- 1Department of Internal Medicine, National Taiwan University College of Medicine, Taipei, Taiwan
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 3Division of Environmental Health and Occupational Medicine of the National Health Research Institutes, Zhunan, Taiwan
Background and aims: Animal studies suggested that vildagliptin might exert a beneficial effect on cognitive function. The present study evaluated whether the use of vildagliptin in patients with type 2 diabetes mellitus might affect dementia risk.
Methods: The database of Taiwan’s National Health Insurance was used to enroll an unmatched cohort and a propensity score-matched-pair cohort of ever and never users of vildagliptin from patients with newly diagnosed diabetes mellitus during 2002-2014. The patients should be alive on January 1, 2015 and were followed up for dementia diagnosis until December 31, 2016. Unadjusted and multivariate-adjusted hazard ratios (HR) and their 95% confidence intervals (CI) were estimated for vildagliptin ever versus never users, for cumulative duration and cumulative dose of vildagliptin therapy categorized into tertiles versus never users, and for cumulative duration and cumulative dose treated as continuous variables.
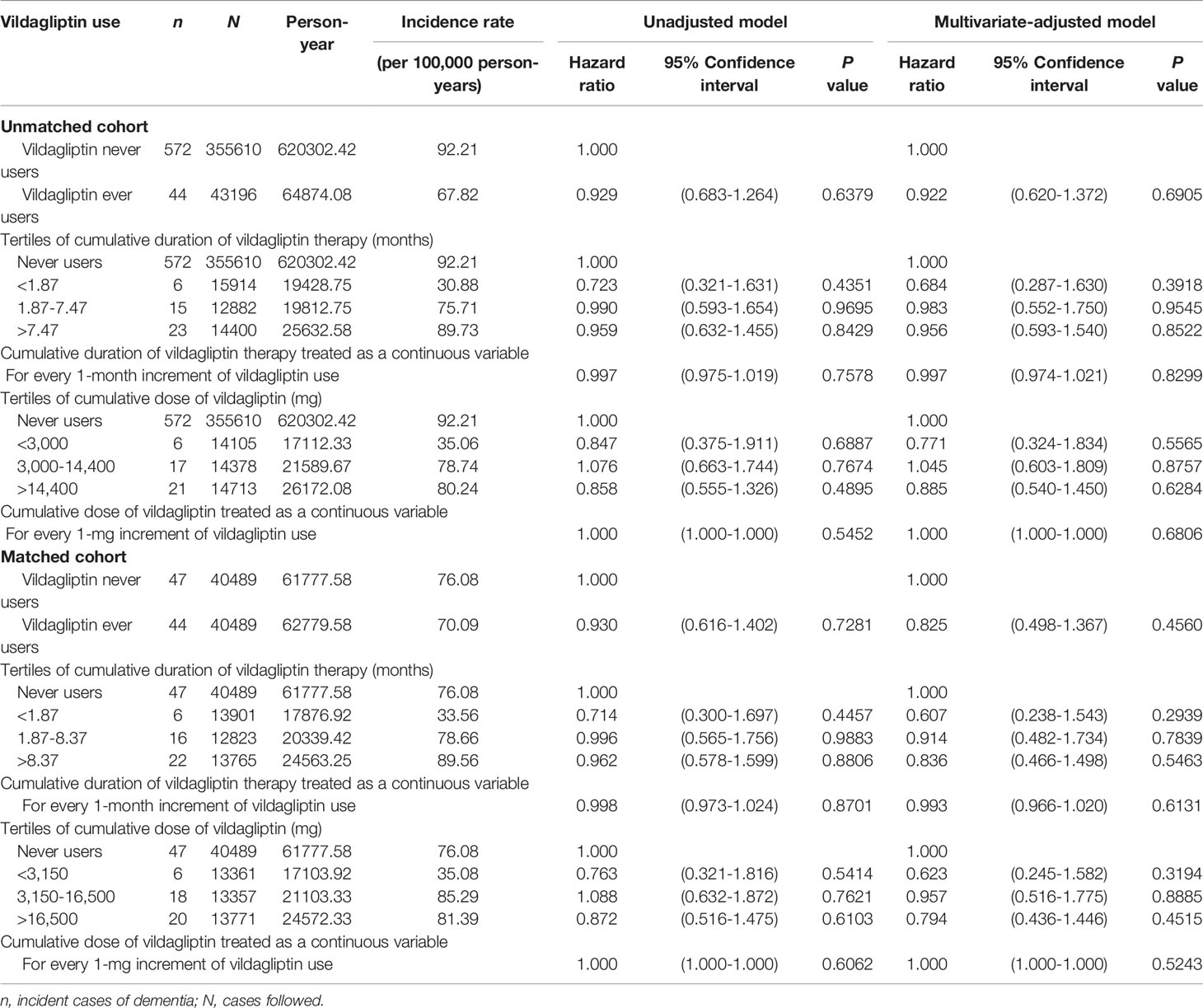
Results: There were 355610 never users and 43196 ever users in the unmatched cohort and 40489 never users and 40489 ever users in the matched cohort. In the unmatched cohort, unadjusted HR (95% CI) was 0.929 (0.683-1.264) and the multivariate-adjusted HR (95% CI) was 0.922 (0.620-1.372). In the matched cohort, the unadjusted HR (95% CI) was 0.930 (0.616-1.402) and the multivariate-adjusted HR (95% CI) was 0.825 (0.498-1.367). None of the analyses conducted for cumulative duration and cumulative dose was significant, either being treated as tertile cutoffs or as continuous variables, in either the unmatched cohort or the matched cohort.
Conclusions: This study showed a neutral effect of vildagliptin on dementia risk.
Introduction
Both diabetes and dementia affect hundreds of millions of the world population. The International Diabetes Federation estimates that 463 million people or 1 in every 11 adults aged 20 to 79 years have diabetes mellitus over the world (1). On the other hand, the World Health Organization estimates that around 50 million people are suffering from dementia over the world and every year there are nearly 10 million new cases of dementia (2). Diabetes mellitus and dementia are closely linked and diabetes patients may have a significantly higher risk of dementia. According to a meta-analysis that included 20 studies, diabetes mellitus is associated with an approximately 70% higher risk of all types of dementia (3). Studies conducted in Taiwan using the reimbursement database of the National Health Insurance (NHI) showed a similarly increased risk of dementia of 50% (4) to 60% (5) in the diabetes patients. Dementia can be resulted from either a vascular etiology or a neurodegenerative disease known as Alzheimer’s disease (which contributes to 60-70% of the cases of dementia) (2). The two disease entities may share common pathophysiological changes of impaired insulin expression and insulin resistance, leading to the coining of “type 3 diabetes” for Alzheimer’s disease (6). The higher risk of dementia in diabetes patients may also be explained by vascular and metabolic changes associated with hyperglycemia and diabetes-related comorbidities, including atherosclerosis, increased deposition of advanced glycation end-products, dysregulation of lipid metabolism, and augmented status of inflammation and oxidative stress (6, 7).
Dipeptidyl peptidase-4 (DPP4) inhibitors are commonly used oral antidiabetic drugs that stimulate insulin secretion by prolonging the half-life of glucagon like peptide-1 and glucose dependent insulinotropic polypeptide (8). Vildagliptin is one of the drugs in the class of DPP4 inhibitors. Previous in vitro and animal studies suggested that vildagliptin might improve cognitive dysfunction, exert neuroprotective effect and prevent the development of Alzheimer’s disease or dementia (9–17). A recent animal study from China suggested that vildagliptin might alleviate cognitive deficits of spatial learning and memory by using the Morris water maze in streptozotocin-induced diabetes in male Wistar rats (18). Such a beneficial effect might be exerted through reducing the levels of apoptosis-related proteins in the hippocampus, probably via reversing diabetes-induced decrease in the phosphorylated (p)-protein kinase B (Akt) and p-glycogen synthase kinase 3β (18). However, whether this neuroprotective effect observed in in vitro and animal studies could be applied to millions of patients with type 2 diabetes mellitus who had been treated with vildagliptin has not been answered. The purpose of the present study was to compare the dementia risk in patients with type 2 diabetes mellitus who had been treated with vildagliptin to those who had never been treated with vildagliptin by using the reimbursement database of the Taiwan’s NHI.
Materials and Methods
This is a retrospective cohort study that used the longitudinal reimbursement database of Taiwan’s NHI, which has been implemented since March 1995. The NHI is a unique and compulsive healthcare system covering >99.9% of Taiwan’s population. The Bureau of NHI signed contracts with all in-hospitals and 93% of all medical settings throughout Taiwan.
The database is managed by the Ministry of Health and Welfare and keeps all records of disease diagnoses, medication prescriptions and performed procedures. It can be used for academic research after ethics review and the study was approved by the Research Ethics Committee C of the National Taiwan University Hospital (NTUH-REC No. 201805002WC). On-site analyses were conducted at the Health and Welfare Data Center of the Ministry. Informed consent was not required according to local regulations because the database had been de-identified before release for analyses for the protection of privacy.
Throughout the study period, diabetes mellitus was coded 250.XX according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and dementia was coded as abridged codes of A210 or A222, or as ICD-9-CM codes of 290.0, 290.1, 290.2, 290.4, 294.1, 331.0–331.2, or 331.7–331.9.
The procedures used to create an unmatched cohort and a cohort of 1:1 matched pairs of ever and never users of vildagliptin are shown in Figure 1. At first, 1,031,243 patients who had newly diagnosed diabetes mellitus during 2002-2014 and had been prescribed antidiabetic drugs for 3 or more times within one year were identified from the outpatient clinics. Patients who had a diagnosis of diabetes mellitus in 2001 or before were excluded to ensure a new diagnosis after 2002. The following patients were then excluded: 1) 449,985 patients who had ever used other incretin-based therapies [including sitagliptin (n = 321,767), saxagliptin (n = 116,563), linagliptin (n = 124,151), alogliptin (n=467) and glucagon like peptide-1 receptor agonists (n = 8,458)] and/or sodium-glucose cotransporter-2 inhibitors (n = 32,862); 2) 22,567 patients with a diagnosis of dementia before start of follow-up; 3) 3,212 patients who died (n = 9) or censored ( n= 3,212) before start of follow-up; 4) 5,154 patients with type 1 diabetes mellitus; 5) 1,557 patients with missing data; 6) 53,166 patients who had been diagnosed of any cancer before start of follow-up (cancer patients were excluded because they might have shortened lifespan and might have distorted follow-up time, and dementia could be misdiagnosed from the clinical presentations of malignancy); 7) 1,509 patients aged <25 years at start of follow-up; 8) 78,322 patients aged >75 years at start of follow-up [the life expectancy of the Taiwan population at the beginning of the study in the year 2000 was approximately 75 years (19), therefore inclusion of older patients might tend to suffer from “healthy survivor” bias (20)]; and 9) 16,965 patients with a follow-up duration <6 months. As a result, 43,196 ever users and 355,610 never users of vildagliptin were identified (unmatched original cohort). A cohort of 1:1 matched pairs of 40,489 ever users and 40,489 never users (the matched cohort) was created by matching on propensity score based on the Greedy 8→1 digit match algorithm (21). Logistic regression was used to create the propensity score from all characteristics listed in Table 1.
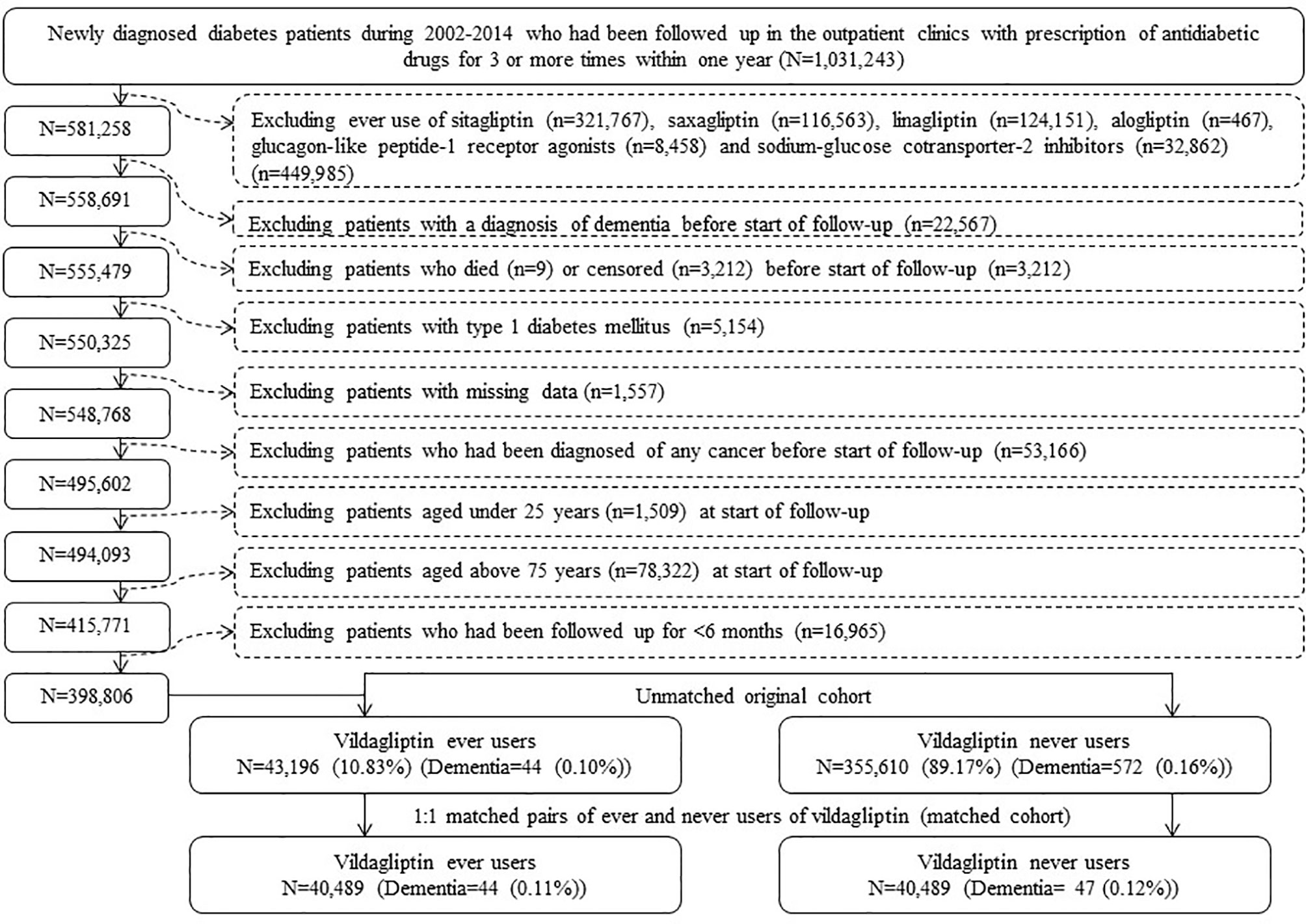
Figure 1 Flowchart showing the procedures followed in creating the unmatched original cohort and a cohort of 1:1 matched pairs of ever users and never users of vildagliptin from the reimbursement database of the National Health Insurance.
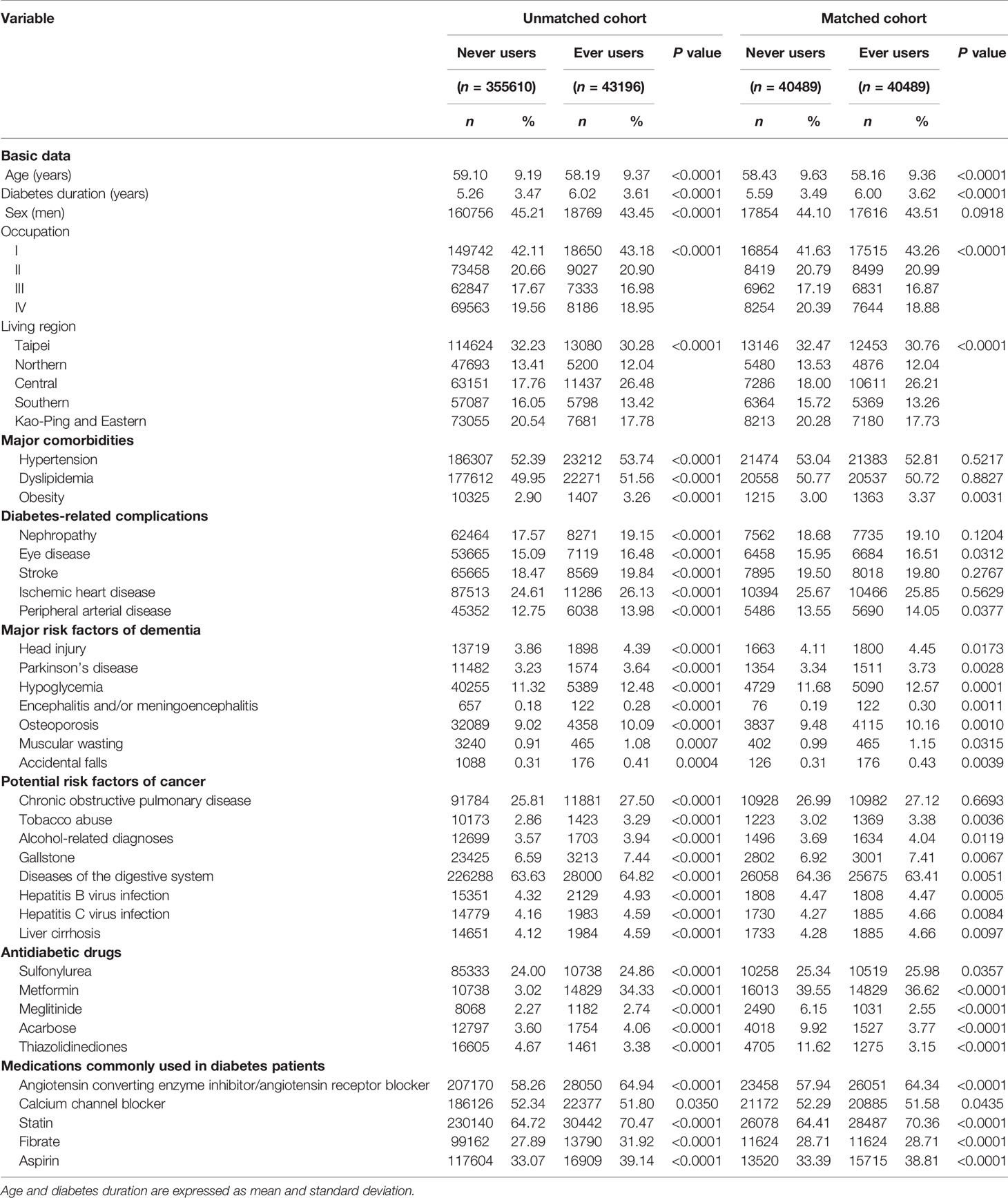
Table 1 Characteristics of never users and ever users of vildagliptin in the unmatched original cohort and in the matched cohort.
Cumulative duration (in months) and cumulative dose (in mg) of vildagliptin therapy were calculated from the database. Potential confounders included the following categories: basic data, major comorbidities associated with diabetes mellitus, diabetes-related complications, major risk factors of dementia, potential risk factors of cancer, antidiabetic drugs and medications commonly used in diabetes patients. Basic data included age, diabetes duration, sex, occupation and living region (classified as Taipei, Northern, Central, Southern, and Kao-Ping/Eastern). Occupation was classified as class I (civil servants, teachers, employees of governmental or private businesses, professionals and technicians), class II (people without a specific employer, self-employed people or seamen), class III (farmers or fishermen) and class IV (low-income families supported by social welfare, or veterans). Major comorbidities associated with diabetes mellitus included hypertension (ICD-9-CM 401-405), dyslipidemia (272.0-272.4) and obesity (278). Major diabetes-related complications included nephropathy (580-589), eye diseases (250.5: diabetes with ophthalmic manifestations, 362.0: diabetic retinopathy, 369: blindness and low vision, 366.41: diabetic cataract, and 365.44: glaucoma associated with systemic syndromes), stroke (430-438), ischemic heart disease (410-414) and peripheral arterial disease (250.7, 785.4, 443.81 and 440-448). Potential risk factors of dementia included head injury (959.01), Parkinson’s disease (332), hypoglycemia (251.0, 251.1 and 251.2), encephalitis and/or meningoencephalitis (323, 062, 063, 064 and 054.3), osteoporosis (733.00), muscular wasting (728.2) and accidental falls (E880-E888). Potential risk factors of cancer included chronic obstructive pulmonary disease (a surrogate for smoking, 490-496), tobacco abuse (305.1, 649.0 and 989.84), alcohol-related diagnoses (291, 303, 535.3, 571.0-571.3 and 980.0), gallstone (574.00, 574.01, 574.10, 574.11, 574.20, 574.21 and A348), diseases of the digestive system (520-579), hepatitis B virus infection (070.22, 070.23, 070.32, 070.33 and V02.61), hepatitis C virus infection (070.41, 070.44, 070.51, 070.54 and V02.62), and liver cirrhosis (571.5). Antidiabetic drugs included sulfonylurea, metformin, meglitinide, acarbose and thiazolidinediones. Commonly used medications in diabetes patients included angiotensin converting enzyme inhibitor/angiotensin receptor blocker, calcium channel blocker, statin, fibrate and aspirin.
Student’s t test compared the difference of age and diabetes duration between never and ever users and Chi-square test was used for other variables.
Incidence density of dementia was calculated for never users, ever users and tertiles of cumulative duration and cumulative dose of vildagliptin therapy. The numerator was the case number of newly diagnosed dementia identified during follow-up and the denominator was the follow-up duration in person-years. Follow-up started on January 1, 2015 and ended on December 31, 2016, at the time of a new diagnosis of dementia, or on the date of death or the last reimbursement record, whichever occurred first.
Cox proportional hazards model was used to estimate the unadjusted and multivariate-adjusted hazard ratios and their 95% confidence intervals for ever users versus never users, for users categorized according to tertiles of cumulative duration and cumulative dose versus never users, and for cumulative duration (every 1-month increment) and cumulative dose (every 1-mg increment) of vildagliptin therapy being treated as continuous variables. Analyses were conducted in the unmatched cohort and the matched cohort, respectively. In the multivariate-adjusted models, all characteristics listed in Table 1 were considered as potential confounders.
To examine whether the findings might be consistent for patients enrolled during three different periods of time, i.e., 2002-2005, 2006-2009 and 2010-2014, multivariate-adjusted models were created for the unmatched cohort enrolled during the three periods.
Analyses were conducted using SAS statistical software, version 9.4 (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
Results
Table 1 shows the characteristics of never users and ever users of vildagliptin in the unmatched cohort and the matched cohort, respectively.
The incidence of dementia and the unadjusted and multivariate-adjusted hazard ratios by vildagliptin exposure are shown in Table 2. The overall hazard ratio comparing ever versus never users suggested a null association. Neither the cumulative duration nor the cumulative dose of vildagliptin therapy was significantly associated with the risk of dementia when these parameters were categorized into tertiles or treated as continuous variables. The findings consistently supported a null association in the unmatched cohort and the matched cohort.
Table 3 shows the overall multivariate-adjusted hazard ratios comparing ever versus never users of vildagliptin analyzed in patients enrolled during three different periods, i.e., 2002-2005, 2006-2009 and 2010-2014. The results suggested a null association in all subgroups.
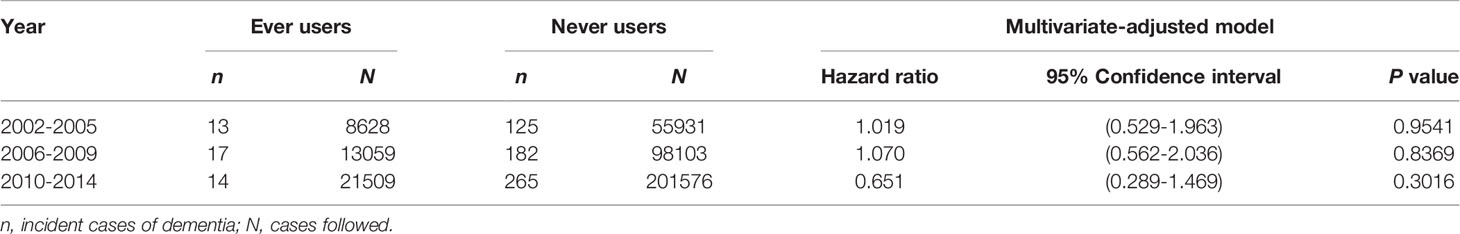
Table 3 Incidence of dementia comparing ever versus never users of vildagliptin in patients enrolled during three different periods of time in the unmatched cohort.
Discussion
The findings suggested that vildagliptin use has a null association with dementia risk in patients with type 2 diabetes mellitus (Tables 2 and 3).
Antidiabetic drugs are being used by thousands of millions of diabetes patients. Therefore, the safety and potential pleiotropic effects or benefits of antidiabetic drugs are clinically important, especially for dementia, a disease that affects millions of patients and has a close link with diabetes mellitus. This study may have some clinical and research significance. First, neuroprotective findings of vildagliptin observed in in vitro, in vivo and animal studies should not be readily interpreted as a potential protection against dementia in humans without consideration of its accessibility to human brain. It is interesting that vildagliptin alleviated cognitive deficits of spatial learning and memory in rats with streptozotocin-induced diabetes (18), but this benefit could not be similarly demonstrated in humans in the present study (Tables 2 and 3). One of the possible explanations is that most DPP4 inhibitors cannot readily pass through the blood-brain barrier in humans (22). However, vildagliptin, a small molecule with a molecular weight of 303.4 Daltons (g/mol) (23), might cross the blood-brain barrier more efficiently in streptozotocin-induced diabetes rats because streptozotocin administration may cause a progressive increase in the blood-brain barrier permeability (especially significant in the midbrain) of small molecules [using vascular space markers ranging from 342 to 65,000 Daltons (g/mol) from 28 to 90 days] (24). In the animal study conducted by Zhang et al., vildagliptin was administered for 4 consecutive weeks after successful induction of diabetes by streptozotocin for 10 weeks (18). This time frame just met the time of streptozotocin-induced progressive increase of blood-brain barrier permeability observed by Huber et al. (24). Although diabetes mellitus has been claimed to affect the permeability of blood-brain barrier, findings derived from human studies are still lacking and the conclusions remain controversial (25). Therefore, the findings derived from streptozotocin-induced diabetes might not be readily applied to patients with diabetes mellitus if the blood-brain barrier remains intact. More in-depth studies are required to explore the possible effect of vildagliptin on the risk of dementia in humans. Second, patients with type 2 diabetes mellitus in East Asia are characterized by more remarkable β‐cell dysfunction and less insulin resistance than in Caucasians, and DPP4 inhibitors seem to exert better glycemic control in East Asians (26). In Japan, DPP4 inhibitors have become the first-line antidiabetic drugs and more than 70% of the diabetes patients are being treated with incretin-based therapies (26). Although major clinical trials suggested a neutral cardiovascular effect of DPP4 inhibitors (27) and the present study did not favor a beneficial effect of vildagliptin on dementia, DPP4 inhibitors can at least be safely used for glycemic control in older patients because of a high tolerability and a lack of hypoglycemic risk (28). Third, recent studies suggested that DPP4 inhibitors (especially vildagliptin and linagliptin) are associated with a higher risk of bullous pemphigoid (29–31). According to an observational study conducted in Taiwan, the risk factors of bullous pemphigoid in patients with type 2 diabetes mellitus seemed to be associated with using DPP4 inhibitors, having dementia and taking spironolactone (32). Therefore, DPP4 inhibitors should better be avoided in diabetes patients with dementia and/or taking spironolactone.
Some potential biases commonly seen in pharmacoepidemiological studies such as selection bias, prevalent user bias, immortal time bias and confounding by indication have been addressed in the present study. Selection bias would not be a problem because of the use of the nationwide database that covers more than 99.9% of the population. Prevalent user bias was avoided by including patients with new-onset type 2 diabetes mellitus and new users of vildagliptin (Figure 1).
Immortal time refers to the follow-up period when the outcome cannot happen. When the treatment status or follow-up time is inappropriately assigned, immortal time bias can be introduced (33). We tried to exclude patients with ambiguous diagnosis of diabetes mellitus by enrolling only patients who had been prescribed antidiabetic drugs for 3 or more times within one year (Figure 1). In the universal healthcare system in Taiwan, the information of all prescriptions in the NHI was complete during the whole follow-up period and misclassification of treatment status was not likely. Therefore, inappropriate assignment of treatment status was unlikely in the present study.
To avoid the inappropriate assignment of follow-up time, we first enrolled only patients who had been treated with antidiabetic drugs and they were followed up only after a certain period of antidiabetic treatment (Figure 1). This avoided the immortal time between diabetes diagnosis and the start of the use of antidiabetic drugs (i.e., a certain period when the patients could have been put on diet control or exercise and antidiabetic drugs were not used). We then excluded patients with a short follow-up duration of <6 months (Figure 1) to avoid the enrollment of patients with such an immortal time in the calculation of person-years. It should be pointed out that the immortal time during the waiting period between drug prescription and dispense at hospital discharge as described by Lévesque et al. (33) would not happen in Taiwan because all discharge drugs can be obtained at the hospital when the patient is discharged.
To examine whether the results might be affected by potential confounding by indication, we compared the findings between the unmatched cohort and the matched cohort based on propensity score and between the unadjusted and multivariate-adjusted models (Table 2). The findings seemed to be very consistent in different analyses. Analyses in subgroups of patients categorized by the tertiles of exposure parameters and by treating these parameters as continuous variables (Table 2) also supported a lack of association between vildagliptin and dementia. The indications and recommendations for the use of antidiabetic drugs for the treatment of type 2 diabetes mellitus have evolved over the past decades following the introduction of newer classes of antidiabetic drugs and according to the results of novel clinical trials. The ever-changing recommendations for the indications and uses of different classes of antidiabetic drugs would not confound the finding of a neutral effect of vildagliptin on dementia risk while patients enrolled during three different periods of time were analyzed separately (Table 3).
The present study has some other merits. First, the findings can be readily generalized to the whole population because the NHI database covers >99.9% of the Taiwan’s population. Second, self-reporting bias and recall bias could be avoided by using the medical records. Third, although detection bias because of different socioeconomic status could be a severe problem in some countries, this would not be the case in our study because the drug cost-sharing is low and can always be waived in patients with low-income, in veterans and when the patients receive prescription refills for chronic disease in our NHI healthcare system.
There are several limitations in the present study. First, we could only use the ICD-9-CM codes for disease diagnoses and no additional support from laboratory examinations was available in the database. The accuracy of the diagnosis of dementia was not known. If the misdiagnosis was non-differential between ever users and never users of vildagliptin, the estimated effect would be expected to bias towards to null (34) and a true positive or negative effect could not be shown. Therefore, the findings of the present study should better be considered as preliminary and future studies with well-verified cases are required to confirm our findings of a null effect. Second, we did not have measured data of some confounders like blood levels of glucose and insulin, fluctuation of blood glucose, indicators of insulin resistance and β-cell function, anthropometric factors, dietary pattern, nutritional status, lifestyle, smoking, alcohol drinking, family history and genetic parameters. It is recognized that the application of propensity score matching in a retrospective cohort study can never adjust for unmeasured confounders as a randomized control trial can do (35). It is impossible for the present study to assess whether the impact of unmeasured confounders could be substantial and the estimates could be misleading. Therefore, if possible, the findings of the present study should better be confirmed by a randomized control trial in the future. Third, we were not able to discern the two major types of dementia, i.e., vascular or degenerative type, because of lack of sufficient laboratory data. If the effects of vildagliptin were not the same for these two types of dementia, the estimates would be misleading by including different types of dementia. Additionally, to our knowledge, although the accuracy of diabetes diagnosis and most other comorbidities in the NHI database has been validated in previous studies (36, 37), the accuracy of dementia diagnosis remains to be validated. It would be a good future research topic to validate the related diagnostic codes of dementia in the database. Fourth, the mean age of the patients was around 58-59 years old at the start of follow-up (Table 1). The incidence of dementia in these patients might not be high enough to have sufficient power to detect a significant difference. It would be better to include a cohort of older age for additional study in the future. Fifth, the follow-up duration might be too short and therefore the findings should be confirmed by studies with longer follow-up duration. Finally, because the study excluded users of other incretin-based therapies, whether the findings can be applied to other DPP4 inhibitors or to glucagon like peptide-1 receptor agonists is not known.
In conclusion, the present study finds a neutral effect of vildagliptin on the association with dementia risk in Taiwanese patients with type 2 diabetes mellitus. More studies are warranted to clarify the neuroprotective effects of vildagliptin or other DPP4 inhibitors observed in in vitro or animal studies.
Data Availability Statement
The datasets presented in this article are not readily available because public availability of the dataset is restricted by local regulations to protect privacy. Requests to access the datasets should be directed to C-HT,Y2NrdHNoQG1zNi5oaW5ldC5uZXQ=.
Ethics Statement
The studies involving human participants were reviewed and approved by The Research Ethics Committee C of the National Taiwan University Hospital (NTUH-REC No. 201805002WC). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
The study was supported partly by the Ministry of Science and Technology (MOST 107-2221-E-002-129-MY3) of Taiwan and by Novartis Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
References
1. IDF Diabetes Atlas 9th edition (2019). Available at: https://www.diabetesatlas.org/en/ (Accessed April 2, 2021).
2. World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/dementia (Accessed April 2, 2021).
3. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes Mellitus and Risk of Dementia: A Meta-Analysis of Prospective Observational Studies. J Diabetes Investig (2013) 4:640–50. doi: 10.1111/jdi.12087
4. Fan YC, Hsu JL, Tung HY, Chou CC, Bai CH. Increased Dementia Risk Predominantly in Diabetes Mellitus Rather Than in Hypertension or Hyperlipidemia: A Population-Based Cohort Study. Alzheimers Res Ther (2017) 9:7. doi: 10.1186/s13195-017-0236-z
5. Cheng PY, Sy HN, Wu SL, Wang WF, Chen YY. Newly Diagnosed Type 2 Diabetes and Risk of Dementia: A Population-Based 7-Year Follow-Up Study in Taiwan. J Diabetes Complicat (2012) 26:382–7. doi: 10.1016/j.jdiacomp.2012.06.003
6. de la Monte SM, Tong M, Wands JR. The 20-Year Voyage Aboard the Journal of Alzheimer’s Disease: Docking At ‘Type 3 Diabetes’, Environmental/Exposure Factors, Pathogenic Mechanisms, and Potential Treatments. J Alzheimers Dis (2018) 62:1381–90. doi: 10.3233/JAD-170829
7. Li X, Song D, Leng SX. Link Between Type 2 Diabetes and Alzheimer’s Disease: From Epidemiology to Mechanism and Treatment. Clin Interv Aging (2015) 10:549–60. doi: 10.2147/CIA.S74042
8. Mulvihill EE. Dipeptidyl Peptidase Inhibitor Therapy in Type 2 Diabetes: Control of the Incretin Axis and Regulation of Postprandial Glucose and Lipid Metabolism. Peptides (2018) 100:158–64. doi: 10.1016/j.peptides.2017.11.023
9. D’Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, et al. Long-Term Inhibition of Dipeptidyl Peptidase-4 in Alzheimer’s Prone Mice. Exp Gerontol (2010) 45:202–7. doi: 10.1016/j.exger.2009.12.004
10. Matteucci E, Giampietro O. Mechanisms of Neurodegeneration in Type 2 Diabetes and the Neuroprotective Potential of Dipeptidyl Peptidase 4 Inhibitors. Curr Med Chem (2015) 22:1573–81. doi: 10.2174/0929867322666150227153308
11. Pintana H, Tanajak P, Pratchayasakul W, Sa-Nguanmoo P, Chunchai T, Satjaritanun P, et al. Energy Restriction Combined With Dipeptidyl Peptidase-4 Inhibitor Exerts Neuroprotection in Obese Male Rats. Br J Nutr (2016) 17:1–9. doi: 10.1017/S0007114516003871
12. Sripetchwandee J, Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP-4 Inhibitor and PPARγ Agonist Restore the Loss of CA1 Dendritic Spines in Obese Insulin-Resistant Rats. Arch Med Res (2014) 45:547–52. doi: 10.1016/j.arcmed.2014.09.002
13. Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, et al. Vildagliptin: An Anti-Diabetes Agent Ameliorates Cognitive Deficits and Pathology Observed in Streptozotocin-Induced Alzheimer’s Disease. J Pharm Pharmacol (2013) 65:1773–84. doi: 10.1111/jphp.12148
14. Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Pparγ Agonist Improves Neuronal Insulin Receptor Function in Hippocampus and Brain Mitochondria Function in Rats With Insulin Resistance Induced by Long Term High-Fat Diets. Endocrinology (2012) 153:329–38. doi: 10.1210/en.2011-1502
15. Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-Inhibitor Improves Neuronal Insulin Receptor Function, Brain Mitochondrial Function and Cognitive Function in Rats With Insulin Resistance Induced by High-Fat Diet Consumption. Eur J Neurosci (2013) 37:839–49. doi: 10.1111/ejn.12088
16. Zheng T, Qin L, Chen B, Hu X, Zhang X, Liu Y, et al. Association of Plasma DPP4 Activity With Mild Cognitive Impairment in Elderly Patients With Type 2 Diabetes: Results From the GDMD Study in China. Diabetes Care (2016) 39:1594–601. doi: 10.2337/dc16-0316
17. Nath S, Ghosh SK, Choudhury Y. A Murine Model of Type 2 Diabetes Mellitus Developed Using a Combination of High Fat Diet and Multiple Low Doses of Streptozotocin Treatment Mimics the Metabolic Characteristics of Type 2 Diabetes Mellitus in Humans. J Pharmacol Toxicol Methods (2017) 84:20–30. doi: 10.1016/j.vascn.2016.10.007
18. Zhang DD, Shi N, Fang H, Ma L, Wu WP, Zhang YZ, et al. Vildagliptin, a DPP4 Inhibitor, Alleviates Diabetes-Associated Cognitive Deficits by Decreasing the Levels of Apoptosis-Related Proteins in the Rat Hippocampus. Exp Ther Med (2018) 15:5100–6. doi: 10.3892/etm.2018.6016
19. Taiwan Life Expectancy 1950-2021. Available at: https://www.macrotrends.net/countries/TWN/taiwan/life-expectancy (Accessed April 2, 2021).
20. Galatas C, Afilalo J. Transcatheter Aortic Valve Replacement Over Age 90: Risks vs Benefits. Clin Cardiol (2020) 43:156–62. doi: 10.1002/clc.23310
21. Parsons LS. Performing a 1:N Case-Control Match on Propensity Score. Available at: http://www2.sas.com/proceedings/sugi29/165-29.pdf (Accessed April 2, 2021).
22. Deacon CF. Dipeptidyl Peptidase-4 Inhibitors in the Treatment of Type 2 Diabetes: A Comparative Review. Diabetes Obes Metab (2011) 13:7–18. doi: 10.1111/j.1463-1326.2010.01306.x
23. PubChem. US National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/cid-5251896 (Accessed April 2, 2021).
24. Huber JD, VanGilder RL, Houser KA. Streptozotocin-Induced Diabetes Progressively Increases Blood-Brain Barrier Permeability in Specific Brain Regions in Rats. Am J Physiol Heart Circ Physiol (2006) 291:H2660–8. doi: 10.1152/ajpheart.00489.2006
25. Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J Pharmacovigil (2014) 2:125. doi: 10.4172/2329-6887.1000125
26. Seino Y, Kuwata H, Yabe D. Incretin-Based Drugs for Type 2 Diabetes: Focus on East Asian Perspectives. J Diabetes Investig (2016) Suppl 1:102–9. doi: 10.1111/jdi.12490
27. Home P. Cardiovascular Outcome Trials of Glucose-Lowering Medications: An Update. Diabetologia (2019) 62:357–69. doi: 10.1007/s00125-018-4801-1
28. Ling J, Cheng P, Ge L, Zhang DH, Shi AC, Tian JH, et al. The Efficacy and Safety of Dipeptidyl Peptidase-4 Inhibitors for Type 2 Diabetes: A Bayesian Network Meta-Analysis of 58 Randomized Controlled Trials. Acta Diabetol (2019) 56:249–72. doi: 10.1007/s00592-018-1222-z
29. Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl Peptidase-4 Inhibitor-Associated Bullous Pemphigoid. Front Immunol (2019) 10:1238. doi: 10.3389/fimmu.2019.01238
30. Nishie W. Dipeptidyl Peptidase IV Inhibitor-Associated Bullous Pemphigoid: A Recently Recognized Autoimmune Blistering Disease With Unique Clinical, Immunological and Genetic Characteristics. Immunol Med (2019) 42:22–8. doi: 10.1080/25785826.2019.1619233
31. Kridin K, Bergman R. Association of Bullous Pemphigoid With Dipeptidyl-Peptidase 4 Inhibitors in Patients With Diabetes: Estimating the Risk of the New Agents and Characterizing the Patients. JAMA Dermatol (2018) 154:1152–8. doi: 10.1001/jamadermatol.2018.2352
32. Guo JY, Chen HH, Yang YC, Wu PY, Chang MP, Chen CC. The Association of Dipeptidyl Peptidase IV Inhibitors and Other Risk Factors With Bullous Pemphigoid in Patients With Type 2 Diabetes Mellitus: A Retrospective Cohort Study. J Diabetes Complicat (2020) 34:107515. doi: 10.1016/j.jdiacomp.2019.107515
33. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of Immortal Time Bias in Cohort Studies: Example Using Statins for Preventing Progression of Diabetes. BMJ (2010) 340:b5087. doi: 10.1136/bmj.b5087
34. Kesmodel US. Information Bias in Epidemiological Studies With a Special Focus on Obstetrics and Gynecology. Acta Obstet Gynecol Scand (2018) 97:417–23. doi: 10.1111/aogs.13330
35. Reiffel JA. Propensity Score Matching: The ‘Devil is in the Details’ Where More may be Hidden Than You Know. Am J Med (2020) 133:178–81. doi: 10.1016/j.amjmed.2019.08.055
36. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of Diabetes Diagnosis in Health Insurance Claims Data in Taiwan. J Formos Med Assoc (2005) 104:157–63.
Keywords: vildagliptin, dementia, diabetes mellitus, pharmacoepidemiology, Taiwan
Citation: Tseng C-H (2021) Vildagliptin Has a Neutral Association With Dementia Risk in Type 2 Diabetes Patients. Front. Endocrinol. 12:637392. doi: 10.3389/fendo.2021.637392
Received: 04 December 2020; Accepted: 12 April 2021;
Published: 30 April 2021.
Edited by:
Szu-Tah Chen, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Jung Lung Hsu, Chang Gung University, TaiwanHorng-Yih Ou, National Cheng Kung University Hospital, Taiwan
Copyright © 2021 Tseng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Hsiao Tseng, Y2NrdHNoQG1zNi5oaW5ldC5uZXQ=
 Chin-Hsiao Tseng
Chin-Hsiao Tseng