- 1Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan, China
- 3Shandong Key Laboratory of Reproductive Medicine, Jinan, China
- 4Shandong Provincial Clinical Research Center for Reproductive Health, Jinan, China
- 5National Research Center for Assisted Reproductive Technology and Reproductive Genetics, Shandong University, Jinan, China
- 6School of Basic Medical Science, Shandong First Medical University, Jinan, China
Background: Diminished ovarian reserve (DOR) is one of the most intractable clinical issues in human reproduction and is reported to be associated with raised risk of recurrent pregnancy loss and aneuploid blastocysts. In this study, we aimed to explore whether DOR was also associated with maternal and neonatal complications in in-vitro fertilization/intracytoplasmic sperm injection cycles.
Methods: A retrospective cohort study including women below 40 years of age who achieved singleton live birth after fresh embryo transfer in in-vitro fertilization/intracytoplasmic sperm injection cycles in a single center from January 2012 to June 2019 was conducted. Participants with DOR, defined as basal follicle-stimulating hormone (FSH) ≥ 10IU/L and antimullerian hormone (AMH) < 1.2ng/ml, were enrolled as the study group. The controls were 1:2 matched by age and body mass index with FSH < 10IU/L and AMH ≥ 1.2ng/ml. Maternal and neonatal complications were compared between the DOR group and the controls.
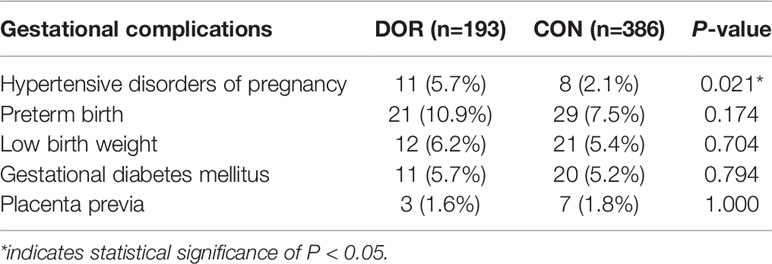
Results: A total of 579 women, 193 in the DOR group and 386 matched as controls, were included in this study. Compared to controls, the incidence of hypertensive disorders of pregnancy was significantly increased in the DOR group (5.7% vs. 2.1%, P = 0.021). DOR patients also presented slightly higher incidences of preterm birth (10.9% vs. 7.5%, P = 0.174) and low birthweight (6.2% vs. 5.4%, P = 0.704) yet without statistical significances. The incidences of gestational diabetes mellitus and placenta previa were comparable between the two groups.
Conclusion: Compared to women with normal ovarian reserve, women with diminished ovarian reserve might have elevated incidence of hypertensive disorders of pregnancy. Patients with diminished ovarian reserve might need more strict antenatal care.
Introduction
According to the American Society for Reproductive Medicine, diminished ovarian reserve (DOR) is defined as women of reproductive age having impaired ovarian reserve and/or poor ovarian response to gonadotropin stimulation (1), clinically characterized by elevated concentration of basal follicle-stimulating hormone (FSH), reduced antimullerian hormone (AMH) level as well as declined antral follicle count (AFC). Distinct from the low prevalence of premature ovarian insufficiency, the prevalence of DOR was relatively high, which increased from 19% to 26% from 2004 to 2011 according to a survey among the United States assisted reproductive technology population (2). For DOR patients, the impaired ovarian reserve always leads to fewer oocytes retrieved, less embryos or even no good-quality embryos acquired, which results in poor pregnancy outcomes. Additionally, with the irreversibility of reduced ovarian reserve, DOR becomes one of the most intractable situations in the clinical practice of assisted reproductive technology.
As reported, DOR is associated with raised risk of recurrent pregnancy loss and aneuploid blastocysts (3, 4); however, whether DOR is associated with maternal or neonatal complications remains uncertain. Ovarian aging has been correlated with abnormalities in luteal phase function (5), and recent studies demonstrated that luteal phase defect was related to altered maternal vascular health and higher risk of preeclampsia (6, 7). So, it is worth investigating whether the incidences of hypertensive disorders of pregnancy (HDP) and other perinatal complications are elevated among DOR patients.
The aim of the study was to explore the incidences for adverse maternal and neonatal outcomes, assessed as HDP, preterm birth (PTB), low birthweight (LBW), gestational diabetes mellitus (GDM) and placenta previa in women with DOR after in-vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) treatment.
Materials and Methods
Study Design and Participants
This was a retrospective cohort study of women who achieved singleton live birth after fresh embryo transfer in IVF/ICSI cycles in Center for Reproductive Medicine, Shandong University from January 2012 to June 2019. All participants were below 40 years old. Participants with DOR, defined as basal FSH ≥ 10IU/L measured at least twice and AMH < 1.2ng/ml, were enrolled as the study group. The controls were matched by age and body mass index (BMI) with FSH < 10IU/L and AMH ≥ 1.2ng/ml. To be specific, we stratified age by an interval of 5 years and in each age subgroup we stratified BMI by the category according to the World Health Organization criteria (8) (<18.5 kg/m2; ≥18.5kg/m2, <23 kg/m2; ≥23kg/m2, <27.5 kg/m2; ≥27.5kg/m2), then the controls were 1:2 enrolled. Data in this study were collected from the electronic medical record system in our center. Since female age makes huge impact on the adverse maternal and neonatal outcomes as well as the ovarian reserve, the participants were then divided into two subgroups by age with the cutoff value of 35 years old in order to further explore the associations between DOR and various pregnancy complications in different age groups. Patients with diabetes mellitus or preconceptional fasting glucose (PFG) ≥ 7.0 mmol/L, preconceptional hypertension, polycystic ovary syndrome (PCOS), thyroid dysfunction, hyperprolactinemia, endometriosis, chromosomal abnormalities, chemo-/radio-therapy or autoimmune disorders were excluded from the study. The study was approved by the Institutional Review Board (IRB) of the Center for Reproductive Medicine, Shandong University. All the participants enrolled in this study had signed written informed consent.
Assessment of Maternal and Neonatal Complications
Maternal and neonatal complications analyzed included HDP, PTB, LBW, GDM and placenta previa. Hypertensive disorders were diagnosed according to the International Society for the Study of Hypertension in Pregnancy (ISSHP) criteria (9). HDP in this study included gestational hypertension and preeclampsia and excluded chronic hypertension. PTB was defined as live birth before 37 gestational age. LBW referred to birth weight of full-term delivered newborns below 2500 g. According to WHO criteria, GDM was diagnosed when met one or more of the following criteria: fasting plasma glucose ≥ 7.0 mmol/l; 2-h plasma glucose ≥ 11.1 mmol/l following a 75 g oral glucose load; random plasma glucose ≥ 11.1 mmol/l in the presence of diabetes symptoms (10). Placenta praevia was diagnosed when the placenta lies directly over the internal os by transvaginal ultrasound (11).
Statistical Analysis
The baseline characteristics of patients were summarized using means ± standard deviations for continuous variables and frequencies and percentages for categorical variables. The between-group differences of continuous data were assessed by student’s t-test. Categorical data were tested by χ² analysis, with Fisher’s exact test for expected frequencies less than five. A conditional logistic regression model was also applied to adjust the factors manifesting significant differences between the DOR and control groups including AFC, basal LH concentration, days of ovarian stimulation, total gonadotropin dose and estradiol level on human chorionic gonadotropin (hCG) trigger day. All the data were analyzed by SPSS 21.0 (SPSS Inc, Chicago, IL). P < 0.05 was considered statistically significant.
Results
A total of 579 women, 193 with DOR and 386 as controls, were enrolled in this study. Demographic and ovarian stimulation characteristics were presented in Table 1. Due to the disease attributes, between-group significant differences were observed as expected among factors including AFC (5.70 vs. 11.52, P < 0.001), AMH (0.56 vs. 2.92ng/ml, P < 0.001), basal FSH (13.77 vs. 6.86IU/L, P < 0.001), luteinizing hormone (LH, 6.01 vs. 4.97IU/L, P < 0.001), days of stimulation (9.65 vs. 10.99, P < 0.001), total gonadotropin dose (2367.68 vs. 1975.32IU, P < 0.001) and estradiol level on hCG trigger day (1468.70 vs. 2953.50pg/ml, P < 0.001).
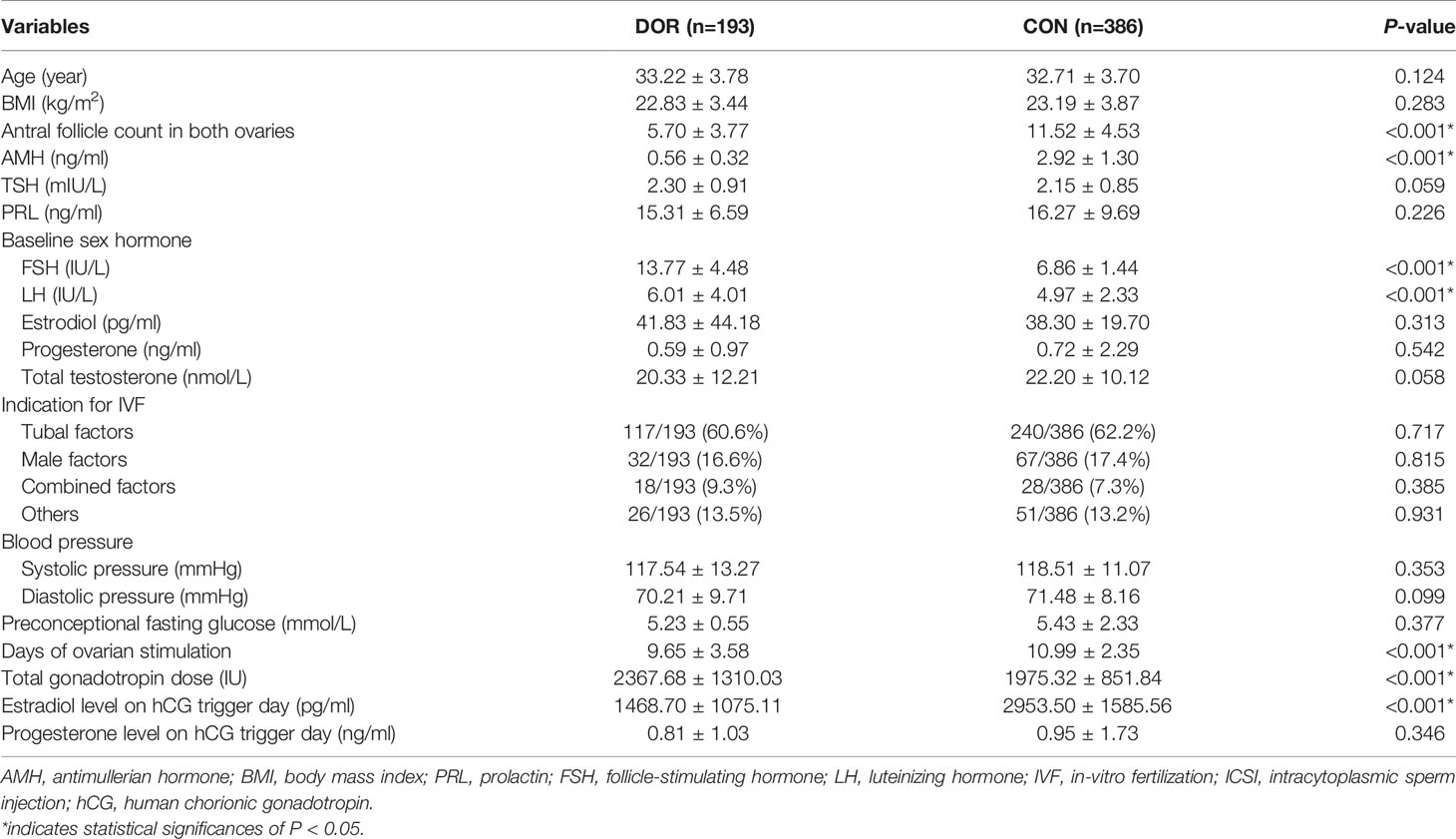
Table 1 Demographic and ovarian stimulation characteristics of patients and controls during IVF/ICSI treatment.
Maternal and neonatal complications were listed in Table 2 and presented in Figure 1. Compared to controls, the incidence of HDP was significantly elevated in the DOR group (5.7% vs. 2.1%, P = 0.021). DOR patients also presented slightly increased incidences of PTB (10.9% vs. 7.5%, P = 0.174) and LBW (6.2% vs. 5.4%, P = 0.704) yet without statistical significances. The incidences of GDM and placenta previa were comparable between the two groups. After the adjustment, the DOR group still exhibited a raised incidence of HDP compared to the controls (adjusted OR 2.63, 95%CI 1.01-6.84, P=0.045). None of the other maternal or neonatal complications had significantly different incidences between the DOR and non-DOR groups after the adjustment.
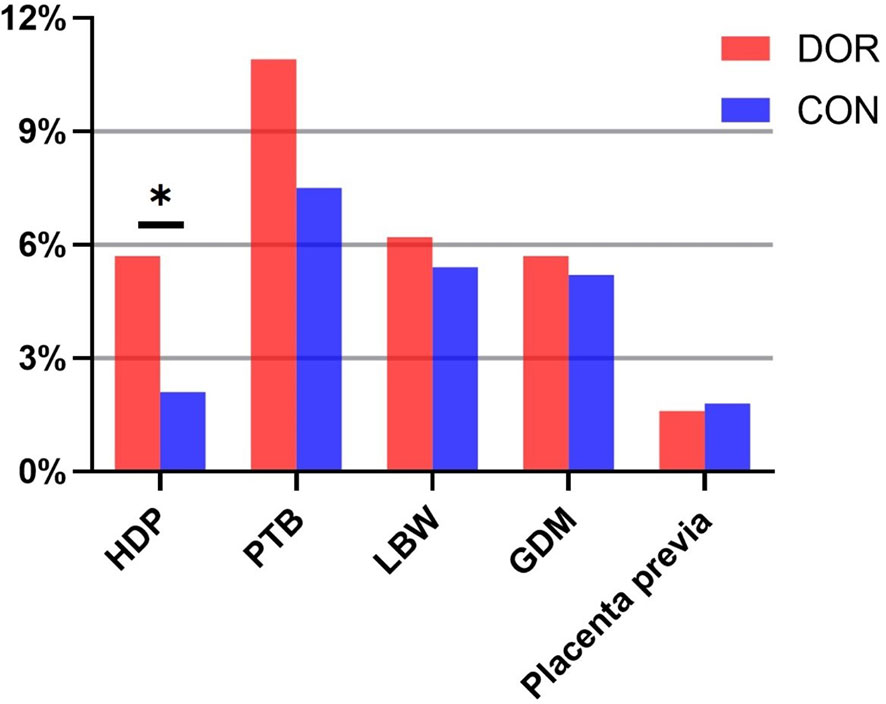
Figure 1 Gestational complications in DOR patients and controls. HDP, hypertensive disorders of pregnancy; PTB, preterm birth; LBW, low birthweight; GDM, gestational diabetes mellitus. *indicates statistical significance of P < 0.05.
Table 3 demonstrated the gestational complications when stratified by age. There were 119 DOR women and 238 controls below 35 years old, leaving 74 DOR patients and 148 age matched controls in the advanced age subgroup. Although lacking of statistical differences, in younger patients with DOR, the incidences of HDP (5.0% vs. 1.3%, P = 0.065), PTB (11.8% vs. 6.3%, P = 0.075) and LBW (6.7% vs. 5.0%, P = 0/515) were increased compared with controls. However, among women over 35 years of age, only the incidence of HDP represented a rising trend in the DOR group (6.8% vs. 3.3%, P = 0.307). The incidences of all other complications were similar between the two groups in women of advanced ages.
Discussion
This study analyzed the risk of maternal and neonatal complications, including HDP, PTB, LBW, GDM and placenta previa in women with DOR after IVF/ICSI treatment. The results suggested that women with impaired ovarian function had higher incidence of HDP. And the incidences of PTB and LBW also had an elevated trend. Our study indicates that patients with DOR are supposed to be under more careful antenatal examinations for any signs of pregnancy complications.
With decreased oocyte quantity, DOR is a common status of ovarian aging. According to the committee opinion of the American Society for Reproductive Medicine, ovarian aging is associated with abnormalities of luteal phase function (5). The progesterone and estradiol metabolites production during luteal phase was decreased greatly among advanced aged women (12, 13). It has been reported that vascular problems and hypertensive disorders of pregnancy was related with luteal phase dysfunction and lacking of relaxin (6, 7).
Similar findings were reported that hormone replace therapy cycles with no corpus luteum in frozen embryo transfer had increased incidence of hypertensive disorders of pregnancy (7, 14, 15). And von Versen-Hoynck et al (6, 7) revealed that relaxin concentration in the hormone replace therapy cycles with no corpus luteum was significantly lower than that in the natural cycles, and maternal vascular health and aortic compliance was impaired remarkably in the hormone replace therapy group as well. In our study, patients with DOR, who were supposed to have poor luteal function producing less relaxin, therefore presented with elevated incidence of HDP.
Relaxin is a potent vasodilator produced only by corpus luteum throughout gestation (16). Relaxin has crucial impacts on the vascular health during pregnancy. First, lacking of or low level of relaxin in the DOR patients may results in impaired decidualization, which is the key pathological process of preeclampsia. Second, dramatic remodeling of the uterine artery wall occurs during pregnancy and relaxin is the leading candidate molecule inducing compositional and geometric remodeling of arteries (17, 18). The arterial remodeling fuels the changes of arterial passive mechanical properties which benefits the arterial compliance. Third, relaxin also has vasodilatory effect acting through increased expression of inducible nitric oxide generation (19, 20).
Another explanation for the elevated HDP incidence in women with impaired ovarian reserve could be ascribed to the deteriorative status of oxidative stress. Two cross-sectional studies indicated that serum levels of inducible nitric oxide synthase, total oxidant status, and oxidative stress index in patients with premature ovarian insufficiency were elevated significantly (21, 22). Oxidative stress could decrease the biodisponibility of nitric oxide and prostacyclin and result in increased vasoconstriction and reduced endothelium-dependent vasodilation (23). Excessive oxidative stress is proved to be associated with severe hypertension and target organ damage (24) in human and mice.
Our study also provided the clinicians with more informative clues for antenatal managements of patients with impaired ovarian reserve. HDP is one of the major causes of maternal and neonatal morbidity and mortality (25). Thus, it is crucial to identify the risk factors for HDP and take preventative measures. Since DOR is a risk factor for HDP, obstetricians need to be vigilant on patients’ ovarian reserve evaluation and pay special attention to their blood pressure for patients with DOR. For example, frequent home blood pressure monitoring will be helpful, and weight management during pregnancy is recommended as obese is a known risk factor for preeclampsia. Low-dose aspirin for prevention of preeclampsia may also be considered for DOR patients, especially for those who have other risk factors for eclampsia.
Previous studies always focused on the pregnancy outcomes of DOR patients except for one study (26) analyzing the perinatal outcomes as well; however, they only included DOR patients under 35 years old. Calhoun et al’ s study (27) was also a retrospective study focusing on the association between FSH and obstetric outcomes including PTB and LBW among women underwent IVF. With a large cohort, their study categorized the participants into three groups (defined by the serum FSH concentration of 1–5, 6–8 and ≥ 9 mIU/ml) and revealed that the incidences of PTB and LBW were not significantly different among the groups. In our study we strictly defined the women with DOR as basal FSH ≥ 10IU/L measured at least twice as well as AMH < 1.2ng/ml, which meant that we homogenized the patients with impaired ovarian reserve and aimed to figure out the characteristics of this specific population. Both two studies failed to reveal a significant association between ovarian reserve and the incidences of PTB and LBW, which might indicate that poor ovarian reserve did not affect PTB or LBW even taken AMH into consideration or lift the FSH cut-off values. The major limitation of our study was its retrospective design. The levels of relaxin and estrogen concentration during pregnancy were lacking. Information of other potential confounders including second-hand smoking exposure, income levels, nutritional status, physical activity, and sleep during pregnancy could not be collected as well. Prospective studies with larger size of patients are needed in the future.
Conclusion
In conclusion, diminished ovarian reserve might be a potential risk factor for hypertensive disorders of pregnancy. Although it still needs further exploration with larger sample size to confirm this conclusion, patients with DOR might be benefit from more careful pregnancy surveillance.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of the Center for Reproductive Medicine, Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
In this research, PL and SH contributed to the conception and design. YZ, SH and QG were involved in data collection and analysis. PL, YQ and SH drafted and modified the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Natural Science Foundation of Shandong Province (ZR2019PH009), Shanghai Commission of Science and Technology (no. 19410760300) and Taishan Scholars Program for Young Experts of Shandong Province (tsqn20161069).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Practice Committee of the American Society for Reproductive M. Testing and Interpreting Measures of Ovarian Reserve: A Committee Opinion. Fertil Steril (2015) 103:e9–e17. doi: 10.1016/j.fertnstert.2014.12.093
2. Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished Ovarian Reserve in the United States Assisted Reproductive Technology Population: Diagnostic Trends Among 181,536 Cycles From the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril (2015) 104:612–19 e3. doi: 10.1016/j.fertnstert.2015.05.017
3. Bunnewell SJ, Honess ER, Karia AM, Keay SD, Al Wattar BH, Quenby S. Diminished Ovarian Reserve in Recurrent Pregnancy Loss: A Systematic Review and Meta-Analysis. Fertil Steril (2020) 113:818–27 e3. doi: 10.1016/j.fertnstert.2019.11.014
4. Shahine LK, Marshall L, Lamb JD, Hickok LR. Higher Rates of Aneuploidy in Blastocysts and Higher Risk of No Embryo Transfer in Recurrent Pregnancy Loss Patients With Diminished Ovarian Reserve Undergoing in Vitro Fertilization. Fertil Steril (2016) 106:1124–8. doi: 10.1016/j.fertnstert.2016.06.016
5. Practice Committee of the American Society for Reproductive M. Current Clinical Irrelevance of Luteal Phase Deficiency: A Committee Opinion. Fertil Steril (2015) 103:e27–32. doi: 10.1016/j.fertnstert.2014.12.128
6. von Versen-Hoynck F, Narasimhan P, Selamet Tierney ES, Martinez N, Conrad KP, Baker VL, et al. Absent or Excessive Corpus Luteum Number is Associated With Altered Maternal Vascular Health in Early Pregnancy. Hypertension (2019) 73:680–90. doi: 10.1161/HYPERTENSIONAHA.118.12046
7. von Versen-Hoynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased Preeclampsia Risk and Reduced Aortic Compliance With in Vitro Fertilization Cycles in the Absence of a Corpus Luteum. Hypertension (2019) 73:640–9. doi: 10.1161/HYPERTENSIONAHA.118.12043
8. WHO Expert Consultation. Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
9. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The Classification, Diagnosis and Management of the Hypertensive Disorders of Pregnancy: A Revised Statement From the ISSHP. Pregnancy Hypertens (2014) 4:97–104. doi: 10.1016/j.preghy.2014.02.001
10. World health organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy: A World Health Organization Guideline. Diabetes Res Clin Pract (2014) 103:341–63. doi: 10.1016/j.diabres.2013.10.012
11. Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-Top Guideline No. 27a. BJOG (2019) 126:e1–e48. doi: 10.1111/1471-0528.15306
12. Mersereau JE, Evans ML, Moore DH, Liu JH, Thomas MA, Rebar RW, et al. Luteal Phase Estrogen is Decreased in Regularly Menstruating Older Women Compared With a Reference Population of Younger Women. Menopause (2008) 15:482–6. doi: 10.1097/gme.0b013e31815982cf
13. Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of Reproductive Hormonal Dynamics in the Perimenopause. J Clin Endocrinol Metab (1996) 814:1495–501. doi: 10.1210/jcem.81.4.8636357
14. Ginstrom Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and Maternal Outcome After Frozen Embryo Transfer: Increased Risks in Programmed Cycles. Am J Obstet Gynecol (2019) 221:126 e1– e18. doi: 10.1016/j.ajog.2019.03.010
15. Zong L, Liu P, Zhou L, Wei D, Ding L, Qin Y. Increased Risk of Maternal and Neonatal Complications in Hormone Replacement Therapy Cycles in Frozen Embryo Transfer. Reprod Biol Endocrinol (2020) 18:36. doi: 10.1186/s12958-020-00601-3
16. Singh B, Reschke L, Segars J, Baker VL. Frozen-Thawed Embryo Transfer: The Potential Importance of the Corpus Luteum in Preventing Obstetrical Complications. Fertil Steril (2020) 113:252–7. doi: 10.1016/j.fertnstert.2019.12.007
17. Leo CH, Jelinic M, Ng HH, Marshall SA, Novak J, Tare M, et al. Vascular Actions of Relaxin: Nitric Oxide and Beyond. Br J Pharmacol (2017) 174:1002–14. doi: 10.1111/bph.13614
18. Osol G, Moore LG. Maternal Uterine Vascular Remodeling During Pregnancy. Microcirculation (2014) 21:38–47. doi: 10.1111/micc.12080
19. Nistri S, Bani D. Relaxin Receptors and Nitric Oxide Synthases: Search for the Missing Link. Reprod Biol Endocrinol (2003) 1:5. doi: 10.1186/1477-7827-1-5
20. Baccari MC, Bani D. Relaxin and Nitric Oxide Signalling. Curr Protein Pept Sci (2008) 96:638–45. doi: 10.2174/138920308786733921
21. Ağaçayak E, Yaman Görük N, Küsen H, Yaman Tunç S, Başaranoğlu S, İçen MS, et al. Role of Inflammation and Oxidative Stress in the Etiology of Primary Ovarian Insufficiency. Turk J Obstet Gynecol (2016) 13:109–15. doi: 10.4274/tjod.00334
22. Tokmak A, Yıldırım G, Sarıkaya E, Çınar M, Boğdaycıoğlu N, Yılmaz FM, et al. Increased Oxidative Stress Markers May Be a Promising Indicator of Risk for Primary Ovarian Insufficiency: A Cross-Sectional Case Control Study. Rev Bras Ginecol Obstet (2015) 37:411–6. doi: 10.1590/SO100-720320150005397
23. Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative Stress and Human Hypertension: Vascular Mechanisms, Biomarkers, and Novel Therapies. Can J Cardiol (2015) 31:631–41. doi: 10.1016/j.cjca.2015.02.008
24. Dikalov SI, Ungvari Z. Role of Mitochondrial Oxidative Stress in Hypertension. Am J Physiol Heart Circ Physiol (2013) 305:H1417–27. doi: 10.1152/ajpheart.00089.2013
25. Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, et al. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J Am Coll Cardiol (2020) 75:2323–34. doi: 10.1016/j.jacc.2020.03.028
26. Hu S, Xu B, Jin L. Perinatal Outcome in Young Patients With Diminished Ovarian Reserve Undergoing Assisted Reproductive Technology. Fertil Steril (2020) 114:118–24 e1. doi: 10.1016/j.fertnstert.2020.02.112
Keywords: diminished ovarian reserve, maternal and neonatal complications, in-vitro fertilization, hypertensive disorders of pregnancy, ovarian aging
Citation: Han S, Zhai Y, Guo Q, Qin Y and Liu P (2021) Maternal and Neonatal Complications in Patients With Diminished Ovarian Reserve in In-Vitro Fertilization/Intracytoplasmic Sperm Injection Cycles. Front. Endocrinol. 12:648287. doi: 10.3389/fendo.2021.648287
Received: 31 December 2020; Accepted: 14 April 2021;
Published: 29 April 2021.
Edited by:
Qiongjie Zhou, Fudan University, ChinaReviewed by:
Borut Kovacic, Maribor University Medical Centre, SloveniaRossella Mazzilli, Sapienza University of Rome, Italy
Dan Zhang, Zhejiang University, China
Copyright © 2021 Han, Zhai, Guo, Qin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peihao Liu, ZHJwZWloYW9AMTYzLmNvbQ==
 Shuang Han
Shuang Han Yiwei Zhai
Yiwei Zhai Qingqing Guo
Qingqing Guo Yiming Qin6
Yiming Qin6 Peihao Liu
Peihao Liu