- Department of Reproductive Medicine Center, The Affiliated Changzhou Maternal and Child Health Care Hospital of Nanjing Medical University, Changzhou, China
Background: To determine whether the embryo developmental stage affects biochemical or clinical pregnancy loss in young women undergoing frozen-thawed embryo transfer (FET) and to investigate the underlying mechanism.
Methods: This was a retrospective study including a total of 18,34 β-HCG (human chorionic gonadotropin)-positive FET cycles. According to the morphological appearance (MA) of transferred blastocysts, FET cycles with blastocysts were divided into two groups: Group A: morphologically good (MG) blastocysts only, and Group B: at least one morphologically non-good (MNG) blastocyst. FET cycles with day 3 cleavage embryos were assigned as Group C. Biochemical and clinical pregnancy loss were the main outcome measures.
Result(s): We predicted 78% in vivo-formed MG and 53.9% in vivo-formed day 5 blastocysts in Group C. (a) Including cases in Group A and Group B for binary logistic regression, we showed that Group B and day 6 blastocysts had significantly higher rates of BPL and CPL than Group A and day 5 blastocysts, respectively. (b) Including cases in Group A, Group B, and Group C for binary logistic regression, we showed that Group C had a significantly higher rate of BPL than Group A and day 5 blastocysts and a similar rate of BPL as Group B and day 6 blastocysts. Group C had a higher rate of CPL than Group A (p=0.071) and day 5 blastocysts (p=0.039), and a lower rate of CPL than Group B (p=0.199) and day 6 blastocysts (p=0.234).
Conclusion(s): (1) MA and days of usable blastocysts could serve as independent factors affecting the occurrence of BPL and CPL. (2) Transfer of day 3 cleavage embryos may produce “unusable blastocysts” in vivo, which significantly increased the rate of BPL. (3) The rate of CPL resulting from the transfer of day 3 embryos may depend on the rate of in vivo-formed MG or day 5 blastocysts. Our study indicated that the difference in the BPL or CPL between transfer of blastocysts and day 3 cleavage embryos may largely depend on the quality of embryos transferred.
Introduction
The physiological process of pregnancy begins with the event of fertilization. The fertilized zygote develops into a Day 3 cleavage embryo, which further becomes a blastocyst. Then, the blastocyst initiates the embryo–utero interaction and secretion of β-HCG (1). A positive serum β-HCG test indicates biochemical pregnancy (2). With successful implantation and subsequent further development, gestational sacs can be found by ultrasound examination, indicating the achievement of clinical pregnancy. However, biochemical pregnancy loss (BPL) or clinical pregnancy loss (CPL) often occurs during pregnancy, resulting in a failed pregnancy (3, 4). Previously, Day 3 cleavage embryos were the most commonly used for transfer in in vitro fertilization (IVF). In recent years, extended culture of Day 3 embryos to the blastocyst stage has been widely adopted in most reproductive centers worldwide. The transfer of blastocysts can achieve better clinical outcomes (5). One of the most important reasons for this is that extended culture can eliminate embryos with low developmental potential (DP) (6, 7). In addition, would blastocyst transfer result in reduced pregnancy loss (BPL or CPL) compared to Day 3 cleavage embryo transfer?
However, few studies have been designed to compare the rate of BPL or CPL between the transfer of Day 3 embryos and blastocysts. Two previous systematic reviews with low evidence indicated that the rates of CPL resulting from the transfer of Day 3 embryos and blastocysts were similar (8, 9). Opposite results were reported regarding the rate of BPL between transfer of Day 3 embryos and blastocysts. Zeadna et al. indicated that the developmental stage of embryos is associated with the occurrence of BPL (10). A recent study conducted by Alberto et al. showed a similar rate of BPL between transfer of Day 3 embryos and blastocysts (11). Therefore, this issue needs to be further investigated.
Commonly, according to the morphological appearance (MA), blastocysts can be classified into three types: morphologically good (MG) blastocysts with high DP, morphologically non-good (MNG) blastocysts with relatively low DP, and morphologically bad (MB) blastocysts with very limited DP (MB blastocysts will be abandoned in most reproductive centers) (12). In addition to the morphological score, blastocyst days are also an important indicator reflecting the DP of blastocysts (13, 14). According to the days of blastocysts, blastocysts can also be classified into three types: Day 5 blastocysts with high DP, Day 6 blastocysts with relatively low DP, and Day 7 blastocysts with very limited DP (in clinical practice, very few embryos will be cultured in vitro for 7 days).
For transfer of Day 3 embryos, a confirmation of biochemical pregnancy indicates that the transferred Day 3 embryo successfully reached the blastocyst stage. However, we do not know what kind of blastocyst it is in vivo. There is no doubt that transfer of Day 3 embryos may result in different qualities of blastocysts (MG/MNG or Day 5/Day 6 blastocysts) in vivo for implantation and subsequent development. However, for blastocyst transfer, blastocysts (MG/MNG or Day 5/Day 6) can be selected subjectively. The key point that helps to clarify whether there is a difference in the rate of BPL or CPL between transfer of Day 3 cleavage embryos and blastocysts is to investigate whether the quality of blastocysts (mainly reflected by MA or days of blastocysts) affects the rate of BPL or CPL. If the quality of blastocysts transferred has an impact on the rate of BPL or CPL, the expected rate of BPL or CPL from transfer of Day 3 embryos may be in the middle of the BPL or CPL from transfer of good and non-good quality of blastocysts; or, we expect a similar rate of BPL or CPL between transfer of blastocysts and Day 3 cleavage embryos.
In the present study, we determined whether the quality of blastocysts (MA or days of blastocysts) affects the rate of BPL or CPL. Then, we compared the rate of BPL or CPL among transfer of Day 3 embryos, transfer of “good” quality blastocysts, and transfer of non-good quality blastocysts. Our study may advance our understanding of the occurrence of BPL and CPL between transfer of blastocysts and Day 3 cleavage embryos.
Materials and Methods
Study Design
This was a retrospective study. The data generated in the present study were collected from January 2017 to May 2020 in the reproductive center of Nanjing Medical University Affiliated Changzhou Maternal and Child Health Care Hospital. FET cycles with biochemical pregnancy were included for analysis. According to the developmental stage of embryos and morphological score of blastocysts, FET cycles were divided into three groups: FET cycles with only MG blastocysts (Group A); FET cycles with at least one MNG blastocyst (Group B); and FET cycles with Day 3 cleavage embryos (Group C). A total of 1,834 β-HCG-positive FET cycles were included. The baseline characteristics of FET cycles and patients were compared. The primary measurements were BPL and CPL. A detailed flowchart of the present study design is presented in Figure 1.
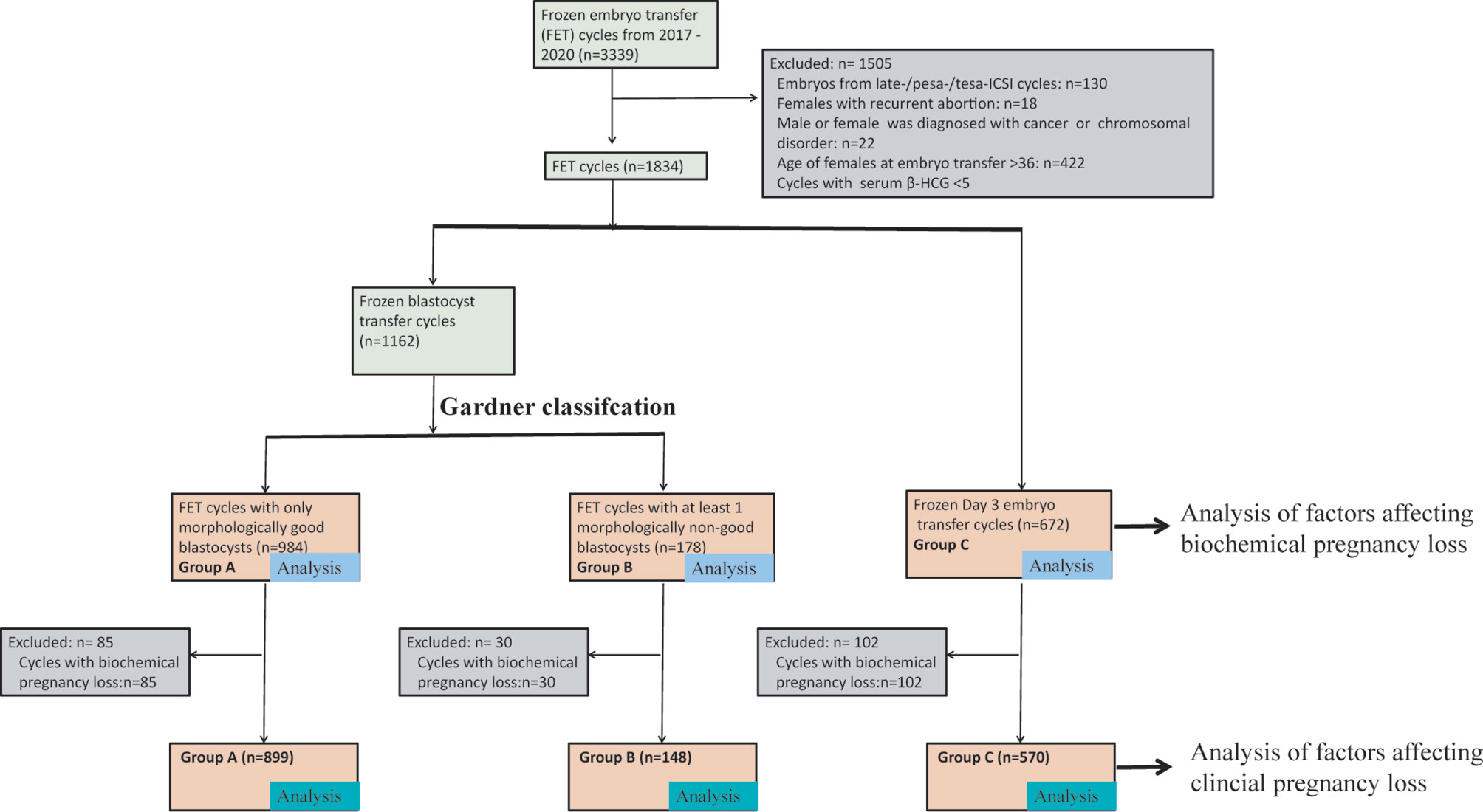
Figure 1 A flow chart of the study design. We initially included all FET cycles that were performed in Changzhou Maternal and Child Health Care Hospital from 2017 to 2019. According to the exclusion criteria, we excluded 1,505 FET cycles. The remaining 1,834 cycles were divided into three groups: Group A: FET cycles with only MG blastocysts; Group B: FET cycles with at least one MNG blastocyst; and Group C: Day 3 cleavage embryo transfer. Cases in Group A, Group B, and/or Group C were used to analyze factors affecting BPL. We further excluded FET cycles with BPL, and cases in Group A, Group B, and/or Group C were used to analyze factors affecting CPL. BPL, biochemical pregnancy loss; CPL, clinical pregnancy loss. FET, frozen-thawed embryo transfer.
Inclusion/Exclusion Criteria
a. FET cycles with biochemical pregnancy (serum β-HCG >5) were included.
b. Cycles with embryos from late-/pesa-/tesa-ICSI cycles were excluded.
c. Females with recurrent abortion or couples in which either the male or female was diagnosed with cancer or chromosomal disorder were excluded from the analysis.
d. Age of females at embryo transfer <=36. Commonly, females at 35 years old may experience a sharp reduction in the quantity and quality of oocytes. Age may be the most important factor affecting pregnancy loss for women with advanced age. Furthermore, women with advanced age undergoing IVF/ICSI treatment may have a relatively small number of available embryos for extended culture. Therefore, in the present study, we focused on young patients.
Embryo Culture Procedures
Controlled ovarian stimulation protocols, including the long protocol, antagonist protocol, PPOS protocol, and mini-stimulation protocols, were used as previously described (15). Thirty-six hours after the trigger, oocytes were retrieved. Oocytes were fertilized by conventional IVF or the ICSI method. G1 and G2 plus medium (Vitrolife, Sweden) was used for embryo culture. Fertilization was evaluated on Day 1, and embryos were morphologically graded on Day 3 and Day 5/6.
Morphological Score of Embryos
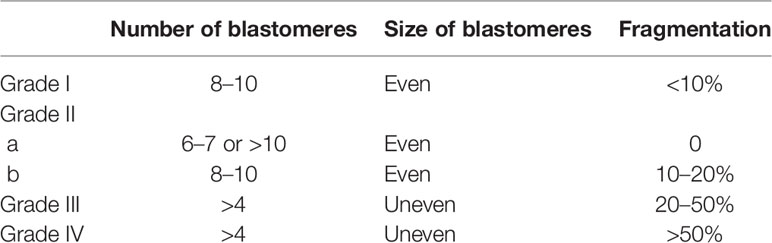
Day 3 cleavage embryos were scored as in a previous study (16). Blastocysts were evaluated based on the degree of blastocyst expansion, inner cell mass (ICM), and trophectoderm (TE) (12). The details of scoring for Day 3 cleavage embryos are described in Table 1, and those for blastocysts are described in Table 2. In our reproductive center, for Day 3 cleavage embryos, grade I and grade II embryos were taken as good cleavage embryos, grade III embryos were taken as non-good embryos, and grade IV embryos were abandoned.
For blastocysts, MG blastocysts were defined as blastocysts with a degree of blastocyst expansion over 3 and an A or B score of ICM and TE.
MNG blastocysts were defined as blastocysts with a degree of blastocyst expansion over 3, a C score of ICM or TE, and a corresponding A or B score of TE or ICM.
MB blastocysts were defined as blastocysts with ICM and TE C scores.
Day 7 blastocysts were defined as the formation of MG or MNG blastocysts on Day 7.
Usable blastocysts were defined as MG or MNG blastocysts on Day 5 or on Day 6.
Unusable blastocysts were defined as MB blastocysts or Day 7 blastocysts.
In our reproductive center, (a) no embryo is cultured for 7 days; (b) blastocysts with a degree of blastocyst expansion <=3 are abandoned; and (c) only usable blastocysts are transferred. In addition, morphological evaluation of embryos in our reproductive center was performed by YW, who has more than 10 years of work experience as an embryologist.
Biochemical and Clinical Pregnancy
Serum β-HCG was measured 11 or 14 days after frozen Day 3 cleavage embryo or blastocyst transfer, respectively. Biochemical pregnancy was defined as serum β-HCG >5. Clinical pregnancy was confirmed by the presence of a gestational sac with fetal heartbeat (14 days after biochemical pregnancy).
The Main Outcome Measures
BPL was defined as pregnancy loss after a confirmation of biochemical pregnancy.
CPL was defined as pregnancy loss after a confirmation of clinical pregnancy.
Endometrial Preparation Protocols
Modified Natural Cycle
On the 10th–12th day of the menstrual cycle, follicles were monitored by transvaginal ultrasonography. When the dominant follicle reached a mean diameter of >17 mm, serum LH was <20 IU/L, endometrial thickness reached =>8 mm, and endometrium presented as pattern A, human chorionic gonadotropin (hCG, 10,000 IU, Lizhu Pharmaceutical Trading Co., China) was used as a trigger. Two days after the hCG trigger, patients were administered progesterone.
Artificial Cycle
On the first day of the menstrual cycle, patients received estradiol valerate tablets at a dose of 2 mg for 3 days, 4 mg for 6 days, and subsequently 6 mg for 5 days. On the 15th day of the menstrual cycle, transvaginal ultrasound was performed to ascertain the endometrial thickness and pattern. If endometrial thickness reached =>8 mm and endometrium presented as pattern A, patients were administered progesterone. If endometrium presented as pattern A while endometrial thickness reached <8 mm, patients would still take oral estradiol valerate tablets until the endometrial thickness reached =>8 mm.
Ovarian Stimulation Cycle
On the 3rd–5th day of the menstrual cycle, patients were administered letrozole (Hengrui Medicine Co., China) for 5 consecutive days. On the 10th day of menstrual cycles, if the diameter of the dominant follicle was <14 mm, human menopausal gonadotropin (Fengyuan Pharmaceutical Co. Anhui, China, 75 IU) was added every other day. When the dominant follicle reached a mean diameter of >17 mm, serum LH was <20 IU/L, endometrial thickness reached =>8 mm, and endometrium presented as pattern A, hCG was used (10,000 IU, Lizhu Pharmaceutical Trading Co., China) as a trigger. Two days after the hCG trigger, patients were administered progesterone.
Statistical Analysis
All analyses were performed using SPSS software (Version 21, IBM). For a comparison of the constituent ratio, the chi-square test was employed; for a comparison of the continuous data, the data were first examined by the normality and lognormality test. If data fit the pattern of normal distribution, Student’s t test was employed; if not, the Mann–Whitney U test was employed.
In the present study, for Table 3, there were 9.6 events per independent variable (EPV) for the analysis of factors affecting BPL, while there were 14 EPVs for the analysis of factors affecting CPL. For Table 4, there were 19.7 EPVs for analysis of factors affecting BPL in manner a and 18.1 EPVs for analysis of factors affecting BPL in manner b; there were 24.9 EPVs for analysis of factors affecting CPL in manner a and 22.8 EPVs for analysis of CPL in manner b. According to the principle of 10 EPVs (17), the sample size in the present study was appropriate.
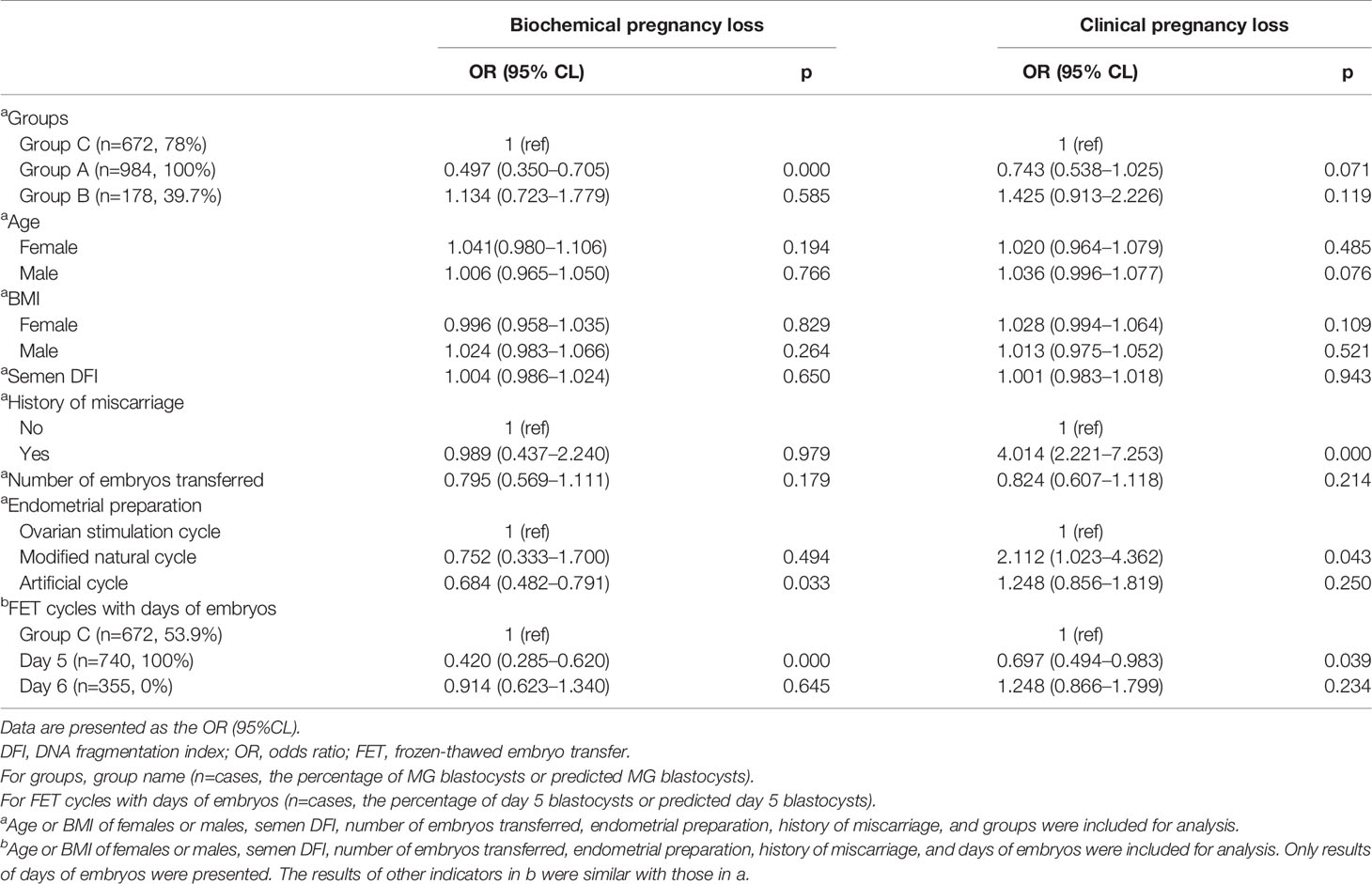
Table 4 Association between factors and BPL/CPL for frozen-thawed blastocyst and day 3 cleavage embryo transfer.
Binary logistic regression analyses were used to evaluate the association between factors (including MA of blastocysts, days of blastocysts, developmental stage of embryos, age of males and females, BMI of females and males, semen DFI, and protocols of endometrial preparation) and BPL or CPL.
A p value of <0.05 was considered a statistically significant difference.
Results
Baseline Characteristics of Patients and FET Cycles
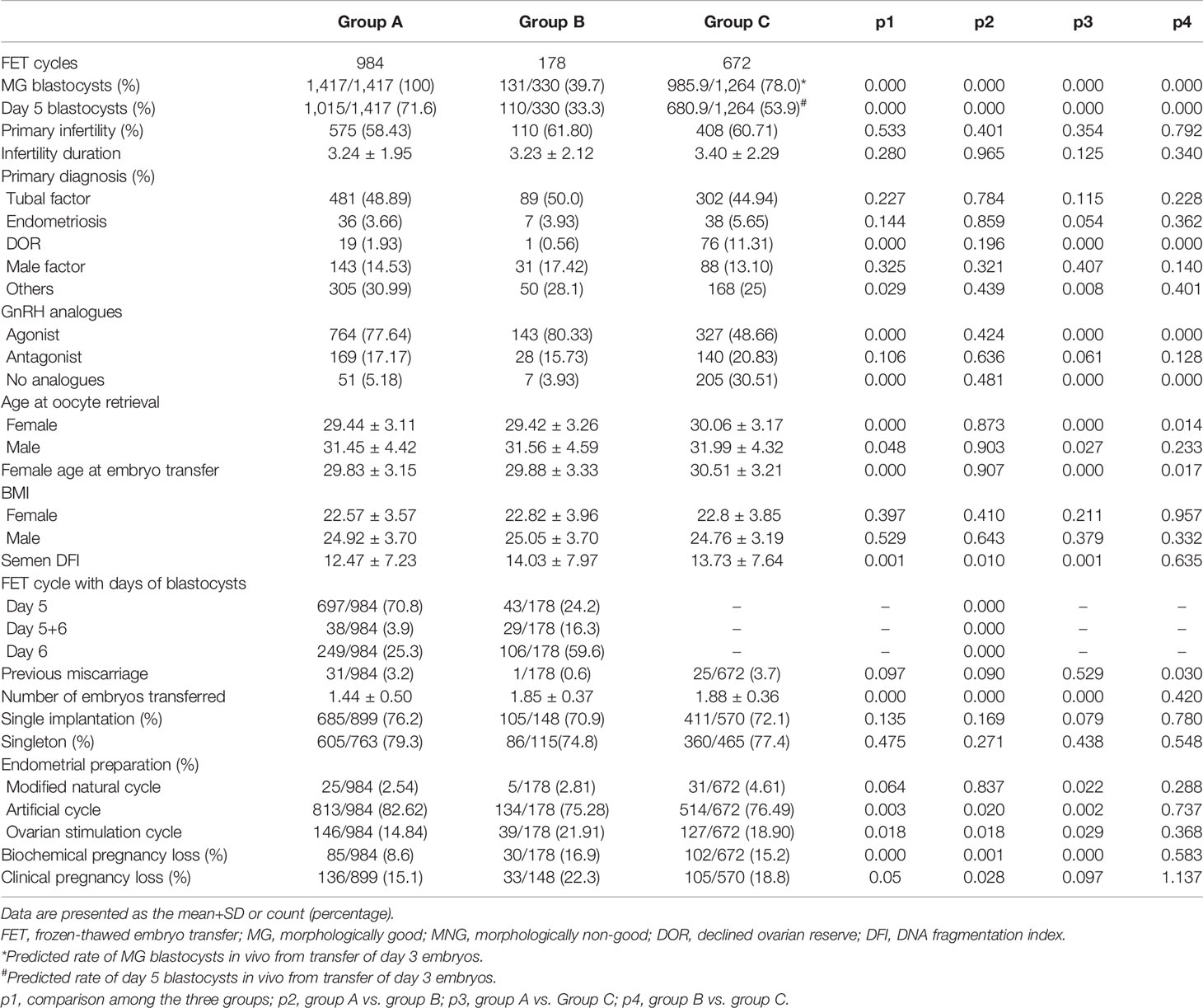
According to the in vitro cultural data of Day 3 cleavage embryos, the ratio of usable MG blastocysts formed from grade I embryos was 88.9%, from grade II embryos was 78.5%, and from grade III embryos was 45.7%, while the ratio of usable Day 5 blastocysts formed from grade I embryos was 62.7%, from grade II embryos was 54.2%, and from grade III embryos was 28.9% (Table 5). Here, we assumed that all the Day 3 embryos transferred in the present study formed useable blastocysts in vivo. This was a rough evaluation without taking the “unusable blastocysts” for consideration. Based on these results, we predicted that 78% of in vivo-formed MG blastocysts and 53.9% of in vivo-formed Day 5 blastocysts resulted from the transfer of Day 3 cleavage embryos in the present study (Table 6).
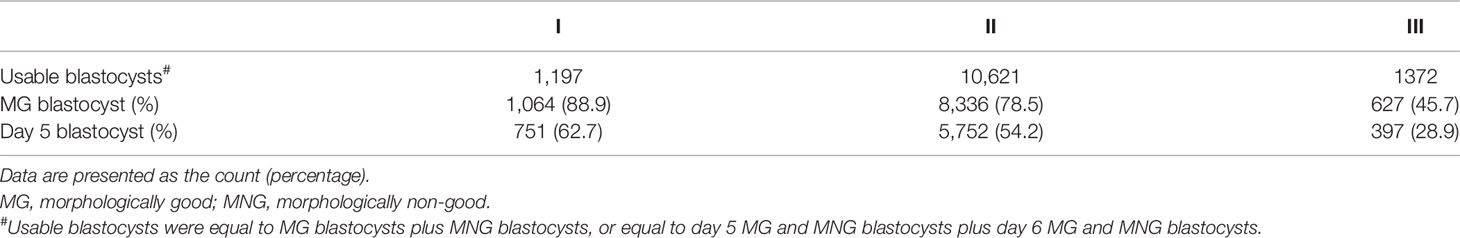
Table 5 The ratio of formed MG or day 5 blastocysts to formed usable blastocysts from extended culture of different grading of Day 3 embryos.
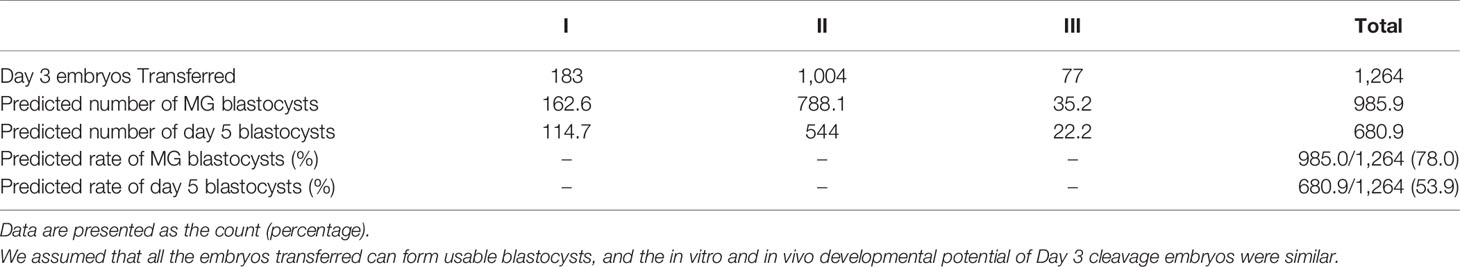
Table 6 Predicted in vivo-formed number and ratio of MG and Day 5 blastocysts to in vivo-formed usable blastocysts for Day 3 cleavage embryos transferred in the present study.
The percentage of primary infertility, primary diagnosis of tubal factors, endometriosis, and male factors, FET cycle-originated fresh cycles with GnRH antagonist, previous miscarriage, single implantation and singleton, BMI of males and females, and infertility duration were comparable among groups (Table 7). The percentage of MG blastocysts and Day 5 blastocysts transferred, the percentage of primary diagnosis of DOR, the percentage of FET cycle-originated fresh cycles with GnRH agonist/without GnRH analogs, the age of males and females at oocyte retrieval, the female age at embryo transfer, the semen DFI, the number of embryos transferred, the protocols of endometrial preparation, and the rate of BPL and CPL among groups were significantly different (Table 7). Furthermore, the percentage of FET cycles with different days of blastocysts between Group A and Group B was significantly different (Table 7).
MA or Days of Blastocysts Were Associated With BPL or CPL
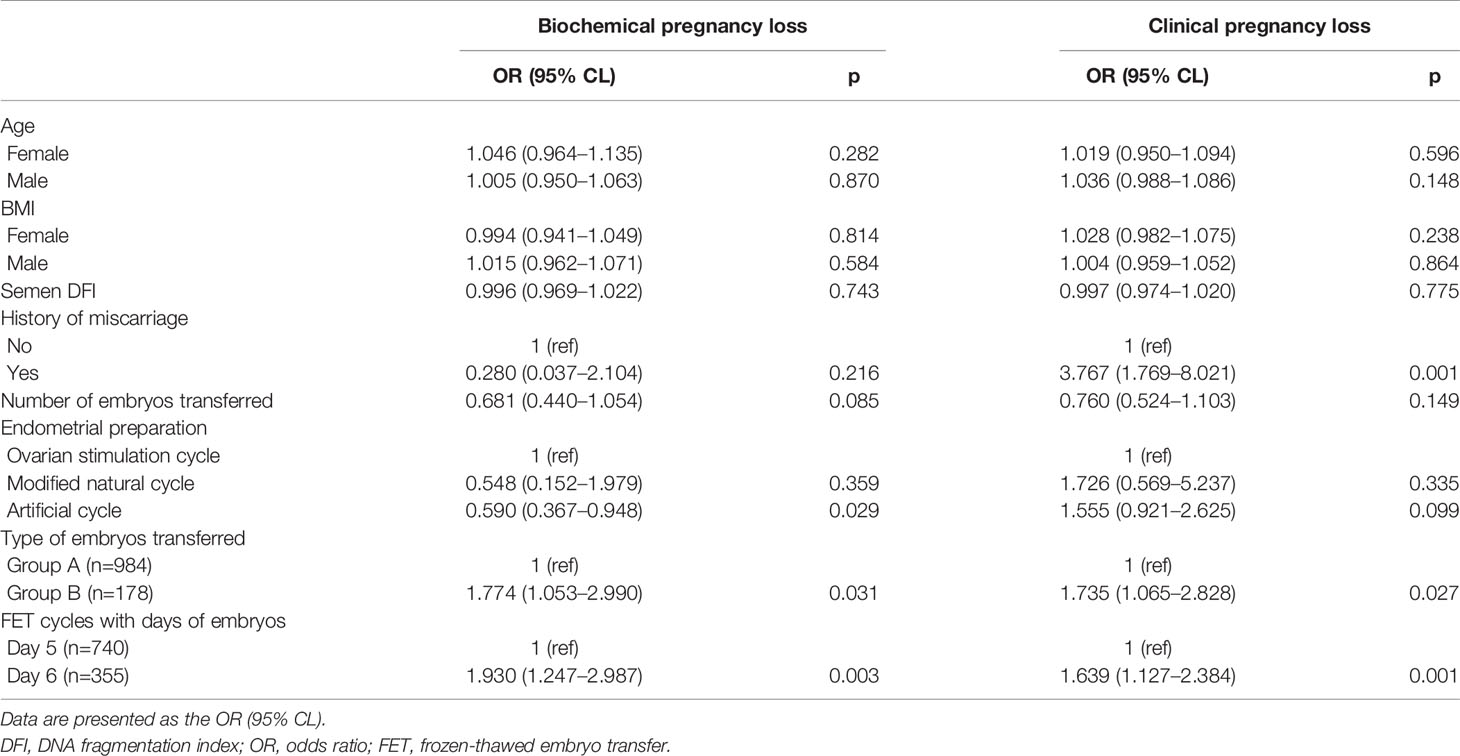
The age or BMI of females or males, semen DFI, and number of embryos transferred had no influence on the occurrence of BPL and CPL (Table 3). A history of miscarriage significantly and positively affected the occurrence of CPL, not BPL (Table 3). Compared to the ovarian stimulation cycle, the artificial cycle showed a significantly reduced rate of BPL (Table 3). Group B had significantly higher rates of BPL and CPL than Group A (Table 3). Similarly, FET cycles with Day 6 blastocysts had significantly higher rates of BPL and CPL than FET cycles with Day 5 blastocysts (Table 3).
Comparison of BPL or CPL Between Transfer of Blastocysts and Transfer of Day 3 Cleavage Embryos
After adding Group C for analysis, we also found that age or BMI of females or males, semen DFI, and number of embryos transferred had no influence on the occurrence of BPL or CPL, and a history of miscarriage significantly and positively affected the occurrence of CPL, not BPL (Table 4). Compared to the ovarian stimulation cycle, the artificial cycle showed a significantly reduced rate of BPL, while the natural cycle showed a significantly higher rate of CPL (Table 4). After controlling factors including age and BMI of females and males, semen DFI, number of embryos transferred, and protocols of endometrial preparation, we found that Group C had a significantly higher rate of BPL than Group A (p=0.00) and a similar rate of BPL as Group B (p=0.585) (Table 4). However, Group C had a higher rate of CPL than Group A (p=0.071) and a reduced rate of CPL compared to Group B (p=0.119) (Table 4). Similarly, after controlling factors including age or BMI of females or males, semen DFI, and number of embryos transferred and protocols of endometrial preparation, we found that Group C had a significantly higher rate of BPL than FET cycles with Day 5 blastocysts (p=0.000) and a similar rate of BPL as FET cycles with Day 6 blastocysts (p=0.645) (Table 4). However, Group C had a significantly higher rate of CPL than FET cycles with Day 5 blastocysts (p=0.039) and a reduced rate of CPL compared to FET cycles with Day 6 blastocysts (p=0.24) (Table 4).
We further tested whether the OR value of Group C (compared to Groups A and B or FET cycles with Day 5 blastocysts and Day 6 blastocysts) fitted the findings from the transfer of blastocysts, and we performed some analyses. The rates of MG blastocysts in Groups A, C, and B were 100 vs. 78 vs. 39.7%, while the rates of Day 5 blastocysts in Groups A, C, and B were 100 vs. 53.9 vs. 0% (Figure 2). Considering that MA and days of blastocysts correlated with the occurrence of BPL and CPL for the transfer of blastocysts, we assumed a linear relationship between the percentage of MG or Day 5 blastocysts and the rate of BPL or CPL in each group. According to the rate of MG or Day 5 blastocysts and the corresponding rate of BPL or CPL for transfer of blastocysts, we predicted the OR value in Group C. For BPL, the predicted OR value (yellow circle) for Group C was obviously lower than the observed OR value grouped by either MA (0.73 vs. 1) or days of blastocyst (0.65 vs. 1); for CPL, the predicted OR value (yellow circle) for Group C was similar to the observed OR value grouped by either MA (0.99 vs. 1) or days of blastocyst (0.95 vs. 1) (Figure 2). Obviously, CPL, not BPL, resulting from the transfer of Day 3 embryos met our expectation.
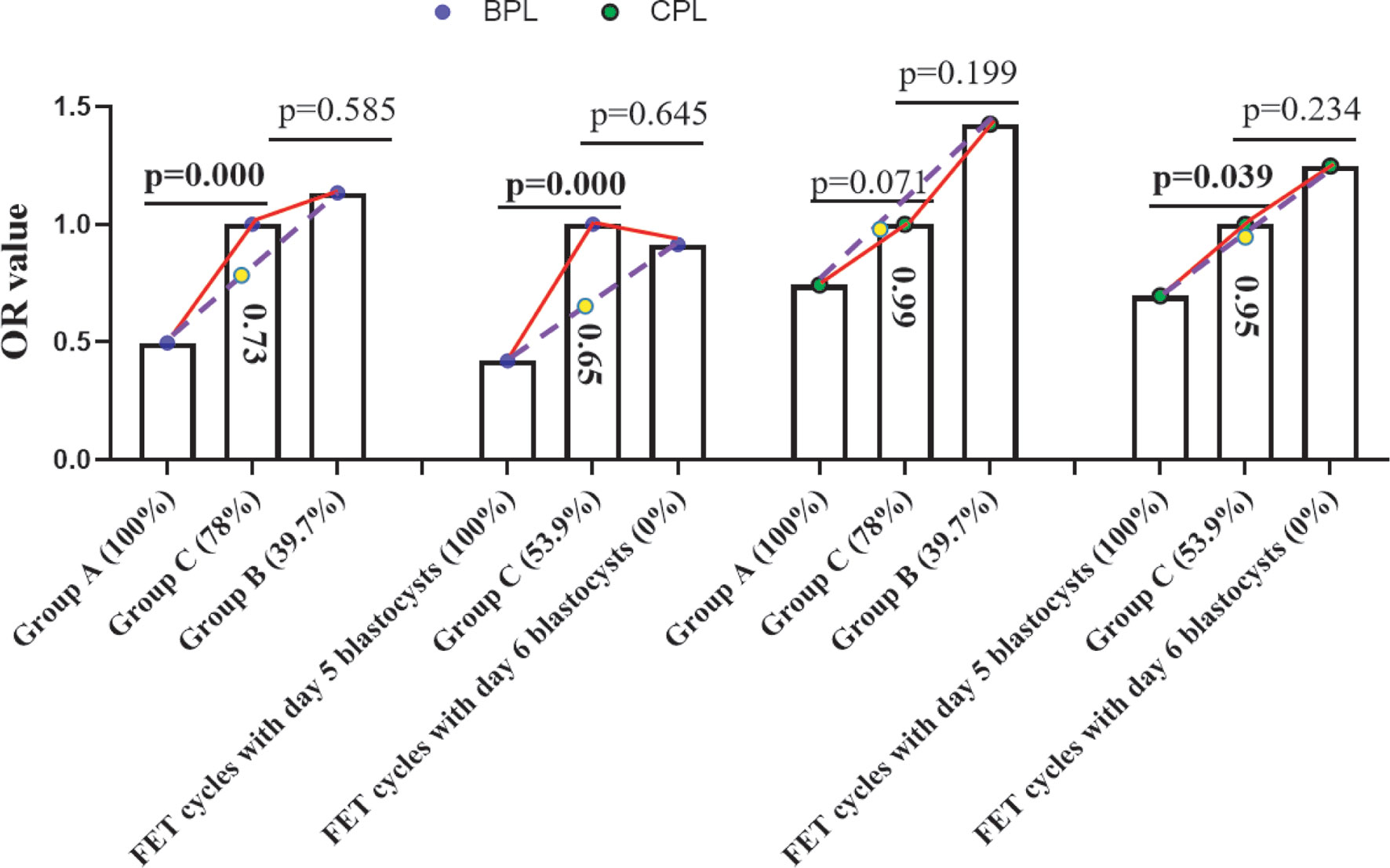
Figure 2 Prediction of the OR value of Group C. According to the OR value of Group A and Group B or the OR value of FET cycles with Day 5/Day 6 blastocysts and the rate of MG blastocysts in Group A and Group B or the rate of Day 5 blastocysts in FET cycles with Day 5 and Day 6 blastocysts, we predicted the OR value of Group C (the predicted OR value is in the column of Group C). The blue circle indicates the OR value of BPL, the green circle indicates the OR value of CPL, and the yellow circle indicates the predicted OR value of BPL or CPL in group C. Values in brackets indicate the percentage of MG or Day 5 blastocysts in each group. BPL, biochemical pregnancy loss; CPL, clinical pregnancy loss; MG, morphologically good; MNG, morphologically non-good; FET, frozen embryo transfer; OR, odds ratio.
Discussion
The argument of pregnancy loss between the transfer of cleavage embryos and blastocysts needs to be clarified. In the present study, our results indicated that the quality of blastocysts obviously affected the occurrence of BPL or CPL, and the difference in the rate of BPL or CPL between transfer of blastocysts and Day 3 cleavage embryos depends on the quality of blastocysts and cleavage embryos transferred. In addition, our study also highlights the increased rate of BPL in the transfer of Day 3 cleavage embryos.
There is consensus that blastocysts are a specific embryo stage that initiates the interaction between embryos and utero (1). Viewed from the perspective of embryos, at least four events account for a failed live birth resulting from transfer of a Day 3 cleavage embryo in IVF: (a) Embryos cannot develop into the blastocyst stage. (b) Blastocyst fails to interact with utero. (c) Blastocyst fails to complete implantation. (d) Abortion. The DP of embryos increased from a to d; c and d were investigated in the present study. Therefore, only β-HCG-positive FET cycles were included in the present study.
In IVF, Day 3 cleavage embryos and blastocysts are the most commonly used for transfer. The biggest difference between the transfer of Day 3 embryos and blastocysts is the further development of Day 3 embryos. For transfer of Day 3 embryos, this development occurs in utero, while for transfer of blastocysts, development occurs in the culture medium. Extended culture allows us to evaluate blastocysts and select blastocysts of good quality for transfer. However, it is impossible to evaluate the blastocysts in vivo when performing transfer of Day 3 embryos. In the present study, we summarized the data of extended culture in our reproductive center and predicted 78% of in vivo-formed MG blastocysts and 53.9% of Day 5 blastocysts for transfer of Day 3 cleavage embryos (Group C). This prediction was based on the assumptions that the in vitro and in vivo blastulation abilities of Day 3 embryos were similar, and all transferred Day 3 embryos formed usable blastocysts and resulted in biochemical pregnancy.
Because few embryos could be retrieved from patients with declined ovarian reserve (DOR) and extended culture may result in no embryos for transfer for those patients, patients with DOR were prone to receive Day 3 cleavage embryo transfer. This is why there were more patients with DOR in Group C. Consequently, these patients were treated with mild ovarian stimulation protocols. A previous study demonstrated that oocytes from young women with normal and decreased ovarian reserve show similar quality (18). Therefore, we deduced that decreased ovarian reserve may not have influenced BPL or CPL in the present study.
Many factors that were reported to possibly affect the occurrence of BPL or CPL were taken as confounding factors for analysis in the present study, including age at oocyte retrieval/BMI of males or females (19–25), semen DFI (26, 27), history of miscarriage (21, 28), number of embryos transferred (29–31), and endometrial preparation protocols (32). In the present study, we found that most of these factors were not related to the occurrence of BPL or CPL. However, we ascertained that a history of miscarriage was tightly associated with the occurrence of CPL but not BPL, indicating the difference between the occurrence of BPL and CPL. A previous study showed that the pregnancy loss rate was significantly higher with ovarian stimulation cycles or artificial cycles than with natural cycles (32). However, our study supported the use of artificial endometrial preparation. The most commonly used endometrial preparation protocol in our center was artificial cycles, and cases in the natural cycle or ovarian stimulation cycles were very limited. Therefore, this conclusion still needs to be verified by large datasets.
To our knowledge, no studies have been conducted to investigate whether MA or days of blastocysts can predict BPL. In the present study, we found that Group B and FET cycles with Day 6 blastocysts had significantly higher rates of BPL than Group A and FET cycles with Day 5 blastocysts, respectively. Due to limited cases of β-HCG-positive FET cycles with MNG blastocysts only, we included FET cycles with at least one MNG blastocyst. Group B had 39.7% of MG blastocysts. Therefore, we deduced that FET cycles with MNG blastocysts only may further increase the rate of BPL. These results indicated that MA or days of blastocyst could serve as an independent factor affecting the occurrence of BPL.
Inconsistent with our prediction, we found that Group C had a significantly higher rate of BPL than Group A or FET cycles with Day 5 blastocysts and a similar rate of BPL as Group B or FET cycles with Day 6 blastocysts. However, this result was consistent with a previous study showing that early pregnancy loss was significantly higher after Day 3 single embryo transfer than after Day 5 single blastocyst transfer (33). For blastocyst transfer, usable blastocysts (only MG or MNG blastocysts) will be selected for use. In contrast, for transfer of Day 3 cleavage embryos, the cleavage embryos not only produce usable blastocysts but also “unusable blastocysts” in vivo. Several studies have demonstrated the DP of “unusable blastocysts”, including MB and Day 7 blastocysts (34–36). Therefore, for Day 3 embryo transfer, biochemical pregnancy may result not only from MG or MNG blastocysts but also from MB or Day 7 blastocysts in the present study. Previous studies revealed that the rate of BPL was similar between transfer of MB or Day 5 blastocysts and transfer of MB or Day 7 blastocysts (34, 37). In their studies, the rate of BPL was defined as the number of FET cycles with BPL to total FET cycles, not to β-HCG-positive FET cycles. Obviously, this definition is not scientific and correct, as most Day 7 or MB blastocysts will not result in biochemical pregnancy. Therefore, the rate of BPL should be defined as the rate of FET cycles with BPL to β-HCG-positive FET cycles. After reanalyzing the data in the two published articles, we found that the rate of BPL of transfer of MB/Day 7 blastocysts was significantly higher (3 times) than that of the transfer of MG/Day 5 blastocysts. Therefore, we deduced that the biochemical pregnancy resulting from in vivo-formed unusable blastocysts may account for the higher BPL for the transfer of Day 3 cleavage embryos. This could explain the similar rate of BPL between transfer of MNG or Day 6 blastocysts and transfer of Day 3 cleavage embryos in the present study.
Numerous studies have been conducted to investigate the role of MA or days of blastocysts in predicting clinical pregnancy and live birth (38–40). Few studies have focused on investigating the effect of MA or the day of blastocyst on the occurrence of pregnancy loss. Previous studies have investigated the relationship between the quality of TE or ICM and miscarriage; however, no consistent conclusions have been drawn (41, 42). A recent study reported no statistical correlation between miscarriage and the grade of ICM or TE (43). In that study, they only included blastocysts with grade A or B ICM and without grade C ICM; therefore, it is not strange that no association of ICM grade with miscarriage was observed (43). However, that study showed a trend that the C score of TE was related to a higher rate of miscarriage (43). A common drawback of these studies is that they only considered a part of the blastocyst (TE or ICM) and did not take the blastocyst as a whole for analysis. However, our morphological classification of blastocysts in the present study could avoid this. In the present study, we found that Group B and FET cycles with Day 6 blastocysts had a significantly higher rate of CPL than Group A and FET cycles with Day 5 blastocysts, respectively. Part of these results was consistent with previous reports that transfer of Day 6 blastocysts resulted in a significantly higher miscarriage rate than that of Day 5 blastocysts (43, 44). These results indicated that MA or days of blastocyst could serve as an independent factor affecting the occurrence of CPL.
Consistent with our prediction, we found that Group C had a higher rate of CPL than Group A and FET cycles with Day 5 blastocysts and a lower rate of CPL than Group B and FET cycles with Day 6 blastocysts. However, some comparisons failed to reach statistical significance. Similar to biochemical pregnancy, it is possible that MB or Day 7 embryos contributed to clinical pregnancy for the transfer of Day 3 embryos in the present study. However, we did not observe an obviously higher rate of CPL for transfer of Day 3 embryos, indicating that the possibly implanted “unusable blastocysts” produced by Day 3 cleavage embryos in vivo did not result in a significantly higher rate of CPL compared to transfer of MG or Day 5 blastocysts. This is consistent with previous studies reporting that the CPL between transfer of MG/Day 5 blastocysts and transfer of MB/Day 7 blastocysts was similar (34, 37). However, due to the too limited implantation ability of “unusable blastocysts”, whether “unusable blastocysts” would not result in a significantly higher rate of CPL remains to be investigated by using large data.
Taking all the results as a whole for analysis, we concluded that (1) MA or days of blastocyst served as an independent factor affecting the occurrence of BPL and CPL. (2) Transfer of Day 3 cleavage embryos may result in “unusable blastocysts”, which may significantly increase the rate of BPL. (3) The rate of CPL resulting from the transfer of Day 3 embryos may depend on the rate of in vivo-formed MG or Day 5 blastocysts (to in vivo-formed useable blastocysts). Therefore, when comparing the rate of BPL or CPL between transfer of blastocysts and Day 3 cleavage embryos, the quality of embryos transferred, including blastocysts or cleavage embryos, should be taken into consideration. Our study suggested that morphologically good Day 5 blastocysts should be first considered for transfer, not only because of the high implantation potential, as a previous study reported (45), but also because of the low rate of pregnancy loss. Furthermore, our study may help to explain a recently published study indicating that FET cycles with blastocysts arising from poor-quality cleavage embryos showed a significantly higher miscarriage rate (46). As we showed in the present study, the rate of Day 5 blastocysts from grade III Day 3 embryos was 29%, while that from grade I embryos was 62.7%. Although the MA of transferred blastocysts among groups was similar in that study, it was more likely that Day 5 blastocysts were selected for transfer in the grade I group, thereby resulting in a difference in the miscarriage rate. The comprehensive analysis of the present study advanced our understanding of the occurrence of BPL and CPL between transfer of blastocysts or Day 3 cleavage embryos. Therefore, our study is clinically and theoretically important.
Due to the retrospective nature of the present study, limitations were presented, including possible confounding factors that were not considered in the present study. Our study included FET cycles with double embryos, which may introduce bias. FET cycles with two embryos may result in two implantations and one live birth. However, these FET cycles were not taken as cycles with CPL or possible BPL. Furthermore, the sample size was not large enough to reach statistical significance for some comparisons. Additionally, data from a single center may dampen the confidence of the present study. Therefore, our study needs to be confirmed by a large study.
Conclusion
We concluded that (1) MA or days of blastocyst served as an independent factor affecting the occurrence of BPL or CPL. (2) Transfer of Day 3 cleavage embryos may produce “unusable blastocysts”, which may significantly increase the rate of BPL. (3) The rate of CPL resulting from the transfer of Day 3 embryos may depend on the rate of in vivo-formed MG or Day 5 blastocysts. In conclusion, the difference in BPL or CPL between transfer of blastocysts and Day 3 cleavage embryos depends on the quality of embryos transferred. Our study advanced our understanding of the occurrence of BPL and CPL between transfer of blastocysts and Day 3 cleavage embryos.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Statement
All of the included patients read and signed the informed consent form. This retrospective study was approved by the Ethics Committee of Changzhou Maternal and Child Health Care Hospital and Nanjing Medical University.
Author Contributions
Substantial contribution to conception and design: LC, XD, and YW. Data acquisition: TG, XX, and FC. Data analysis: TG, XX, FC, CY, TL, and LL. Data interpretation: All authors. Drafting the article: XD and YW. Critical revision of the article for important intellectual content: All authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81901436, to XD), Changzhou Health Committee Funded Young Investigator Training Project (CZQM2020094 to XD and CZQM2020099 to TG), Key Program of Changzhou Municipal Health Commission (ZD201921 to LC), Program of Jiangsu Province’s Key Provincial Talents of Women and Child Health Care (FRC 201751 to LC), and Jiangsu Population Institute funded program (JSPA2019017 to TL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schumacher A, Zenclussen AC. Human Chorionic Gonadotropin-Mediated Immune Responses That Facilitate Embryo Implantation and Placentation. Front Immunol (2019) 10:2896. doi: 10.3389/fimmu.2019.02896
2. Lopata A, Hay DL. The Potential of Early Human Embryos to Form Blastocysts, Hatch From Their Zona and Secrete HCG in Culture. Hum Reprod (1989) 4(8 Suppl):87–94. doi: 10.1093/humrep/4.suppl_1.87
3. Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of Early Loss of Pregnancy. N Engl J Med (1988) 319(4):189–94. doi: 10.1056/NEJM198807283190401
4. Macklon NS, Geraedts JP, Fauser BC. Conception to Ongoing Pregnancy: The 'Black Box' of Early Pregnancy Loss. Hum Reprod Update (2002) 8(4):333–43. doi: 10.1093/humupd/8.4.333
5. Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In Vitro Fertilization With Single Blastocyst-Stage Versus Single Cleavage-Stage Embryos. N Engl J Med (2006) 354(11):1139–46. doi: 10.1056/NEJMoa053524
6. Olivennes F, Hazout A, Lelaidier C, Freitas S, Fanchin R, de Ziegler D, et al. Four Indications for Embryo Transfer at the Blastocyst Stage. Hum Reprod (1994) 9(12):2367–73. doi: 10.1093/oxfordjournals.humrep.a138454
7. Plachot M, Belaisch-Allart J, Mayenga JM, Chouraqui A, Serkine AM, Tesquier L. Blastocyst Stage Transfer: The Real Benefits Compared With Early Embryo Transfer. Hum Reprod (2000) 15 Suppl 6:24–30.
8. Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C. Blastocyst vs Cleavage-Stage Embryo Transfer: Systematic Review and Meta-Analysis of Reproductive Outcomes. Ultrasound Obstet Gynecol (2017) 49(5):583–91. doi: 10.1002/uog.17327
9. Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage Stage Versus Blastocyst Stage Embryo Transfer in Assisted Reproductive Technology. Cochrane Database Syst Rev (2016) 6):CD002118. doi: 10.1002/14651858.CD002118.pub5
10. Zeadna A, Son WY, Moon JH, Dahan MH. A Comparison of Biochemical Pregnancy Rates Between Women Who Underwent IVF and Fertile Controls Who Conceived Spontaneouslydagger. Hum Reprod (2015) 30(4):783–8. doi: 10.1093/humrep/dev024
11. Vaiarelli A, Cimadomo D, Patrizio P, Venturella R, Orlando G, Soscia D, et al. Biochemical Pregnancy Loss After Frozen Embryo Transfer Seems Independent of Embryo Developmental Stage and Chromosomal Status. Reprod BioMed Online (2018) 37(3):349–57. doi: 10.1016/j.rbmo.2018.05.019
12. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst Score Affects Implantation and Pregnancy Outcome: Towards a Single Blastocyst Transfer. Fertil Steril (2000) 73(6):1155–8. doi: 10.1016/s0015-0282(00)00518-5
13. Tubbing A, Shaw-Jackson C, Ameye L, Colin J, Rozenberg S, Autin C. Increased Live Births After Day 5 Versus Day 6 Transfers of Vitrified-Warmed Blastocysts. J Assist Reprod Genet (2018) 35(3):417–24. doi: 10.1007/s10815-017-1097-x
14. Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A Comparison of Day 5 and Day 6 Blastocyst Transfers. Fertil Steril (2001) 75(6):1126–30. doi: 10.1016/s0015-0282(01)01771-x
15. Dai X, Wang Y, Yang H, Gao T, Yu C, Cao F, et al. AMH has No Role in Predicting Oocyte Quality in Women With Advanced Age Undergoing IVF/ICSI Cycles. Sci Rep (2020) 10(1):19750. doi: 10.1038/s41598-020-76543-y
16. Li M, Wang H, Ma C, Shi J. Transferring Two Grades I Cleavage-Stage Embryo Might Not Be a Good Protocol. Gynecol Endocrinol (2017) 33(7):557–9. doi: 10.1080/09513590.2017.1302420
17. Stoltzfus JC. Logistic Regression: A Brief Primer. Acad Emerg Med (2011) 18(10):1099–104. doi: 10.1111/j.1553-2712.2011.01185.x
18. Morin SJ, Patounakis G, Juneau CR, Neal SA, Scott RT, Seli E. Diminished Ovarian Reserve and Poor Response to Stimulation in Patients <38 Years Old: A Quantitative But Not Qualitative Reduction in Performance. Hum Reprod (2018) 33(8):1489–98. doi: 10.1093/humrep/dey238
19. Salumets A, Suikkari AM, Makinen S, Karro H, Roos A, Tuuri T. Frozen Embryo Transfers: Implications of Clinical and Embryological Factors on the Pregnancy Outcome. Hum Reprod (2006) 21(9):2368–74. doi: 10.1093/humrep/del151
20. du Fosse NA, van der Hoorn MP, van Lith JMM, le Cessie S, Lashley E. Advanced Paternal Age Is Associated With an Increased Risk of Spontaneous Miscarriage: A Systematic Review and Meta-Analysis. Hum Reprod Update (2020) 26(5):650–69. doi: 10.1093/humupd/dmaa010
21. Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Haberg SE. Role of Maternal Age and Pregnancy History in Risk of Miscarriage: Prospective Register Based Study. BMJ (2019) 364:l869. doi: 10.1136/bmj.l869
22. Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of Maternal Age on the Outcomes of In Vitro Fertilization and Embryo Transfer (IVF-Et). Sci China Life Sci (2012) 55(8):694–8. doi: 10.1007/s11427-012-4357-0
23. Ghimire PR, Akombi-Inyang BJ, Tannous C, Agho KE. Association Between Obesity and Miscarriage Among Women of Reproductive Age in Nepal. PloS One (2020) 15(8):e0236435. doi: 10.1371/journal.pone.0236435
24. Cavalcante MB, Sarno M, Peixoto AB, Araujo Junior E, Barini R. Obesity and Recurrent Miscarriage: A Systematic Review and Meta-Analysis. J Obstet Gynaecol Res (2019) 45(1):30–8. doi: 10.1111/jog.13799
25. Cozzolino M, Garcia-Velasco JA, Meseguer M, Pellicer A, Bellver J. Female Obesity Increases the Risk of Miscarriage of Euploid Embryos. Fertil Steril (2021) 115(6):1495–502. doi: 10.1016/j.fertnstert.2020.09.139
26. Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA Damage Is Associated With an Increased Risk of Pregnancy Loss After IVF and ICSI: Systematic Review and Meta-Analysis. Hum Reprod (2008) 23(12):2663–8. doi: 10.1093/humrep/den321
27. Zhang Z, Zhu L, Jiang H, Chen H, Chen Y, Dai Y. Sperm DNA Fragmentation Index and Pregnancy Outcome After IVF or ICSI: A Meta-Analysis. J Assist Reprod Genet (2015) 32(1):17–26. doi: 10.1007/s10815-014-0374-1
28. Wilcox AJ, Weinberg CR, Baird DD. Risk Factors for Early Pregnancy Loss. Epidemiology (1990) 1(5):382–5. doi: 10.1097/00001648-199009000-00008
29. Tomas C, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy Loss After Frozen-Embryo Transfer–A Comparison of Three Protocols. Fertil Steril (2012) 98(5):1165–9. doi: 10.1016/j.fertnstert.2012.07.1058
30. Zhang Q, Zhang J, Zhou X, Li Y, Chen Y, Chen X, et al. Association of Number and Quality of Embryos Transferred With Early Pregnancy Loss in Infertile Women at an Advanced Age Undergoing Frozen-Thawed Embryo Transfer. Nan Fang Yi Ke Da Xue Xue Bao (2021) 41(7):1050–5. doi: 10.12122/j.issn.1673-4254.2021.07.12
31. Kamath MS, Mascarenhas M, Kirubakaran R, Bhattacharya S. Number of Embryos for Transfer Following In Vitro Fertilisation or Intra-Cytoplasmic Sperm Injection. Cochrane Database Syst Rev (2020) 8:CD003416. doi: 10.1002/14651858.CD003416.pub5
32. Li C, He YC, Xu JJ, Wang Y, Liu H, Duan CC, et al. Perinatal Outcomes of Neonates Born From Different Endometrial Preparation Protocols After Frozen Embryo Transfer: A Retrospective Cohort Study. BMC Pregnancy Childbirth (2021) 21(1):341. doi: 10.1186/s12884-021-03791-9
33. Papanikolaou EG, Camus M, Fatemi HM, Tournaye H, Verheyen G, Van Steirteghem A, et al. Early Pregnancy Loss is Significantly Higher After Day 3 Single Embryo Transfer Than After Day 5 Single Blastocyst Transfer in GnRH Antagonist Stimulated IVF Cycles. Reprod BioMed Online (2006) 12(1):60–5. doi: 10.1016/s1472-6483(10)60981-9
34. Li M, Yin M, Wu L, Yan Z, Lyu Q, Yan Z, et al. Pregnancy and Neonatal Outcomes of Morphologically Grade CC Blastocysts: Are They of Clinical Value? Arch Gynecol Obstet (2020) 302(6):1511–21. doi: 10.1007/s00404-020-05741-w
35. Richter KS, Ginsburg DK, Shipley SK, Lim J, Tucker MJ, Graham JR, et al. Factors Associated With Birth Outcomes From Cryopreserved Blastocysts: Experience From 4,597 Autologous Transfers of 7,597 Cryopreserved Blastocysts. Fertil Steril (2016) 106(2):354–62.e2. doi: 10.1016/j.fertnstert.2016.04.022
36. Su Y, Li JJ, Wang C, Haddad G, Wang WH. Aneuploidy Analysis in Day 7 Human Blastocysts Produced by In Vitro Fertilization. Reprod Biol Endocrinol (2016) 14:20. doi: 10.1186/s12958-016-0157-x
37. Du T, Wang Y, Fan Y, Zhang S, Yan Z, Yu W, et al. Fertility and Neonatal Outcomes of Embryos Achieving Blastulation on Day 7: Are They of Clinical Value? Hum Reprod (2018) 33(6):1038–51. doi: 10.1093/humrep/dey092
38. Ai J, Jin L, Zheng Y, Yang P, Huang B, Dong X. The Morphology of Inner Cell Mass Is the Strongest Predictor of Live Birth After a Frozen-Thawed Single Embryo Transfer. Front Endocrinol (Lausanne) (2021) 12:621221. doi: 10.3389/fendo.2021.621221
39. Zhao YY, Yu Y, Zhang XW. Overall Blastocyst Quality, Trophectoderm Grade, and Inner Cell Mass Grade Predict Pregnancy Outcome in Euploid Blastocyst Transfer Cycles. Chin Med J (Engl) (2018) 131(11):1261–7. doi: 10.4103/0366-6999.232808
40. Bourdon M, Pocate-Cheriet K, Finet de Bantel A, Grzegorczyk-Martin V, Amar Hoffet A, Arbo E, et al. Day 5 Versus Day 6 Blastocyst Transfers: A Systematic Review and Meta-Analysis of Clinical Outcomes. Hum Reprod (2019) 34(10):1948–64. doi: 10.1093/humrep/dez163
41. Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm Morphology Significantly Affects the Rates of Ongoing Pregnancy and Miscarriage in Frozen-Thawed Single-Blastocyst Transfer Cycle In Vitro Fertilization. Fertil Steril (2012) 98(2):361–7. doi: 10.1016/j.fertnstert.2012.05.014
42. Van den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJ, Klein BM, et al. Association Between Blastocyst Morphology and Outcome of Single-Blastocyst Transfer. Reprod BioMed Online (2013) 27(4):353–61. doi: 10.1016/j.rbmo.2013.07.006
43. Shi D, Xu J, Zhang M, Niu W, Shi H, Yao G, et al. Association Between the Quality of Inner Cell Mass and First Trimester Miscarriage After Single Blastocyst Transfer. Reprod Biol Endocrinol (2020) 18(1):43. doi: 10.1186/s12958-020-00595-y
44. Park DS, Kim JW, Chang EM, Lee WS, Yoon TK, Lyu SW. Obstetric, Neonatal, and Clinical Outcomes of Day 6 vs. Day 5 Vitrified-Warmed Blastocyst Transfers: Retrospective Cohort Study With Propensity Score Matching. Front Endocrinol (Lausanne) (2020) 11:499. doi: 10.3389/fendo.2020.00499
45. Yerushalmi GM, Shavit T, Avraham S, Youngster M, Kedem A, Gat I, et al. Day 5 Vitrified Blastocyst Transfer Versus Day 6 Vitrified Blastocyst Transfer in Oocyte Donation Program. Sci Rep (2021) 11(1):10715. doi: 10.1038/s41598-021-90238-y
Keywords: embryo developmental stage, biochemical pregnancy loss, clinical pregnancy loss, frozen-thawed embryo transfer, young women
Citation: Dai X, Gao T, Xia X, Cao F, Yu C, Li T, Li L, Wang Y and Chen L (2021) Analysis of Biochemical and Clinical Pregnancy Loss Between Frozen-Thawed Embryo Transfer of Blastocysts and Day 3 Cleavage Embryos in Young Women: A Comprehensive Comparison. Front. Endocrinol. 12:785658. doi: 10.3389/fendo.2021.785658
Received: 29 September 2021; Accepted: 01 December 2021;
Published: 24 December 2021.
Edited by:
Alessandro Conforti, University of Naples Federico II, ItalyReviewed by:
Joaquin Llacer, Ginefiv-GeneraLife, SpainKatja Hummitzsch, University of Adelaide, Australia
Copyright © 2021 Dai, Gao, Xia, Cao, Yu, Li, Li, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Chen, Y3pyY2NoZW5saUAxMjYuY29t; Yufeng Wang, NDA2NjU0MjZAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Xiuliang Dai
Xiuliang Dai Tingting Gao†
Tingting Gao† Chunmei Yu
Chunmei Yu Li Chen
Li Chen