- 1Mental Health Centre Copenhagen, Copenhagen Research Center for Mental Health (CORE) Research Unit, Copenhagen University Hospital, Hellerup, Denmark
- 2Department of First Episode Psychosis, Psychiatric Centre, Glostrup, Denmark
- 3Department of Endocrinology and Metabolism, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
Background and Aims: Weight gain is a major adverse effect of antipsychotic medication, negatively affecting physical and mental well-being. The objective of this study was to explore if dose reduction, discontinuation, switch to a partial agonist, or switch from polypharmacy to monotherapy will lead to weight loss.
Methods: Controlled and uncontrolled studies reporting the effects of discontinuation, dose reduction, switch to a partial agonist, or switch from polypharmacy to monotherapy on weight were included. Primary outcome was difference in weight compared to maintenance groups based on controlled studies. Secondary outcome was change in weight from initiation of one of the included interventions until follow-up in a pre-post analysis.
Results: We identified 40 randomized controlled trials and 15 uncontrolled studies including 12,279 individuals. The effect of the interventions, i.e. dose reduction, drug discontinuation, or switch to a partial agonis, reduced the weight with 1.5 kg (95% CI −2.03 to −0.98; P < 0.001) compared to maintenance treatment. The weight change from pre to post was a reduction of 1.13 kg (95% CI −1.36 to −0.90; P < 0.001).
Conclusion: We found a significant but small reduction in weight, suggesting that antipsychotic-induced weight gain can be reversed to some degree. Only a few studies were designed to address the question as primary outcome, which limits the generalizability of our findings.
Introduction
Shared decision making is the cornerstone of modern, evidence-based medicine and requires transparency about risks and benefits associated with any treatment decisions. Second generation antipsychotic medication is the mainstay of treatment of psychotic disorders (1) and is increasingly prescribed for other indications like bipolar disorder and severe depression and off-label indications such as sleeplessness and anxiety (2, 3). Weight gain is a major adverse effect of second-generation antipsychotics, affecting quality of life (4), personal recovery, and somatic morbidity and is a common reason for antipsychotic discontinuation (5). Therefore, knowledge about the reversibility of antipsychotic weight gain is important to qualify the informed decision of both initiation and discontinuation.
Shared decision-making refers to a process where expert knowledge, data from the literature, and the patient’s personal values and preferences are integrated in a final decision. Information about the risk of weight gain during treatment is important, and it is well documented in the scientific literature (6). However, knowledge about the reversibility of weight gain when the antipsychotic medication is reduced or discontinued remains scarce. To fully inform decisions to start or to stop medication, information about reversibility is crucial. Many may expect adverse effects to be reversible upon discontinuation, but this is not always the case with antipsychotic medication, as exemplified by tardive dyskinesia (7). Non-pharmacological interventions to reduce weight in a general population have shown only transient effect on obesity (8). While decades have passed with different trials of behavioral interventions, it is increasingly realized that temporary lost weight is normally rapidly regained, and obesity is now defined as: “chronic, relapsing, multi-factorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.” (https://obesitymedicine.org/obesity-algorithm/).
If antipsychotic-induced obesity is an irreversible adverse effect, it has consequences for the shared decision making and potentially also for the off-label prescribing. If there is a risk of developing life long, severe obesity, some might find the risk–benefit balance to tip against medication. On the other hand, if the patient considers discontinuing antipsychotic medication to lose weight, it is important to know if this is likely to happen, to balance against the risk of relapse.
None of the second-generation antipsychotics are weight neutral, but some are more obesogenic than others, with olanzapine and clozapine having the highest obesogenic properties. Substantial effort has been put into understanding the molecular mechanisms underlying the obesogenic properties of antipsychotic medication, without clear answers (9). While the dopamine blockade produces the antipsychotic effect, several other neurotransmitters like the muscarinic, serotonergic, and histaminergic systems account for the metabolic effects, but no definite explanations have been established (10). Recently, intense focus has been put on the gut microbiota, which is linked to antipsychotic medication, obesity, and even to clinical variables of schizophrenia (11), suggesting that the effect of antipsychotic drugs is mediated via an effect on the gut microbiome (12) and then affecting psychotic symptoms and obesity.
Peculiarly, little research has been focusing on the potential reversibility of weight gain if the antipsychotic drug is reduced, discontinued, or switched to an agent with lower obesogenic properties, but case reports (13, 14) and a few studies of patients with intellectual disabilities and children with bipolar disorder (15) suggest that at least some weight can be lost after discontinuation.
How the Interventions May Work
Reducing or discontinuing second generation antipsychotic medication may cause weight loss due to negative energy balance or directly through affecting various neurotransmitters and neuroendocrine signaling. Negative energy balance could be achieved by decreased appetite and by increased physical activity due to lower level of sedation. The effect is likely mediated via neurotransmitter signaling involving histamine and serotonin (16), and some suggest that thyroid function is associated with antipsychotic medication (17). Similarly, switch to partial agonists may affect weight through lower affinity to histamine receptors and lower level of sedation.
Objective
The aim of this systematic review was to report the effect of dose reduction/discontinuation of second-generation antipsychotic, switch to a partial dopamine agonist, or switch from polypharmacy to monotherapy on weight in individuals diagnosed with severe mental illness. We hypothesized that any of the four interventions would lead to a reduction in weight, in relative values when compared to maintenance treatment and in absolute values when compared to weight before interventions.
Method
Eligibility
Inclusion Criteria
1. Patients diagnosed with major depression, schizophrenia, psychosis, bipolar disorder or schizoaffective psychosis, aged above 17 years old.
2. Interventions evaluating the effect of the following interventions were included:
a. Dose reduction, defined as interventions where dose was gradually reduced until completely stopped, or a smaller dose was kept due to re-emergence of symptoms.
b. Switch from antipsychotic polypharmacy to monotherapy, defined as any intervention where all participants were abruptly or gradually switched from two antipsychotic medications at any dose, where at least one was a second-generation antipsychotic to one antipsychotic drug.
c. Discontinuation, defined as interventions where all participants where gradually or abruptly discontinued.
d. Switch from any second generation antipsychotic to a partial dopamine agonist: aripiprazole, brexpiprazole, or cariprazine (18).
3. A minimum of four weeks exposure to a second-generation antipsychotic prior to discontinuation.
4. Control condition was defined as maintenance treatment with antipsychotic medication.
5. Outcomes for weight should be reported in kg or lbs. as endpoint or change scores.
6. Clinical studies on humans, including cohort studies, non-randomized controlled trials, and randomized controlled trials reporting either between groups at end point or pre- post change scores.
Exclusion Criteria
1. Interventions evaluating the effect of intermittent treatment.
2. Studies where only clinically significant weight change was reported as dichotomous outcome.
Information Sources
The bibliographical search was performed on 18th of February 2021 and included a search of PUBMED, Scopus, Lilac, Embase, and Web of Science using medical subject headings (MESH or similar) when possible and text word terms: (Major depression OR schizophrenia OR bipolar Or schizoaffective OR psychosis OR severe mental disorder) AND (deprescription OR deprescribing OR discontinuation OR dose reduction OR cessation OR reduction OR switching OR tapering OR polypharmacy OR dose reduction OR reducing medication OR ceasing medication OR switch to monotherapy OR monotherapy OR withdrawal OR switch to aripiprazole OR relapse prevention OR maintenance) AND (second generation antipsychotic OR antipsychotic OR neuroleptic OR olanzapine OR clozapine OR aripiprazole OR amisulpride OR zotepine OR second generation antipsychotic OR quetiapine OR paliperidone OR lurasidone OR risperidone OR ziprasidone OR sertindole OR brexpiprazole OR cariprazine OR asenapine OR iloperidone).
Study Selection
Two authors (HS, CW) independently examined the remaining full list, selected relevant abstracts and examined the relevant full text determining compliance with inclusion criteria using the software from Covidence.
Data Extraction
Two authors (HS, CW) independently extracted data using Covidence software. The authors were not blinded to study results, authors, or institutions. Data extraction included data on weight and additional information regarding age, gender, number of patients, duration of treatment, type of treatment, baseline weight, type of discontinuation and duration of follow up. HS and CW independently conducted risk of bias applying Rob-2 tool (19).
Outcomes
Primary outcome was defined as mean difference in weight between any of the methods of: (discontinuation, dose reduction, switch to partial agonist, switch to monotherapy) as described in the Introduction, compared to maintenance treatment measured on a continuous scale in kg. Results for each of the four interventions will be reported both pooled and separately. Secondary outcome was pre-post change in weight from initiation of intervention to end of follow-up measured on a continuous scale in kg reported pooled and separately for each of the four groups. This analysis included non-controlled intervention studies pooled with the intervention groups from the controlled trials. Type of intervention, diagnoses and difference in duration of exposure in active versus placebo medication was tested as potential moderators of effect.
Data Synthesis
In order to include a maximum of studies, we combined end-scores and change scores for primary outcomes. Thereby, we abstain from calculating standardized measures, as the combination of dispersion of end- and change scores cannot be combined in a standardized effect size (20). If change scores as well as end scores were reported, end-scores were preferred. All results were reported with 95% confidence intervals and 95% prediction intervals (21). Missing measures of dispersion were imputed as recommended by Cochrane (20). Random effects were reported, assuming underlying heterogeneity of effects due to variations in the interventions. The degree of heterogeneity was quantified using the I2 statistic, which can be interpreted as the percentage of variation observed between the trials attributable to between-trial differences, rather than sampling error (chance). Heterogeneity was explored by analyses of subgroups and meta-regressions. Results from randomized clinical trials and uncontrolled studies were analyzed and reported separately. Results from RCTs were pooled with pre-post studies if data for these were available.
Data was analyzed using Comprehensive Meta-Analysis v. 3.11; p-values <0.05 were considered significant. Prisma reporting guidelines were followed (22).
Deviations From the Protocol
1) We changed the switch to partial agonist from only including aripiprazole to also including brexpiprazole and cariprazine. 2) We changed one moderator from study duration to difference in exposure to drug in active versus placebo drug. 3) An improved search strategy was applied.
Results
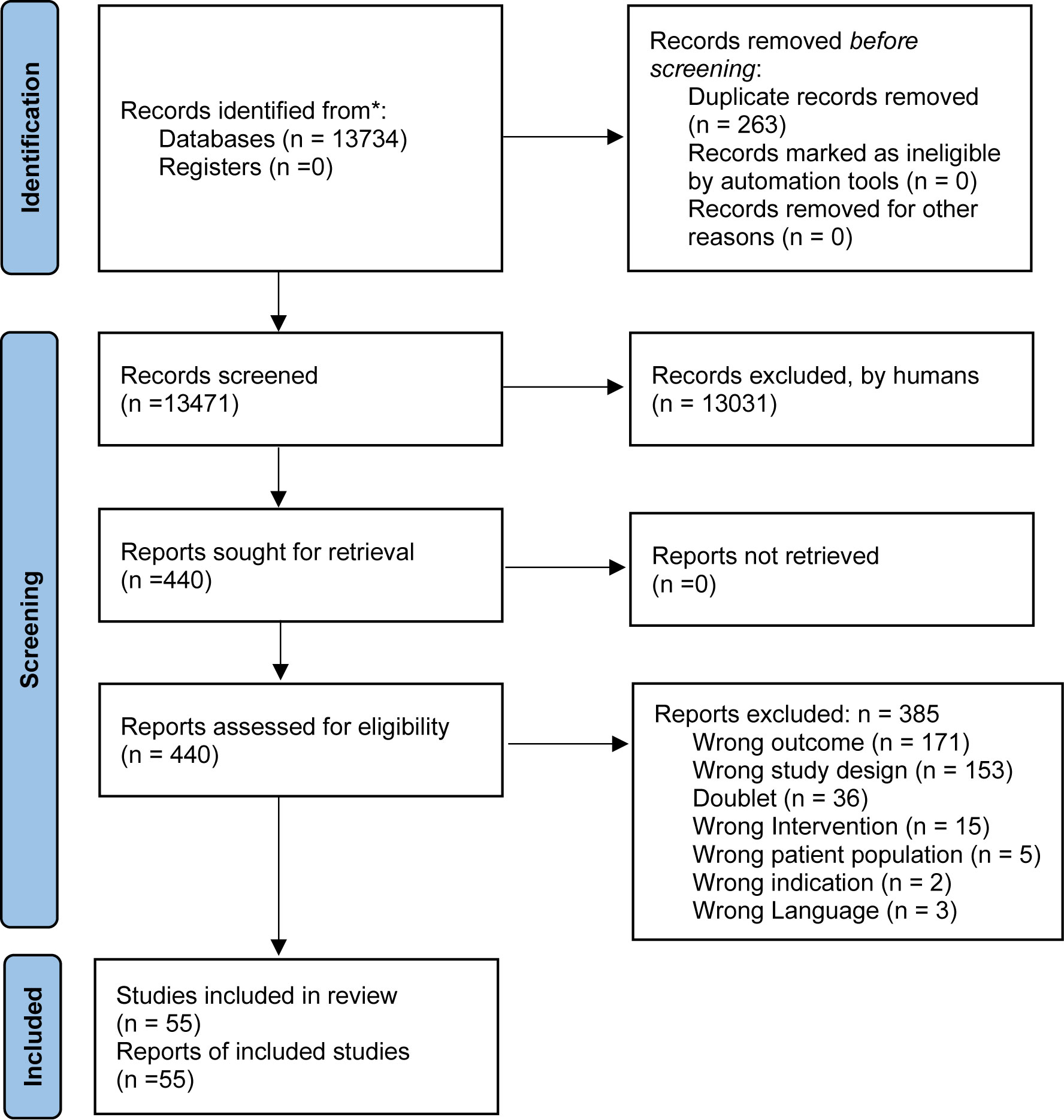
The main bibliographical search was conducted on 18th February 2021. As illustrated in Figure 1, we identified 55 studies (23–76) fulfilling the inclusion criteria and none of the exclusion criteria (Table 1). Of these, 40 were RCTs and 15 were uncontrolled studies. Of these, 33 reported on the effect of discontinuation, seven on dose reduction, 18 on switch to a partial agonist, and no studies on switch from polypharmacy to monotherapy. A total of 12,279 (mean participants 224 per study) participants were included, with a mean age of 40 years old, and the mean baseline weight of 69.6 kg.
Risk of Bias
The risk of bias assessment is presented in Table S1 (19). We found a low risk of bias in 26 trials, and some concerns in 14 trials. All the included trials were blinded, but as weight was reported as adverse events, the methods to handle missing data were often not clearly described. Visual inspection of Funnel plots (Figures S1 and S2) did not reveal any signs of publication bias.
Primary and Secondary Outcomes
For the primary outcome (Figure 2), weight change in intervention groups compared to maintenance treatment, based on 40 randomized trials, we found a weight reduction of -1.51 kg (95% CI -1.95 to -1.06) in groups who had their medication discontinued/reduced/switched to partial agonist, compared to control groups who continued maintenance treatment (Table 2). The corresponding 95% prediction interval was −4.71 to 1.61. In the subgroup analyses, the effect for discontinuation (N = 31) was −1.60 kg (95% CI −2.25 to −0.95; P < 0.001), for dose reduction (N = 7) −0.46 kg (95% CI −2.0 to 1.07; P = 0.56), and for switch to partial agonist (N = 2) it was −3.19 kg (95% CI −3.43 to −2.96; P < 0.001).
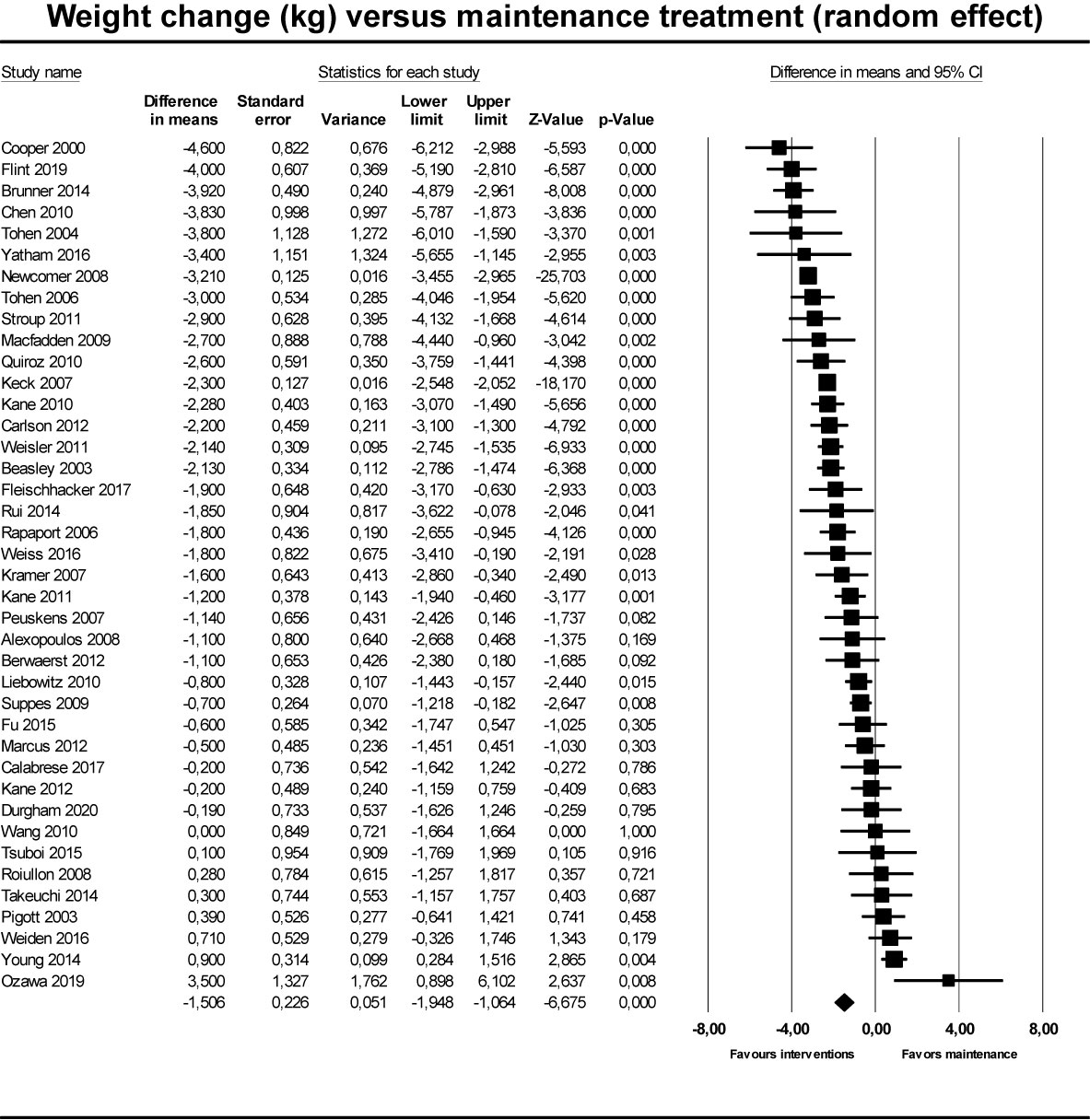
Figure 2 Forest plot showing results from studies comparing maintenaince treatment to dose reduction, switch or discontinuation.
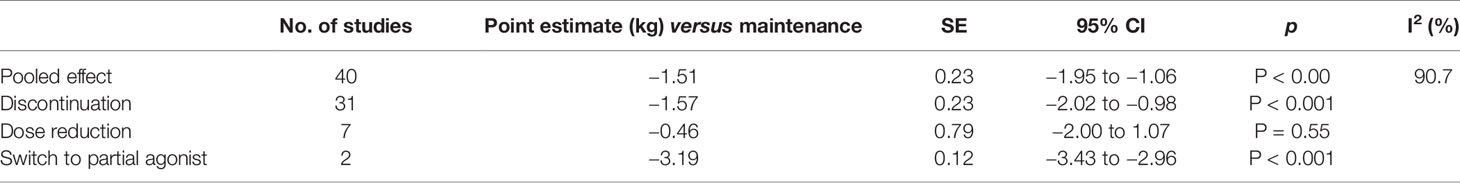
Table 2 Pooled results of primary outcomes, pooled and grouped by type of intervention) measured in kilograms (kg) with standard error (SE).
For the secondary outcome (Figure 3), pre-post analyses based on 55 studies reporting on 58 groups (three studies had two intervention groups), the pooled results of interventions including discontinuation, dose reduction, and switch to partial agonist, found a weight reduction of 1.13 kg (95% CI −1.36 to −0.90; P < 0.001) at end of follow-up compared to baseline values (Table 3). In the subgroup analyses, the effect of discontinuation (N = 33) was −0.86 kg (95% CI −1.34 to −0.38; P < 0.001), of dose reduction (N = 7) was −1.25 kg (95% CI −2.66 to 0.17; P = 0.084), and of switch to partial agonist (N = 18) was −.57 kg (95% CI −1.46 to −0.83; P<0.001).
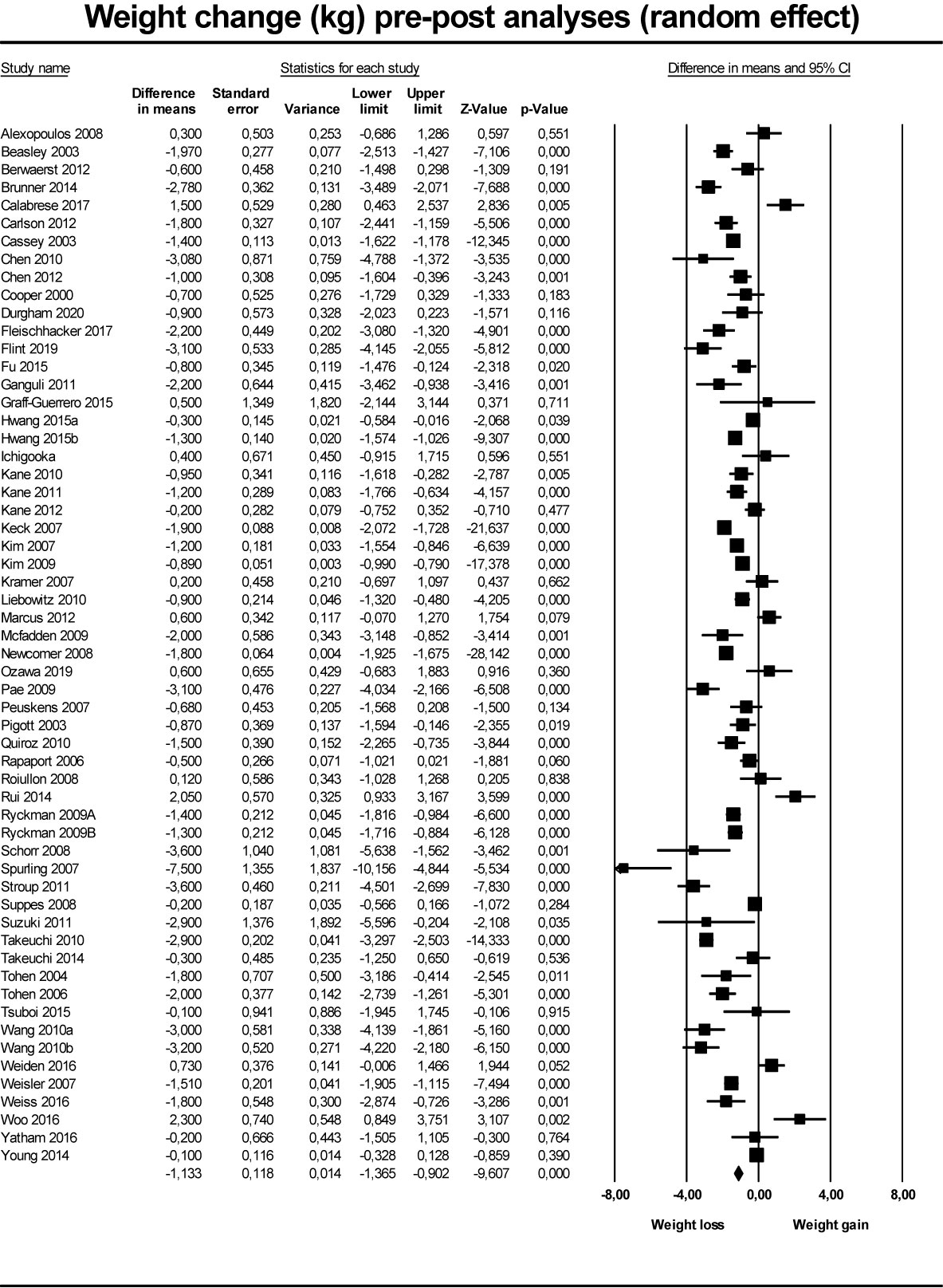
Figure 3 Forest plot showing results from studies comparing pre- to post intervention change in weight.
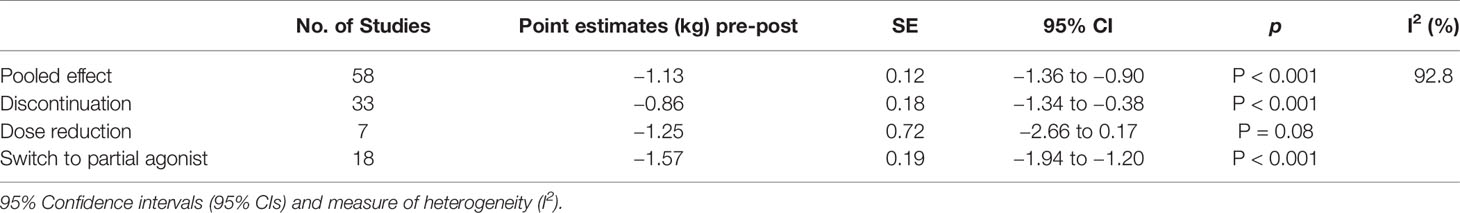
Table 3 Pooled results of secondary outcomes, pooled and grouped by type of intervention) measured in kilograms (kg) with standard error (SE).
Heterogeneity, Subgroup Analyses, and Meta-Regression
The I2 was 90.7% for the primary outcome and 97.8% for the secondary outcome, suggesting substantial heterogeneity. This was expected, as we pooled different diagnoses, different interventions, and different designs. We explored heterogeneity by testing categorical variables (diagnoses, controlled vs. uncontrolled studies) and one continuous variable (differences in exposure between active and placebo), Table 4.
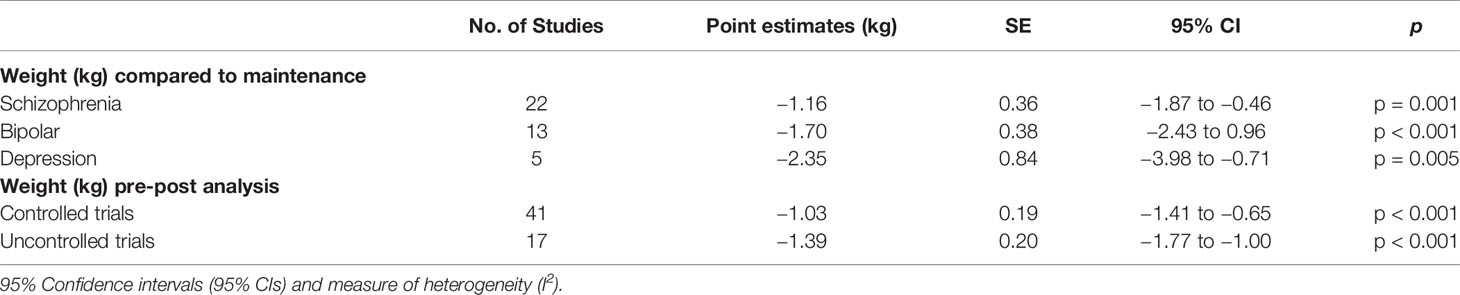
Table 4 Results for subgroup analyses and meta-regressions, including weight in kilogram (kg) grouped by diagnoses,and type of study design, standard error (SE), 95% confidence intervals (95% CI).
For the pooled primary outcome, comparing groups based on diagnoses revealed similar effects where studies on schizophrenia (N = 22) had a weight reduction of −2.24 kg (95% CI −0.46 to −3.26; p = 0.001) on bipolar (N = 13) a weight reduction of −1.70 kg (95% CI −2.43 to −0.896 p < 0.001) and on major depression (N = 5) a weight reduction of −2.35 kg (95% CI −3.98 to −0.71; p = 0.005). The differences in exposure between active drug and placebo in the RCTs (N = 17) explained 9% of variation, but this was not significant (p = 0.27).
For the secondary outcome, there was no difference in effect when comparing groups from randomized trials to uncontrolled studies; −1.03 kg versus −1.39 kg (p = 0.20).
Discussion
We found that the pooled effect of discontinuation, dose reduction, and switch to partial agonists reduced the weight with 1.54 kg compared to maintenance treatments with a second generation antipsychotic drug. The 95% CI interval was −2.08 to −1.2, allowing us to reject the null hypothesis, but the corresponding 95% prediction interval was −4.71 to 1.61, which does not exclude the probability of null effect in future trials, but the results could be as high as −4.7 kg. The subgroup results suggest that the effect of switch to partial agonists was larger than discontinuation and dose reduction, but this may reflect trial methodology rather than true differences, as discussed beneath. In the pooled pre-post analysis, we found a weight reduction of 1.13 (p < 0.001), with no substantial differences in subgroups.
The primary outcome, based on controlled trials, and secondary outcomes, based on uncontrolled trials, address two fundamentally different questions with different inherent methodological limitations to the answers. The primary outcome asks the clinical question: Will my weight be lower if I choose a dose reduction/discontinuation strategy, compared to if I continue the same dose? But the outcome does not inform about a weight loss compared to baseline, as the observed difference may be explained by a larger weight increase in the maintenance group. On the other hand, the secondary outcome, the change in weight form baseline to post intervention, addresses the question: Will I lose weight over time if I reduce dose/discontinue medication?
Our results suggest that a weight loss does occur, but substantial methodological considerations limit the validity of our findings. A major limitation is that most of the trials included in the primary analysis were designed to evaluate relapse prevention of antipsychotic drugs in individuals who received treatment during a short stabilization phase and were defined as responders. It is likely that individuals with severe weight gain were excluded in the stabilization phase due to adverse effects, which could explain the low mean baseline weight, which again could affect the generalizability to a real-world population. It could be argued that the short duration of placebo-treatment is insufficient to evaluate weight loss, as many patients relapse and thus have withdrawn from the study. On the other hand, high degrees of relapses are observed in real world data, and these findings therefore reflect the effectiveness rather than efficacy, as complete adherence is often not realistic. Next, the duration of exposure to study drug, active or placebo, differed due to earlier relapse in placebo groups, leaving shorter time to weight change in placebo versus active treatment. Data on duration of exposure was available for 17 of the included trials and did not explain a significant proportion of the variance, although this could be a type II error. Weight change was reported as an adverse event for the large majority of RCTs, and it was generally difficult to assess how missing data were handled, and if the way of reporting weight was pre-registered to avoid any multiple possible methods (continuous, dichotomized, BMI, 7% change) could be affected by financial conflicts of interest, as many of the RCTs were industry sponsored. This possible bias is likely to deflate effect size, as most sponsors would be interested in reporting a lower weight gain in the maintenance groups. Analyses of publication bias did not confirm any concerns but cannot exclude selective reporting. Finally, the generalizability of the population could be affected by the short duration of exposure in the open label, stabilization phase prior to randomization, as duration of prior exposure might be important, as pointed out by Kim et al. (30).
This allows only a short period of weight gain, which could deflate effect sizes compared to real-world populations. On the other hand, it may be easier to lose weight if the time being overweight is shorter, which could oppositely inflate effect size.
By comparing the results with the pre-post analysis, it is tempting to conclude that a weight loss is occurring, and that the observed effect cannot be ascribed to weight gain in the maintenance group. However, important caveats in inferring from pre-post studies should be kept in mind. Results could overestimate the effect due to regression to the mean, skewed loss to follow-up; it could also be confounded by lifestyle or other types of medication. Many of the studies reporting on the effect of switching to partial agonists were uncontrolled with metabolic disturbances being the primary outcome, which increases the risk that the participants engaged in parallel weight reducing behaviors, seen as a manualized co-treatment in one study (77), which could inflate the effect.
We did not identify any studies evaluating the effect of switching from antipsychotic polypharmacy to monotherapy on weight. This is surprising, as there has been increased focus on the lack of evidence supporting superior effect of polypharmacy compared to monopharmacy, and therefore safety in switching (78).
Even though the weight reduction is highly statistically significant, it should be discussed if this weight reduction is clinically important. For the subset of individuals who gain tens of kilograms, the moderate reduction is likely to be ignorable. On the other hand, the magnitude is similar to the magnitude of weight lost from lifestyle interventions (79, 80) and by adding topiramate or metformin to current antipsychotic medication, all being recommended in clinical guidelines (81). Furthermore, the observed effects should be interpreted considering the short duration of most of the trials, with some individuals only receiving placebo treatment for a few weeks before showing signs of impending relapse and subsequently withdrew from the study. Thus, it cannot be excluded that the weight loss could continue over time. The largest weight loss was achieved by switching to partial agonists, which could be explained by longer duration of exposure to antipsychotic medication, making risk of relapse in this group lower compared to trial where an active treatment is compared to placebo.
None of the included studies were designed directly to address the reversibility of antipsychotic-induced weight gain, but some studies have been published in other populations, not included in this paper: Upadhyay et al. (15) followed a large sample of children and adolescents (N = 537) with bipolar disorder who had experienced weight gain while treated with psychotropic medication. At 12 months follow-up after discontinuation of psychotropic medication, weight loss was stabilized, but never returned to baseline, and the authors conclude that it is likely that those who gained significant weight during treatment will stay overweight or obese. Significant improvement in weight was found by De Kuijpers et al. (82) in individuals with intellectual disabilities, where a reduction of dose was associated with weight loss. An improvement in metabolic factors and with no psychiatric deterioration, was reported by Hulvershorn et al. (83) who evaluated the effect of antipsychotic discontinuation in youth with disruptive behavior. However, the majority of these were prescribed medication to treat ADHD, which, in itself will induce weight loss. Finally, case reports describing severe cases of rapid weight gain and metabolic disturbances confirm reversibility, at least to some degree (14, 84).
Limitations
Adding to the limitations of using primarily relapse prevention studies, there are important limitations in the conduction of this meta-analysis that should be kept in mind. First, we did not pre-register the protocol at Prospero. The protocol was circulated in the author group, and all agreed on the design before the literature search was started. Second, we a-priori decided to measure weight change as continuous variable, as dichotomizing continuous variable may inflate effect sizes and decrease power (85). When extracting data, we were surprised to identify a large number of papers only reporting >7% weight gain and not supplementing with 7% weight loss or a mean weight change. It is possible that excluding these papers has introduced a selection bias. Third, we did not assess potential adverse events associated with dose reduction, switch, or discontinuation. To fully inform a clinical decision, potential risks, such as relapses or worsening of substance abuse, are just as important. However, as we chose only to include studies where weight was reported, reporting risks of adverse events on this basis would not provide the full picture of the available literature that has been done by others (86–89). A general finding was that the relative risk of relapse was doubled after discontinuation [27% vs placebo 64%; risk ratio (RR) 0.40, 95% CI 0·33–0·49] (90) on short term. However, it has been argued that relapse rates converge with time, and the chance of recovery is increased after dose reduction. Based on very few trials, switch to monotherapy does not seem to increase relapse rates (78, 91). Finally, the lack of access to individual data precludes the possibility of performing subgroup analyses based on personal characteristics like magnitude of weight gain during treatment or type of antipsychotic medication. Thus, it cannot be excluded that individuals with severe gain also have an equivalent weight loss, but that these are hidden in the mean.
Implications for Research
The lack of direct studies on the reversibility of weight gain calls for future research directly focusing on this topic in rigorously designed studies. Most important, the subgroups with substantial weight gain, for whom this issue may be especially important, need to be included. Ideally, RCTs should be conducted, but the feasibility in conducting these is challenging (92), and observational designs could be an alternative (93). Close monitoring of clinical characteristics during treatment in early intervention services could provide valuable information if interpreted correctly. In order to address, specifically, the question of causality, careful considerations should be put on the potential confounders, and we suggest that transparent frameworks, such as directed acyclic graphs (DAGs) (94), could be applied to make causal inferences based on observational data.
Implications for Clinical Practice
Any health care decisions are based on information about potential risks and benefits. As weight gain and potential loss are important to many people, it should be shared that little is known about the reversibility of antipsychotic-induced weight gain. This lack of knowledge could affect the decision, especially when drugs are prescribed as off-label (3), where the potential benefits are less clear. Some may worry that patients with severe mental illness may reject the medication in fear of substantial and irreversible weight gain, and thereby put themselves at risk of relapse. However, as long as we regard the patients as having decision capacity (95) and therefore able to provide informed consent, it is our obligation to inform them about potential adverse effects, including what we currently do not know, if this could affect the decision making. In four RCTs determining the effect of dose reduction, a paradoxical weight increase was seen in the reduced group (47, 56, 73, 96), suggesting that metabolic adverse effects may not be dose dependent, which is also found in other studies (97). This underlines the importance of reconsidering the current off-label prescribing trend, as also small doses may lead to metabolic disturbances.
Conclusion
The main finding of this study is a paucity of studies designed to directly evaluate the reversibility of antipsychotic-induced weight gain. This lack of knowledge is problematic, as individuals making an informed decision has the right to know if they run a risk of lifelong obesity. In spite of the limitations discussed above, our findings do suggest that at least some weight gain is reversible, even though there is doubtfully any health benefit in this small effect size when balanced against the risk of relapse. The lack of dose-related effect is important and should lead to increased awareness of off-label use of low dose second generation antipsychotic for indications like sleep and anxiety, as this might create equally serious weight problems and is increasingly being prescribed (98).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
HS conceived the idea and made the analysis plan. HS and CW performed the bibliographic search, extracted data, performed the final analyses and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We want to thank Dr. Nina Vindegaard Sørensen for valuable input during the writing process.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.577919/full#supplementary-material
References
1. Kuipers E, Yesufu-Udechuku A, Taylor C, Kendall T. Management of Psychosis and Schizophrenia in Adults: Summary of Updated NICE Guidance. BMJ (2014) 348:g1173. doi: 10.1136/bmj.g1173
2. Taylor DM, Cornelius V, Smith L, Young AH. Comparative Efficacy and Acceptability of Drug Treatments for Bipolar Depression: A Multiple-Treatments Meta-Analysis. Acta Psychiatr Scand (2014) 130:452–69. doi: 10.1111/acps.12343
3. Højlund M, Andersen JH, Andersen K, Correll CU, Hallas J. Use of Antipsychotics in Denmark 1997–2018: A Nation-Wide Drug Utilisation Study With Focus on Off-Label Use and Associated Diagnoses. Epidemiol Psychiatr Sci (2021) 30:e28. doi: 10.1017/S2045796021000159
4. Seeman MV. Obesity in Schizophrenia. J Obes Manag (2017) 1:10–24. doi: 10.14302/issn.2574-450x.jom-16-1039
5. Hugenholtz GW, Heerdink ER, Meijer WE, Stolker J-J, Egberts AC, Nolen WA. Reasons for Switching Between Antipsychotics in Daily Clinical Practice. Pharmacopsychiatry (2005) 38:122–4. doi: 10.1055/s-2005-864122
6. Bak M, Fransen A, Janssen J, Van Os J, Drukker M. Almost All Antipsychotics Result in Weight Gain: A Meta-Analysis. PloS One (2014) 9:10–2. doi: 10.1371/journal.pone.0094112
7. Stroup TS, Gray N. Management of Common Adverse Effects of Antipsychotic Medications. World Psychiatry (2018) 17:341–56. doi: 10.1002/wps.20567
8. Hall KD, Kahan S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med Clin North Am (2018) 102:183–97. doi: 10.1016/j.mcna.2017.08.012
9. Grajales D, Ferreira V, Valverde ÁM. @ of Glucose Metabolism: Beyond Weight Gain. Cells (2019) 8(11):1336. doi: 10.3390/cells8111336
10. Ersland KM, Myrmel LS, Fjære E, Berge RK, Madsen L, Steen VM, et al. One-Year Treatment With Olanzapine Depot in Female Rats: Metabolic Effects. Int J Neuropsychopharmacol (2019) 22:358–69. doi: 10.1093/ijnp/pyz012
11. Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making Sense of ... the Microbiome in Psychiatry. Int J Neuropsychopharmacol (2019) 22:37–52. doi: 10.1093/ijnp/pyy067
12. Bretler T, Weisberg H, Koren O, Neuman H. The Effects of Antipsychotic Medications on Microbiome and Weight Gain in Children and Adolescents. BMC Med (2019) 17:1–12. doi: 10.1186/s12916-019-1346-1
13. Dibben CRM, Kalavalapalli SS, Linnington HEJ, Hynes FA, Dinneen SF, Adler AI, et al. Diabetes Associated With Atypical Antipsychotic Treatment may Be Severe But Reversible: Case Report. Int J Psychiatry Med (2005) 35:307–11. doi: 10.2190/B9RD-VMC4-CU8C-KFDD
14. Sahoo S, Mishra B, Akhtar S. Dose-Dependent Acute Excessive Weight Gain and Metabolic Changes in a Drug-Naive Patient on Risperidone are Reversible With Discontinuation: A Case Report [4]. Br J Clin Pharmacol (2007) 64:715–6. doi: 10.1111/j.1365-2125.2007.02941.x
15. Upadhyay N, Patel A, Chan W, Aparasu RR, Ochoa-Perez M, Sherer JT, et al. Reversibility of Psychotropic Medication Induced Weight Gain Among Children and Adolescents With Bipolar Disorders. Psychiatry Res (2019) 276:151–9. doi: 10.1016/j.psychres.2019.05.005
16. Libowitz MR, Nurmi EL. The Burden of Antipsychotic-Induced Weight Gain and Metabolic Syndrome in Children. Front Psychiatry (2021) 12:623681. doi: 10.3389/fpsyt.2021.623681
17. Calarge CA, Nicol G, Schlechte JA, Burns TL. Cardiometabolic Outcomes in Children and Adolescents Following Discontinuation of Long-Term Risperidone Treatment. J Child Adolesc Psychopharmacol (2014) 24:120–9. doi: 10.1089/cap.2013.0126
18. Tuplin EW, Holahan MR. Aripiprazole A. Drug That Displays Partial Agonism and Functional Selectivity. Curr Neuropharmacol (2017) 15:1192–207. doi: 10.2174/1570159X15666170413115754
19. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
20. Higgins JPT, Green S eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011] (2011). doi: 10.1002/jrsm.38
21. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for Routinely Presenting Prediction Intervals in Meta-Analysis. BMJ Open (2016) 6:e010247. doi: 10.1136/bmjopen-2015-010247
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
23. Beasley CM, Sutton VK, Hamilton SH, Walker DJ, Dossenbach M, Taylor CC, et al. A Double-Blind, Randomized, Placebo-Controlled Trial of Olanzapine in the Prevention of Psychotic Relapse. J Clin Psychopharmacol (2003) 23:582–94. doi: 10.1097/01.jcp.0000095348.32154.ec
24. Chen EYH, Hui CLM, Lam MML, Chiu CPY, Law CW, Chung DWS, et al. Maintenance Treatment With Quetiapine Versus Discontinuation After One Year of Treatment in Patients With Remitted First Episode Psychosis: Randomised Controlled Trial. BMJ (2010) 341:c4024. doi: 10.1136/bmj.c4024
25. Weisler RH, Nolen WA, Neijber A, Hellqvist Å, Paulsson B. Continuation of Quetiapine Versus Switching to Placebo or Lithium for Maintenance Treatment of Bipolar I Disorder (Trial 144: A Randomized Controlled Study). J Clin Psychiatry (2011) 72:1452–64. doi: 10.4088/JCP.11m06878
26. Casey DE, Carson WH, Saha AR, Liebeskind A, Ali MW, Jody D, et al. Switching Patients to Aripiprazole From Other Antipsychotic Agents: A Multicenter Randomized Study. Psychopharmacology (Berl) (2003) 166:391–9. doi: 10.1007/s00213-002-1344-3
27. Spurling RD, Lamberti JS, Olsen D, Tu X, Tang W. Changes in Metabolic Parameters With Switching to Aripiprazole From Another Second-Generation Antipsychotic: A Retrospective Chart Review. J Clin Psychiatry (2007) 68:406–9. doi: 10.4088/JCP.v68n0308
28. Kim SH, Ivanova O, Abbasi FA, Lamendola CA, Reaven GM, Glick ID. Metabolic Impact of Switching Antipsychotic Therapy to Aripiprazole After Weight Gain: A Pilot Study. J Clin Psychopharmacol (2007) 27:365–8. doi: 10.1097/JCP.0b013e3180a9076c
29. Pae C-U, Serretti A, Chiesa A, Mandelli L, Lee C, Lee C, et al. Immediate Versus Gradual Suspension of Previous Treatments During Switch to Aripiprazole: Results of a Randomized, Open Label Study. Eur Neuropsychopharmacol (2009) 19:562–70. doi: 10.1016/j.euroneuro.2009.04.002
30. Kim S-W, Shin I-S, Kim J-M, Lee J-H, Lee Y-H, Yang S-J, et al. Effectiveness of Switching to Aripiprazole From Atypical Antipsychotics in Patients With Schizophrenia. Clin Neuropharmacol (2009) 32:243–9. doi: 10.1097/WNF.0b013e31819a68b5
31. Takeuchi H, Uchida H, Suzuki T, Watanabe K, Kashima H. Changes in Metabolic Parameters Following a Switch to Aripiprazole in Japanese Patients With Schizophrenia: One-year Follow-Up Study. Psychiatry Clin Neurosci (2010) 64:104–6. doi: 10.1111/j.1440-1819.2009.02036.x
32. Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, Alexopoulos GS, Rudorfer MV, et al. Effect of Continuing Olanzapine vs. Placebo on Relapse Among Patients With Psychotic Depression in Remission: The STOP-PD II Randomized Clinical Trial. JAMA - J Am Med Assoc (2019) 322:622–31. doi: 10.1001/jama.2019.10517
33. Quiroz JA, Yatham LN, Palumbo JM, Karcher K, Kushner S, Kusumakar V. Risperidone Long-Acting Injectable Monotherapy in the Maintenance Treatment of Bipolar I Disorder. Biol Psychiatry (2010) 68:156–62. doi: 10.1016/j.biopsych.2010.01.015
34. Takeuchi H. Lack of Effect of Risperidone or Olanzapine Dose Reduction on Metabolic Parameters, Prolactin, and Corrected QT Interval in Stable Patients With Schizophrenia. J Clin Psychopharmacol (2014) 34:517–20. doi: 10.1097/JCP.0000000000000142
35. Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG. Aripiprazole for the Prevention of Relapse in Stabilized Patients With Chronic Schizophrenia: A Placebo-Controlled 26-Week Study. J Clin Psychiatry (2003) 64:1048–56. doi: 10.4088/jcp.v64n0910
36. Berwaerts J, Melkote R, Nuamah I, Lim P. A Randomized, Placebo- and Active-Controlled Study of Paliperidone Extended-Release as Maintenance Treatment in Patients With Bipolar I Disorder After an Acute Manic or Mixed Episode. J Affect Disord (2012) 138:247–58. doi: 10.1016/j.jad.2012.01.047
37. Calabrese JR, Sanchez R, Jin N, Amatniek J, Cox K, Johnson B, et al. Efficacy and Safety of Aripiprazole Once-Monthly in the Maintenance Treatment of Bipolar I Disorder: A Double-Blind, Placebo-Controlled, 52-Week Randomized Withdrawal Study. J Clin Psychiatry (2017) 78:324–31. doi: 10.4088/JCP.16m11201
38. Macfadden W, Alphs L, Haskins JT, Turner N, Turkoz I, Bossie C, et al. A Randomized, Double-Blind, Placebo-Controlled Study of Maintenance Treatment With Adjunctive Risperidone Long-Acting Therapy in Patients With Bipolar I Disorder Who Relapse Frequently. Bipolar Disord (2009) 11:827–39. doi: 10.1111/j.1399-5618.2009.00761.x
39. Tohen M, Chengappa KNR, Suppes T, Baker RW, Zarate CA, Bowden CL, et al. Relapse Prevention in Bipolar I Disorder: 18-Month Comparison of Olanzapine Plus Mood Stabiliser V. Mood Stabiliser Alone. Br J Psychiatry (2004) 184:337–45. doi: 10.1192/bjp.184.4.337
40. Weiden PJ, Manning R, Wolfgang CD, Ryan JM, Mancione L, Han G, et al. A Randomized Trial of Iloperidone for Prevention of Relapse in Schizophrenia: The REPRIEVE Study. CNS Drugs (2016) 30:735–47. doi: 10.1007/s40263-016-0345-4
41. Brunner E, Tohen M, Osuntokun O, Landry J, Thase ME. Efficacy and Safety of Olanzapine/Fluoxetine Combination vs. Fluoxetine Monotherapy Following Successful Combination Therapy of Treatment-Resistant Major Depressive Disorder. Neuropsychopharmacology (2014) 39:2549–59. doi: 10.1038/npp.2014.101
42. Kramer M, Simpson G, Maciulis V, Kushner S, Vijapurkar U, Lim P, et al. Paliperidone Extended-Release Tablets for Prevention of Symptom Recurrence in Patients With Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. J Clin Psychopharmacol (2007) 27:6–14. doi: 10.1097/JCP.0b013e31802dda4a
43. Kane JM, Detke HC, Naber D, Sethuraman G, Lin DY, Bergstrom RF, et al. Olanzapine Long-Acting Injection: A 24-Week, Randomized, Double-Blind Trial of Maintenance Treatment in Patients With Schizophrenia. Am J Psychiatry (2010) 167:181–9. doi: 10.1176/appi.ajp.2009.07081221
44. Kane JM, Mackle M, Snow-Adami L, Zhao J, Szegedi A, Panagides J. A Randomized Placebo-Controlled Trial of Asenapine for the Prevention of Relapse of Schizophrenia After Long-Term Treatment. J Clin Psychiatry (2011) 72:349–55. doi: 10.4088/JCP.10m06306
45. Rapaport MH, Gharabawi GM, Canuso CM, Mahmoud RA, Keller MB, Bossie CA, et al. Effects of Risperidone Augmentation in Patients With Treatment-Resistant Depression: Results of Open-Label Treatment Followed by Double-Blind Continuation. Neuropsychopharmacology (2006) 31:2505–13. doi: 10.1038/sj.npp.1301113
46. Fu D-J, Bossie C, Turkoz I, Mahalchick L, Alphs L. Paliperidone Palmitate Treatment Response in Early and Chronic Illness Schizoaffective Disorder Patients. Schizophr Bull (2015) 41:S312. doi: 10.1093/schbul/sbv010
47. Tsuboi T, Suzuki T, Bies RR, Remington G, Pollock BG, Mimura M, et al. Challenging the Need for Sustained Blockade of Dopamine D2 Receptor Estimated From Antipsychotic Plasma Levels in the Maintenance Treatment of Schizophrenia: A Single-Blind, Randomized, Controlled Study. Schizophr Res (2015) 164:149–54. doi: 10.1016/j.schres.2015.03.025
48. Tohen J, Sachs GS, Banov MD, Detke HC, Ph D, Risser R, et al. Maintenance Therapy in Patients With Bipolar I Disorder Responding to Acute Treatment With Olanzapine. Am Psychiat (2006) 163(2):247–56. doi: 10.1176/appi.ajp.163.2.247
49. Hwang TJ, Lo WM, Chan HY, Lin CF, Hsieh MH, Liu CC, et al. Fast Versus Slow Strategy of Switching Patients With Schizophrenia to Aripiprazole From Other Antipsychotics. J Clin Psychopharmacol (2015) 35:635–44. doi: 10.1097/JCP.0000000000000426
50. Keck PEJ, Calabrese JR, McIntyre RS, McQuade RD, Carson WH, Eudicone JAM, et al. Aripiprazole Monotherapy for Maintenance Therapy in Bipolar I Disorder: A 100-Week, Double-Blind Study Versus Placebo. J Clin Psychiatry (2007) 68:1480–91. doi: 10.4088/JCP.v68n1003
51. Alexopoulos GS, Canuso CM, Gharabawi GM, Bossie CA, Greenspan A, Turkoz I, et al. Placebo-Controlled Study of Relapse Prevention With Risperidone Augmentation in Older Patients With Resistant Depression. Am J Geriatr Psychiatry (2008) 16:21–30. doi: 10.1097/JGP.0b013e31813546f2
52. Young AH, McElroy SL, Olausson B, Paulsson B. A Randomised, Placebo-Controlled 52-Week Trial of Continued Quetiapine Treatment in Recently Depressed Patients With Bipolar I and Bipolar II Disorder. World J Biol Psychiatry (2014) 15:96–112. doi: 10.3109/15622975.2012.665177
53. Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Long-Term Cariprazine Treatment for the Prevention of Relapse in Patients With Schizophrenia: A Randomized , Double-Blind. Schizophr Res (2020) 176:264–71. doi: 10.1016/j.schres.2016.06.030
54. Peuskens J, Trivedi J, Malyarov S, Brecher M, Svensson O, Miller F, et al. Prevention of Schizophrenia Relapse With Extended Release Quetiapine Fumarate Dosed Once Daily: A Randomized, Placebo-Controlled Trial in Clinically Stable Patients. Psychiatry (Edgmont) (2007) 4:34–50.
55. Fleischhacker WW, Hobart M, Ouyang J, Forbes A, Pfister S, McQuade RD, et al. Efficacy and Safety of Brexpiprazole (OPC-34712) as Maintenance Treatment in Adults With Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. Int J Neuropsychopharmacol (2017) 20:11–21. doi: 10.1093/ijnp/pyw076
56. Rouillon F, Chartier F, Gasquet I. Strategies of Treatment With Olanzapine in Schizophrenic Patients During Stable Phase: Results of a Pilot Study. Eur Neuropsychopharmacol (2008) 18:646–52. doi: 10.1016/j.euroneuro.2008.04.012
57. Kane JM, Sanchez R, Perry PP, Jin N, Johnson BR, Forbes RA, et al. Aripiprazole Intramuscular Depot as Maintenance Treatment in Patients With Schizophrenia: A 52-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Psychiatry (2012) 73:617–24. doi: 10.4088/JCP.11m07530
58. Wang C-Y, Xiang Y-T, Cai Z-J, Weng Y-Z, Bo Q-J, Zhao J-P, et al. Risperidone Maintenance Treatment in Schizophrenia: A Randomized, Controlled Trial. Am J Psychiatry (2010) 167:676–85. doi: 10.1176/appi.ajp.2009.09030358
59. Graff-Guerrero A, Rajji TK, Mulsant BH, Nakajima S, Caravaggio F, Suzuki T, et al. Evaluation of Antipsychotic Dose Reduction in Late-Life Schizophrenia: A Prospective Dopamine D2/3 Receptor Occupancy Study. JAMA Psychiatry (2015) 72:927–34. doi: 10.1001/jamapsychiatry.2015.0891
60. Suzuki Y, Ono S, Sugai T, Fukui N, Watanabe J, Tsuneyama N, et al. Dose-Dependent Effects of Olanzapine on QT Intervals and Plasma Prolactin Levels in Japanese Patients With Stable Schizophrenia. Hum Psychopharmacol (2011) 26:440–3. doi: 10.1002/hup.1218
61. Schorr SG, Slooff CJ, Postema R, Van Oven W, Schilthuis M, Bruggeman R, et al. A 12-Month Follow-Up Study of Treating Overweight Schizophrenic Patients With Aripiprazole. Acta Psychiatr Scand (2008) 118:246–50. doi: 10.1111/j.1600-0447.2008.01232.x
62. Yatham LN, Beaulieu S, Schaffer A, Kauer-Sant’Anna M, Kapczinski F, Lafer B, et al. Optimal Duration of Risperidone or Olanzapine Adjunctive Therapy to Mood Stabilizer Following Remission of a Manic Episode: A CANMAT Randomized Double-Blind Trial. Mol Psychiatry (2016) 21:1050–6. doi: 10.1038/mp.2015.158
63. Carlson BX, Ketter TA, Sun W, Timko K, McQuade RD, Sanchez R, et al. Aripiprazole in Combination With Lamotrigine for the Long-Term Treatment of Patients With Bipolar I Disorder (Manic or Mixed): A Randomized, Multicenter, Double-Blind Study (CN138-392). Bipolar Disord (2012) 14:41–53. doi: 10.1111/j.1399-5618.2011.00974.x
64. Suppes T, Vieta E, Liu S, Brecher M, Paulsson B. Maintenance Treatment for Patients With Bipolar I Disorder: Results From a North American Study of Quetiapine in Combination With Lithium or Divalproex (Trial 127). Am J Psychiatry (2009) 166:476–88. doi: 10.1176/appi.ajp.2008.08020189
65. Weiss C, Weiller E, Hobart M, Ouyang J. Effect of Brexpiprazole on Weight and Metabolic Parameters: Analysis of a Maintenance Trial in Schizophrenia. Eur Neuropsychopharmacol (2016) 26:S556–. doi: 10.1002/central/CN-01303749/full
66. Ishigooka J, Kato T, Miyajima M, Watabe K, Masuda T, Hagi K, et al. Lurasidone in the Long-Term Treatment of Bipolar I Depression: A 28-Week Open Label Extension Study. J Affect Disord (2021) 281:160–7. doi: 10.1016/j.jad.2020.12.005
67. Ryckmans V, Kahn JP, Modell S, Werner C, McQuade RD, Kerselaers W, et al. Switching to Aripiprazole in Outpatients With Schizophrenia Experiencing Insufficient Efficacy and/or Safety/Tolerability Issues With Risperidone: A Randomized, Multicentre, Open-Label Study. Pharmacopsychiatry (2009) 42:114–21. doi: 10.1055/s-0028-1112134
68. Rui Q, Wang Y, Liang S, Liu Y, Wu Y, Wu Q, et al. Relapse Prevention Study of Paliperidone Extended-Release Tablets in Chinese Patients With Schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2014) 53:45–53. doi: 10.1016/j.pnpbp.2014.02.007
69. Chen Y, Bobo WV, Watts K, Jayathilake K, Tang T, Meltzer HY. Comparative Effectiveness of Switching Antipsychotic Drug Treatment to Aripiprazole or Ziprasidone for Improving Metabolic Profile and Atherogenic Dyslipidemia: A 12-Month, Prospective, Open-Label Study. J Psychopharmacol (2012) 26:1201–10. doi: 10.1177/0269881111430748
70. Marcus R, Khan A, Rollin L, Morris B, Timko K, Carson W, et al. Efficacy of Aripiprazole Adjunctive to Lithium or Valproate in the Long-Term Treatment of Patients With Bipolar I Disorder With an Inadequate Response to Lithium or Valproate Monotherapy: A Multicenter, Double-Blind, Randomized Study. Bipolar Disord (2011) 13:133–44. doi: 10.1111/j.1399-5618.2011.00898.x
71. Liebowitz M, Lam RW, Lepola U, Datto C, Sweitzer D, Eriksson H. Efficacy and Tolerability of Extended Release Quetiapine Fumarate Monotherapy as Maintenance Treatment of Major Depressive Disorder: A Randomized, Placebo-Controlled Trial. Depress Anxiety (2010) 27:964–76. doi: 10.1002/da.20740
72. Newcomer JW, Campos JA, Marcus RN, Breder C, Berman RM, Kerselaers W, et al. A Multicenter, Randomized, Double-Blind Study of the Effects of Aripiprazole in Overweight Subjects With Schizophrenia or Schizoaffective Disorder Switched From Olanzapine. J Clin Psychiatry (2008) 69:1046–56. doi: 10.4088/JCP.v69n0702
73. Ozawa C, Bies RR, Pillai N, Suzuki T, Mimura M, Uchida H. Model-Guided Antipsychotic Dose Reduction in Schizophrenia: A Pilot, Single-Blind Randomized Controlled Trial. J Clin Psychopharmacol (2019) 39:329–35. doi: 10.1097/JCP.0000000000001046
74. Woo YS, Bahk WM, Park YM, Chung S, Yoon BH, Won S, et al. Effects of Switching to Aripiprazole From Current Atypical Antipsychotics on Subsyndromal Symptoms and Tolerability in Patients With Bipolar Disorder. Int Clin Psychopharmacol (2016) 31:275–86. doi: 10.1097/YIC.0000000000000136
75. Cooper SJ, Butler A, Tweed J, Welch C, Raniwalla J. Zotepine in the Prevention of Recurrence: A Randomised, Double-Blind, Placebo-Controlled Study for Chronic Schizophrenia. Psychopharmacology (Berl) (2000) 150:237–43. doi: 10.1007/s002130000452
76. Ganguli R, Brar JS, Garbut R, Chang CCH, Basu R. Changes in Weight and Other Metabolic Indicators in Persons With Schizophrenia Following a Switch to Aripiprazole. Clin Schizophr Relat Psychoses (2011) 5:75–9. doi: 10.3371/CSRP.5.2.3
77. Stroup TS, Byerly MJ, Nasrallah HA, Ray N, Khan AY, Lamberti JS, et al. Effects of Switching From Olanzapine, Quetiapine, and Risperidone to Aripiprazole on 10-Year Coronary Heart Disease Risk and Metabolic Syndrome Status: Results From a Randomized Controlled Trial. Schizophr Res (2013) 146:190–5. doi: 10.1016/j.schres.2013.01.013
78. Baandrup L. Polypharmacy in Schizophrenia. Basic Clin Pharmacol Toxicol (2020) 126:183–92. doi: 10.1111/bcpt.13384
79. Speyer H, Jakobsen AS, Westergaard C, Nørgaard HCB, Pisinger C, Krogh J, et al. Lifestyle Interventions for Weight Management in People With Serious Mental Illness: A Systematic Review With Meta-Analysis, Trial Sequential Analysis, and Meta-Regression Analysis Exploring the Mediators and Moderators of Treatment Effects. Psychother Psychosom (2019) 88:350–62. doi: 10.1159/000502293
80. Speyer H, Christian H, Nørgaard B, Birk M, Karlsen M, Jakobsen AS, et al. The CHANGE Trial: No Superiority of Lifestyle Coaching Plus Care Coordination Plus Treatment as Usual Compared to Treatment as Usual Alone in Reducing Risk of Cardiovascular Disease in Adults With Schizophrenia Spectrum Disorders and Abdominal Obesity. World Psychiatry (2016) 15:155–65. doi: 10.1002/wps.20318
81. Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, et al. BAP Guidelines on the Management of Weight Gain , Metabolic Disturbances and Cardiovascular Risk Associated With Psychosis and Antipsychotic Drug Treatment. J Psychopharmacol (2016) 30:717–48. doi: 10.1177/0269881116645254
82. de Kuijper G, Mulder H, Evenhuis H, Visser F, Hoekstra PJ. Effects of Controlled Discontinuation of Long-Term Used Antipsychotics on Weight and Metabolic Parameters in Individuals With Intellectual Disability. J Clin Psychopharmacol (2013) 33:520–4. doi: 10.1097/JCP.0b013e3182905d6a
83. Hulvershorn L, Parkhurst S, Jones S, Dauss K, Adams C. Improved Metabolic and Psychiatric Outcomes With Discontinuation of Atypical Antipsychotics in Youth Hospitalized in a State Psychiatric Facility. J Child Adolesc Psychopharmacol (2017) 27:897–907. doi: 10.1089/cap.2017.0040
84. Wysokiński A, Sobów T. Improvements in Body Composition, Anthropometric Measurements and Lipid Profile Following Discontinuation of Clozapine. Nord J Psychiatry (2016) 70:156–60. doi: 10.3109/08039488.2015.1056225
85. Altman DG, Royston P. The Cost of Dichotomising Continuous Variables. BMJ (2006) 332:1080. doi: 10.1136/bmj.332.7549.1080
86. Leucht S, Tardy M, Komossa K, Heres S, Kissling W, JM D. Maintenance Treatment With Antipsychotic Drugs for Schizophrenia. Cochrane Database Syst Rev (2012). doi: 10.1002/14651858.CD008016.pub2
87. Tani H, Uchida H, Suzuki T, Fujii Y, Mimura M, Tani H, et al. Interventions to Reduce Antipsychotic Polypharmacy: A Systematic Review. Schizophr Res (2013) 143:215–20. doi: 10.1016/j.schres.2012.10.015
88. Bowtell M, Eaton S, Thien K, Bardell-Williams M, Downey L, Ratheesh A, et al. Rates and Predictors of Relapse Following Discontinuation of Antipsychotic Medication After a First Episode of Psychosis. Schizophr Res (2018) 195:231–6. doi: 10.1016/j.schres.2017.10.030
89. Correll CU, Shi L, Weiss C, Hobart M, Eramo A, Duffy RA, et al. Successful Switching of Patients With Acute Schizophrenia From Another Antipsychotic to Brexpiprazole: Comparison of Clinicians’ Choice of Cross-Titration Schedules in a Post Hoc Analysis of a Randomized, Double-Blind, Maintenance Treatment Study. CNS Spectr (2018) 24(5):507–17. doi: 10.1017/S1092852918001086
90. Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, et al. Antipsychotic Drugs Versus Placebo for Relapse Prevention in Schizophrenia: A Systematic Review and Meta-Analysis. Lancet (2012) 379:2063–71. doi: 10.1016/S0140-6736(12)60239-6
91. Matsui K, Tokumasu T, Takekita Y, Inada K, Kanazawa T, Kishimoto T, et al. Switching to Antipsychotic Monotherapy vs. Staying on Antipsychotic Polypharmacy in Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr Res (2019) 209:50–7. doi: 10.1016/j.schres.2019.05.030
92. Speyer H, Begemann M, Albert N, Weller A, Nordentoft M, Veling W, et al. Discontinuation of Antipsychotic Medication — Time to Rethink Trial Design. Lancet Psychiatry (2020) 7:841–2. doi: 10.1016/S2215-0366(20)30340-0
93. Luykx JJ, Tiihonen J. Antipsychotic Discontinuation: Mind the Patient and the Real-World Evidence. Lancet Psychiatry (2021) 8(7):555–7. doi: 10.1016/S2215-0366(21)00159-0
94. Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol (2016) 183:758–64. doi: 10.1093/aje/kwv254
95. Peisah C, Sorinmade OA, Mitchell L, Hertogh CMPM. Decisional Capacity: Toward an Inclusionary Approach. Int Psychogeriatr (2013) 25:1571–9. doi: 10.1017/S1041610213001014
96. Takeuchi H, Fervaha G, Lee J, Agid O, Remington G. Effectiveness of Different Dosing Regimens of Risperidone and Olanzapine in Schizophrenia. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol (2015) 25:295–302. doi: 10.1016/j.euroneuro.2014.12.008
97. Citrome L, Stauffer VL, Chen L, Kinon BJ, Kurtz DL, Jacobson JG, et al. Olanzapine Plasma Concentrations After Treatment With 10, 20, and 40 mg/d in Patients With Schizophrenia: An Analysis of Correlations With Efficacy, Weight Gain, and Prolactin Concentration. J Clin Psychopharmacol (2009) 29:278–83. doi: 10.1097/JCP.0b013e3181a289cb
Keywords: antipsychotic medication, weight loss, obesity, antipsychotic induced weight gain, discontinuation, dose reduction
Citation: Speyer H, Westergaard C, Albert N, Karlsen M, Stürup AE, Nordentoft M and Krogh J (2021) Reversibility of Antipsychotic-Induced Weight Gain: A Systematic Review and Meta-Analysis. Front. Endocrinol. 12:577919. doi: 10.3389/fendo.2021.577919
Received: 30 June 2020; Accepted: 07 June 2021;
Published: 28 July 2021.
Edited by:
Roger Chen, St Vincent’s Hospital Sydney, AustraliaReviewed by:
Danijela Gnjidic, The University of Sydney, AustraliaDan Siskind, The University of Queensland, Australia
Copyright © 2021 Speyer, Westergaard, Albert, Karlsen, Stürup, Nordentoft and Krogh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helene Speyer, aGVsZW5lLnNwZXllckByZWdpb25oLmRr
†These authors have contributed equally to this work and share first authorship
 Helene Speyer
Helene Speyer Casper Westergaard
Casper Westergaard Nikolai Albert
Nikolai Albert Mette Karlsen1
Mette Karlsen1