- 1Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Henan Key Laboratory of Reproduction and Genetics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Henan Provincial Obstetrical and Gynecological Diseases (Reproductive Medicine) Clinical Research Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: Previous studies have described the effects of different drugs in preventing ovarian hyperstimulation syndrome (OHSS). However, the efficacies of those drugs in preventing OHSS remain inconclusive.
Methods: We searched the PubMed, Web of Science, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases. A network meta-analysis of randomized controlled trials (RCTs) was performed up to August 2021. We investigated the following drugs in our study: aspirin, albumin, metformin, calcium, cabergoline, quinagolide, letrozole, hydroxyethyl starch (HES), and glucocorticoids. The primary outcome was the incidence rate of moderate-to-severe OHSS, with the results presented as risk ratios (RRs) with 95% confidence intervals (CIs).
Results: The incidence of moderate-to-severe OHSS was significantly reduced by calcium administration (risk ratios [RR] 0.14, 95% confidence interval [CI]: 0.04, 0.46) (grade: high), HES (RR 0.25, 95% CI 0.07, 0.73) (grade: high), and cabergoline (RR 0.43, 95% CI 0.24, 0.71) (grade: moderate). The surface under the cumulative ranking curve (SUCRA) indicated that calcium (SUCRA, 92.4%) was the most effective intervention for preventing moderate-to-severe OHSS. These drugs were safe and did not affect clinical pregnancy, miscarriage, or live birth rates.
Conclusion: Calcium, HES, and cabergoline could effectively and safely prevent moderate-to-severe OHSS, with calcium as the most effective intervention.
Introduction
Ovarian hyperstimulation syndrome (OHSS) is a common complication of controlled ovulation stimulation (COS). A review reported that the incidence of OHSS was 6%–11% for in vitro fertilisation or intracytoplasmic sperm injection (IVF/ICSI) (1). To date, several risk factors have been reported for OHSS including high serum anti-Mullerian hormone levels (> 3.4 ng/mL), high peak estradiol (E2) (> 3500 pg/mL), multiple follicle development (> 25), and a high number of retrieved oocytes (> 24) (2). Women with polycystic ovarian syndrome (PCOS) are at higher risks of developing OHSS during COS. In addition, our previous studies have shown that the incidence of OHSS is related to temperature and is higher in extreme climates (summer and winter) (3). Mild OHSS is characterised by slightly enlarged ovaries. Moderate OHSS is characterised by abdominal distension, nausea, vomiting, and diarrhoea. Pleural fluid, ascites, abnormal kidney function, and coagulation abnormalities, which could be life threatening, can be observed in severe OHSS patients (4). Furthermore, OHSS increases the cycle cancellation rate and financial burden, which prolongs the treatment time. Therefore, OHSS prevention is a key clinical concern.
Currently, several approaches have been used to prevent OHSS, such as using gonadotropin-releasing hormone (GnRH) antagonist protocols for COS, replacing the human chorionic gonadotropin (HCG) with GnRH-agonist for trigger, and replacing the fresh embryo transfers to whole embryo cryopreservation (2). Previous studies have described the effects of numerous drugs in preventing OHSS, including calcium (5–9), glucocorticoids (10–12), hydroxyethyl starch (HES) (13, 14), albumin (13, 15–23), aspirin (24, 25), cabergoline (6–9, 12, 15, 22, 23, 26–32), letrozole (24, 33), metformin (34–43), and quinagolide (31, 44). However, the efficacies of these drugs in preventing OHSS remain controversial or inconclusive.
Given the lack of direct comparisons among studies, the optimal drugs for preventing OHSS remain unclear. In this network meta-analysis, we present direct and indirect evidence regarding multiple drug comparisons (45). Furthermore, we considered the efficacy and safety of different drugs in preventing OHSS during COS. The aim of this study was to investigate drugs that can prevent OHSS and to compare the efficacy of different interventions to provide clinical guidance for women at high-risk of OHSS.
Methods
Inclusion and Exclusion Criteria
Our study population was at high risk of OHSS based on any of the following conditions: ≥ 20 retrieved oocytes, E2 > 3000 pg/mL on HCG day, ≥18 follicles on HCG day, and polycystic ovary syndrome (PCOS) or polycystic ovary (9, 18, 34, 38). We included the following drugs in our study: aspirin, albumin, metformin, calcium, cabergoline, quinagolide, letrozole, HES, glucocorticoids, bromocriptine, and progesterone. Placebo, blank group, or other drugs were used as controls. We excluded studies if treatment was performed using the same medicine, with only the dose and duration of use differing between groups. Moreover, we excluded studies that combined two or more drugs. The outcomes included the incidence rate of moderate-to-severe OHSS, clinical pregnancy rate, miscarriage rate, and live birth rate. We only included RCTs regarding the prevention of OHSS during IVF/ICSI, and excluded retrospective studies, reviews, case reports, conference papers, meta-analyses, duplicate published studies, studies lacking the required outcome indicators and incomplete data regarding outcome indicators, studies lacking the appropriate design, and studies with poor quality assessment. Since this study was a network meta-analysis, formal ethical approval was not required.
Search Strategy and Screening
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (46). We searched for eligible RCTs in the PubMed, EMBASE, Web of Science, and Cochrane Library databases published from database establishment to 22 August 2021. The search strategy and adjustment to the syntax for the databases are presented in Supplemental 1. Additionally, we searched for relevant articles in the references. Two researchers independently screened the articles (D. W. and H. S.), with disagreements being resolved by a third researcher (T. Y.).
Data Extraction and Efficacy Measures
We designed a special table for this analysis after a thorough assessment of all studies. We extracted the following data: first author (year), country, demographic characteristics (inclusion criteria for patients, diagnostic criteria for OHSS, age, body mass index [BMI]), sample size, COS protocol, details regarding drug use, number of moderate-to-severe OHSS, number of clinical pregnancies, number of miscarriages, number of live births, risk bias, and randomisation method. Additionally, we contacted the authors via calls or emails to obtain original data.
Primary and Secondary Outcomes
The primary outcome was the incidence rate of moderate-to-severe OHSS. Moderate-to-severe OHSS is mainly characterised by abdominal distension, nausea, vomiting, diarrhoea, hydrothorax, blood clotting disorders, and abnormal kidney function (4). Secondary outcomes included the clinical pregnancy rate, miscarriage rate, and live birth rate.
Risk-of-Bias Assessment
The risk of bias was assessed based on the Cochrane Collaboration Handbook for Quality Assessment (47). The tools used to evaluate the risk of bias included the following factors: allocation concealment, random sequence generation, blinding of participants and personnel, blinding of outcome assessment, selective reporting, incomplete outcome data, and other sources of bias. Each iteration was assessed; additionally, the risk of bias was classified as low, high, or unclear. Since this was a meta-analysis, formal ethical approval was not required.
Quality of Evidence
This meta-analysis assessed the quality of evidence-based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group (48). The risk of bias, inconsistency (heterogeneity), imprecision, indirectness, and publication bias for pairwise comparisons were assessed. The quality of evidence was ranked as high (high confidence in the evidence), moderate (moderate confidence), low (some confidence), and very low (little confidence).
Statistical Analysis
In this network-meta analysis, analyses were performed using the Bayesian theory. We used the following analytics and graphing software: R (version 4.1.1), Stata (version 14.0, Stata Corp LP, 4905 Lakeway Drive, College Station, TX, USA), and JAGS (version 4.3.0). Furthermore, we used the gemtc package (49). The network meta-analysis was performed a priori using a Markov chain Monte Carlo method. Moreover, we used a generalised linear model with four chains, performing 50,000 iterations followed by 10,000 adjustments. Inconsistency was assessed using the node split method, with the results being analysed and ranked using the consistency and non-consistency models if the difference was insignificant and significant (p > 0.05), respectively (50). The study outcomes were binary variables; furthermore, the results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Statistical significance was set at P < 0.05. Among-study heterogeneity was assessed using the I2 test (0%–30%, mild heterogeneity; 30%–60%, moderate heterogeneity; > 60%, substantial heterogeneity). Fixed-effects and random-effects models were used when heterogeneity was absent and present, respectively. In the case of a closed loop, the heterogeneity results were expressed as RR and its 95% CI, with 1 (RR) and P > 0.05 representing no heterogeneity; otherwise, heterogeneity was present. Forest and league plots were used to present the results of pairwise comparisons in the network meta-analysis. To determine the ranking of drug efficacy, we used Bayesian analysis to obtain the surface under the cumulative ranking curve (SUCRA) (45, 51). The effectiveness of each intervention is presented as a percentage, which allows identification of the best intervention. The SUCRA value is directly proportional to the effectiveness of the intervention (51, 52). Publication bias was assessed using a comparison-adjusted funnel plot.
Results
Literature Search Results
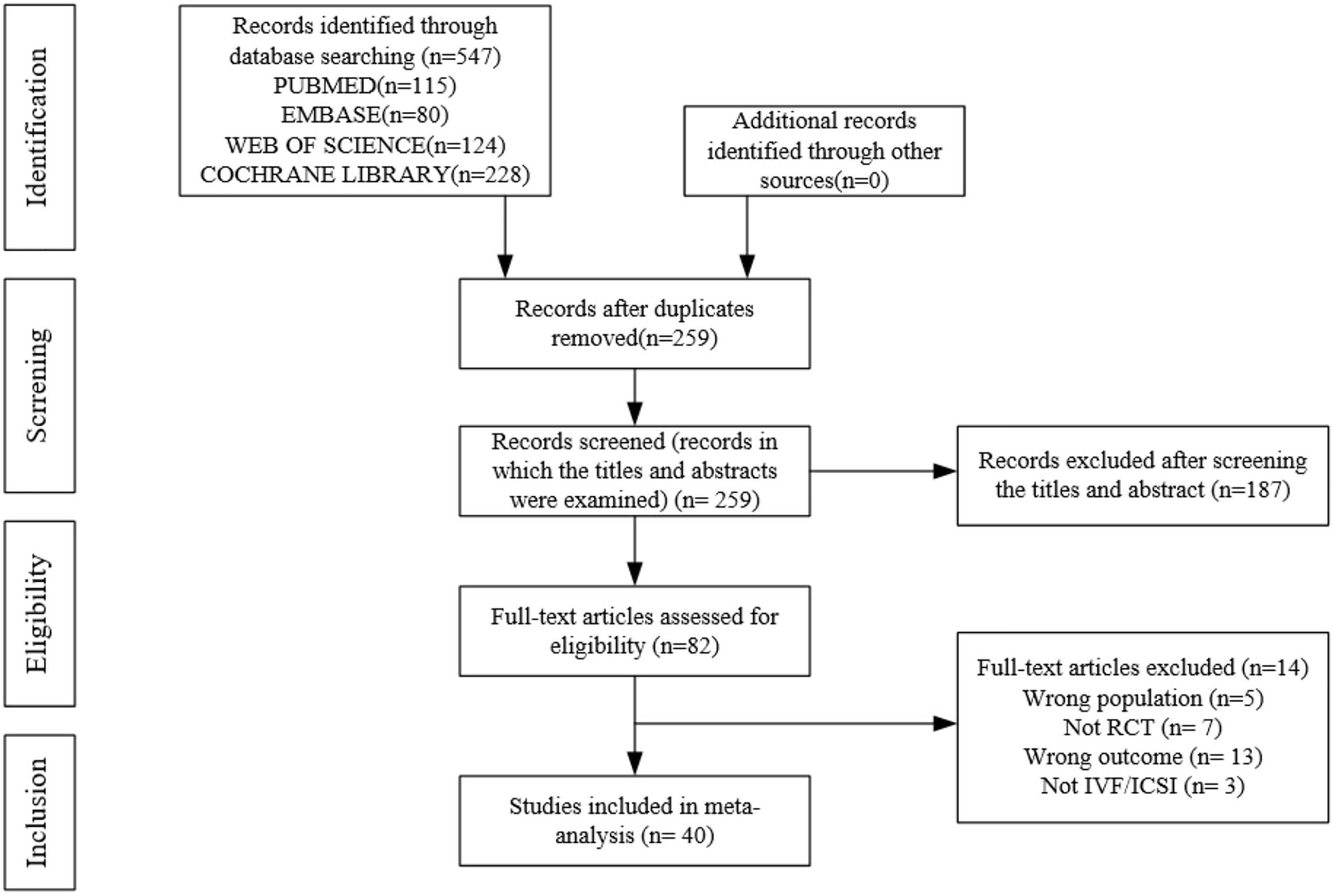
A preliminary search of multiple databases yielded 259 articles. After browsing the titles and abstracts, 82 studies were included. After a full-text review, 40 articles (n = 5849) were included. Figure 1 shows the flow chart. The included studies were presented in Supplemental 2.
Characteristics of Included Studies
We did not analyse the effect of bromocriptine and progesterone on OHSS since they were not assessed in the included RCTs. Therefore, nine interventions were analysed, including aspirin, letrozole, cabergoline, albumin, HES, metformin, calcium, glucocorticoids, and quinagolide. Thirteen RCTs included women with PCOS. The included RCTs used various criteria for classifying OHSS (Supplemental Table 1); The definitions for moderate-to-severe OHSS were similar (Supplemental 3.1). Most RCTs used Golan’s criteria for OHSS classification (4). Only 31 studies (n = 4964) with clear OHSS classification criteria and distinct reporting of moderate-to-severe OHSS were included in the analysis of the preventative effects of drugs on moderate-to-severe OHSS. The remaining nine RCTs were only analysed for safety outcomes (7, 22, 35, 36, 38–41, 43). Supplemental Table 1 presents the characteristics of the study population. The effects of eight drugs on clinical pregnancy were analysed in 29 RCTs (n = 3965). Cabergoline was the most frequently studied drug, followed by albumin and metformin. There were no significant differences of baseline characteristics (Supplemental Table 1).
Quality of Evidence
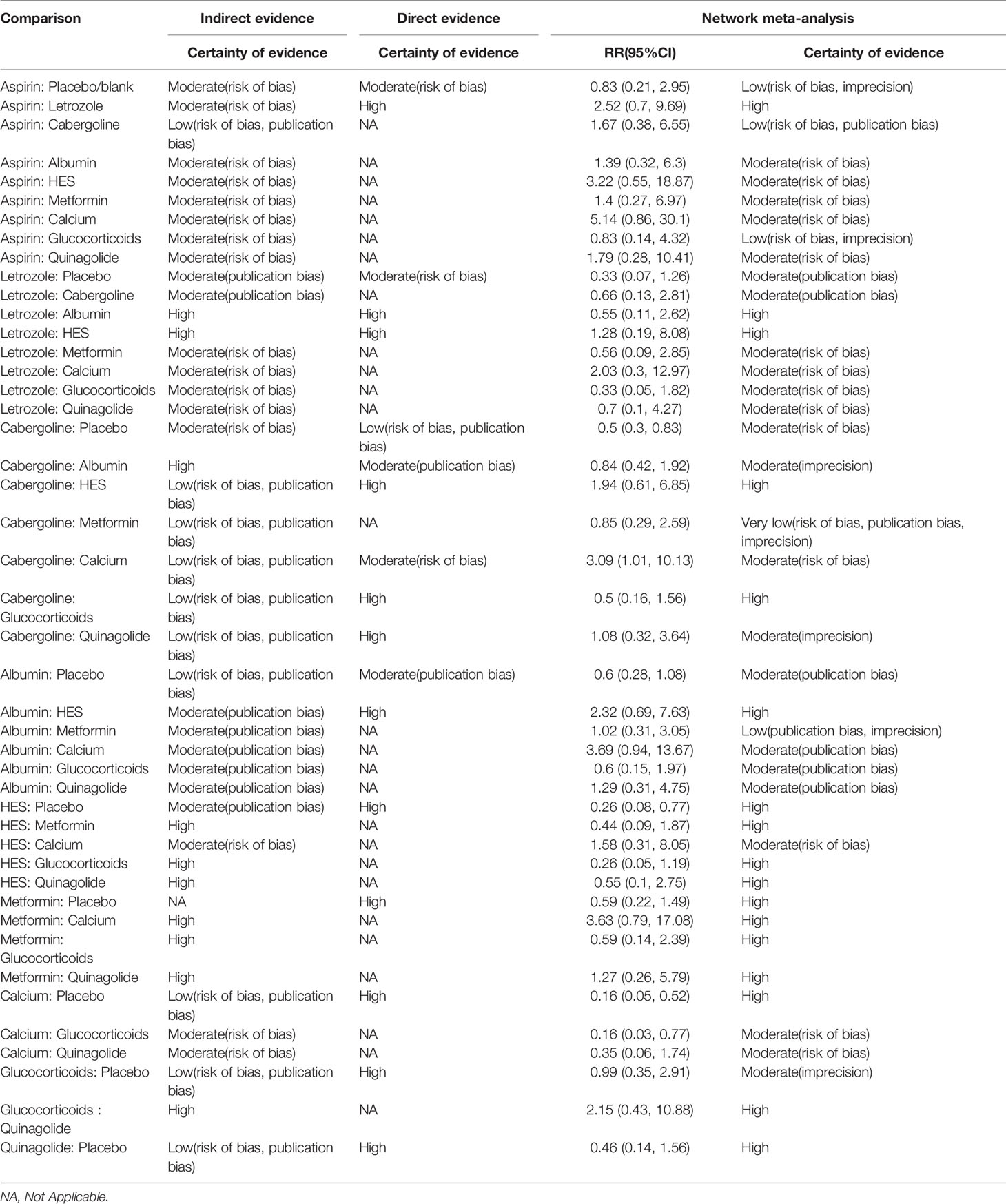
Table 1 shows the GRADE assessment of interventions for preventing moderate-to-severe OHSS. Nearly half of the evidence was moderate, with little evidence being low.
Primary Outcome Measure
Supplemental 3.2 shows a network plot for preventing moderate-to-severe OHSS. Most studies on the preventative effects of drugs on moderate-to-severe OHSS directly compared interventions with blank/placebo groups. One study directly compared aspirin and letrozole (24), while two studies directly compared cabergoline and albumin (15, 22). Another study directly compared cabergoline and HES (22), while two studies directly compared cabergoline and calcium (6, 8). Additionally, one study directly compared cabergoline and glucocorticoids (12), while two studies directly compared HES and albumin (13, 22), and another study directly compared cabergoline and quinagolide (31). Calcium (RR 0.14, 95% CI 0.04, 0.46) (grade: high), HES (RR 0.25, 95% CI 0.07, 0.73) (grade: high), and cabergoline (RR 0.43, 95% CI 0.24, 0.71) (grade: moderate) significantly prevented moderate-to-severe OHSS compared with placebo or blank control. Contrastingly, letrozole (grade: moderate), aspirin (grade: low), albumin (grade: moderate), metformin (grade: high), glucocorticoids (grade: moderate), and quinagolide (grade: high) could not prevent moderate-to-severe OHSS (P > 0.05). Figure 2A shows the forest plot.
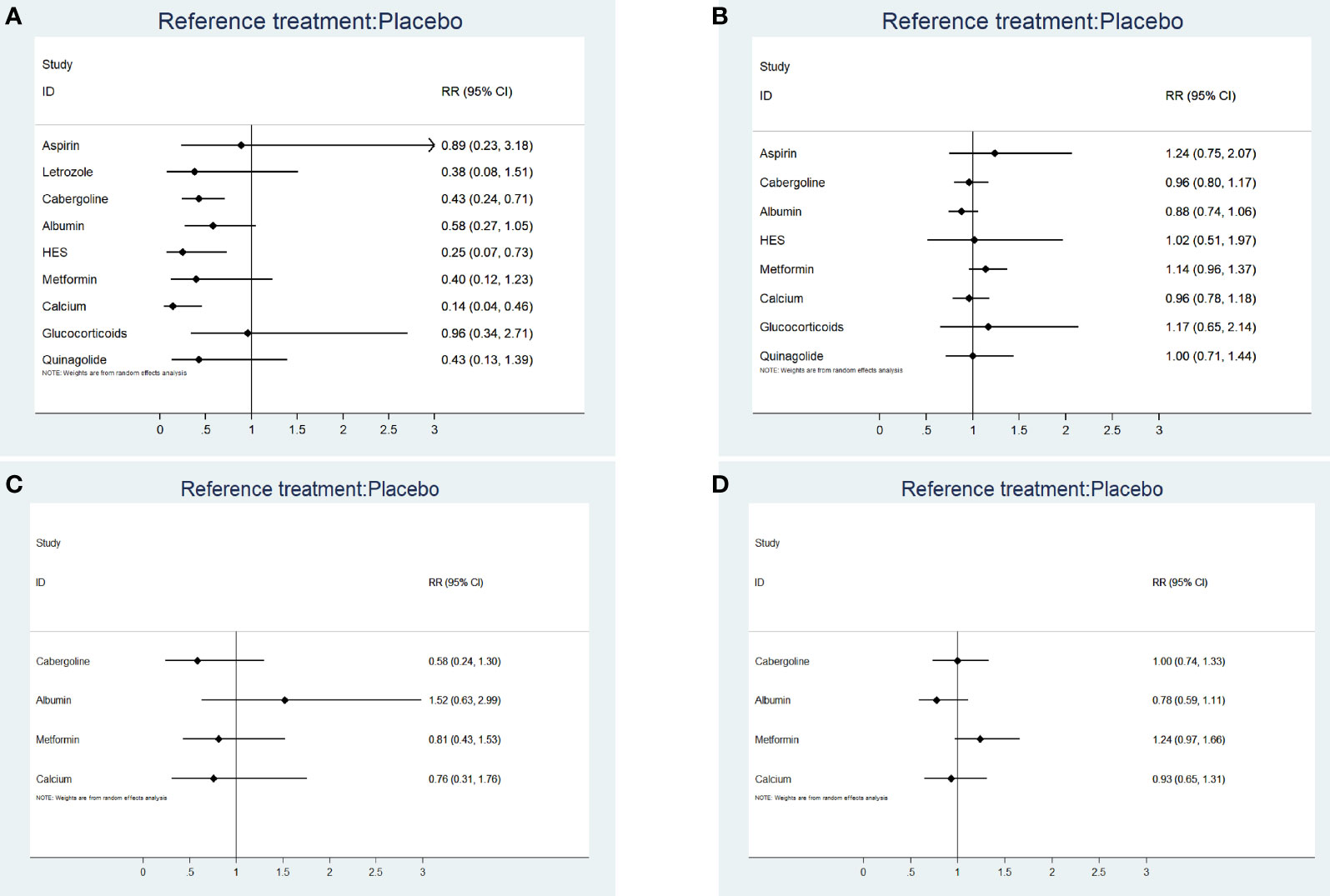
Figure 2 (A) The forest plot for incidence rate of moderate-to-severe OHSS. (B) The forest plot for clinical pregnancy rate. (C) The forest plot for miscarriage rate. (D) The forest plot for live birth rate.
According to the SUCRA results, the rankings in terms of effectiveness in preventing moderate-to-severe OHSS were as follows: calcium (SUCRA, 92.4%), HES (SUCRA, 78.6%), letrozole (SUCRA, 61.3%), metformin (SUCRA, 58.8%), cabergoline (SUCRA, 57.4%), quinagolide (SUCRA, 55.7%), albumin (SUCRA, 41.2%), aspirin (SUCRA, 23.0%), and glucocorticoids (SUCRA, 18.9%) (Figure 3). The league plot showed that calcium was significantly more effective in preventing OHSS than aspirin and albumin (grade: moderate, moderate, respectively). There was no significant difference among the remaining drugs (Figure 4). Node splitting revealed inconsistencies in the direct and indirect comparisons between the quinagolide and placebo/blank control groups. As shown in Supplemental 3.3, there were no significant inconsistencies between the remaining individual comparisons. There were heterogeneities of 64.7% (I2.pair) and 67.3% (I2.cons), which represented substantial heterogeneity. The forest plots of each drug were shown in Supplemental 3.4.
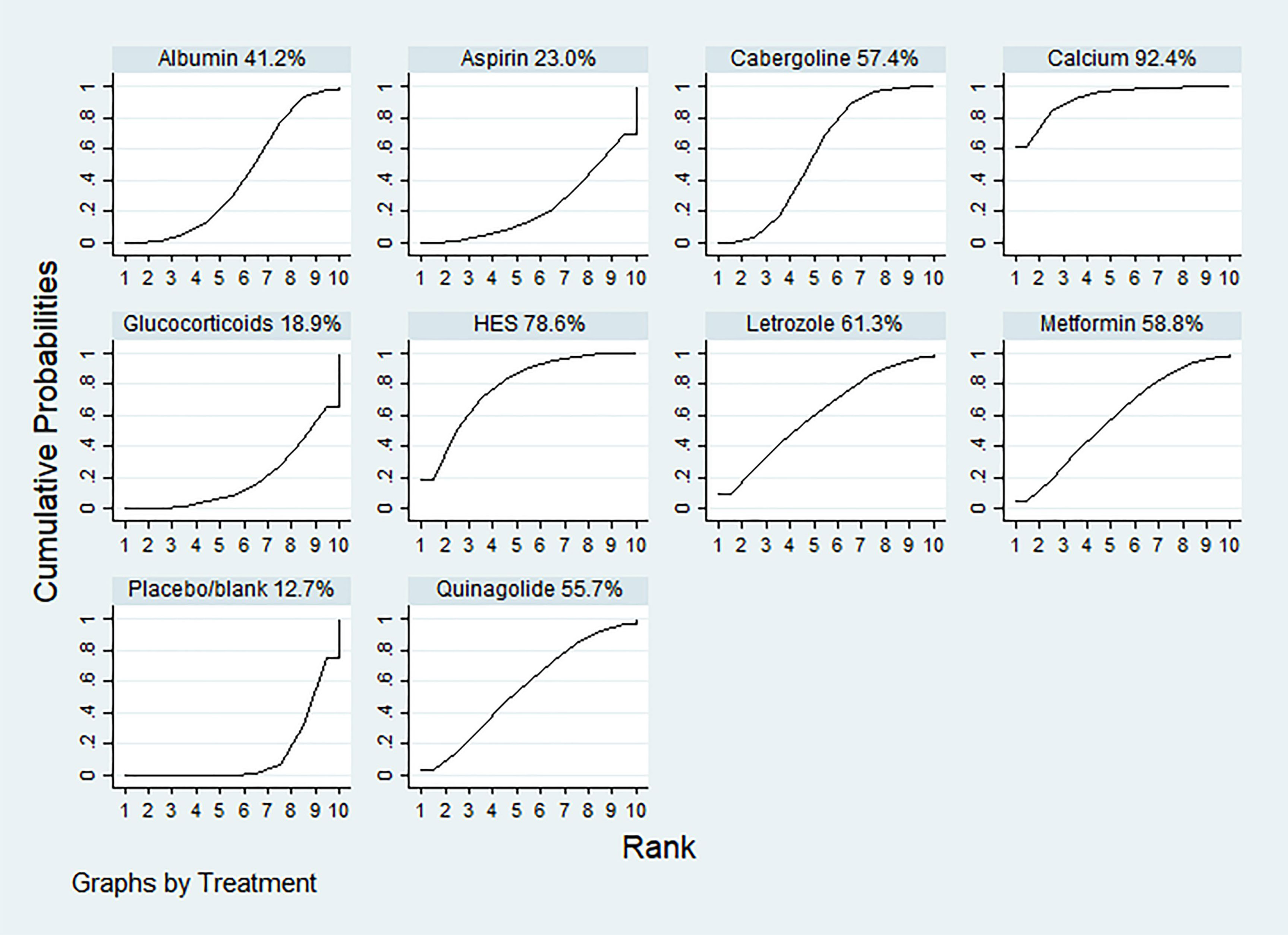
Figure 3 The ranking of interventions for primary outcomes: the incidence rate of moderate-to-severe OHSS. The surface under the cumulative ranking curve values for calcium, hydroxyethyl starch (HES), letrozole, metformin, cabergoline, quinagolide, albumin, aspirin, glucocorticoids and placebo/blank was 92.4, 78.6, 61.3, 58.8, 57.4, 55.7, 41.2 23.0, 18.9 and 12.7%, respectively.
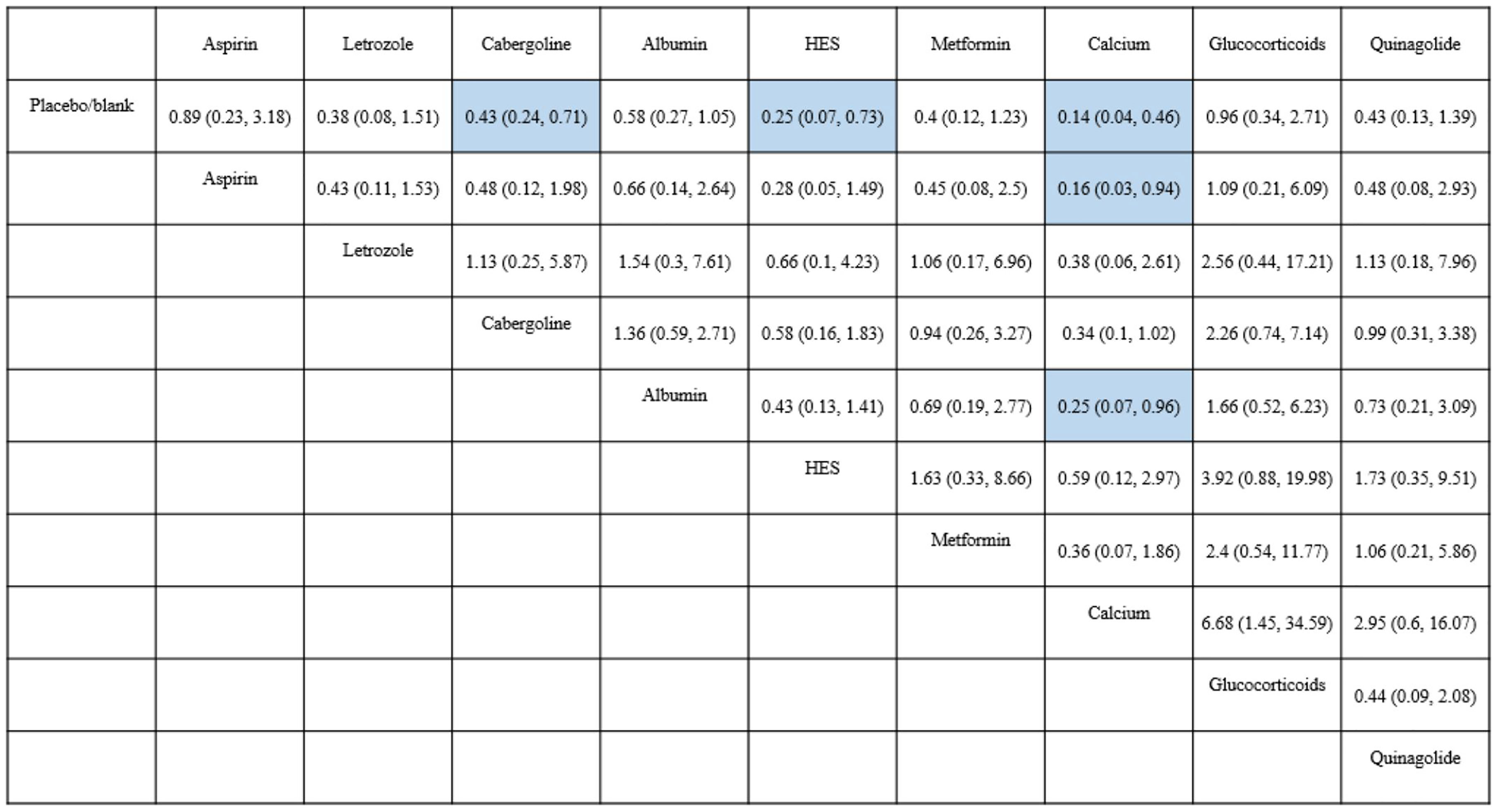
Figure 4 Results of network meta-analysis for moderate-to-severe OHSS. Results are shown as RR (95%CI), representing column-defining treatment versus row-defining treatment. HES, Hydroxyethyl starch. Statistically significant results are shown in blue.
Secondary Outcome Measures
Clinical Pregnancy Rate
A total of 29 RCTs (n = 3965) were included. The league plot showed that none of the eight drugs affected the clinical pregnancy rate. Albumin significantly reduced clinical pregnancy rates compared with metformin (RR 0.77, 95% CI 0.60, 0.99). There were no significant differences in the other between-drug comparisons (Supplemental 3.5). Figure 2B shows the forest plot. There were heterogeneities of 0% (I2.pair) and 0% (I2.cons), which indicated no heterogeneity.
Miscarriage Rate
A total of 18 RCTs (n = 2816) were included. The league plot showed that none of the four drugs (cabergoline, albumin, metformin, and calcium) affected the miscarriage rate. There were no significant differences in the between-drug comparisons (Supplemental 3.6). Figure 2C shows the forest plot. The heterogeneities were 0% (I2.pair) and 0% (I2.cons), which represented no heterogeneity.
Live Birth Rate
A total of 17 RCTs (n = 2616) were included. The league plot showed that none of the four drugs (cabergoline, albumin, metformin, and calcium) affected the live birth rate. There were no significant differences in the between-drug comparisons (Supplemental 3.7). Figure 2D shows the forest plot. The heterogeneities were 35.0% (I2.pair) and 22.6% (I2.cons), which represented mild heterogeneity.
Risk of Bias
A few studies did not report allocation concealment and whether double blinding was used; therefore, they were judged as having an unclear risk. The plot of the risk of bias is shown in Supplemental 3.8. Supplemental 3.9 shows a comparison-adjusted funnel plot for the prevention of moderate-to-severe OHSS. Funnel plots for clinical pregnancies, miscarriages, and live births are shown in Supplemental 3.10-12. All funnel plots showed good symmetry, with none having publication bias.
Discussion
There remains inconclusive evidence regarding the best intervention for preventing OHSS given the lack of head-to-head studies for different interventions. This network meta-analysis included 40 RCTs assessing the effectiveness and safety of nine drugs for the prevention of moderate-to-severe OHSS. We found that calcium, HES, and cabergoline significantly reduced the incidence of moderate-to-severe OHSS (grade: high, high, and moderate, respectively). These drugs did not significantly affect the clinical pregnancy rates.
Our network meta-analysis showed that intravenous or oral calcium significantly prevented moderate-to-severe OHSS and was safe for patients. The SUCRA ranking showed that calcium was the most effective intervention for preventing OHSS. One RCT concluded that 200 mL saline containing 10 mL of 10% calcium gluconate for three consecutive days after oocyte retrieval significantly prevented OHSS compared to the placebo group (5). Intravenous or oral calcium was significantly more effective than cabergoline in preventing OHSS (6, 7). Vascular endothelial growth factor (VEGF) is a crucial factor in OHSS development and is associated with follicular growth, luteal function, angiogenesis, and vascular endothelial stimulation (2). The possible underlying mechanism of calcium preventing OHSS could be by inhibiting cyclic adenosine monophosphate-stimulated renin secretion, which reduces the production of angiotensin-converting enzyme II (53–55). This subsequently reduces VEGF expression in human luteinized granulosa cells (53–55).
This network meta-analysis showed that HES significantly prevented moderate-to-severe OHSS and demonstrated good safety. However, HES is less effective than calcium. A previous meta-analysis showed that administering 1000 ml of 6% HES on the day of oocyte retrieval effectively prevented OHSS (56). HES could prevent OHSS due to its large molecular mass of approximately 200,000kDa (14). HES increases the intravascular volume and osmotic pressure as well as reduces blood viscosity (14). Furthermore, HES inhibits platelet aggregation and reduces blood coagulation, which ultimately prevents OHSS development (14).
This network meta-analysis showed that oral administration of 0.5 mg cabergoline for 8 consecutive days starting from the HCG triggering day was safe and effective in preventing moderate-to-severe OHSS. Consistent with previous meta-analyses, cabergoline prevented moderate-to-severe OHSS and did not affect the clinical pregnancy rates, miscarriage rates, live birth rates, or multiple birth rates (57). Cabergoline is a dopamine receptor agonist that selectively binds to dopamine receptors to promote endocytosis of VEGF receptors (58). It blocks VEGF binding, interferes with the VEGF/VEGFR-2 pathway, reduces neovascularization, and decreases vessel permeability, which ultimately prevents OHSS development (58). However, subgroup analysis revealed that cabergoline did not prevent OHSS in women with PCOS. Few studies have investigated OHSS prevention using cabergoline in women with PCOS. Moreover, we found that quinagolide, another dopamine agonist, did not prevent moderate-to-severe OHSS.
Our analysis showed that metformin did not prevent OHSS. Currently, the efficacy of metformin in preventing OHSS remains controversial. The results of this RCT are consistent with our results (37). However, other studies suggest that metformin can prevent OHSS in patients with PCOS (41, 42). Metformin may prevent OHSS by reducing the levels of VEGF, insulin, and E2 on the HCG triggering day (59).
This network meta-analysis showed that letrozole did not prevent OHSS development. The effect of letrozole in preventing OHSS remains unclear. Letrozole is a third-generation aromatase inhibitor used to treat oestrogen receptor-positive breast cancer and as a first-line ovulation induction agent in assisted reproductive technology (60). Mai et al. concluded that using 5.0 mg letrozole daily for 5 days was superior to aspirin in preventing OHSS (24). However, another study showed that using 5.0 mg letrozole daily for 5 days from the day of oocyte retrieval did not prevent OHSS (61). One study compared the use of 7.5 mg and 5.0 mg letrozole daily for preventing moderate-to-severe OHSS (33). They found that 7.5 mg, but not 5.0 mg, per day effectively prevented OHSS (33). However, these findings should be interpreted with caution given the small number of studies and the wide CIs of the results.
Our analysis showed that aspirin did not prevent OHSS or PCOS, which is consistent with the findings of Namavar et al. (25). Consistent with a previous meta-analysis, albumin did not prevent OHSS (62). There are inconsistent reports regarding the role of glucocorticoids in preventing OHSS (10, 11, 63). Our analysis showed that glucocorticoids did not prevent OHSS. However, Revelli et al. found that acetylsalicylic acid combined with glucocorticoids could prevent severe OHSS and increase the number of oocytes retrieved (63).
This network meta-analysis has several advantages. It analysed the effects of nine drugs on the prevention of OHSS, clarified that three drugs (calcium, HES, and cabergoline) were able to prevent OHSS, and to the best of our knowledge, is the first of its kind to compare the effectiveness of different drugs. The effects of eight drugs (except letrozole) on clinical pregnancy rates were also analysed. It was conducted in strict accordance with the recommendations of the Cochrane Handbook and PRISMA statement. Furthermore, we conducted a comprehensive search of different databases to extract all relevant RCTs. However, this study has several limitations. First, there were missing direct comparative results between some interventions, which could have led to bias in the study results even though consistency models were used for fitting. Given the large number of studies that did not report the BMI, we could not conduct subgroup analysis based on the BMI. The SUCRA curve was used to estimate the ranking of effectiveness between the different interventions; however, it has limitations and the results should be interpreted with caution. The included RCTs reported few miscarriages and live births.
Conclusions
In conclusion, calcium, HES, and cabergoline are safe and effective in preventing moderate-to-severe OHSS. SUCRA showed that calcium is the most effective intervention for preventing moderate-to-severe OHSS. Given the limitations of this study, the aforementioned conclusions should be validated by large-scale multi-centre RCTs. Determining the effectiveness of various drugs for OHSS prevention could facilitate the establishment of the best protocol for OHSS prevention.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
JZ, DW, and HS were the principal investigators. They formulated the meta-analysis and wrote the manuscript. DW and JZ contributed to the acquisition of the data and the manuscript writing process. TY helped in the acquisition of the data. YY created the statistical analysis. All authors also contributed to the critical revision of the intellectual content and approved the final version of the paper.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82071649) and the Key Scientific Research Projects of Higher Education Institutions in Henan Province (Grant No. 22A320025).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.808517/full#supplementary-material.
References
1. Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-Releasing Hormone Antagonists for Assisted Reproductive Technology. Cochrane Database Syst Rev (2016) 4(4):CD001750. doi: 10.1002/14651858.CD001750.pub4
2. Practice Committee of the American Society for Reproductive Medicine. Electronic Address:QVNSTUBBc3JtLk9yZw==; Practice Committee of the American Society for Reproductive Medicine. Prevention and Treatment of Moderate and Severe Ovarian Hyperstimulation Syndrome: A Guideline. Fertil Steril (2016) 106(7):1634–47. doi: 10.1016/j.fertnstert.2016.08.048
3. Cao Y, Shi H, Ma Y, Ma L, Zhai J. Effect and Relationship of Seasons on the High Risk of Ovarian Hyperstimulation Syndrome After Oocyte Retrieval in Patients With Polycystic Ovary Syndrome. Front Endocrinol (2021) 11:610828. doi: 10.3389/fendo.2020.610828
4. Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian Hyperstimulation Syndrome: An Update Review. Obstet Gynecol Surv (1989) 44:430–40. doi: 10.1097/00006254-198906000-00004
5. El-Khayat W, Elsadek M. Calcium Infusion for the Prevention of Ovarian Hyperstimulation Syndrome: A Double-Blind Randomized Controlled Trial. Fertil Steril (2015) 103:101–5. doi: 10.1016/j.fertnstert.2014.09.046
6. Elnory MA, Elmantwe ANM. Comparison of Cabergoline Versus Calcium Infusion in Ovarian Hyperstimulation Syndrome Prevention: A Randomized Clinical Trial. Middle East Fertil S (2018) 23:357–62. doi: 10.1016/j.mefs.2018.05.001
7. Samy Saad A, Abd Aziz Mohamed K. Calcium Dobesilate Versus Cabergoline for Prevention of Ovarian Hyper Stimulation Syndrome. Reprod System Sexual Disord: Curr Res (2017) 6:1–5. doi: 10.4172/2161-038X.1000204
8. Naredi N, Karunakaran S. Calcium Gluconate Infusion is as Effective as the Vascular Endothelial Growth Factor Antagonist Cabergoline for the Prevention of Ovarian Hyperstimulation Syndrome. J Hum Reprod Sci (2013) 6:248–52. doi: 10.4103/0974-1208.126293
9. Fouda UM, Elshaer HS, Youssef GG, Hanafy A, Mehrem WM, Youssef MA, et al. Cabergoline Versus Calcium Infusion in the Prevention of Ovarian Hyperstimulation Syndrome: A Randomised Controlled Study. J Obstet Gynaecol (2021) 42:122–8. doi: 10.1080/01443615.2020.1870944
10. Mohammadi Yeganeh L, Moini A, Shiva M, Mirghavam N, Bagheri Lankarani N. Methylprednisolone for Prevention of Ovarian Hyperstimulation Syndrome in Patients With Polycystic Ovarian Syndrome Undergoing in-Vitro Fertilisation: A Randomised Controlled Trial. J Obstet Gynaecol (2018) 38(2):241–6. doi: 10.1080/01443615.2017.1346593
11. Tan SL, Balen A, Hussein E, Campbell S, Jacobs HS. The Administration of Glucocorticoids for the Prevention of Ovarian Hyperstimulation Syndrome in In Vitro Fertilization: A Prospective Randomized Study. Fertil Steril (1992) 58(2):378–83. doi: 10.1016/s0015-0282(16)55223-6
12. Salah AM, El-Helew Y. Can Cabergoline Prevent Ovarian Hyperstimulation Syndrome in Polycystic Ovarian Patients Undergoing Gonadotropin Stimulation? Evid Based Women's Health J (2012) 2:56–9. doi: 10.1097/01.EBX.0000413115.90725.a7
13. Gokmen O UM, Ekin M, Keles G, Turan C, Oral H. Intravenous Albumin Versus Hydroxyethyl Starch for the Prevention of Ovarian Hyperstimulation in an in-Vitro Fertilization Programme a Prospective Randomized Placebo Controlled Study. Eur J Obstet Gynecol Reprod Biol (2001) 96:187–92. doi: 10.1016/s0301-2115(00)00452-8
14. König E, Bussen S, Sütterlin M, Steck T. Prophylactic Intravenous Hydroxyethyle Starch Solution Prevents Moderate-Severe Ovarian Hyperstimulation in in-Vitro Fertilization Patients: A Prospective, Randomized, Double-Blind and Placebo-Controlled Study. Hum Reprod (1998) 13(9):2421–4. doi: 10.1093/humrep/13.9.2421
15. Tehraninejad ES, Hafezi M, Arabipoor A, Aziminekoo E, Chehrazi M, Bahmanabadi A. Comparison of Cabergoline and Intravenous Albumin in the Prevention of Ovarian Hyperstimulation Syndrome: A Randomized Clinical Trial. J Assist Reprod Genet (2012) 29:259–64. doi: 10.1007/s10815-011-9708-4
16. Shalev E GY, Matilsky M, Ben-Ami M. Decreased Incidence of Severe Ovarian Hyperstimulation Syndrome in High Risk in-Vitro Fertilization Patients Receiving Intravenous Albumin a Prospective Study. Hum Reprod (1995) 10(6):1373–6. doi: 10.1093/humrep/10.6.1373
17. Isikoglu M, Berkkanoglu M, Senturk Z, Ozgur K. Human Albumin Does Not Prevent Ovarian Hyperstimulation Syndrome in Assisted Reproductive Technology Program: A Prospective Randomized Placebo-Controlled Double Blind Study. Fertil Steril (2007) 88:982–5. doi: 10.1016/j.fertnstert.2006.11.170
18. Bellver J, Muñoz EA, Ballesteros A, Soares SR, Bosch E, Simón C, et al. Intravenous Albumin Does Not Prevent Moderate-Severe Ovarian Hyperstimulation Syndrome in High-Risk IVF Patients: A Randomized Controlled Study. Hum Reprod (2003) 18:2283–8. doi: 10.1093/humrep/deg451
19. Shoham Z WA, Barash A, Borenstein R, Schachter M, Insler V. Intravenous Albumin for the Prevention of Severe Ovarian Hyperstimulation Syndrome in an In Vitro Fertilization Program a Prospective, Randomized, Placebo-Controlled Study. Fertil Steril (1994) 62(1):137–42. doi: 10.1016/s0015-0282(16)56829-0
20. Isik AZ GO, Zeyneloglu HB, Kara S, Keles G, Gulekli B. Intravenous Albumin Prevents Moderate-Severe Ovarian Hyperstimulation in in-Vitro Fertilization Patients a Prospective, Randomized and Controlled Study. Eur J Obstet Gynecol Reprod Biol (1996) 70(2):179–83. doi: 10.1016/s0301-2115(95)02603-7
21. Ben-Chetrit A, Eldar-Geva T, Gal M, Huerta M, Mimon T, Algur N, et al. The Questionable Use of Albumin for the Prevention of Ovarian Hyperstimulation Syndrome in an IVF Programme a Randomized Placebo-Controlled Trial. Hum Reprod (2001) 16(9):1880–4. doi: 10.1093/humrep/16.9.1880
22. Torabizadeh A VF, Ghorbanpour Z. Comparison of Albumin and Cabergoline in the Prevention of Ovarian Hyperstimulation Syndrome a Clinical Trial Study. Iran J Reprod Med (2013) 11(10):837–42.
23. Ghahiri A MN, Movahedi M, Hosseini N. Evaluation of Intravenous Hydroxylethyl Starch, Intravenous Albumin 20%, and Oral Cabergoline for Prevention of Ovarian Hyperstimulation Syndrome in Patients Undergoing Ovulation in Patients Undergoing Ovulation Induction. J Res Med Sci (2015) 20(7):692–6. doi: 10.4103/1735-1995.166228
24. Mai Q, Hu X, Yang G, Luo Y, Huang K, Yuan Y, et al. Effect of Letrozole on Moderate and Severe Early-Onset Ovarian Hyperstimulation Syndrome in High-Risk Women: A Prospective Randomized Trial. Am J Obstet Gynecol (2017) 216(1):42.e1–42.e10. doi: 10.1016/j.ajog.2016.08.018
25. Namavar Jahromi B, Zolghadri J, Rahmani E, Alipour S, Anvar Z, Zarei A, et al. Effect of Low-Dose Aspirin on the Development of Ovarian Hyperstimulation Syndrome and Outcomes of Assisted Reproductive Techniques in the Women With PCOS, a Randomized Double-Blinded Clinical Trial. Taiwan J Obstet Gynecol (2019) 58:255–60. doi: 10.1016/j.tjog.2019.01.016
26. Carizza C AV, Abdelmassih S, Ravizzini P, Salgueiro L, Salgueiro PT, Jine LT, et al. Cabergoline Reduces the Early Onset of Ovarian Hyperstimulation Syndrome a Prospective Randomized Study. Reprod BioMed Online (2008) 17(6):751–5. doi: 10.1016/s1472-6483(10)60401-4
27. Shaltout A, Shohyab A, Youssef MA. Can Dopamine Agonist at a Low Dose Reduce Ovarian Hyperstimulation Syndrome in Women at Risk Undergoing ICSI Treatment Cycles? A Randomized Controlled Study. Eur J Obstet Gynecol Reprod Biol (2012) 165:254–8. doi: 10.1016/j.ejogrb.2012.08.008
28. Jellad S, Haj Hassine A, Basly M, Mrabet A, Chibani M, Rachdi R. Vascular Endothelial Growth Factor Antagonist Reduces the Early Onset and the Severity of Ovarian Hyperstimulation Syndrome. J Gynecol Obstet Hum Reprod (2017) 46:87–91. doi: 10.1016/j.jgyn.2016.04.002
29. Alvarez C, Martí-Bonmatí L, Novella-Maestre E, Sanz R, Gómez R, Fernández-Sánchez M, et al. Dopamine Agonist Cabergoline Reduces Hemoconcentration and Ascites in Hyperstimulated Women Undergoing Assisted Reproduction. J Clin Endocrinol Metab (2007) 92:2931–7. doi: 10.1210/jc.2007-0409
30. Amir H YD, Hasson J, Amit A, Gordon D, Azem F. Cabergoline for Reducing Ovarian Hyperstimulation Syndrome in Assisted Reproductive Technology Treatment Cycles. A Prospective Randomized Controlled Trial. J Reprod Med (2015) 60(1-2):48–54.
31. Taheripanah R, Vasef M, Zamaniyan M, Taheripanah A. Comparison of Cabergoline and Quinagolide in Prevention of Severe Ovarian Hyperstimulation Syndrome Among Patients Undergoing Intracytoplasmic Sperm Injection. Fertil Steril (2018) 12:1–5. doi: 10.22074/ijfs.2018.5259
32. Kilic N, Ozdemir O, Basar HC, Demircan F, Ekmez F, Yucel O. Cabergoline for Preventing Ovarian Hyperstimulation Syndrome in Women at Risk Undergoing In Vitro Fertilization/Intracytoplasmic Sperm Injection Treatment Cycles: A Randomized Controlled Study. Avicenna J Med (2015) 5:123–7. doi: 10.4103/2231-0770.165121
33. He Q, Liang L, Zhang C, Li H, Ge Z, Wang L, et al. Effects of Different Doses of Letrozole on the Incidence of Early-Onset Ovarian Hyperstimulation Syndrome After Oocyte Retrieval. Syst Biol Reprod Med (2014) 60:355–60. doi: 10.3109/19396368.2014.957879
34. Swanton A, Lighten A, Granne I, McVeigh E, Lavery S, Trew G, et al. Do Women With Ovaries of Polycystic Morphology Without Any Other Features of PCOS Benefit From Short-Term Metformin Co-Treatment During IVF? A Double-Blind, Placebo-Controlled, Randomized Trial. Hum Reprod (2011) 26:2178–84. doi: 10.1093/humrep/der120
35. Abdalmageed OS, Farghaly TA, Abdelaleem AA, Abdelmagied AE, Ali MK, Abbas AM. Impact of Metformin on IVF Outcomes in Overweight and Obese Women With Polycystic Ovary Syndrome: A Randomized Double-Blind Controlled Trial. Reprod Sci (2019) 26:1336–42. doi: 10.1177/1933719118765985
36. Qublan HS, Al-Khaderei S, Abu-Salem AN, Al-Zpoon A, Al-Khateeb M, Al-Ibrahim N, et al. Metformin in the Treatment of Clomiphene Citrate-Resistant Women With Polycystic Ovary Syndrome Undergoing In Vitro Fertilisation Treatment: A Randomised Controlled Trial. J Obstet Gynaecol (2009) 29:651–5. doi: 10.1080/01443610903147576
37. Palomba S, Falbo A, Carrillo L, Villani MT, Orio F, Russo T, et al. Metformin Reduces Risk of Ovarian Hyperstimulation Syndrome in Patients With Polycystic Ovary Syndrome During Gonadotropin-Stimulated In Vitro Fertilization Cycles: A Randomized, Controlled Trial. Fertil Steril (2011) 96:1384–90.e4. doi: 10.1016/j.fertnstert.2011.09.020
38. Kjøtrød SB, von Düring V, Carlsen SM. Metformin Treatment Before IVF/ICSI in Women With Polycystic Ovary Syndrome; a Prospective, Randomized, Double Blind Study. Hum Reprod (2004) 19:1315–22. doi: 10.1093/humrep/deh248
39. Onalan G, Pabuccu R, Goktolga U, Ceyhan T, Bagis T, Cincik M. Metformin Treatment in Patients With Polycystic Ovary Syndrome Undergoing In Vitro Fertilization: A Prospective Randomized Trial. Fertil Steril (2005) 84:798–801. doi: 10.1016/j.fertnstert.2005.03.043
40. Cheraghi E, Mehranjani MS, Shariatzadeh MA, Esfahani MH, Ebrahimi Z. N-Acetylcysteine Improves Oocyte and Embryo Quality in Polycystic Ovary Syndrome Patients Undergoing Intracytoplasmic Sperm Injection: An Alternative to Metformin. Reprod Fertil Dev (2016) 28:723–31. doi: 10.1071/RD14182
41. An Y, Sun Z, Zhang Y, Liu B, Guan Y, Lu M. The Use of Berberine for Women With Polycystic Ovary Syndrome Undergoing IVF Treatment. Clin Endocrinol (2014) 80:425–31. doi: 10.1111/cen.12294
42. Tang T, Glanville J, Orsi N, Barth JH, Balen AH. The Use of Metformin for Women With PCOS Undergoing IVF Treatment. Hum Reprod (2006) 21:1416–25. doi: 10.1093/humrep/del025
43. Kjotrod SB, Carlsen SM, Rasmussen PE, Holst-Larsen T, Mellembakken J, Thurin-Kjellberg A, et al. Use of Metformin Before and During Assisted Reproductive Technology in non-Obese Young Infertile Women With Polycystic Ovary Syndrome: A Prospective, Randomized, Double-Blind, Multi-Centre Study. Hum Reprod (2011) 26:2045–53. doi: 10.1093/humrep/der154
44. Busso C, Fernández-Sánchez M, García-Velasco JA, Landeras J, Ballesteros A, Muñoz E, et al. The non-Ergot Derived Dopamine Agonist Quinagolide in Prevention of Early Ovarian Hyperstimulation Syndrome in IVF Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Hum Reprod (2010) 25:995–1004. doi: 10.1093/humrep/deq005
45. Salanti G. Indirect and Mixed-Treatment Comparison, Network, or Multiple-Treatments Meta-Analysis: Many Names, Many Benefits, Many Concerns for the Next Generation Evidence Synthesis Tool. Res Synth Methods (2012) 3:80–97. doi: 10.1002/jrsm.1037
46. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med (2015) 162:777–84. doi: 10.7326/M14-2385
47. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
48. Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates From Network Meta-Analysis. BMJ (2014) 349:g5630. doi: 10.1136/bmj.g5630
49. Van Valkenhoef G, Kuiper J. Gemtc: Network Meta-Analysis Using Bayesian Methods: R Package Version0.8. Available at: http://cran.r-project.org/web/packages/gemtc/index.html.
50. Dias S WN, Caldwell DM, Ades AE. Checking Consistency in Mixed Treatment Comparison Meta-Analysis. Stat Med (2010) 29:932–44. doi: 10.1002/sim.3767
51. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphicaltools for Network Meta-Analysis in STATA. PloS One (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
52. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to Interpreting and Choosing the Best Treatments in Network Meta-Analyses. Syst Rev (2017) 6:79. doi: 10.1186/s13643-017-0473-z
53. Herr D, Duncan WC, Hack G, Konrad R, Kreienberg R, Wulff C. Regulated Expression of the Renin-Angiotensin-System in Human Granulosa Lutein Cells: Angiotensin II Increases VEGF Expression But its Synthesis is Reduced by Hcg. Arch Gynecol Obstet (2010) 281:409–16. doi: 10.1007/s00404-009-1135-8
54. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased Intracellular Calcium Stimulates Renin Release via Calcium-Inhibitable Adenylyl Cyclase. Hypertension (2007) 49:162–9. doi: 10.1161/01.HYP.0000250708.04205.d4
55. Beierwaltes WH. The Role of Calcium in the Regulation of Renin Secretion. Am J Physiol Renal Physiol (2010) 298:F1–F11. doi: 10.1152/ajprenal.00143.2009
56. Youssef MA A-IH, Evers JL, Aboulghar M. Intra-Venous Fluids for Prevention of Severe Ovarian Hyperstimulation Syndrome. Cochrane Database Syst Rev (2011) 2:CD001302. doi: 10.1002/14651858.CD001302.pub2
57. Tang H, Mourad SM, Wang A, Zhai SD, Hart RJ. Dopamine Agonists for Preventing Ovarian Hyperstimulation Syndrome. Cochrane Database Syst Rev (2021) 4:Cd008605. doi: 10.1002/14651858.CD008605.pub3
58. Basu S NJ, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, et al. The Neurotransmitter Dopamine Inhibits Angiogenesis Induced by Vascular Permeability Factor Vascular Endothelial Growth Factor. Nat Med (2001) 7(5):569–74. doi: 10.1038/87895
59. Wu Y, Tu M, Huang Y, Liu Y, Zhang D. Association of Metformin With Pregnancy Outcomes in Women With Polycystic Ovarian Syndrome Undergoing In Vitro Fertilization: A Systematic Review and Meta-Analysis. JAMA Netw Open (2020) 3(8):e2011995. doi: 10.1001/jamanetworkopen.2020.11995
60. Mejia RB, Summers KM, Kresowik JD, Van Voorhis BJ. A Randomized Controlled Trial of Combination Letrozole and Clomiphene Citrate or Letrozole Alone for Ovulation Induction in Women With Polycystic Ovary Syndrome. Fertil Steril (2019) 111:571–8e1. doi: 10.1016/j.fertnstert.2018.11.030
61. Wang YQ, Luo J, Xu WM, Xie QZ, Yan WJ, Wu GX, et al. Can Steroidal Ovarian Suppression During the Luteal Phase After Oocyte Retrieval Reduce the Risk of Severe OHSS? J Ovarian Res (2015) 8:63. doi: 10.1186/s13048-015-0190-y
62. Jee BC, Suh CS, Kim YB, Kim SH, Choi YM, Kim JG, et al. Administration of Intravenous Albumin Around the Time of Oocyte Retrieval Reduces Pregnancy Rate Without Preventing Ovarian Hyperstimulation Syndrome: A Systematic Review and Meta-Analysis. Gynecol Obstet Invest (2010) 70:47–54. doi: 10.1159/000286379
Keywords: ovarian hyperstimulation syndrome, various drugs, controlled ovulation stimulation, network meta-analysis, OHSS
Citation: Wu D, Shi H, Yu Y, Yu T and Zhai J (2022) Comparison of the Effectiveness of Various Medicines in the Prevention of Ovarian Hyperstimulation Syndrome: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 13:808517. doi: 10.3389/fendo.2022.808517
Received: 03 November 2021; Accepted: 03 January 2022;
Published: 26 January 2022.
Edited by:
Wenpei Xiang, Huazhong University of Science and Technology, ChinaReviewed by:
Shibin Chao, Jiangxi University of Traditional Chinese Medicine, ChinaJinhua Fu, Qingdao Jinhua Gynecology Hospital, China
Xue Wang, Peking Union Medical College Hospital (CAMS), China
Copyright © 2022 Wu, Shi, Yu, Yu and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhai, YmVzdHpoYWkyMDA1QDE2My5jb20=
†These authors have contributed equally to this work
 Di Wu
Di Wu Hao Shi
Hao Shi Yiping Yu1,2,3
Yiping Yu1,2,3 Ting Yu
Ting Yu Jun Zhai
Jun Zhai