- 1Department of Endocrinology and Metabolism, Hacettepe University Faculty of Medicine, Ankara, Turkey
- 2Department of Endocrinology and Metabolism, Koc University Faculty of Medicine, Istanbul, Turkey
- 3Department of Endocrinology and Metabolism, Istanbul University Istanbul Faculty of Medicine, Istanbul, Turkey
- 4Department of Endocrinology and Metabolism, Istanbul University Cerrahpasa Faculty of Medicine, Istanbul, Turkey
- 5Department of Endocrinology and Metabolism, Gazi University Faculty of Medicine, Ankara, Turkey
- 6Department of Endocrinology and Metabolism, Florence Nightingale Hospital, Istanbul, Turkey
This expert panel of diabetes specialists aimed to provide guidance to healthcare providers on the best practice in the use of innovative continuous glucose monitoring (CGM) techniques through a practical and implementable document that specifically addresses the rationale for and also analysis and interpretation of the new standardized glucose reporting system based on standardized CGM metrics and visual ambulatory glucose profile (AGP) data. This guidance document presents recommendations and a useful algorithm for the use of a standardized glucose reporting system in the routine diabetes care setting.
Introduction
Limitations of glycated hemoglobin (HbA1c) per se, underutilization of continuous glucose monitoring (CGM), and lack of an easy to interpret and standardized system for glucose reporting are considered among the key contributors to continued suboptimal glycemic control in diabetes patients despite advances in therapeutics (1).
The main drawbacks of self-monitoring blood glucose (SMBG) systems include inadequate patient compliance to intermittent capillary sampling, unreliability of patient-recorded data, inability to capture and store large amounts of glycemic data including hypoglycemic episodes for extended periods of time (2). In this regard, as strongly advocated by recent consensus statements, the use of CGM via a standardized metric reporting, using the ambulatory glucose profile (AGP) for data visualization, is considered a part of the evolving standard of diabetes care, supplementing periodic HbA1c testing in addition to accurate evaluation of glycemic variability, and the identification of nocturnal hypoglycemic episodes (1–6).
CGM, involving the real-time CGM (rtCGM) and the flash glucose monitoring (FGM) also known as intermittently scanned CGM (isCGM), has become an increasingly used method with technical improvements over time (i.e., in sensor accuracy, convenience and ease of use, and reimbursement conditions) (7). However, despite the increased CGM adoption in insulin-requiring diabetes care as recognized by national and international medical organizations in many countries, successful utilization of CGM data in real-life diabetes care remains relatively low (1, 7).
The recent innovations in sensor technology combined with standardization and simplification of the analysis of glucose data via AGP enable an improved method for retrospective analysis of rtCGM/FGM profiles (1, 8). However, it may remain underutilized in clinical practice due to concerns regarding the complexity and inconvenience of CGM use among physicians and patients and also the clinician’s reluctance due to lack of experience in interpreting CGM data.
This expert panel of diabetes specialists therefore aimed to provide guidance to healthcare providers on the best practice in the use of innovative CGM techniques through a practical and implementable document that specifically addresses the analysis and interpretation of the new standardized glucose reporting system based on standardized CGM metrics and visual AGP data, and to provide consensus recommendations and a practical algorithm for the potential use of this reporting system in the routine diabetes care setting.
Methods
The present expert panel involved seven diabetes specialists who are key opinion leaders with at least 15 years of experience in dealing with diabetes in Turkey. The panel critically analyzed recommendations from existing guidelines, consensus statements and data from systematic reviews, meta-analyses and literature review of articles published on glycemic control and blood glucose testing in type 1 (T1DM) and type 2 (T2DM) patient populations and agreed on a series of statements supported by scientific evidence and expert clinical opinions to assist healthcare providers on the best practice in analysis and interpretation of the new standardized glucose reporting system based on standardized CGM metrics plus AGP data in diabetes care.
Therefore the main areas addressed by this consensus document include a) an overview of glycemic parameters and diabetes-related complications, b) methods for glucose testing/monitoring (limitations of HbA1c and SBMG, CGM in relation to brief history of technology advancement, indications and currently available systems, published evidence on international practice and limitations), c) basics of new standardized glucose reporting system (rationale and utility, standardized CGM metrics, glucose statistics and targets, and analysis and interpretation of visual AGP data), and d) use of new standardized glucose reporting system in routine clinical practice (consensus recommendations and treatment and follow-up algorithm)
Glycemic Parameters and Diabetes-Related Complications
Long-term hyperglycemia (reflected by elevated HbA1c), glycemic variability (inter-day variations in blood glucose), and glycemic instability (intra-day variations in blood glucose) are considered the major barriers to suboptimal glycemic control, while the risk of hypoglycemia is also increased by both glycemic variability and instability (5). Maintenance of adequate glycemic control is of critical value in the diabetes management, as associated with reduced risk of long-term morbidity and mortality (9, 10). The long-term hyperglycemia and glycemic variability are considered to increase the likelihood of diabetes related microvascular and macrovascular complications in T1DM and T2DM patients (11–13).
Additionally, while intensive glycemic control reduces the onset and severity of microvascular complications along with long-term cardiovascular benefits in T1DM and T2DM patients (14–16), hypoglycemia is also identified as a key determinant of increased risk of mortality in case of aggressively-targeted HbA1c (17, 18).
Hence, intensive glycemic control based on HbA1c targets without considering glycemic variability and instability profile is considered to be associated with increased risk of hypoglycemia, while glycemic variability rather than prolonged hyperglycemia is considered to be responsible for symptoms related to poor glycemic control (5, 18).
Thus, reducing glucose variability, accepted as a clinically valuable marker of glycemic control, is suggested to a valid therapeutic objective per se, alongside correction of elevated HbA1c (19–23).
Glycemic variability, in terms of both the amplitude and the timing of blood glucose fluctuations, has been associated with increased risk of hypoglycemia and hyperglycemia in diabetes patients (23, 24). This emphasizes the use of an accurate and standardized tool for glucose data collection and analysis that would reveal not only the overall glycemic patterns but also dynamic glycemic patterns and timing of deviations along with the hyperglycemic excursions (i.e., after meals) and potentially dangerous hypoglycemia (i.e., nocturnal) (1, 23, 25).
Methods for Glucose Testing/Monitoring
While HbA1c, SMBG, and CGM are the three methods of testing glucose levels, the first two methodologies have been associated with significant drawbacks limiting their use in diabetes care (26).
HbA1c has been the key parameter in glycemic control assessment and the key surrogate marker for the development of long-term diabetes complications in T1DM and T2DM patients (7). However, given that HbA1c reflects the mean blood glucose over the life-span (~120 days) of red blood cells, it is not considered a good indicator of day-to-day diabetes control, glycemic variability, acute glycemic excursions, and associated risks of hypoglycemia or hyperglycemia (5, 7, 23, 25). The failure of HbA1c to reflect the diurnal glucose patterns is a major drawback considering the critical role of these patterns in making safe, effective, and timely insulin adjustment (1). In addition, HbA1c measurement is considered not reliable in certain confounding conditions such as pregnancy, hemoglobinopathies, anemia, and iron deficiency (7).
In contrast to HbA1c measurement, the use of CGM enables the direct observation of glycemic excursions and daily profiles and implementation of related therapy decisions and/or lifestyle modifications along with its ability to identify glucose variability and patterns of hypoglycemia and hyperglycemia (7, 23). Nonetheless, despite its limitations, HbA1c is the only prospectively evaluated parameter in assessment of the risk for diabetes-related complications, and thus should be used as a complementary method to CGM-based glycemic measurements (7).
SMBG, the intermittent finger-tip capillary sampling, is considered the gold standard, cheap, and readily available method for point-of-care glucose measurement (27). Although structured use of SMBG has been associated with improved glycemic control and quality of life (QoL) in diabetes patients (28, 29), it gives just snapshots of blood glucose concentration without capturing enough data points required to provide a complete story of daily glucose control, day-to-day glycemic variability and nocturnal and asymptomatic hypoglycemia and its application is dependent upon the patient’s decision to self-monitor (8, 23, 27, 30, 31). Thus, having limitations in detailed assessment of daily glucose fluctuations to guide the therapy for controlling the glycemic variability, using SMBG data per se may not reveal appropriate therapy decisions (5, 23).
Moreover, patient adherence to routine testing as per guidelines is very poor (only by 44% of T1DM patients and 24% of T2DM patients) due to factors such as fear of blood or needles, concerns about the frequency of application or perception of SMBG as a method used only for the insulin titration (32–35). Accordingly, demanding a self-testing strategy, getting reliable information via SMBG is rarely achieved in routine clinical practice despite proven efficacy in the research setting (5, 36).
However, CGM uses standardized metrics, glucose statistics, and targets to reflect the dynamics of glucose fluctuations and quantify glycemic variability and also hyperglycemic excursions (i.e., after meals) and potentially dangerous hypoglycemia (i.e., nocturnal) (1, 23, 25).
Continuous Glucose Monitoring (CGM)
Brief History of Technology Advancements
CGM emerged by the new millennium as an innovative technology with potential to revolutionize diabetes care (37).
The major developments in the use of CGM in clinical practice included: a) the shift in CGM assessment from a retrospective to a prospective methodology to obtain real-time glucose readings by 2006, b) the identification of a research focus by its introduction to define the purpose of CGM (to characterize diurnal glucose patterns to detect abnormalities, to help identification and success of potential interventions for dysglycemia), c) the introduction of appropriate tools to optimize clinical decision-making by avoiding the errors that minimized the use of SBMG (i.e., incomplete understanding of the purpose, standardization accuracy, and reliability problems) and d) most recently the development of FGM systems that operate without necessitating calibrating interstitial glucose value to the capillary blood glucose and provision of AGP as a scientifically accurate and clinically reasonable method of reporting the dynamic properties of glucose metabolism (37–39).
Accordingly, CGM can provide both the real-time data on glucose levels and trends and the retrospective data on patterns of glycemic control over specified time periods and glucose metrics (1). Being a less-invasive approach than SMBG, CGM is considered to reveal an improved metabolic control with reduced HbA1c and/or the rate of hypoglycemia in T1DM and T2DM patients even in those already utilizing insulin pump therapy and those who have already achieved excellent control (1, 5, 30, 40).
The expert panel recommendations on CGM scope and technology are provided in Box 1.
Box 1. Expert panel recommendations on CGM scope and technology.
● CGM uses standardized metrics, glucose statistics and targets
● Reflects the dynamics of glucose fluctuations and quantify glycemic variability as well as hyperglycemic excursions (i.e. after meals) and potentially dangerous hypoglycemia (i.e. nocturnal)
● Inaccurate blood glucose readings, especially in the hypoglycemic range, and short sensor life have been barriers to the effective use of methods for the CGM previously
● Notably, the most recent advancements in CGM technology include introduction of isCGM systems that operate without necessitating calibrating interstitial glucose value to the capillary blood glucose and provision of AGP as a scientifically accurate, clinically meaningful method of reporting the dynamic properties of glucose metabolism
Indications for CGM
In diabetes care, CGM is used as both a short-term diagnostic tool (retroCGM, or professional CGM, sometimes blinded) and a long-term therapeutic tool (personal CGM) especially for T1DM patients (41), and in any patient on MDI or insulin pump therapy (42).
Indication of CGM is in accordance with its clear benefits regarding the clarification of glucose patterns and previously unknown hypoglycemic or hyperglycemic drifts, particularly for periods (i.e., nocturnal, postprandial) poorly explored by SMBG (41)
CGM is recommended to be used in combination with HbA1c in glycemic status assessment and therapy adjustment in all patients with poorly controlled T1DM or T2DM under intensive insulin therapy, particularly for those experiencing problematic hypoglycemia and/or have hypoglycemia unawareness (23, 42).
There are also miscellaneous indications for CGM with different levels of evidence which include the following (41):
● Brittle diabetes (variability analysis, assessment of potential causes such as premature needle withdrawal, intramuscular injection, and lipodystrophy)
● Flexible insulin therapy [FIT; facilitation of the assessment of FIT algorithms; in terms of basal insulin requirements (after fasting or a carbohydrate-free day test), carbohydrate ratios (for prandial rapid-acting insulin), and sensitivity index (for compensatory rapid-acting insulin)]
● Physical activity (glycemic effect of activity, validation of treatment options to avoid hypoglycemia during physical activity)
● Pregnancy (optimization of glycemic control)
● Discrepancy between HbA1c and SMBG (underestimation of HbA1c in dialysis patients, low/high hemoglobin glycation phenotypes)
● Certain clinical situations leading to variable glucose patterns (i.e., chronic dialysis, shift-work schedules, and defective compliance)
rtCGM devices are recommended to be used as close to daily as possible for maximal benefit, while FGM devices should be scanned frequently, at a minimum once every 8 h (42). CGM-based analysis involves a standardized report on metrics such as time in range, glycemic variability, patterns of hypoglycemia, and hyperglycemia (43). Glycemic variability data should also be considered in overall assessment of glycemic control, while the assessment of hypoglycemia should also include certain factors such as reduced awareness of subsequent hypoglycemia, cardiac arrhythmia, confusion, or abnormal or combative behavior, weight gain, and fear of hypoglycemia (23). Overall, entire CGM data should be evaluated within the context of other variables such as meals, treatments, exercise, illness, insulin boluses, and automated insulin delivery activity (43).
The expert panel recommendations on CGM indications in diabetes care are provided in Box 2.
Box 2. Expert panel recommendations on CGM indications in diabetes care.
● CGM refers to a short-term diagnostic tool (retroCGM, or professional CGM, sometimes blinded) or a long-term therapeutic tool (personal CGM) especially for T1DM patients
● Indications of CGM are in accordance with its clear benefits on identifying glucose patterns and previously unknown hypo- or hyperglycemic drifts, especially during periods poorly explored by SMBG such as night-time and postprandial periods
● CGM (rtCGM or isCGM) is recommended (in conjunction with HbA1c) for glycemic status assessment and therapy adjustment in all insulin-treated patients with T1DM or T2DM who are not achieving glucose targets, who are not meeting glycemic targets, have hypoglycemia unawareness, and/or have episodes of hypoglycemia
● CGM should be considered in all children and adolescents with T1DM, whether using injections or continuous subcutaneous insulin infusion, as an additional tool to help improve glucose control
Currently Available CGM Systems
Currently, the two CGM systems available are rtCGM and FGM [also called isCGM] (8). Both rtCGM [Dexcom G5 and G6 (Dexcom, Inc.) and Medtronic Enlite (Medtronic, Inc.), Eversense (Senseonics, Inc.)] and FGM [Freestyle Libre® system (Abbott Diabetes Care, Alameda, CA)] sensors collect real-time glucose readings continuously, while there are certain differences between two systems (41) (Table 1).
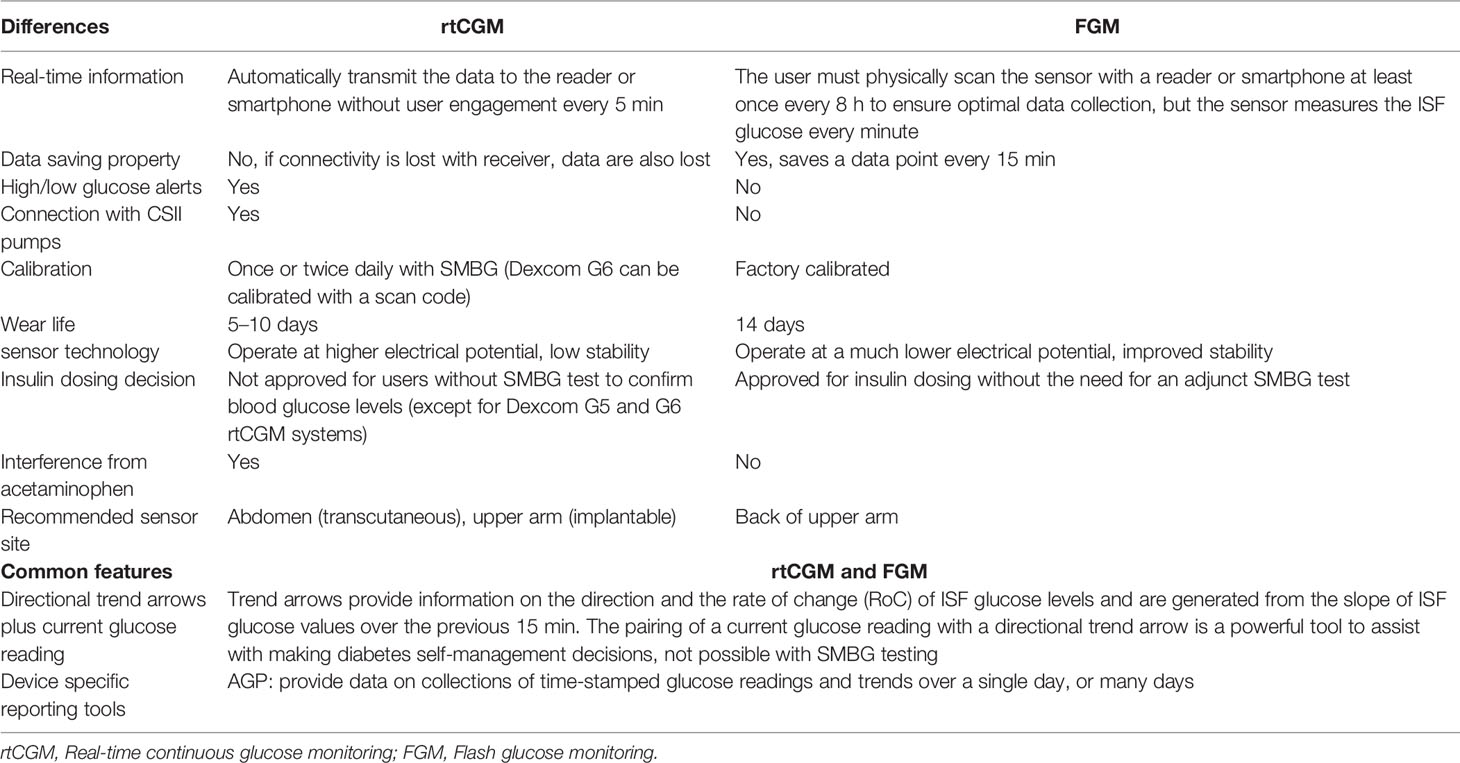
Table 1 Basic features of rtCGM and FGM systems (41).
FGM is widely recognized as a convenient tool for cost effective glucose level monitoring with readings provided upon scanning of a sensor, an advantage for patients to obtain real-time glucose levels without the need to routinely run a finger prick test (30).
CGM in Routine Clinical Practice—Published Evidence
The use of rtCGM in adults and children with T1DM was reported to be associated with significantly reduced HbA1c levels (from 0.4 to 1.0%) in JDRF, DIAMOND, GOLD, and SWITCH studies (44–47), with improved TIR (1.3–2.3 h/day) in JRDF CGM, DIAMOND, SWITCH, IN CONTROL, and REPLACE-BG studies (44, 45, 47–49) and with reduced hypoglycemia risk in DIAMOND, GOLD, HypoDE, and CONTROL studies (45, 46, 48, 50), while a qualitative meta-analysis of rtCGM studies revealed that besides the established healthcare benefits of the method, rtCGM users experience certain physical, emotional, and social issues that should be properly addressed by education and support measures (51).
Referring to the newest technology, the use of FGM plus AGP system has generally been considered favorable with positive feedbacks regarding its ease of use and ability to capture information on glycemic variability and hypoglycemic episodes (2, 52). The FGM has important advantages such as an overall lower cost of acquisition and no need for patient calibration with SMBG, and the utility of FGM as an alternative to both SMBG and other methods of CGM is considered to rise substantially in the near future as a widely recognized convenient tool for a cost-effective blood glucose monitoring (30).
The following examples summarize the published evidence regarding the usefulness of the FGM in insulin-treated T1DM and T2DM patients. Overall, the reduction in HbA1c after using FGM is evidently demonstrated in many studies, particularly in patients with a suboptimal HbA1c and poor adherence to blood glucose monitoring rather than already motivated patients with well-controlled diabetes (30). Most studies revealed a statistically significant improvement in TIR, patients’ QoL and treatment satisfaction along with reduction of time spent in hypoglycemia and frequency of SMBG after the use of flash glucose monitoring, while the change in time spent in hyperglycemia was clinically insignificant (30).
Randomized Controlled Trials
● The IMPACT trial by Bolinder et al. in T1DM patients revealed 38% reduction in time in hypoglycemia and significant reduction in the mean number of SMBG (from 5.5 ± 2.0 to 0.5 ± 0.7) in FGM users (n = 119) as compared with the control (n = 120) group, while there was no significant change in HbA1c between both groups at 6-month follow up (53). There was no significant difference in Diabetes QoL (DQoL) score between both groups, while the Diabetes Treatment Satisfaction Questionnaire (DTSQ) score improved significantly in FGM users (53).
● In the REPLACE trial by Haak et al. covering T2DM patients, a 50% reduction in time in hypoglycemia and a significant reduction in the mean number of SMBG (from 3.9 ± 1.2 to 0.6 ± 1.2) were reported in FGM (n = 139) users vs. control (n = 62) group at 6-month follow up (54).
● The 8-week RCT by Reddy et al. in T1DM patients revealed the reduction in median HbA1c from 55 mmol/mol (159 mg/dl) to 51 mmol/mol (149 mg/dl) and the increase in median percentage time in hypoglycemia from 8.0% (IQR 5.7–10.7) to 8.2% (IQR 6.0–13.2) in FGM users (n = 20) (55).
● The 10-week RCT by Yaron et al. in T2DM patients showed significant reduction in HbA1c in FGM (n = 52) users vs. control (n = 44) group (−0.85% ± 0.45 vs. −0.32% ± 0.39), while no significant difference between groups in terms of frequency of hypoglycemic episodes (56). Mean DTSQ change (DTSQc) score was 2.47 ± 0.77 (FGM users) vs. 2.18 ± 0.83 (control) (p = 0.053). FGM users found it more flexible and would recommend to their counterparts. The Audit of Diabetes-Dependent QoL (ADDQoL) questionnaires scores were not significant between both groups (56).
Prospective Cohort Studies
● In a 12-month study by Paris et al. in 120 T1DM patients using flash glucose monitoring, significant change was noted in HbA1c from 70 mmol/mol ±1.5 (198 mg/dl ± 4.0) to 61 mmol/mol ± 10.4 (176 mg/dl ± 27.3), while the number of hypoglycemic events per month significantly increased from 16.9 ± 1.44 to 22.9 ± 2.03 (57).
● In a 3–6 month study by Heald et al. in 92 T1DM patients using flash glucose monitoring, significant change was noted in mean HbA1c from 83 mmol/mol (233 mg/dl) to 72.3 mmol/mol (205 mg/dl) at 3 months and 66.9 mmol/mol (191 mg/dl) at 6 months (58).
● In a 12-month study by Kramer et al. in 40 T1DM patients using flash glucose monitoring, no significant change was noted in HbA1c [from 57.6 mmol/mol ± 11.4 (166 mg/dl ± 29.9) to 57.1 mmol/mol ± 7.4 (165 mg/dl ± 19.4)], insulin dosing, number of insulin injections and BMI from baseline, while frequency of SMBG significantly decreased from 6.7 ± 4.2 to 0.9 ± 1.8 per day and DTSQc score increased by 12.6 ± 5.5 points (59).
● In a 6-month study by Overend et al. in 40 T1DM patients using flash glucose monitoring, absence of finger prick test was reported to be a major benefit with reduction in frequency and severity of hypoglycemia alongside good glycemic control and positive impact on psychological well-being and self-esteem (60).
● In a 6-month study by Tyndall et al. in T1DM patients, significant reduction was noted in median HbA1c (−4 mmol/mol (−10.5 mg/dl) from baseline) and median number of glucose test strip use per day (from 3.8 to 0.6), while percentage of patients with hospital anxiety and depression scale (HADS) depression (from 7.6 to 15.0%) and anxiety (from 24.9 to 30.9%) scores of >7 were increased from baseline in FGM users, and increase in median BMI was significantly higher in FGM users (n = 750, by 0.3 kg/m2) vs. control (n = 518, by 0.1 kg/m2) group (61).
Retrospective Cohort Studies
● In a 24-week study by Moreno-Fernandez et al. in T1DM patients, significant change in HbA1c (−0.4% vs. 0.1%) and decrease in SMBG per day (from 5.2 ± 2.5 to 2.8 ± 1.7) were noted in FGM users (n = 18) vs. control (n = 18) group with no significant difference between groups in frequency of hypoglycemic episodes (62)
● In a 3–12 months study by Nana et al. in 90 T1DM patients using flash glucose monitoring, significant change in mean HbA1c of −7.29 mmol/mol ± 10.76 (−19.1 mg/dl ± 28.2), 51.86% reduction in hypoglycemic episodes, significant reduction in frequency of SMBG per day and significant improvement in the abbreviated Diabetes Distress Scale (DDS) score were noted after FGM use (63).
Nonetheless, it should be noted that the currently available evidence on usefulness of FGM is drawn from T1DM and T2DM treated with insulin therapy and there is a need for further studies addressing the utility of FGM in non-insulin dependent T2DM patients. Also the impact on prevention of DKA or HHS has not been assessed in any of the studies (30).
In addition, data from a 6-month follow up study by Hermanns et al. indicated significantly improved HbA1c reduction, TIR, diabetes-related distress scores, and satisfaction with the glucose monitoring method among diabetes patients with vs. without participation in structured education and treatment program on FGM (64), while a prospective 12-month follow up study by Pintus et al. in T1DM children indicated significant improvement in patient QoL, reduction of diabetes symptoms, and treatment barriers after patients were trained in the use of the FGM system (65). Accordingly, patient education regarding FGM use is considered to provide an additional significant benefit regarding the reduction of HbA1c and also reduction of diabetes distress and enhanced satisfaction with glucose monitoring and better engagement in diabetes management, compared to the use of FGM technology alone (64, 65).
Current Limitations of rtCGM/FGM Systems
Most of the current devices on the market require finger-pricking via a standard home blood glucose monitoring system to confirm the glucose levels displayed on the CGM to be able to initiate the appropriate and most accurate intervention, which also raises another issue of not completely replacing the finger-pricking (66). However, while inaccurate blood glucose readings, especially in the hypoglycemic range, and short sensor life have been barriers to the effective use of methods for the CGM previously, introduction of longer-lasting, more accurate and cheaper sensors with improvements in sensor technology (FGM) eliminated the need for calibration measurements, as an innovative technology product (5).
However, there are potential drawbacks of rtCGM/FGM use (7, 8, 23, 66):
● Lag time (4 to 27 min, longer in adult vs. adolescent patients) when using a sensor due to physiological lag between interstitial fluid and blood and also the intrinsic effect of the device, greatly affecting the accuracy of the device and placing patients at risk for overdosing on insulin therapy or inadvertently inducing hypoglycemia. Nonetheless, newer algorithms have a shorter lag time.
● The risk of anxiety and consequent accuracy limitations due to requirement of the device to be actively used in order to be effective, particularly with the delay in registering blood glucose changes in dynamic situations,
● The risk of provoking skin allergies (devices like FGM have reduced the incident by removing allergens as IBOA from the sensor adhesive). The technology is not yet widely available in several regions of the world
● Requirement of adequate training on this new wave of technology by both practitioners and their patients to be able to use these medical devices both comfortably and effectively.
Ambulatory Glucose Profile (AGP)
Rationale and Utility of AGP
Despite the benefits of CGM, the utilization of this technology in clinical practice has been suboptimal including only 3% of young T1DM patients (≤25 years) and 14% of older T1DM patients (26–49 years) (1, 67). The lack of software enabling relatively simple and standardized statistical and graphic visualization and interpretation of the glucose data has been a major contributor to the uncertainty and reluctance of clinicians to incorporate CGM into their practices (1, 3, 68, 69).
Thus, for many healthcare providers, the challenges of working with SMBG/CGM data have reinforced the practice of making therapeutic decisions by HbA1c values alone, despite its considerable limitations (69, 70).
Notably, recent improvements in monitoring technologies and establishing tools such as AGP provided a simple and informative method of analysis of the complex glucose data, and thereby a more consistent and standardized approach to the reporting and interpretation of this data in routine clinical practice (1, 8, 71, 72). Moreover, recommendations for the standardization of glucose reporting and analysis of continuous glucose data through use of the AGP were also published recently in order to optimize diabetes care (1, 5, 69).
Accordingly, AGP is currently recognized as an internationally agreed standard for summarizing and interpreting daily glycemic patterns using large amounts of data collected from rtCGM or FGM systems (1, 8, 72). By 2020, world-wide professional diabetes organizations recommended that the major manufacturers adopted the AGP as the primary visual means of representing CGM data for clinical decision-making within the standardized report (1, 8, 37, 42, 43, 73, 74).
The European consensus recommendations on the use of standardized glucose reporting system with AGP data in clinical practice suggest a week 4 review of the patient after the first assessment and then subsequent follow-up visits every 3 to 6 months (2).
The use of standardized glucose reporting system in combination with assessment of the patient’s daily routine and identification of times of day with increased risk of hypoglycemic or hyperglycemic events enables addressing potentially modifiable factors that are central to achieving good glycemic control in diabetes, and thus implementing specific changes to behavior and treatment (5, 25, 36). The AGP, representing the visual component of standardized glucose reporting system, is superior to glucose diaries for assessing hypoglycemic risk, while AGP readouts also provide a platform for constructive dialogue between members of the healthcare team and the patient, with potential for better engagement of patients in the management of diabetes and increased adherence to lifestyle intervention or changes to insulin or other pharmacotherapy (25, 36, 75).
The AGP displays large amounts of glucose data as if all the readings had occurred in a single 24-h period, while a profile can be created from at least 5 days to maximum of 3 months of such data (optimal period for reliability is 14 days) that provide important feedback on hypoglycemia and glucose variability along with information on the impact of insulin doses, meals, exercise, stress over single days or more-extended periods (8).
The AGP meets the three main purposes of CGM, namely, detection, intervention, and outcome (37) through providing data on glucose patterns to determine dysglycemia, to quantify glucose exposure, variability and stability, and to enable evidence-based clinical decision-making (37). Besides, by providing patterns via easily recognizable pictorial display, the use of AGP not only facilitates the analysis by the healthcare team and but also enables the patients with good self-management skills to easily identify and implement the necessary lifestyle of medication changes and thus changes the conversation between the patient and the healthcare team in the clinic (69). The AGP is documented to be a useful procedure for the analysis of glucose values in insulin-treated T1DM and T2DM patients, while evidence on its utility among insulin-naïve T2DM patients is lacking (3). The clinical situations in which AGP is considered useful (3, 25) are summarized in Table 2.
In fact, FGM is able to overcome SMBG’s limitation in glycemic variability detection and serve as a more affordable alternative with excellent accuracy to rtCGM without the need for calibration (30). In addition, the use of novel parameters in AGP analysis such as time in range and time spent in hypoglycemia that refer hyperglycemia or glycemic variability, respectively allow a more comprehensive overview of glycemic control than HbA1c and more informed treatment decisions (64). Thus, the use of AGP plus FGM is considered to represent the foremost innovative technology that has transformed diabetes care and had a positive impact on the psychological wellbeing in patients with diabetes that ultimately enhances patient compliance and ensures better glycemic control (30).
The expert panel recommendations on rationale and standardized reporting of AGP are provided in Box 3.
Box 3. Expert panel recommendations on rationale and standardized reporting of AGP.
● AGP overcomes the previous challenges of working with SMBG/CGM data and meets the need for software enabling relatively simple and standardized statistical and graphic visualization and interpretation of the glucose data to facilitate clinicians to incorporate CGM into their practices.
● Moreover, recommendations for the standardization of glucose reporting and analysis of continuous glucose data through use of the AGP were also published recently in order to optimize diabetes care.
● AGP provides a simple and informative method of analysis of the complex glucose data, and thereby a more consistent and standardized approach to the reporting and interpretation of this data in routine clinical practice.
● AGP-based visual assessment enables to summarize and interprete daily glycemic patterns using large amounts of data collected from rtCGM or FGM systems
● The European consensus recommendations on the use of AGP report in clinical practice suggest a week 4 review of the patient after the first AGP-based assessment and subsequent follow-up visits with analysis of AGP data every 3 to 6 months.
Key CGM Metrics: Visualization, Analysis and Documentation
Understanding and using the CGM generated glucose profiles and patterns are critical to managing diabetes and titrating therapy and is becoming easier given that CGM profile visualization is moving toward a standard AGP (1, 23, 76).
Effective use and appropriate interpretation of CGM data to optimize clinical outcomes is based on common metrics for assessment of glycemic status, graphical visualization of the glucose data and daily profile, and clear clinical targets (7).
Standardized CGM Metrics
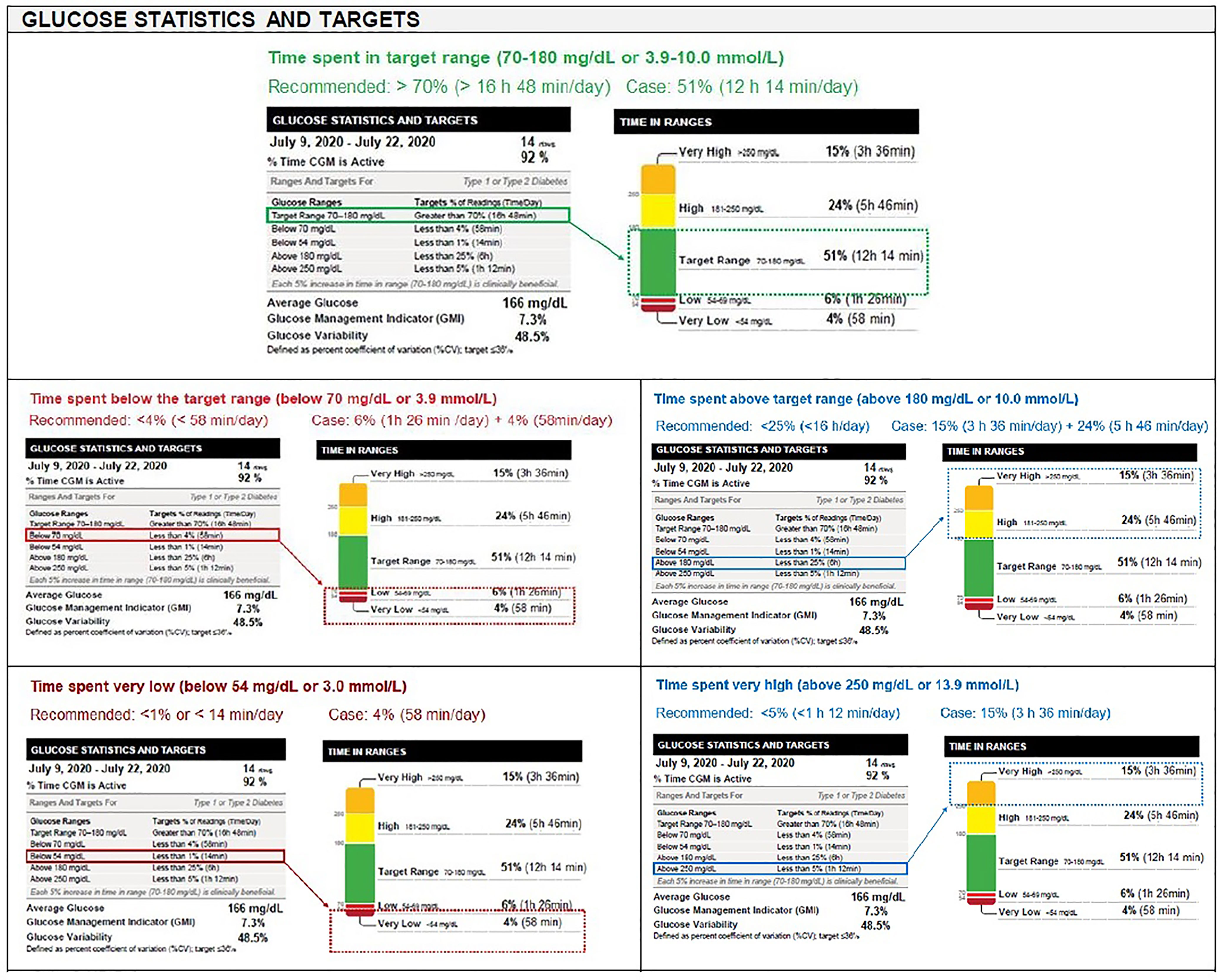
The list of core standardized CGM metrics for the use in clinical practice recommended by the 2019 International Consensus statement (7) is provided in Table 3. The standardized CGM metrics include novel glucose statistics and targets such as time in range (TIR), time above range (TAR; high, very high, dangerously high), time below range (TBR; low, very low, dangerously low), and glucose management indicator (GMI) along with mean glucose and glycemic variability (7, 76).
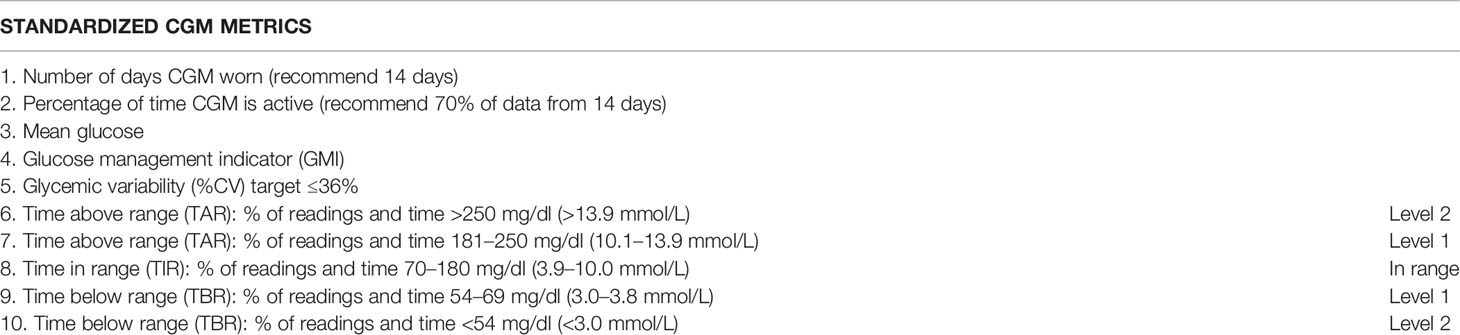
Table 3 Standardized CGM metrics by the 2019 International Consensus recommendation (7).
TIR refers to time spent in target glucose range (70–180 mg/dl, 3.9–10.0 mmol/L), while TBR [low (level 1): 54–69 mg/dl (3.0–3.8 mmol/L), very low (level 2): <54 mg/dl (<3.0 mmol/L)] and TAR [high (level 1): 181–250 mg/dl (10.1–13.9 mmol/L), very high (level 2): >250 mg/dl (>13.9 mmol/L)] are further categorized in two subgroups (Level 1 and Level 2) according to deviation from the target range (7) (Table 3 and Figure 1).
Glucose Statistics and Targets
Time in Range
Recent consensus conferences (ADA, ATTD, and AACE) and publications have recognized that diabetes management needs to go beyond HbA1c and recommend aligning to common metrics on glycemic status including Time in Range (TIR) recommendations, standardization for AGP and daily glucose profiles, glucose Management Indicator (GMI) as a replacement for estimated A1c (eA1c), and is calculated using an updated equation, and clear clinical targets (7) (Figure 1).
TIR refers to one of the key metrics of CGM, providing more comprehensive information on glucose profile (short-term glycemic control) than HbA1c alone, and emerged as a novel metric for assessing glycemic control during recent years (77). TIR overcomes some of the inherent limitations of HbA1c besides its association with diabetes related complications (23, 77, 78).
A logical glycemic goal is thus considered to maximize TIR, while TIR alone is not an adequate description of overall glycemic control, it is also necessary to quantitate the times below (TBR) and above target range (TAR) (1, 23, 71, 79). Hence, a combined use of additional measures that quantify amount and severity of hypoglycemia and hyperglycemia is considered necessary to make TIR more broadly acceptable as a research end point or clinical measure (1, 23, 71, 79). TIRs are useful for a research comparison of interventions and can help patients understand whether the amount of clinically significant hypoglycemia or hyperglycemia they are experiencing is improving over time (4). Breaking out the time in hypoglycemia and hyperglycemia into level 1 (low and high, respectively; monitor and take action if needed) and level 2 (very low and very high, respectively; immediate action required due to the more potentially clinically significant nature of the glucose levels) can guide the urgency and degree of clinical response (23).
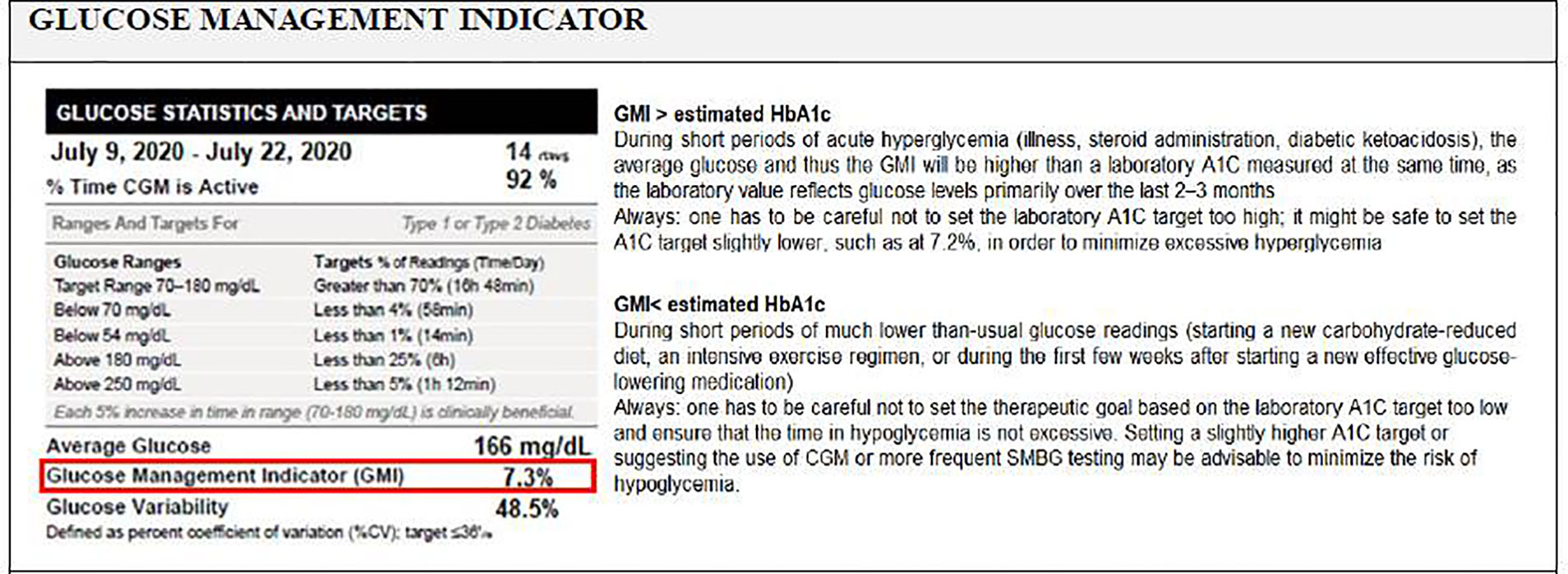
Glucose Management Indicator
Many CGM data reports include an estimate of A1C based on the CGM-measured mean glucose concentration, which might be closer, higher or lower than the actual laboratory-measured A1C (76, 80). This discordance between the eA1C (glucose in interstitial fluid) and the lab measured A1C (hemoglobin-attached glucose) can be confusing for patients and clinicians and the nomenclature of “eA1c” might imply there is a more direct relationship between the two (76). Accordingly, the term GMI, replacing the old term eA1C and calculated using an updated equation, is intended to convey that this is a measure calculated by converting CGM-derived mean glucose to a percentage and can provide an indication of the current state of a person’s glucose management (76).
GMI can help patients and HCPs monitor progress but should not replace lab A1c tests (62). Differences between the GMI and laboratory measured A1C may reflect several conditions as summarized in Figure 2 (76).
The expert panel recommendations on key points and clinical utility of AGP are provided in Box 4.
Box 4. Expert panel recommendations on AGP- key points and clinical utility.
● AGP is used in combination with assessment of the patient's daily routine and identification of times of day with increased risk of hypoglycemic or hyperglycemic events
● AGP and addresses potentially modifiable factors that are central to achieving good glycemic control in diabetes via providing important feedback on hypoglycemia and glucose variability and information on the impact of insulin doses, meals, exercise, stress over single days or more-extended periods (8)
● By providing patterns via easily recognizable pictorial display, AGP readouts also enables a platform for constructive dialogue between members of the healthcare team and the patient thus facilitates implementing specific changes to behavior and treatment.
● Accordingly AGP meets three main purposes of CGM including detection, intervention and outcome (37) through providing data on glucose patterns to determine dysglycemia, to quantity glucose exposure, variability and stability, and to enable evidence-based clinical decision-making,
● Use of novel parameters in the new standardized report including AGP analysis such as time in range and time spent in hypoglycemia that refer hyperglycemia or glycemic variability, respectively allow better overview of glycemic control than HbA1c and more informed treatment decisions
● Clinical utility of AGP involves comparison of the actual glucose values of the patient with the individual target values, analysis of the extent and causes of high glycemic variability review of the suitability and appropriateness of a therapeutic strategy testing the safety of adjusting a dose of insulin and clarifying the causes inconsisteney in HbA1c and glucose profiles
AGP Report
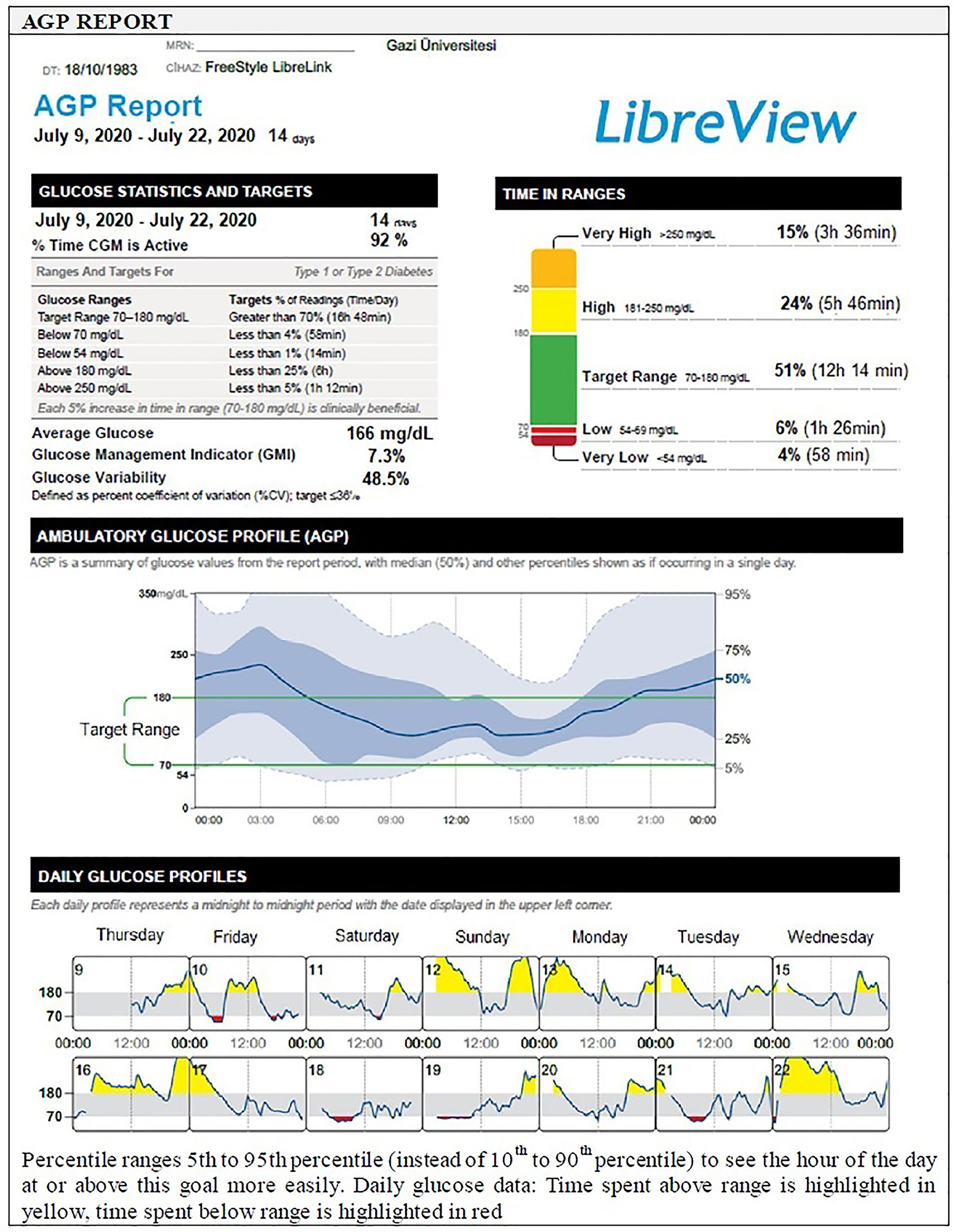
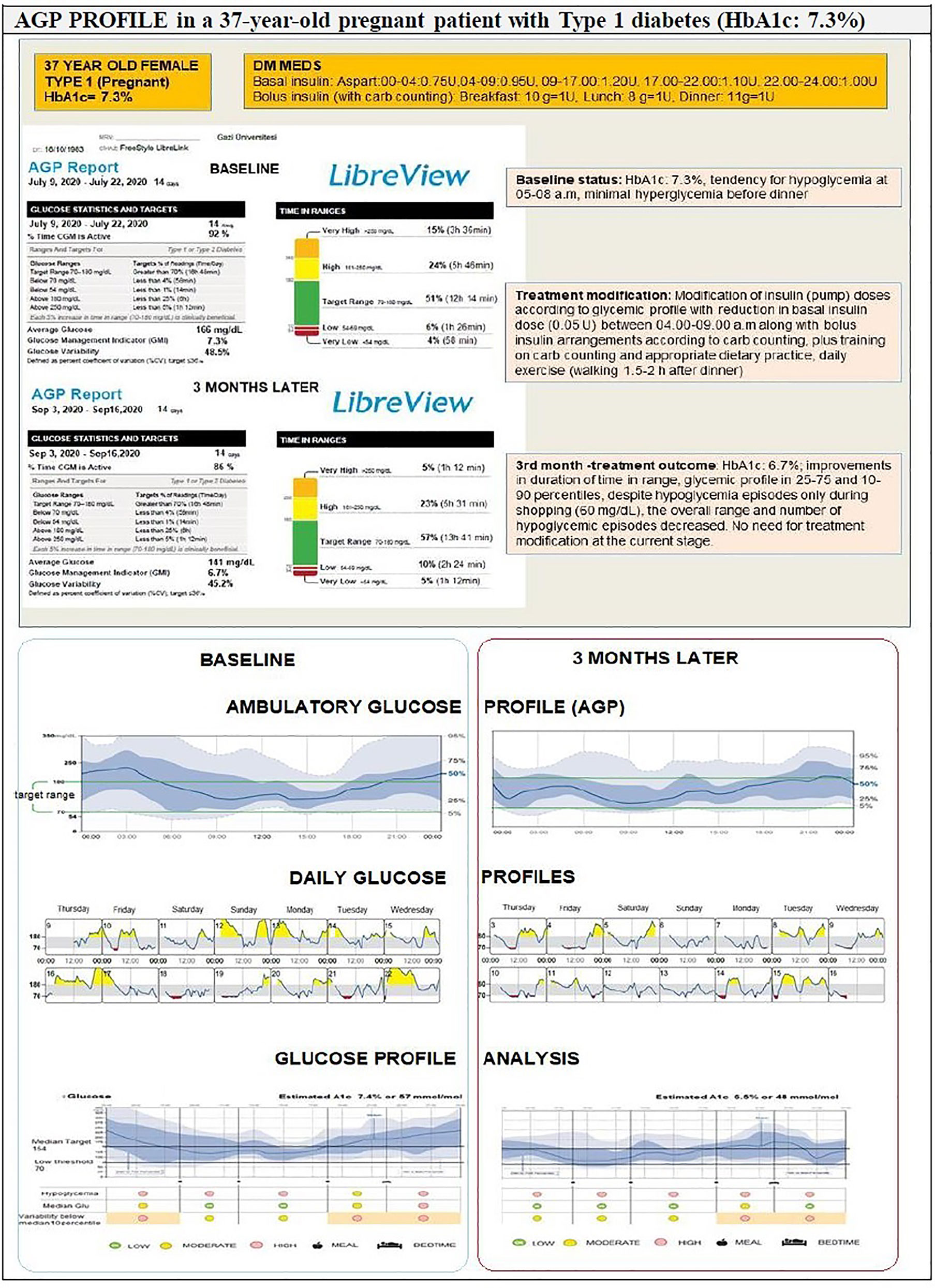
The AGP is a visual report and an easy to interpret graph that converts the readings obtained from CGM into a waveform based on pattern recognition, similar to an electrocardiogram. While the waveform will start to develop after at least 5 days of data collection, 14 days of data collection has been deemed ideal to most accurately reflect glucose control (1, 37, 39) (Figure 3).
Analyzing large amounts of glucose data from a number of separate days of recording collated in a single projection or a modal day, the AGP is presented as a median glucose value that reflects “what usually happens” rather than the mean which might be more strongly affected by outlying values, alongside the 25–75th and 10–90th percentiles (or 5–95th percentiles to see the hour of the day at or above the goal more easily), as calculated from the range of blood or interstitial glucose values at each time point (5). Interpretation is based on assessment of median, inter-quartile (IQR, 25th–75th percentile values) and inter-decile (IDR, 10th and 90th percentile or 5th to 95th percentile values) range curves that represent the central tendency and spread in glucose exposure, variability, and stability, over multiple days (7, 25, 37). The zone between the 25th and 75th percentile curves, accounts for 50% of all glucose values at any time point, the distance between the median blood glucose curve and those for the percentiles increases as the underlying glucose variability increases: the distance between the median and the 25th and 75th percentiles provides an indication of ‘usual’ glucose variability, while the 10th and 90th percentiles provide information on ‘occasional’ glucose excursions (7, 25, 37) (Figure 3).
Hence the AGP software, creates a standardized glucose reporting and analysis similar to electrocardiogram output and help the user to quickly identify areas of concern, namely, hypoglycemia or potential hypoglycemia, overall glucose control (TIR) and mean blood glucose value, and the degree of glycemic variability (5, 23, 69). A minimum of 14 consecutive days of data with approximately 70% of possible CGM readings over those 14 days appear to generate a report that has been validated as sufficient to provide a full analysis of issues relating to glycemic control in any given patient enabling optimal analysis and decision-making (5, 23) (Figure 3).
Expert Recommendations for the Use of AGP in Routine Clinical Practice in Turkey
Main obstacles of diabetes management today are the continued need for effective control of glycaemia, glucose variability and their relationship with diabetes complications, the continued need to limit the incidence of hypoglycemia; and these issues may be controlled by achieving a better understanding of daily glycemic control. Therefore, AGP might be particularly useful in managing patients who have poor exercise planning, who are doing active sports/swimming, who describe nocturnal hypoglycemia, hypoglycemia unawareness, fear of hypoglycemia and related suboptimal treatment adherence, who have pre-gestational diabetes, diabetes with high glycemic variability and/or morning hyperglycemia.
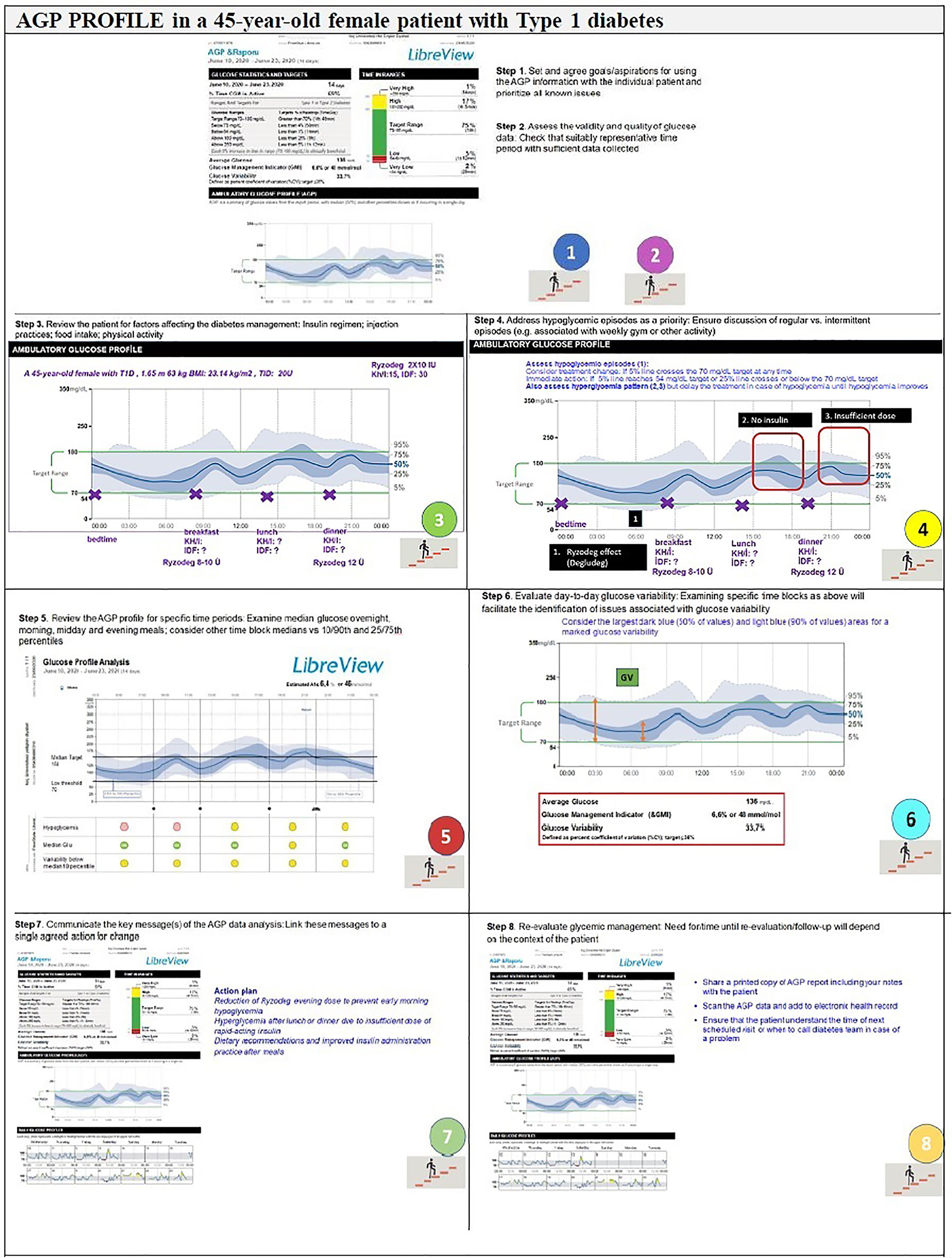
The expert recommendations for the use of AGP in clinical practice include (Figures 4, 5):
Step 1. Agreement on specific goals before starting the AGP analysis: Set and agree goals/aspirations for using the AGP information with the individual patient and prioritize all known issues
Step 2. Assess the validity and quality of glucose data: Check that suitably representative time period with sufficient data collected.
Step 3. Review the patient: Insulin regimen, injection practices, food intake, and physical activity.
Step 4. Address hypoglycemic episodes as a priority: Ensure discussion of regular vs. intermittent episodes (e.g. associated with weekly gym or other activity)
Step 5. Review the AGP profile for specific time periods: Examine median glucose overnight, morning, midday and evening meals; consider other time block medians vs 10/90th and 25/27th percentiles.
Step 6. Evaluate day-to-day glucose variability: Examining specific time blocks as above will facilitate the identification of issues associated with glucose variability.
Step 7. Communicate the key message(s) of the AGP data analysis: Link these messages to a single agreed action for change.
Step 8. Re-evaluate glycemic management: Need for/time until re-evaluation/follow-up will depend on the context of the patient.
Overall, the clinical utility of AGP involves a comparison of the actual glucose values of the patient with the individual target values, an analysis of the extent and causes of high glycemic variability, a review of the suitability and appropriateness of a therapeutic strategy, testing the safety of adjusting a dose of insulin and clarifying the causes of inconsistency in HbA1c and glucose profiles (Figure 6).
Conclusion
This paper prepared by an expert panel, provides a practical document to assist healthcare providers on the best practice with interpretation of a new standardized glucose reporting system based on standardized CGM metrics plus visual AGP data in diabetes care. Experts emphasize that the key factors supporting the clinical utility of standardized glucose reporting system are the comparison of actual versus target glucose values, detailed analysis of the glycemic variability, review of the appropriateness of a therapeutic strategy, test of the safety of adjusting insulin dosages and clarification of the inconsistency in HbA1c and glucose profiles, through an analysis of standardized CGM metrics and visual AGP data.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This expert panel study report was prepared with a workshop supported by the Abbott Diabetes Care Turkey which played a role in organization of expert panel meetings.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Sule Oktay and Cagla Ayhan from the KAPPA Consultancy Training Research Ltd, Istanbul who provided editorial support funded by the Abbott Diabetes Care, Turkey.
References
1. Bergenstal RM, Ahmann AJ, Bailey T, Beck RW, Bissen J, Buckingham B, et al. Recommendations for Standardizing Glucose Reporting and Analysis to Optimize Clinical Decision Making in Diabetes: The Ambulatory Glucose Profile. J Diabetes Sci Technol (2013) 7:562–78. doi: 10.1177/193229681300700234
2. Maran A, Esposito K, Toni S, Giordano C. Ambulatory Glucose Profile Applied to Flash Glucose Monitoring in Real Life: An Expert Opinion. J Diabetes Sci Technol (2017) 11:633–4. doi: 10.1177/1932296816674739
3. Siegmund T, Matthaei S, Reuter M, Reichel A, Kellerer M, Kröger J. Ambulatory Glucose Profile (AGP): Recommendations for Use in Clinical Setting. Diabetes Stoffwechsel und Herz (2015) 24:115–20.
4. Matthaei S, Dealaiz RA, Bosi E, Evans M, Geelhoed-Duijvestijn N, Joubert M. Consensus Recommendations for the Use of Ambulatory Glucose Profile in Clinical Practice. Br J Diabetes Vasc Dis (2014) 14:153–7. doi: 10.15277/bjdvd.2014.046
5. Evans M, Cranston I, Bailey CJ. Ambulatory Glucose Profile (AGP): Utility in UK Clinical Practice. Br J Diabetes (2017) 17:26–33. doi: 10.15277/bjd.2017.121
6. Kröger J, Reichel A, Siegmund T, Ziegler R. Clinical Recommendations for the Use of the Ambulatory Glucose Profile in Diabetes Care. J Diabetes Sci Technol (2019) 14:586–94. doi: 10.1177/1932296819883032
7. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care (2019) 42:1593–603. doi: 10.2337/dci19-0028
8. Bruttomesso D, Laviola L, Avogaro A, Bonora E, Del Prato S, Frontoni S, et al. The Use of Real Time Continuous Glucose Monitoring or Flash Glucose Monitoring in the Management of Diabetes: A Consensus View of Italian Diabetes Experts Using the Delphi Method. Nutr Metab Cardiovasc Dis (2019) 29:421–31. doi: 10.1016/j.numecd.2019.01.018
9. Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is Glucose Control Important for Prevention of Cardiovascular Disease in Diabetes? Diabetes Care (2013) 36(Suppl 2):S259–263. doi: 10.2337/dcS13-2018
10. Nathan DM. DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care (2014) 37:9–16. doi: 10.2337/dc13-2112
11. Orasanu G, Plutzky J. The Pathologic Continuum of Diabetic Vascular Disease. J Am Coll Cardiol (2009) 53(5 Suppl):S35–42. doi: 10.1016/j.jacc.2008.09.055
12. Research Group DCCT, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med (1993) 329:977–86. doi: 10.1056/NEJM199309303291401
13. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of Glycaemia With Macrovascular and Microvascular Complications of Type 2 Diabetes (UKPDS 35): Prospective Observational Study. BMJ (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405
14. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of Intensive Control of Glucose on Cardiovascular Outcomes and Death in Patients With Diabetes Mellitus: A Meta-Analysis of Randomised Controlled Trials. Lancet (2009) 373:1765–72. doi: 10.1016/S0140-6736(09)60697-8
15. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive Diabetes Treatment and Cardiovascular Disease in Patients With Type 1 Diabetes. N Engl J Med (2005) 353:2643–53. doi: 10.1056/NEJMoa052187
16. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year Follow-Up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med (2008) 359:1577–89. doi: 10.1056/NEJMoa0806470
17. Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe Hypoglycaemia and Cardiovascular Disease: Systematic Review and Meta-Analysis With Bias Analysis. BMJ (2013) 347:f4533. doi: 10.1136/bmj.f4533
18. Yakubovich N, Gerstein HC. Serious Cardiovascular Outcomes in Diabetes: The Role of Hypoglycemia. Circulation (2011) 123:342–8. doi: 10.1161/CIRCULATIONAHA.110.948489
19. Hirsch IB, Brownlee M. Should Minimal Blood Glucose Variability Become the Gold Standard of Glycemic Control? J Diabetes Complications (2005) 19:178–81. doi: 10.1016/j.jdiacomp.2004.10.001
20. Suh S, Kim JH. Glycemic Variability: How do We Measure it and Why is it Important? Diabetes Metab J (2015) 39:273–82. doi: 10.4093/dmj.2015.39.4.273
21. American Diabetes Association. (6) Glycemic Targets. Diabetes Care (2015) 38(Suppl):S33–40. doi: 10.2337/dc15-S009
22. Kowalski AJ, Dutta S. It’s Time to Move From A1c to Better Metrics for Diabetes Control. Diabetes Technol Ther (2013) 15:194–6. doi: 10.1089/dia.2013.0060
23. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care (2017) 40:1631–40. doi: 10.2337/dc17-1600
24. Kovatchev BP, Flacke F, Sieber J, Breton MD. Accuracy and Robustness of Dynamical Tracking of Average Glycemia (A1c) to Provide Real-Time Estimation of Hemoglobin A1c Using Routine Self-Monitored Blood Glucose Data. Diabetes Technol Ther (2014) 16:303–9. doi: 10.1089/dia.2013.0224
25. Matthaei S. Assessing the Value of the Ambulatory Glucose Profile in Clinical Practice. Br J Diabetes Vasc Dis (2014) 14:148–52. doi: 10.15277/bjdvd.2014.045
26. Hui Z, He J, Li P, Guo M, Jin H, Shen J, et al. Glucose Screening Measurements and Noninvasive Glucose Monitor Methods. Proc Comput Sci (2018) 139:613–21. doi: 10.1016/j.procs.2018.10.202
27. American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care (2018) 41(Suppl 1):S55–64. doi: 10.2337/dc18-S006
28. Kato N, Cui J, Kato M. Structured Self-Monitoring of Blood Glucose Reduces Glycated Hemoglobin in Insulin-Treated Diabetes. J Diabetes Investig (2013) 4:450–3. doi: 10.1111/jdi.12072
29. Kempf K, Kruse J, Martin S. ROSSO-In-Praxifollow-Up: Long-Term Effects of Self-Monitoring of Blood Glucose on Weight, Hemoglobin A1c, and Quality of Life in Patients With Type 2 Diabetes Mellitus. Diabetes Technol Ther (2012) 14:59–64. doi: 10.1089/dia.2011.0116
30. Ang E, Lee ZX, Moore S, Nana M. Flash Glucose Monitoring (FGM): A Clinical Review on Glycaemic Outcomes and Impact on Quality of Life. J Diabetes Complications (2020) 34:107559. doi: 10.1016/j.jdiacomp.2020.107559
32. Patton SR. Adherence to Glycemic Monitoring in Diabetes. J Diabetes Sci Technol (2015) 9:668–75. doi: 10.1177/1932296814567709
33. Al Hayek AA, Robert AA, Babli S, Almonea K, Al Dawish MA. Fear of Self-Injecting and Self-Testing and the Related Risk Factors in Adolescents With Type 1 Diabetes: A Crosssectional Study. Diabetes Ther (2017) 8:75–83. doi: 10.1007/s13300-016-0221-8
34. Ong WM, Chua SS, Ng CJ. Barriers and Facilitators to Self-Monitoring of Blood Glucose in People With Type 2 Diabetes Using Insulin: A Qualitative Study. Patient Prefer Adherence (2014) 8:237–46. doi: 10.2147/PPA.S57567
35. Polonsky WH, Fisher L, Hessler D, Edelman SV. What is So Tough About Self-Monitoring of Blood Glucose? Perceived Obstacles Among Patients With Type 2 Diabetes. Diabetes Med (2014) 31:40–6. doi: 10.1111/dme.12275
36. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin Adherence Behaviours and Barriers in the Multinational Global Attitudes of Patients and Physicians in Insulin Therapy Study. Diabetes Med (2012) 29:682–9. doi: 10.1111/j.1464-5491.2012.03605.x
37. Mazze R. Advances in Glucose Monitoring: Improving Diabetes Management Through Evidence-Based Medicine. Prim Care Diabetes (2020) 14:515–21. doi: 10.1016/j.pcd.2020.03.001
38. Mazze RS, Strock E, Borgman S, Wesley D, Stout P, Racchini J. Evaluating the Accuracy, Reliability, and Clinical Applicability of Continuous Glucose Monitoring (CGM): Is CGM Ready for Real Time? Diabetes Technol Ther (2009) 11:11–8. doi: 10.1089/dia.2008.0041
39. Mazze RS, Strock E, Wesley D, Borgman S, Morgan B, Bergenstal R, et al. Characterizing Glucose Exposure for Individuals With Normal Glucose Tolerance Using Continuous Glucose Monitoring and Ambulatory Glucose Profile (AGP) Analysis. Diabetes Technol Ther (2008) 10:149–59. doi: 10.1089/dia.2007.0293
40. Poolsup N, Suksomboon N, Kyaw AM. Systematic Review and Meta-Analysis of the Effectiveness of Continuous Glucose Monitoring (CGM) on Glucose Control in Diabetes. Diabetol Metab Syndr (2013) 5:39. doi: 10.1186/1758-5996-5-39
41. Joubert M, Baillot-Rudoni S, Catargi B, Charpentier G, Esvant A, Franc S, et al. Indication, Organization, Practical Implementation and Interpretation Guidelines for Retrospective CGM Recording: A French Position Statement. Diabetes Metab (2015) 41:498–508. doi: 10.1016/j.diabet.2015.07.001
42. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S85–99. doi: 10.2337/dc21-S007
43. Fonseca V, Grunberger G, Anhalt H, Bailey TS, Blevins T, Garg SK, et al. Continuous Glucose Monitoring: A Consensus Conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract (2016) 22:1008–21. doi: 10.4158/EP161392.CS
44. The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, et al. Continuous Glucose Monitoring and Intensive Treatment of Type 1 Diabetes. N Engl J Med (2008) 359:1464–76. doi: 10.1056/NEJMoa0805017
45. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections. The DIAMOND Randomized Clinical Trial. JAMA (2017) 317:371–8. doi: 10.1001/jama.2016.19975
46. Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections. The GOLD Randomized Clinical Trial. JAMA (2017) 317:379–87. doi: 10.1001/jama.2016.19976
47. Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, et al. The Use and Efficacy of Continuous Glucose Monitoring in Type 1 Diabetes Treated With Insulin Pump Therapy: A Randomised Controlled Trial. Diabetologia (2012) 55:3155–62. doi: 10.1007/s00125-012-2708-9
48. van Beers CA, DeVries JH, Kleijer SJ, Smits MM, Geelhoed-Duijvestijn PH, Kramer MH, et al. Continuous Glucose Monitoring for Patients With Type 1 Diabetes and Impaired Awareness of Hypoglycaemia (IN CONTROL): A Randomised, Open-Label, Crossover Trial. Lancet Diabetes Endocrinol (2016) 4:893–902. doi: 10.1016/S2213-8587(16)30193-0
49. Aleppo G, Ruedy KJ, Riddlesworth TD, Kruger DF, Peters AL, Hirsch I, et al. REPLACE-BG: A Randomized Trial Comparing Continuous Glucose Monitoring With and Without Routine Blood Glucose Monitoring in Adults With Well-Controlled Type 1 Diabetes. Diabetes Care (2017) 40:538–45. doi: 10.2337/dc16-2482
50. Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Real-Time Continuous Glucose Monitoring in Adults With Type 1 Diabetes and Impaired Hypoglycaemia Awareness or Severe Hypoglycaemia Treated With Multiple Daily Insulin Injections (HypoDE): A Multicentre, Randomized Controlled Trial. Lancet (2018) 391:1367–77. doi: 10.1016/S0140-6736(18)30297-6
51. Messer LH, Johnson R, Driscoll KA, Jones J. Best Friend or Spy: A Qualitative Meta-Synthesis on the Impact of Continuous Glucose Monitoring on Life With Type 1 Diabetes. Diabetes Care (2018) 35:409–18. doi: 10.1111/dme.13568
52. Heinemann L, Freckmann G. CGM Versus FGM; or, Continuous Glucose Monitoring is Not Flash Glucose Monitoring. J Diabetes Sci Technol (2015) 9:947–50. doi: 10.1177/1932296815603528
53. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel Glucose-Sensing Technology and Hypoglycaemia in Type 1 Diabetes: A Multicentre, non-Masked, Randomised Controlled Trial. Lancet (2016) 388:2254–63. doi: 10.1016/S0140-6736(16)31535-5
54. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of Flash Glucose-Sensing Technology for 12 Months as a Replacement for Blood Glucose Monitoring in Insulin-Treated Type 2 Diabetes. Diabetes Ther (2017) 8:573–86. doi: 10.1007/s13300-017-0255-6
55. Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver N. A Randomized Controlled Pilot Study of Continuous Glucose Monitoring and Flash Glucose Monitoring in People With Type 1 Diabetes and Impaired Awareness of Hypoglycaemia. Diabetes Med (2018) 35:483–90. doi: 10.1111/dme.13561
56. Yaron M, Roitman E, Aharon-Hananel G, Landau Z, Ganz T, Yanuv I, et al. Effect of Flash Glucose Monitoring Technology on Glycemic Control and Treatment Satisfaction in Patients With Type 2 Diabetes. Diabetes Care (2019) 42:1178–84. doi: 10.2337/dc18-0166
57. Paris I, Henry C, Pirard F, Gerard AC, Colin IM. The New FreeStyle Libre Flash Glucose Monitoring System Improves the Glycaemic Control in a Cohort of People With Type 1 Diabetes Followed in Real-Life Conditions Over a Period of One Year. Endocrinol Diabetes Metab (2018) 1:e00023. doi: 10.1002/edm2.23
58. Yadegarfar G, Anderson SG, Khawaja Z, Cortes G, Leivesley K, Metters A. Et.Al. The FreeStyle Libre Flash Glucose Monitoring System: How it has Improved Glycaemic Control for People With Type 1 Diabetes in Eastern Cheshire, UK. Cardiovasc Endocrinol Metab (2020) 9:171–6. doi: 10.1097/XCE.0000000000000216
59. Kramer G, Michalak L, Muller UA, Kloos C, Werner C, Kuniss N. Association Between Flash Glucose Monitoring and Metabolic Control as Well as Treatment Satisfaction in Outpatients With Diabetes Type 1. Exp Clin Endocrinol Diabetes (2021) 129:303–8. doi: 10.1055/a-0875-3988
60. Overend L, Simpson E, Grimwood T. Qualitative Analysis of Patient Responses to the ABCD FreeStyle Libre Audit Questionnaire. Pract Diabetes (2019) 36:45–50. doi: 10.1002/pdi.2213
61. Tyndall V, Stimson RH, Zammitt NN, Ritchie SA, McKnight JA, Dover AR, et al. Marked Improvement in HbA1c Following Commencement of Flash Glucose Monitoring in People With Type 1 Diabetes. Diabetologia (2019) 62:1349–56. doi: 10.1007/s00125-019-4894-1
62. Moreno-Fernandez J, Pazos-Couselo M, Gonzalez-Rodriguez M, Rozas P, Delgado M, Aguirre M, et al. Clinical Value of Flash Glucose Monitoring in Patients With Type 1 Diabetes Treated With Continuous Subcutaneous Insulin Infusion. Endocrinol Diabetes Nutr (2018) 65:556–63. doi: 10.1016/j.endinu.2018.04.003
63. Nana M, Moore SL, Ang E, Lee ZX, Bondugulapati LNR. Flash Glucose Monitoring: Impact on Markers of Glycaemic Control and Patient-Reported Outcomes in Individuals With Type 1 Diabetes Mellitus in the Real-World Setting. Diabetes Res Clin Pract (2019) 157:107893. doi: 10.1016/j.diabres.2019.107893
64. Hermanns N, Ehrmann D, Schipfer M, Kröger J, Haak T, Kulzer B. The Impact of a Structured Education and Treatment Programme (FLASH) for People With Diabetes Using a Flash Sensor-Based Glucose Monitoring System: Results of a Randomized Controlled Trial. Diabetes Res Clin Pract (2019) 150:111–21. doi: 10.1016/j.diabres.2019.03.003
65. Pintus D, Ng SM. Freestyle Libre Flash Glucose Monitoring Improves Patient Quality of Life Measures in Children With Type 1 Diabetes Mellitus (T1DM) With Appropriate Provision of Education and Support by Healthcare Professionals. Diabetes Metab Syndr (2019) 13:2923–6. doi: 10.1016/j.dsx.2019.07.054
66. Olczuk D, Priefer R. A History of Continuous Glucose Monitors (CGMs) in Self-Monitoring of Diabetes Mellitus. Diabetes Metab Syndr (2018) 12:181–7. doi: 10.1016/j.dsx.2017.09.005
67. Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA, et al. The T1D Exchange Clinic Registry. J Clin Endocrinol Metab (2012) 97:4383–9. doi: 10.1210/jc.2012-1561
68. Klonoff DC, Blonde L, Cembrowski G, Chacra AR, Charpentier G, Colagiuri S, et al. Consensus Report: The Current Role of Self-Monitoring of Blood Glucose in Non-Insulin-Treated Type 2 Diabetes. J Diabetes Sci Technol (2011) 5:1529–48. doi: 10.1177/193229681100500630
69. Berard L. The Ambulatory Glucose Profile: What, Why, and How? (2018). Available at: https://diabetes.medicinematters.com/blood-glucose-monitoring/continuous-glucose-monitoring/ambulatory-glucose-profile-what-why-and-how/16063922.
70. Hammond P. Interpreting the Ambulatory Glucose Profile. Br J Diabetes (2016) 16(Suppl 1):10–5. doi: 10.15277/bjd.2016.072
71. Rodbard D. Clinical Interpretation of Indices of Quality of Glycemic Control and Glycemic Variability. Postgrad Med (2011) 123:107–18. doi: 10.3810/pgm.2011.07.2310
72. Mazze RS. Making Sense of Glucose Monitoring Technologies From SMBG to CGM. Diabetes Technol Ther (2005) 7:784–7. doi: 10.1089/dia.2005.7.784
73. Forlenza GP, Pyle LL, Maahs DM, Dunn TC. Ambulatory Glucose Profile Analysis of the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Dataset-Applications to the Pediatric Diabetes Population. Pediatr Diabetes (2017) 18:622–8. doi: 10.1111/pedi.12474
74. Rewers MJ, Pillay K, de Beaufort C, Craig ME, Hanas R, Acerini CL, et al. ISPAD Clinical Practice Consensus Guidelines 2014 Compendium. Assessment and Monitoring of Glycemic Control in Children and Adolescents With Diabetes. Pediatr Diabete (2014) 15(Suppl 20):102–14. doi: 10.1111/pedi.12190
75. Wens J, Vermeire E, Van Royen P, Sabbe B, Denekens J. GPs' Perspectives of Type 2 Diabetes Patients' Adherence to Treatment: A Qualitative Analysis of Barriers and Solutions. BMC Fam Pract (2005) 6:20. doi: 10.1186/1471-2296-6-20
76. Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose Management Indicator (GMI): A New Term for Estimating A1C From Continuous Glucose Monitoring. Diabetes Care (2018) 41:2275–80. doi: 10.2337/dc18-1581
77. Lu J, Ma X, Zhang L, Mo Y, Lu W, Zhu W, et al. Glycemic Variability Modifies the Relationship Between Time in Range and Hemoglobin A1c Estimated From Continuous Glucose Monitoring: A Preliminary Study. Diabetes Res Clin Pract (2020) 161:108032. doi: 10.1016/j.diabres.2020.108032
78. Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, et al. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care (2019) 42:400–5. doi: 10.2337/dc18-1444
79. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Bode B, Beck RW, Xing D, Gilliam L, Hirsch I, et al. Sustained Benefit of Continuous Glucose Monitoring on A1C, Glucose Profiles, and Hypoglycemia in Adults With Type 1 Diabetes. Diabetes Care (2009) 32:2047–9. doi: 10.2337/dc09-0846
Keywords: diabetes care, continuous glucose monitoring, ambulatory glucose profile, expert opinion, clinical utility, algorithm
Citation: Dagdelen S, Deyneli O, Dinccag N, Ilkova H, Osar Siva Z, Yetkin I and Yilmaz T (2022) Expert Panel Recommendations for Use of Standardized Glucose Reporting System Based on Standardized Glucometrics Plus Visual Ambulatory Glucose Profile (AGP) Data in Clinical Practice. Front. Endocrinol. 12:663222. doi: 10.3389/fendo.2021.663222
Received: 02 February 2021; Accepted: 25 November 2021;
Published: 24 January 2022.
Edited by:
Samim Özen, Ege University, TurkeyReviewed by:
Krishna Seshadri, Sri Balaji Vidyapeeth University, IndiaBanshi Saboo, Diabetes Care & Hormone Clinic at Ahmedabad, India
Manoj Chawla, Lina Diabetes Care Center, India
Copyright © 2022 Dagdelen, Deyneli, Dinccag, Ilkova, Osar Siva, Yetkin and Yilmaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Selcuk Dagdelen, c2VsY3VrZGFnZGVsZW5AeWFob28uY29t
 Selcuk Dagdelen
Selcuk Dagdelen Oguzhan Deyneli
Oguzhan Deyneli Nevin Dinccag3
Nevin Dinccag3 Temel Yilmaz
Temel Yilmaz