- 1Department of Clinical Nutrition, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Nutrition, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Nutrition and Dietetics, University of North Florida, Jacksonville, FL, United States
- 5Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Obesity has been reported to be an important contributing factor for precocious puberty, especially in girls. The effect of green tea polyphenols on weight reduction in adult population has been shown, but few related studies have been conducted in children. This study was performed to examine the effectiveness and safety of decaffeinated green tea polyphenols (DGTP) on ameliorating obesity and early sexual development in girls with obesity.
Design: This is a double-blinded randomized controlled trial. Girls with obesity aged 6–10 years old were randomly assigned to receive 400 mg/day DGTP or isodose placebo orally for 12 weeks. During this period, all participants received the same instruction on diet and exercise from trained dietitians. Anthropometric measurements, secondary sexual characteristics, B-scan ultrasonography of uterus, ovaries and breast tissues, and related biochemical parameters were examined and assessed pre- and post-treatment.
Results: Between August 2018 and January 2020, 62 girls with obesity (DGTP group n = 31, control group n = 31) completed the intervention and were included in analysis. After the intervention, body mass index, waist circumference, and waist-to-hip ratio significantly decreased in both groups, but the percentage of body fat (PBF), serum uric acid (UA), and the volumes of ovaries decreased significantly only within the DGTP group. After controlling confounders, DGTP showed a significantly decreased effect on the change of PBF (β = 2.932, 95% CI: 0.214 to 5.650), serum UA (β = 52.601, 95% CI: 2.520 to 102.681), and ovarian volumes (right: β = 1.881, 95% CI: 0.062 to 3.699, left: β = 0.971, 95% CI: 0.019 to 1.923) in girls with obesity. No side effect was reported in both groups during the whole period.
Conclusion: DGTP have shown beneficial effects of ameliorated obesity and postponed early sexual development in girls with obesity without any adverse effects.
Clinical Trial Registration: [https://clinicaltrials.gov/ct2/show/NCT03628937], identifier [NCT03628937].
Introduction
Precocious puberty refers to the appearance of physical and hormonal signs of pubertal development at an age earlier than normal. Female precocious puberty is defined as the development of secondary sexual characteristics before the age of 8 or menarche before the age of 10 (1). Currently, increasing numbers of countries worldwide are experiencing earlier sexual development in children (2–4). In China, a multi-center study in 2013 reported that the prevalence of precocious puberty in children was 0.43%, and the proportion of girls with breast development before the age of 8 was 2.91% (5). Also, a survey in nine major cities in China of about 20,654 girls found that 19.57% of urban girls aged 8 already had breast development (6). Moreover, the prevalence of precocious puberty is higher in girls, 5 to 10 times more common than in boys (7). Early sexual development in children was reported to be associated with rapid bone maturation with decreased adult height, psychological problems, and also increased risk of reproductive tract cancers in adulthoods (8).
The mechanisms of precocious puberty are complicated, which involve genetic, nutritional, psychosocial stress, and environmental toxin interactions (9, 10). Some studies demonstrated that obesity was an important contributing factor to early sexual development, especially for girls (11–16). According to the Third National Health and Nutrition Survey in the United States, Rosenfield et al. (17) reported that girls with high body mass index (BMI) had a higher proportion of breast development and pubic hair development, and the average age of menarche was 10.2 years old, 10 months earlier than the girls in the normal BMI range. Also, Biro’s study (18) suggested that the BMI of girls with precocious puberty was higher than that of normal development girls.
Gonadotropin-releasing hormone agonist therapy is well established as the standard treatment for central precocious puberty worldwide. In addition, some other clinical treatments like traditional Chinese medicine and acupuncture therapy were also used. Though clinically precocious puberty can be treated in some ways, it is important to find approaches to prevent or delay its development in children.
Green tea, originated in China, is made from the leaves of Camellia sinensis and contains high quantities of polyphenols (19). Among the large body of polyphenols, epigallocatechin gallate (EGCG) has been shown effective in reducing body weight, along with decreased adipocyte differentiation and proliferation during lipogenesis (20). A high dose of catechins ingestion (~600 mg/day), has also been reported to lower body fat and cholesterol in adults with obesity (21). Till now, most human studies on green tea polyphenols (GTP) were conducted in adult populations, and only a Japanese study conducted in children (22), which showed that 576 mg catechins for 24 weeks improved obesity and suppressed leptin concentration without raising any safety concerns for children with obesity. It is worth mentioning that the caffeine contained in tea polyphenols might cause main adverse effects for children, such as sleep difficulty (23), mental anxiety and depression (24), and slow heart rate (25). It is probably for this reason that GTP interventions were rarely conducted in children with obesity. Decaffeinated green tea polyphenols (DGTP) thus become more appealing to be tested for their weight reduction effect in children.
Since precocious puberty is a common comorbidity in girls with obesity (26), it is important to find effective ways to manage obesity as well as to prevent precocious puberty. Previously, our group has conducted a pilot experiment in female Sprague Dawley (SD) rats, and reported that GTP could delay the date of vaginal opening of precocious puberty rats (p < 0.05) (27), showing the preliminary effect of GTP. To our best knowledge, the potential effects of GTP or DGTP to delay precocious puberty has not yet been tested in children with obesity. Therefore, this study aimed to examine the effect of DGTP on obesity and sexual development in girls with obesity aged 6–10 years old, and the safety of the study was also assessed.
Materials and Methods
Study Design and Participants
We did a randomized, double-blinded, placebo-controlled trial that enrolled obese girls with a 12-week DGTP or placebo intervention period. This study was registered with a Clinical Trials.gov identifier NCT03628937 and was approved by the Ethics Committee of Xinhua Hospital (Shanghai, China). Participants were recruited from the Clinical Nutrition Department, Children Endocrinology Department, Children Health Care Department in Xinhua Hospital, and some primary schools near the hospital from August 2018 to January 2020. Flyers and posters with the study information were posted in the hospital, primary school campus, and also posted on the hospital and external websites (such as WeChat, which is one of the most common online social platforms in China). The inclusion criteria for this study were as follows: (1) girls with obesity aged 6–10 years old; (2) BMI reached or exceeded the overweight threshold of school-aged girls in China (28) (6 years old, BMI ≥16.3 kg/m2; 7 years old, BMI ≥17.2 kg/m2; 8 years old, BMI ≥18.1 kg/m2; 9 years old, BMI ≥19.0 kg/m2; 10 years old, BMI ≥20.0 kg/m2). Participants with the following conditions were excluded: (1) menarche or central precocious puberty clearly diagnosed; (2) current treatment for obesity; (3) taking medications that would impact obesity, such as the stimulants and some psychotropic drugs; (4) severe systemic, liver, and renal disorders; and (5) judged to be ineligible by the study physician, such as mental disorders.
During recruitment, the study details and possible risks of the study were explained to participants and their guardians both in written form and orally by the investigators. Written informed consent for publication of their clinical details was obtained from each participant or her guardian.
Randomization and Masking
After recruitment, girls with obesity were randomly assigned (on a 1:1 ratio) to the DGTP group or the control group by computer-generated block randomization with block sizes of 4, conducted by the Clinical Research Unit of Shanghai Xinhua Hospital, China. The random results were sealed and given to a staff not involved in the trial investigation. For each new participant, the staff informed doctors the group of the participant orderly. The participants, guardians, outcome assessors, statisticians, conclusion drawers, as well as all doctors or dietitians were masked to the treatment allocation, except for the investigator who contacted DGTP capsule manufacturing companies and distributed the DGTP and placebo capsules to participants. Both DGTP and placebo were packed in the congruent opaque capsules for avoiding light and blinding. The two types of study capsules were assigned to transparent bottles of exactly the identical size and appearance, except for different label numbers on the bottles to distinguish their types. The randomization list and questionnaires were securely stored in a locker, and the data were stored in the researcher’s computer protected by a password.
Procedures
The DGTP were purchased from CHENGDU WAGOTT BIO-TECH CO., LTD (Chengdu City, China), and the capsules, including the placebo capsules, were manufactured by LIAOCHENG AOJIAN BIO-TECH CO., LTD (Shandong Province, China). The capsules were made from food additive gelatin with soybean oil and DGTP as filler, and all materials had certificates of safety testing and analysis.
After the informed consent and randomization, participants in the DGTP group were asked to consume two capsules daily (200 mg/capsule DGTP, EGCG accounted for 50%, caffeine accounted for lower than 0.5%), while the control group was given indistinguishable capsules containing isodose soybean oil as a placebo treatment. The dosage of 400 mg daily DGTP (EGCG accounted for 50%) was verified to be safe for children in a previous study (22). The intervention period would last for 12 weeks, which was consistent with some previous GTP interventions among adults with weight reduction effectively and no adverse effect (21, 29–32). Each participant took two capsules 30 min post-dinner per day for 12 weeks. During the intervention period, besides DGTP or placebo treatments, participants were instructed to consume an almost isocaloric diet and engage in the same level of physical activities every day. Participants were also reminded not to receive other types of obesity management, or consume catechins, or polyphenol-rich foods and beverages during the study period. The participants’ guardians were responsible for providing their children with the recommended diets, and study investigators contacted the guardians regularly to ensure the compliance of participants and also monitor the potential adverse effects of treatment.
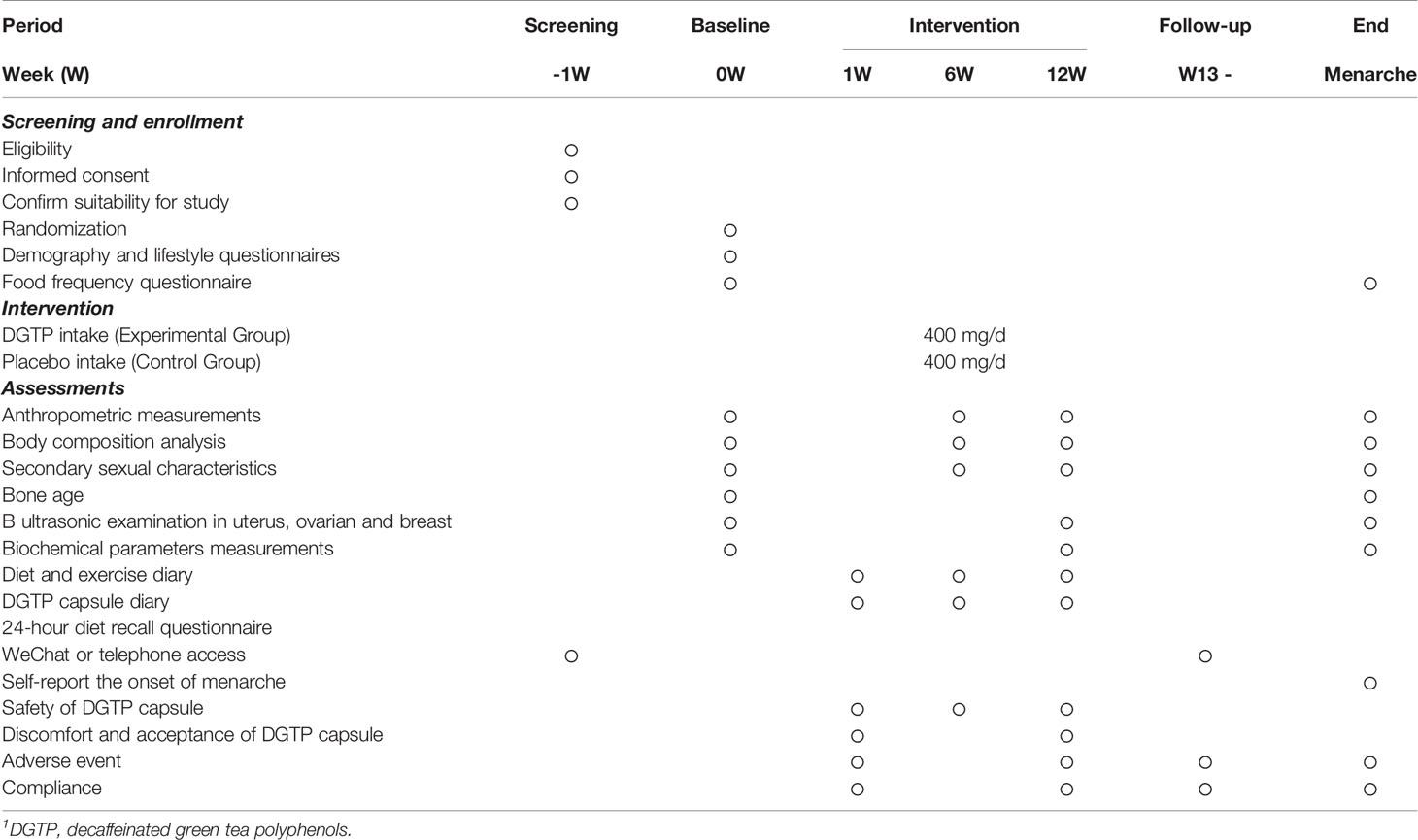
At baseline, participants were asked to complete demographic and lifestyle questionnaires, and bone age measurement. Other assessments were conducted at baseline and at the end of the 12-week intervention period, mainly including anthropometric measurements; body composition analysis; secondary sexual characteristics; B ultrasonic measurements in uterus, ovaries, and breast tissues; biochemical parameter measurements; and dietary assessments. The safety assessments were conducted during the whole study period. A timeline of the study is shown in Table 1.
Outcomes
The primary outcome was the effect of 12-week DGTP on percentage of body fat (PBF) change. The PBF of participants was measured using bioelectrical impedance analyses by whole-body impedance (InBody720, Biospace Inc., Korea) pre- and post-treatment. The onset of menarche was reported to be closely related with the achievement of a certain PBF in girls. Secondary efficacy outcomes included the changes from baseline in BMI, fat-free mass (FFM), serum triglyceride, total cholesterol, and uric acid (UA); secondary sexual characteristics; volume of uterus, ovary, and breast; and sex hormones levels at week 12. The blood samples were collected in the morning with the participants in fasting state, and the serum was sent for laboratory test. Serum triglyceride, total cholesterol, and uric acid values were detected by uricase method using a Hitachi 7600 automatic biochemical analyzer (Hitachi, Tokyo, Japan), and sex hormone levels were measured with a chemiluminescence immuno-metric assay using a UniCell DXI 800 analyzer (Beckman Coulter Inc., Fullerton, CA, USA). Also, the average age of onset of menarche will be followed up and recorded in participants as a secondary outcome.
Participants and their guardians were asked to record the number of capsules participants took every day and all adverse events occurred during the study. Side effects and adverse reactions on sleep or digestive tract were assessed at baseline, week 1, week 6, week 12, and 3 months follow-up after the intervention. Meanwhile, the serum trace elements, hepatic and renal function, and complete blood count of participants were also measured at baseline and week 12 to ensure safety. All participants were free to withdraw at any time during the study.
Statistical Analysis
Data were analyzed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as the mean ± SD, and categorical variables are presented as number and percentages. To compare differences between the DGTP group and the control group, independent sample t-test was performed for continuous variables and χ2 test for categorical variables. To compare differences between baseline and after 12-week intervention in the same groups, paired t-test was performed for continuous variables, and McNemar test was performed for matched categorical variables. In addition, the group differences in changes from week 0 to week 12 were calculated and analyzed using independent sample t-test and χ2 test as well.
Multiple linear regression was employed to evaluate the effect of DGTP intake on the changes of obese and pubertal related outcomes after controlling confounding factors in girls with obesity. All p values were calculated based on two-sided test, and p < 0.05 was considered statistically significant.
Results
After screening, 96 girls with obesity who met the inclusion criteria were invited to participate. Among them, eight girls refused to participate, while four others were excluded due to plans of moving out of town. The remaining 84 participants were enrolled and were randomly allocated into either the DGTP group or the placebo control group. During the study, 13 participants in the DGTP group and 9 in the control group withdrew or dropped out, and the dropout rate was 22/84 (26.2%). Specifically, in the 22 dropouts, 9 could not pay a punctual study visit due to the impact of the coronavirus disease 2019 (COVID-19) in early 2020, which was the period of the initial outbreak of this pandemic, and the original arrangements of follow-up in this study were unavoidably affected. Also, two girls in the DGTP group and one in the control group were diagnosed as precocious puberty and changed to gonadotropin-releasing hormone agonist therapy. Five participants in the DGTP group and five in the control group were considered non-adherent or discontinued the study due to personal reasons like too overload homework to cooperate with. Finally, 62 participants completed the study without any adverse events or side effects reported. The study flowchart is in Figure 1.
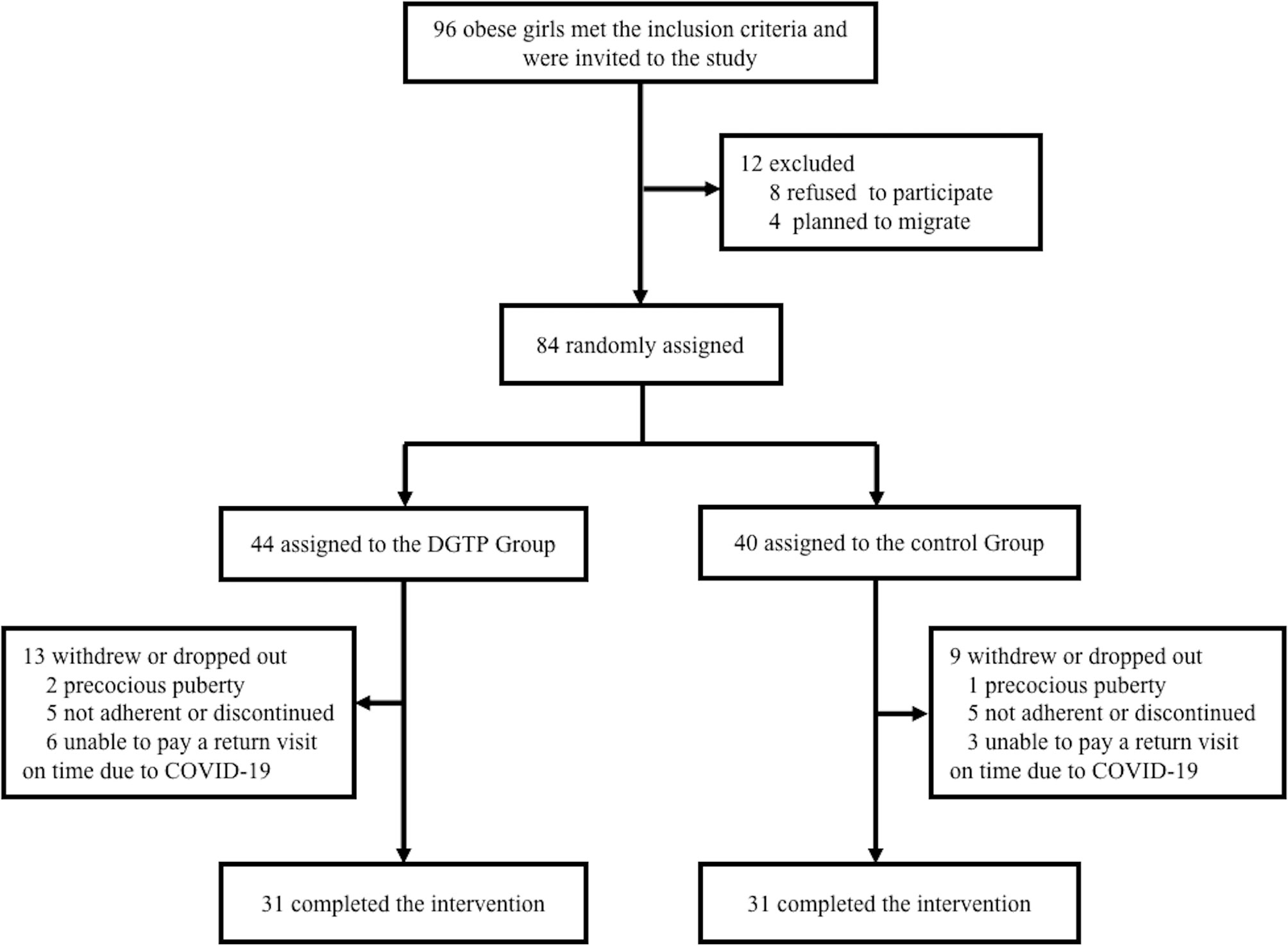
Figure 1 Participant flow diagram showing obese girls aged 6–10 years old from screening to endline analysis. Boxes display number of participants included in the analysis in each stage, along with the reasons for loss of follow-up or exit. COVID-19, Coronavirus Disease 2019; DGTP, decaffeinated green tea polyphenols.
Table 2 presents the demographic and lifestyle data of participants at baseline. There were no significant differences between groups in almost all measures except that a higher percentage of DGTP participants had screen time over 2 h a day than placebo participants (36% vs. 10%; p < 0.05).
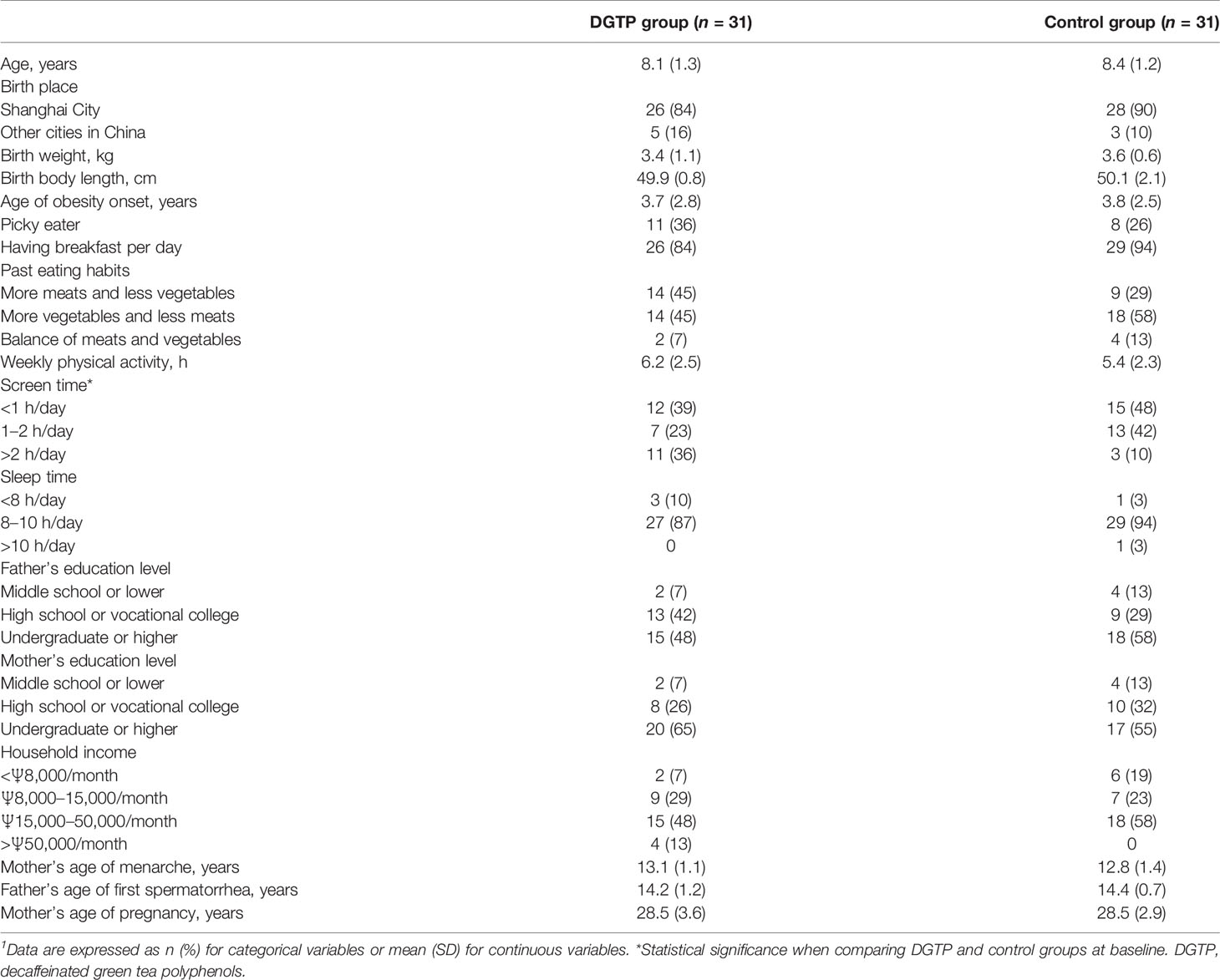
Table 2 Demographic characteristics and lifestyles in both DGTP group and control group at baseline1.
Anthropometric and body composition data are presented in Table 3. Regarding anthropometric data, there was no significant difference between the two groups at baseline and after the intervention. When comparing pre- and post-intervention differences within each group, the height of both groups significantly increased (DGTP group: Δheight: 2.3 ± 0.9 cm, Control group: Δheight: 2.4 ± 0.9 cm), and the BMI, waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) of both groups significantly decreased after 12-week intervention (DGTP group: ΔBMI: −0.8 ± 1.4 kg/m2, Control group: ΔBMI: −0.7 ± 1.4 kg/m2; DGTP group: ΔWC: −2.8 ± 5.4 cm, Control group: ΔWC: −2.5 ± 4.9 cm; DGTP group: ΔWHR: −0.03 ± 0.05, Control group: ΔWHR: −0.03 ± 0.06; DGTP group: ΔWHtR: −0.03 ± 0.04, Control group: ΔWHtR: −0.03 ± 0.04; p < 0.05). No significant changes were found in weight, hip circumference, and blood pressure in either group (Table 3). As for within-group analysis, in the DGTP group, the basal metabolic rate and FFM significantly increased (Δbasal metabolic rate: 18.0 ± 39.9 kcal; ΔFFM: 0.7 ± 1.6kg) while the fat mass and PBF significantly decreased after the 12-week intervention (Δfat mass: 0.7 ± 2.4 kg; ΔPBF: −1.8 ± 4.3%).
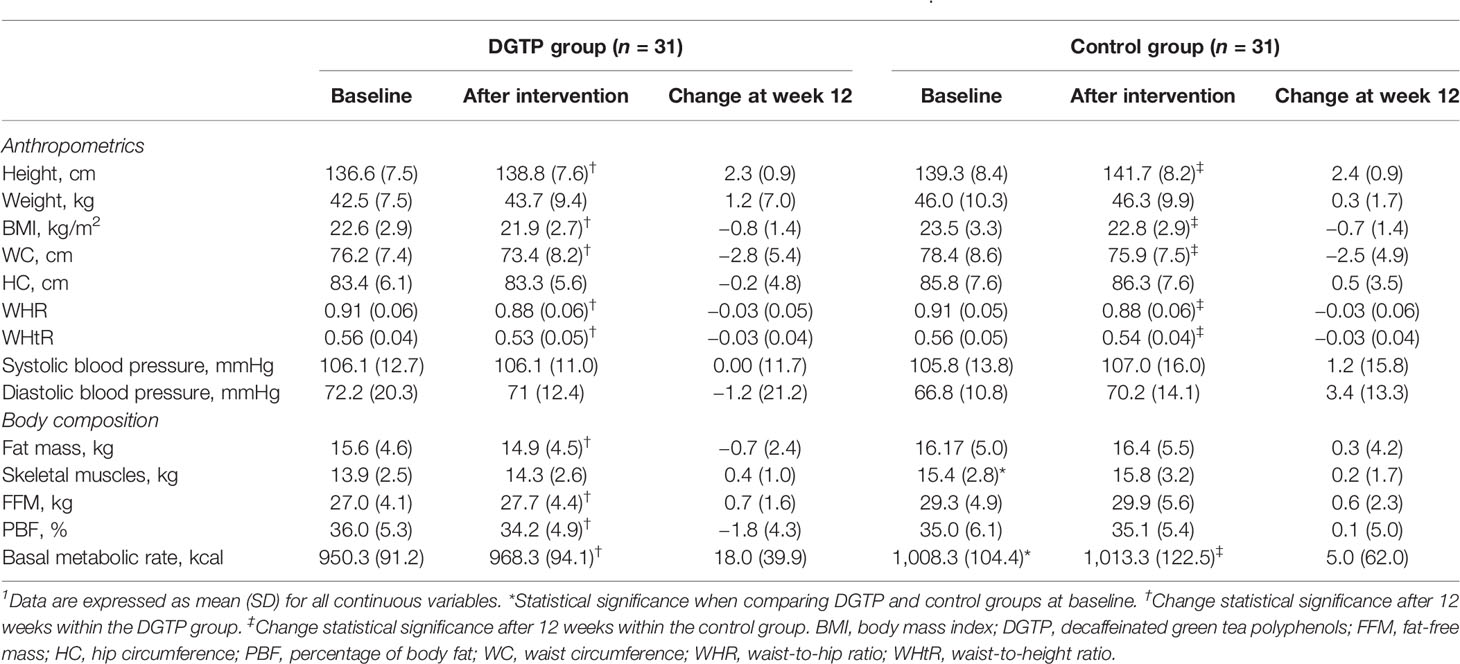
Table 3 Changes in anthropometrics and body composition after daily DGTP intervention for 12 weeks1.
As shown in Table 4, at baseline, the bone age of participants in the DGTP group and the control group were 9.6 ± 1.5 years and 9.7 ± 1.6 years, both higher than their actual age. The difference between bone age and actual age in the two groups was 1.56 ± 1.43 years and 1.48 ± 0.93 years, respectively, with no statistical difference between groups. For the secondary sexual characteristics and ultrasonic examination results, no significant group differences were found either at baseline or after the intervention. When comparing pre- and post-intervention changes, the number of participants with breast Tanner stage II or above and the uterine volume in both groups significantly increased after 12 weeks (DGTP group: Δuterine volume: 0.30 ± 2.20 cm3, Control group: Δuterine volume: 0.46 ± 1.80 cm3). In addition, in the DGTP group, ovarian volumes on both sides significantly decreased (Right: 0.24 ± 1.47 cm3; Left: 0.39 ± 1.24 cm3) after 12 weeks, and the number of participants with diameter >4 mm follicles in the left ovary reduced by three (p < 0.05).
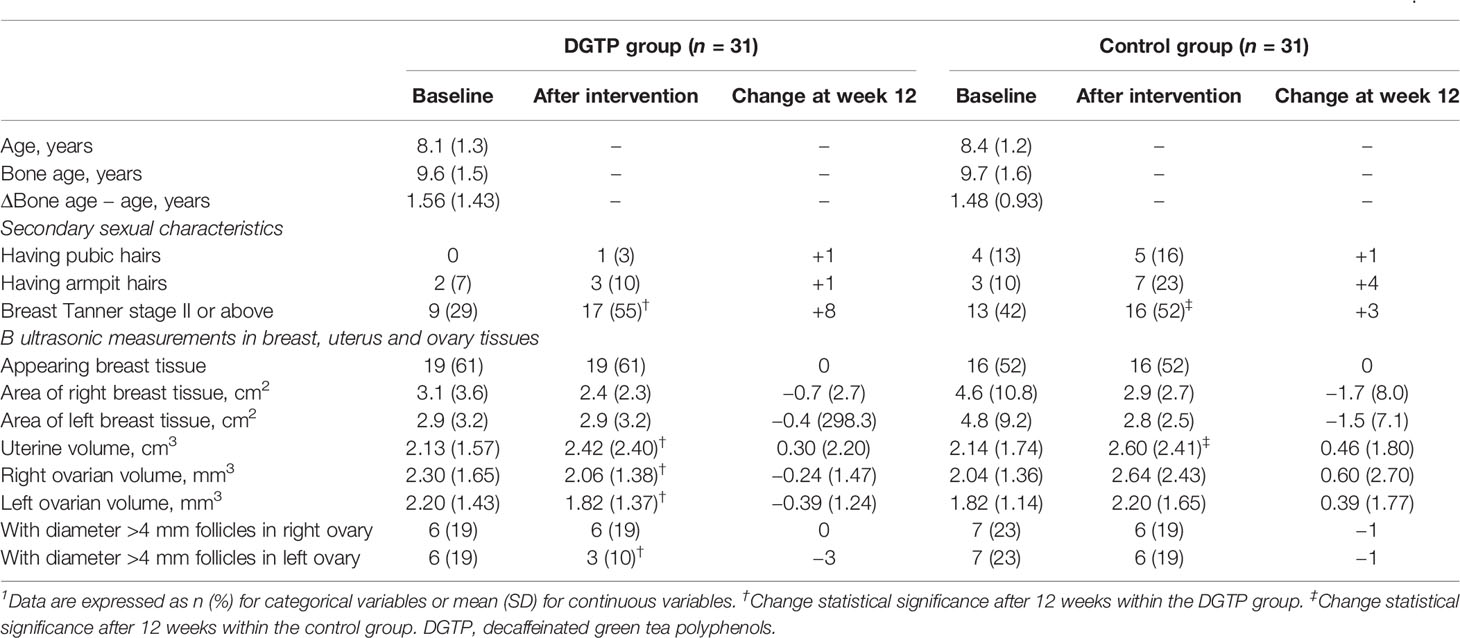
Table 4 Changes in secondary sexual characteristics and ultrasonic examination in breast, uterus, and ovary tissues after daily DGTP intervention for 12 weeks1.
As shown in Table 5, no significant group differences in biomarkers of obesity or sexual development were found at baseline. After 12 weeks, the serum UA concentration in the DGTP group had a significantly higher drop than the control group. When examining the pre- and post-intervention changes within each group, in the DGTP group the serum UA concentration significantly dropped from 376.42 ± 91.08 to 326.65 ± 53.99 μmol/L after 12 weeks. In addition, the concentrations of sex hormones including estradiol, follicle-stimulating hormone, luteinizing hormone in the DGTP group, and estradiol, luteinizing hormone, and testosterone in the control group significantly increased after 12 weeks. However, those concentrations were still relatively low, far below the concentrations of sexual development initiation of girls.
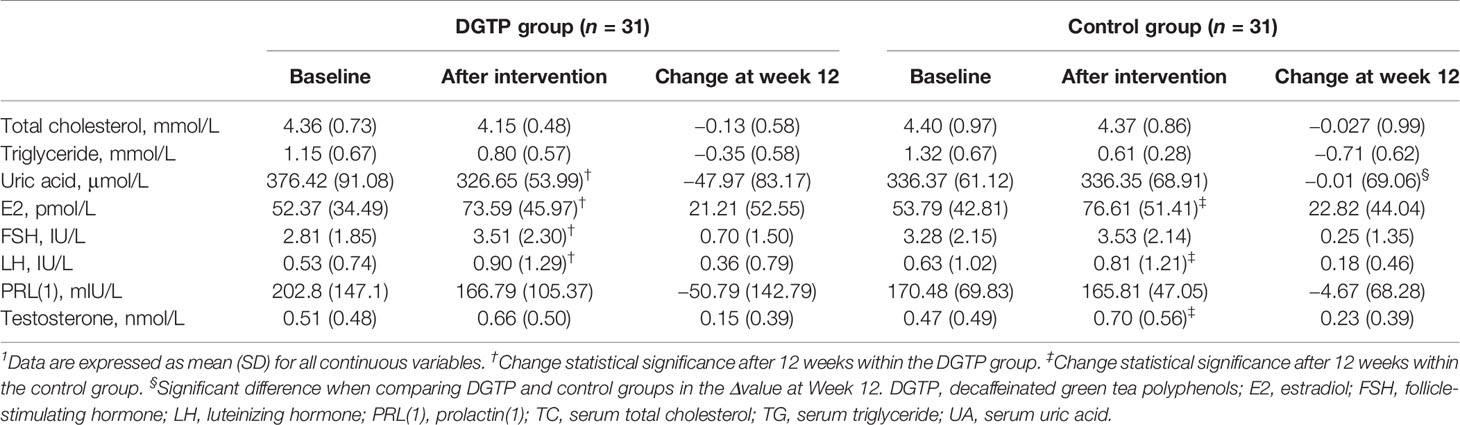
Table 5 Changes in the levels of serum sex hormones, lipid profiles, and uric acid after daily DGTP or placebo intervention for 12 weeks1.
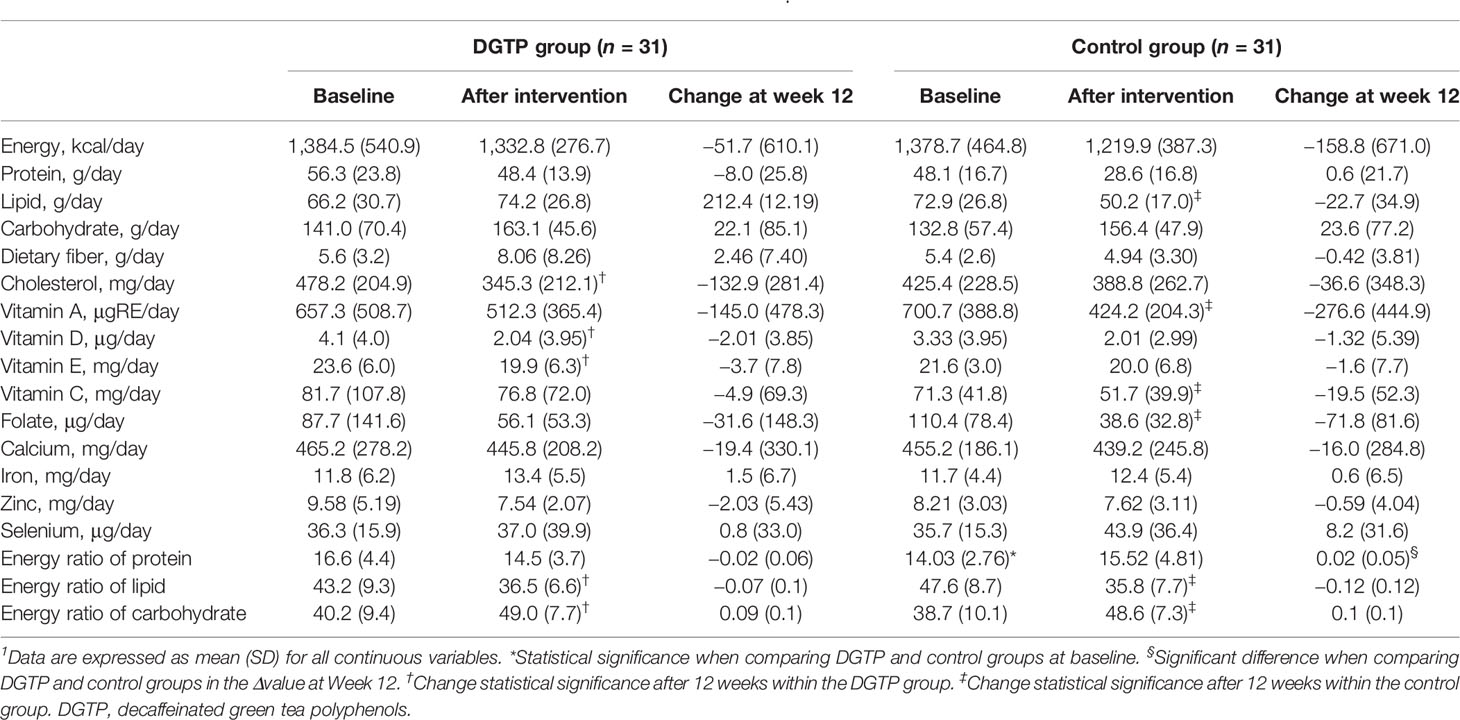
The nutrient intake data are presented in Table 6. No statistical difference in nutrient intakes between the two groups was found before or after intervention except for the energy ratio of protein. After 12 weeks, the energy ratio of lipid decreased, and the energy ratio of carbohydrates increased in both groups. This might be due to the dietary instruction on limiting the excess consumption of lipids, sugars, and beverages during the study. With those changes, the proportion of three macronutrients seemed more balanced compared to the baseline.
After controlling various confounding factors, the association between DGTP intake and the changes of obesity- or sexual development-related parameters after intervention are presented in Table 7. In model 1, DGTP intake was significantly associated with decreased ΔUA (β = 48.747, 95% confidence interval CI: 7.100 to 90.394, p = 0.02) in girls with obesity. In model 2, DGTP intake remained significantly associated with decreased ΔUA (β = 55.172, 95% CI: 6.328 to 104.015, p = 0.03) and also associated with reduced Δright ovarian volume (β = 1.935, 95% CI: 0.336 to 3.533, p = 0.02). Furthermore, in model 3, DGTP intake for 12 weeks was significantly associated with ΔPBF (β = 2.932, 95% CI: 0.214 to 5.650, p = 0.04), ΔUA (β = 52.601, 95% CI: 2.520 to 102.681, p = 0.04), Δright ovarian volume (β = 1.881, 95% CI: 0.062 to 3.699, p = 0.04), and Δleft ovarian volume (β = 0.971, 95% CI: 0.019 to 1.923, p = 0.05).
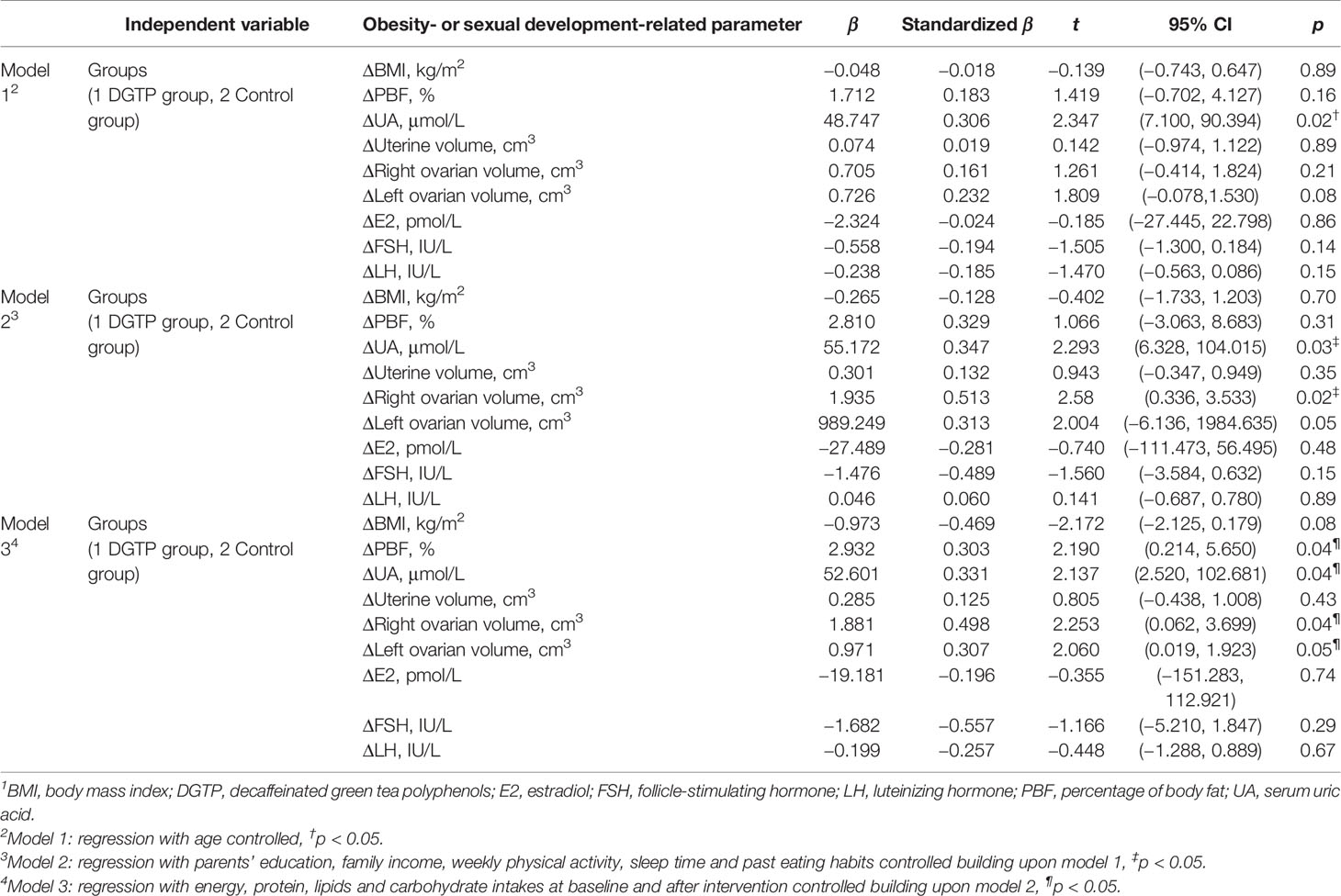
Table 7 Multiple lineal regression results for DGTP intake associated with the change of obesity-related parameters after intervention among obese girls1.
No participants withdrew from the study because of discomfort or adverse events related to the intervention. No adverse reactions and discomfort indigestion were noted and reported in the study period. The changes in their serum trace elements, hepatic and renal function and complete blood count showed no significant differences between the two groups and remained within the normal ranges (not shown in tables). Some participants reported that the size of the capsule was large, which was a little difficult to swallow for them.
Discussion
This study demonstrated that short-term DGTP daily consumption effectively ameliorated obesity and reduced the risk of hyperuricemia among girls with obesity. DGTP might also be effective in preventing early sexual development in girls with obesity. No adverse effects were reported. To our knowledge, this is the first randomized controlled trial study examining the effect of DGTP on obesity and precocious puberty in children, and these findings provided valuable evidence for DGTP clinical application.
In our study, after the intervention, the BMI, WC, WHR, FFM, and PBF in the DGTP group decreased significantly, and DGTP could also reduce ΔPBF after controlling confounders. These findings suggested that DGTP could contribute to a decrease in obesity in children, which were similar with the results of some previous studies (22, 31, 33–35) on GTP for adult weight reduction and its reduction effects on BMI, WHR, and PBF. The mechanisms of GTP in reducing body weight and fat were discussed, such as GTP could inhibit the activities of gastrointestinal enzymes involved in lipids and sugar absorption and metabolism (36–38), or EGCG had an effect on activating like AMP-activated protein kinase, to effectively promote the decomposition and inhibit the formation of body fat (39, 40).
Also, this study reported that DGTP could reduce the concentration of uric acid in children, and in the adjusted model, the significant effect still existed. Some in vitro and in vivo studies (41, 42) showed that GTP might have the effect of lowering uric acid through decreasing uric acid production and increasing uric acid excretion via the inhibition of xanthine oxidase activity and glucose transporter-9 expression and the promotion of organic anion transporter-1 expression.
In the control group, the BMI, WC, and WHR of participants had significant reduction after intervention as well. This change might be due to the professional instruction on diet and physical activities by doctors and dietitians in the Clinical Nutrition Department. With those instructions, participants had developed healthier dietary patterns and habits and had improved health awareness.
In the current study, after DGTP intervention, the ovarian growth of girls with obesity appeared as a “REVERSAL” phenomenon, which is reduced ovarian volumes and reduced number of follicles. This phenomenon might confirm the positive effect of DGTP on postponing early sexual development among girls with obesity. The participants we recruited were simple obese girls and they have not yet entered puberty. Since their hypothalamic–pituitary–gonadal axis has not been officially launched, after the effective intervention, the delayed ovarian development seemed reasonable. In addition, the sex hormone concentrations increased in both groups after 12 weeks significantly but slightly. Overall, they remained at low concentrations, far below the hormone concentration standards of puberty for girls, which also indicated that these girls with obesity have not entered the sexual developmental stage. Certainly, further follow-up is needed to determine whether the final onset of menarche is delayed among girls with obesity. Also, the mechanisms of DGTP to prevent early sexual development in girls with obesity need to be further explored. Some previous studies suggested that leptin was considered likely to be the important signal between obesity and puberty (27, 43, 44), and GTP has been found to suppress the effects of leptin or inhibit the expression of leptin in adipose tissue in obese rats and adults (45, 46). GTP might reduce the expression of leptin in adipose tissue, thereby inhibiting puberty by regulating gonadotrophin-releasing hormone secretion and postponing the initiation of the hypothalamic–pituitary–gonadal axis.
These findings are of great significance in preventing precocious puberty and ameliorating obesity and high uric acid or hyperuricemia among girls with obesity. Green tea polyphenols, as widely accepted natural functional food ingredients, have been further explored and used in a new field of clinical nutrition and pediatric endocrinology. The results of this study also showed great economic value and market of GTP.
However, some limitations also existed in the current study. First, the dropout rate was 26.2% in this study, which was a little high in a RCT. As we described before, 9 of the 22 dropouts could not pay study visits on time due to the COVID-19 pandemic in early 2020 in China. The suddenly new outbreak of COVID-19 hit all the medical resources nationally and people were too scared and observed social distancing strictly, let alone went out to go to the hospital. Our study was unavoidably affected by the situation that the nine participants were way beyond their 12-week study visit when the pandemic alleviated after April 2020; thus, we did not take their post-treatment data into analysis due to the obvious bias. Referring to the study process before the pandemic, we suppose the dropout rate should be much lower without the impact of COVID-19. Second, as this is the first study to identify the effect of DGTP on precocious puberty in children, we have no reference to calculate our sample size. Therefore, referring to the only previous RCT about green tea catechins on children in Japan (22), the sample size of participants was 40 (catechin group; n = 21, control group; n = 19), and we considered our sample size would also be meaningful and, most importantly, provide effective evidence in this field. We also consulted some experts in epidemiology, pediatric endocrinology, and clinical nutrition about our study design and sample size, and they all agreed that this study could provide some preliminary implications if no previous literature helps to calculate the sample size, but small sample size was still one of the limitations. Third, human error may occur during testing and data collection. Participants all started at different times. The testing was done by different clinicians and thus the interpretation of results might be affected by their skills and experiences, e.g., the B-scan ultrasound imaging reports of uterus, ovaries, and breast tissues. Fourth, other parameters, such as sex hormone-binding globulin, could help to further explain the mechanisms between obesity and precocious puberty, but have not been measured in the present study. Finally, the 12-week 400 mg/day DGTP intervention period might not be sufficient to determine the final pubertal change of girls with obesity. This study is a preliminary explorer in this vacant field, and longer follow-up is also needed to determine whether their final age of menarche onset was delayed and the adult heights were as excepted.
Conclusions
This study demonstrated that short-term DGTP daily consumption effectively ameliorated obesity and reduced the risk of hyperuricemia among girls with obesity. DGTP might also be effective in preventing early sexual development in girls with obesity. No adverse effects were reported. These findings provided valuable evidence for the clinical application of DGTP in children.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xinhua Hospital (Shanghai, China). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
LX and XS conceptualized and designed the research, conducted the intervention to this study’s setting, contributed the data collection instruments, collected data, performed statistical analysis, wrote the paper, revised the manuscript, and had primary responsibility for final content. QT conceptualized and designed the study, coordinated and supervised data collection, supported training of data collection staff, and critically reviewed the manuscript for important intellectual content. DY, QG, HZ, and XW supported intervention to the setting and collected data. ZY helped with the initial manuscript and critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the National Natural Science Foundation of China (No. 81773407).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the participating children and their families, and the doctors, dietitians, and staff from Clinical Nutrition Department, Children Endocrinology Department, Children Health Care Department, Ultrasonography Department, Clinical Laboratory, and Clinical Research Unit of Xinhua Hospital, Shanghai, China, for their support during this study.
References
1. Adolescent Health Care Group of Women Health Care Society CPMA. Consensus on Diagnosis and Treatment of Female Precocious Puberty. Chin J Woman Child Health Res (2018) 29(2):135–8. doi: 10.3969/j.issn.1673-5293.2018.02.001
2. Ma HM, Du ML, Luo XP, Chen SK, Liu L, Chen RM, et al. Onset of Breast and Pubic Hair Development and Menses in Urban Chinese Girls. Pediatrics (2009) 124(2):e269–77. doi: 10.1542/peds.2008-2638
3. Jaruratanasirikul S, Chanpong A, Tassanakijpanich N, Sriplung H. Declining Age of Puberty of School Girls in Southern Thailand. World J Pediatr WJP (2014) 10(3):256–61. doi: 10.1007/s12519-014-0472-2
4. Rubin C, Maisonet M, Kieszak S, Monteilh C, Holmes A, Flanders D, et al. Timing of Maturation and Predictors of Menarche in Girls Enrolled in a Contemporary British Cohort. Paediatric perinatal Epidemiol (2009) 23(5):492–504. doi: 10.1111/j.1365-3016.2009.01055.x
5. Mingqiang Z, Junfen F, Li L, Cunxiu G, Feng X, Geli L, et al. Epidemiologic Study on Current Pubertal Development in Chinese School-Aged Children. J ZheJiang University(Medical Science) (2013) 42(4):396–402. doi: 10.3785/j.issn.1008-9292.2013.04.005
6. The Pubertal Study Group of the Society of Pediatric Endocrinology and Genetic Disease CMA. Secondary Sexual Characteristics and Menses in Urban Chinese Girls. Chin J Endocrinol Metab (2010) 26(8):669–75. doi: 10.3760/cma.j.issn.1000-6699.2010.08.011
7. Cesario SK, Hughes LA. Precocious Puberty: A Comprehensive Review of Literature. J Obstetric Gynecologic Neonatal Nurs (2007) 36(3):263–74. doi: 10.1111/j.1552-6909.2007.00145.x
8. Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, et al. Public Health Implications of Altered Puberty Timing. Pediatrics (2008) 121(Suppl 3):S218–30. doi: 10.1542/peds.2007-1813G
9. Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological Mechanisms Underlying Kisspeptin Activation of Gonadotropin-Releasing Hormone (GnRH) Neurons at Puberty. Mol Cell Endocrinol (2010) 324(1-2):45–50. doi: 10.1016/j.mce.2010.01.026
10. Denham M. Relationship of Lead, Mercury, Mirex, Dichlorodiphenyldichloroethylene, Hexachlorobenzene, and Polychlorinated Biphenyls to Timing of Menarche Among Akwesasne Mohawk Girls. Pediatrics (2005) 115(2):e127–34. doi: 10.1542/peds.2004-1161
11. Li W, Liu Q, Deng X, Chen Y, Yang B, Huang X, et al. Association of Prepubertal Obesity With Pubertal Development in Chinese Girls and Boys: A Longitudinal Study. Am J Hum Biol (2018) 30(6):e23195. doi: 10.1002/ajhb.23195
12. Gavela-Perez T, Garces C, Navarro-Sanchez P, Lopez Villanueva L, Soriano-Guillen L. Earlier Menarcheal Age in Spanish Girls is Related With an Increase in Body Mass Index Between Pre-Pubertal School Age and Adolescence. Pediatr Obes (2015) 10(6):410–5. doi: 10.1111/ijpo.277
13. Ramezani Tehrani F, Mirmiran P, Gholami R, Moslehi N, Azizi F. Factors Influencing Menarcheal Age: Results From the Cohort of Tehran Lipid and Glucose Study. Int J Endocrinol Metab (2014) 12(3):e16130. doi: 10.5812/ijem.16130
14. Zhai L, Liu J, Zhao J, Liu J, Bai Y, Jia L, et al. Association of Obesity With Onset of Puberty and Sex Hormones in Chinese Girls: A 4-Year Longitudinal Study. PloS One (2015) 10(8):e0134656. doi: 10.1371/journal.pone.0134656
15. Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, et al. Pubertal Assessment Method and Baseline Characteristics in a Mixed Longitudinal Study of Girls. Pediatrics (2010) 126(3):e583–90. doi: 10.1542/peds.2009-3079
16. Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier Onset of Puberty in Girls: Relation to Increased Body Mass Index and Race. Pediatrics (2001) 108(2):347–53. doi: 10.1542/peds.108.2.347
17. Rosenfield RL, Lipton RB, Drum ML. Thelarche, Pubarche, and Menarche Attainment in Children With Normal and Elevated Body Mass Index. Pediatrics (2009) 123(1):84–8. doi: 10.1542/peds.2008-0146
18. Biro FM, McMahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, Morrison JA, et al. Impact of Timing of Pubertal Maturation on Growth in Black and White Female Adolescents: The National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr (2001) 138(5):0–643. doi: 10.1067/mpd.2001.114476
19. Kao YH, Hiipakka RA, Liao S. Modulation of Endocrine Systems and Food Intake by Green Tea Epigallocatechin Gallate. Endocrinology (2000) 141(3):980–7. doi: 10.1210/endo.141.3.7368
20. Wolfram S, Wang Y, Thielecke F. Anti-Obesity Effects of Green Tea: From Bedside to Bench. Mol Nutr Food Res (2006) 50(2):176–87. doi: 10.1002/mnfr.200500102
21. Nagao T, Hase T, Tokimitsu I. A Green Tea Extract High in Catechins Reduces Body Fat and Cardiovascular Risks in Humans. Obes (Silver Spring Md) (2007) 15(6):1473–83. doi: 10.1038/oby.2007.176
22. Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I. Catechin Safely Improved Higher Levels of Fatness, Blood Pressure, and Cholesterol in Children. Obes (Silver Spring Md) (2008) 16(6):1338–48. doi: 10.1038/oby.2008.60
23. Watson EJ, Banks S, Coates AM, Kohler MJ. The Relationship Between Caffeine, Sleep, and Behavior in Children. J Clin Sleep Med Jcsm Off Publ Am Acad Sleep Med (2017) 13(04):533–43. doi: 10.5664/jcsm.6536
24. Richards G, Smith A. Caffeine Consumption and Self-Assessed Stress, Anxiety, and Depression in Secondary School Children. J Psychopharmacol (2015) 29(12):1236–47. doi: 10.1177/0269881115612404
25. Turley KR, DeSisso T, Gerst JW. Effects of Caffeine on Physiological Responses to Exercise: Boys Versus Men. Pediatr Exercise Sci (2007) 19(4):481–92. doi: 10.1123/pes.19.4.481
26. Teilmann G. Prevalence and Incidence of Precocious Pubertal Development in Denmark: An Epidemiologic Study Based on National Registries. Pediatrics (2005) 116(6):1323–8. doi: 10.1542/peds.2005-0012
27. Wu Y, Wang J, Cai W, Shen X. Could Tea Polyphenols be Beneficial for Preventing the Precocious Puberty? Med Hypotheses (2016) 95:24–6. doi: 10.1016/j.mehy.2016.07.017
28. Force GoCOT. Body Mass Index Reference Norm for Screening Overweight and Obesity in Chinese Children and Adolescents. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi (2004) 25(2):97–102. doi: 10.3760/j.issn:0254-6450.2004.02.003
29. Chen IJ, Liu CY, Chiu JP, Hsu CH. Therapeutic Effect of High-Dose Green Tea Extract on Weight Reduction: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Clin Nutr (2016) 35(3):592–9. doi: 10.1016/j.clnu.2015.05.003
30. Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green Tea Extract Reduces Blood Pressure, Inflammatory Biomarkers, and Oxidative Stress and Improves Parameters Associated With Insulin Resistance in Obese, Hypertensive Patients. Nutr Res (2012) 32(6):421–7. doi: 10.1016/j.nutres.2012.05.007
31. Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, et al. Green Tea Catechin Consumption Enhances Exercise-Induced Abdominal Fat Loss in Overweight and Obese Adults. J Nutr (2009) 139(2):264–70. doi: 10.3945/jn.108.098293
32. Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of Green Tea Extract on Obese Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Clin Nutr (2008) 27(3):363–70. doi: 10.1016/j.clnu.2008.03.007
33. Nagao T, Meguro S, Hase T, Otsuka K, Yamamoto K. A Catechin-Rich Beverage Improves Obesity and Blood Glucose Control in Patients With Type 2 Diabetes. Obesity (2008) 17(2):310–7. doi: 10.1038/oby.2008.505
34. Tomonori N, Yumiko K, Satoko S, Shinichi M, Tadashi H, Yukitaka T, et al. Ingestion of a Tea Rich in Catechins Leads to a Reduction in Body Fat and Malondialdehyde-Modified LDL in Men. Amjclinnutr (2005) 81(1):122–9. doi: 10.1079/PHN2004668
35. Tsuchida T, Itakura H, Nakamura H. Reduction of Body Fat in Humans by Long-Term Ingestion of Catechins. Le Progrés Médical (2002) 22(9):2189–203.
36. Glisan SL, Grove KA, Yennawar NH, Lambert JD. Inhibition of Pancreatic Lipase by Black Tea Theaflavins: Comparative Enzymology and in Silico Modeling Studies. Food Chem (2017) 216:296–300. doi: 10.1016/j.foodchem.2016.08.052
37. Koo SI, Noh SK. Green Tea as Inhibitor of the Intestinal Absorption of Lipids: Potential Mechanism for its Lipid-Lowering Effect. J Nutr Biochem (2007) 18(3):179–83. doi: 10.1016/j.jnutbio.2006.12.005
38. Satoh T, Igarashi M, Yamada S, Takahashi N, Watanabe K. Inhibitory Effect of Black Tea and its Combination With Acarbose on Small Intestinal α-Glucosidase Activity. J Ethnopharmacology (2015) 161:147–55. doi: 10.1016/j.jep.2014.12.009
39. Wang S, Huang Y, Xu H, Zhu Q, Sheng J. Oxidized Tea Polyphenols Prevent Lipid Accumulation in Liver and Visceral White Adipose Tissue in Rats. Eur J Nutr (2016) 56(6):1–12. doi: 10.1007/s00394-016-1241-x
40. Rocha A, Bolin AP, Cardoso CAL, Otton R. Green Tea Extract Activates AMPK and Ameliorates White Adipose Tissue Metabolic Dysfunction Induced by Obesity. Eur J Nutr (2015) 55(7):1–14. doi: 10.1007/s00394-015-1033-8
41. Li F, Liu Y, Xie Y, Liu Z, Zou G. Epigallocatechin Gallate Reduces Uric Acid Levels by Regulating Xanthine Oxidase Activity and Uric Acid Excretion In Vitro and In Vivo. Ann palliative Med (2020) 9(2):331–8. doi: 10.21037/apm.2019.11.28
42. Chen G, Tan ML, Li KK, Leung PC, Ko CH. Green Tea Polyphenols Decreases Uric Acid Level Through Xanthine Oxidase and Renal Urate Transporters in Hyperuricemic Mice. J Ethnopharmacol (2015) 175:14–20. doi: 10.1016/j.jep.2015.08.043
43. Chehab FF, Lim ME, Lu R. Correction of the Sterility Defect in Homozygous Obese Female Mice by Treatment With the Human Recombinant Leptin. Nat Genet (1996) 12(3):318–20. doi: 10.1038/ng0396-318
44. Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, et al. Leptin is a Metabolic Signal to the Reproductive System. Endocrinology (1996) 137(7):3144–7. doi: 10.1210/endo.137.7.8770941
45. Shen C-L, Cao JJ, Dagda RY, Chanjaplammootil S, Lu C, Chyu M-C, et al. Green Tea Polyphenols Benefits Body Composition and Improves Bone Quality in Long-Term High-Fat Diet–Induced Obese Rats. Nutr Res (2012) 32(6):448–57. doi: 10.1016/j.nutres.2012.05.001
Keywords: obesity, precocious puberty, decaffeinated green tea polyphenol, girls, randomized controlled trial
Citation: Xie L, Tang Q, Yao D, Gu Q, Zheng H, Wang X, Yu Z and Shen X (2021) Effect of Decaffeinated Green Tea Polyphenols on Body Fat and Precocious Puberty in Obese Girls: A Randomized Controlled Trial. Front. Endocrinol. 12:736724. doi: 10.3389/fendo.2021.736724
Received: 05 July 2021; Accepted: 20 September 2021;
Published: 12 October 2021.
Edited by:
George Paltoglou, National and Kapodistrian University of Athens, GreeceReviewed by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroHyun Wook Chae, Yonsei University, South Korea
Copyright © 2021 Xie, Tang, Yao, Gu, Zheng, Wang, Yu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuhua Shen, c3JhY2hlbEAxMjYuY29t
 Luyao Xie
Luyao Xie Qingya Tang1
Qingya Tang1 Zhiping Yu
Zhiping Yu Xiuhua Shen
Xiuhua Shen