- 1Department of Endocrinology, Endocrinology and Metabolism Institute, Cleveland Clinic, Cleveland, OH, United States
- 2Department of Surgery, The James Comprehensive Cancer Center, The Ohio State University, Columbus, OH, United States
- 3Department of Surgery, UT Southwestern Medical Center, Dallas, TX, United States
- 4Wexner Medical Center, The Ohio State University, Columbus, OH, United States
- 5Lake Cumberland Regional Hospital, Somerset, KY, United States
- 6Endocrinology Center of Lake Cumberland, Somerset, KY, United States
- 7Department of Pathology, Mid-Atlantic Permanente Medical Group, Rockville, MD, United States
- 88Department of Research and Development, Veracyte, South San Francisco, CA, United States
- 9Department of Medical Affairs, Veracyte, South San Francisco, CA, United States
- 10Department of Clinical Affairs, Veracyte, South San Francisco, CA, United States
- 11Department of Endocrine Surgery, Endocrinology and Metabolism Institute, Cleveland Clinic, Cleveland, OH, United States
Objectives: To evaluate the frequency and risk of malignancy of TSHRpI568T mutations discovered in indeterminate thyroid nodules (ITN) within the Veracyte CLIA laboratory undergoing Afirma® Genomic Sequencing Classifier (GSC) testing, and to evaluate a broader cohort of TSHR variants and their categorization as Afirma GSC benign (GSC-B) or suspicious (GSC-S). Finally, we seek to assess the risk of malignancy (ROM) of this group of TSHR mutated ITN in the GSC-S category.
Methods: ITN submitted to Veracyte for Afirma GSC testing between October 2017 and February 2022 were analyzed for TSHR variants and rates of GSC-B and GSC-S were calculated based upon BIII or IV cytology, by TSHR variant codon amino acid (AA) substitution, age, and gender. For GSC-S samples, surgical pathology reports were requested, and the rate of malignancy was calculated.
Results: Five percent of the ITN samples harbored an isolated TSHR variant and 5% of those were classified as GSC-S. Among TSHRpI568T samples, 96% were GSC-B and of the GSC-S samples, 21% were malignant. Among an unselected group of TSHR, absent TSHRpI568T mutations, 16.3% of GSC-S samples were malignant, all but one with codon mutations in the transmembrane subdomains of the TSHR. This prompted a dedicated evaluation of transmembrane codons which revealed a malignancy rate of 10.7% among GSC-S nodules. In total, 13/85 (15.3%) TSHR mutated ITN with Afirma GSC-S results were found to be malignant.
Conclusions: TSHR variants are rare in ITN, and most are categorized as benign under Afirma GSC testing which carries a < 4% risk of malignancy. For GSC-S ITN with TSHR mutations, the risk of malignancy is ≥= 15%, which is clinically meaningful and may alter treatment or monitoring recommendations for patients.
Introduction
The thyroid stimulating hormone receptor (TSHR) is a member of the large superfamily of G-protein-coupled receptors that is expressed primarily in thyroid follicular cells as well as non-thyroidal sites including fibroblasts, adipocytes, brain, and other sites (1–3). Structurally, the receptor has seven transmembrane domains with a long extracellular amino acid chain leading to the NH2-terminal peptide and a shorter intracellular chain leading to the carboxy terminus (4, 5). TSH binding of the receptor leads primarily to activation of adenylyl cyclase and a resultant cyclic AMP cascade that regulates growth, differentiation, and hormone secretion of thyroid follicular cells (3, 6).
Mutations of the TSHR have been associated most with thyroid autonomy and toxic adenomas (3, 7, 8). Though it is often taught that toxic thyroid nodules are benign, and hyperthyroidism is correlated with a low risk of thyroid cancer, several surgical series show a higher-than-expected incidence of thyroid cancer. Mohamed et al. reported a 21% malignancy rate in patients who underwent surgery with toxic multinodular goiter or toxic adenomas, though there was not specification of the presence of thyroid cancers within the autonomous thyroid tissue (9). Tam et al. found a similar 19% malignancy rate in toxic nodular goiter resections with specification that half (9.5%) of these malignancies arose from the autonomous nodule/s (10).
There have been at least two studies looking specifically at the clinicopathological correlation of TSHR variants in indeterminate thyroid nodules (ITN). Mon et al. evaluated 703 ITNs or nodules with benign cytology and suspicious clinical features with the Thyroseq® v2 next generation sequencing (NGS) platform (11). TSHR variants were detected in 4.4% of thyroid nodules. Of 16 samples with known histopathology, 4 were malignant though one of those sample had a concurrent BRAFV600E mutation. Therefore, 3/15 (20%) ITN with an isolated TSHR variant were malignant. Interestingly, 2 of the 3 samples had initial benign cytology reads and three of those patients had a suppressed TSH. All were follicular thyroid carcinomas (FTC). Guan et al. reported on 388 ITN samples sent for Thyroseq v2 analysis and 4.7% of nodules were found to harbor TSHR variants (4). Of the 7 nodules that were resected, none were malignant.
The current study was influenced by an interesting case report of an aggressive thyroid malignancy with a TSHRpI568T mutation (12). Briefly, a 53-year-old female underwent a fine needle aspiration biopsy (FNAB) of a thyroid nodule with resultant Bethesda V cytology and Afirma® Xpression Atlas (XA) testing that revealed a TSHRpI568T mutation (13). Based on ultrasound and clinical findings, a total thyroidectomy was performed and revealed a 2 cm papillary thyroid carcinoma with posterior invasion and with a need for a partial resection of 2 tracheal rings. As most literature describes TSHR mutated nodules as benign and when malignant, low risk, a query was made to Veracyte, Inc regarding the prevalence and risk of malignancy of TSHRpI568T mutated thyroid nodules (14).
The objectives of this study are to evaluate the frequency and risk of malignancy of TSHRpI568T mutations discovered in ITN within the Veracyte CLIA laboratory from FNAB samples undergoing Afirma Genomic Sequencing Classifier (GSC) testing, and to evaluate a broader cohort of TSHR variants and their categorization as Afirma GSC benign (GSC-B) or suspicious (GSC-S) (15). Finally, we seek to assess the risk of malignancy (ROM) of this group of TSHR mutated ITN in the GSC-S category.
Materials and methods
Afirma testing analyzes aspirate material from ITN placed in an RNA protect reagent and processed as previously described (13, 15–17). Samples with isolated TSHR variants discovered by Afirma Xpression Atlas (XA) (which reports on 905 gene variants and 235 fusion pairs from RNA-sequencing of 593 genes) testing were evaluated in Afirma GSC-B and GSC-S samples across The Bethesda System for Reporting Thyroid Cytopathology categories III and IV (BIII and BIV) that were submitted to Veracyte as part of routine clinical care (13, 15, 18). The Afirma GSC utilizes an ensemble model of 1115 differentially expressed core genes, and over 10,000 genes in total, which manages the benign versus suspicious classifier categorization (15). XA identifies over 80 TSHR variants, including mutations at codons within the extracellular, transmembrane, and intracellular domains of the TSHR. The analyzed samples were collected between October 2017 and February 2022. As described in the Afirma XA analytical and clinical validation study, for research purposes only, molecular variants can be detected in the GSC-B category though these are not reported clinically (13).
The rates of GSC-B and GSC-S for TSHR variants were calculated based upon BIII or IV cytology, by TSHR variant codon amino acid (AA) substitution, age, and gender. For GSC-S samples, surgical pathology reports were requested under an IRB protocol (WCG IRB protocol # DHF 005-044) and the rate of malignancy was calculated. Non-invasive follicular neoplasm with papillary like nuclear features (NIFTP) was considered malignant as these lesions are not categorized histologically as benign and surgical resection is required to make the diagnosis (19).
Statistical analyses were performed using R statistical software version 3.2.3 (https://www.r-project.org). Continuous variables were compared using T test, categorical variables were compared using Fisher exact test. All confidence intervals are 2-sided 95% CIs and were computed using the exact binomial test. Multiple hypotheses were corrected using Benjamini-Hochberg Procedure.
Results
TSHR prevalence
There were 8881 Afirma GSC samples of ITN (Bethesda III/IV only) with isolated TSHR variants. This represents 5.4% of all samples collected over the study period. Of these nodules, 8,437 were categorized as GSC-B and 444 as GSC-S (GSC-S = 5.0% [95% CI 4.6%-5.5%]) (Figure 1). Among TSHR mutated nodules that were GSC-S, Bethesda III cytology represented 378/7935 (4.8%) and Bethesda IV 66/946 (7.0%) (p<0.01). There were no specific TSHR codon changes with statistically higher GSC-B vs GSC-S results nor were any subdomains (transmembrane vs intracellular vs extracellular) of the TSHR overrepresented as GSC-B or GSC-S.
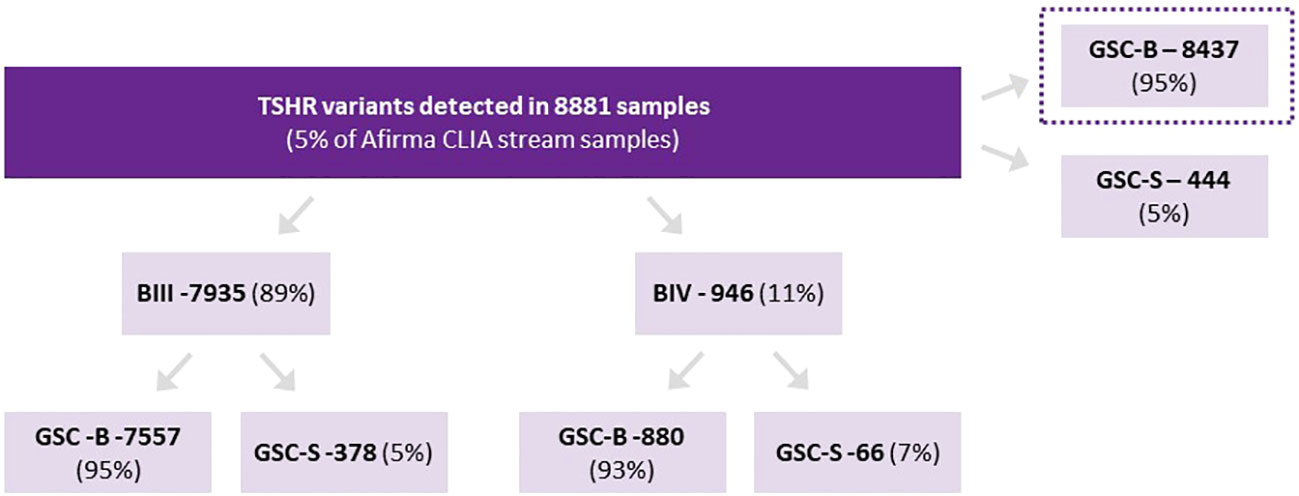
Figure 1 Proportion of TSHR variants assigned as Afirma GSC-B or GSC-S as well as the breakdown of ITN Bethesda categories and Afirma result for each cytology type.
In females, 370/7807 (4.7%) ITNs with TSHR variants were GSC-S and in males, 74/1072 (6.9%) were GSC-S, which was statistically higher (p<0.01) (2 samples did not have gender stated on the test request form and were excluded). Age younger than 55 years also had a statistically higher rate of GSC-S TSH variants than age > 55 years 215/3695 (5.8%) and <55 years 229/5186 (4.4%) respectively (p<0.01) (Table 1).
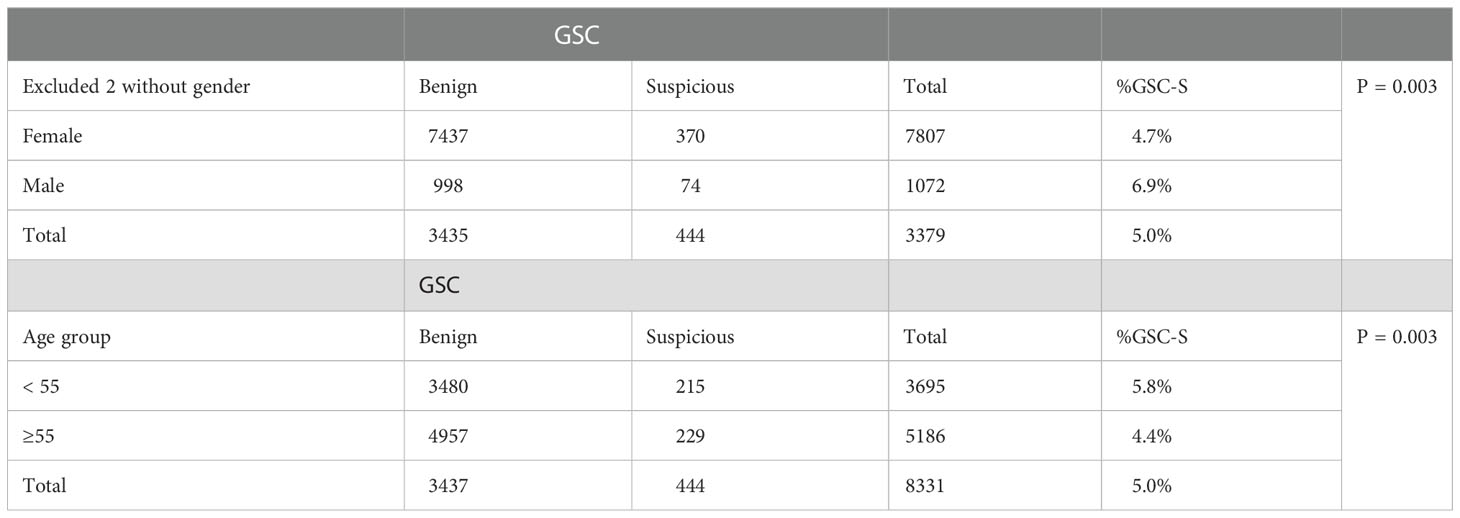
Table 1 Proportion of TSHR variant distribution by gender and age amongst Afirma GSC-B or GSC-S results.
TSHR I568T prevalence and ROM
A total of 427 ITN samples harbored the TSH I568T variant, 383 from BIII (89.7%) and 44 from BIV (10.3%) cytology. GSC-S was resulted in 17/383 BIII (4.4%) and 8/44 BIV nodules (18.2%) (p<0.01) (Figure 2). Therefore, 402 samples (94%) were categorized as GSC-B. Of the 25 GSC-S samples, 14 final histopathology reports were obtained (8 patients had no follow up data or record of surgery and 3 pathology reports could not be obtained). Final pathology showed 11 benign results which were (when described) comprised of follicular adenomas, one Hurthle cell adenoma and one infarcted nodule; and 3 malignancies indicating a 21.4% risk of malignancy [95% CI 4.7-50.8%]. The tumors included a 1.2 cm NIFTP, a 2.0 cm primary infiltrative follicular variant of papillary thyroid carcinoma (FV-PTC) that was multifocal, and a 0.8 cm PTC (Table 2). If we make the extreme assumption that the 11 GSC-S samples without histopathology are all benign or malignant, the malignancy risk ranges from 3/25 (12%) to 14/25 (56%). When assuming all GSC-B nodules are true negatives lesions and excluding the 11 GSC-S nodules for which histopathology reports were not obtained, the risk of malignancy in the overall TSHR I568T mutated ITN population is 3/416 (0.72% [95% CI 0.15-2.1%]).
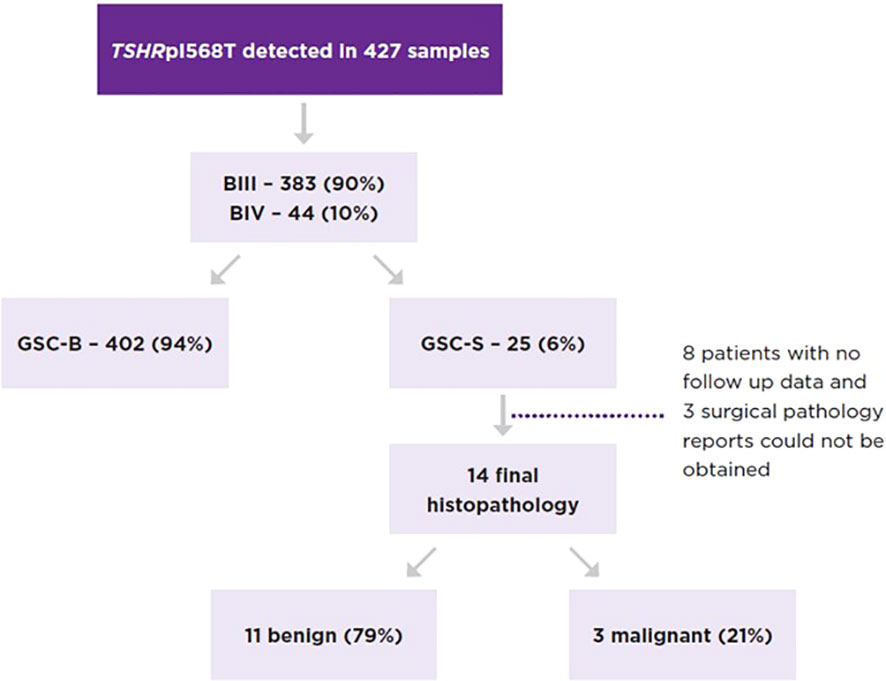
Figure 2 Proportion of TSHRpI568T mutated nodules by Bethesda category and Afirma GSC category as well as the malignancy rate of GSC-S nodules.
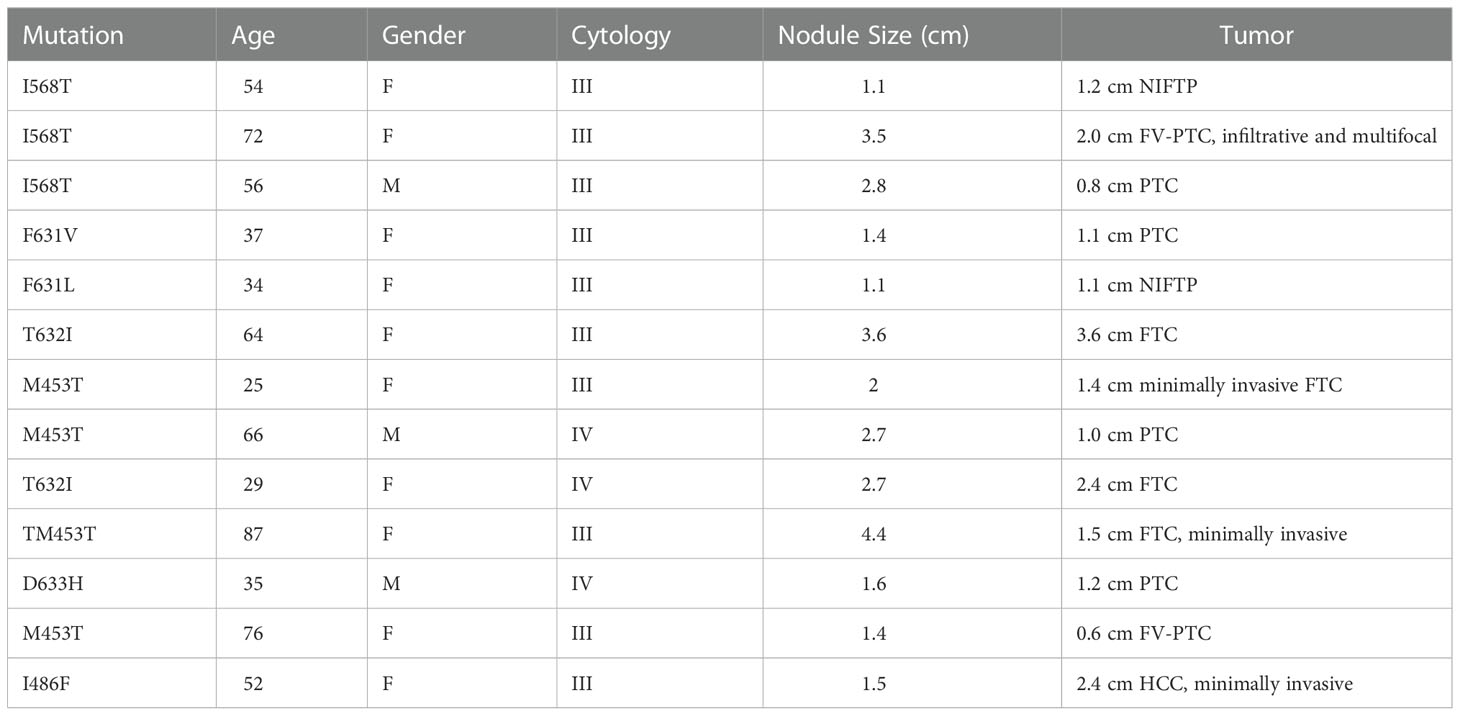
Table 2 Patient and tumor characteristics of malignancies discovered within Afirma GSC-S TSHR variants.
TSHR variant ROM in GSC-S ITN
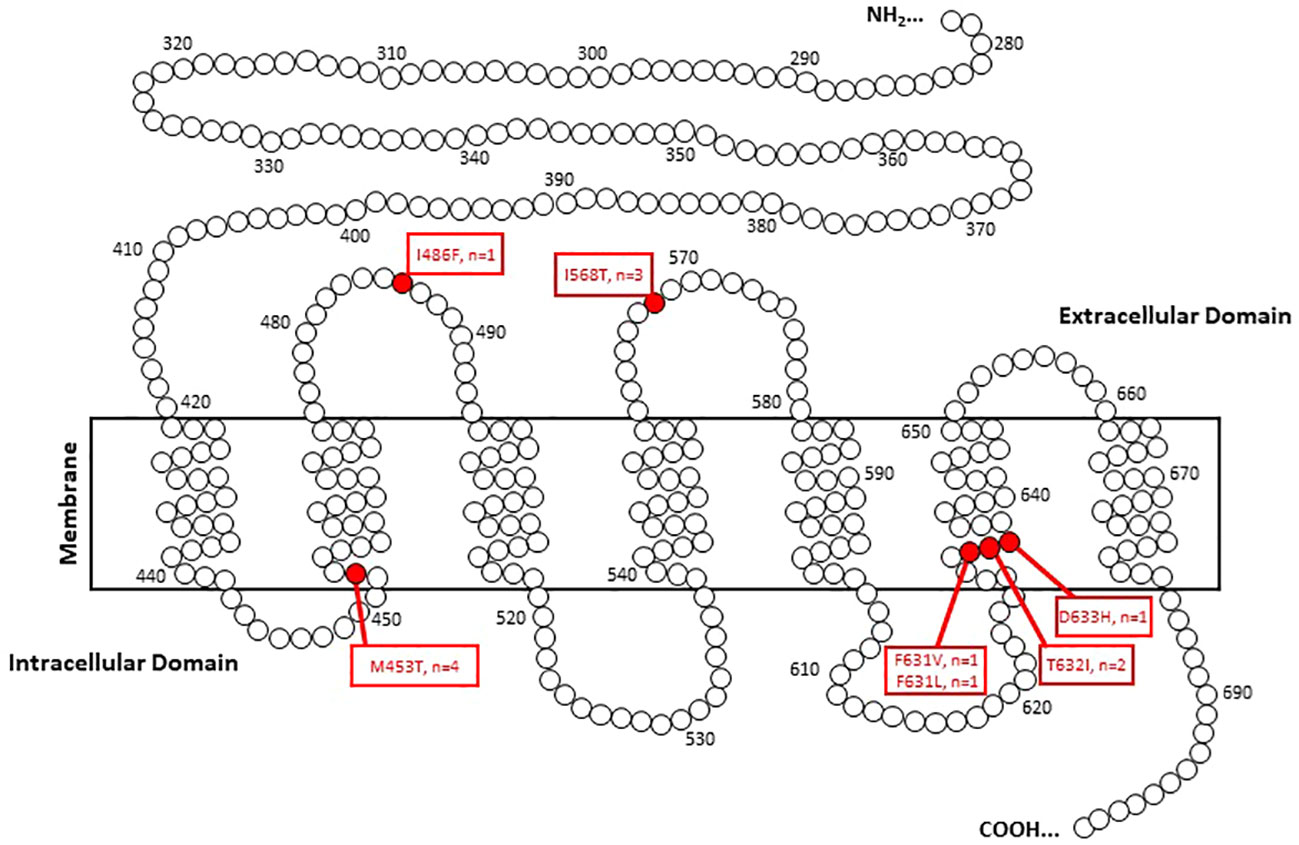
To assess the ROM of TSHR variants that were not mutations at the 568 codon, 43 pathology reports were obtained where 7 malignancies were discovered (7/43 = 16.3% [95% CI 6.8%-30.7%]). These malignancies included a 1.1cm classical PTC, a 1.1 cm NIFTP, a 3.6 cm widely invasive Follicular Thyroid Carcinoma (FTC), a 1.4 cm minimally invasive FTC, a 1.0 cm PTC with multifocality, a 2.4 cm FTC and a 2.4 cm minimally invasive Hurthle cell cancer with an associated 0.1 cm PTC (Table 2). All but one malignancy in this cohort were associated with transmembrane subdomain codon variants at codons 631, 632, and 453 (Figure 3). Therefore, 28 more pathology reports from GSC-S nodules with TSHR variants in transmembrane domain codons were obtained from the primary study cohort to see if there was enrichment of malignancy at these receptor locations. This analysis revealed 3/28 (10.7% [95% CI 2.3-28.2%]) malignancies and included a 1.5 cm minimally invasive FTC, a 1.2 cm PTC with oncocytic cell changes, and a 0.6 cm FV-PTC (Table 2 and Figure 3). Benign histology (when reported) included follicular adenomas, Hurthle cell adenomas, multinodular hyperplasia, hyperplastic nodules with hashimoto’s thyroiditis, and one colloid nodule.
When assessing the overall cohort for which pathology reports were available, there were 13/85 malignancies within the GSC-S result of ITNs (15.3% [95% CI 8.4-24.7%]). There was no significant difference in malignant vs benign samples with respect to nodule size, age, or gender. The median nodule size was 2.3 cm [0.9, 6.6], 2.0 cm for malignant and 2.3 cm for benign. The median age was 58 years [20, 91], 47 years for malignant and 59 years from benign. If we assume all GSC-B samples are true negatives, the ROM by detection of a TSHR variant alone is <1.5%.
Discussion
In the present study, we have investigated the epidemiology of TSHR variants in ITN submitted for molecular analysis by the Afirma GSC. Additionally, the malignancy rate in 85 GSC-S ITN with TSHR variants was assessed which represents the largest cohort published to date. We have demonstrated that the prevalence of isolated TSHR variants in ITN is 5%, similar to other published rates (4, 11). A very small subset of nodules was co-mutated with better characterized thyroid cancer driver mutations from the RAS family and BRAFV600E as previously described, and they were excluded from this analysis in favor of analyzing the risk of malignancy in ITN with isolated TSHR variants as detected by the Afirma XA (11, 20). We also show that Afirma only categorizes 5% of TSHR mutated ITNs as GSC-S. Though there are statistically significant differences shown for GSC-S rates of TSHR mutated ITNs by Bethesda cytology category (IV>III), gender (M>F), and age (more frequent in those under 55 years), they are likely not clinically significant differences to confidently risk stratify patients. Additionally, there does not seem to be a specific codon or subdomain region of the TSHR mutation that consistently predicts malignancy.
This study was inspired by a query after the management of an aggressive case of PTC where the index lesion was positive for a TSHRpI568T mutation (12). We showed that this variant is extremely rare, representing only ~0.7% of all molecular findings in the Afirma database. Additionally, the overall risk of malignancy in nodules harboring TSHRpI568T mutations is very low (< 2%) when analyzed by its presence alone. However, when categorized as GSC-S, the risk of malignancy has a point estimate of 21% based on a limited number of pathology reports. This observation highlights the value of the Afirma GSC to significantly alter the risk of malignancy in ITNs with its benign vs suspicious categorization, even in the presence of low-risk mutations.
As shown in Table 2, not all malignancies associated with TSHR mutated ITN are low risk. Cancers reported include widely invasive FTC and a 2 cm infiltrative FV-PTC with multifocality. It is unclear if there were clinical indicators of these more aggressive tumors pre-operatively. One of the limitations of this study is not having the clinical and ultrasound findings available to know how TSH level, ATA or TIRADs ultrasound risk stratification or how individual patient risk factors may have influenced the decision to monitor or refer patients for surgery (21–23). Another limitation of this study is the assumption that all GSC-B TSHR mutated ITN are true negatives. Given the 96% negative predictive value of an Afirma GSC-B result and the known overall low risk TSHR mutated ITN, it is very unlikely there are many false negative TSHR mutated thyroid nodules (15).
The only other commercially available thyroid nodule molecular diagnostic test in the United States that reports TSHR variants is the Thyroseq Thyroid Genomic Classifier (Thyroseq) (24). Thyroseq is a targeted next generation sequencing test whose current iteration (v3) evaluates point mutations, gene fusions, copy number alterations and abnormal gene expression in 112 thyroid cancer related genes (24). Thyroseq reports TSHR mutations as “currently negative” with a <10% probability of a low-risk cancer or NIFTP and a recommendation of active surveillance (25). An exhaustive literature search to ascertain the ROM in operated TSHR mutated ITNs reveals 3/29 malignancies reported for a point estimate of 10% cancer rate (4, 11, 26–35). Almost all these studies utilized the Thyroseq platform, thus it is reasonable to assume there were other concerning clinical or ultrasound features prompting a recommendation for surgery given the low risk ascribed to TSHR mutations. The allelic frequency of the TSHR variant may influence malignancy risk. Mon et al. describe nodules with isolated TSHR variants and < 36% allelic frequency as all being histologically benign. However, among 5 ITN with allelic frequency between 36-45%, 3 were FTC and 2 were benign, indicating a poor discrimination of malignancy risk at this allelic frequency range (11).
Based on this largest survey of TSHR mutated ITNs, it is true that the overall malignancy risk is low. Only 5% of ITN undergoing Afirma testing harbor a TSHR variant and only 5% of that subset are categorized as GSC-S. The Afirma GSC’s ability to assign a benign or suspicious call provides value for risk stratifying patients with this low-risk mutation (15). As opposed to a reported risk of < 10% with a recommendation for active surveillance, Afirma will call 95% of TSHR mutated ITNs as GSC-B, with an extrapolated ≤= 4% risk of malignancy, and likely lower based on the known overall low risk of TSHR mutated nodules. Therefore, these patients can be monitored conservatively, like those with a Bethesda II cytology result (18). Alternatively, when assigned a GSC-S result, the risk of malignancy is >= 15%, a clinically significant difference that likely leads to a very different shared decision-making conversation with patients. At this relatively low risk of malignancy, dependent on clinical and ultrasound features, some may be comfortable with an active surveillance approach. Given this risk is similar to the baseline risk of an ITN, many may feel a diagnostic lobectomy is more appropriate, which may also be adequately therapeutic. If a TSHR variant is detected by molecular testing, the above clinical decisions should be made in the context of thyroid stimulating hormone (TSH) testing (if not done prior to nodule FNA) and assessment of thyroid autonomy when the TSH is suppressed.
Population based risk assessments of thyroid nodule mutations provide beneficial guidance for a general approach to patients with ITN and thyroid cancer. However, physicians treat individuals, and this study demonstrates the utility of the Afirma GSC to risk stratify patients with TSHR mutated ITN in a clinically meaningful way. An Afirma GSC-B was reported for nearly all nodules with an isolated TSHR variant, and they likely avoided diagnostic surgery. Conversely, a minority of patients with an isolated TSHR variant received an Afirma GSC-S result. An Afirma GSC-S result is the foundation that informs further risk assessment based on XA results and may optimize the approach to initial therapy in patients with ITN (13).
Data availability statement
The datasets presented in this article are not readily available because TSHR variant sequence information is proprietary information of Veracyte, Inc. Requests to access the datasets should be directed toam9zaHVhLmtsb3BwZXJAdmVyYWN5dGUuY29t.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JPK, RTK, SW, YH, JH and GCK designed the study. DW, JEP, SH, BO’D, JN, DJ, AC and JS review clinical records and collected data. JPK, SW, and YH prepared the manuscript. All authors reviewed the manuscript. JPK, DW, RTK, JS and YH revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
SW, YH, JH, JPK, RTK, and GCK are employees and equity owners of Veracyte, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bockmann J, Winter C, Wittkowski W, Kreutz MR, Bockers TM. Cloning and expression of a brain-derived TSH receptor. Biochem Biophys Res Commun (1997) 238:173–8. doi: 10.1006/bbrc.1997.7268
2. Davies TF, Teng CS, McLachlan SM, Smith BR, Hall R. Thyrotropin receptors in adipose tissue, retro-orbital tissue and lymphocytes. Mol Cell Endocrinol (1978) 9:303–10. doi: 10.1016/0303-7207(78)90072-2
3. Kopp P. The TSH receptor and its role in thyroid disease. Cell Mol Life Sci (2001) 58:1301–22. doi: 10.1007/PL00000941
4. Guan H, Matonis D, Toraldo G, Lee SL. Clinical significance of thyroid-stimulating hormone receptor gene mutations and/or sodium-iodine symporter gene overexpression in indeterminate thyroid fine needle biopsies. Front Endocrinol (2018) 9:566. doi: 10.3389/fendo.2018.00566
5. Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev (1992) 13:596–611. doi: 10.1210/edrv-13-3-596
6. Dumont JE, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev (1992) 72:667–97. doi: 10.1152/physrev.1992.72.3.667
7. Krohn K, Paschke R. Clinical review 133: Progress in understanding the etiology of thyroid autonomy. J Clin Endocrinol Metab (2001) 86:3336–45.
8. Trulzsch B, Krohn K, Wonerow P, Chey S, Holzapfel HP, Ackermann F, et al. Detection of thyroid-stimulating hormone receptor and gsalpha mutations: in 75 toxic thyroid nodules by denaturing gradient gel electrophoresis. J Mol Med (Berl) (2001) 78:684–91. doi: 10.1007/s001090000170
9. Mohamed TZ, Sultan A, Tag El-Din M, Mostafa AAE, Nafea MA, Kalmoush AE, et al. Incidence and risk factors of thyroid malignancy in patients with toxic nodular goiter. Int J Surg Oncol (2022) 2022:1054297. doi: 10.1155/2022/1054297
10. Tam AA, Ozdemir D, Alkan A, Yazicioglu O, Yildirim N, Kilicyazgan A, et al. Toxic nodular goiter and thyroid cancer: Is hyperthyroidism protective against thyroid cancer? Surgery (2019) 166:356–61. doi: 10.1016/j.surg.2019.03.012
11. Mon SY, Riedlinger G, Abbott CE, Seethala R, Ohori NP, Nikiforova MN, et al. Cancer risk and clinicopathological characteristics of thyroid nodules harboring thyroid-stimulating hormone receptor gene mutations. Diagn cytopathology (2018) 46:369–77. doi: 10.1002/dc.23915
12. Nguyen J, Joseph D. Locally invasive classical papillary thyroid carcinoma with TSH receptor I568T mutation: case report. Endocrinol Diabetes Metab Case Rep (2022) 2022. doi: 10.1530/EDM-21-0192
13. Angell TE, Wirth LJ, Cabanillas ME, Shindo ML, Cibas ES, Babiarz JE, et al. Analytical and clinical validation of expressed variants and fusions from the whole transcriptome of thyroid FNA samples. Front Endocrinol (2019) 10:612. doi: 10.3389/fendo.2019.00612
14. Goldner WS, Angell TE, McAdoo SL, Babiarz J, Sadow PM, Nabhan FA, et al. Molecular variants and their risks for malignancy in cytologically indeterminate thyroid nodules. Thyroid (2019) 29:1594–605. doi: 10.1089/thy.2019.0278
15. Patel KN, Angell TE, Babiarz J, Barth NM, Blevins T, Duh QY, et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg (2018) 153:817–24. doi: 10.1001/jamasurg.2018.1153
16. Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. New Engl J Med (2012) 367:705–15. doi: 10.1056/NEJMoa1203208
17. Hao Y, Choi Y, Babiarz JE, Kloos RT, Kennedy GC, Huang J, et al. Analytical verification performance of afirma genomic sequencing classifier in the diagnosis of cytologically indeterminate thyroid nodules. Front Endocrinol (2019) 10:438. doi: 10.3389/fendo.2019.00438
18. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid (2017) 27:1341–6. doi: 10.1089/thy.2017.0500
19. Rosario PW, Mourao GF. Follow-up of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Head Neck (2019) 41:833–4. doi: 10.1002/hed.25550
20. Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PloS Genet (2016) 12:e1006239. doi: 10.1371/journal.pgen.1006239
21. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016 26:1–133. doi: 10.1089/thy.2015.0020
22. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid ultrasound reporting lexicon: White paper of the ACR thyroid imaging, reporting and data system (TIRADS) committee. J Am Coll Radiol (2015) 12:1272–9. doi: 10.1016/j.jacr.2015.07.011
23. Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab (2008) 93:809–14. doi: 10.1210/jc.2007-2215
24. Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: A prospective blinded multicenter study. JAMA Oncol (2019) 5:204–12. doi: 10.1001/jamaoncol.2018.4616
25. Ohori NP, Landau MS, Carty SE, Yip L, LeBeau SO, Manroa P, et al. Benign call rate and molecular test result distribution of ThyroSeq v3. Cancer cytopathology (2019) 127:161–8. doi: 10.1002/cncy.22088
26. Pagan M, Kloos RT, Lin CF, Travers KJ, Matsuzaki H, Tom EY, et al. The diagnostic application of RNA sequencing in patients with thyroid cancer: an analysis of 851 variants and 133 fusions in 524 genes. BMC Bioinf (2016) 17 Suppl 1:6. doi: 10.1186/s12859-015-0849-9
27. Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer (2014) 120:3627–34. doi: 10.1002/cncr.29038
28. Valderrabano P, Khazai L, Thompson ZJ, Leon ME, Otto KJ, Hallanger-Johnson JE, et al. Impact of oncogene panel results on surgical management of cytologically indeterminate thyroid nodules. Head Neck (2018) 40:1812–23. doi: 10.1002/hed.25165
29. Livhits MJ, Kuo EJ, Leung AM, Rao J, Levin M, Douek ML, et al. Gene expression classifier vs targeted next-generation sequencing in the management of indeterminate thyroid nodules. J Clin Endocrinol Metab (2018) 103:2261–8. doi: 10.1210/jc.2017-02754
30. Maerki J, Klein M, Chau K, Gimenez C, Fishbein J, Khutti S, et al. Determining the molecular test for indeterminate thyroid nodules best suited for our practice: A quality assurance study. Diagn cytopathology (2019) 47:259–67. doi: 10.1002/dc.24091
31. Song Y, Xu G, Ma T, Zhu Y, Yu H, Yu W, et al. Utility of a multigene testing for preoperative evaluation of indeterminate thyroid nodules: A prospective blinded single center study in China. Cancer Med (2020) 9:8397–405. doi: 10.1002/cam4.3450
32. Li W, Justice-Clark T, Cohen MB. The utility of ThyroSeq((R)) in the management of indeterminate thyroid nodules by fine-needle aspiration. Cytopathology (2021) 32:505–12. doi: 10.1111/cyt.12981
33. Torrecillas V, Sharma A, Neuberger K, Abraham D. Utility of mutational analysis for risk stratification of indeterminate thyroid nodules in a real-world setting. Clin Endocrinol (2022) 96:637–45. doi: 10.1111/cen.14601
34. Dublin JC, Papazian M, Zan E, Oweity T, Sun W, Jacobson A, et al. Predictive value of a genomic classifier in indeterminate thyroid nodules based on nodule size. JAMA otolaryngology– Head Neck Surg (2022) 148:53–60. doi: 10.1001/jamaoto.2021.3080
Keywords: thyroid nodule, molecular diagnosis, TSH receptor (TSHR), Afirma ®, thyroid cancer
Citation: Whitmer D, Phay JE, Holt S, O’Donnell B, Nguyen J, Joseph D, Chi A, Wu S, Hao Y, Huang J, Klopper JP, Kloos RT, Kennedy GC and Shin J (2022) Risk of malignancy in cytologically indeterminate thyroid nodules harboring thyroid stimulating hormone receptor mutations. Front. Endocrinol. 13:1073592. doi: 10.3389/fendo.2022.1073592
Received: 18 October 2022; Accepted: 09 December 2022;
Published: 22 December 2022.
Edited by:
Vasyl Vasko, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Cristiane Jeyce Gomes-Lima, MedStar Health Research Institute (MHRI), United StatesMaria Cecilia Mendonca Torres, Uniformed Services University of the Health Sciences, United States
Copyright © 2022 Whitmer, Phay, Holt, O’Donnell, Nguyen, Joseph, Chi, Wu, Hao, Huang, Klopper, Kloos, Kennedy and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorota Whitmer, V0hJVE1FREBjY2Yub3Jn
 Dorota Whitmer
Dorota Whitmer John E. Phay
John E. Phay Shelby Holt3
Shelby Holt3 Jay Nguyen
Jay Nguyen Dennis Joseph
Dennis Joseph Yangyang Hao
Yangyang Hao Joshua P. Klopper
Joshua P. Klopper Richard T. Kloos
Richard T. Kloos