, Guzmán JM, Reutelingsperger SJ, Mancera JM, Flik G. Low salinity acclimation and thyroid hormone metabolizing enzymes in gilthead seabream (Sparus auratus). Gen Comp Endocrinol (2007) 152(2–3):215–22. doi: 10.1016/j.ygcen.2007.02.010
300. Ruiz–Jarabo I, Klaren PHM, Louro B, Martos–Sitcha JA, Pinto PIS, Vargas–Chacoff L, et al. Characterization of the peripheral thyroid system of gilthead seabream acclimated to different ambient salinities. Comp Biochem Physiol –Part A Mol Integr Physiol (2017) 203:24–31. doi: 10.1016/j.cbpa.2016.08.013
301. Peyghan R, Enayati A, Sabzevarizadeh M. Effect of salinity level on TSH and thyroid hormones of grass carp, Ctenophayngodon idella. Vet Res Forum (2013) 4(3):175–8.
302. Hammerschlag N. Osmoregulation in elasmobranchs: a review for fish biologists, behaviourists and ecologists. Marine Freshwater Behav Physiol (2006) 39(3):209–28. doi: 10.1080/10236240600815820
303. de Vlaming VL, Sage M, Beitz B. Pituitary, adrenal and thyroid influences on osmoregulation in the euryhaline elasmobranch, Dasyatis sabina. Comp Biochem Physiol Part A: Physiol (1975) 52(3):505–13. doi: 10.1016/S0300-9629(75)80073-9
304. Thornburm CC, Matty AJ. The effect of thyroxine on some aspects of nitrogen metabolism in the goldfish (Carassius auratus) and the trout (Salmo trutta). Comp Biochem Physiol (1963) 8(1):1–12. doi: 10.1016/0010-406X(63)90064-1
305. Hoar WS. Effects of synthetic thyroxine and gonadal steroids on the metabolism of goldfish. Can J Zool (1958) 36(2):113–21. doi: 10.1139/z58-011
306. Rønnestad I, Gomes AS, Murashita K, Angotzi R, Jönsson E, Volkoff H. Appetite–controlling endocrine systems in teleosts. Front Endocrinol (2017) 8:73. doi: 10.3389/fendo.2017.00073
307. Klockars A, Levine AS, Olszewski PK. Hypothalamic integration of the endocrine signaling related to food intake. Curr Topics Behav Neurosci (2019) 43:239–69. doi: 10.1007/7854_2018_54
308. Volkoff H. The neuroendocrine regulation of food intake in fish: A review of current knowledge. Front Neurosci (2016) 10:1–31. doi: 10.3389/fnins.2016.00540
309. Amin A, Dhillo WS, Murphy KG. The central effects of thyroid hormones on appetite. J Thyroid Res (2011) 2011:1–7. doi: 10.4061/2011/306510
310. Kouidhi S, Clerget–Froidevaux M–S. Integrating thyroid hormone signaling in hypothalamic control of metabolism: Crosstalk between nuclear receptors. Int J Mol Sci (2018) 19(7):2017. doi: 10.3390/ijms19072017
311. Lin MT, Chu PC, Leu SY. Effects of TSH, TRH, LH and LHRH on thermoregulation and food and water intake in the rat. Neuroendocrinology (1983) 37:206–11. doi: 10.1159/000123544
312. Vijayan E, McCann SM. Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH). Endocrinology (1977) 100:1727–9. doi: 10.1210/endo-100-6-1727
313. Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Triiodothyronine (T3) stimulates food intake via enhanced hypothalamic AMP–activated kinase activity. Regul Peptides (2008) 151:164–9. doi: 10.1016/j.regpep.2008.07.007
314. Kong WM, Martin NM, Smith KL, Gardiner JV, Connoley IP, Stephens DA, et al. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology (2004) 145:5252–8. doi: 10.1210/en.2004-0545
315. Blake NG, Eckland DJA, Foster OJF, Lightman SL. Inhibition of hypothalamic thyrotropin–releasing hormone messenger ribonucleic acid during food deprivation. Endocrinology (1991) 129(5):2714–8. doi: 10.1210/endo-129-5-2714
316. Rondeel JMM, Heide R, De Greef WJ, Van Toor HV, Van Haasieren GAC, Klootwijk W, et al. Effect of starvation and subsequent refeeding on thyroid function and release of hypothalamic thyrotropin–releasing hormone. Neuroendocrinology (1992) 56(3):348–53. doi: 10.1159/000126248
317. Toni R, Jackson IM, Lechan RM. Neuropeptide–Y–immunoreactive innervation of thyrotropin–releasing hormone–synthesizing neurons in the rat hypothalamic paraventricular nucleus. Endocrinology (1990) 126(5):2444–53. doi: 10.1210/endo-126-5-2444
318. Zhang X, van den Pol AN. Thyrotropin–releasing hormone (TRH) inhibits melanin–concentrating hormone neurons: Implications for TRH–mediated anorexic and arousal actions. J Neurosci (2012) 32(9):3032 LP–43. doi: 10.1523/JNEUROSCI.5966-11.2012
319. Moslemipour F, Khazali H, Emami MA. Study of the effects of Neuropeptide Y injections on plasma concentrations of thyroxine and triiodothyronine in goat. Physiol Pharmacol (2006) 10:219–28.
320. Cote–Vélez A, Martínez Báez A, Lezama L, Uribe RM, Joseph–Bravo P, Charli J–L. A screen for modulators reveals that orexin–A rapidly stimulates thyrotropin releasing hormone expression and release in hypothalamic cell culture. Neuropeptides (2017) 62:11–20. doi: 10.1016/j.npep.2017.01.005
321. Mitsuma T, Hirooka Y, Mori Y, Kayama M, Adachi K, Rhue N, et al. Effects of orexin A on thyrotropin–releasing hormone and thyrotropin secretion in rats. Hormone Metab Res (1999) 31(11):606–9. doi: 10.1055/s-2007-978805
322. Hollenberg AN. The role of the thyrotropin–releasing hormone (TRH) neuron as a metabolic sensor. Thyroid (2008) 18(2):131–9. doi: 10.1089/thy.2007.0251
323. Sternson SM, Shepherd GMG, Friedman JM. Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci (2005) 8(10):1356–63. doi: 10.1038/nn1550
324. Decherf S, Seugnet I, Kouidhi S, Lopez–Juarez A, Clerget–Froidevaux M–S, Demeneix BA. Thyroid hormone exerts negative feedback on hypothalamic type 4 melanocortin receptor expression. Proc Natl Acad Sci (2010) 107(9):4471 LP–6. doi: 10.1073/pnas.0905190107
325. Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Hypothalamic neuropeptide y/y1 receptor pathway activated by a reduction in circulating leptin, but not by an increase in circulating ghrelin, contributes to hyperphagia associated with triiodothyronine–induced thyrotoxicosis. Neuroendocrinology (2003) 78(6):321–30. doi: 10.1159/000074885
326. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB–R. Cell (1995) 83(7):1263–71. doi: 10.1016/0092-8674(95)90151-5
327. Fekete C, Légrádi G, Mihály E, Huang Q–H, Tatro JB, Rand WM, et al. α–melanocyte–stimulating hormone is contained in nerve terminals innervating thyrotropin–releasing hormone–synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting–induced suppression of prothyrotropin–releasing hormone gene expression. J Neurosci (2000) 20(4):1550 LP–8. doi: 10.1523/JNEUROSCI.20-04-01550.2000
328. Kristensen K, Pedersen SB, Langdahl BL, Richelsen B. Regulation of leptin by thyroid hormone in humans: Studies in vivo and in vitro. Metabolism (1999) 48(12):1603–7. doi: 10.1016/S0026-0495(99)90252-4
329. Sreenan S, Caro JF, Refetoff S. Thyroid dysfunction is not associated with alterations in serum leptin levels. Thyroid (1997) 7(3):407–9. doi: 10.1089/thy.1997.7.407
330. Gross WL, Fromm PO, Roelofs EW. Relationship between thyroid and growth in green sunfish,Lepomis cyanellus (Rafinesque). Trans Am Fish Soc(1963) 92:401–8. doi: 10.1577/1548-8659(1963)92[401:RBTAGI]2.0.CO;2
331. Li D, Liu Z, Xie C. Effect of stocking density on growth and serum concentrations of thyroid hormones and cortisol in Amur sturgeon, Acipenser schrenckii. Fish Physiol Biochem (2012) 38(2):511–20. doi: 10.1007/s10695-011-9531-y
332. Pavlov ED, Zvezdin AO, Pavlov DS. Effect of thiourea on migratory activity of climbing perch Anabas testudineus and food consumption. J Ichthyol (2019) 59(5):810–4. doi: 10.1134/S0032945219050126
333. Milne RS, Leatherland JF, Holub BJ. Changes in plasma thyroxine, triiodothyronine and cortisol associated with starvation in rainbow trout (Salmo gairdneri). Environ Biol Fish (1979) 4:185–90. doi: 10.1007/BF00005452
334. Buckley C, MacDonald EE, Tuziak SM, Volkoff H. Molecular cloning and characterization of two putative appetiteregulators in winter flounder (Pleuronectes americanus): Preprothyrotropin–releasing hormone (TRH) and preproorexin (OX). Peptides (2010) 31(9):1737–47. doi: 10.1016/j.peptides.2010.05.017
335. Huising MO, Geven EJW, Kruiswijk CP, Nabuurs SB, Stolte EH, Spanings FAT, et al. Increased leptin expression in common carp (Cyprinuscarpio) after food intake but not after fasting or feeding to satiation. Endocrinology (2006) 147(12):5786–97. doi: 10.1210/en.2006-0824
336. Abbott M, Volkoff H. Thyrotropin Releasing Hormone (TRH) in goldfish (Carassiusauratus): Role in the regulation of feeding and locomotor behaviors and interactions with the orexin system and cocaine– and amphetamine regulated transcript (CART). Hormones Behav (2011) 59(2):236–45. doi: 10.1016/j.yhbeh.2010.12.008
337. Lu R–H, Zhou Y, Yuan X–C, Liang X–F, Fang L, Bai X–L, et al. Effects of glucose, insulin and triiodothyroxine on leptin andleptin receptor expression and the effects of leptin on activities of enzymes related to glucose metabolism in grass carp (Ctenopharyngodon idella) hepatocytes. Fish Physiol Biochem (2015) 41(4):981–9. doi: 10.1007/s10695-015-0063-8
338. Liu Y–Y, Brent GA. Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation. Trends Endocrinol Metab (2010) 21(3):166–73. doi: 10.1016/j.tem.2009.11.004
339. Oppenheimer JH, Schwartz HL, Lane JT, Thompson MP. Functional relationship of thyroid hormone–induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Invest (1991) 87(1):125–32. doi: 10.1172/JCI114961
340. Hapon MB, Varas SM, Jahn GA, Giménez MS. Effects of hypothyroidism on mammary and liver lipid metabolism in virgin and late–pregnant rats. J Lipid Res (2005) 46(6):1320–30. doi: 10.1194/jlr.M400325-JLR200
341. Souza LL, Nunes MO, Paula GSM, Cordeiro A, Penha–Pinto V, Neto JFN, et al. Effects of dietary fish oil on thyroid hormone signaling in the liver. J Nutr Biochem (2010) 21(10):935–40. doi: 10.1016/j.jnutbio.2009.07.008
342. Kucukkurt I, Dundar Y. Effects of dietary Yucca schidigera supplementation on plasma leptin, insulin, iodated thyroid hormones and some biochemical parameters in rats. Rev Méd Vétérinaire (2013) 164(7):362–7.
343. Plisetskaya E, Woo NYS, Murat J–C. Thyroid hormones in cyclostomes and fish and their role in regulation of intermediary metabolism. Comp Biochem Physiol Part A: Physiol (1983) 74(2):179–87. doi: 10.1016/0300-9629(83)90586-8
344. Sheridan MA. Effects of thyroxin, cortisol, growth hormone, and prolactin on lipid metabolism of coho salmon, Oncorhynchus kisutch, during smoltification. Gen Comp Endocrinol (1986) 64(2):220–38. doi: 10.1016/0016-6480(86)90007-9
345. Abdollahpour H, Falahatkar B, Efatpanah I, Meknatkhah B, Van Der Kraak G. Hormonal and physiological changes in Sterlet sturgeon Acipenser ruthenus treated with thyroxine. Aquaculture (2019) 507:293–300. doi: 10.1016/j.aquaculture.2019.03.063
346. Degani G, Dosoretz C. The effect of 3,3′,5–triiodo–L–thyronine and 17–α–methyltestosterone on growth and body composition of the glass stage of the eel (Anguilla anguilla L). Fish Physiol Biochem (1986) 1(3):145–51. doi: 10.1007/BF02290255
347. Tripathi G, Verma P. Differential effects of thyroxine on metabolic enzymes and other macromolecules in a freshwater teleost. J Exp Zool Part A: Comp Exp Biol (2003) 296A(2):117–24. doi: 10.1002/jez.a.10218
348. Woo NYS, Chung ASB, Ng TB. Influence of oral administration of 3,5,3′–triiodo–thyronine on growth, digestion, food conversion and metabolism in the underyearling red sea bream, Chrysophrys major (Temminck & Schlegel). J Fish Biol (1991) 39(4):459–68. doi: 10.1111/j.1095-8649.1991.tb04378.x
349. Vargas–Chacoff L, Ruiz–Jarabo I, Arjona FJ, Laiz–Carrión R, Flik G, Klaren PHM, et al. Energy metabolism of hyperthyroid gilthead sea bream Sparus aurata L. Comp Biochem Physiol Part A: Mol Integr Physiol (2016) 191:25–34. doi: 10.1016/j.cbpa.2015.09.014
350. Matty AJ, Lone KP. The Hormonal Control of Metabolism and Feeding. In: Tytler P, Calow P, editors. Fish Energetics: New Perspectives. Dordrecht: Springer Netherlands (1985). p. 185–209.
351. Leung LY, Woo NYS. Effects of growth hormone, insulin–like growth factor I, triiodothyronine, thyroxine, and cortisol on gene expression of carbohydrate metabolic enzymes in sea bream hepatocytes. Comp Biochem Physiol Part A: Mol Integr Physiol (2010) 157(3):272–82. doi: 10.1016/j.cbpa.2010.07.010
352. Eales JG, MacLatchy DL, Higgs DA, Dosanjh BS. The influence of dietary protein and caloric content on thyroid function and hepatic thyroxine 5′–monodeiodinase activity in rainbow trout. Oncorhynchus mykiss Can J Zool (1992) 70(8):1526–35. doi: 10.1139/z92-210
353. Higgs DA, Fagerlund UHM, McBride JR, Eales JG. Influence of orally administered L –thyroxine or 3,5,3′–triiodo– L –thyronine on growth, food consumption, and food conversion of underyearling coho salmon (Oncorhynchus kisutch). Can J Zool (1979) 57:1974–9. doi: 10.1139/z79-261
354. Leatherland JF, Cho Y, Hilton J. Effect of diet on serum thyroid hormone levels in rainbow trout (Salmo gairdneri Richardson). Comp Biochem Physiol Part A: Physiol (1984) 78(3):601–5. doi: 10.1016/0300-9629(84)90604-2
355. Higgs DA, Fagerlund UHM, Eales JG, McBride JR. Application of thyroid and steroid hormones as anabolic agents in fish culture. Comp Biochem Physiol Part B: Comp Biochem (1982) 73(1):143–76. doi: 10.1016/0305-0491(82)90206-1
356. Sadler J, Pankhurst PM, King HR. High prevalence of skeletal deformity and reduced gill surface area in triploid Atlantic salmon (Salmo salar L.). Aquaculture (2001) 198(3):369–86. doi: 10.1016/S0044-8486(01)00508-7
357. Opstad I, Fjelldal PG, Karlsen Ø, Thorsen A, Hansen TJ, Taranger GL. The effect of triploidization of Atlantic cod (Gadus morhua L.) on survival, growth and deformities during early life stages. Aquaculture (2013), 388–391:54–9. doi: 10.1016/j.aquaculture.2013.01.015
358. Yamano K. The role of thyroid hormone in fish development with reference to aquaculture. Japan Agric Res Quarterly: JARQ (2005) 39(3):161–8. doi: 10.6090/jarq.39.161
359. Nayak PK, Mishra T, Mishra J, Pandey AK. Effect of combined thyroxine and cortisol treatment on hatching of eggs, post–embryonic growth and survival of larvae of Heteropneustes fossilis. J Indian Fish Assoc (2004)31:125–37. 2004,31:125–37.
360. Nayak PK, Mahapatra CT, Mishra J, Mishra TK. Effect of treatment of eggs with thyroxin and cortisol on larval morphometry and survival in the freshwater carp, Catla catla. Indian J Fish (2000) 47(4):337–42.
361. Brown CL, Kim BG. Combined application of cortisol and triiodothyronine in the culture of larval marine finfish. Aquaculture (1995) 135(1):79–86. doi: 10.1016/0044-8486(95)01016-5
362. Barrington EJW, Barron N, Piggins DJ. The influence of thyroid powder and thyroxine upon the growth of rainbow trout (Salmo gairdnerii). Gen Comp Endocrinol (1961) 1(2):170–8. doi: 10.1016/0016-6480(61)90045-4
363. Lam TJ, Juario JV, Banno J. Effect of thyroxine on growth and development in post–yolk–sac larvae of milkfish, Chanos chanos. Aquaculture (1985) 46(3):179–84. doi: 10.1016/0044-8486(85)90203-0
364. Dales S, Hoar WS. Effects of thyroxine and thiourea on the early development of chum salmon (Oncorhynchus keta). Can J Zool (1954) 32(3):244–51. doi: 10.1139/z54-024
365. Parra MAL. Efeito da triiodotironina (T3) no desenvolvimento embrionário e no desempenho das larvas de pintado (Pseudoplatystoma coruscans), piracanjuba (Brycon orbignyanus) e dourado (Salminus maxillosus). Universidade Estadual Paulista, Centro de Aquicultura (2003).
366. Roche G, Leblond CP. Effect of thyroid preparation and iodide on salmonidae. Endocrinology (1952) 51(6):524–45. doi: 10.1210/endo-51-6-524
367. Nacario JF. The effect of thyroxine on the larvae and fry of Sarotherodon niloticus L. (Tilapia nilotica). Aquaculture (1983) 34:73–83. doi: 10.1016/0044-8486(83)90292-2
368. Yoo JH, Takeuchi T, Tagawa M, Seikai T. Effect of thyroid hormones on the stage–specific pigmentation of the Japanese flounder Paralichthys olivaceus. Zool Sci (2000) 17(8):1101–6. doi: 10.2108/zsj.17.1101
369. Zohar Y. Endocrinology and fish farming: Aspects in reproduction, growth, and smoltification. Fish Physiol Biochem (1989) 7(1):395–405. doi: 10.1007/BF00004734
370. Prunet P, Boeuf G, Bolton JP, Young G. Smoltification and seawater adaptation in Atlantic salmon (Salmo salar): Plasma prolactin, growth hormone, and thyroid hormones. Gen Comp Endocrinol (1989) 74(3):355–64. doi: 10.1016/S0016-6480(89)80031-0
371. Wagner HH. Photoperiod and temperature regulation of smolting in steelhead trout (Salmo gairdneri). Can J Zool (1974) 52(2):219–34. doi: 10.1139/z74-026
372. Zydlewski G, Haro A, McCormick S. Evidence for cumulative temperature as an initiating and terminating factor in downstream migratory behavior of Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci (2005) 62:68–78. doi: 10.1139/f04-179
373. Langdon JS. Smoltification Physiology in the Culture of Salmonids. In: Muir JF, Roberts RJ, editors. Recent Advances in Aquaculture: Volume 2. Boston, MA: Springer US (1985). p. 79–118.
374. Jørgensen EH, Jobling M. Feeding and growth of exercised and unexercised juvenile Atlantic salmon in freshwater, and performance after transfer to seawater. Aquaculture Int (1994) 2(3):154–64. doi: 10.1007/BF00231512
375. Carr JW, Whoriskey FG, Courtemanche DA eds. Aquatic telemetry: advances andapplications: Landlocked Atlantic salmon: movements to sea by a putative freshwater life history form. Proceedings of the Fifth Conference on Fish Telemetry, Ustica, Italy. Rome, Italy: Food and Agriculture Organization of the United Nations (2003).
376. Tilson MB. Thyroid–induced chemical imprinting in early life stages andassessment of smoltification in kokanee salmon: Implications for operating lake roosevelt kokaneesalmon hatcheries. Portland, OR: U.S. Department of Energy, Bonneville Power Administration, Division of Fish & Wildlife (1994).
377. Usher ML, Talbot C, Eddy FB. Effects of transfer to seawater on growth and feeding in Atlantic salmon smolts (Salmo salar L.). Aquaculture (1991) 94(4):309–26. doi: 10.1016/0044-8486(91)90176-8
378. Morin PP, Hara TJ, Eales JG. T4 depresses olfactory responses to L–alanine and plasma T3 and T3 production in smoltifying Atlantic salmon. Am J Physiol Regul Integr Comp Physiol (1995) 269(6):R1434–R40. doi: 10.1152/ajpregu.1995.269.6.R1434
379. Zhang Y. Husbandry and dietary effects on sturgeon (Acipenser transmontanus) farmedfor caviar. Ann Arbor, MI: California Sea Grant College Program (2011).
380. Tachihara K, El–Zibdeh MK, Ishimatsu A, Tagawa M. Improved seed production of goldstriped amberjack Seriola lalandi under hatchery conditions by injection of triiodothyronine (T3) to broodstock fish. J World Aquaculture Soc (1997) 28(1):34–44. doi: 10.1111/j.1749-7345.1997.tb00959.x
381. El–Zibdeh MK, Tachihara K, Tsukashima Y, Tagawa M, Ishimatsu A. Effect of triiodothyronine injection of broodstock fish on seedproduction in cultured seawater fish. Aquaculture Sci (1996) 44(4):487–96. doi: 10.11233/aquaculturesci1953.44.487
382. Brown CL, Doroshov SI, Cochran MD, Bern HA. Enhanced survival in striped bass fingerlings after maternal triiodothyronine treatment. Fish Physiol Biochem (1989) 7(1):295–9. doi: 10.1007/BF00004720
383. Soyano K, Saito T, Nagae M, Yamauchi K. Effects of thyroid hormone on gonadotropin–induced steroid production in medaka, Oryzias latipes, ovarian follicles. Fish Physiol Biochem (1993) 11(1):265–72. doi: 10.1007/BF00004574
384. Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri EA, et al. Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci (2018) 19(5):1501–. doi: 10.3390/ijms19051501
385. Leemans M, Couderq S, Demeneix B, Fini J–B. Pesticides with potential thyroid hormone–disrupting effects: A review of recent data. Front Endocrinol (2019) 10:743–. doi: 10.3389/fendo.2019.00743
386. Turyk ME, Anderson HA, Persky VW. Relationships of thyroid hormones with polychlorinated biphenyls, dioxins, furans, and DDE in adults. Environ Health Perspect (2007) 115(8):1197–203. doi: 10.1289/ehp.10179
387. Nugegoda D, Kibria G. Effects of environmental chemicals on fish thyroid function: Implications for fisheries and aquaculture in Australia. Gen Comp Endocrinol (2017) 244:40–53. doi: 10.1016/j.ygcen.2016.02.021
388. Cuesta A, Meseguer J, Esteban MÁ. Immunotoxicological effects of environmental contaminants in teleost fishreared for aquaculture. Pesticides in the Modern World–Risks and Benefits. IntechOpen (2011). p. 241–66.
389. Ricard AC, Daniel C, Anderson P, Hontela A. Effects of subchronic exposure to cadmium chloride on endocrine and metabolic functions in rainbow trout Oncorhynchus mykiss. Arch Environ Contamination Toxicol (1998) 34(4):377–81. doi: 10.1007/s002449900333
390. Teles M, Pacheco M, Santos MA. Physiological and genetic responses of European eel (Anguilla anguilla L.) to short–term chromium or copper exposure—Influence of preexposure to a PAH–like compound. Environ Toxicol (2005) 20(1):92–9. doi: 10.1002/tox.20082
391. Mishra AK, Mohanty B. Effect of acute hexavalent chromium exposure onpituitary–thyroid axis of a freshwater fish, Channa punctatus (Bloch). Environ Toxicol (2015) 30(6):621–7. doi: 10.1002/tox.21939
392. Bhattacharya T, Bhattacharya S, Ray AK, Dey S. Influence of industrial pollutants on thyroid function in Channa punctatus (Bloch). Indian J Exp Biol (1989) 27(1):65–8.
393. Hedayati A, Zare P, Abarghouei S. Effect of environmental mercury on some hormonal parameters of the main mariculture fish of Persian Gulf. Global Veterinaria (2012) 8(1):43–50.
394. Dey C, Saha SK. A comparative study on the acute toxicity bioassay of dimethoate andlambda–cyhalothrin and effects on thyroid hormones of freshwater teleost fish Labeo rohita (Hamilton). Int J Environ Res (2014) 8(4):1085–92. doi: 10.22059/IJER.2014.802
395. Khatun N, Mahanta R. A study on the effect of chlorpyrifos (20% EC) on thyroid hormonesin freshwater fish, Heteropneustes fossilis (Bloch.) by using EIAtechnique. Sci Probe (2014) 2(2):8–16.
396. Besson M, Feeney WE, Moniz I, François L, Brooker RM, Holzer G, et al. Anthropogenic stressors impact fish sensory development and survival via thyroid disruption. Nat Commun (2020) 11(1):3614. doi: 10.1038/s41467-020-17450-8
397. Zhang X, Tian H, Wang W, Ru S. Exposure to monocrotophos pesticide causes disruption of the hypothalamic–pituitary–thyroid axis in adult male goldfish (Carassius auratus). Gen Comp Endocrinol (2013) 193:158–66. doi: 10.1016/j.ygcen.2013.08.003
398. Ortiz–Delgado JB, Funes V, Sarasquete C. The organophosphate pesticide –OP– malathion inducing thyroidal disruptions and failures in the metamorphosis of the Senegalese sole, Solea senegalensis. BMC Vet Res (2019) 15(1):57–. doi: 10.1186/s12917-019-1786-z
399. Schnitzler JG, Klaren PHM, Bouquegneau J–M, Das K. Environmental factors affecting thyroid function of wild sea bass (Dicentrarchus labrax) from European coasts. Chemosphere (2012) 87(9):1009–17. doi: 10.1016/j.chemosphere.2011.11.039
400. Leatherland JF, Sonstegard RA. Lowering of serum thyroxine and triiodothyronine levels in yearling coho salmon, Oncorhynchus kisutch, by Dietary Mirex and PCBs. J Fish Res Board Canada (1978) 35(10):1285–9. doi: 10.1139/f78-202
401. Leatherland JF, Sonstegard RA. Effect of dietary polychlorinated biphenyls (PCBs) or mirex in combination with food deprivation and testosterone administration on serum thyroid hormone concentration and bioaccumulation of organochlorines in rainbow trout, Salmo gairdneri. J Fish Dis (1980) 3:115–24. doi: 10.1111/j.1365-2761.1980.tb00194.x
402. Besselink HT, van Beusekom S, Roex E, Vethaak AD, Koeman JH, Brouwer A. Low hepatic 7–ethoxyresorufin–O–deethylase (EROD) activity and minor alterations in retinoid and thyroid hormone levels in flounder (Platichthys flesus) exposed to the polychlorinated biphenyl (PCB) mixture, Clophen A50. Environ Pollut (1996) 92(3):267–74. doi: 10.1016/0269-7491(95)00116-6
403. Volkoff H. Feeding and its regulation. In: Woo PTK, lwama GK, editors. Climate Change and Non–infectious Fish Disorders. Oxfordshire, United Kingdom: CABI (Centre for Agriculture and Biosciences International (2020).
404. Barange M, Bahri T, Beveridge MCM, Cochrane KL, Funge–Smith S, Poulain F. Impacts of climate change on fisheries and aquaculture: synthesis ofcurrrent knowledge, adaptation and mitigation options. Rome, ITL: FAO (2018).
405. Little AG, Loughland I, Seebacher F. What do warming waters mean for fish physiology and fisheries? J Fish Biol (2020) 97(2):328–40. doi: 10.1111/jfb.14402
406. Le Roy A, Seebacher F. Mismatched light and temperature cues disrupt locomotion andenergetics via thyroid–dependent mechanisms. Conserv Physiol(2020) 8(1):1–8. doi: 10.1093/conphys/coaa051
407. Fenkes M, Shiels HA, Fitzpatrick JL, Nudds RL. The potential impacts of migratory difficulty, including warmer waters and altered flow conditions, on the reproductive success of salmonid fishes. Comp Biochem Physiol Part A: Mol Integr Physiol (2016) 193:11–21. doi: 10.1016/j.cbpa.2015.11.012
408. Lee S, Ji K, Choi K. Effects of water temperature on perchlorate toxicity to the thyroid and reproductive system of Oryzias latipes. Ecotoxicol Environ Safety (2014) 108:311–7. doi: 10.1016/j.ecoenv.2014.07.016
409. Cyr DG, Idler DR, Audet C, McLeese JM, Eales JG. Effects of long–term temperature acclimation on thyroid hormone deiodinase function, plasma thyroid hormone levels, growth, and reproductive status of male Atlantic cod. Gadus morhua Gen Comp Endocrinol (1998) 109(1):24–36. doi: 10.1006/gcen.1997.6994
410. Subhash Peter MC, Rejitha V. Interactive effects of ambient acidity and salinity on thyroid function during acidic and post–acidic acclimation of air–breathing fish (Anabas testudineus Bloch). Gen Comp Endocrinol (2011) 174(2):175–83. doi: 10.1016/j.ygcen.2011.08.018
411. Brown JA, Edwards D, Whitehead C. Cortisol and thyroid hormone responses to acid stress in the brown trout, Salmo trutta L. J Fish Biol (1989) 35(1):73–84. doi: 10.1111/j.1095-8649.1989.tb03394.x
412. Brown SB, Evans RE, Majewski HS, Sangalang GB, Klaverkamp JF. Responses of plasma electrolytes, thyroid hormones, and gill histology in Atlantic salmon (Salmo salar) to acid and limed river waters. Can J Fish Aquat Sci (1990) 47(12):2431–40. doi: 10.1139/f90-271
413. Senese R, Cioffi F, de Lange P, Goglia F, Lanni A. Thyroid: biological actions of ‘nonclassical’ thyroid hormones. J Endocrinol (2014) 221(2):R1–12. doi: 10.1530/JOE-13-0573
Keywords: fish, thyroid, hormones, reproduction, osmoregulation, feeding, metabolism
Citation: Deal CK and Volkoff H (2020) The Role of the Thyroid Axis in Fish. Front. Endocrinol. 11:596585. doi: 10.3389/fendo.2020.596585
Received: 19 August 2020; Accepted: 13 October 2020;
Published: 06 November 2020.
Edited by:
Gustavo M. Somoza, CONICET Instituto Tecnológico de Chascomús (INTECH), ArgentinaReviewed by:
Marco António Campinho, University of Algarve, PortugalTakashi Hasebe, Nippon Medical School, Japan
Copyright © 2020 Deal and Volkoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helene Volkoff, aHZvbGtvZmZAbXVuLmNh
†ORCID: Cole Deal, orcid.org/0000-0003-1677-2729
Helene Volkoff, orcid.org/0000-0003-1344-3428
 Cole K. Deal
Cole K. Deal Helene Volkoff
Helene Volkoff