- 1Department of Obstetrics and Gynaecology, West China Second University Hospital of Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital of Sichuan University, Chengdu, China
- 3Key Laboratory of Chronobiology (Sichuan University), National Health Commission (NHC), West China Second University Hospital of Sichuan University, Chengdu, China
Background: Some female thyroid cancer survivors wish to become pregnant following their cancer treatment. Current studies have shown inconsistent results on pregnancy outcomes in these survivors; however, detailed information on the pathological type, treatment, and gestational thyroid function of these patients are not yet well documented, making the refined assessment of the influence of a history of thyroid cancer and related treatments on pregnancy outcomes challenging.
Objective: To investigate the risk of adverse pregnancy outcomes in thyroid cancer survivors.
Methods: This was a retrospective cohort study. We included all women aged between 19 and 45 years old who delivered between January 2019 and June 2020 in West China Second University Hospital of Sichuan University. Women with tumors other than thyroid cancer or other thyroid diseases were excluded. The included women were divided into survivors of thyroid cancer (survivors) and women without any history of thyroid disease (controls). Propensity score matching and logistic regression were used to control confounding variables.
Results: All 18,332 women who met the inclusion criteria were included in the study (96 survivors of papillary thyroid cancer and 18,236 controls). After propensity score matching, 96 survivors and 192 controls were included. The survivors had higher levels of free thyroxine (15.47 [13.61–17.67] vs. 14.38 [13.20–15.81] pmol/mL; P<0.001) and higher levels of thyroid peroxidase antibodies (TPOAb) (43.55 [31.43–71.43] vs. 35.95 [28.00–48.03] U/mL; P=0.008) but similar levels of thyroid stimulating hormone (1.46 [0.56–3.15] vs. 1.36 [0.81–1.92] mIU/mL; P=0.142) than the controls. There were no significant differences in adverse pregnancy outcomes between survivors and controls. Fetal macrosomia was lower among survivors (OR: 0.077, 95% CI: 0.009–0.668. P=0.020) than controls. Additionally, survivors had reduced weight gain during pregnancy (13.0 [10.0–15.0] vs. 14.00 [11.00–16.00] kg, P=0.005) and reduced placental weight (563.0 [514.5–620.0] vs. 572.0 [520.0–650.0] g, P=0.019), albeit with small absolute differences. Thyroidectomy or radioiodine therapy did not adversely affect pregnancy outcomes.
Conclusion: A history of treated papillary thyroid cancer was not associated with adverse pregnancy outcomes.
Introduction
Thyroid cancer has become the most common malignant endocrine cancer among women of childbearing age, and patients usually have a favorable prognosis (1–5). Some female survivors wish to become pregnant following their thyroid cancer treatment. However, existing thyroid cancer-related treatments may have adverse effects on pregnancy outcomes.
According to the current guidelines, thyroidectomy with or without radioiodine therapy (RAIT) and postoperative thyroid-stimulating hormone (TSH) suppressive therapy with levothyroxine (LT4) are the standard treatments for thyroid cancer (5–7). During pregnancy, thyroxine is vital in maintaining maternal health and fetal development (7, 8). However, both hypothyroidism caused by thyroidectomy and hyperthyroidism after TSH suppressive therapy may lead to adverse pregnancy outcomes, including pre-eclampsia, gestational diabetes mellitus, preterm delivery, and miscarriage (9–11). In addition, studies have shown that RAIT might lead to gonadal dysfunction and follicular atresia (12). Impaired gonadal function may contribute to adverse pregnancy outcomes, especially miscarriage.
Two national database studies have shown different results. A Korean research study by Cho et al. found a higher risk of postpartum hemorrhage (PPH) among survivors (13). A US-based study by Spiegel et al. showed a higher incidence of thromboembolism and blood transfusion among survivors (14). The other three smaller-sized studies (15–17) generally showed no adverse fetal outcomes. However, PPH was mentioned in Clark et al.’s study (17). Detailed information on the pathological type, treatment, and gestational thyroid function of thyroid cancer survivors are still not well understood, making the refined assessment of the impact of a history of thyroid cancer and related treatments on pregnancy outcomes challenging.
Therefore, we conducted this retrospective cohort study among women with treated thyroid cancer to evaluate the gestational thyroid function of the survivors and determine the potential adverse maternal or fetal outcomes of thyroid cancer-related treatments.
Materials and Methods
Study Design
This study was approved by the Ethics Committee of West China Second University Hospital of Sichuan University (No. 2021098) and performed in accordance with the tenets of the Declaration of Helsinki. Informed consent was not required as we analyzed data anonymously without divulging any personal information.
Excluding those with a history of other tumors or other thyroid diseases, we included all women aged between 19 and 45 years who delivered between January 2019 and June 2020 in West China Second University Hospital of Sichuan University. The group was divided into survivors and controls based on the presence or absence of a history of thyroid cancer, respectively.
Baseline information, pregnancy outcomes, and neonatal data were obtained from the electronic medical records (EMR) of West China Second University Hospital of Sichuan University. Thyroid cancer-related data including pathological type, treatment modality, and duration were collected with patients’ consent from the EMR of the hospital where the patients were diagnosed and/or treated. Patients’ blood samples were routinely collected between 0800 and 1130 hours. Thyroid function tests including TSH, free thyroxine (fT4), and thyroid peroxidase antibodies (TPO-Ab) were performed between gestational weeks 10 and 14. TSH, fT4, and TPO-Ab were measured in the laboratory of West China Second University Hospital of Sichuan University by chemiluminescence (Siemens ADVIA Centaur CP, Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA). The reference range was 0.1–2.5 mIU/L for TSH (intra-assay coefficient of variation [CV]=2.1%, inter-assay CV=1.9%), 11.5–22.7 pmol/L for fT4 (intra-assay CV=2.22%, inter-assay CV=3.48%), and <60 U/mL for TPO-Ab (intra-assay CV=7.8%, inter-assay CV=7.3%).
Outcome Measures
Maternal outcomes in this study included PPH (≥1000 mL for cesarean delivery or ≥500 mL for vaginal delivery); preterm delivery (between gestational weeks 28 and 36 + 6); pre-eclampsia (hypertension after week 20 with concomitant proteinuria); gestational diabetes mellitus (GDM, diagnosed according to a the 75-g oral glucose tolerance test between week 24 and 28, with plasma glucose thresholds for fasting, 1, and 2 h being 5.1, 10.0, and 8.5 mmol/L, respectively); late miscarriage (between gestational weeks 14 and week 27 + 6); cesarean section; anemia (hemoglobin<100 g/L); weight gain during pregnancy; and the blood pressure in the third trimester of pregnancy.
The neonatal outcomes of this study included macrosomia (newborn birthweight >4000 g); small for gestational age (SGA), i.e., birth weight below the 10th percentile for sex-specific gestational age; fetal death (after week 20); malformation; placental weight; birth weight; birth length; offspring sex; and Apgar score.
Statistical Analysis
Continuous data were presented as the mean (standard deviation) for normally distributed data and as median (first quartile, third quartile) for non-normally distributed data. Categorical data were presented as percentages. Descriptive statistics were analyzed between groups by the independent samples t-test and the Mann–Whitney U test (if data were not normally distributed). Pearson’s chi-square test was applied for categorical data with the Fisher’s exact test for expected frequencies <5.
We used propensity score matching (PSM) to eliminate possible confounding factors and selection bias with SPSS 22.0 (IBM Corp., Armonk, NY, USA) embedded with the PSM plug-in (IBM Corporation, Armonk, NY, USA). A 1:2 matching was performed on the propensity score (PS) with a maximum caliber of 0.05. The PS was calculated using multivariable logistic regression models. Matching variables are presented in Table 1. We adjusted the P-values and odds ratios for the matching variables and other possible confounders by unconditional logistic regression models. Pre-pregnancy hypertension and diabetes mellitus were not involved as neither was present in both groups after PSM.
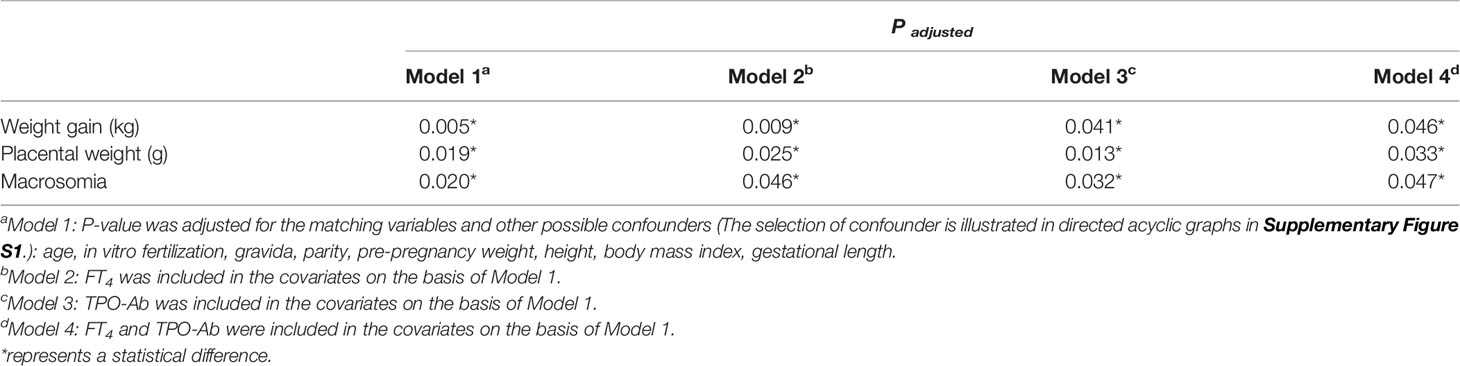
The selection of confounders is presented in directed acyclic graphs (Supplementary Figures S1A–C). Intermediate factors (fT4, TSH, TPO-Ab) were not adjusted to estimate the total effect of thyroid cancer (Model 1). FT4 and TPO-Ab were then included based on Model 1 to explore their effects (Table 4). All data were analyzed with SPSS 22.0. A two-tailed P-value <0.050 was considered to indicate statistically significant differences.
Subgroup analysis of treatment modality was performed between survivors and the corresponding PS-matched controls using regression analysis. Chi-square analysis and ANOVA analysis were performed to compare the pregnancy outcomes among the survivors with different treatment modalities.
In order to explore the impact of the time interval between conception and thyroidectomy, the survivors were trisected as Group T1, Group T2, Group T3. Logistic regression was used to calculate the P-trend across groups.
The study of time interval between conception and RAIT was not allowed due to the small sample size (n=33).
Results
Participants
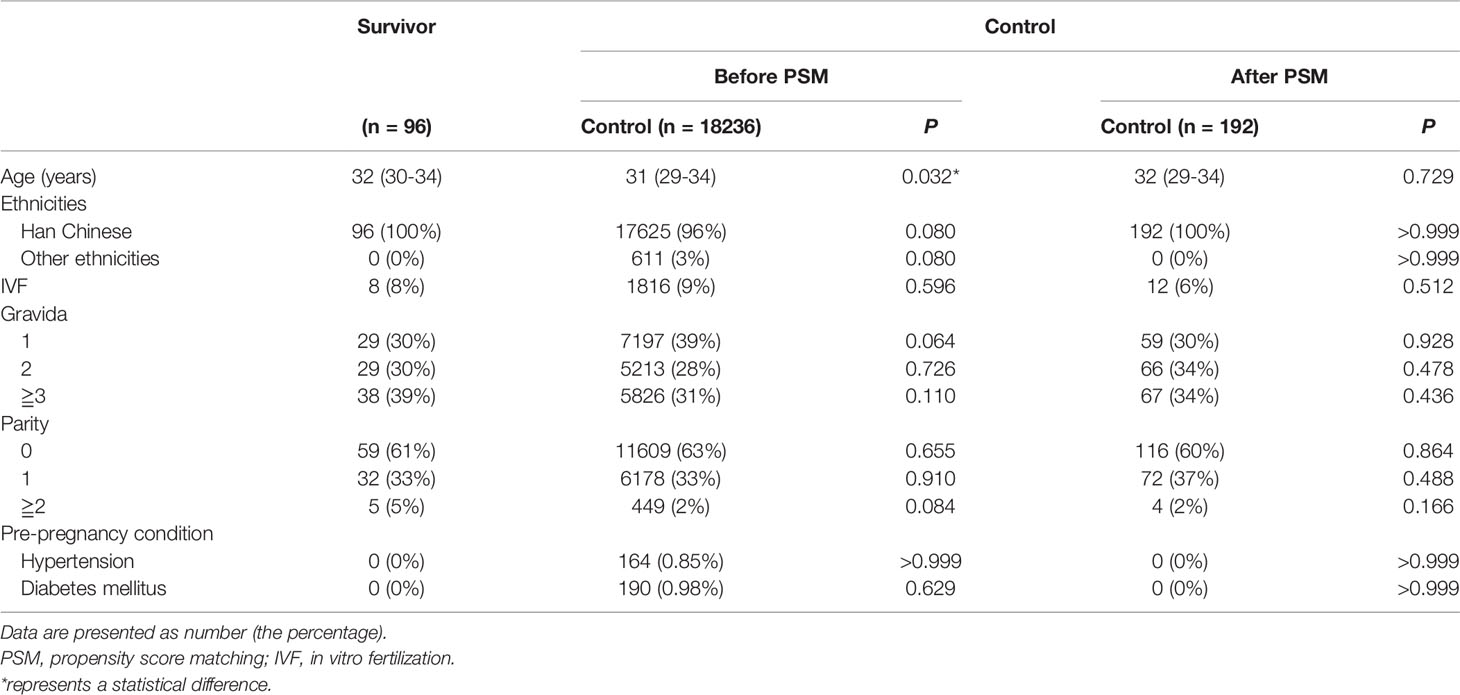
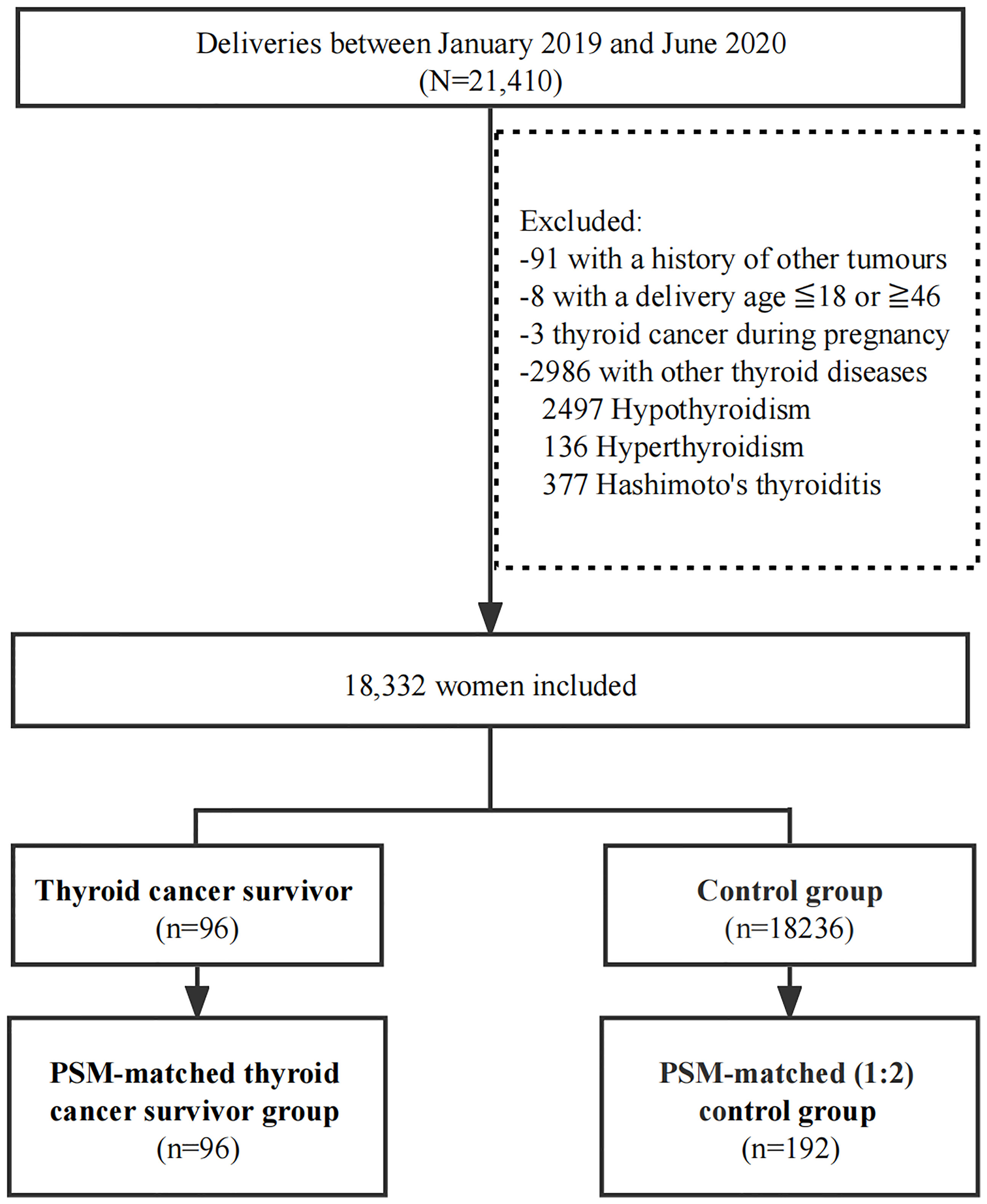
Between January 2019 and June 2020, 21,410 women gave birth in West China Second University Hospital of Sichuan University. Ninety-nine women had a history of thyroid cancer, but three of them were excluded for being diagnosed while pregnant. All 18,332 women who met the inclusion criteria were included in the study (96 in the thyroid cancer survivor group and 18,236 in the controls according to the subgroup criteria) (Figure 1).
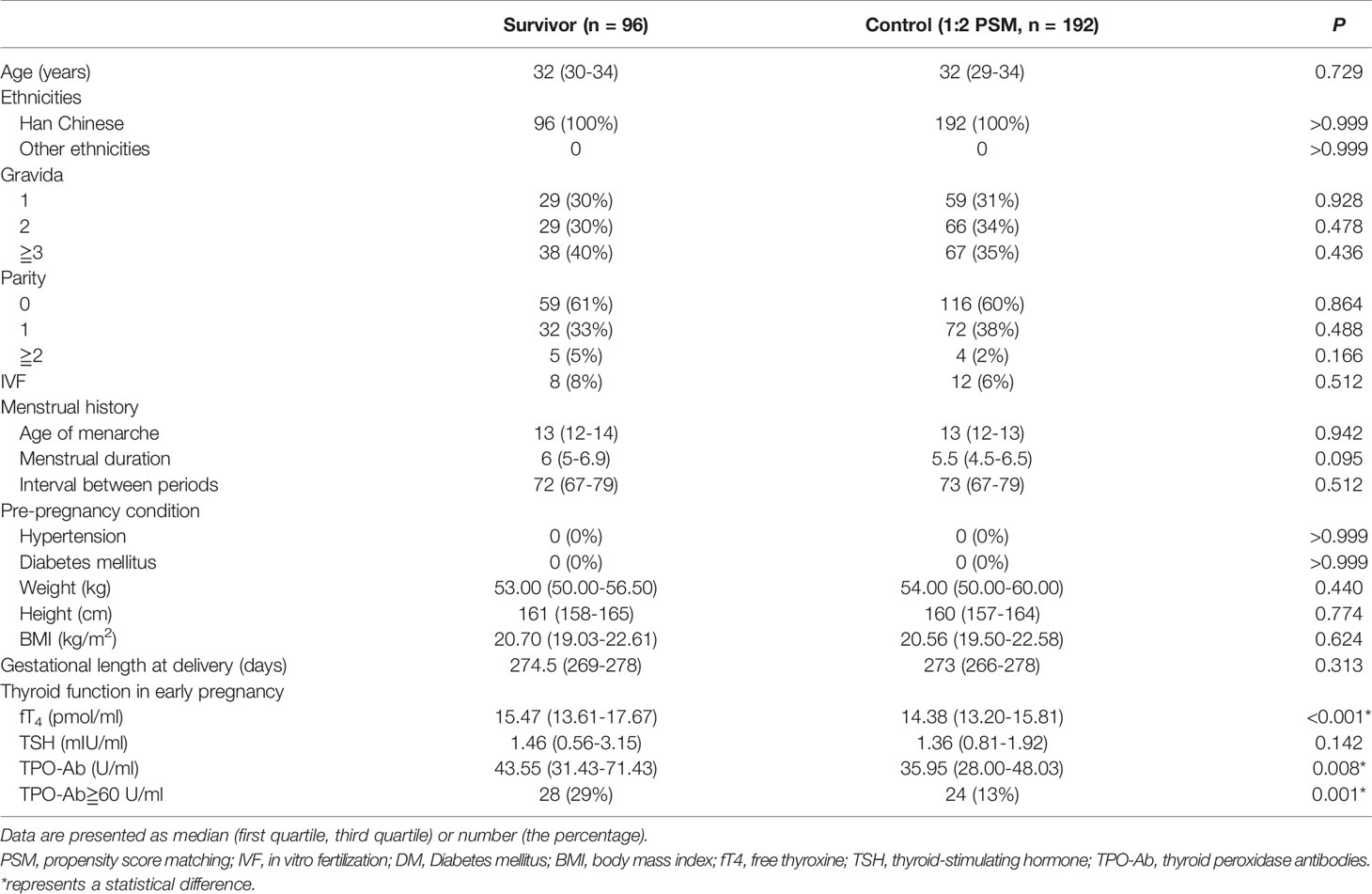
A 1:2 PSM was performed to obtain matched controls (n=192). The baseline characteristics between groups were comparable after PSM (Tables 1, 2).
All 96 survivors were pathologically diagnosed with papillary thyroid cancer. Of these 96 women, 25% (24/96) received hemithyroidectomy, 41% (39/96) received total-thyroidectomy, and 33% (33/96) received total-thyroidectomy combined RAIT (Table S1).
There were statistical intergroup differences in fT4 and TPO-Ab. The survivors had higher levels of fT4 (15.47 [13.61–17.67] vs. 14.38 [13.20–15.81] pmol/mL; P<0.001) and TPO-Ab (43.55 [31.43–71.43] vs. 35.95 (28.00–48.03) U/mL, P=0.008] (Table 2). The incidence of abnormal TPO-Ab (≧60 U/mL) was also higher in the survivors (P<0.001) at 29% (28/96) vs. 13% (24/192). However, in both groups, the mean values of TPO-Ab were still within the reference range. No significant difference was found in TSH (1.46 [0.56–3.15] vs. 1.36 [0.81–1.92] mIU/mL, P=0.142].
Maternal Outcomes
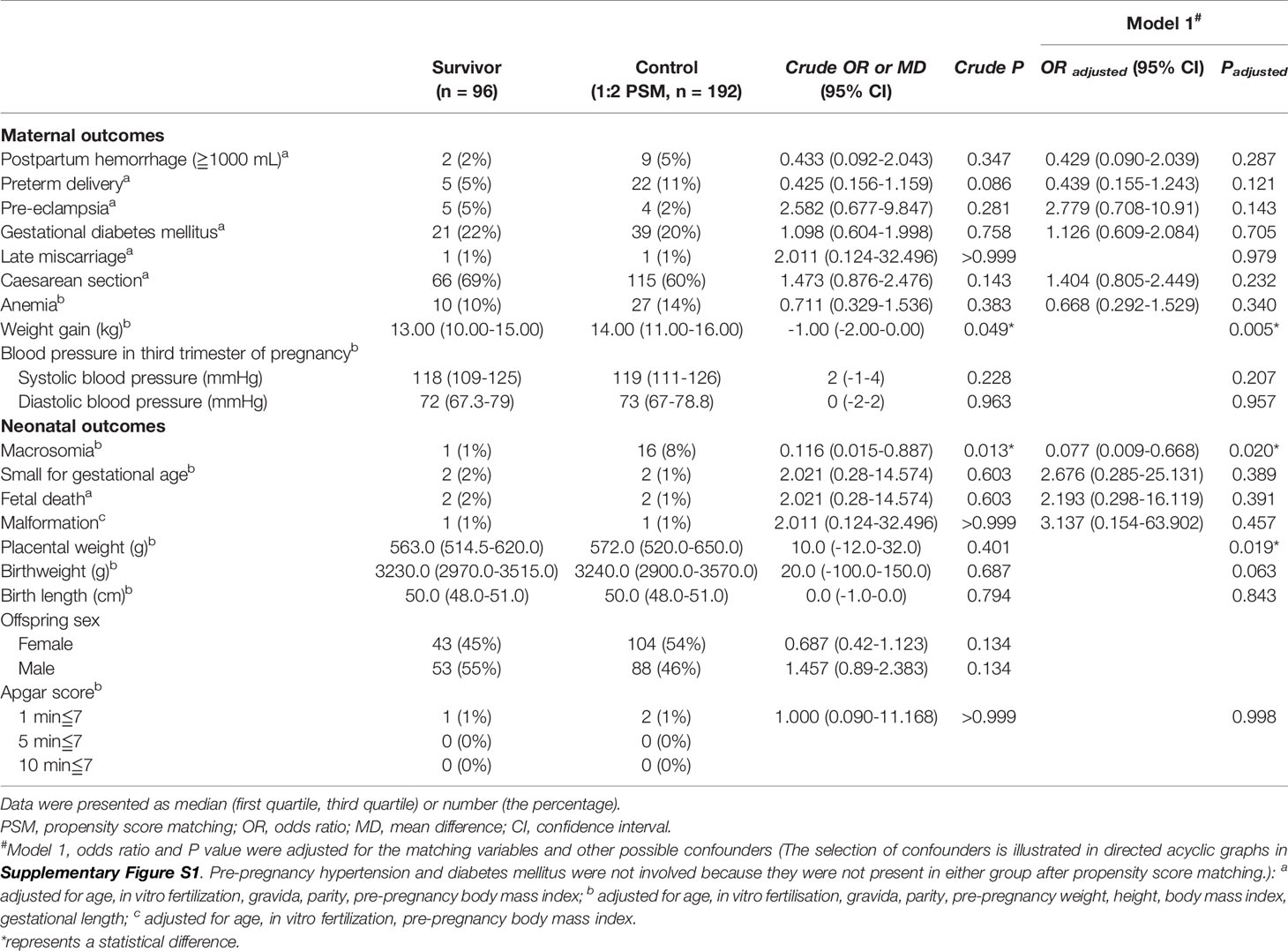
After PSM and the adjustment of potential confounders, no differences were found in the incidence of PPH (OR=0.429, 95%CI=0.090–2.039, P=0.287); preterm delivery (OR=0.439, 95%CI=0.155–1.243, P=0.121); pre-eclampsia (OR=2.779, 95%CI=0.708–10.91, P=0.143); and GDM (OR=1.126, 95%CI=0.609–2.084, P=0.705) (Table 3). The weight gain during pregnancy of thyroid cancer survivors was 1.0 kg lesser than that of the controls (13.00 [10.00–15.00] vs. 14.00 [11.00–16.00] g, P=0.005).
Neonatal Outcomes
For neonatal outcomes, placental weight was 9 g lighter in survivors than in controls (563.0 [514.5–620.0] vs. 572.0 [520.0–650.0] g, P=0.019] (Table 3). We also found a lower risk of macrosomia among survivors (OR=0.077, 95%CI=0.009–0.668, P=0.020) (Table 3). After further adjustment of fT4 and TPO-Ab (Model 2, Model 3, and Model 4), the differences were still significant (Table 4).
Subgroup Analysis and Comparison of Different Treatment Modalities
No statistical differences in pregnancy outcomes were found in subgroup analysis between survivors receiving specific treatment modality and the corresponding PS-matched controls (Table 5). The comparison of the three treatment modalities found no differences in pregnancy outcomes as well (Table 5).
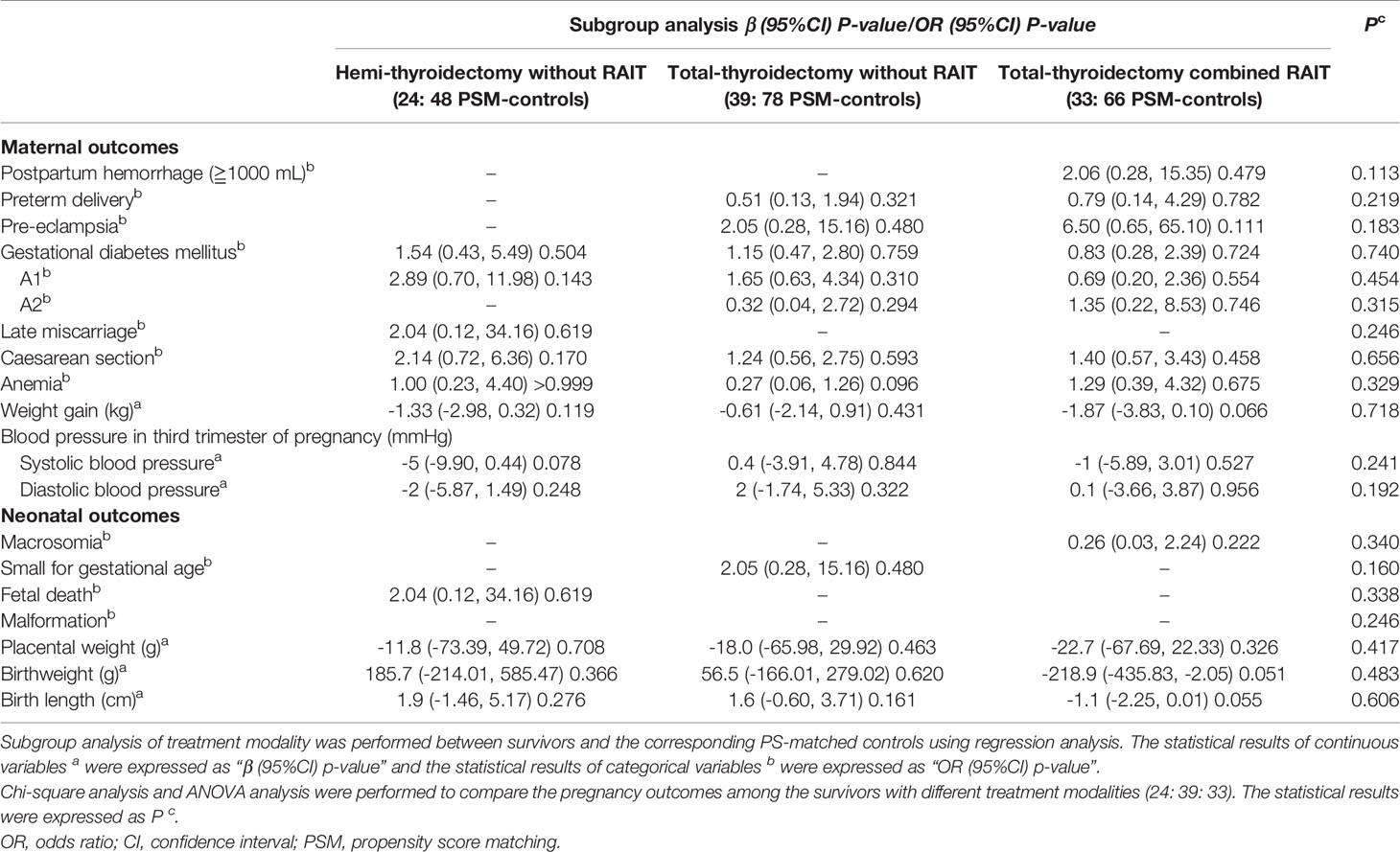
Table 5 Subgroup analysis and comparison of different treatments modality among thyroid cancer survivors.
Impact of the Time Interval between Conception and Thyroidectomy
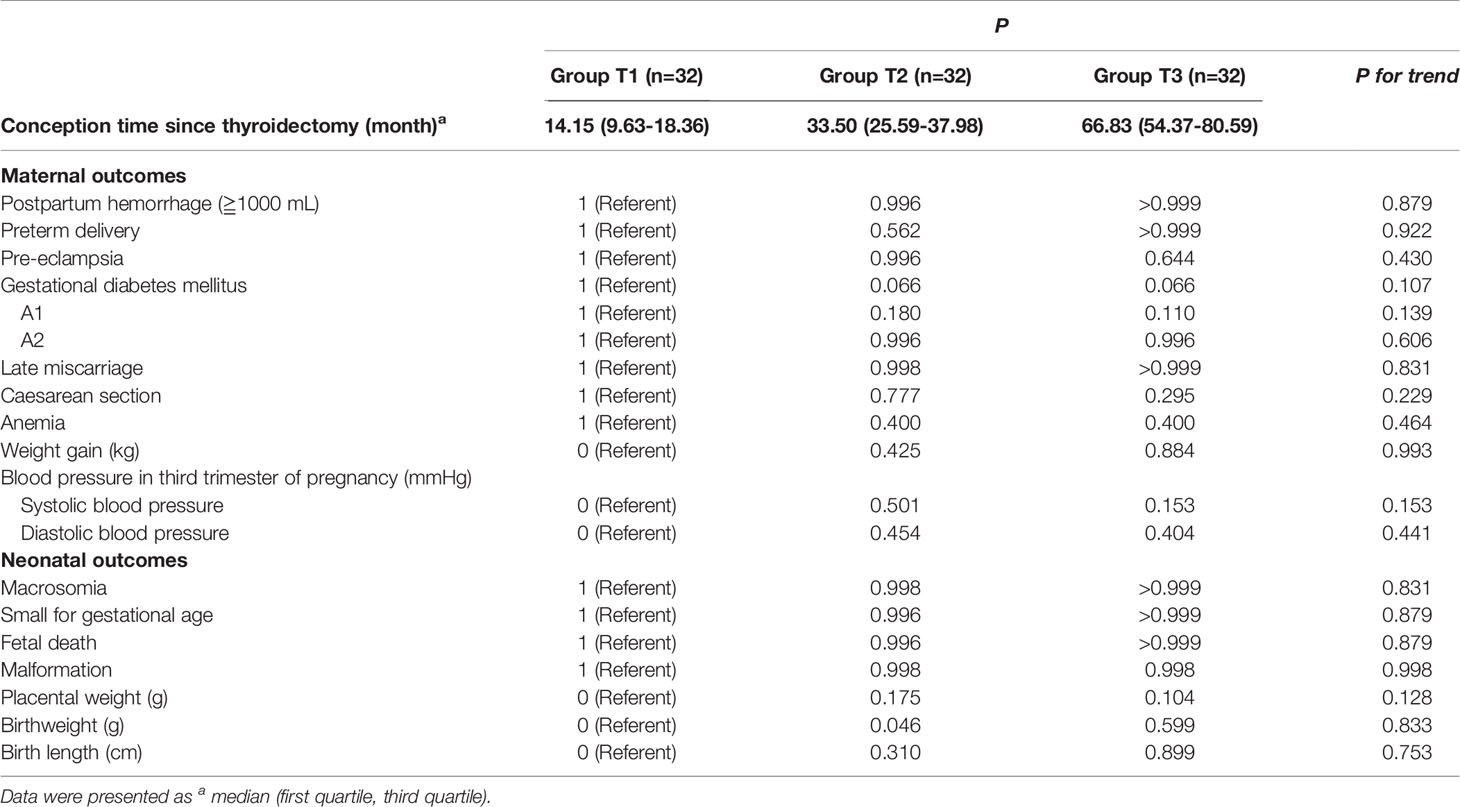
The survivors were trisected according to the time interval between conception and thyroidectomy: Group T1 (n =32, 14.15 [9.63-18.36] months), Group T2 (n =32, 33.50 [25.59-37.98] months), Group T3 (n =32, 66.83 [54.37-80.59] months). Compared with Group T1, no differences in pregnancy outcomes were found in Group T2 and T3. There were no statistically significant change trends in the trend test (Table 6).
Discussion
In this PS-matched cohort study, only reduced weight gain, decreased placental weight, and a lower risk of macrosomia were found in the survivors. Overall, no significant increased adverse pregnancy outcomes were detected in women with treated papillary thyroid cancer.
A higher incidence of pre-pregnancy hypertension and diabetes mellitus had previously been found in thyroid cancer survivors by two national database studies (13, 14). Nonetheless, the mean age of the survivors was much higher than that of the controls in those two studies. In our study, the survivors were also older than the controls before PSM (P=0.032). However, the baseline characteristics including the incidence of pre-pregnancy hypertension and pre-pregnancy diabetes mellitus were consistent between the two groups after PSM.
No significant difference was found in TSH, and the TSH of most survivors was not low. Therefore, the effects of TSH suppressive therapy on pregnancy outcomes could not be assessed in the present study. TPO-Ab levels and the incidence of abnormal TPO-Ab (≧60 U/mL) were higher in survivors (the mean values of TPO-Ab were still within the reference range). One possible explanation is that the release of thyroid antigens may occur during thyroidectomy and induce thyroid autoantibodies.21 Higher levels of fT4 were found in the survivors. Although there was a statistical significance (P<0.001), the difference was small (1 pmol/mL). In addition, we should interpret the results with caution, because the effect of diurnal variation (since patients’ blood samples were generally drawn between 0800 and 1130 hours) and CV (<4% for both intra- and inter-assay CV) could not be determined.
According to the results, there were no significant differences between women with a history of thyroid cancer and controls in adverse pregnancy outcomes, including pre-eclampsia, GDM, preterm delivery, and late miscarriage. These findings are consistent with two national database-based studies (13, 14) and three smaller-sized studies (15–17). The Korean study by Cho et al. (13) found a higher risk of PPH in the survivors, but no such difference was noted in our study. Different diagnostic standards, regions, and healthcare quality may have contributed to unmeasured confounding factors in the database-based studies, especially when based on ICD-10 diagnosis codes, which is mainly used for insurance and reimbursement purposes. In addition, postpartum blood loss is usually subjectively estimated and inaccurate. Consistent with our results, a recent study more accurately evaluated PPH with original records of blood loss amount and found no difference between thyroid cancer survivors and controls (16). It has been reported that RAIT is associated with the decrease of hemoglobin that is thought to be one of the risk factors for PPH. However, our result showed no difference in anemia risk between the two groups. Given that the interval between RAIT and conception is rather long in our study (34.70 [19.15–59.93] months), it might already have little effect on hemoglobin and PPH.
Interestingly, reduced weight gain, decreased placental weight, and a lower risk of macrosomia were found in the survivors. Initially, we thought that the changes in metabolic status caused by thyroid cancer treatment and TSH suppressive therapy altered the body’s metabolic status and reduced maternal, infant, and placental weight gain. However, TPO-Ab may also have played a role, as recent studies have shown that placental and fetal birth weights were lower in TPO-Ab-positive subjects (18–20). Therefore, we adjusted for fT4 and TPO-Ab separately. However, the differences were still significant after adjustment. This suggests the role of factors other than thyroid function. Other studies have confirmed that thyroid cancer survivors experienced greater psychological stress during pregnancy (21, 22). Moreover, a prospective cohort including 1,308 women found that stress and anxiety were associated with lower gestational weight gain (23). Therefore, the mental health status of thyroid cancer survivors may account for the results of our study but requires further research.
The survivors had reduced gestational weight gain, but it was still within the recommended range by the American Institute of Medicine (24). Although it has been reported that gestational weight gain may be related to the risk of pregnancy complications (25, 26), no increased incidence of major adverse pregnancy outcomes were detected among the survivors in our study.
The placental weight difference (9 g) was small, although statistically different. Moreover, since there was no statistically significant difference in birthweight or birthweight/placental weight (BW/PW, the ratio of fetal birth weight to placental weight, which indicates how successfully the placenta has adapted to the growing needs of the fetus [data not shown]), we believe that these carry little clinical significance.
Subgroup analysis showed no differences in pregnancy outcomes between survivors and the corresponding PS-matched controls. However, the significant differences in gestational weight gain, placental weight and the incidence of macrosomia between the survivors and the controls no longer exist, which may be caused by the reduced sample size. In terms of RAIT, the number of included women was small but is consistent with a large-scale real-world cohort study, thereby indicating that RAIT did not appear to be associated with increased adverse pregnancy outcomes (27).
Our study on time interval between conception and thyroidectomy found no significant differences or change trends among trisected time interval groups in pregnancy outcomes. However, due to the relatively large overall time interval and small sample size, this requires more large-scale studies to confirm.
Strength and Limitation
Our study has some limitations. First, compared with studies based on national databases, the sample size was small, which limited the ability to detect minor differences. Second, women in our cohort were mainly from a tertiary referral hospital, limiting the generalizability of results. Third, all included survivors had papillary thyroid cancer and are therefore not representative of other pathological types. Last, thyroid function was not checked after 10–14 weeks of pregnancy.
The strengths of our study are as follows: First, confounders between the two groups were controlled as much as possible using PSM and logistic regression. Second, based on the original EMR, our data are more reliable than that from national databases based on ICD diagnosis codes alone. Third and perhaps the greatest strength of our study was that we provided detailed information on the pathological type, treatment, and gestational thyroid function of each thyroid cancer survivor. We believe that this study contributes to an improved understanding of the impact of treated thyroid cancer on pregnancy outcomes.
Conclusion
In this retrospective cohort study involving 96 thyroid cancer survivors and 192 PS-matched control women, a history of papillary thyroid cancer was not associated with adverse pregnancy outcomes. These findings provide reassurance for women of childbearing age with a history of treated papillary thyroid cancer.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of West China Second University Hospital of Sichuan University (No.2021098). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
QC and WH made substantial contributions to the study’s conception and design, acquisition of data, and analysis. QC, HZ, and JZ made significant contributions to the interpretation of data and drafting of the manuscript. JZ made contributions to the acquisition of data. HZ and YL were involved in revising it critically for important intellectual content. All the authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agree to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.816132/full#supplementary-material
Supplementary Figure S1 | (A–C). Directed acyclic graph illustrating confounder selection. IVF, in vitro fertilization; BMI, body mass index; fT4, free thyroxine; TSH, thyroid-stimulating hormone; TPO-Ab, thyroid peroxidase antibodies; GDM, gestational diabetes mellitus; PPH, postpartum hemorrhage.
References
1. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
2. Kitahara CM, Sosa JA. The Changing Incidence of Thyroid Cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
3. Park S, Oh C-M, Cho H, Lee JY, Jung K-W, Jun JK, et al. Association Between Screening and the Thyroid Cancer “Epidemic” in South Korea: Evidence From a Nationwide Study. BMJ (Clinical Res Ed) (2016) 355:i5745. doi: 10.1136/bmj.i5745
4. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw (2018) 16(12):1429–40. doi: 10.6004/jnccn.2018.0089
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
6. Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJG, et al. Guidelines for Radioiodine Therapy of Differentiated Thyroid Cancer. Eur J Nucl Med Mol Imaging (2008) 35(10):1941–59. doi: 10.1007/s00259-008-0883-1
7. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid (2017) 27(3):315–89. doi: 10.1089/thy.2016.0457
8. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and Magnitude of Increases in Levothyroxine Requirements During Pregnancy in Women With Hypothyroidism. N Engl J Med (2004) 351(3):241–9. doi: 10.1056/NEJMoa040079
9. Mannisto T, Mendola P, Grewal J, Xie Y, Chen Z, Laughon SK. Thyroid Diseases and Adverse Pregnancy Outcomes in a Contemporary US Cohort. J Clin Endocrinol Metab (2013) 98(7):2725–33. doi: 10.1210/jc.2012-4233
10. Medici M, Korevaar TI, Schalekamp-Timmermans S, Gaillard R, de Rijke YB, Visser WE, et al. Maternal Early-Pregnancy Thyroid Function is Associated With Subsequent Hypertensive Disorders of Pregnancy: The Generation R Study. J Clin Endocrinol Metab (2014) 99(12):E2591–8. doi: 10.1210/jc.2014-1505
11. Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, et al. Hypothyroxinemia and TPO-Antibody Positivity Are Risk Factors for Premature Delivery: The Generation R Study. J Clin Endocrinol Metab (2013) 98(11):4382–90. doi: 10.1210/jc.2013-2855
12. Ceccarelli C, Bencivelli W, Morciano D, Pinchera A, Pacini F. 131I Therapy for Differentiated Thyroid Cancer Leads to an Earlier Onset of Menopause: Results of a Retrospective Study. J Clin Endocrinol Metab (2001) 86(8):3512–5. doi: 10.1210/jcem.86.8.7719
13. Cho GJ, Kim SY, Lee HC, Lee KM, Han SW, Oh MJ, et al. Risk of Adverse Obstetric Outcomes and the Abnormal Growth of Offspring in Women With a History of Thyroid Cancer. Thyroid (2019) 29(6):879–85. doi: 10.1089/thy.2018.0283
14. Spiegel E, Spence AR, Czuzoj-Shulman N, Abenhaim HA. Pregnancy Outcomes After Thyroid Cancer. J Perinat Med (2019) 47(7):710–6. doi: 10.1515/jpm-2019-0039
15. Boucek J, de Haan J, Halaska MJ, Plzak J, Van Calsteren K, de Groot CJM, et al. Maternal and Obstetrical Outcome in 35 Cases of Well-Differentiated Thyroid Carcinoma During Pregnancy. Laryngoscope (2018) 128(6):1493–500. doi: 10.1002/lary.26936
16. Liu D, Wei Y, Zhao Y, Li R, Yan J, Qiao J. Obstetric Outcomes in Thyroid Cancer Survivors: A Retrospective Cohort Study. Int J Gynaecol Obstet (2020) 16:119–24. doi: 10.1002/ijgo.13571
17. Clark H, Kurinczuk JJ, Lee AJ, Bhattacharya S. Obstetric Outcomes in Cancer Survivors. Obstet Gynecol (2007) 110(4):849–54. doi: 10.1097/01.AOG.0000284458.53303.1c
18. Ushijima J, Furukawa S, Sameshima H. The Presence of Thyroid Peroxidase Antibody Is Associated With Lower Placental Weight in Maternal Thyroid Dysfunction. Tohoku J Exp Med (2019) 249(3):231–6. doi: 10.1620/tjem.249.231
19. Chen L-M, Zhang Q, Si G-X, Chen Q-S, Ye E-l, Yu L-C, et al. Associations Between Thyroid Autoantibody Status and Abnormal Pregnancy Outcomes in Euthyroid Women. Endocrine (2015) 48(3):924–8. doi: 10.1007/s12020-014-0420-x
20. Plowden TC, Schisterman EF, Sjaarda LA, Perkins NJ, Silver R, Radin R, et al. Influence of Positive Serum Thyroid Peroxidase Antibody on Pregnancy Outcomes. Am J Obstet Gynecol (2017) 217(6):697.e1–e7. doi: 10.1016/j.ajog.2017.09.001
21. Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having Children After Cancer. A Pilot Survey of Survivors' Attitudes and Experiences. Cancer (1999) 86(4):697–709. doi: 10.1002/(SICI)1097-0142(19990815)86:4<697::AID-CNCR20>3.0.CO;2-J
22. Bresner L, Banach R, Rodin G, Thabane L, Ezzat S, Sawka AM. Cancer-Related Worry in Canadian Thyroid Cancer Survivors. J Clin Endocrinol Metab (2015) 100(3):977–85. doi: 10.1210/jc.2014-3169
23. Harvey MW, Braun B, Ertel KA, Pekow PS, Markenson G, Chasan-Taber L. Stress and Anxiety are Associated With Lower Gestational Weight Gain in Hispanic Women. Womens Health Issues (2020) 30(6):409–15. doi: 10.1016/j.whi.2020.08.003
24. Medicine IO. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (2009).
25. Mamun AA, Mannan M, Doi SAR. Gestational Weight Gain in Relation to Offspring Obesity Over the Life Course: A Systematic Review and Bias-Adjusted Meta-Analysis. Obes Rev (2014) 15(4):338–47. doi: 10.1111/obr.12132
26. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-Analysis. JAMA (2017) 317(21):2207–25. doi: 10.1001/jama.2017.3635
Keywords: thyroid cancer, thyroidectomy, radioiodine therapy, gestational thyroid function, pregnancy outcomes
Citation: Cao Q, Zhu H, Zhang J, Li Y and Huang W (2022) Pregnancy Outcomes in Thyroid Cancer Survivors: A Propensity Score-Matched Cohort Study. Front. Endocrinol. 13:816132. doi: 10.3389/fendo.2022.816132
Received: 16 November 2021; Accepted: 21 January 2022;
Published: 17 February 2022.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Stephanie Behringer-Massera, Icahn School of Medicine at Mount Sinai, United StatesLaura Sterian Ward, State University of Campinas, Brazil
Copyright © 2022 Cao, Zhu, Zhang, Li and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, d2VpaHVhbmc2NEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Qi Cao
Qi Cao Huili Zhu1,2†
Huili Zhu1,2† Jiani Zhang
Jiani Zhang Yujing Li
Yujing Li